SUMMARY
Cellular processes function through multi-step pathways that are reliant on the controlled association and disassociation of sequential protein complexes. While dynamic action is critical to propagate and terminate work, the mechanisms used to disassemble biological structures are not fully understood. Here, we show that the p23 molecular chaperone initiates disassembly of protein-DNA complexes and that the GCN5 acetyltransferase prolongs the dissociated state through lysine acetylation. By modulating the DNA bound-state, we found that the conserved and essential joint activities of p23 and GCN5 impacted transcription factor activation potential and response time to an environmental cue. Notably, p23 and GCN5 were required to maintain open chromatin regions along the genome indicating that dynamic protein behavior is a critical feature of various DNA-associated events. Our data support a model in which p23 and GCN5 regulate diverse multi-step pathways by controlling the longevity of protein-DNA complexes.
INTRODUCTION
To achieve and maintain physiological balance a cell requires a multitude of biological pathways to operate efficiently, including DNA-associated events. The complexity of the processes working along the genome is only surpassed by the variety of employed factors (Lemon and Tjian, 2000; Bell and Dutta, 2002; Sancar et al., 2004). Yet within this ostensible chaos, the separate parts routinely and efficiently organize into multi-step pathways to transduce signals or accomplish requisite work (Misteli, 2001). Further challenging cell systems are a multitude of fluctuating internal and external signals that must be monitored to initiate, continue or halt various cellular processes (Freeman and Yamamoto, 2001). Although cooperative interactions provide a mechanism for rapid and precise assembly, the inherent stability of such organized structures would interfere with the proper timing of biological events. Hence, factors are needed to destabilize protein assemblies in order to efficiently transition between structures along a sequential path or to terminate action (DeZwaan and Freeman, 2008).
Stress signaling pathways culminating in a transcriptional response are exemplary paradigms highlighting the challenges encountered by many cellular processes. For example, glucocorticoid stress hormones are bound by cytosolic Glucocorticoid Receptors (GR) to trigger a nuclear translocation where the holoreceptors bind to specific genomic sites termed Glucocorticoid Response Elements (GREs) (Yamamoto, 1985). This relatively simple transcription complex recruits numerous, distinct coactivating complexes (e.g., histone modifying, chromatin remodeling, etc.) to mark and remove nearby nucleosomes followed by nucleation of the RNA polymerase machinery to the target gene (Lemon and Tjian, 2000). Even with all apparent complexities, production of specific RNA transcripts occurs within minutes (Ucker and Yamamoto, 1984). As importantly, the transduction pathway terminates rapidly (<5 min) upon hormone withdrawal (Zaret and Yamamoto, 1984). The quick responses happen in vivo despite intrinsically stable holoreceptor-RE structures in vitro (e.g., GR:GRE complex t1/2 ~110 min) (Perlmann et al., 1990). Clarifying this apparent dichotomy was the discovery that holoreceptors interact dynamically with regulated promoters in vivo (t1/2 ~1–2 seconds) (McNally et al., 2000). The expeditious promoter binding and release provides the receptor with key features including: 1) an ability to rapidly recruit all the necessary coactivating complexes and 2) the capacity to monitor the levels of signal by continuously sampling the cellular environment (Freeman and Yamamoto, 2001). Several factors, including molecular chaperones and chromatin remodelers, have been implicated in coordinating the difference between the in vitro and in vivo DNA binding kinetics of hormone receptors (Hager et al., 2009). Notably, hormone receptors are not unique in this regard since most chromatin-associated proteins transiently interact with DNA in vivo (Phair et al., 2004). Hence, cells must contain a general system for creating a dynamic environment along the genome.
Molecular chaperones have been shown to promote the assembly and disassembly of diverse protein DNA complexes, including transcription and telomere structures (DeZwaan and Freeman, 2010). Chaperones are good candidates to initiate rapid behavior for a wide range of proteins, as they are highly abundant and have evolved to act promiscuously (Echtenkamp and Freeman, in press). To offset the broad binding capacity and to avoid interfering with the functional activity of a client, chaperones typically have short-lived, low affinity interactions with proteins (Ellis, 1987). While molecular chaperones are ideal factors to coordinate protein movements, the transient nature of the chaperone-client interaction is likely insufficient to terminate work or, perhaps, even to transition between different structures along a pathway. Hence, additional components are needed to prolong the dissociated state of protein-DNA assemblies. In the presented study, we show that the p23 molecular chaperone works in conjunction with the GCN5 acetyltransferase to mediate the dynamics of heterologous protein-DNA complexes, which impact both RNA transcription and open chromatin processes.
RESULTS
p23 and GCN5 Display Compensatory Expression Patterns
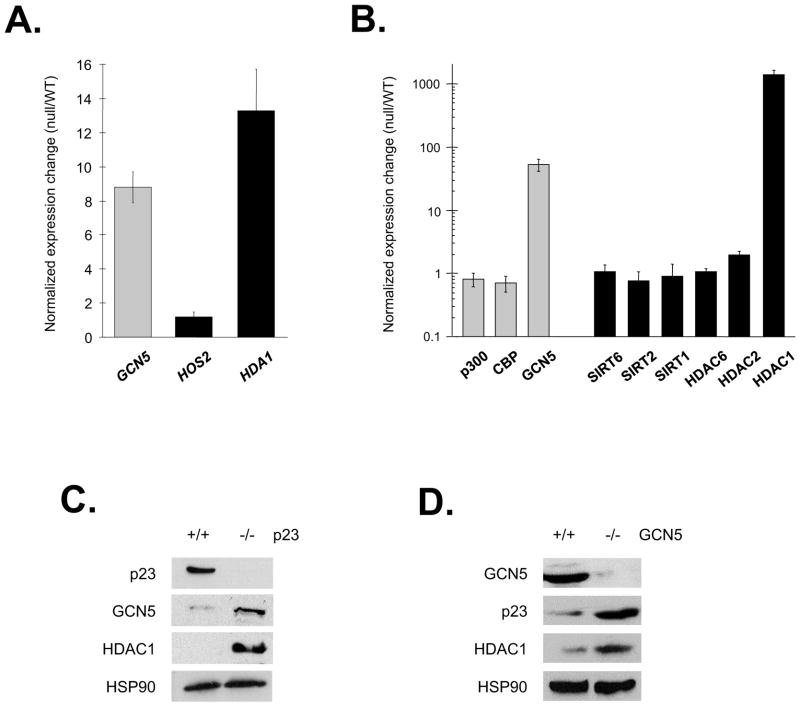
In a study designed to reveal the cellular activities working in parallel with the yeast p23 molecular chaperone Sba1 we identified a synthetic genetic lethal interaction between SBA1 and the lysine acetyltransferase encoding gene GCN5 and synthetic sick phenotypes with the deacetyltransferase genes HDA1 and HOS2 (Echtenkamp et al., 2011). Intriguingly, we found that the expression levels of GCN5 and HDA1 were elevated in sba1Δ yeast implying a compensatory relationship (Figure 1A). To determine if the link is conserved, we examined the expression of various acetylases and deacetylases in parental and p23 null mouse embryonic fibroblasts (MEFs) (Grad et al., 2006). We observed that GCN5 and HDAC1 mRNA levels were significantly increased in p23 null cells, as were the steady-state amounts of GCN5 and HDAC1 proteins (Figure 1B and 1C). Notably, the expression linkage also was apparent upon loss of GCN5 (Bu et al., 2007), as p23 and HDAC1 proteins were elevated in GCN5 null MEFs (Figure 1D). The conserved and shared expression changes indicate a physiologically significant connection, but how might a chaperone relate to the acetylation process?
Figure 1.
GCN5 and deacetylase levels were increased in sba1Δ yeast and p23 null MEF cells. (A) The expression of the HDA1, HOS2 and GCN5 loci in sba1Δ yeast relative to the parental strain was established by real time RT-PCR. Error bars represent the standard error of the mean. (B) The relative enrichment in the steady-state mRNA amounts of the indicated acetylases (grey bars) and deacetylases (black bars) were determined using p23 null and parental MEFs. The steady-state levels of the indicated proteins in p23 null (−/−) (C) or GCN5 null (−/−) (D) MEFs relative to the respective parental (+/+) cells were investigated by immunoblot analysis.
