Abstract
Platinum (Pt)-based antitumor agents are widely used in cancer chemotherapy. Drug resistance is a major obstacle to the successful use of these agents because once drug resistance develops, other effective treatment options are limited. Recently, we have conducted a clinical trial using a copper (Cu)-lowering agent to overcome Pt drug resistance in ovarian cancer patients and the preliminary results are encouraging. In supporting this clinical study, using three pairs of cisplatin (cDDP)-resistant cell lines and two ovarian cancer cell lines derived from patients who had failed in Pt-based chemotherapy, we demonstrated that cDDP resistance associated with reduced expression of the high affinity copper transporter (hCtr1) which is also a cDDP transporter, can be preferentially re-sensitized by copper-lowering agents due to enhanced hCtr1 expression, as compared with their drug-sensitive counterparts. Such a preferential induction of hCtr1 expression in cDDP-resistant variants by Cu chelation can be explained by the mammalian Cu homeostasis regulatory mechanism. Enhanced cell-killing efficacy by a Cu-lowering agent was also observed in animal xenografts bearing cDDP-resistant cells. Finally, by analyzing a public gene expression dataset, we found that ovarian cancer patients with elevated levels of hCtr1 in their tumors, but not ATP7A and ATP7B, had more favorable outcomes after Pt-drug treatment than those expressing low hCtr1 levels. This study reveals the mechanistic basis for using Cu chelation to overcome cDDP resistance in clinical investigations.
Keywords: Cisplatin, high-affinity copper transporter, Cu-lowering agents, drug-resistance
Introduction
Platinum (Pt)-based drugs have been widely used in chemotherapy for many human solid cancers. Drug resistance is a major barrier to the successful use of these agents. Multiple mechanisms are involved in Pt drugs resistance and reduction of transport capacity has long been recognized as one of the important mechanisms (1, 2). Expression of the high-affinity copper transporter 1 (Ctr1) (SCLC31A1 or hCtr1 for humans), which also transports Pt drugs including cisplatin (cDDP) (3) and carboplatin (4), are frequently reduced in the cDDP-resistant (cDDPR) variants (5, 6).
The first demonstration that Cu chelation can sensitize the cell killing capacity by cDDP through the upregulation of hCtr1 transporter was observed in cell lines that overproduce glutathione (GSH) established by transfecting recombinant DNA encoding the heavy subunit of γ-glutamylcysteine synthetase (γ-GCSh), a rate-limiting enzyme for biosynthesis of GSH. GSH is an abundant physiologic Cu chelator and elevated GSH levels enhanced hCtr1 expression and cDDP transport (7). Induction of hCtr1 expression by Cu-chelation was also reported using other Cu-lowering agents (8–10). We recently conducted a clinical study using a Cu-lowering agent (trientine) in combination of carboplatin for five Pt-resistant high grade epithelial ovarian cancer patients and encouraging results were found (11). However, the mechanistic basis by which Cu chelation can overcome Pt drug resistance remains to be elucidated.
In the present study, we demonstrated in multiple cell models that expression of hCtr1 in cDDPR variants can be preferentially upregulated by Cu-lowering agents as compared with those in their drug-sensitive counterparts, providing greater sensitivity to the killing by Pt drugs. Enhanced cDDP efficacy by a Cu-lowering agent was also observed in animal tumor xenografts bearing cDDPR cells. Furthermore, we analyzed a public dataset and found that ovarian cancer patients with elevated expression levels of hCtr1 in their tumors had more favorable treatment outcomes after Pt-drug treatment than did those with low hCtr1 levels. Together, our findings provide the mechanistic basis for overcoming cDDP resistance using Cu chelation strategy.
Materials and Methods
Cell lines, reagents, and recombinant DNA
59M, OVCAR3, IGROV1, and SKOV3 and HEK293 cells were obtained from ATCC (Manassas, VA), 2008 and 2008-Cp cells were from Dr. Z. Siddik (MD Anderson Cancer Center, Houston, TX), A172 and A172-Cp cells were from Dr. A. Gomi (Jichi Univ., Tokyo, Japan), small cell lung cancer (SCLC) cell line and it cDDPR variant SR2 were from N. Savaraj, (University of Miami). hCtr1-wt and hCtr1-DN cell lines were described previously (10, 12). No authentication of these cell lines was done by the authors. cDDP, carboplatin, oxaliplatin, tetrathiomolybdate (TM), trientine, and D-pencillamide (D-pen) were purchased from Sigma-Aldrich (St Louis, MO). Cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum at 37°C in a 5% CO2 atmosphere.
Preparation of anti-hCtr1 antibody, confocal immunofluorescence microscopy, and blockage of Cu and cDDP transport assay
A 50-amino acid peptide from the N-terminus of hCtr1 was synthesized and conjugated to the keyhole limpet hemocyanin carrier protein and was used to immunize rabbits. The anti-peptide antibody was affinity purified. IGROV-1 cells grown on sterile cover glass slides were immunostained by anti-hCtr1 antibody followed the standard procedure. The cells were counterstained with propidium iodine (PI) and visualized with a Nikon confocal microscope.
For assaying the blockage of Cu and cDDP transport, 5 × 105 IGROV-1 cells per well were cultured in the Corning 6-well plates for 16 h. The cultures were then replaced with a fresh medium containing various concentrations of anti-hCtr1 antibody or control rabbit IgG and cells and were grown for another 2 h. The culture medium was removed, and new medium containing either 10 µM cDDP or 40 µM CuSO4 was added. After incubation of 1 h, the cells were washed three times with PBS and the amounts of Pt and Cu in the cells were measured by atomic absorption spectroscopy.
Animal experiments
Female athymic NCR nu/nu-nude mice (aged 5 weeks, NCI-Frederick Cancer Research and Development Center, Frederick, MD) were housed in a pathogen-free environment with an approved protocol by the Institutional Animal Care and Use Committee. The animals were inoculated subcutaneously with 5 × 106 SR2 cells on the dorsal lumbosacral region. When the tumor volumes reached 150-250 mm3, the animals were randomly divided into four groups, four animals per group. The first group was treated with 100 µl of 0.85% NaCl by gavage. The second group was treated with 100 µl of 400 mM D-pen (in 0.85% NaCl) by daily gavage for 28 days. The third group was intravenously injected with cDDP (5 mg/kg) four times at intervals of 7 days. The fourth group was treated with combined D-pen and cDDP following the schedules for the second and third groups, respectively.
Tumor growth rate was evaluated weekly using the described formular (13). One week after the last treatment, two mice from each group were euthanized and necropsied. Blood samples were collected for hematology and biochemistry analysis prior to termination. At necropsy, the liver, kidney, heart, lung, submandibular and mesenteric lymph nodes, spleen, and the femur and sternum with the bone marrow were examined grossly and collected in 10% neutral buffered formalin for microscopic examination. Total body weight of the mice and the weights of the kidney, spleen, and liver, were recorded for each animal at necropsy. Formalin-fixed tissues were processed in 4 µm-thick sections on glass slides that were stained with hematoxylin and eosin (H&E). The pathologist examined microscopically the H&E stained slides and evaluated all pathology data including hematology, biochemistry, and organs weight results.
Determination of hCtr1 expression and treatment outcomes in the ovarian cancer database
Microarray gene expression data published by Tothill et al (14) was used for determination of whether ovarian cancer patients with higher levels of hCtr1 expression in their tumors would have better treatment outcomes with cDDP-based regimens. We used a Cox proportional hazard regression model for univariate survival analysis. All statistical analysis and Kaplan-Meier survival plots were performed using R Software (15).
