Abstract
OBJECTIVE
Due to the increased lifetime risk of endometrial cancer (EC), guidelines recommend that women with Lynch syndrome (LS) age ≥35 undergo annual EC surveillance or prophylactic hysterectomy (PH). The aim of this study was to examine the uptake of these risk-reducing strategies.
METHODS
The study population included women meeting clinical criteria for genetic evaluation for LS. Data on cancer risk-reducing behaviors were collected from subjects enrolled in two distinct studies: (1) a multicenter cross-sectional study involving completion of a one-time questionnaire, or (2) a single-center longitudinal study in which subjects completed questionnaires before and after undergoing genetic testing. The main outcome was uptake of EC risk-reducing practices.
RESULTS
In the cross-sectional cohort, 58/77 (75%) women at risk for LS-associated EC reported engaging in EC risk-reduction. Personal history of genetic testing was associated with uptake of EC surveillance or PH (OR 17.1; 95% CI 4.1–70.9). Prior to genetic testing for LS, 26/40 (65%) women in the longitudinal cohort reported engaging in EC risk-reduction. At one-year follow-up, 16/16 (100%) mismatch repair (MMR) gene mutation carriers were adherent to guidelines for EC risk-reduction, 9 (56%) of whom had undergone PH. By three-year follow-up, 11/16 (69%) MMR mutation carriers had undergone PH. Among women with negative or uninformative genetic test results, none underwent PH after testing.
CONCLUSIONS
Genetic testing for LS is strongly associated with uptake of EC risk-reducing practices. Women found to have LS in this study underwent prophylactic gynecologic surgery at rates comparable to those published for BRCA1/2 mutation carriers.
INTRODUCTION
Lynch syndrome (LS), also known as hereditary nonpolyposis colorectal cancer (HNPCC), is a highly-penetrant genetic condition associated with an increased risk of gastrointestinal, gynecologic, and other malignancies.[1–6] Individuals with LS carry a germline mutation in one of the DNA mismatch repair (MMR) genes (hMLH1, hMSH2, hMSH6, hPMS2, and TACSTD1).[7–12] After colorectal carcinoma (CRC), endometrial carcinoma (EC) is the second most common LS-associated malignancy among female mutation carriers, and 2% of all EC diagnoses are attributable to LS.[1–3, 13–15] Prior studies have estimated that women with LS have a 16–71% lifetime risk of EC with a median age of diagnosis below 50 years.[1, 2, 12–15]
Published guidelines recommend that women with LS undergo annual EC surveillance with endometrial biopsy and/or transvaginal ultrasound (TVUS) starting at age 30–35, and/or consider prophylactic hysterectomy (PH) after the completion of childbearing.[16–18] Although prior studies have suggested that women with LS underestimate their risk for extracolonic cancers compared to CRC,[19] there are few data regarding the uptake of EC risk-reducing strategies. The aim of our study was to examine factors associated with uptake of EC risk-reduction practices among women at risk for LS-associated EC.
METHODS
We conducted a survey of health behaviors in two distinct cohorts of patients:
Cohort 1 – Multicenter cross-sectional cohort
Individuals with a personal or family history of cancer fulfilling Bethesda guidelines[20] for evaluation of LS were identified through cancer genetics clinics at four different sites (Dana-Farber Cancer Institute, Boston, MA; Massachusetts General Hospital Cancer Center, Boston, MA; University of California San Francisco, San Francisco, CA; and University of Michigan, Ann Arbor, MI) between 2002–2006. Potential subjects included individuals who personally visited one of the clinics or who were referred to the study by a family member who had been seen at one of the clinics. Participants were limited to those ≥18 years of age who could read and write in English. Subjects completed a onetime written self-administered questionnaire, which collected standard demographic information as well as data regarding their medical history, family history, cancer surveillance practices, and personal history of genetic evaluation.[21] All subjects provided informed consent and the study was approved by the Institutional Review Board at all participating sites.
Cohort 2 – Single-center longitudinal cohort
Individuals referred for genetic testing for LS at a single center (Dana-Farber Cancer Institute, Boston, MA) were invited to enroll in a prospective longitudinal study between 2003–2009. Eligible subjects included individuals ≥18 years of age who met clinical criteria for evaluation for LS and chose to proceed with genetic testing. Participants completed self-administered questionnaires prior to genetic testing, and at one-year intervals for up to three years after the disclosure of test results. All individuals who underwent genetic testing met with a physician and genetic counselor before testing and received additional counseling following the disclosure of test results. For subjects whose testing confirmed a diagnosis of LS, post-test counseling included discussion about the risks of LS-associated malignancies, recommendations about cancer surveillance guidelines, and discussion about potential surgical options to reduce cancer risks. All subjects provided informed consent and the study was approved by the Dana-Farber Cancer Institute’s Institutional Review Board.
In addition to standard demographic information, questionnaires collected data regarding participants’ medical history, use of specific cancer screening practices (including colonoscopy, TVUS, and endometrial biopsy), and prior surgical history. Subjects reporting a prior hysterectomy and/or salpingo-oophorectomy (BSO) were asked whether these had been performed “for cancer prevention,” “for cancer treatment,” or “for reasons other than cancer treatment or prevention.” Annual follow-up questionnaires asked if participants had undergone any screening tests and/or surgeries in the preceding 12 months. At baseline, participants provided a detailed family history, including specific cancer diagnoses among first-, second-, and third-degree relatives. Subjects were asked whether any family member had previously had genetic testing which identified a pathogenic MMR gene mutation.
Study population
Subjects in both cohorts were considered to be at risk for LS if their family history (1) fulfilled Amsterdam I or II criteria[22, 23] or (2) included a known, pathogenic MMR gene mutation. Cohort 1 participants with a personal history of prior genetic testing confirming that they did not carry the family’s known MMR gene mutation (“true negative” test results) were excluded; individuals in Cohort 2, by definition, had not had prior genetic testing for LS. For the purposes of examining risk-reducing behaviors among women at risk for LS-associated EC, subjects were excluded if they had previously been diagnosed with EC and/or reported having a prior hysterectomy for reasons other than cancer prevention.
Outcomes
For both cohorts, the primary outcome measure of uptake of EC risk-reduction was defined as having had at least one of the following: (1) a prophylactic hysterectomy (“for cancer prevention”), (2) annual surveillance endometrial biopsy, or (3) annual TVUS. Women age ≥35 who did not undergo at least one of these procedures were classified as not engaging in EC risk-reduction. Since published guidelines[16–18] recommend that women with LS initiate EC surveillance between ages 30–35, all women age <35 in our cohorts were classified as engaging in EC risk-reduction. Since all subjects were considered to be at risk for having LS, adequate CRC surveillance was defined as undergoing colonoscopy at least every 2 years for individuals age >25.