Recent reports implicate acetylation status as a means to positively or negatively regulate various transcription factor activities including DNA binding (Sims and Reinberg, 2008; Yang and Seto, 2008). For example, Sirtuin 1 (SIRT1) impacts the heat shock response by deacetylating a lysine (K80) residue within the DNA binding domain (DBD) of Heat Shock Factor 1 (HSF1)—acetylation of K80 blocks DNA binding (Westerheide et al., 2009). Additionally, glucocorticoid-responsive loci are modulated by the constitutively promoter-associated deacetylase HDAC1 that functions as a GR coactivator through an undefined, histone-independent mechanism (Mulholland et al., 2003; Qiu et al., 2006). Despite knowing the identities of the deacetylases, the involved acetylase(s) had not been revealed (Qiu et al., 2006; Westerheide et al., 2009). Given the common expression profiles (Figure 1) and influences of chaperone and acetylase activities on DNA binding activities, we focused on how p23 and GCN5 might functionally intersect with DNA-associated events.
p23 and GCN5 both Impede Transcription by Inhibiting DNA Binding
We investigated the impact of p23 and GCN5 on the transcriptional activation potentials of GR or HSF1 in response to hormone or heat-stress, respectively. Overexpression of either p23 or GCN5 reduced activation of both GR- and HSF1-controlled endogenous loci in the human embryonic kidney cell line 293T without affecting the steady-state levels of either transcription factor (Figure 2A and 2B). Similarly, overexpression of p23 or GCN5 impeded the up-regulation of transiently transfected glucocorticoid- or heat shock-responsive plasmid reporters suggesting that the p23 and GCN5 effects are independent of chromatin promoter events (Supplementary Figure 1). Mutants that either abolished GCN5 acetyltransferase (E575Q) or p23 chaperone (p23Δ35) activity had no apparent effect on GR or HSF1 transactivation (Supplementary Figure 1) (Weaver et al., 2000; Yanagisawa et al., 2002). Unexpectedly, GR and HSF1 transcriptional activities were unaffected upon co-overexpression of p23 and GCN5 (Figure 2A and 2B). While we had anticipated either no impact (same pathway) or an enhanced inhibition (parallel paths) upon coexpression, the cessation of a negative influence indicates that p23 and GCN5 share a complicated regulatory relationship.
Figure 2.
GR and HSF1 transcriptional and DNA binding activities were inhibited by p23 or GCN5 but unaltered upon co-overexpression of p23 and GCN5. The effect of p23 and/or GCN5 overexpression on endogenous genes regulated by either activated GR (A) or HSF1 (B) was determined (graphs). Error bars represent the standard error of the mean. In addition, the steady-state levels of the indicated proteins were determined by immunoblot analysis (right panels) along with the loading control protein HSP90. The impact of increased p23 and/or GCN5 on hormone-induced GR DNA binding (C) or heat-stimulated HSF1 DNA binding (D) was investigated in p23 null MEFs by EMSA using GRE or HSE oligonucleotides, respectively. (E) The influence of p23 and GCN5 overexpression on the HSF1 heat-stress DNA binding attenuation period was tested in transiently transfected human cells by EMSA.
Next, we examined the effect of p23 and GCN5 on the DNA binding activities of GR and HSF1. Correlating with the transcription data, overexpression of either p23 or GCN5 resulted in declined GR or HSF1 DNA binding (Figure 2C and 2D). Expression of GCN5 E575Q or p23Δ35 mutants did not affect GR or HSF1 DNA binding and coexpression of p23 and GCN5 abrogated the negative effect imposed by the individual proteins (Figure 2C and 2D). Thus, p23 and GCN5 separately affect transcription factors at the level of DNA binding. Ideally, we wanted to test the influence p23 or GCN5 reduction (i.e., RNAi) on GR and HSF1 activities, but unfortunately the compensatory changes observed in the null cells (Figure 1C and 1D) were apparent under these conditions.
The HSF1 Signal Transduction Pathway is Modulated by p23 and GCN5
The fundamental purpose of HSF1 and GR is to transduce environmental signals into specific gene programs by binding to cognate response elements that control the inducible genes (Freeman and Yamamoto, 2001). Mammalian cells cultured at 37°C will undergo a transient HSF1-mediated stress-response when exposed to 42°C in which HSF1 will display maximal DNA binding after 1–2 h that dissipates by 4 h (Mosser et al., 1988). Upon overexpression of either p23 or GCN5 the attenuation rate to a 42°C heat shock was accelerated (Figure 2E). Hence, p23 and GCN5 regulate the responsiveness of the signal transduction pathway governed by HSF1.
p23 Regulates GCN5 Acetylase Activity
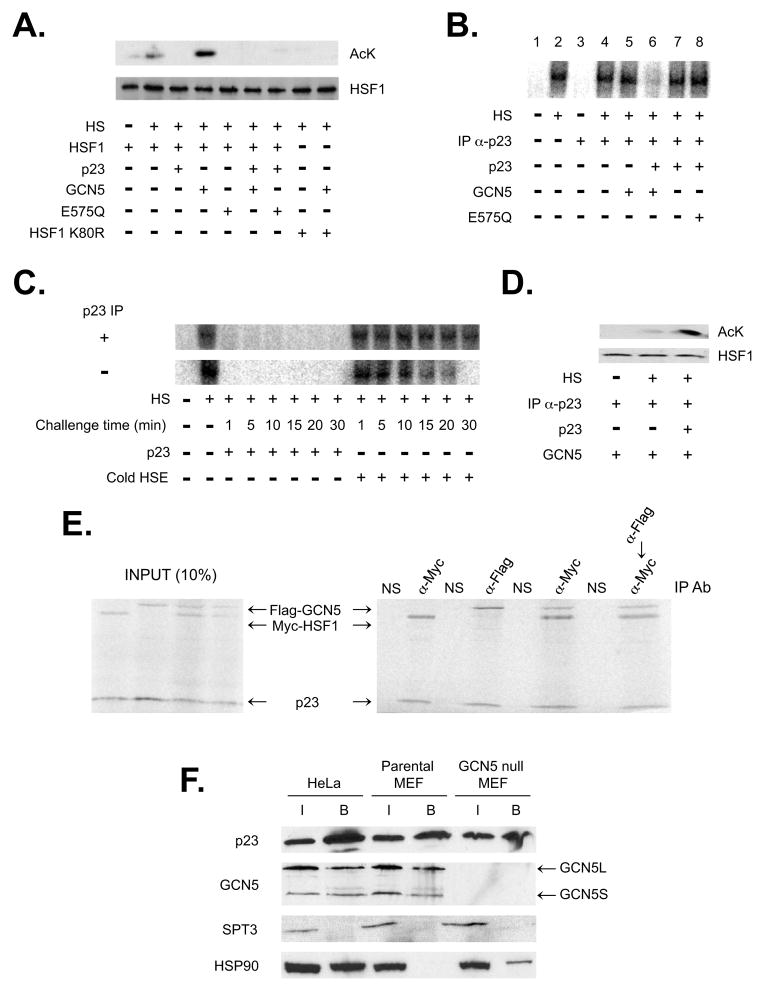
To refine how p23 and GCN5 modulate protein-DNA interactions we exploited rabbit reticulolysate. Reticulolysate has limited acetylase activities relative to typical mammalian cells, supports efficient in vitro heat-activated HSF1 DNA binding, and coexpression of either p23 or GCN5 was sufficient to impair HSF1 DNA binding (Sarge et al., 1991) (Supplementary Figure 2A). Correlating with the DNA binding levels was the HSF1 acetylation status—GCN5 expression led to HSF1 hyper-acetylation whereas coexpression of p23 impeded HSF1 acetylation (Figure 3A). The apparent inhibition of GCN5 by p23 explains, in part, why joint overexpression of p23 and GCN5 does not affect HSF1 (i.e., if GCN5 cannot acetylate HSF1 then no effect would be anticipated).