Other Procedures
Procedures for determinations of 64Cu (MIR Radiological Sciences, St. Louis, MO) (16) and Pt contents, drug sensitivity (IC50), RNase protection assay (RPA), Western blotting using anti-hCtr1 and anti-Sp1 antibodies (Santa Cruz Biotech. Santa Cruz, CA) (10, 12) were described previously. All statistical analyses were performed from at least four measurements using the two-tailed t-test and the results were expressed as mean ± standard deviation. A p< 0.05 was considered as statistically significant.
Results
The magnitudes of hCtr1 regulation by Cu stresses are constrained within upper and lower limits and depend upon the basal hCtr1 levels
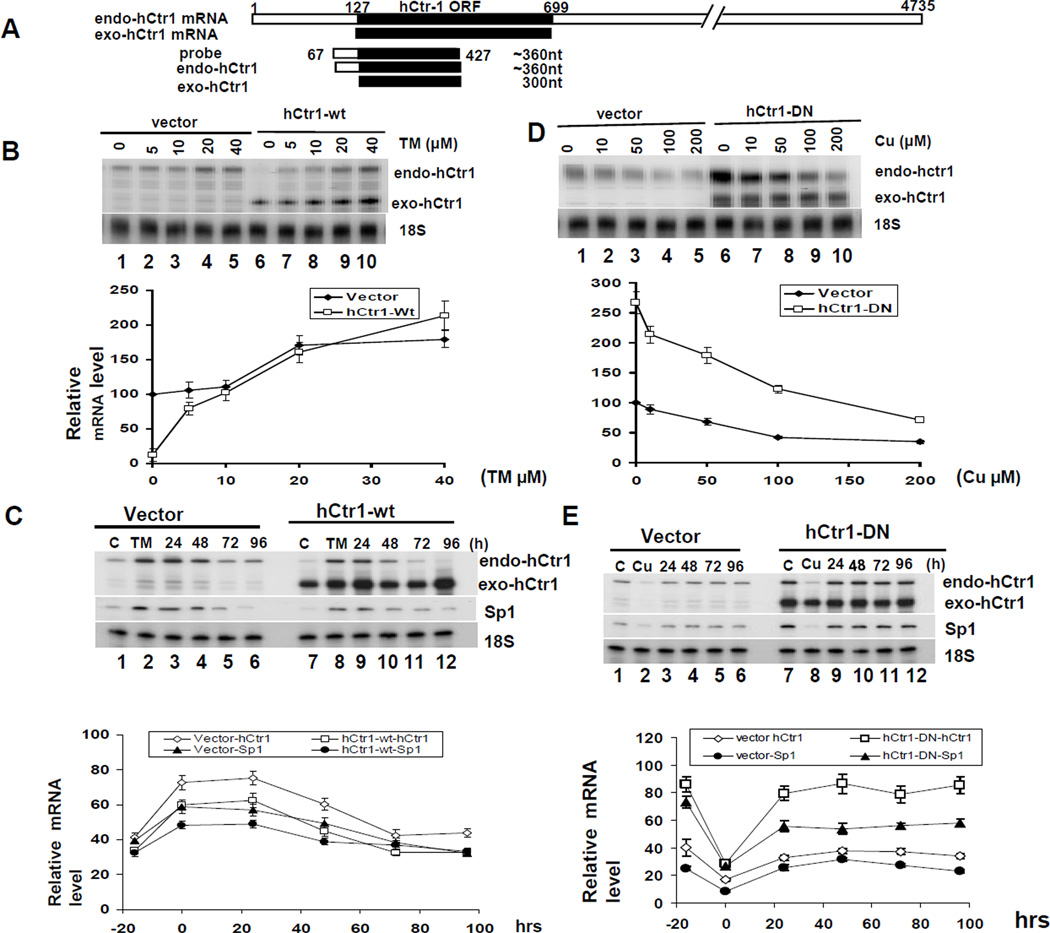
Using specifically designed probe in the RPA that can differentiate transcripts between the transfected (exogenous, exo-hCtr1) and the endogenous (endo) hCtr1 alleles (Fig. 1A), we previously demonstrated that overexpressing the wild-type exo-hCtr1 mRNA in SCLC cells downregulates endo-hCtr1 mRNA expression (10) (~85% reduction, Fig. 1B, comparing lanes 1 and 6). In contrast, expression of dominant-negative (dn-) hCtr1 upregulates endo-hCtr1 expression (12) (2–7-fold, Fig. 1D). We used these genetically engineered cell lines to investigate whether different endo-hCtr1 background would affect the magnitudes of hCtr1 regulation by Cu stresses. Fig. 1B shows that treating hCtr1-wt- and vector-transfected cells with a Cu chelator (TM) resulted in a concentration-dependent upregulation of endo-hCtr1 mRNA with maximal induction of ~20-fold for the former and ~80% for the latter at 20 µM TM. These results demonstrated that magnitudes of hCtr1 induction by Cu chelation depend upon the basal endo-hCtr1 levels. These results also demonstrated that maximal levels of endo-hCtr1 induction between these two cell lines were very similar.
Fig. 1.
hCtr1 regulation by Cu stresses is constrained by upper and lower limits and the magnitudes of regulation depend upon the basal hCtr1 levels. (A) A schematic diagram showing the design of the hybridization probe for the simultaneous detection of endo-hCtr1 and exo-hCtr1 mRNA levels by the RPA (see ref. (9)). (B) hCtr1-wt or vector-transfected SCLC cells were treated with different concentrations of TM for 16 h. Endo-hCtr1 mRNA and exo-hCtr1 mRNA levels were determined by the RPA using probe as indicated in (A). 18S RNA was used as a loading control. Lower panel, densitometric measurements of the RPA results. (C) hCtr1-wt-transfected or vector-transfected SCLC cells were treated with TM for 16 h and then were grown in medium without TM for different time intervals as indicated. Endo- and exo-hCtr1 and endo-Sp1 mRNA, and 18S RNA were measured. Densitometric measurements of RPA results are shown in the lower panel. (D) hCtr1-DN-transfected or vector-transfected cells were treated with CuSO4 as indicated for 16 h. Endo- and exo-hCtr1 mRNA levels were determined by RPA. Lower panel shows densitometric measurements. (E) hCtr1-DN or vector–transfected cells were treated with CuSO4 for 16 h then were then grown in CuSO4-free medium for different time intervals. Endo- and exo-hCtr1 and Sp1 mRNA levels were determined. Lower panel, densitometric measurements of the results.
We then tested the reversibility of TM-induced hCtr1 upregulation. We first treated the cells with 40 µM TM for 16 h and then allowed them to grown in a medium without TM for different lengths of time. The endo-hCtr1 mRNA levels in these two cell lines were gradually reduced with similar kinetics and reached levels comparable to those in the respective untreated controls 96 h after the removal of TM (Fig. 1C). These results further support that the influence of basal hCtr1 mRNA levels in hCtr1 regulation by Cu-depleting agent.
To determine whether basal hCtr1 mRNA levels also regulate hCtr1 downregulation by Cu overload, we used hCtr1-dn-transfected cells. Fig. 1D shows that CuSO4 treatment induces greater reduction of endo-hCtr1 mRNA in hCtr1-dn-transfected cells (~2.6-fold) then in the vector-transfected cells (~60%), but reaching to levels close to each other when high concentration of CuSO4 was used. The reversibility test revealed that Cu-induced hCtr1 mRNA downregulation was restored within 24 h after Cu removal for both cell lines, reduced to levels similar to their respective untreated controls (Fig. 1E). Figures 1C and 1E also show that the expression of Sp1 and hCtr1 was coordinated, consistent with the inter-regluatory relationship between Sp1 and hCtr1 in response to Cu concentration changes (9). These results, taken together, show that there are homeostatic hCtr1 limits that dictate the amplitudes of hCtr1 regulation by Cu stresses.