Statistical analysis
Potential relationships between clinical and demographic factors and uptake of EC risk-reduction were examined using univariate tests of association (Fisher’s exact and t-tests). Age was studied as both a continuous and categorical (<40, 40–49, and ≥50 years old) variable. Factors found to be statistically significant on univariate analysis were considered for inclusion in multivariable logistic regression models in order to detect those with an independent association with uptake of EC risk-reduction. Generalized estimating equations were used to account for potential clustering of results among members of the same family. Analyses were performed using SAS Version 9.1 (Cary, NC) software. All p-values were two-sided and p-values <0.05 were considered statistically significant.
RESULTS
Cohort 1
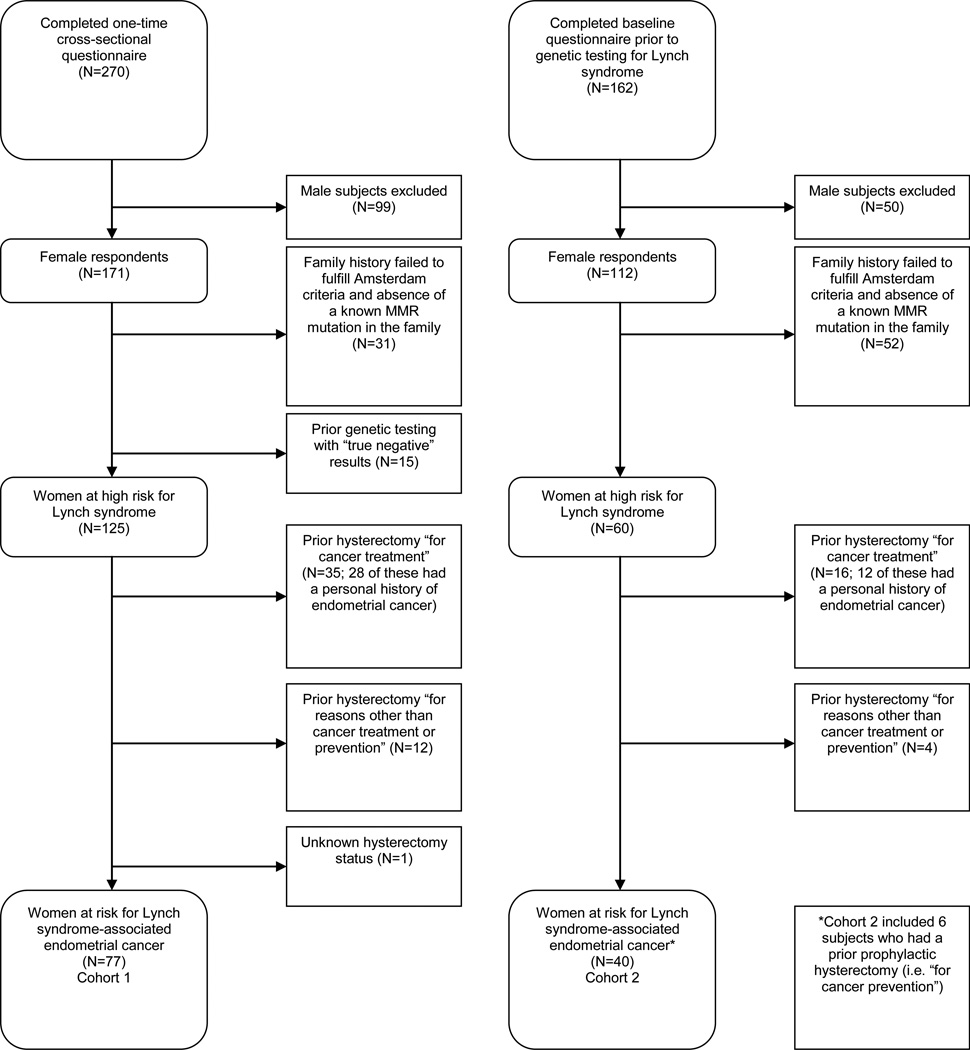
Two-hundred seventy (58%) of the 462 potentially eligible individuals who were invited to participate in the study due to a personal and/or family history fulfilling Bethesda guidelines[20] completed the one-time questionnaire.[21] Of these, 77/270 (29%) were at risk for LS-associated EC (Figure 1) and were included in the analysis. Subject demographics, personal history and health practices, family history, and prior genetic evaluation data are presented in Table 1.
Figure 1.
CONSORT diagram for Cohort 1 and Cohort 2.
Table 1.
