Abstract
Background
Granulocyte colony stimulating factor (G-CSF) and erythropoietin stimulating agents (ESA) may be used to support patients during chemotherapy. We assessed whether G-CSF or ESA were associated with progression or death in patients with ovarian cancer.
Methods
Patients with ovarian cancer following surgery, were on a protocol to evaluate bevacizumab with chemotherapy. Guidelines for administering G-CSF and ESA were specified in the protocol. Overall survival (OS) was analyzed with landmark procedures and multivariate, time-dependent hazard models.
Results
Eighteen-hundred-seventy-three women were enrolled, with no differences in clinical and pathologic variables among treatment group. Performance status, hemoglobin, and white cell counts were associated with G-CSF and/or ESA usage during treatment. Nine patients received no protocol directed therapy, leaving 1,864 patients for this review. One-thousand-one-hundred-twenty-five patients received neither ESA nor G-CSF; 311 received G-CSF but no ESA; 241 received ESA but no G-CSF; and 187 received both. Median survival following a five month landmark from the start of treatment was 34 versus 38 months for those who did versus did not receive ESA (multivariate hazard ratio: 0.989; 95% confidence interval: 0.849–1.15) and 40 versus 37 months for those who did versus did not receive G-CSF (multivariate hazard ratio: 0.932; 95% confidence interval: 0.800–1.08).
Conclusions
Neither ESA nor G-CSF had a negative impact on survival after adjustment of prognostic factors among patients with ovarian cancer receiving chemotherapy. ESA may appear to be associated with shorter survival in univariate analyses because factors prognostic for ESA use are also prognostic for progression-free survival.
Keywords: erythropoietin, cytokines, ovarian cancer
INTRODUCTION
Approximately 22,000 women in the United States annually will be diagnosed with ovarian cancer, and nearly 14,000 will die [1]. Ovarian cancer ranks as the second most lethal malignancy affecting women. After operation, patients with advanced disease are treated with cytotoxic chemotherapy which can induce significant hematologic toxicity.
Erythropoietin-stimulating agents (ESA) have been shown to increase hemoglobin levels, reduce the need for blood transfusions, and improve quality of life [2,3]. These benefits are particularly needed during chemotherapy. It has been suggested that ESA stimulate cancer cell growth, however, this has not been consistently supported [4,5].
A recent multi-institutional, retrospective review of women treated for ovarian cancer appeared to show negative impact on survival when ESA were used [6]. These authors recommend that since patients who receive ESA are more likely to experience recurrence, death, and decreased survival, the use of ESA should be carefully considered. However, the negative impact on overall survival (OS) may be due to confounding patient characteristics, such as age, pre-existing anemia or advanced stage [2,3,6]. Multivariate assessment of these confounding variables has been limited by small sample sizes.
We sought to evaluate the association between growth factor use and survival outcomes for women with ovarian cancer. To do this we performed an analysis of patients treated on GOG 218, a prospective randomized trial of chemotherapy with or without bevacizumab (given during and/or as consolidation) [7,8]. Data from GOG-0218 provide an opportunity to confirm the observations of Rocconi et al [6]. The larger sample size and the standardized data collection permits a multivariate assessment of the association between ESA and time to progression or death while adjusting for potential confounding from known prognostic factors.
The objectives of this study were to evaluate the factors associated with the usage of ESA and G-CSF during treatment of patients with ovarian cancer; and to evaluate the hypothesis that ESA or G-CSF are associated with an increased risk of progression or death in this patient population.
METHODS
Participants
Enrollment criteria included previously untreated stage III–IV epithelial ovarian, primary peritoneal or fallopian tube cancer after standard abdominal operation with maximal effort at tumor debulking. Eligible patients had a GOG performance status (PS) of 0–2 and no history of either significant vascular events or evidence of intestinal obstruction requiring parenteral hydration or nutrition. All participants gave informed consent according to institutional and federal guidelines before enrollment. Details of the primary objectives and the results from that trial have been reported.8
Study Design
This was a double-blind, placebo-controlled phase III trial. The study regimens consisted of 22 three-week cycles-the first six cycles including standard chemotherapy and the remaining 16 a continuation phase. Regimen 1 consisted of chemotherapy with intravenous (IV) carboplatin at an “area-under-the-curve” (AUC) of six and paclitaxel at 175 mg/m2 (CT) plus concurrent placebo (P), followed by placebo. Regimen 2 consisted of CP plus concurrent BEV at 15 mg/kg, followed by placebo. Regimen 3 consisted of CT plus concurrent BEV followed by BEV. In all treatment groups, BEV or placebo was initiated with cycle 2, to reduce the risk of wound complications. Treatment was continued for a total of 22 cycles, or discontinued for disease progression, unacceptable toxicity or voluntary withdrawal.
Disease was assessed prior to cycle 1 by physical examination, CA-125 assay and either computed tomography or magnetic resonance imaging. In the absence of progression, repeat assessments were to be performed following cycles 3, 6, 10, 14, 18, 22 and at the completion of protocol. Following completion of study treatment, disease assessments were repeated every three months for two years, then every six months for three years, then annually. In cases of treatment discontinuation for reasons other than disease progression, disease assessments were performed at time points projected based on participants' study calendars.