Resensitization of cDDP-resistant cell lines to Pt drugs by Cu-lowering agents
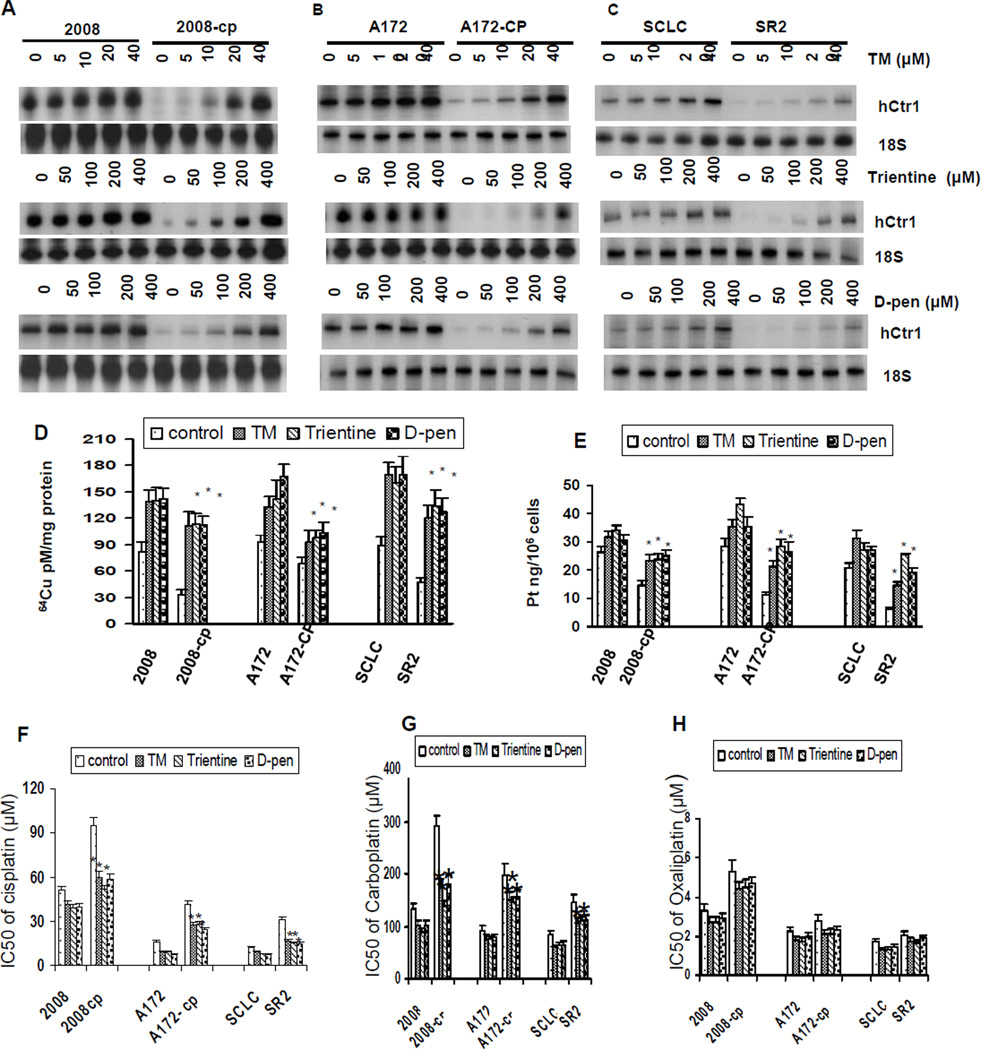
Given the observations that cDDPR variants often display reduced hCtr1 expression (5, 6), we then tested whether hCtr1 expression in cDDPR variants would be greatly induced by Cu-lowering agents as compared with their cDDPS counterparts. Three cDDPR/cDDPS pairs, i.e. 2008-Cp (ovarian cancer cells), A172-Cp (glioma cells), and SR2 (SCLC), and three different Cu chelators (TM, trientine, and D-pen) were used.
All these cDDPR cells exhibited reduced hCtr1 expression in reference to their corresponding parental cells (Fig. 2A – 2C, comparing the non-treated lanes in each pairs). These cDDPR cells also show reduced Pt accumulation (Fig. 2E), therefore, these are Pt transport variants. Treating pairwise cDDPR/cDDPS cell lines with different Cu depletors enhanced hCtr1 mRNA expression. Strikingly, hCtr1 mRNA expression levels were increased more in the cDDPR cells (ranging from 15- to 20-fold increase from basal levels) than in their respective drug-sensitive cells (< 50% increase) by densitometry analysis (data not shown). Higher hCtr1 expression in the treated cells generally correspond with larger increases in 64Cu (Fig. 2D) and cDDP (Fig. 2E) uptake, although there may not be strictly correlated in all cases, suggesting the complexity of cDDP resistance mechanisms. Preferentially enhanced hCtr1 expression is generally correlated with an increased sensitivity to cDDP (Fig. 2F) and carboplatin albeit to a lesser extent (Fig. 2G), but much reduced sensitivity to oxaliplatin (Fig. 2H).
Fig. 2.
hCtr1 mRNA levels in cDDPR cells and their parental cells treated with different Cu-lowering agents. (A – C) RPA of endo-hCtr1 mRNA expression levels in three pairs of cells, i.e., 2008 (cDDPS)/2008-cp (cDDPR), A172 (cDDPs)/A172-CP (cDDPR), and SCLC (cDDPS)/SR2 (cDDPR), treated with different concentrations of TM (upper row), trientine (middle), or D-pen (lower) as indicated for 16 h. 18S RNA was used as an internal control. (D) Measurements of 64Cu uptake in three pairs of cDDPR/cDDPS cell lines with or without Cu-lowering agent added. (E) cDDP uptake measurements. Cells were treated with TM (5 µM), trientine or D-Pen (100 µM each) for 16 h. Greater sensitization of cDDPR cells to cDDP (F), carboplatin (G), but not oxaliplatin (H) than their parental cells, after treatment with Cu-lowering agents was shown *, p ≤ 0.05, Student’s t-test.