Demographics of two distinct cohort of women at high risk for Lynch syndrome (LS) -associated endometrial cancer (EC)
| Variable | Cohort 1 (cross-sectional cohort) N=77 Number (column %) |
Cohort 2 at baseline (longitudinal cohort) N=40 Number (column %) |
|---|---|---|
| Demographics | ||
| Mean age (range) | 41.4 (20–70) | 43.6 (20–70) |
| <40 Years old | 35 (45) | 13 (33) |
| 40–49 Years old | 24 (31) | 18 (45) |
| ≥50 Years old | 17 (22) | 9 (23) |
| Unknown/missing | 1(1) | 0 (0) |
| Educational level | ||
| Less than college graduate | 23 (30) | 3 (8) |
| At least college graduate | 54 (70) | 26 (90) |
| Unknown/missing | 0 (0) | 1 (3) |
| Annual income | ||
| <$50,000 | 15 (19) | 5 (13) |
| >$50,000 | 57 (74) | 33 (83) |
| Unknown/missing | 5 (6) | 2 (5) |
| Marital status | ||
| Married | 58 (75) | 30 (75) |
| Not married | 18 (23) | 9 (23) |
| Unknown/missing | 1 (1) | 1 (3) |
| Health insurance | ||
| Yes | 76 (99) | 40 (100) |
| No | 1 (1) | 0 (0) |
|
Personal history and health practices |
||
| Personal cancer history | ||
| Have had any cancer | 30 (39) | 17 (43) |
| Have not had any cancer | 47 (61) | 22 (55) |
| Unknown/missing | 0 (0) | 1 (3) |
| Personal colorectal cancer (CRC) history |
||
| Have had CRC | 27 (35) | 9 (23) |
| Have not had CRC | 50 (65) | 31 (78) |
| Physician visits in the past year | ||
| 0 | 1 (1) | 1 (3) |
| 1–6 | 58 (75) | 26 (65) |
| ≥7 | 18 (23) | 11 (28) |
| Unknown/missing | 0 (0) | 2 (5) |
| Physician discussed cancer risk | ||
| Yes | 66 (86) | 33 (83) |
| No | 11 (14) | 7 (18) |
| CRC surveillance practicesa | ||
| Adequate CRC surveillancea | 55 (71) | 21 (53) |
| Inadequate CRC surveillancea | 22 (29) | 19 (48) |
| Family history | ||
| Fulfills Amsterdam Criteria | ||
| Yes | 65 (84) | 33 (83) |
| No | 12 (16) | 7 (18) |
| Known MMR mutation in the family |
||
| Yes | 49 (64) | 19 (48) |
| No | 28 (36) | 21 (53) |
| Family history of any LS cancer | ||
| Yes | 73 (95) | 39 (98) |
| No | 4 (5) | 1 (3) |
| 1st/2nd degree relative with EC | ||
| Yes | 33 (43) | 16 (40) |
| No | 44 (57) | 24 (60) |
| Genetic evaluation | ||
| Visited high-risk clinic | ||
| Yes | 64 (83) | 40 (100) |
| No | 13 (17) | 0 (0) |
| Genetic testing status | ||
| Had genetic testing | 58 (75) | 0 (0) |
| Have not had genetic testing | 19 (25) | 40 (100) |
| Genetic test result | ||
| Positive | 45 (58) | 0 (0) |
| Indeterminate/variant | 13 (17) | 0 (0) |
| True negative | 0 (0) | 0 (0) |
| Have not had genetic testing | 19 (25) | 40 (100) |
“Adequate colorectal cancer surveillance” defined as having colonoscopy at least every 2 years or being age <25
Overall, 58/77 (75%) women at risk for LS-associated EC reported engaging in EC risk-reduction with 42 reporting annual EC surveillance with TVUS and/or endometrial biopsy, and 16 reporting having undergone PH. Twelve (75%) of the 16 women who had PH carried a pathogenic MMR gene mutation. Compared to those reporting annual EC surveillance, women who had undergone PH were more likely to have had a prior cancer diagnosis (non-EC) (p=0.02) and be compliant with CRC surveillance (p=0.03). Only one woman under age 40 had undergone PH.
Factors associated with uptake of EC risk-reduction on univariate analysis (Table 2) included having visited a cancer genetics/high-risk clinic (p=0.01), having undergone genetic testing (p<0.01), testing positive for a pathogenic MMR gene mutation (p=0.04), having a known pathogenic MMR gene mutation in the family (p=0.01), and uptake of LS-specific guidelines for colonoscopic surveillance (p=0.03). Subjects’ educational level, income, marital status, health insurance carriage, personal history of cancer, and family history of EC were not significantly associated with uptake of EC risk-reduction.
Table 2.
Factors associated with fulfillment of endometrial cancer (EC) risk-reducing guidelinesa among women at risk for Lynch syndrome (LS)-associated EC (Cohort 1 - cross-sectional cohort)
| Variable | Fulfilling guidelines a Number (row %) |
Not fulfilling guidelines a Number (row %) |
P valueb |
|---|---|---|---|
| Total cohort 1 (N=77) | 58 (75) | 19 (25) | |
| Demographics | |||
| Mean age (± SD) | 39.5 ± 11.1 | 47.3 ±9.9 | 0.01 |
| <40 Years old | 30 (86) | 5 (14) | 0.04 |
| 40–49 Years old | 18 (75) | 6 (25) | |
| ≥50 Years old | 9 (53) | 8 (47) | |
| Unknown/missing | 1(--) | 0(--) | |
| Educational level | |||
| Less than college graduate | 14 (61) | 9 (39) | 0.08 |
| At least college graduate | 44 (81) | 10 (19) | |
| Annual income | |||
| <$50,000 | 12 (80) | 3 (20) | 0.75 |
| ≥$50,000 | 42 (74) | 15 (26) | |
| Unknown/missing | 4(--) | 1(--) | |
| Marital status | |||
| Married | 46 (79) | 12 (21) | 0.13 |
| Not married | 11 (61) | 7 (39) | |
| Unknown/missing | 1 (--) | 0 (--) | |
|
Personal history and health practices |
|||
| Personal cancer history | |||
| Have had any cancer | 25 (83) | 5 (17) | 0.28 |
| Have not had any cancer | 33 (70) | 14 (30) | |
| Personal colorectal cancer (CRC) history |
|||
| Have had CRC | 22 (81) | 5 (19) | 0.42 |
| Have not had CRC | 36 (72) | 14 (28) | |
| Physician visits in the past year |
|||
| 0 | 1 (100) | 0 (0) | 0.82 |
| 1–6 | 44 (76) | 14 (24) | |
| ≥7 | 13 (72) | 5 (28) | |
| Physician discussed cancer risk |
|||
| Yes | 48 (73) | 18 (27) | 0.28 |
| No | 10 (91) | 1 (9) | |
| CRC surveillance practicesc |
|||
| Adequate CRC surveillancec | 47 (85) | 8 (15) | 0.03 |
| Inadequate CRC surveillancec | 11 (50) | 11 (50) | |
| Family history | |||
| Fulfills Amsterdam | |||
| Criteria | |||
| Yes | 48 (74) | 17 (26) | 0.72 |
| No | 10 (83) | 2 (17) | |
| Known MMR mutation in the family |
|||
| Yes | 42 (86) | 7 (14) | 0.01 |
| No | 16 (57) | 12 (43) | |
| 1st/2nd degree relative with EC |
|||
| Yes | 27 (82) | 6 (18) | 0.30 |
| No | 31 (70) | 13 (30) | |
| Genetic evaluation | |||
| Visited high-risk clinic | |||
| Yes | 52 (81) | 12 (19) | 0.01 |
| No | 6 (46) | 7 (54) | |
| Genetic testing status | |||
| Had genetic testing | 51 (88) | 7 (12) | <0.001 |
| Have not had genetic testing | 7 (37) | 12 (63) | |
| Genetic test result | |||
| Positive | 42 (93) | 3 (7) | 0.04 |
| Indeterminate/variant | 9 (69) | 4 (31) |
“Fulfilling guidelines” defined as either (1) having had a prophylactic hysterectomy, (2) reporting annual EC surveillance by transvaginal ultrasound and/or endometrial biopsy, and/or (3) age <35 years old
Fisher’s exact test was used for categorical values, and t-test was used for continuous variables
“Adequate colorectal cancer surveillance” defined as having colonoscopy at least every 2 years or being age <25
In our multivariable logistic regression, the final model was limited to two variables due to constraints imposed by the cohort’s size. We used “genetic testing status” (“have had genetic testing” versus “have not had genetic testing”) as a surrogate for other correlated variables that were found to have a significant association on univariate analysis (“genetic testing status”, “genetic test result”, “visit to a high-risk clinic”, and “known mutation in the family”) to avoid overfitting the model. Controlling for subject age, “genetic testing status” remained strongly associated with uptake of EC risk-reduction (OR 17.1, 95% CI 4.1–70.9; p<0.01). Age as a categorical variable (<40, 40–49, and ≥50 years old) was not significant on multivariable analysis (p=0.10).
