Abstract
In spite of evidence to the contrary, concern that substances injected into the fourth ventricle (4V) reach forebrain structures challenges the validity of using these injections to evaluate the role of hindbrain structures. Injection of AngII into the lateral ventricle (LV) increases water intake, but a similar response is not observed after injection into the 4V. This alone suggests the requirement of forebrain structures, but the potential for a counteracting, anti-dipsogenic pressor response to hindbrain AngII allows for lingering concern that this competing effect of AngII, rather than lack of forebrain access, underlies the negative result. Here, we used a double cannulation approach (LV and 4V) to evaluate the effect of the AngII receptor antagonist, losartan, on the drinking response to AngII injected into the LV. Injections of losartan into the LV blocked the dipsogenic response to AngII given 5 min later into the LV. There was no effect, however, when losartan was injected into 4V, even when we used a dose of losartan that was 25 times greater than needed when injected into the LV. Collectively, these experiments suggest that concerns about diffusion from hindbrain ventricles to forebrain structures are overstated and can be circumvented using proper dose and timing of injections. Moreover, these data provide additional support to the existing literature showing that forebrain structures are key sites in the stimulation of drinking behavior by AngII.
Keywords: angiotensin II, drinking, thirst, cerebral ventricles, hindbrain
1. Introduction
The cerebral ventricles are often used as an injection route when evaluating the potential for CNS action of peptides or small molecules. Many studies have used injections into the fourth ventricle (4V) to test for the existence of a sensitive substrate in the caudal brainstem (for examples, see Faulconbridge et al., 2003; Grill et al., 1998; Grill et al., 2000; Kinzig et al., 2006; McKay et al., 2011). Nevertheless, these studies leave open the possibility, however small, that the substance injected into the hindbrain ventricle traveled through the ventricular space to reach sensitive sites in the forebrain parenchyma.
Concern that substances injected into the 4V can reach forebrain substrates persists in spite of much evidence to the contrary. For example, obstruction of the cerebral aqueduct that connects the third and fourth ventricles does not change the response to 4V injection of many substances including cocaine- and amphetamine-regulated transcript, ghrelin, or 5-thioglucose into the 4V (Aja et al., 2001; Faulconbridge et al., 2005; Ritter et al., 1981). Nevertheless, activation of putative leptin receptorspecific signaling pathways has been observed in forebrain structures in response to 4V injections of leptin (Ruiter et al., 2010). Accordingly, there appears to be lingering concern that experiments using 4V injections do not rule out the possibility of the injectant reaching forebrain substrates.
Angiotensin II (AngII) is a potent dipsogen when injected into the lateral ventricle (LV). Injections of AngII into the 4V, however, do not cause drinking, even when very large doses are given (Fitts and Masson, 1989). This alone suggests the requirement of forebrain structures and suggests that AngII cannot flow from the 4V to forebrain sites that produce a dipsetic response to AngII. Even in this case, however, it remains possible that the lack of drinking to 4V injections was not because AngII failed to reach the required sites in the forebrain, but because 4V injections could be more effective at stimulating an anti-dipsogenic pressor response given the role of the area postrema in the response and its proximity to the 4V (Joy and Lowe, 1970; Joy, 1971).
In the present studies, we used an alternate strategy to test the hypothesis that a substance injected into the 4V can reach substances near forebrain ventricles to affect a behavioral response. Specifically, we used a double cannulation approach (LV and 4V) to evaluate the effect of the AngII receptor antagonist, losartan, on the drinking response to AngII injected into the LV.
2. Results
2.1 Experiment 1: Effect of losartan in the LV or 4V on water intake stimulated by LV injection of AngII
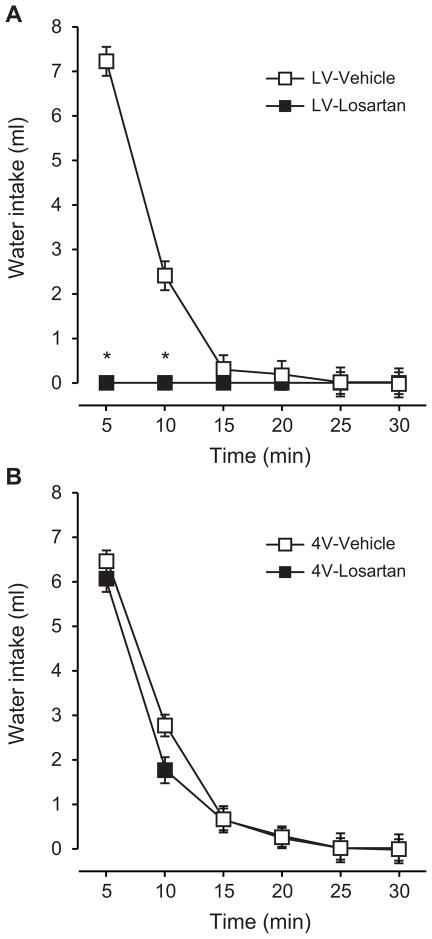
A first set of experiments was used to test the effect of losartan, injected into the LV or 4V, on drinking stimulated by AngII injected into the LV. These initial experiments used injections of 10 μg of losartan injected 5 min before the LV injection of 10 ng AngII. As expected, injection of losartan into the LV virtually abolished any drinking stimulated by AngII injected into the same ventricle 5 min later (Figure 1A). Analysis of non-cumulative intake in 5-min time bins over the 30-min test using a two-way repeated measures ANOVA (Time x Drug) revealed a significant main effect of Time (F5,30=158.3, p<0.05) and Drug (F1,6=28.0, p<0.05) and a significant Time x Drug interaction (F5,30=24.4, p<0.05). SNK post hoc tests revealed that differences in water intake by vehicle- and losartan-pretreated rats were statistically significant at 5 and 10 min of the intake test, after which vehicle-pretreated rats stopped drinking and intake was, therefore, the same as it was in rats given losartan.