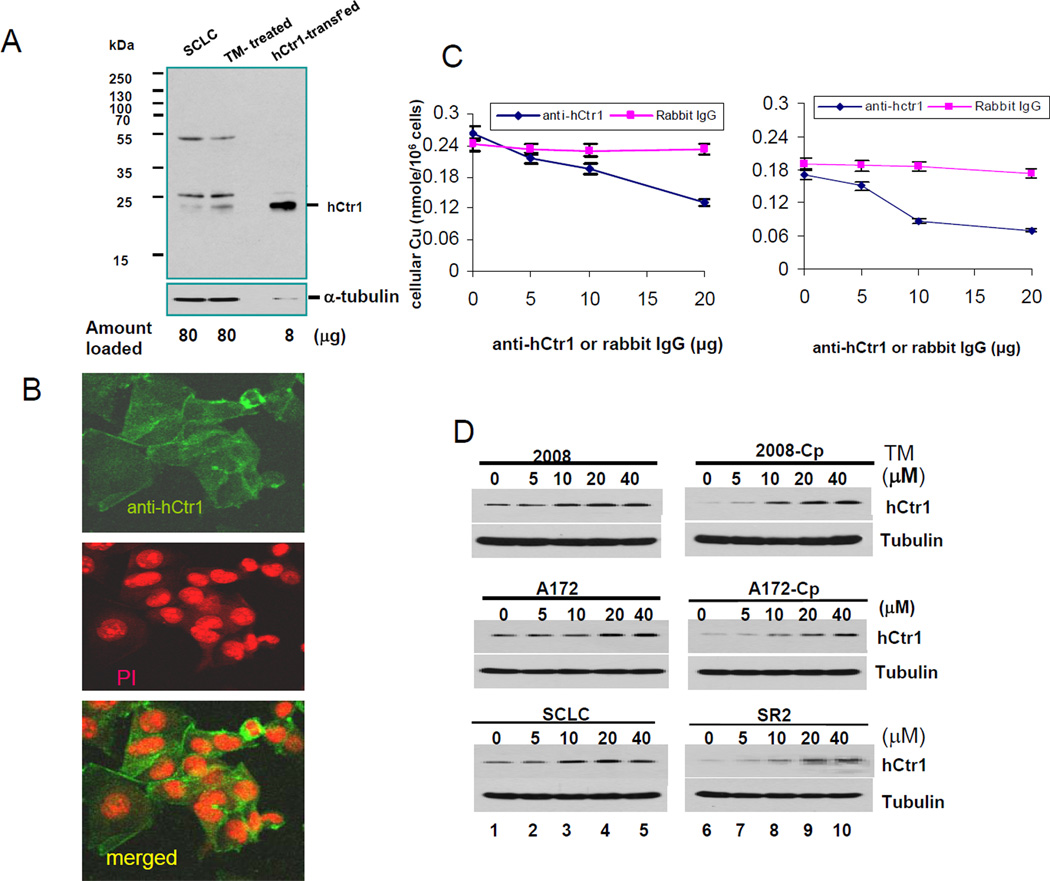
We prepared a polyclonal antibody using the N-terminal 50 amino acid residues of hCtr1 as antigen. This peptide sequence is extracellularly located and is important for the hCtr1-mediated Cu and cDDP transport (12, 17). Three protein species (~55, 28, and 23 kDa) were seen in the Western blots of whole cell extracts prepared from SCLC and TM-treated SCLC cells by this antibody (Fig. 3A). The intensity of 23-kDa signal, which corresponds to the molecular mass of unmodified monomeric hCtr1 protein, was increased in the TM-treated sample. This 23-kDa protein is highly expressed in the hCtr1-wt-transfected cells. Further characterizations of this anti-hCtr1 antibody included Immunofluorescent confocal microscopy on cultured cells which revealed that this anti-hCtr1 antibody heavily stains the cell membrane, with minor staining inside the cytoplasm, consistent with primary cytologic location of hCtr1 (Fig. 3B). Furthermore, this antibody can block Cu and cDDP transport in concentration-dependent manner using non-immunized rabbit IgG as a control (Fig. 3C), demonstrating the functionality of this antibody. Whether the 55-kDa and 28-kDa signals represent posttranslationally modified hCtr1 or non-specific proteins have yet to be determined. Given the consideration that Ctr1 is an evoluationarily conserved protein and good anti-hCtr1 antibody is difficult to obtain (18, 19), our hCtr1 antibody can be reliably used for Western blotting analyses.
Fig. 3.
Characterization of anti-hCtr1 antibody. (A) Western blotting of hCtr1 expression in cell extracts prepared from different cells as indicated. The hCtr1-transfected sample was purposely under-loaded (0.1x the amount). (B) Confocal immunofluorescence microscopy of hCtr1 staining by anti-hCtr1 antibody (upper), PI staining (middle) and merged (lower). (C) Functional demonstration of the concentration-dependent suppression of Cu (left) and cDDP (right) transport by the hCtr1 antibody. (D) Western blotting determinations of hCtr1 expression in three pairs of cDDPR vs their corresponding sensitive cells treated with various concentrations of TM as indicated for 16 h.
Western blottings of hCtr1 expression in three pairs of cDDPR vs cDDPS cells treated with various concentrations of TM for 16 h were performed. hCtr1 levels in all three cDDPR variants were markedly less than those in the corresponding cDDPS cell lines (Fig. 3D, between lanes 1 and 6) and all the CDDPR cell lines exhibited greater hCtr1 induction (~20 fold above basal levels) than their corresponding parental cell lines (< 2-fold) (Fig. 3D). Overall, the hCtr1 protein results were consistent with the mRNA results (Figs. 2A ~ 2C). No induction of Cu exporters ATP7A and ATP 7B at mRNA and protein levels between 2008 and 2008-Cp were seen by the RPA and Western blotting, respectively (Supplementary Fig. 1S).
Cu-lowering agents preferentially sensitize cDDPR ovarian cancer cell lines derived from patients failed cDDP-based chemotherapy
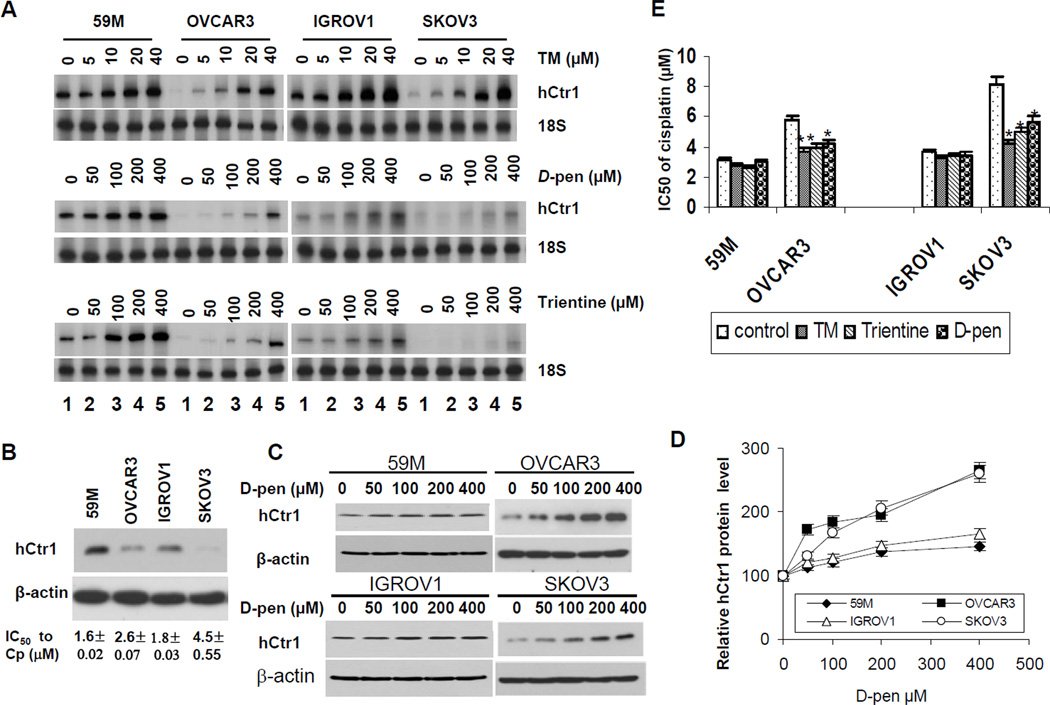
We further asked whether cells lines derived from ovarian cancer patients who failed cDDP-based treatments would contain reduced hCtr1 levels, and if so, whether these cells could be preferentially re-sensitized to cDDP treatment by Cu-lowering agents in comparison with those derived from patients had not been treated with Pt drugs. We randomly chose four cell lines including, the 59M cell line which was established from the ascites of an ovarian cancer patient had never been treated with cDDP but failed isoflamide/melphane treatment (20), the IGROV1 line which was established from a patient prior to chemotherapy (21), and the OVCAR3 (22) and the SKOV3 (23) cell lines which were established from patients with relapsed cDDP-based treatments.