Cohort 2
Of the 162 subjects who completed a baseline questionnaire prior to genetic testing for LS, 40 (25%) were at risk for LS-associated EC (Figure 1) and included in the analysis. Subject demographics, medical history and health practices, family history, and prior genetic evaluation data are presented in Table 1.
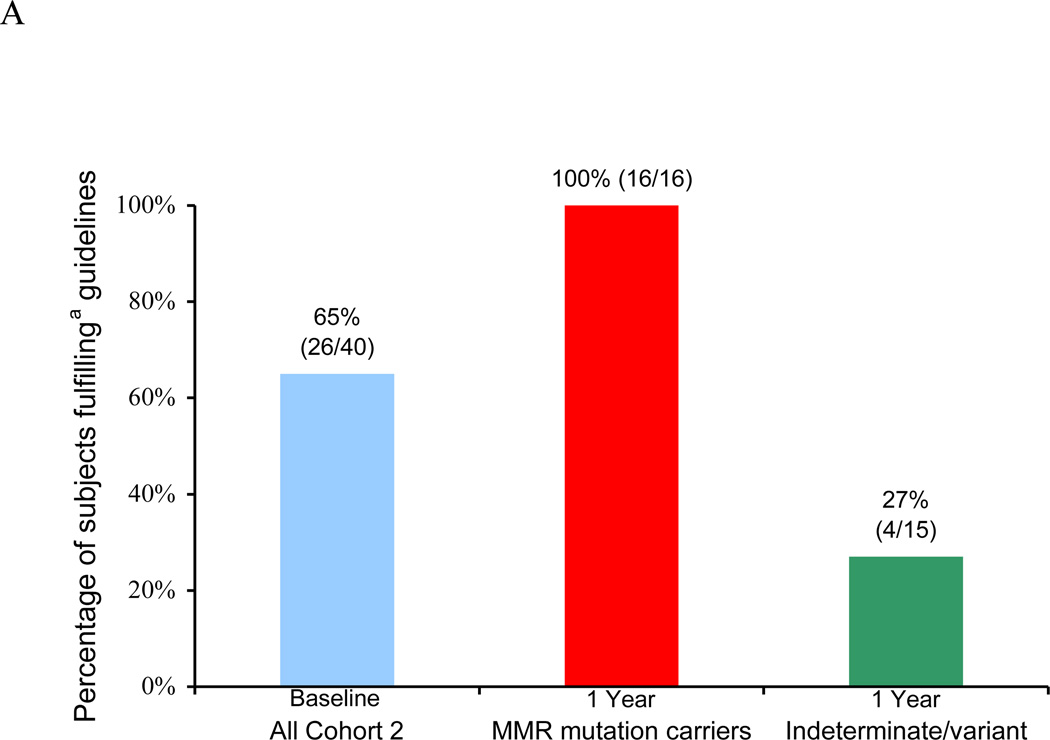
Of these 40 women, 26 (65%) engaged in EC risk-reduction prior to undergoing genetic testing for LS: 6 (15%) reported a prior PH, 13 (33%) were undergoing annual EC surveillance with TVUS and/or endometrial biopsy, and an additional 7 (18%) were age <35 and had not reached the age at which EC surveillance or prophylactic surgery would be recommended (Figure 2A).
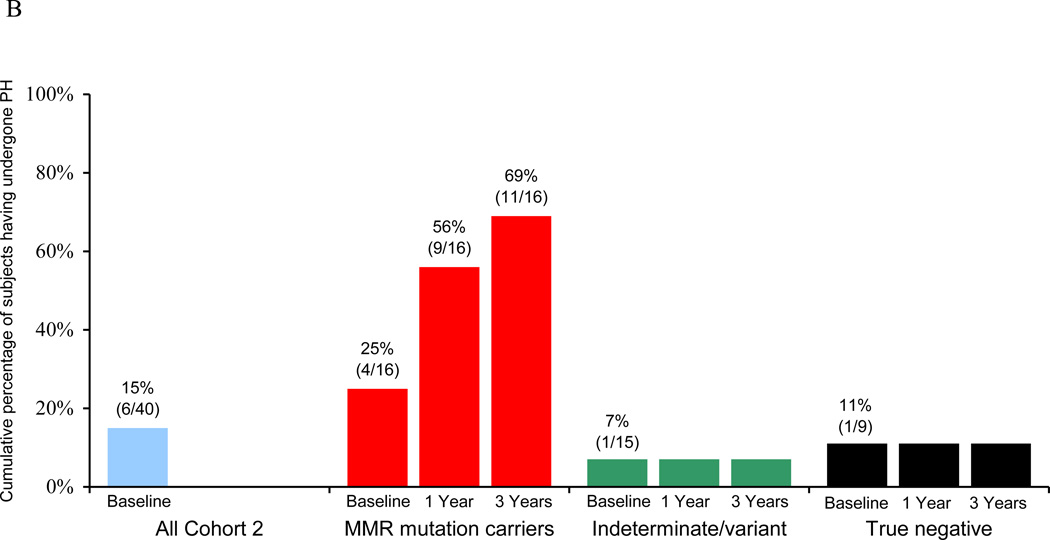
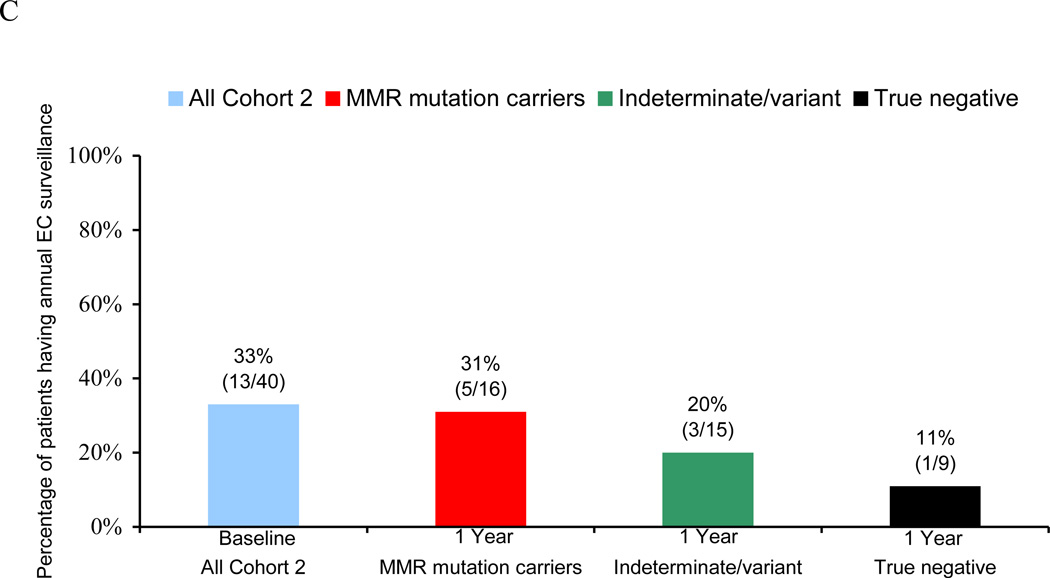
Figure 2.