Safety was monitored through physical and laboratory assessments following each treatment cycle. Treatment decisions were based on the absolute neutrophil count (ANC) rather than the total white cell count (WBC). Patients who were delayed more than seven days were allowed to begin with ANC ≥ 1000 cells/mm3. The use of G-CSF was permitted only in the management of complicated neutropenia (febrile neutropenia or grade 4 neutropenia persisting ≥7 days) and prophylaxis for subsequent treatment cycles (Table 1). In general, patients were not to receive G-CSF unless they experienced treatment delays or recurrent neutropenic complications after treatment modifications as specified. Hematopoietic growth factors were not prescribed per protocol to avoid initial chemotherapy dose modifications. G-CSF was discontinued when the ANC exceeded 10,000/mm3 and not used within 72 hours of a subsequent dose of chemotherapy.
Table 1.
Treatment Cycle When Cytokine Was Initiated
| Treatment Cycle | Erythropoietin | GCSF |
|---|---|---|
| 1 | 85 | 71 |
| 2 | 101 | 92 |
| 3 | 74 | 119 |
| 4 | 61 | 84 |
| 5 | 54 | 79 |
| 6 | 40 | 48 |
| 7 | 8 | 3 |
| 8 | 3 | 1 |
| 9 | 1 | 0 |
| 12 | 1 | 1 |
|
| ||
| Total | 428 | 498 |
| Fraction and percent of patients initiating ESAs or GCSF by Initial Performance Status | ||
|---|---|---|
| Erythropoietin | ||
| Performance Status | ESA | GCSF |
| 0 | 174/928 (18.7) | 235/928 (25.3) |
| 1 | 209/805 (26.0) | 219/805 (27.2) |
| 2 | 45/131 (34.3) | 44/131 (33.6) |
| Total | 428/1864 | 498/1864 |
| Number of patients initiating GCSF or Erythropoietin during study | |||
|---|---|---|---|
| Erythropoietin | Total | ||
| GCSF | No | Yes | |
| No | 1125(82.4) | 241 (17.6) | 1366 |
| Yes | 311 (62.4) | 187 (37.5) | 498 |
| Total | 1436 | 428 | 1864 |
Patients were not to receive thrombopoietic agents unless they experienced recurrent Grade 4 thrombocytopenia after treatment modifications as specified.
Patients could receive ESA, iron supplements, and/or transfusions as clinically indicated for management of anemia at their treating physician's discretion.
Statistical Considerations
Study participants were stratified by stage of disease (stage III versus IV), maximum size of residual disease following primary surgery (≤1 cm versus >1 cm), and initial PS (0 versus 1 versus 2). Following enrollment, the study regimen was dynamically allocated using a minimization procedure which tended to allocate each of the study regimens with equal frequency within each stratum-level [9].
The analysis used an indicator for ESA and G-CSF usage during study treatment which was recorded following each cycle of treatment. These data, as well as the patient characteristics, were electronically recorded by the clinic staff managing each patient. The association between ESA and G-CSF usage and patient demographic and disease characteristics was assessed with a logisitic model [10]. Since the guidelines and policies for administering cytokines varied from institution to institution, these logisitic models were stratifed by clinic [11].
The Landmark analysis consisted of selecting an initial time interval during which patients were monitored for ESA (or G-CSF) usage and then classified as either exposed or not exposed, based on whether they initiated a cytokine within this interval [12,13]. For the purpose of this report, each patient's landmark period was defined as the five months following her enrollment onto the study, capturing the chemotherapy phase of treatment for most participants. For the Landmark analyses, OS and progression-free survival (PFS) were measured from the end of the landmark period to the date of death or last contact, if the participant was alive. The interval of PFS was terminated on the date of first radiographic evidence of increasing disease or new disease by Response Evaluation Criteria in Solid Tumors (RECIST) criteria, CA-125 progression, global deterioration or death due to any cause [14,15]. For those participants who were progression-free when last contacted, the duration of PFS was censored at the date of last contact
The Kaplan-Meier procedure was used to estimate the cumulative probability of OS or PFS following the landmark period and the logrank procedure was used to provide a univariate assessment of the hypothesis that the death rate is independent of cytokine usage [16,17]. The Kaplan-Meier plots measure risk-time from date the patient enrolled onto the study and therefore display the landmark period.
A proportional hazards model with cytokine exposure included as time-dependent covariates was used to provide multivariate estimates of the relative death rates [18]. In this case, all participants were initially classified as unexposed. Once an ESA (or G-CSF) was initiated, the participant was moved into the exposed category for calculating the contribution to the partial likelihood due to each subsequent death. This approach includes all events, particularly those that occur during the landmark period, and it permits an estimate of the relative hazard adjusted for other potentially confounded factors. A time-dependent proportional hazards model was also used to evaluate the hypothesis that ESA and G-CSF multiplicatively interact to further increase the death rate. All reported p-values are two-sided.
Adverse events were classified and graded for severity according to National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 3, and were reported up to 30 days following last study treatment [19].
RESULTS
Study Conduct
There were 1,873 women enrolled between 2005 and 2009. Nine patients who did not receive any study-directed therapy are not included in this report, leaving 1,864 women who initiated study treatment.
Of these 1,864 women, 1,125 received neither G-CSF nor ESA; 311 received G-CSF but no ESA; 241 received ESA but no G-CSF; and 187 received both G-CSF and ESA. Therefore, 428 (22.9%) received an ESA and 498 (26.7%) women received G-CSF. Only nine patients initiated a cytokine following the five-month landmark period (Table 2). These nine individuals are classified as unexposed only for the landmark analyses.
Table 2.