These cells lines were treated with various concentrations of TM, D-pen, or trientine for 16 h and following results were obtained: (i) Without treatment, levels of hCtr1 mRNA (Fig. 4A, lanes 1) and protein (Fig. 4B) were lower in cell lines derived from patients who had been treated with Pt-drug (OVCAR3 and SKOV3) than those derived from patients without Pt-drug exposure (M59 and IGROV1). Although it is tempting to infer that the in situ low hCtr1 expression was associated with the cDDP-refractory outcome in patients, we realize that these cell lines have been in cultures for so long and results must be treated with great care. (ii) The sensitivities of these tumor cell lines to cDDP treatment were inversely correlated with their hCtr1 expression levels (Fig. 4B bottom, IC50 values), implicating that cell lines derived from non-cDDP-treated patients were more sensitive to cDDP than those derived from patients that had been treated with cDDP. (iii) RPA assays showed that hCtr1 mRNA levels were induced in all four ovarian cancer cell lines by all three Cu-lowering agents; and all the Cu-lowering agents induces more hCtr1 mRNA expression in the low hCtr1-expressing cells (OVACAR3 and SKOV3, 9- to 15-fold) than in the high hCtr1-expressing cells (59M and IGROV1, 1.5- to 2-fold) (Fig. 4A). (iv) D-Pen induced hCtr1 protein expression in all four cell lines (Fig. 4C), and greater induction of protein expression was found in low-hCtr1 expressing cells (OVCAR3 and SKOV3, 2- to 3-fold) than in the high hCtr1-expressing cells (59M and GROV1, ~50% increases) (Fig. 4D). Although the fold-changes in protein expression analyzed by Western blotting were not strictly agreeable with the mRNA results, the trend that the Cu-lowering agent induces hCtr1 more in the low hCtr1-expressing cells than in the high hCtr1-expressing cells remained consistent. The discrepancy in the fold of induction determined between Western blotting and RNA assays may reflect differences in sensitivities of the two assay systems and/or possible involvement of translational/post-translational regulation. And (v), we also found that greater induction of hCtr1 expression was correlated with greater sensitivities to cDDP (Fig. 4E). These results, collectively, suggest that hCtr1 expression levels are correlated to cDDP sensitivity and that Cu-lowering agents can preferentially re-sensitize ovarian cancer cells with reduced hCtr1 expression from patients-derived cell lines.
Fig. 4.
hCtr1 expression levels and re-sensitization to cDDP in four ovarian cancer cell lines treated with Cu-lowering agents. (A) RPA of hCtr1 mRNA levels in cells treated with different concentrations of TM (upper), D-pen (middle), and trientine (lower). (B) Western blotting analysis of hCtr1 expression (top) and sensitivity of these cell lines to cDDP (IC50 values shown in bottom). (C) Western blotting of ovarian cancer cell lines treated with D-Pen as indicated using β-actin as loading control. (D) Densitometric analyses of hCtr1 expression blotting results as shown in (C). (E) Sensitivity of ovarian cancer cells to cDDP in the presence and absence of Cu-lowering agents as indicated.
Cu-lowering agent enhances the antitumor activity of cDDP in an animal tumor model
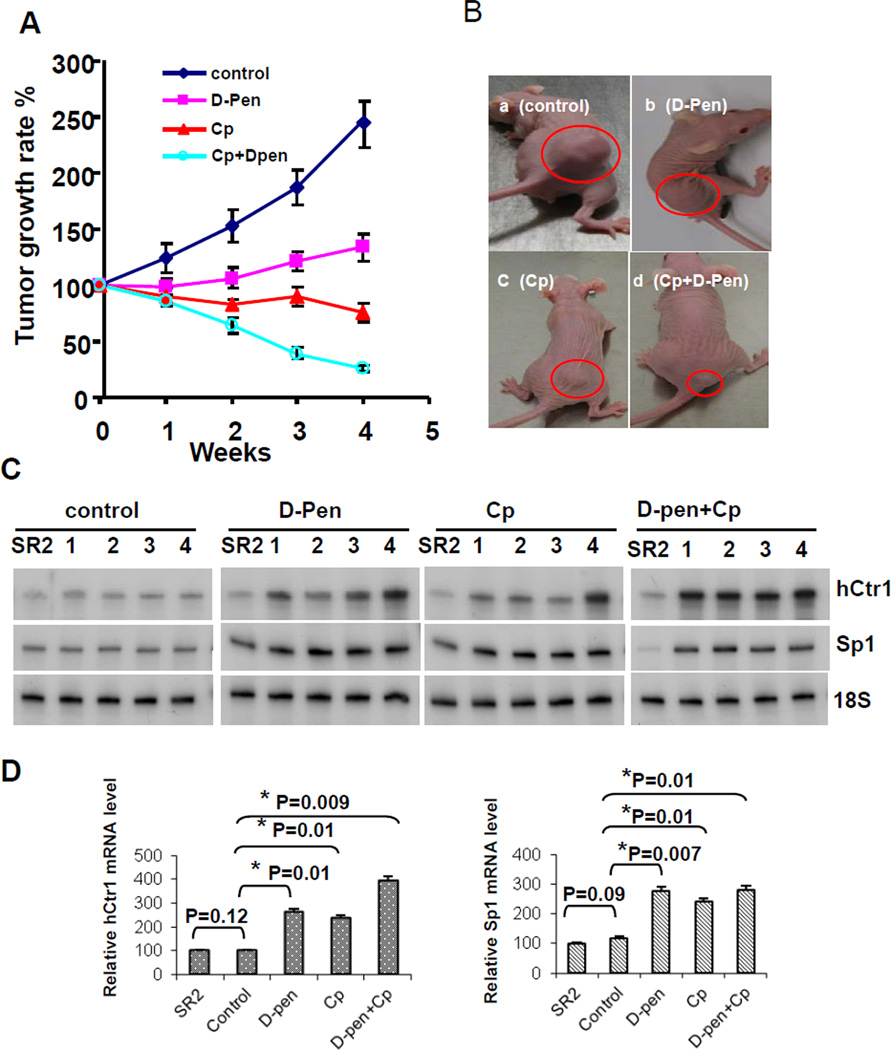
We established SR2 tumor xenografts in nu/nu mice. These animals were treated with vehicle, D-pen, cDDP, or D-pen plus cDDP over 4 weeks. In comparison with those in the untreated group, tumor growth rates in the D-pen-treated group were reduced, consistent with previous findings that anti-Cu agents have antitumor activities (24). Tumor growth rates were also reduced in animals treated with cDDP alone, and strikingly, were further reduced in the group treated with D-pen and cDDP combination (Fig. 5A). Examples of tumor sizes in each group at the end of treatment are shown in Fig. 5B. These results show that D-pen can enhance the antitumor activity of cDDP in this animal model.
Fig. 5.