Endometrial cancer (EC) risk-reducing practices in a longitudinal cohort of women before and after genetic testing for Lynch syndrome. (A) Overall percentage of subjects engaging in EC risk-reductiona at baseline and one yearb after genetic testing. (B) Cumulative rates of prophylactic hysterectomy (PH) at baseline and at one- and three-year follow-up. (C) Rates of annual EC surveillance at baseline and at one-year follow-up.
a “Engaging in EC risk-reduction” defined as either (1) having had a PH, (2) reporting annual EC surveillance by transvaginal ultrasound and/or endometrial biopsy, and/or (3) age <35 years old.
b Uptake in EC risk-reduction at one-year follow-up is not reported for subjects found to have “true negative” genetic test results, since they would no longer be considered at risk for Lynch-associated EC.
Genetic test results were available for all 40 women. Sixteen (40%) were found to carry a pathogenic MMR gene mutation (“positive” results), 9 (23%) tested negative for their family’s known mutation (“true negative” results), 14 (35%) had indeterminate results, and 1 (3%) was found to have a variant of uncertain significance. At the time of analysis, one-, two-, and three-year follow-up questionnaires were available on 30 (75%), 21 (53%), and 8 (20%) of the 40 subjects, respectively. No subjects reported being diagnosed with EC at any of the follow-up intervals.
At one-year follow-up after genetic testing, all 16 MMR gene mutation carriers were adherent to EC risk-reducing guidelines. Four (25%) of the 16 mutation carriers had undergone PH prior to testing, 5 (31%) underwent PH in the year following testing (4 of whom had reported no EC surveillance at baseline), 5 (31%) reported undergoing EC surveillance in the year after testing, and the remaining 2 (13%) were under age 35 (Figure 2A–C). Three mutation carriers did not return one-year questionnaires but were still considered adherent to EC risk-reducing guidelines since two of them had undergone PH prior to genetic testing and the third would have been 23 years old at the time of one year follow-up. By three-year follow-up, an additional 2 subjects reported having undergone PH, thereby giving a cumulative total of 11/16 (69%) mutation carriers who underwent PH (Figure 2B).
Four (27%) of the 15 women found to have indeterminate or variant genetic test results reported engaging in EC risk-reduction at one-year follow-up with 3 (20%) having annual EC surveillance (1 of whom was not having EC surveillance at baseline) and 1 (7%) having undergone PH prior to testing. None reported having undergone PH on follow-up.
One (11%) of the 9 women who received “true negative” test results reported continuing her EC surveillance in the year after testing. Although 1/9 (11%) had undergone PH prior to testing, none of the remaining women who received “true negative” results underwent PH on follow-up.
DISCUSSION
Our study found that uptake of interventions to reduce EC risk is high among women at risk for LS. In the cross-sectional cohort, personal history of genetic testing was strongly associated with uptake of surveillance and/or prophylactic surgery for EC risk-reduction. In our longitudinal cohort, all subjects confirmed to carry a MMR gene mutation were compliant with guidelines for EC risk-reduction in the first year after genetic testing, with nearly 70% opting for PH by three-year follow-up. In contrast, the majority of women who received negative or uninformative genetic test results did not continue EC surveillance and none underwent PH. We believe that our study provides an important and updated contribution to our understanding of how women at risk for LS-associated EC choose to manage this risk, since the increase in EC risk-reduction was driven largely by uptake of PH among mutation carriers.
Most of the existing data regarding the uptake of prophylactic gynecologic surgery among women with hereditary cancer syndromes come from studies of BRCA1 and BRCA2 gene mutation carriers. Women with such mutations have an estimated 18–62% lifetime risk of ovarian, fallopian tube, and primary peritoneal cancer.[24–26] Although gynecologic cancer surveillance is considered to be ineffective, studies have shown that prophylactic BSO significantly reduces the risk of gynecologic cancer, and women with BRCA1/2 mutations are thus counseled to undergo BSO at age 35–40.[24–29] In a prospective, multi-center, United States study, Kauff et al reported that 509/792 (64%) BRCA1/2 mutation carriers underwent prophylactic BSO after genetic testing, with most having surgery in the year following testing.[30] Numerous smaller studies have described similar rates of uptake of prophylactic BSO among BRCA1/2 mutation carriers after genetic testing.[31, 32] In our own cohort of women undergoing genetic testing for BRCA1/2 mutations (studied in parallel with the longitudinal cohort (Cohort 2) reported here), we found that 18/30 (60%) BRCA1/2 mutation carriers underwent prophylactic BSO in the year following testing (unpublished data).