Patient Characteristics
| Patient Characteristic | −E −G (n=1125) n % | −E +G (n=311) n % | +E −G (n=241) n % | +E +G (n=187) n % | Total | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age Group (Years) | <40 | 42 | 3.7 | 11 | 3.5 | 4 | 1.7 | 6 | 3.2 | 63 |
| 40–49 | 166 | 14.8 | 43 | 13.8 | 26 | 10.8 | 28 | 15.0 | 263 | |
| 50–59 | 367 | 32.6 | 103 | 33.1 | 80 | 33.2 | 53 | 28.3 | 603 | |
| 60–69 | 364 | 32.4 | 101 | 32.5 | 80 | 33.2 | 64 | 34.2 | 609 | |
| 70–79 | 173 | 15.4 | 47 | 15.1 | 39 | 16.2 | 35 | 18.7 | 294 | |
| >=80 | 13 | 1.2 | 6 | 1.9 | 12 | 5.0 | 1 | 0.5 | 32 | |
| Race | Non Hispanic Black | 48 | 4.3 | 11 | 3.5 | 13 | 5.4 | 8 | 4.3 | 80 |
| Non Hispanic White | 939 | 83.5 | 247 | 79.4 | 214 | 88.8 | 161 | 86.1 | 1561 | |
| Hispanic | 53 | 4.7 | 8 | 2.6 | 5 | 2.1 | 6 | 3.2 | 72 | |
| Asian | 64 | 5.7 | 38 | 12.2 | 7 | 2.9 | 6 | 3.2 | 115 | |
| Pacific Islander | 6 | 0.5 | 2 | 0.6 | 1 | 0.4 | 0 | 0.0 | 9 | |
| A. Indian/Alaska N. | 3 | 0.3 | 0 | 0.0 | 1 | 0.4 | 3 | 1.6 | 7 | |
| Other/Not specified | 12 | 1.1 | 5 | 1.6 | 0 | 0.0 | 3 | 1.6 | 20 | |
| Performance Status | 0 | 593 | 52.7 | 161 | 51.8 | 100 | 41.5 | 75 | 40.1 | 929 |
| 1 | 467 | 41.5 | 130 | 41.8 | 120 | 49.8 | 88 | 47.1 | 805 | |
| 2 | 65 | 5.8 | 20 | 6.4 | 21 | 8.7 | 24 | 12.8 | 130 | |
| Initial Weight (kgs) | < 60 | 295 | 26.2 | 124 | 39.9 | 68 | 28.2 | 63 | 33.7 | 550 |
| 60–80 | 515 | 45.8 | 141 | 45.3 | 106 | 44.0 | 86 | 46.0 | 848 | |
| > 80 | 315 | 28.0 | 46 | 14.8 | 67 | 27.8 | 38 | 20.3 | 466 | |
| Primary Site | Ovary | 933 | 82.9 | 270 | 86.8 | 195 | 80.9 | 155 | 82.9 | 1553 |
| Fallopian Tube | 21 | 1.9 | 11 | 3.5 | 2 | 0.8 | 2 | 1.1 | 36 | |
| Primary Peritoneum | 171 | 15.2 | 30 | 9.6 | 44 | 18.3 | 30 | 16.0 | 275 | |
| Cell Type | Papillary Serous | 935 | 83.1 | 258 | 83.0 | 219 | 90.9 | 163 | 87.2 | 1575 |
| Endometrioid | 39 | 3.5 | 12 | 3.9 | 4 | 1.7 | 5 | 2.7 | 60 | |
| Clear Cell Carcinoma | 35 | 3.1 | 10 | 3.2 | 8 | 3.3 | 1 | 0.5 | 54 | |
| Mucinous Adenocarcinoma | 12 | 1.1 | 3 | 1.0 | 0 | 0.0 | 4 | 2.1 | 19 | |
| Other/Not specified | 104 | 9.2 | 28 | 9.0 | 10 | 4.1 | 14 | 7.5 | 156 | |
| Histologic Grade | 1 | 59 | 5.2 | 11 | 3.5 | 7 | 2.9 | 12 | 6.4 | 89 |
| 2 | 178 | 15.8 | 49 | 15.8 | 46 | 19.1 | 31 | 16.6 | 304 | |
| 3 | 829 | 73.7 | 227 | 73.0 | 177 | 73.4 | 139 | 74.3 | 1372 | |
| Clear cell/not specified | 59 | 5.2 | 24 | 7.7 | 11 | 4.6 | 5 | 2.7 | 99 | |
| Stage/Residual size | Ill-optimal | 436 | 38.8 | 103 | 33.1 | 54 | 22.4 | 44 | 23.5 | 637 |
| III-suboptimal | 436 | 38.8 | 117 | 37.6 | 112 | 46.5 | 80 | 42.8 | 745 | |
| IV | 253 | 22.5 | 91 | 29.3 | 75 | 31.1 | 63 | 33.7 | 482 | |
| Initial HGB | <10 | 94 | 8.4 | 14 | 4.5 | 37 | 15.4 | 20 | 10.7 | 165 |
| 10 – 12 | 553 | 49.2 | 163 | 52.4 | 142 | 58.9 | 116 | 62.0 | 974 | |
| > 12 | 478 | 42.5 | 134 | 43.1 | 62 | 25.7 | 51 | 27.3 | 725 | |
| Initial HCT | < 30 | 86 | 7.6 | 16 | 5.1 | 31 | 12.9 | 22 | 11.8 | 155 |
| 30 – 36 | 520 | 46.2 | 150 | 48.2 | 137 | 56.8 | 111 | 59.4 | 918 | |
| > 36 | 517 | 46.0 | 145 | 46.6 | 73 | 30.3 | 54 | 28.9 | 789 | |
| Not Specified | 2 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 2 | |
| Initial WBC | < 4,000 | 32 | 2.8 | 13 | 4.2 | 7 | 2.9 | 4 | 2.1 | 56 |
| 4,000 – 6,000 | 242 | 21.5 | 104 | 33.4 | 38 | 15.8 | 48 | 25.7 | 432 | |
| > 6,000 | 851 | 75.6 | 194 | 62.4 | 196 | 81.3 | 135 | 72.2 | 1376 | |
| Randomized Treatment | CT+P->P | 356 | 31.6 | 102 | 32.8 | 94 | 39.0 | 69 | 36.9 | 621 |
| CT+Bev->P | 398 | 35.4 | 97 | 31.2 | 73 | 30.3 | 56 | 29.9 | 624 | |
| CT+Bev->Bev | 371 | 33.0 | 112 | 36.0 | 74 | 30.7 | 62 | 33.2 | 619 | |
+E/−E = Did/did not receive erythropoiesis-stimulating agent.