Figure 3.
GCN5 relies on p23 to modify a DNA-bound transcription factor. (A) The effect of GCN5 and p23 on the acetylation status of HSF1 was investigated in vitro. Myc-HSF1 wild type or K80R was supplemented with p23, GCN5 or GCN5 E575Q and the reactions were incubated at 37°C (−HS) or 43°C (+HS), as marked. The Myc-HSF1 or acetyl lysine was detected by immunoblot analysis. (B) Aliquots of in vitro translated HSF1 were incubated at 37°C (−HS) or 43°C (+HS) for 30 min with radiolabeled HSE oligonucleotide and reactions were either mock treated or p23-immunodepleted (IP α-p23). GCN5, GCN5 E575Q and/or p23 were added, as marked, and the samples were analyzed by EMSA. (C) The stability of preformed HSF1:HSE (radiolabeled oligonucleotide) complexes before or after immunodepletion of rabbit p23 were tested by the addition of either p23 (2x normal levels) or unlabeled HSE oligonucleotide. The reactions were resolved by native gel electrophoresis at the indicated time points following addition of either challenger. (D) The rabbit p23 was immunodepleted from preformed HSF1-HSE complexes, GCN5 alone or GCN5 and recombinant p23 was added, as indicated, and the Myc-HSF1 or acetyl lysine levels were determined by immunoblot analysis. (E) p23 was in vitro cotranslated with Myc-HSF1, Flag-GCN5 or both Myc-HSF1 and Flag-GCN5 (left panel represents 10% of IP input). The reactions were subjected to immunoprecipitation (right panel) using α-IgG (NS), α-Myc (HSF1) and/or α-Flag (GCN5) antibodies and the 35S-labeled proteins were visualized following resolution by denaturing gel electrophoresis. (F) p23 was immunoprecipitated from nuclear extract prepared from HeLa, parental MEF or GCN5 null MEF cells. The presence of p23, GCN5, SPT3 or HSP90 in the pull-downs was determined by immunoblot analysis, as marked.
The ability of GCN5 to modify HSF1 was dependent upon lysine 80 since the HSF1 point mutant K80R was not acetylated (Figure 3A). Lysine 80 is in the DNA binding cleft of HSF1, it is critical for DNA binding activity, K80 is 1 of 8 lysines acetylated by p300 and it is the target residue deacetylated by SIRT1 (Westerheide et al., 2009). In contrast to p300, GCN5 selectively targets HSF1 K80.
GCN5 Acetylates HSF1 following p23-Mediated Release from DNA
Upon further study, we found that the connection between p23 and GCN5 extended beyond acetylase control. If p23 was removed by immunodepletion from reactions containing preformed HSF1-heat shock element (HSE) complexes, little effect on HSF1 DNA binding was observed (Figure 3B lane 2 vs. 4). Yet following p23 removal, GCN5 addition no longer influenced HSF1 DNA interactions (Figure 3B lane 5). Thus, the ability of GCN5 to alter HSF1 DNA binding was dependent upon the presence of p23. In support of this notion, if we supplemented an immunodepleted reaction with recombinant p23 to normal reticulolysate levels then HSF1 DNA binding was inhibited by GCN5 (Figure 3B lane 6). In the absence of GCN5 reconstituting p23 levels to normal had no apparent effect on DNA interactions (Figure 3B lane 7). As expected, the addition of p23 to amounts higher than normal decreased HSF1 DNA binding (Supplementary Figure 2B).
Significantly, p23 initiates the disassembly process by accelerating the off-rate of HSF1-HSE complexes. By challenging preformed HSF1-HSE complexes with either p23 or unlabeled HSE oligonucleotides a significant difference in the longevity of DNA-bound structures was observed (Figure 3C). Immunodepletion of p23 from the reticulolysate further stabilized the HSF1-HSE complexes when challenged with HSE oligonucleotides (Figure 3C). To modulate binding p23 does not directly target the DBD but rather it involves the central regulatory domain (RD) of HSF1 (Supplementary Figure 2C), which has been shown to negatively control the transcription activation domain of HSF1 (Shi et al., 1995).
To confirm whether p23-mediated release of HSF1 from DNA was necessary for modification by GCN5, we examined the HSF1 acetylation state by immunoblot analysis. Following immunodepletion of p23 from HSF1-HSE complexes, GCN5 addition did not lead to HSF1 hyper-acetylation (Figure 3D). However, hyper-acetylation was observed upon a return of normal p23 levels and co-supplementation with GCN5 (Figure 3D). Thus, GCN5 regulates HSF1 through protein acetylation that is only possible after p23 releases HSF1 from the DNA.
Taken together, our data indicate that p23 regulates both GCN5 acetylase and HSF1 DNA binding activities. To confirm this potential, we performed order of addition experiments using HSF1-HSE complexes immunodepleted of p23 and a short incubation period (2 minutes) to capture the immediate impact of the chaperone. If GCN5 was mixed with p23 (normal reticulolysate levels) prior to adding the proteins to HSF1-HSE complexes then no apparent effect on DNA binding was observed (i.e., p23 inhibited GCN5) (Supplementary Figure 2D lane 6). However, as previously shown, if HSF1-HSE was first exposed to normal p23 amounts and then to GCN5, the HSF1-HSE complex was dissociated (Supplementary Figure 2D lane 7). Hence, both GCN5 and HSF1 are regulated by p23 and it is the sequence of interactions that dictate the functional outcome.
While the order of addition work supports our contention that p23 directly controls GCN5, the decline in HSF1 acetylation also might result from a manipulation of HSF1’s conformation into a state disfavoring the modification. To test whether p23 regulates the acetylation-target or if it modulates GCN5, we incorporated peptides derived from the established GCN5-substrates histone H3 and H4 (Kuo et al., 1996). As the short peptides (21 amino acids) lack substantial structure, any loss of acetylation would require direct inhibition of GCN5 by p23. Importantly, p23 impeded GCN5’s ability to efficiently acetylate either the H3 or H4 peptides (Supplementary Figure 3A).
p23 and GCN5 Associate Independent of Established Cofactors
The presented functional data suggest p23 and GCN5 interact to control transcription factors. Using in vitro translated proteins and immunoprecipitation (IP) assays we found that p23 binds to either GCN5 or HSF1 individually and also can be part of a tripartite p23-GCN5-HSF1 complex, as determined by single- and sequential-precipitations (Figure 3E). To control acetylase activity, p23 utilized the ADA2-association domain (AAD) of GCN5 (Supplementary Figure 3B and 3C). In contrast to p23, ADA2 stimulates the acetyltransferase activity of GCN5 (Grant et al., 1997). Hence, a simple mode of regulation is envisioned in which ADA2 activates and p23 inhibits GCN5 through the shared ADA2-association domain.
Significantly, a p23-GCN5 interaction comprised of either the short or long form of GCN5 was detected in both human and mouse nuclear extracts by IP (Figure 3F). The p23-GCN5 assembly is distinct from the established GCN5-containing STAGA/TFTC structures, which modify histones (Nagy and Tora, 2007), since the SPT3 and TRRAP subunits did not pull-down with p23 (Figure 3F; Supplementary Figure 3D). In contrast, GCN5 precipitated with STAGA/TFTC components along with p23 (Supplementary Figure 3D). Perhaps notably, p23 formed a stable interaction with GCN5 in nuclear extract prepared from wild type MEFs but not with the HSP90 chaperone (Figure 3F). Typically, p23 is considered an HSP90 cochaperone (Echtenkamp and Freeman, 2012). In GCN5 null MEFs (Bu et al., 2007), however, an interaction between p23 and HSP90 was observed (Figure 3F). In contrast to the parental MEFs, HSP90 was associated with p23 in HeLa nuclear extract (Figure 3F). Whether the difference in HSP90 interactions is species- or cancer-cell specific has yet to be determined. Minimally, p23 and GCN5 form a distinct and stable regulatory complex.