Enhanced cDDP-mediated inhibition of tumor growth by D-pen. (A) Growth rates measurements in four groups of animals. (B) Representative tumors in the treated animals from each group at the end of the treatment (circled). (C) hCtr1 and Sp1 mRNA expression in the residual tumors in each animal as determined by the RPA. (D) Statistical analyses of the hCtr1 (left) and Sp1 (right) expression results of RPA shown in (C).
hCtr1 mRNA and human Sp1 mRNA levels in the residual tumors from each animal at the end of treatments in reference to those in the SR2 cells were determined. The human hCtr1 and Sp1 probes do not cross-react with the murine counterparts (data not shown). No significant difference of hCtr1 and Sp1 expression levels between tumors from the untreated animals and those in SR2 cells was found (Fig. 5C and 5D). Tumors from all four animals in the D-pen- and cDDP-treated groups showed higher expression of hCtr1 and Sp1 than those in the untreated group, demonstrating that hCtr1/Sp1 expression can be induced by D-pen and cDDP in the xenograft model. The observation of cDDP treatment alone can also induce hCtr1/Sp1 expression can be explained that cDDP functions as an antagonist for hCtr1-mediated Cu transport (Liang et al., manuscript in preparation). We found further induction of hCtr1 expression in the D-pen plus cDDP-treated group, although no apparent additional induction of Sp1 mRNA expression as determined by densitometry (Fig. 5C and 5D). These results are consistent with the observed additional anti-tumoric effects in combination therapy using D-pen and cDDP (Fig. 5A). Therefore, we conclude that hCtr1 expression can be induced in animal tumor model by a Cu-lowering agent. hCtr1 protein levels could not be conclusively determined in the tumor tissues because our anti-hCtr1 antibody cross-reacts with the mouse counterpart.
No treatment-related abnormalities were observed clinically during the in-life period of animals of this study. Postmortem pathological investigations from each group of treated animals revealed no differences in hematological and biochemical results among the four groups of animals. Furthermore, gross and microscopic investigations of liver, kidney, heart, lung, submandibular and mesenteric lymph nodes, spleen, and bone marrow of the femur and sternum revealed no significant treatment-related adverse effects in animals of this study. Only a mild to moderate reduction of erythropoiesis of bone marrow was observed in 50% of the mice from groups treaded with cDDP and cDDP plus D-pen but not in mice from D-pen treated or control groups. Overall, the pathology results support the no observed adverse effect level (NOAEL) of the D-pen, cDDP, and combination of cDDP plus D-pen treatments in athymic nude mice under the doses and conditions described in this study.
Role of hCtr1 expression in sensitivity to cDDP chemotherapy in ovarian cancer patients
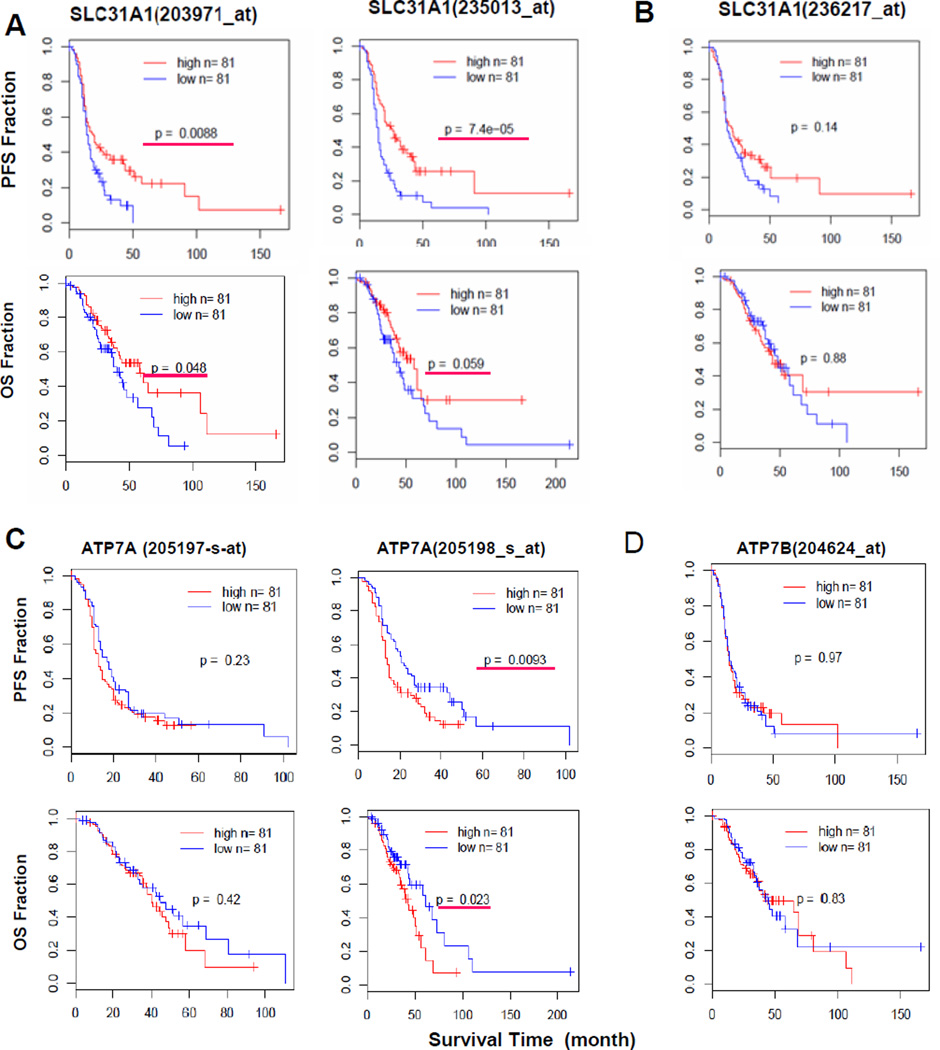
Ishida et al (8) analyzed an array-based hCtr1 expression dataset consisting of 91 patients with stage III or IV serous epithelial ovarian cancer who had been treated with a cytoreductive surgery followed by adjuvant chemotherapy of a Pt drug and a taxane deposited in The Cancer Genome Atlas. The investigators reported that high expression of hCtr1 was associated with longer disease-free survival in these patients. The therapeutic values of hCtr1 and other potential biomarkers for cDDP in clinical settings require additional substantiation. To this end, we analyzed an independent dataset derived from 285 serous and endometrioid tumors of ovary, peritoneum and fallopian tube using Affymetrix U133 Plus 2 arrays (14). The patient characteristics and clinicopathologic features were presented (14). Of the 285 patients, 243 patients received first-line Pt/taxane-based chemotherapy and were analyzed in the present study. While three SLC31A1 probes which encodes hCtr1 (203971_at, 235013_at, and 236217_at) were included in the microarray, however, using the BLAST tool from NCBI, we found that only 203971_at and 235013_at probes contain hCtr1 mRNA-encoded sequences located at the 3’-untranslated region; whereas probe 236271_at contains a sequence outside the transcriptional unit of SLC31A1 locus. Therefore, 236271_at probe is not an hCtr1 probe, but we used it as a negative control for the comparison.
To determine the relationship between the treatment outcomes and hCtr1 expression levels, we compared patients whose hCtr1 expression levels were in the upper 30th percentile (hereafter referred to a high hCtr1) with those in the lower 30th percentile (low hCtr1). We arbitrarily chose these cut-off lines so that the possibility of overlap between the high-hCtr1 and low-hCtr1 groups would be minimized. Fig. 6A shows that patients with high hCtr1 expression levels had significantly longer progression-free survival (PFS, upper panels) and overall survival (OS, lower panels) times than those with low hCtr1 expression detected by both 203971_at and 235013_at probes but not by the 236271_at probe (Fig. 4B).