+G/−G = Did/did not receive Granulocyte colony-stimulating factor (GCSF).
* 9 patients who refused study treatment are not included in this report.
The percentages in this table are column percentages.
Characteristics of Study Population
The baseline clinical and pathologic characteristics of the study population are detailed in Table 3. The median age for all patients was 60.0 years (1st and 3rd quartiles 52.4 and 67.0, respectively). The median weight for all patients was 67.7 kg (1st and 3rd quartiles 57.9 and 80.2, respectively). Forty percent had stage III disease with surgical residual intra-abdominal tumor implants >1 cm in diameter, and 26% had stage IV disease.
Table 3.
Select Adverse Events*
| AE Category | Treatment Group | 0 | 1 | 2 | 3 | 4 | 5 | Total | % Grade 3 or worse |
|---|---|---|---|---|---|---|---|---|---|
| Neutrophils/AGC†† | −E −G | 75 | 21 | 82 | 314 | 633 | 0 | 1125 | 84 |
| −E +G | 12 | 1 | 11 | 55 | 232 | 0 | 311 | 92 | |
| +E −G | 4 | 4 | 16 | 52 | 165 | 0 | 241 | 90 | |
| +E +G | 6 | 1 | 7 | 47 | 126 | 0 | 187 | 92 | |
| Hemoglobin†† | −E −G | 110 | 424 | 482 | 97 | 12 | 0 | 1125 | 10 |
| −E +G | 16 | 78 | 165 | 49 | 3 | 0 | 311 | 17 | |
| +E −G | 3 | 41 | 156 | 36 | 5 | 0 | 241 | 17 | |
| +E +G | 3 | 17 | 117 | 44 | 6 | 0 | 187 | 27 | |
| Febrile Neutropenia†† | −E −G | 1099 | 0 | 0 | 23 | 3 | 0 | 1125 | 2 |
| −E +G | 268 | 0 | 0 | 40 | 3 | 0 | 311 | 14 | |
| +E −G | 234 | 0 | 0 | 6 | 1 | 0 | 241 | 3 | |
| +E +G | 167 | 0 | 0 | 17 | 3 | 0 | 187 | 11 | |
| Venous TE† | −E −G | 1115 | 0 | 1 | 4 | 5 | 0 | 1125 | 1 |
| −E +G | 310 | 0 | 0 | 0 | 1 | 0 | 311 | < 1 | |
| +E −G | 237 | 0 | 1 | 2 | 1 | 0 | 241 | 1 | |
| +E +G | 181 | 0 | 0 | 1 | 5 | 0 | 187 | 3 | |
| Other TE | −E −G | 1047 | 0 | 12 | 32 | 32 | 2 | 1125 | 6 |
| −E +G | 292 | 1 | 3 | 9 | 6 | 0 | 311 | 5 | |
| +E −G | 262 | 0 | 1 | 9 | 5 | 0 | 241 | 6 | |
| +E +G | 170 | 1 | 4 | 7 | 5 | 0 | 187 | 6 |
Common Terminology Criteria for Adverse Events version 3.0. Onset of adverse event between start of study treatment and 30 days after date of last cycle of treatment.
p < 0.05,
p <0.001 for a two-sided exact test of the hypothesis that the incidence of a grade 3 or higher adverse event is independent of the treatment group.
The institution-stratified odds of initiating a cytokine during treatment was not associated with the randomly assigned study regimen (p=0.21). Age at enrollment, race, Hispanic ethnicity, PS, tumor debulking level, histologic cell type, and tumor grade, were similarly distributed across the groups determined by cytokine usage. There were some differences between the four groups of G-CSF/ESA users. Patients who received ESA were more likely to have a poorer initial PS (p=0.009), higher stage of disease (p<0.003) and to start chemotherapy with anemia (p<0.001). Patients who received G-CSF tended to have a poorer initial PS (p=0.006), higher stage of disease (p=0.007) and lower pretreatment white blood counts (p<0.001). The percentages of patients completing 6 cycles of chemotherapy were 90.2% vs 87.6% for those prescribed an ESA and vs no ESA and 88.1% vs 88.5% for those prescribed G-CSF vs no G-CSF. These differences are not statistically significant.
Adverse Events
Overall, 99% of the grade 3 or higher hematologic adverse events and 76% of the grade 3 or higher non-hematologic adverse events occurred during the chemotherapy phase even though the duration of this phase comprised less than 30% of the entire planned treatment regimen. Table 4 shows the frequency of selected clinically relevant adverse events. Participants who received ESA were more likely to have experienced anemia and the patients who received G-CSF were more likely to have experienced neutropenia.