Diverse DNA Binding Proteins are Influenced by p23 and GCN5
In addition to the transcription factors, we found that p23 affected the binding status of the DNA replication factor CDC6 and DNA repair protein RAD51 (Supplementary Figure 4A and 4B) whereas GCN5 only inhibited CDC6 (Supplementary Figure 4A). As both of these proteins mediate complex DNA events, we suspect a dynamic behavior facilitates the timely and proper function of the associated pathways. For example, recent reports show that GCN5 is a positive regulator of origins of DNA replication that includes acetylation of CDC6 to control its cellular localization (Paolinelli et al., 2009; Espinosa et al., 2010). Our data suggest that the influence of GCN5 on CDC6 begins with the inhibition of DNA binding providing a controlled means to prevent CDC6-mediated reassembly of DNA pre-replicative complexes. Not all proteins were influenced since the telomere-protein TRF2 was unaffected (Supplementary Figure 4C). However, given the influence on several unrelated proteins (GR, HSF1, CDC6 and RAD51), we wanted to determine the range of p23 and GCN5.
DNase I Hypersensitive Site Maintenance is p23 and GCN5 Dependent
To reveal the impact of p23 and GCN5 on genome-wide DNA binding activities we exploited a yeast deep-sequencing assay (DNase-Seq) that identifies DNA footprints within DNase I Hypersensitive Sites (DHSs) (Hesselberth et al., 2009). DHSs are associated with areas of protein action including active gene promoters where nucleation of chromatin-modifying complexes by DNA-bound transcription factors displace nearby histone octamers, thereby increasing DNase I accessibility (Bell et al., 2011). Importantly, the DNase-Seq assay in conjunction with a DNA-motif scan identifies all the transcription factors bound within hypersensitive areas, as the cognate binding sites are protected from DNase I digestion. Hence, by comparing the DNA footprint patterns in WT, sba1Δ and gcn5Δ yeast the transcription factors regulated by Sba1 or Gcn5 are identified.
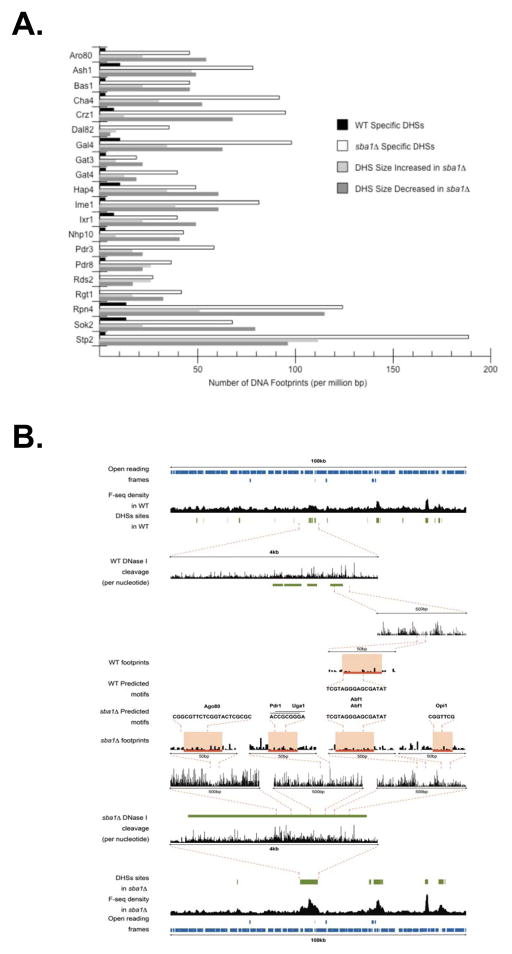
As an initial step, we mapped the DHSs in the different strains. Unexpectedly, the number of hypersensitive sites was reduced in sba1Δ, as we identified 3260 DHSs in WT cells, 3062 in gcn5Δ, and 2439 in sba1Δ (Figure 4A). While the ~25% reduction in the total DHSs in sba1Δ is significant, it is even more notable that ~50% of the WT DHSs are lost upon deletion of sba1; however, the overall decline is less drastic since 869 novel sites appear (Figure 4A). In contrast, ~20% of the WT sites disappear in gcn5Δ but 561 new sites form and therefore the total remains relatively constant (Figure 4A). We were surprised by the lower impact of gcn5Δ, as we had anticipated a more prominent effect given the projected role of histone acetylation in marking nucleosomes for removal. We suspect deletion of sba1 has a more pronounced impact since p23 directly mediates the release of transcription factors whereas Gcn5 is coordinating the downstream maintenance of the dissociated state and therefore its effect on DHSs is not as pronounced.
Figure 4.
Yeast p23 and GCN5 are DHSs maintenance factors. (A) DHSs were identified by deep-sequencing samples following limited DNase I digestion of chromatin within nuclei prepared from wild type, sba1Δ and gcn5Δ yeast. The green bars represent DHSs unique to wild type, blue bars are DHSs specific to gcn5Δ, red bars are select to sba1Δ and grey bars are the remaining sites with the number of overlaps indicated in parentheses. (B) The typical size of DHSs increased in sba1Δ relative to wild type or gcn5Δ. In the top graph, the yellow bars represent the 25–75% quantiles (bottom to top, respectively), the error bars mark the maximal and minimal sizes, and the closed circles represent atypical points. In the bottom graph, the size distribution of DHSs in wild type (grey), sba1Δ (red) and gcn5Δ (blue) yeast is shown. The size distribution of smaller (<500 bp) and larger (>500 bp) DHSs is marked with single and double asterisks, respectively.
Besides the loss of DHSs, the typical size of the hypersensitivity regions in sba1Δ was extended relative to WT or gcn5Δ (Figure 4B). DHSs specific to WT averaged 198 nucleotides in size whereas sba1Δ unique sites were 440 bases in mean width. A comparable fold-difference was found when contrasting the common sites, as the overlapping DHSs averaged 585 bp in WT but were 972 bp wide in sba1Δ. We had anticipated a correlation between the types of underlying occupied response elements (i.e., resident transcription factors) and the influence of deleting sba1 on the various DHSs. However, no relationship was apparent to rationalize how a DHS might change in sba1Δ (Supplementary Table 1). Rather, the DHSs, independent of strain background, were linked to an established wide-array of transcription factors (Hesselberth et al., 2009).
The Occupancy of Diverse DNA Footprints is Altered in sba1Δ Yeast
A prominent feature within the DHSs affected in sba1Δ was a significant increase in the number of identified DNA footprints (Figure 5A; Supplementary Table 1). Of the 110 transcription factors linked to DHSs, 71 displayed a >2-fold increase in the quantity of DNA elements bound in sba1Δ. Notably, several transcription factors (e.g., Rgt1, Rds2, etc.) were not associated with WT-specific DHSs yet were abundantly found in the sba1Δ-select sites. Importantly, the robust expansion in DNA footprints was unrelated to the change in DHS size (Figure 5A; Supplementary Table 1). Often, the newly occupied sites appeared at preexisting DHSs along with a correlative increase in DNase I sensitivity (Figure 5B). Alternatively, DNA footprints materialized in zones of hypersensitivity that were specific to sba1Δ. In either case, the occupancies of diverse DNA binding sites were altered in the absence of Sba1.
Figure 5.
Transcription factor DNA residency increases in the sba1Δ yeast. (A) The number of DNA footprints associated with numerous transcription factors is shown. The average occupancy (per 106 bp) of each motif has been categorized according the type of DHSs including WT specific (black), sba1Δ specific (white), increased in sba1Δ (light grey) and decreased in sba1Δ (dark grey). (B) Shown is the DNase I cleavage densities from the WT and sba1Δ data sets across two 100-kilobase regions of chromosome X (coordinates 245345–345345) with magnification of 5 kilobase sectors, followed by further enlargement of 500 base pair sections down to 50 base segments that visualize the positions and frequencies of the DNase I cleavage events, which reveal the individual DNase I footprints. The identities of the underlying motifs within the DNA footprints (red bar) are indicated.