Fig. 6.
Correlation between Cu expression and the treatment outcomes in ovarian cancer patient using different gene probes. (A) hCtr1, (B) hCtr1-unrelated DNA sequence, (C) ATP7A, and (D) ATP7B. In all cases, probes for the given genes are indicated in parentheses, PFS results are shown in the upper panels and the corresponding OS results are shown in the lower panels, high expressers in each panel are shown as red lines and lower expressers are shown in blue, *, p ≤0.05 is considered as significant and is underlined by a red line.
The roles of Cu/cDDP efflux exporters ATP7A and ATP7B were similarly analyzed. Two ATP7A probes were present in the dataset, but only one (204624_at) probe but not the other (205197_s_at) showed a statistically significant in correlation between reduced ATP7A expression and longer PFS and OS in the patients (Fig. 6C). Only one ATP7B probe (204624_at) is present in the dataset, but its expression has no significance for the treatment outcomes (Fig. 6D). We also analyzed the following cDDP resistance biomarkers (25) but no significant correlation was observed. These include the redox regulator γ-glutamycysteine synthetase (two probes, 1555330_at and 202922_at), ABCB1 (205197_s_at and 243951_at) and ABCC1 (202804-at and 202805-at) which encode MDR transporters, BRCA1 (204531_s_at and 211851_x_at) and DNA polymerase δ (208070_s_at and 208070_s_at) encoding proteins for repair/tolerance of cDDP-induced DNA damages (data not shown). These results, taken together, support the association of hCtr1 expression with favorable treatment outcomes in ovarian cancer patients receiving the cDDP/taxane protocol.
Discussion
In this communication we used multiple cultured cell models including genetically engineered, established cDDPR, and patient-derived cell lines to demonstrate that reduced hCtr1-associated cDDP resistance can be overcome by Cu-lowering agents. We also present supportive results from animal work and bioinformatics from independent ovarian cancer patient dataset demonstrating the role of hCtr1 expression and cDDP sensitivity. The mechanism underlying differential upregulation of hCtr1 in cDDPR variants over cDDPS cells by Cu-lowering agents can be explained by the transcriptional regulation of hCtr1 expression within the context of the overall Cu homeostasis regulation network which consists of Cu-Sp1-hCtr1 loop (9, 18). Here, we demonstrated that Cu homeostatic regulation is confined within upper and lower ranges that constrain the magnitudes of hCtr1 regulation in response to Cu stressed conditions. cDDPR cells with reduced hCtr1 levels possess high capacity of hCtr1 upregulation (~20-fold) by Cu chelation; whereas in cDDPS cells which already express high hCtr1 levels, only limited capacity bywhich hCtr1 can be further upregulated (general <2-fold). These findings provide the mechanistic basis for the use of Cu chelation in overcoming cDDP resistance.
Our current findings may explain some seemingly contradictory published results. It has been reported that no induction of rCtr1 expression in the livers and intestines (26) in the rats fed Cu-deficient diets, despite these organs showed >69% reduced Cu contents as correspondingly compared with those in animals fed Cu adequate diet. In another study, levels of mCtr1 expression were elevated in cervical tumors developed in the HPV16/E2 transgenic mice as compared with those in the cervix of wild-type animals. TM treatment did not further induce mCtr1 expression in these tumors (8). These results can be explained because these tissues already produce elevated Ctr1 levels. Alternatively, it remains possible that different Ctr1 regulation mechanisms may exist between in vivo and in vitro systems, and between tumor tissues and normal counterparts. Further studies are needed to address these important issues.
hCtr1 is located on human chromosome 9q32. Another transcription unit with opposite direction encoding an FK506-binding protein-like transcript is located −201 bp upstream of the transcription start site of hCtr1 locus (10). This intergenic sequence controls the expression of both genes in response of Cu bioavailability (our unpublished data). While we found no single nucleotide polymorphism associated with the Sp1 binding sites within this region from the NCBI database; however, whether other genetic polymorphisms in the promoter region of hCtr1 that may contribute to differential regulation of hCtr1 by Cu chelation, particularly in the patient-derived ovarian cancer cells used in this study, remains to be critically evaluated.
Posttranslational regulation of hCtr1 expression by Cu stresses has also been described (18, 27). Nonetheless, this study demonstrated that hCtr1 mRNA levels are mostly correlated with hCtr1 protein levels which in turn correlated with cDDP sensitivity, although we also observed no strict correlations in some cases. These results suggest that transcriptional regulation may remain the major mechanism of hCtr1 regulation by Cu chelation.
While Cu chelation strategy targets cDDPR tumor cells with reduced hCtr1 expression. It is important to note that mechanisms of cDDP resistance are multifactoral (28, 29). Notably, it has been reported that hCtr2 (for Cu storage) and ATP7A/ATP7B (for Cu efflux) can also transport cDDP (5) and their overexpression is associated with cDDP resistance (5, 30, 31). The complex cDDP resistance mechanisms suggest the need of using hCtr1 expression level as a guidance for the Cu chelation strategy in Pt drug chemotherapy.
Cu-lowering agents have been used for treating Wilson’s disease resulting from Cu accumulation because of genetic defect in ATP7B. These chelating agents have also been used in clinical studies by targeting tumor angiogenesis (32, 33) because many angiogenic modulators require Cu as cofactor (34, 35). Combination therapy using Cu-lowering agents and Pt drugs may have additive antitumor effects but may also produces additional adverse cytotoxicities (18). Carboplatin which is the second generation antitumor Pt drug has reduced cytotoxicities in many organs as compared with cDDP. The major adverse event in the trientine/carboplatin trial is myelosuppression but is medically manageable (11). Several approaches have been proposed for improving the therapeutic index of Cu chelation therapy by enhancing the treatment efficacies of Pt drug through upregulating hCtr1 expression and reducing the unwanted adverse events (18). Further research in this area may eventually lead to the development of effective strategies for using Cu chelation to combate Pt drug resistance in cancer chemotherapy.
Supplementary Material
Acknowledgement
Grant Support: Supported in part by ROI-CA152197 (M. T. Kuo) from the National Cancer Institute, the National Science Council, Taiwan (NSC97-2314-B-006-043) and the National Cheng Kung University Hospital (NCKUH9904005), Taiwan, to H.H.W. Chen.
We thank Drs. Zahid Siddik and Akira Gomi for the cell lines.
Footnotes
Conflicts of Interest: No potential conflicts of interest to disclose.