Table 4.
| Estimated hazard ratios from a proportional hazards model of overall survival with erythropoietin exposure considered a time-dependent factor | |||
|---|---|---|---|
| Covariate | Hazard ratio | 95% Confidence interval | p-value |
| Erythropoietin (time-dependent) | 0.989 | 0.849 – 1.15 | 0.892 |
| Performance Status 0 | 1.00 | < 0.001 | |
| Performance Status 1 | 1.37 | 1.19 – 1.58 | |
| Performance Status 2 | 2.37 | 1.84 – 2.95 | |
| Stage III (≤ 1 cm residual) | 1.00 | < 0.001 | |
| Stage III (> 1 cm residual) | 1.43 | 1.21 – 1.70 | |
| Stage IV | 1.60 | 1.33 – 1.926 | |
| HGB < 10 | 1.00 | 0.093 | |
| 10 ≤ HGB ≤ 12 | 1.01 | 0.798 – 1.286 | |
| HGB > 12 | 0.863 | 0.672 – 1.109 | |
| Estimated hazard ratios from a proportional hazards model of overall survival with GCSF exposure considered a time-dependent factor | |||
|---|---|---|---|
| Covariate | Hazard ratio | 95% Confidence interval | p-value |
| GCSF (time-dependent) | 0.932 | 0.800 – 1.08 | 0.363 |
| Performance Status 0 | 1.00 | < 0.001 | |
| Performance Status 1 | 1.36 | 1.18 – 1.56 | |
| Performance Status 2 | 2.37 | 1.88 – 2.99 | |
| Stage III (≤ 1 cm residual) | 1.00 | < 0.001 | |
| Stage III (> 1 cm residual) | 1.43 | 1.21 – 1.70 | |
| Stage IV | 1.60 | 1.33 – 1.92 | |
| WBC < 10 | 1.00 | 0.027 | |
| 10 ≤ WBC ≤ 12 | 1.24 | 0.780 – 1.98 | |
| WBC > 12 | 1.50 | 0.956 – 2.34 | |
CTCAE grade 3 or higher venous thrombotic events (VTE) occurred more often among those treated with an ESA (9 of 428 (2.1%) versus 10 of 1436 (0.7%); p<0.020). While the absolute risk of a serious thrombotic event is small, after adjusting for treatment with bevacizumab, the risk was three times greater among those treated with ESA (relative odds=3.31 95% CI: 1.15–9.52).
Progression-Free Survival
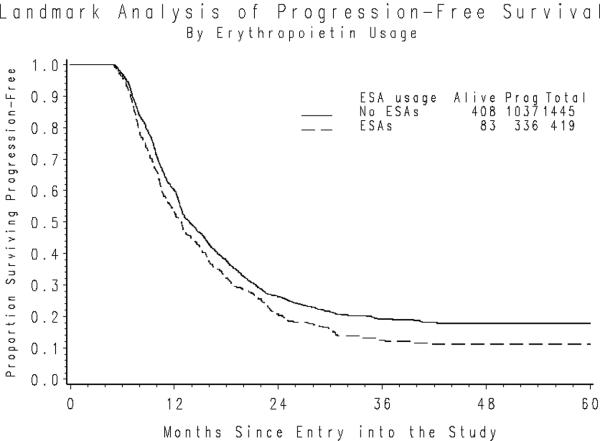
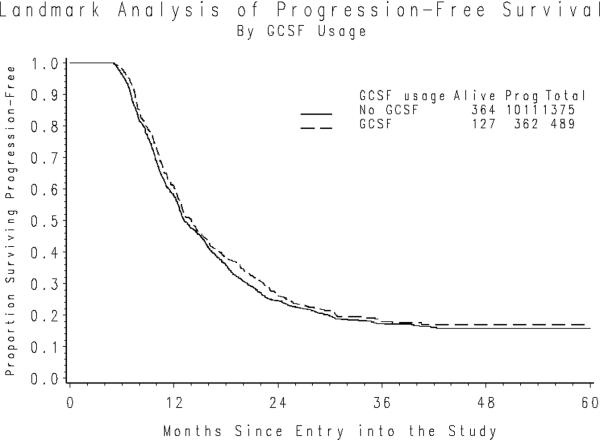
The median duration of PFS following the five-month landmark period was 9.0 and 8.2 months for those who did and did not initiate ESAs during the landmark period. After adjusting for initial PS, stage of disease, size of residual disease and initial hemoglobin level the hazard of first progression or death was similar for those using ESA compared to those who did not (hazard ratio (HR)=1.06; 95% confidence interval (CI): 0.937–1.19; p=0.364) (Figure 1a). The median PFS following the landmark period was 7.8 and 8.8 months for those who did and did not initiate G-CSF. The hazard of first progression or death adjusted for initial PS, stage, residual disease size and pretreatment WBC was also similar for G-CSF users compared to non-users HR=0.920; 95% CI 0.819 – 1.03; p=0.157) (Figure 1b).
Figure 1a. PFS following the Landmark Period by use of ESA Usage.
*Nine patients initiated cytokines following the landmark period.
Figure 1b. PFS following the Landmark Period by use of G-CSF Usage.
*Nine patients initiated cytokines following the landmark period.