Sba1 Controls Mcm1 DNA Binding Activity
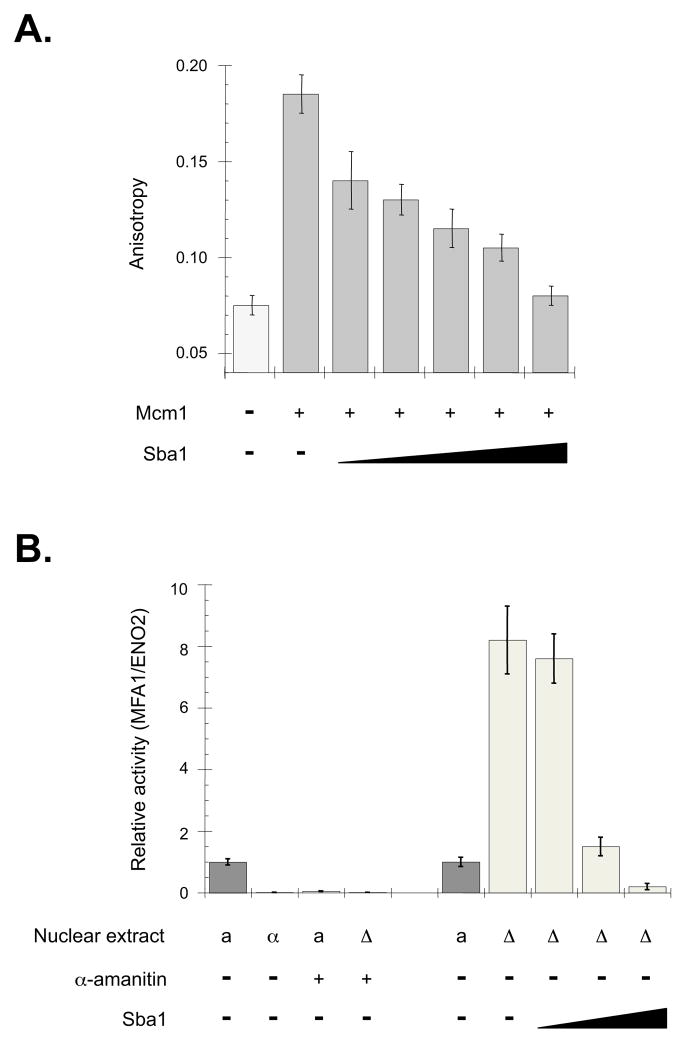
To corroborate that the increase in DNA footprints correlates with an ability of yeast p23 to control DNA binding proteins, we examined the impact of Sba1 on Mcm1 as an example. Mcm1 is a classic transcription factor that regulates the activities of many promoters including genes involved in cell cycle and mating-type decisions (Zhong et al., 1999). In sba1Δ-specific DHSs the occurrence of Mcm1 footprints increased ~3.5-fold relative to WT-select DHSs (Supplementary Table 1) suggesting that Sba1 negatively modulates Mcm1 DNA interactions. To test this premise we checked the influence of recombinant Sba1 on the DNA binding activity of purified Mcm1 using fluorescence anisotropy. Sba1 readily inhibited Mcm1 DNA binding by accelerating the off-rate of Mcm1-DNA complexes (Figure 6A; Supplementary Figure 5A).
Figure 6.
Yeast p23 (Sba1) regulates the Mcm1 transcription factor. (A) DNA binding of purified Mcm1 (250 nM) was measured by fluorescence anisotropy and a fluorescein-labeled oligonucleotide representing the Mcm1 response element (MRE) from the MFA1 promoter. Reactions were supplemented with recombinant Sba1 (0, 0.5, 1.0, 2.0, 4.0 or 8.0 μM), as indicated. (B) Sba1 regulates the activity of the Mcm1-controlled promoter MFA1 in vitro. As controls, the activity of MFA1 promoter was checked using nuclear extract (NE) prepared from WT a-mating type (a), WT α-mating type (α) and sba1Δ a-mating type yeast. To check that the products were RNA polymerase-dependent, NEs were supplemented with α-amanitin (10 μg mL−1 final), as marked. The impact of Sba1 on MFA1 promoter activity was determined by supplementing the Δsba1 extracts with 0.01, 0.1 or 1.0 μg of recombinant Sba1 corresponding to 0.1x, 1x and 10x the WT levels of Sba1. For all reactions, the MFA1 RNA signal was normalized to the levels of ENO2 RNA produced from an independent ENO2 DNA template. Error bars represent the standard error of the mean.
Paralleling the effects of human p23 on endogenous GR- or HSF1-controlled genes (Figure 2), overexpression of Sba1 dampened steady-state transcript levels of the Mcm1-regulated gene MFA1 whereas MFA1 RNA levels increased in sba1Δ yeast (Supplementary Figure 5B). Use of the MFA1 locus as a template for in vitro transcription showed a similar pattern with higher RNA levels being produced using nuclear extract prepared from sba1Δ yeast relative to WT extract (Figure 6B). Titration of recombinant Sba1 into the sba1Δ extract repressed transcript production indicating that Sba1 was the causative factor for the expression changes. Hence, p23 chaperones share an ability to modulate gene promoter activities by dissociating the regulatory transcription factors.
DISCUSSION
The presented work demonstrates that the p23 molecular chaperone facilitates diverse DNA-associated processes including transcriptional activation, signal transduction and open chromatin maintenance by turning otherwise stable DNA complexes into dynamic machinery capable of rapid action. In our model, DNA pathways move forward using high affinity interactions between low abundant proteins, which are specific for a particular path, and transitions between the diverse structures are mediated by transient, low affinity interactions with the abundant p23 molecular chaperone. In cases requiring a prolonged dissociation (e.g., termination) acetylation by GCN5 provides a decisive means to control the longevity of separation.
Merit of a dynamic genomic environment
The physiological need for pathways to work both efficiently and selectively within the crowded milieu of the cell interior presents great challenges for achieving homeostasis (DeZwaan and Freeman, 2008). Cooperative interactions between subunits help drive paths forward by fostering rapid assembly (Garel et al., 1984). However, the inherent high-stability of such organized structures would interfere with the proper timing of biological systems (Misteli, 2001). Further complicating appropriate pathway function is the nature of the cell interior: it is densely packed and often contains multiple binding partners for each protein—both features increase the probability for non-productive or off-pathway interactions (Echtenkamp and Freeman, in press). To appropriately build each structure along a multi-step path and to foster efficient transitions between the complexes cellular factors must have evolved to destabilize biological assemblies.
While it is possible that unique cofactors developed with individual structures, the probability of this scheme is slight, as it would require thousands of additional proteins. Instead, we suggest that molecular chaperones provide an ideal means to promote a general dynamic protein behavior that is critical for proper cell function. The promiscuous, low-affinity interactions of the abundant molecular chaperones would be used to continuously reshape protein complexes forming through high-affinity cooperative interactions between the individual subunits unique to each structure. Hence, a chaperone-governed system creates an environment in which the speed and efficiency of cellular processes are significantly accelerated while the nature of the accomplished work is dictated by the individual components that sequentially assemble along each pathway.
Modulating DNA binding proteins through covalent post-translational modifications
The inclusion of a covalent modification (i.e., acetylation) into the disassembly mechanism is beneficial since it adds the potential for long-term control, which would be key for pathway cessation. For example, the ability of GCN5 to acetylate the HSF1 lysine side-chain required to form a stable DNA complex provides a rigorous means to dictate the DNA-bound state (Figure 3A). The ability to modulate transcription factor DNA binding likely accounts for the biased localization of GCN5 to response elements across the genome (Robert et al., 2004). As the recruitment of GCN5 to initiating promoters is delayed relative to other HATs, a function aside from marking histones is envisioned (Métivier et al., 2003). One plausible role would be to prolong the released-state of a transcription factor and to facilitate efficient transitions between coregulatory complexes. Minimally, protein acetylation by GCN5 provides a measured way to control DNA binding activities. Given the diversity of post-translation modifications, a broad use in mediating long-term protein-DNA and protein-protein interactions is probable (Sims and Reinberg, 2008).