Prior studies examining the uptake of EC risk-reducing behaviors among women with LS have observed comparably lower rates of prophylactic gynecologic surgery. In a cohort of 17 Australian women confirmed to have LS between 1998–2002, Collins et al reported that 0, 8 (47%), and 9 (53%) went on to have PH, TVUS, and endometrial biopsy, respectively, in the year following genetic testing.[33] In a study of 103 Finnish women found to carry a MMR gene mutation between 1995–1999 and managed through a centralized registry, Jarvinen at el found that 97% of their cohort had EC surveillance at some point after genetic testing.[5] By a median follow-up of 11 years after testing, 48/103 (47%) had undergone a hysterectomy, although half of these might not be considered prophylactic in nature since they were performed for management of specific gynecologic diagnoses, including ovarian cancer and endometrial hyperplasia.[5] By contrast, in the only prior study of EC risk-reducing practices among women with LS in the United States, Hadley et al found that, among a cohort of 28 women confirmed to have LS on genetic testing performed between 1997–2002, only 2 (7%) and 15 (54%) underwent PH and EC surveillance, respectively, after testing.[19]
With nearly 70% of genetically-confirmed women with LS in our longitudinal cohort undergoing PH, our study population had a strikingly higher rate of prophylactic gynecologic surgery compared to prior reports, with an uptake comparable to what has been described among BRCA1/2 mutation carriers. While this high uptake of PH could simply be a result of institutional bias, an alternate hypothesis is that our findings reflect an evolving understanding as to how women with LS can best reduce their risk of EC. Numerous studies over the past decade have failed to demonstrate any meaningful efficacy from EC surveillance in women with LS with regards to cancer detection or survival outcomes.[34–40] A recent systematic literature review[40] concluded that available published data are insufficient to provide evidence-based recommendations about the benefit of EC surveillance in women with LS, due to the observational nature of most of the relevant studies, their lack of a control group, and a paucity of information on subjects’ compliance with surveillance.[34–38] Likewise, guidelines put forth by the National Comprehensive Cancer Network (NCCN) cite a lack of evidence to support routine EC surveillance in women with LS.[41]
While the value of EC surveillance in women with LS remains unproven, evidence supporting the efficacy of prophylactic gynecologic surgery continues to emerge. Most notably, in their landmark 2006 study, Schmeler et al reported that 0/61 women with LS in a retrospective cohort were diagnosed with EC after undergoing PH (with or without BSO), whereas 69/210 (33%) women who had not had prophylactic surgery were eventually diagnosed with EC.[42] Although subsequent data have shown that PH with BSO is not 100% protective against LS-associated gynecologic cancer,[43] this nonetheless remains the strongest evidence to date supporting the efficacy of prophylactic gynecologic surgery in LS. Furthermore, recent cost-effectiveness analyses have supported the use of PH as a primary component of the management of women with LS.[44, 45]
Our study’s main strength is its use of both cross-sectional and longitudinal designs for studying the behaviors of women at risk for LS-associated EC. Our cross-sectional cohort enabled us to identify that having had genetic testing was strongly associated with uptake of EC risk-reduction, and then our longitudinal cohort allowed us to prospectively study the impact of genetic testing on medical decision-making among women at risk for LS and demonstrate the sustainability of this effect through three years of follow-up.
We recognize that our study has limitations. Although our sample size is comparable to prior studies of women with LS, our cohorts’ small size hinders our ability to detect significant associations between various clinical factors and the uptake of EC risk-reducing practices. The study’s questionnaire format is also inherently reliant on subjects’ self-reporting of their health behaviors, and we were unable to verify their accuracy by medical record review. Our study population was demographically homogeneous with a significant majority having health insurance, a college education, and relatively high income, and was presumably enriched with highly-motivated subjects with a particular interest in their personal and/or family history of cancer. We also recognize that LS-associated EC is generally a much less lethal disease than BRCA1/2-associated gynecologic cancer, which could theoretically lead to a higher degree of institutional variability in how patients’ LS-associated gynecologic cancer risk is managed. We acknowledge that using Amsterdam criteria to define subjects at risk for LS is likely to also capture individuals with Familial Colorectal Cancer Type X who would not be at increased risk of gynecologic cancer.[46] Uncertainty regarding EC risk along with questions regarding the effectiveness of EC screening may have contributed to a lower uptake of EC risk-reducing measures among women without a genetically-confirmed diagnosis.
In summary, women at risk for LS-associated EC are more likely to follow published EC risk-reduction guidelines if they have undergone genetic testing. Among women confirmed to carry a pathogenic MMR gene mutation in our study, adherence to guidelines was high with nearly 70% opting for PH by three years after genetic testing. The uptake of PH among women with LS in our study is markedly higher than in previous reports, but is comparable to rates of prophylactic BSO among BRCA1/2 carriers. We hypothesize that these findings might reflect an emerging trend of prophylactic surgery, rather than cancer surveillance, being the preferred method of gynecologic cancer risk-reduction among women with LS.
Highlights.
-
➢
We studied endometrial cancer risk-reducing practices in women at risk for LS.
-
➢
Having genetic testing for LS is strongly associated with adherence to guidelines.
-
➢
Women confirmed to have LS have high uptake of prophylactic hysterectomy.
Acknowledgments
Funding support: NIH/NCI K07CA120448-5 (E. Stoffel), NIH/NCI K24113433 (S. Syngal)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statements:
Dr. Gruber reports serving as a consultant for Myriad Genetics.
Dr. Syngal reports serving as an advisor and consultant for Quest Diagnostics, Inc. and for Archimedes, Inc.
All other authors report no conflicts of interest.
Previous publication: A previous version of this manuscript’s abstract was presented as an oral presentation at the 15th annual meeting of the Collaborative Group of the Americas on Inherited Colorectal Cancer, held October 10–11, 2011, in Montreal, QC, Canada.