Overall Survival
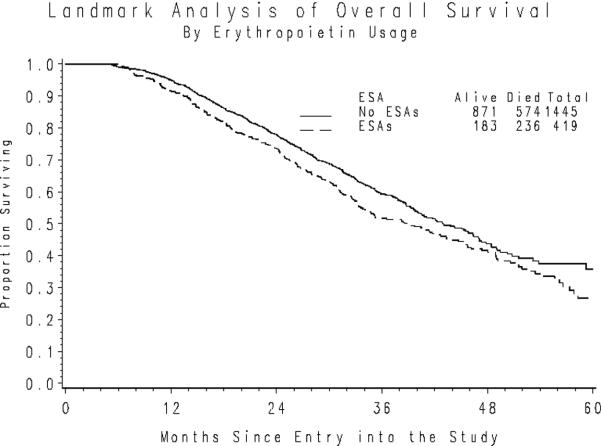
Fifty-six percent of the participants were alive at the time of this analysis. The median duration of follow-up for those patients alive at last contact is 30 months. The median OS following a five-month landmark period was 34 months versus 38 months for those who did versus did not receive an ESA and 40 versus 37 months for those who did versus did not receive G-CSF. Sixty-two patients died during their landmark period. An unadjusted comparison of OS (Figure 2a) indicates that ESA usage is associated with a 19% increase in the death rate (HR= 1.19; 95% CI=1.02–1.39; p=0.024). However, after accounting for confounding due to the patients' initial PS, stage of disease, size of residual disease and initial hemoglobin level, the death rates appear to be independent of ESA usage (HR=0.989; 95% CI=0.849 – 1.15; p=0.892).
Figure 2a. Overall Survival following the Landmark Period by ESA Usage.
*Nine patients initiated cytokines following the landmark period.
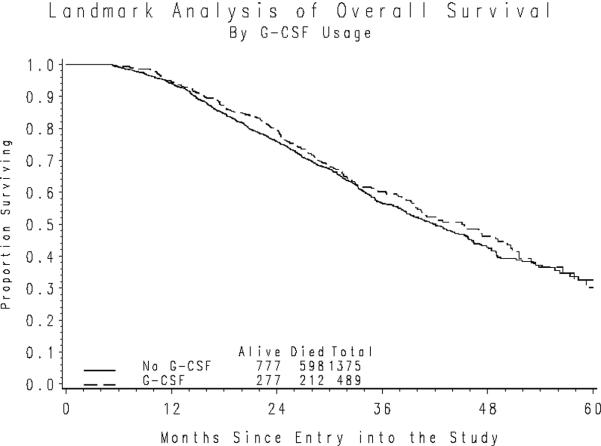
On the other hand, an unadjusted comparison indicates that G-CSF usage does not appreciably alter the duration of OS following the landmark period (Figure 2b). The results from a time-dependent proportional hazards model, which accounted for the patients' initial PS, stage of disease, size of residual disease following primary surgery, and initial WBC also indicated that there was no appreciable difference in the death rate associated with G-CSF usage (HR=0.932; 95% CI=0.800–1.085).
Figure 2b. Overall Survival following the Landmark Period by G-CSF Usage.
*Nine patients initiated cytokines following the landmark period.
A test for a multiplicative interaction in the proportional hazards model between ESA and G-CSF indicates that G-CSF usage does not modify the estimated effect of ESA usage on the risk of death (or visa-versa). Also, there was no statistically significant evidence that the effect of either ESAs or G-CSF markedly varied across the randomized treatment groups. Specifically, it does not appear that bevacizumab's effect of on survival is appreciably modified by either G-CSF or ESA administration, or visa-versa.
DISCUSSION
As early as 2003, the oncology community recognized that ESA might have unexpected complications, and should be used with caution [20,21]. n 2008, the United States Food and Drug Administration (FDA) Oncologic Drug Advisory Committee met and reviewed study results on the risks of ESA when administered to patients with cancer. While there was no clear evidence of tumor progression, there was an unexplained increase in mortality in investigational studies which included ESA [22]. The manufacturers had changed labeling in 2007, but on March 7, 2008 they jointly disseminated new prescribing information to inform healthcare professionals about ESA. This FDA assessment of risks lacks the scientific rigor utilized for evidence to assess efficacy. The GOG cervix trial (GOG-0191) was described as having decreased three year PFS in the ESA arm [21]. The actual PFS results were 59% vs 62%, HR 1.06 (CI=0.58–1.90), p=0.856 by log-rank test. No adjustments were made for known prognostic factors. A recently completed trial did not show any decrease in relapse-free survival or OS with ESA [23].
The question of whether or not ESAs stimulate tumor cell growth was addressed in a meta-analysis by Bohlius et al [24]. These authors obtained clinical data from 53 trials, 38 of which included a chemotherapy regimen. They observed an association between ESA use and all-cause mortality (HR 1.17, CI 1.06–1.30). However, when they examined only the trials which included chemotherapy there was no significant association (HR 1.10, CI 0.98–1.24, p=0.263). The authors concluded that this increase in risk was compatible with random variation. When analyzed by site of primary tumor, significance was found only for breast cancer trials. These investigators estimated the mortality rate might be increased by 18% with ESAs (HR=1.18, 95% CI: 0.72 – 1.94) used in the management of gynecological cancers [24]. Their test for homogeneity across trials limited statistical power to detect clinically important differences.
Rocconi et al studied 581 women with ovarian cancer, of whom 229 (39%) received ESA with treatment and 352 (61%) did not [6]. After a median 27 month follow-up period (similar to this report, 30 months), they reported a higher probability of recurrence among those who had received ESA (56% vs 80%, p<0.001). The median PFS was 16 months for ESA, and 24 months for patients not receiving ESA. The probability of death was also higher among those who received ESA (46% vs 59%, p=0.002).
Another report including only patients with ovarian cancer focused on improvements in hemoglobin levels, decrease in transfusions and improved quality of life [3]. More ESA treated patients had progression of disease, but this was attributed to imbalance in stage distribution. Neither of these reports attempted to adjust for disproportions in known confounding risk factors.