Differential impact of p23 on the size of open chromatin regions
Deletion of yeast p23 had a significant effect on DNase I Hypersensitive sites (Figure 4). In a seemingly paradoxical manner, areas of open chromatin either collapsed or enlarged following loss of sba1. While there are several potential reasons for the differential impact, a probable rationale is the use of distinct mechanisms (single vs. multi-step pathways) to maintain the two classes of DHSs. If the associated transcription factors recruit sequential complexes (i.e., multi-step) to maintain a DHS then deletion of sba1 would favor collapse since progression through the multiple steps would be inefficient without rapid turnover of the transcription factors.
For the DHSs that persisted in sba1Δ the average widths doubled relative to WT. In conjunction with the expanded hypersensitive areas was the appearance of additional DNA footprints (Figure 5; Supplementary Table 1). To account for these observations, we suspect that a pioneering transcription factor using a lone cofactor complex creates a nominal DHS. Following loss of Sba1, the DNA occupancy of the pioneering factor increases leading to an expansion of the hypersensitive site through the actions of the associated histone-removing cofactor. As the area of open chromatin expands, the underlying binding sites are exposed allowing the other transcription factors to engage the DNA. Alternatively, the increase in the number of bound transcription factors might permit the local concurrent association of multiple cofactor complexes bypassing the necessity of a multi-step pathway. Either way, a longer residency time of DHS-promoting structures also would explain the formation of novel DHSs in sba1Δ since Sba1 is not available to dissociate the complex—both the larger and sba1Δ-specific DHSs correlate with the appearance of additional DNA footprints indicating that more transcription factors are stably bound (Figure 5).
Hence, in the absence of Sba1 the DNA occupancy times of client transcription factors increases along with any associated cofactors (e.g., chromatin remodelers), which are yet to be identified. The enlarged DHS size likely explains the genomic instability found in sba1Δ, as decondensed DNA increases the probability of recombination (Ouspenski et al., 1999). While other plausible models exist to explain the observed effects, our data minimally demonstrate that different DNA regions use distinct methods to maintain DHSs. The requirement for dynamic transcription factor action to support proper DHSs provides valuable insight into the nature of open chromatin maintenance.
Summary
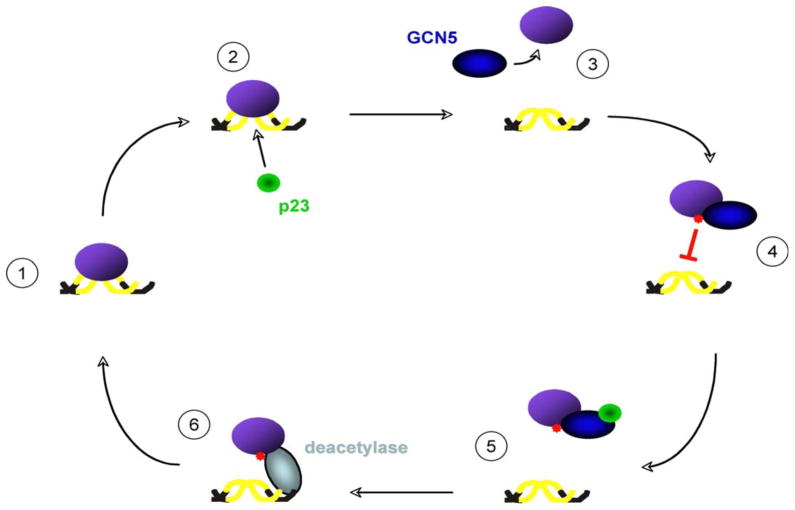
Taken together, our data reveal a novel regulatory network for controlling the dynamics of protein-DNA interactions (Figure 7). In brief, p23 initiates the disassembly process by accelerating the off-rate of protein-DNA complexes. As binding between a chaperone and client is transient, an additional mechanism is required to prolong the dissociated state. The incorporation of a covalent modification by GCN5 (i.e., lysine acetylation) provides a controlled means to determine the longevity of release. To permit DNA rebinding, p23 targets GCN5 and provides a ‘feed-back’ regulatory step to prevent chronic acetylation of a client by GCN5. If further DNA interactions are favored (i.e., activating signal is still present), we believe that a DNA-associated deacetylase (e.g., HDAC1 or SIRT1) displaces GCN5 from the target protein and removes the inhibitory acetyl-group to allow DNA rebinding (Qiu et al., 2006; Westerheide et al., 2009). Together, p23 and the acetyl/deacetyltransferase activities create a dynamic behavior for proteins that nucleate transient complexes critical for the functional capacity of multi-step pathways such as RNA transcription or open chromatin maintenance.
Figure 7.
The p23 molecular chaperone and GCN5 acetyltransferase cooperate to modulate the stabilities of a wide range of protein-DNA complexes. The presented data support a model in which 1) a DNA binding protein (purple) forms an intrinsically long-lived DNA-bound complex; 2) p23 (green) disassembles the protein-DNA structure; 3) GCN5 (blue) acetylates the released protein; 4) acetylation (red) of a lysine within the DNA binding cleft of the target prevents rebinding; 5) p23 impedes GCN5 to avoid constitutive acetylation; 6) a promoter engaged deacetylase (grey) (e.g., HDAC1 or Hda1) removes the inhibitory acetyl group to allow the target to rebind its cognate DNA site.
EXPERIMENTAL PROCEDURES
Yeast strains
The parent strain used for the work presented in Figure 1 was YPH499 (MATa) and the SBA1 disrupted strain was YBF100 (MATa, sba1::HIS3). The strains used for the DNase-Seq experiments were the parent BY4741 (MATa) along with sba1Δ and gcn5Δ isolates (Open BioSystems). Yeast cells were grown in rich medium supplemented with 2% glucose.
Electrophoretic Mobility Shift Assay (EMSA)
EMSA analysis was performed using Buffer C extracts (10 μg) from p23 null MEFs or in vitro transcribed/translated rabbit reticulolysate (Promega) fractions (5 μL). The proteins were incubated with poly dI-dC (Sigma) and 32P-end labeled oligonucleotides: HSE (5′CTAGAAGCTTCTAGAAGCTTCTAG), GRE (5′AGTAGCTAGAAACCCTGTACCGTCGA) along with the complimentary primer. The protein-DNA complexes were resolved by native polyacrylamide gel (4%) electrophoresis and the dried gels were visualized using a PhosphorImager (Molecular Dynamics).
Antibodies
Antibodies were α-p23 (JJ3 and JJ6), α-GCN5 (SCBT sc-20698 or sc-6303), α-HDAC1 (SCBT sc-81598), α-TRRAP (SCBT sc-11411), α-Hsp90 (90H10), α-AcK (Cell Signaling 9411), α-Myc (SCBT sc-47694), α-Flag M1 (Sigma A4596), α-GFP (Covance MMS-118), RAD51 (SCBT sc-53428) and α-HA (Abcam ab27029).
DNase-Seq Assay and Analysis
The DNase-Seq protocol was adapted from an established method (Hesselberth et al., 2009) with the minor modification that following digestion of the chromatin with DNase I the DNA was size fractionated on a 1% agarose gel and the 100–500 bp fragments were purified using microspin columns (Qiagen). The DNA libraries were constructed using the TruSeq DNA Sample Prep kit (Illumina) with the following modifications: 10 ng per sample was used as input, adaptors were diluted 1:20 and final amplification of the adaptored DNA was for 12 cycles. The libraries were quantitated by qPCR (Library Quantification Kit, Kapa Biosystems), equimolar amounts were combined and sequenced on 1 lane for 100 cycles with an Illumina HiSeq2000 and TruSeq SBS sequencing kits version 3. The data were analyzed with Casava1.8 (pipeline 1.9).