References
- 1.Bast RC, Jr, Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer. 2009;9:415–428. doi: 10.1038/nrc2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 3.Ishida S, Lee J, Thiele DJ, Herskowitz I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc Natl Acad Sci U S A. 2002;99:14298–14302. doi: 10.1073/pnas.162491399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holzer AK, Manorek GH, Howell SB. Contribution of the major copper influx transporter CTR1 to the cellular accumulation of cisplatin, carboplatin, and oxaliplatin. Mol Pharmacol. 2006;70:1390–1394. doi: 10.1124/mol.106.022624. [DOI] [PubMed] [Google Scholar]
- 5.Howell SB, Safaei R, Larson CA, Sailor MJ. Copper transporters and the cellular pharmacology of the platinum-containing cancer drugs. Mol Pharmacol. 2010;77:887–894. doi: 10.1124/mol.109.063172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuo MT, Chen HH, Song IS, Savaraj N, Ishikawa T. The roles of copper transporters in cisplatin resistance. Cancer Metastasis Rev. 2007;26:71–83. doi: 10.1007/s10555-007-9045-3. [DOI] [PubMed] [Google Scholar]
- 7.Chen HH, Song IS, Hossain A, Choi MK, Yamane Y, Liang ZD, et al. Elevated glutathione levels confer cellular sensitization to cisplatin toxicity by up-regulation of copper transporter hCtr1. Mol Pharmacol. 2008;74:697–704. doi: 10.1124/mol.108.047969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishida S, McCormick F, Smith-McCune K, Hanahan D. Enhancing tumor-specific uptake of the anticancer drug cisplatin with a copper chelator. Cancer Cell. 2010;17:574–583. doi: 10.1016/j.ccr.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang ZD, Tsai WB, Lee MY, Savaraj N, Kuo MT. Specificity protein 1 (Sp1) oscillation is involved in copper homeostasis maintenance by regulating human high-affinity copper transporter 1 expression. Mol Pharmacol. 2012;81:455–464. doi: 10.1124/mol.111.076422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song IS, Chen HH, Aiba I, Hossain A, Liang ZD, Klomp LW, et al. Transcription factor Sp1 plays an important role in the regulation of copper homeostasis in mammalian cells. Mol Pharmacol. 2008;74:705–713. doi: 10.1124/mol.108.046771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu S, Naing A, Fu C, Kuo MT, Kurzrock R. Overcoming Platinum Resistance Through the Use of a Copper-Lowering Agent. Mol Cancer Ther. doi: 10.1158/1535-7163.MCT-11-0864. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang ZD, Stockton D, Savaraj N, Tien Kuo M. Mechanistic comparison of human high-affinity copper transporter 1-mediated transport between copper ion and cisplatin. Mol Pharmacol. 2009;76:843–853. doi: 10.1124/mol.109.056416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Euhus DM, Hudd C, LaRegina MC, Johnson FE. Tumor measurement in the nude mouse. J Surg Oncol. 1986;31:229–234. doi: 10.1002/jso.2930310402. [DOI] [PubMed] [Google Scholar]
- 14.Tothill RW, Tinker AV, George J, Brown R, Fox SB, Lade S, et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin Cancer Res. 2008;14:5198–208. doi: 10.1158/1078-0432.CCR-08-0196. ( www.ncbi.nlm.nih.gov/geo, accession #GSE9899). [DOI] [PubMed] [Google Scholar]
- 15.The R project for statistical computing. ( http://www.r-project.org/) [Google Scholar]
- 16.Song IS, Savaraj N, Siddik ZH, Liu P, Wei Y, Wu CJ, et al. Role of human copper transporter Ctr1 in the transport of platinum-based antitumor agents in cisplatinsensitive and cisplatin-resistant cells. Mol Cancer Ther. 2004;3:1543–1549. [PubMed] [Google Scholar]
- 17.Kim BE, Nevitt T, Thiele DJ. Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol. 2008;4:176–185. doi: 10.1038/nchembio.72. [DOI] [PubMed] [Google Scholar]
- 18.Kuo MT, Fu S, Savaraj N, Chen HHW. Role of the human high-affinity copper transporter in copper homeostasis regulation and cisplatin sensitivity in cancer chemotherapy. Cancer Res. 2012;71:1–6. doi: 10.1158/0008-5472.CAN-12-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta A, Lutsenko S. Human copper transporters: mechanism, role in human diseases and therapeutic potential. Future Med Chem. 2009;1:1125–1142. doi: 10.4155/fmc.09.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hills CA, Kelland LR, Abel G, Siracky J, Wilson AP, Harrap KR. Biological properties of ten human ovarian carcinoma cell lines: calibration in vitro against four platinum complexes. Br J Cancer. 1989;59:527–534. doi: 10.1038/bjc.1989.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benard J, Da Silva J, De Blois MC, Boyer P, Duvillard P, Chiric E, et al. Characterization of a human ovarian adenocarcinoma line, IGROV1, in tissue culture and in nude mice. Cancer Res. 1985;45:4970–4979. [PubMed] [Google Scholar]
- 22.Saunders DE, Christensen C, Williams JR, Boyer P, Duvillard P, Chiric E, et al. Inhibition of breast and ovarian carcinoma cell growth by 1,25-dihydroxyvitamin D3 combined with retinoic acid or dexamethasone. Anticancer Drugs. 1995;6:562–569. doi: 10.1097/00001813-199508000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Fraifeld V, Seidman R, Sagi O, Muradian K, Wolfson M. Aurintricarboxylic acid decreases proliferative potential of SKOV3 and MCF7 human carcinoma cells. Anticancer Res. 2001;21:1975–1978. [PubMed] [Google Scholar]
- 24.Brewer GJ. Anticopper therapy against cancer and diseases of inflammation and fibrosis. Drug Discov Today. 2005;10:1103–1109. doi: 10.1016/S1359-6446(05)03541-5. [DOI] [PubMed] [Google Scholar]
- 25.Stewart DJ. Mechanisms of resistance to cisplatin and carboplatin. Crit Rev Oncol Hematol. 2007;63:12–31. doi: 10.1016/j.critrevonc.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Lee J, Prohaska JR, Dagenais SL, Glover TW, Thiele DJ. Isolation of a murine copper transporter gene, tissue specific expression and functional complementation of a yeast copper transport mutant. Gene. 2000;254:87–96. doi: 10.1016/s0378-1119(00)00287-0. [DOI] [PubMed] [Google Scholar]
- 27.van den Berghe PV, Klomp LW. Posttranslational regulation of copper transporters. J Biol Inorg Chem. 2010;15:37–46. doi: 10.1007/s00775-009-0592-7. [DOI] [PubMed] [Google Scholar]
- 28.Hall MD, Okabe M, Shen DW, Liang XJ, Gottesman MM. The role of cellular accumulation in determining sensitivity to platinum-based chemotherapy. Annu Rev Pharmacol Toxicol. 2008;48:495–535. doi: 10.1146/annurev.pharmtox.48.080907.180426. [DOI] [PubMed] [Google Scholar]
- 29.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 30.Blair BG, Larson CA, Adams PL, Abada PB, Safaei R, Howell SB. Regulation of copper transporter 2 expression by copper and cisplatin in human ovarian carcinoma cells. Mol Pharmacol. 2009;77:912–921. doi: 10.1124/mol.109.062836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dmitriev OY. Mechanism of tumor resistance to cisplatin mediated by the copper transporter ATP7B. Biochem Cell Biol. 2011;89:138–147. doi: 10.1139/o10-150. [DOI] [PubMed] [Google Scholar]
- 32.Henry NL, Dunn R, Merjaver S, Pan Q, Pienta KJ, Brewer G, et al. Phase II trial of copper depletion with tetrathiomolybdate as an antiangiogenesis strategy in patients with hormone-refractory prostate cancer. Oncology. 2006;71:168–175. doi: 10.1159/000106066. [DOI] [PubMed] [Google Scholar]
- 33.Yu Y, Wong J, Lovejoy DB, Kalinowski DS, Richardson DR. Chelators at the cancer coalface: desferrioxamine to Triapine and beyond. Clin Cancer Res. 2006;12:6876–6883. doi: 10.1158/1078-0432.CCR-06-1954. [DOI] [PubMed] [Google Scholar]
- 34.Gupte A, Mumper RJ. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat Rev. 2009;35:32–46. doi: 10.1016/j.ctrv.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Kuo HW, Chen SF, Wu CC, Chen DR, Lee JH. Serum and tissue trace elements in patients with breast cancer in Taiwan. Biol Trace Elem Res. 2002;89:1–11. doi: 10.1385/BTER:89:1:1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.