Factors such as age, PS, and stage are associated with both ESA use and adverse outcomes. In a retrospective evaluation of 343 ovarian cancer patients treated with a variety of chemotherapy regimens before and after the FDA black box warnings were issued showed no deleterious relationship between ESA use and disease-specific OS (HR, 0.82; P=0.25). Their analysis of covariates suggested that higher disease stage at diagnosis and lack of surgical staging significantly increased the risk of death. Patients receiving ESA were more likely to be older and have stage IV disease [25]. Patients receiving ESA's were more likely to suffer a VTE; this is consistent with other reports (21,26).
The strengths of the present study include the large sample size, the homogeneity of the sample and treatments, prospective accrual and randomization to standard treatments, central quality control and the application of a multivariate time-dependent model to adjust for known prognostic factors. Whether a patient initiates cytokines in the future, and whether a patient is observed to progress (or die) are both patient outcomes. The typical analytic procedures that are used to analyze exposure-outcome relationships are susceptible to known biases when they are used to assess outcome-outcome relationships [13].
There are two sources of bias to consider. First, it may seem reasonable to begin measuring survival (or PFS) from the date when cytokines were initiated, but a comparable date is not defined for those who never initiated cytokines. It may also seem reasonable to measure survival from the date of initiating chemotherapy. However, this would bias survival in favor of cytokines users, because they cannot die until after they begin cytokines. In other words, very early deaths that occur among those who would have initiated cytokines, but died before cytokines could be initiated, could count against the unexposed group. Landmark analysis addresses this potential for bias, by using a common start time for all patients which occurs after the time when most would have started cytokines. A second source of bias arises from imbalances in important prognostic factors between the exposed and unexposed groups. Since cytokine treatments were not randomly assigned, imbalances in prognostic factors should be expected. The time-dependent multivariate proportional hazards model was used in this study to address both of these sources of biases simultaneously. We believe that our conclusions are generalizible to the overall population of ovarian cancer patients.
A weakness of the current study may be that cytokine usage was monitored only during the period that each patient received first-line treatment (up to 22 cycles). Some patients may have initiated cytokines during subsequent lines of anti-cancer therapy. This study has the same shortcomings as any non-randomized prospective cohort study; the model can only account for known prognostic factors. Those patients who eventually require cytokine support tend also to be more frail and likely to progress or die even before they initiate their anti-cancer treatment. The model used in this analysis assumes that the substantive differences in prognosis between groups are captured by the patients' stages of disease, residual tumor size, initial PS, pretreatment hemoglobin and WBC.
CONCLUSIONS
The results from this study do not support existing literature which suggests that ESA or G-CSF use may be associated with adverse ovarian cancer progression and death. We recommend that ESA not be used prophylactically to prevent anemia and they should be used with caution for the treatment of chemotherapy-associated anemia in ovarian cancer patients, since usage may increase the risk of VTE in this population.
Supplementary Material
RESEARCH HIGHLIGHTS
In analysis of a large randomized trial in ovarian cancer patients, the use of neither erythropoietin nor granulocyte colony stimulating agents had a negative impact on survival.
Adjustments were made for other factors known to impact survival such as stage of disease, residual tumor size, and performance status.
Landmark analysis was employed since patients may have received cytokine at any time over the duration of their chemotherapy.
Acknowledgments
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA 27469), the Gynecologic Oncology Group Statistical and Data Center (CA 37517). The following institutions participated in this study: Roswell Park Cancer Institute, University of Alabama at Birmingham, Duke University Medical Center, Abington Memorial Hospital, Walter Reed Army Medical Center, Wayne State University, University of Minnesota Medical School, Mount Sinai School of Medicine, Northwestern Memorial Hospital, University of Mississippi Medical Center, Colorado Gynecologic Oncology Group P.C., University of California at Los Angeles, University of Washington, University of Pennsylvania Cancer Center, Milton S. Hershey Medical Center, University of Cincinnati, University of North Carolina School of Medicine, University of Iowa Hospitals and Clinics, University of Texas Southwestern Medical Center at Dallas, Indiana University School of Medicine, Wake Forest University School of Medicine, University of California Medical Center at Irvine, Rush-Presbyterian-St. Luke's Medical Center, Magee Women's Hospital, SUNY Downstate Medical Center, University of Kentucky, University of New Mexico, The Cleveland Clinic Foundation, State University of New York at Stony Brook, Washington University School of Medicine, Memorial Sloan-Kettering Cancer Center, Cooper Hospital/University Medical Center, Columbus Cancer Council, MD Anderson Cancer Center, University of Massachusetts Medical School, Fox Chase Cancer Center, Women's Cancer Center, University of Oklahoma, University of Virginia Health Sciences Center, University of Chicago, Mayo Clinic, Case Western Reserve University, Tampa Bay Cancer Consortium, Yale University, GOG Japan-Saitama Medical University International Medical Center, University of Wisconsin Hospital, Cancer Trials Support Unit, University of Texas - Galveston, Women and Infants Hospital, Korean Gynecologic Oncology Group, The Hospital of Central Connecticut, Georgia Core, GYN Oncology of West Michigan, PLLC, Aurora Women's Pavilion of West Allis Memorial Hospital, and Community Clinical Oncology Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
Dr. James T. Thigpen is a speaker/consultant for Amgen, Jansen Biotech and Genentech. Dr. Robert Burger has participated in advisory board meetings for Roche/Genentech. All other co-authors have no conflicts of interest.