The generated sequence data were evaluated and aligned to the yeast reference genome (sacCer3, UCSC) using Bowtie. A stringent quality filter was applied and only high-quality, uniquely mapping reads were used for further analyses (Supplementary Table 2). The DNase I hypersensitive regions were identified using the F-seq Peak Caller v1.84 (Supplementary Table 3). DNA footprints within the DHSs were identified using a described algorithm (Hesselberth et al., 2009). Enriched motifs in the different classes of DNase I Hypersensitive Sites were determined by scanning the identified footprints with Regulatory Sequence Analysis Tools (RSAT) using the 177 JASPAR yeast DNA motifs with the exception of the binding motif for HSF1, which used the MacIsaac binding motif (Supplementary Table 1).
Yeast in vitro transcription and DNA anisotropy assays
Mcm1 DNA binding was measured by fluorescence anisotropy using Mcm1 (250 nM) prepared from wild type a-mating type yeast using an established protocol (Mai et al., 2002) and a fluorescein labeled oligonucleotide representing the Mcm1 response element from the MFA1 promoter (fl-AATTACCCAAAAAGGAAATTT).
The MFA1 and ENO2 loci (promoter and coding regions) templates for the in vitro transcription reactions were amplified from YPH499 genomic DNA; the upstream primer was biotin-labeled. The in vitro transcription reactions contained immobilized template (100 ng of each template) and nuclear extracts (100 μg) prepared from either wild type mating type a (YPH499), wild type mating type α (YPH500), or Δsba1 mating type a (YBF100) cells. RNA transcripts were detected by qPCR using oligonucleotides select for either the MFA1 or ENO2 RNAs following production of the first-strand cDNA by reverse transcription.
Supplementary Material
Highlights.
p23 and GCN5 regulate numerous, heterologous DNA binding proteins
p23 initiates disassembly of protein-DNA complexes by accelerating the off-rates
GCN5 prolongs the dissociated state by acetylating lysines used to contact DNA
p23 influences the genome-wide maintenance of DNase I hypersensitive sites
Acknowledgments
We thank Charles Miller 3rd (Tulane University) and Didier Picard (Geneve University) for the parental and p23 null mouse embryonic fibroblast cells, Sharon Dent (University of Texas) for the parental and GCN5 null mouse embryonic fibroblast cells, Richard Morimoto (Northwestern University) for the HSF1 plasmids, Andrew Belmont (UIUC) for the GCN5 plasmids, Titia De Lange (Rockefeller University) for the TRF2 vector, Supriya Prasanth (UIUC) for the CDC6 vector and Maria Spies (UIUC) for her generous gift of purified RAD51. We thank Yuka Bannai for her contributions with Mcm1. We are grateful to William Brieher and Frank Echtenkamp for comments on the manuscript. Support by the Public Service grants DK074270 and CA155333.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bell SP, Dutta A. DNA replication in eukaryotic cells. Annu Rev Biochem. 2002;71:333–374. doi: 10.1146/annurev.biochem.71.110601.135425. [DOI] [PubMed] [Google Scholar]
- Bell O, Tiwari VK, Thomä NH, Schübeler D. Determinants and dynamics of genome accessibility. Nat Rev Genet. 2011;12:554–564. doi: 10.1038/nrg3017. [DOI] [PubMed] [Google Scholar]
- Bu P, Evrard YA, Lozano G, Dent SY. Loss of Gcn5 acetyltransferase activity leads to neural tube closure defects and exencephaly in mouse embryos. Mol Cell Biol. 2007;27:3405–3416. doi: 10.1128/MCB.00066-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeZwaan DC, Freeman BC. Hsp90: The Rosetta Stone of cellular protein dynamics? Cell Cycle. 2008;7:1006–1012. doi: 10.4161/cc.7.8.5723. [DOI] [PubMed] [Google Scholar]
- DeZwaan DC, Freeman BC. Hsp90 manages the ends. Trends Biochem Sci. 2010;35:384–391. doi: 10.1016/j.tibs.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echtenkamp FJ, Woo JI, Oxelmark E, Zelin E, Andrews B, Garabedian MJ, Freeman BC. Global functional map of the yeast p23 homolog reveals extensive nuclear molecular chaperone activities. Mol Cell. 2011;43:229–241. doi: 10.1016/j.molcel.2011.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echtenkamp FJ, Freeman BC. Expanding the cellular molecular chaperone network through the ubiquitous cochaperones. Biochim Biophys Acta. 2012;1823:668–673. doi: 10.1016/j.bbamcr.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Echtenkamp FJ, Freeman BC. Molecular chaperone-mediated nuclear dynamics. Curr Prot Pept Sci. doi: 10.2174/1389203715666140331112230. (in press) [DOI] [PubMed] [Google Scholar]
- Ellis J. Proteins as molecular chaperones. Nature. 1987;328:378–379. doi: 10.1038/328378a0. [DOI] [PubMed] [Google Scholar]
- Ellis RJ. Protein misassembly: macromolecular crowding and molecular chaperones. Adv Exp Med Biol. 2007;594:1–13. doi: 10.1007/978-0-387-39975-1_1. [DOI] [PubMed] [Google Scholar]
- Espinosa MC, Rehman MA, Chisamore-Robert P, Jeffery D, Yankulov K. GCN5 is a positive regulator of origins of DNA replication in Saccharomyces cerevisiae. PLoS One. 2010;5:e8964. doi: 10.1371/journal.pone.0008964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman BC, Felts SJ, Toft DO, Yamamoto KR. The p23 molecular chaperones act at a late step in intracellular receptor action to differentially affect ligand efficacies. Genes Dev. 2000;14:422–434. [PMC free article] [PubMed] [Google Scholar]
- Freeman BC, Yamamoto KR. Disassembly of transcriptional regulatory complexes by molecular chaperones. Science. 2002;296:2232–2235. doi: 10.1126/science.1073051. [DOI] [PubMed] [Google Scholar]
- Freeman BC, Yamamoto KR. Continuous recycling: a mechanism for modulatory signal transduction. Trends Biochem Sci. 2001;26:285–290. doi: 10.1016/s0968-0004(01)01834-5. [DOI] [PubMed] [Google Scholar]
- Garel JR, Martel A, Muller K, Ikai A, Morishima N, Sutoh K. Role of subunit interactions in the self-assembly of oligomeric proteins. Adv Biophys. 1984;18:91–113. doi: 10.1016/0065-227x(84)90008-x. [DOI] [PubMed] [Google Scholar]
- Grad I, McKee TA, Ludwig SM, Hoyle GW, Ruiz P, Wurst W, Floss T, Miller CA, 3rd, Picard D. The Hsp90 cochaperone p23 is essential for perinatal survival. Mol Cell Biol. 2006;26:8976–8983. doi: 10.1128/MCB.00734-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant PA, Duggan L, Côté J, Roberts SM, Brownell JE, Candau R, Ohba R, Owen-Hughes T, Allis CD, Winston F, Berger SL, Workman JL. Yeast Gcn5 functions in two multisubunit complexes to acetylate nucleosomal histones: characterization of an Ada complex and the SAGA (Spt/Ada) complex. Genes Dev. 1997;11:1640–1650. doi: 10.1101/gad.11.13.1640. [DOI] [PubMed] [Google Scholar]
- Guertin MJ, Lis JT. Chromatin landscape dictates HSF binding to target DNA elements. PLoS Genet. 2010;6:e1001114. doi: 10.1371/journal.pgen.1001114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager GL, McNally JG, Misteli T. Transcription dynamics. Mol Cell. 2009;35:741–753. doi: 10.1016/j.molcel.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselberth JR, Chen X, Zhang Z, Sabo PJ, Sandstrom R, Reynolds AP, Thurman RE, Neph S, Kuehn MS, Noble WS, Fields S, Stamatoyannopoulos JA. Global mapping of protein-DNA interactions in vivo by digital genomic footprinting. Nat Methods. 2009;6:283–289. doi: 10.1038/nmeth.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John S, Sabo PJ, Thurman RE, Sung MH, Biddie SC, Johnson TA, Hager GL, Stamatoyannopoulos JA. Chromatin accessibility pre-determines glucocorticoid receptor binding patterns. Nat Genet. 2011;43:264–268. doi: 10.1038/ng.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo MH, Brownell JE, Sobel RE, Ranalli TA, Cook RG, Edmonson DG, Roth SY, Allis CD. GCN5p, a yeast nuclear histone acetyltransferase, acetylates specific lysines in histone H3 and H4 that differ from deposition-related acetylation sites. Nature. 1996;383:269–272. doi: 10.1038/383269a0. [DOI] [PubMed] [Google Scholar]