REFERENCES
- 1.Stoffel E, Mukherjee B, Raymond VM, Tayob N, Kastrinos F, Sparr J, et al. Calculation of risk of colorectal and endometrial cancer among patients with Lynch syndrome. Gastroenterology. 2009;137:1621–1627. doi: 10.1053/j.gastro.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aarnio M, Mecklin JP, Aaltonen LA, Nystrom-Lahti M, Jarvinen HJ. Life-time risk of different cancers in hereditary non-polyposis colorectal cancer (HNPCC) syndrome. Int J Cancer. 1995;64:430–433. doi: 10.1002/ijc.2910640613. [DOI] [PubMed] [Google Scholar]
- 3.Boland CR, Shike M. Report from the Jerusalem workshop on Lynch syndromehereditary nonpolyposis colorectal cancer. Gastroenterology. 2010;138:2197, e1–e7. doi: 10.1053/j.gastro.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunlop MG, Farrington SM, Carothers AD, Wyllie AH, Sharp L, Burn J, et al. Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet. 1997;6:105–110. doi: 10.1093/hmg/6.1.105. [DOI] [PubMed] [Google Scholar]
- 5.Jarvinen HJ, Renkonen-Sinisalo L, Aktan-Collan K, Peltomaki P, Aaltonen LA, Mecklin JP. Ten years after mutation testing for Lynch syndrome: cancer incidence and outcome in mutation-positive and mutation-negative family members. J Clin Oncol. 2009;27:4793–4797. doi: 10.1200/JCO.2009.23.7784. [DOI] [PubMed] [Google Scholar]
- 6.Hampel H, de la Chapelle A. The search for unaffected individuals with Lynch syndrome: do the ends justify the means? Cancer Prev Res (Phila) 2011;4:1–5. doi: 10.1158/1940-6207.CAPR-10-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fishel R, Lescoe MK, Rao MR, Copeland NG, Jenkins NA, Garber J, et al. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell. 1993;75:1027–1038. doi: 10.1016/0092-8674(93)90546-3. [DOI] [PubMed] [Google Scholar]
- 8.Nicolaides NC, Papadopoulos N, Liu B, Wei YF, Carter KC, Ruben SM, et al. Mutations of two PMS homologues in hereditary nonpolyposis colon cancer. Nature. 1994;371:75–80. doi: 10.1038/371075a0. [DOI] [PubMed] [Google Scholar]
- 9.Miyaki M, Konishi M, Tanaka K, Kikuchi-Yanoshita R, Muraoka M, Yasuno M, et al. Germline mutation of MSH6 as the cause of hereditary nonpolyposis colorectal cancer. Nat Genet. 1997;17:271–272. doi: 10.1038/ng1197-271. [DOI] [PubMed] [Google Scholar]
- 10.Akiyama Y, Sato H, Yamada T, Nagasaki H, Tsuchiya A, Abe R, et al. Germ-line mutation of the hMSH6/GTBP gene in an atypical hereditary nonpolyposis colorectal cancer kindred. Cancer Res. 1997;57:3920–3923. [PubMed] [Google Scholar]
- 11.Ligtenberg MJ, Kuiper RP, Chan TL, Goossens M, Hebeda KM, Voorendt M, et al. Heritable somatic methylation and inactivation of MSH2 in families with Lynch syndrome due to deletion of the 3' exons of TACSTD1. Nat Genet. 2009;41:112–117. doi: 10.1038/ng.283. [DOI] [PubMed] [Google Scholar]
- 12.Bronner CE, Baker SM, Morrison PT, Warren G, Smith LG, Lescoe MK, et al. Mutation in the DNA mismatch repair gene homologue hMLH1 is associated with hereditary non-polyposis colon cancer. Nature. 1994;368:258–261. doi: 10.1038/368258a0. [DOI] [PubMed] [Google Scholar]
- 13.Hampel H, Panescu J, Lockman J, Sotamaa K, Fix D, Comeras I, et al. Comment on: Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res. 2007;67:9603. doi: 10.1158/0008-5472.CAN-07-2308. [DOI] [PubMed] [Google Scholar]
- 14.Aarnio M, Sankila R, Pukkala E, Salovaara R, Aaltonen LA, de la Chapelle A, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer. 1999;81:214–218. doi: 10.1002/(sici)1097-0215(19990412)81:2<214::aid-ijc8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 15.Bonadona V, Bonaiti B, Olschwang S, Grandjouan S, Huiart L, Longy M, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305:2304–2310. doi: 10.1001/jama.2011.743. [DOI] [PubMed] [Google Scholar]
- 16.Vasen HF, Moslein G, Alonso A, Bernstein I, Bertario L, Blanco I, et al. Guidelines for the clinical management of Lynch syndrome (hereditary non-polyposis cancer) J Med Genet. 2007;44:353–362. doi: 10.1136/jmg.2007.048991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindor NM, Petersen GM, Hadley DW, Kinney AY, Miesfeldt S, Lu KH, et al. Recommendations for the care of individuals with an inherited predisposition to Lynch syndrome: a systematic review. JAMA. 2006;296:1507–1517. doi: 10.1001/jama.296.12.1507. [DOI] [PubMed] [Google Scholar]
- 18.Burke W, Petersen G, Lynch P, Botkin J, Daly M, Garber J, et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer. I. Hereditary nonpolyposis colon cancer. Cancer Genetics Studies Consortium. JAMA. 1997;277:915–919. [PubMed] [Google Scholar]
- 19.Hadley DW, Jenkins JF, Steinberg SM, Liewehr D, Moller S, Martin JC, et al. Perceptions of cancer risks and predictors of colon and endometrial cancer screening in women undergoing genetic testing for Lynch syndrome. J Clin Oncol. 2008;26:948–954. doi: 10.1200/JCO.2007.13.0575. [DOI] [PubMed] [Google Scholar]
- 20.Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Ruschoff J, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoffel EM, Mercado RC, Kohlmann W, Ford B, Grover S, Conrad P, et al. Prevalence and predictors of appropriate colorectal cancer surveillance in Lynch syndrome. Am J Gastroenterol. 2010;105:1851–1860. doi: 10.1038/ajg.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vasen HF, Mecklin JP, Khan PM, Lynch HT. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC) Dis Colon Rectum. 1991;34:424–425. doi: 10.1007/BF02053699. [DOI] [PubMed] [Google Scholar]
- 23.Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999;116:1453–1456. doi: 10.1016/s0016-5085(99)70510-x. [DOI] [PubMed] [Google Scholar]
- 24.Kauff ND, Satagopan JM, Robson ME, Scheuer L, Hensley M, Hudis CA, et al. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2002;346:1609–1615. doi: 10.1056/NEJMoa020119. [DOI] [PubMed] [Google Scholar]
- 25.Finch A, Beiner M, Lubinski J, Lynch HT, Moller P, Rosen B, et al. Salpingooophorectomy and the risk of ovarian, fallopian tube, and peritoneal cancers in women with a BRCA1 or BRCA2 Mutation. JAMA. 2006;296:185–192. doi: 10.1001/jama.296.2.185. [DOI] [PubMed] [Google Scholar]