REFERENCES
- 1.American Cancer Society . Cancer Facts & Figures 2011. American Cancer Society; Atlanta: 2011. http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/docum ent/acspc-029771.pdf. [Google Scholar]
- 2.Seidenfeld J, Piper M, Flamm C, Hasselblad V, Armitage JO, Bennett CL, et al. Epoetin treatment of anemia associated with cancer therapy; a systematic review and meta-analysis of controlled clinical trials. J Natl Cancer Inst. 2001;93:1204–14. doi: 10.1093/jnci/93.16.1204. [DOI] [PubMed] [Google Scholar]
- 3.Wilkinson PM, Antonopoulos M, Lahousen M, Lind M, Kosmidis P, EPO-INT-45 Study Group Epoetin alfa in platinum treated ovarian cancer patients: results of a multinational, multicenter, randomized trial. Brit J Cancer. 2006;94:947–54. doi: 10.1038/sj.bjc.6603004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swift S, Ellison AR, Kassner P, McCaffery I, Rossi J, Sinclair AM, et al. Absence of functional EPOR expression in human tumor cell lines. Blood. 2010;115:4254–63. doi: 10.1182/blood-2009-10-248674. [DOI] [PubMed] [Google Scholar]
- 5.Sinclair AM, Coxon A, McCaddery I, Kaufman S, Paweletz K, Liu L, et al. Functional erythropoietin receptor is undetectable in endothelial, cardiac, neuronal, and renal cells. Blood. 2010;115:4264–72. doi: 10.1182/blood-2009-10-248666. [DOI] [PubMed] [Google Scholar]
- 6.R Rocconi, Long B, Sullivan P, Long B, Blaize M, Brown J, et al. Treatment of chemotherapy-induced anemia in patients with ovarian cancer: Does the use of erythropoiesis-stimulating agents worsen survival? Int J Gynecological Cancer. 2012;22:786–91. doi: 10.1097/IGC.0b013e31825104f4. [DOI] [PubMed] [Google Scholar]
- 7.Burger RA, Sill MW, Monk BJ, Greer Be, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:5165–71. doi: 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]
- 8.Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–83. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 9.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–15. [PubMed] [Google Scholar]
- 10.Cox DR. The analysis of binary data, Methuen, London. 1970 [Google Scholar]
- 11.Wright JD, Neugut AI, Wilde T, Buono DL, Malin J, Tsai WY, et al. Physician characteristics and variability of erythropoiesis-stimulating agent use among Medicare patients with cancer. J Clin Oncol. 2011;29:3408–18. doi: 10.1200/JCO.2010.34.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dafni U. Landmark analysis at the 25-year landmark point. Circ Cardiovasc Qual Outcomes. 2011;4:363–71. doi: 10.1161/CIRCOUTCOMES.110.957951. [DOI] [PubMed] [Google Scholar]
- 13.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1:710–9. doi: 10.1200/JCO.1983.1.11.710. [DOI] [PubMed] [Google Scholar]
- 14.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 15.Rustin GJ, Marples M, Nelstrop AE, Mahmoudi M, Meyer T. Use of CA-125 to define progression of ovarian cancer in patients with persistently elevated levels. J Clin Oncol. 2001;9:4054–7. doi: 10.1200/JCO.2001.19.20.4054. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J the Amer Stat Assoc. 1958;53:457–81. [Google Scholar]
- 17.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–70. [PubMed] [Google Scholar]
- 18.Cox DR. Regression models and life tables (with discussion) J Royal Stat Soc. B. 1972;74:187–220. [Google Scholar]
- 19. Common Terminology Criteria for Adverse Events (CTCAE) v3.0. 2006. (Accessed at http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_30.)
- 20.Wun T, Law L, Harvey D, Sieracki B, Scudder SA, Ryu JK. Increased incidence of symptomatic venous thrombosis in patients with cervical carcinoma treated with concurrent chemotherapy, radiation and erythropoietin. Cancer. 2003;98:1514–20. doi: 10.1002/cncr.11700. [DOI] [PubMed] [Google Scholar]
- 21.Thomas G, Ali S, Hoebers FJ, Darcy KM, Rodgers WH, Patel M, et al. Phase III trial to evaluate the efficacy of maintaining hemoglobin levels above 12.0 g/dL with erythropoietin vs above 10.0 g/dL without erythropoietin in anemic patients receiving radiation and cisplatin for cervical cancer. Gynecol Oncol. 2008;108:317–25. doi: 10.1016/j.ygyno.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.US Food and Drug Administration: FDA Drug Safety Communication: Erythropoiesis-Stimulating Agents (ESAs) – Procrit®, Epogen®, and Aranesp®. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/103234s5199lbl.pdf.
- 23.Blohmer JU, Paekpe S, Sehouli J, Boehmer D, Kolben M, Würschmidt F, et al. Randomized phase III trial of sequential adjuvant chemoradiotherapy with or without erythropoietin Alfa in patients with high-risk cervcical cancer: Results of the NOGGOAGO Intergroup Study. J Clin Oncol. 2011;29:3791–7. doi: 10.1200/JCO.2010.30.4899. [DOI] [PubMed] [Google Scholar]
- 24.Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, et al. Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta-analysis of randomized trials. Lancet. 2009;373:1532–42. doi: 10.1016/S0140-6736(09)60502-X. [DOI] [PubMed] [Google Scholar]
- 25.Cantrell LA, Westin SN, Van Le L. The use of recombinant erythropoietin for the treatment of chemotherapy-induced anemia in patients with ovarian cancer does not affect progression-free or overall survival. Cancer. 2011;117:122–6. doi: 10.1002/cncr.25590. [DOI] [PubMed] [Google Scholar]
- 26.Wun T, Law L, Harvey D, Sieracki B, Scudder SA, Ryu JK. Increased incidence of symptomatic venous thrombosis in patients with cervical carcinoma treated with concurrent chemotherapy, radiation, and erythropoietin. Cancer. 2003;98:1514–20. doi: 10.1002/cncr.11700. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.