- Lemon B, Tjian R. Orchestrated response: a symphony of transcription factors for gene control. Genes Dev. 2000;14:2551–2569. doi: 10.1101/gad.831000. [DOI] [PubMed] [Google Scholar]
- Mai B, Miles S, Breeden LL. Characterization of the ECB binding complex responsible for the M/G(1)-specific transcription of CLN3 and SWI4. Mol Cell Biol. 2002;22:430–441. doi: 10.1128/MCB.22.2.430-441.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally JG, Muller WG, Walker D, Wolford R, Hager GL. The glucocorticoid receptor: rapid exchange with regulatory sites in living cells. Science. 2000;287:1262–1265. doi: 10.1126/science.287.5456.1262. [DOI] [PubMed] [Google Scholar]
- Metivier R, Penot G, Hubner MR, Reid G, Brand H, Kos M, Gannon F. Estrogen Receptor-α directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell. 2003;115:751–763. doi: 10.1016/s0092-8674(03)00934-6. [DOI] [PubMed] [Google Scholar]
- Minton AP. How can biochemical reactions within cells differ from those in test tubes? J Cell Sci. 2006;119:2863–2869. doi: 10.1242/jcs.03063. [DOI] [PubMed] [Google Scholar]
- Misteli T. The concept of self-organization in cellular architecture. J Cell Biol. 2001;155:181–185. doi: 10.1083/jcb.200108110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DD, Theodorakis NG, Morimoto RI. Coordinate changes in heat shock element-binding activity and HSP70 gene transcription rates in human cells Mol. Cell Biol. 1988;8:4736–4744. doi: 10.1128/mcb.8.11.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland NM, Soeth E, Smith CL. Inhibition of MMTV transcription by HDAC inhibitors occurs independent of changes in chromatin remodeling and increased histone acetylation. Oncogene. 2003;22:4807–4818. doi: 10.1038/sj.onc.1206722. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Tora L. Distinct GCN5/PCAF-containing complexes function as co-activators and are involved in transcription factor and global histone acetylation. Oncogene. 2007;26:5341–5357. doi: 10.1038/sj.onc.1210604. [DOI] [PubMed] [Google Scholar]
- Ouspenski II, Elledge SJ, Brinkley BR. New yeast genes important for chromosome integrity and segregation identified by dosage effects on genome stability. Nucleic Acids Res. 1999;27:3001–3008. doi: 10.1093/nar/27.15.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolinelli R, Mendoza-maldonado R, Cereseto A, Giacca M. Acetylation by GCN5 regulates CDC6 phosphorylation in the S phase of the cell cycle. Nat Struct Mol Biol. 2009;16:412–420. doi: 10.1038/nsmb.1583. [DOI] [PubMed] [Google Scholar]
- Perlmann T, Eriksson P, Wrange O. Quantitative analysis of the glucocorticoid receptor-DNA interaction at the mouse mammary tumor virus glucocorticoid response element. J Biol Chem. 1990;265:17222–17229. [PubMed] [Google Scholar]
- Phair RD, Scaffidi P, Elbi C, Vecerová J, Dey A, Ozato K, Brown DT, Hager G, Bustin M, Misteli T. Global nature of dynamic protein-chromatin interactions in vivo: three-dimensional genome scanning and dynamic interaction networks of chromatin proteins. Mol Cell Biol. 2004;24:6393–6402. doi: 10.1128/MCB.24.14.6393-6402.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Zhao Y, Becker M, John S, Parekh BS, Huang S, Hendarwanto A, Martinez ED, Chen Y, Lu H, Adkins NL, Stavreva DA, Wiench M, Georgel PT, Schiltz RL, Hager GL. HDAC1 acetylation is linked to progressive modulation of steroid receptor-induced gene transcription. Mol Cell. 2006;22:669–679. doi: 10.1016/j.molcel.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Robert F, Pokholok DK, Hannett NM, Rinaldi NJ, Chandy M, Rolfe A, Workman JL, Gifford DK, Young RA. Global position and recruitment of HATs and HDACs in the yeast genome. Mol Cell. 2004;16:199–209. doi: 10.1016/j.molcel.2004.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A, Lindsey-Boltz LA, Unsal-Kaçmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- Sarge K, Zimarino V, Holm K, Wu C, Morimoto RI. Cloning and characterization of two mouse heat shock factors with distinct inducible and constitutive DNA-binding ability. Genes Dev. 1991;5:1902–1911. doi: 10.1101/gad.5.10.1902. [DOI] [PubMed] [Google Scholar]
- Shi Y, Kroeger PE, Morimoto RI. The carboxyl-terminal transactivation domain of heat shock factor 1 is negatively regulated and stress responsive. Mol Cell Biol. 1995;15:4309–4318. doi: 10.1128/mcb.15.8.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims RJ, Reinberg D. Is there a code embedded in proteins that is based on post-translational modifications? Nat Rev Mol Cell Biol. 2008;9:815–820. doi: 10.1038/nrm2502. [DOI] [PubMed] [Google Scholar]
- Ucker DS, Yamamoto KR. Early events in the stimulation of mammary tumor virus RNA synthesis by glucocorticoids. Novel assays of transcription rates. J Biol Chem. 1984;259:7416–7420. [PubMed] [Google Scholar]
- Wang JC, Derynck MK, Nonaka DF, Khodabakhsh DB, Haqq C, Yamamoto KR. Chromatin immunoprecipitation (ChIP) scanning identifies primary glucocorticoid receptor target genes. Proc Nat l Acad Sci USA. 2004;101:15603–15608. doi: 10.1073/pnas.0407008101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver AJ, Sullivan WP, Felts SJ, Owen BA, Toft DO. Crystal structure and activity of human p23, a heat shock protein 90 co-chaperone. J Biol Chem. 2000;275:23045–23052. doi: 10.1074/jbc.M003410200. [DOI] [PubMed] [Google Scholar]
- Westerheide SD, Anckar J, Stevens SM, Sistonen L, Morimoto RI. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science. 2009;323:1063–1066. doi: 10.1126/science.1165946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto KR. Steroid receptor regulated transcription of specific genes and gene networks. Annu Rev Genet. 1985;19:209–252. doi: 10.1146/annurev.ge.19.120185.001233. [DOI] [PubMed] [Google Scholar]
- Yanagisawa J, Kitagawa H, Yanagida M, Wada O, Ogawa S, Nakagomi M, Oishi H, Yamamoto Y, Nagasawa H, McMahon SB, Cole MD, Tora L, Takahashi N, Kato S. Nuclear receptor function requires a TFTC-type histone acetyl transferase complex. Mol Cell. 2002;9:553–562. doi: 10.1016/s1097-2765(02)00478-1. [DOI] [PubMed] [Google Scholar]
- Yang XJ, Seto E. Lysine acetylation: codified crosstalk with other posttranslational modifications. Mol Cell. 2008;31:449–461. doi: 10.1016/j.molcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaret KS, Yamamoto KR. Reversible and persistent changes in chromatin structure accompany activation of a glucocorticoid-dependent enhancer element. Cell. 1984;38:29–38. doi: 10.1016/0092-8674(84)90523-3. [DOI] [PubMed] [Google Scholar]
- Zhong H, McCord R, Vershon AK. Identification of target sites of the alpha2-Mcm1 repressor complex in the yeast genome. Genome Res. 1999;9:1040–1047. doi: 10.1101/gr.9.11.1040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.