- 26.Domchek SM, Friebel TM, Singer CF, Evans DG, Lynch HT, Isaacs C, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304:967–975. doi: 10.1001/jama.2010.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tung N. Management of women with BRCA mutations: a 41-year-old woman with a BRCA mutation and a recent history of breast cancer. JAMA. 2011;305:2211–2220. doi: 10.1001/jama.2011.678. [DOI] [PubMed] [Google Scholar]
- 28.Rebbeck TR, Lynch HT, Neuhausen SL, Narod SA, Van't Veer L, Garber JE, et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med. 2002;346:1616–1622. doi: 10.1056/NEJMoa012158. [DOI] [PubMed] [Google Scholar]
- 29.Hermsen BB, Olivier RI, Verheijen RH, van Beurden M, de Hullu JA, Massuger LF, et al. No efficacy of annual gynaecological screening in BRCA1/2 mutation carriers; an observational follow-up study. Br J Cancer. 2007;96:1335–1342. doi: 10.1038/sj.bjc.6603725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kauff ND, Domchek SM, Friebel TM, Robson ME, Lee J, Garber JE, et al. Riskreducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: a multicenter, prospective study. J Clin Oncol. 2008;26:1331–1337. doi: 10.1200/JCO.2007.13.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz MD, Isaacs C, Graves KD, Poggi E, Peshkin BN, Gell C, et al. Longterm outcomes of BRCA1/BRCA2 testing: risk reduction and surveillance. Cancer. 2012;118:510–517. doi: 10.1002/cncr.26294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skytte AB, Gerdes AM, Andersen MK, Sunde L, Brondum-Nielsen K, Waldstrom M, et al. Risk-reducing mastectomy and salpingo-oophorectomy in unaffected BRCA mutation carriers: uptake and timing. Clin Genet. 2010;77:342–349. doi: 10.1111/j.1399-0004.2009.01329.x. [DOI] [PubMed] [Google Scholar]
- 33.Collins V, Meiser B, Gaff C, St. John DJB, Halliday J. Screening and preventive behaviors one year after predictive genetic testing for hereditary nonpolyposis colorectal carcinoma. Cancer. 2005;104:273–281. doi: 10.1002/cncr.21183. [DOI] [PubMed] [Google Scholar]
- 34.Dove-Edwin I, Boks D, Goff S, Kenter GG, Carpenter R, Vasen HF, et al. The outcome of endometrial carcinoma surveillance by ultrasound scan in women at risk of hereditary nonpolyposis colorectal carcinoma and familial colorectal carcinoma. Cancer. 2002;94:1708–1712. doi: 10.1002/cncr.10380. [DOI] [PubMed] [Google Scholar]
- 35.Renkonen-Sinisalo L, Butzow R, Leminen A, Lehtovirta P, Mecklin JP, Jarvinen HJ. Surveillance for endometrial cancer in hereditary nonpolyposis colorectal cancer syndrome. Int J Cancer. 2007;120:821–824. doi: 10.1002/ijc.22446. [DOI] [PubMed] [Google Scholar]
- 36.Rijcken FE, Mourits MJ, Kleibeuker JH, Hollema H, van der Zee AG. Gynecologic screening in hereditary nonpolyposis colorectal cancer. Gynecol Oncol. 2003;91:74–80. doi: 10.1016/s0090-8258(03)00371-8. [DOI] [PubMed] [Google Scholar]
- 37.Lecuru F, Le Frere Belda MA, Bats AS, Tulpin L, Metzger U, Olschwang S, et al. Performance of office hysteroscopy and endometrial biopsy for detecting endometrial disease in women at risk of human non-polyposis colon cancer: a prospective study. Int J Gynecol Cancer. 2008;18:1326–1331. doi: 10.1111/j.1525-1438.2007.01183.x. [DOI] [PubMed] [Google Scholar]
- 38.Gerritzen LH, Hoogerbrugge N, Oei AL, Nagengast FM, van Ham MA, Massuger LF, et al. Improvement of endometrial biopsy over transvaginal ultrasound alone for endometrial surveillance in women with Lynch syndrome. Fam Cancer. 2009;8:391–397. doi: 10.1007/s10689-009-9252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lecuru F, Huchon C, Metzger U, Bats AS, Le Frere Belda MA, Olschwang S, et al. Contribution of ultrasonography to endometrial cancer screening in patients with hereditary nonpolyposis colorectal cancer/Lynch syndrome. Int J Gynecol Cancer. 2010;20:583–587. doi: 10.1111/IGC.0b013e3181d7283a. [DOI] [PubMed] [Google Scholar]
- 40.Auranen A, Joutsiniemi T. A systematic review of gynecological cancer surveillance in women belonging to hereditary nonpolyposis colorectal cancer (Lynch syndrome) families. Acta Obstet Gynecol Scand. 2011;90:437–444. doi: 10.1111/j.1600-0412.2011.01091.x. [DOI] [PubMed] [Google Scholar]
- 41.Burt RW, Barthel JS, Dunn KB, David DS, Drelichman E, Ford JM, et al. NCCN clinical practice guidelines in oncology. Colorectal cancer screening. J Natl Compr Canc Netw. 2010;8:8–61. doi: 10.6004/jnccn.2010.0003. [DOI] [PubMed] [Google Scholar]
- 42.Schmeler KM, Lynch HT, Chen LM, Munsell MF, Soliman PT, Clark MB, et al. Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. N Engl J Med. 2006;354:261–269. doi: 10.1056/NEJMoa052627. [DOI] [PubMed] [Google Scholar]
- 43.Schmeler KM, Daniels MS, Soliman PT, Broaddus RR, Deavers MT, Vu TM, et al. Primary peritoneal cancer after bilateral salpingo-oophorectomy in two patients with Lynch syndrome. Obstet Gynecol. 2010;115:432–434. doi: 10.1097/AOG.0b013e3181b6f4f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang KY, Caughey AB, Little SE, Cheung MK, Chen LM. A cost-effectiveness analysis of prophylactic surgery versus gynecologic surveillance for women from hereditary non-polyposis colorectal cancer (HNPCC) families. Fam Cancer. 2011;10:535–543. doi: 10.1007/s10689-011-9444-z. [DOI] [PubMed] [Google Scholar]
- 45.Kwon JS, Sun CC, Peterson SK, White KG, Daniels MS, Boyd-Rogers SG, et al. Cost-effectiveness analysis of prevention strategies for gynecologic cancers in Lynch syndrome. Cancer. 2008;113:326–335. doi: 10.1002/cncr.23554. [DOI] [PubMed] [Google Scholar]
- 46.Lindor NM, Rabe K, Petersen GM, Haile R, Casey G, Baron J, et al. Lower cancer incidence in Amsterdam-I criteria families without mismatch repair deficiency: familial colorectal cancer type X. JAMA. 2005;293:1979–1985. doi: 10.1001/jama.293.16.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]