Figure 1.
Effect of LV or 4V injections of losartan in rats given 10 ng AngII into the LV. Losartan (10 μg) was injected into the LV or 4V 5 min before AngII was injected into the LV. Data from rats injected with losartan into the LV are shown in panel A and data from 4V injections of losartan are in panel B. Losartan potently decreased drinking caused by AngII when the losartan was injected into the LV, but had no effect on drinking when it was injected into the 4V. * p<0.05 compared with vehicle within a given time and injection route. Data are mean ± SEM.
In contrast to the strong effect of losartan injections into the LV, we observed no effect of losartan when it was injected into the 4V 5 min before injection of AngII in the LV. As shown in Figure 1B, water intake by rats given injections of losartan into the 4V was virtually identical to that by rats in the control group. Indeed, analysis of these data using a two-way repeated measure ANOVA found a main effect of Time (F5,40=106.6, p<0.05), but detected neither a main effect of Drug (F1,8=0.6, p=0.47) nor a Time x Drug interaction (F5,40=1.65, p=0.17).
Statistical analyses of total intake in each test confirmed the results from the non-cumulative analyses. Specifically, when total intake by rats injected with losartan or vehicle into the LV was analyzed, we found a significant effect of Drug (F1,6=276.8, p<0.05), but we failed to find a similar effect of Drug when intake from rats given injections into the 4V was analyzed (main effect of Drug: F1,8=0.9, p=0.36).
2.2. Experiment 2: Effect of various doses of losartan injected into the LV or 4V 5 min before injection of AngII into the LV
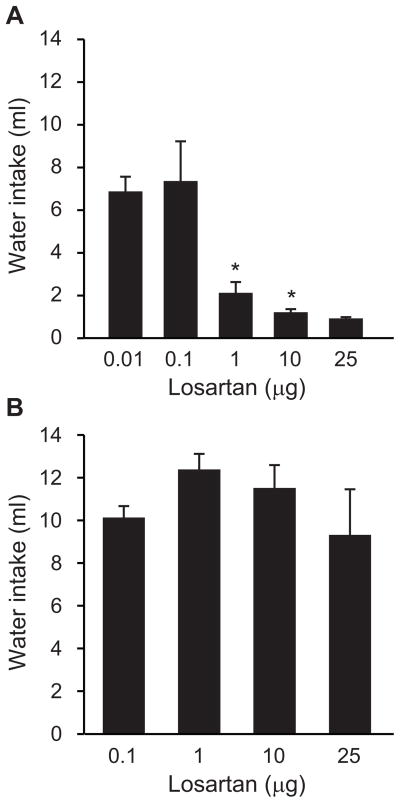
To evaluate the possibility that other doses of losartan would be able to affect drinking induced by injection of AngII into the LV, we conducted additional experiments using the same approach as in Experiment 1 (losartan given 5 min before AngII), but with higher and lower doses of losartan. As shown in Figure 2A, the 3 higher doses of losartan injected into the LV reliably reduced water intake stimulated by LV injection of AngII. Specifically, ANOVA revealed a between-subjects effect of dose (F4,33=7.76, p<0.05) and post hoc tests found that rats injected with 0.01 μg or 0.1 μg drank similar amounts of water after an injection of AngII into the LV 5 min later. Rats given 1μg, 10 μg, or 25 μg, did not differ from each other in their water intake, but all drank less than rats in either the 0.01 μg or 0.1 μg groups.
Figure 2.
Water intake after losartan was injected into the LV (A) or 4V (B) of rats given LV injections of AngII. Different amounts of losartan were injected 5 min before AngII in all cases. *p<0.05 compared with corresponding vehicle-pretreated rats. Data from the entire 30-min intake test are shown as mean + SEM.
In contrast, when a separate group of rats was given injections of various amounts of losartan into the 4V before the LV injection of AngII, drinking after AngII was unaffected even at the highest doses of losartan used. As shown in Figure 2B and confirmed by a one-way ANOVA, all groups of rats drank similar amounts of water (F3,17=0.94, p=0.44).
2.3. Experiment 3: Effect of different times between injection of losartan and injection of AngII
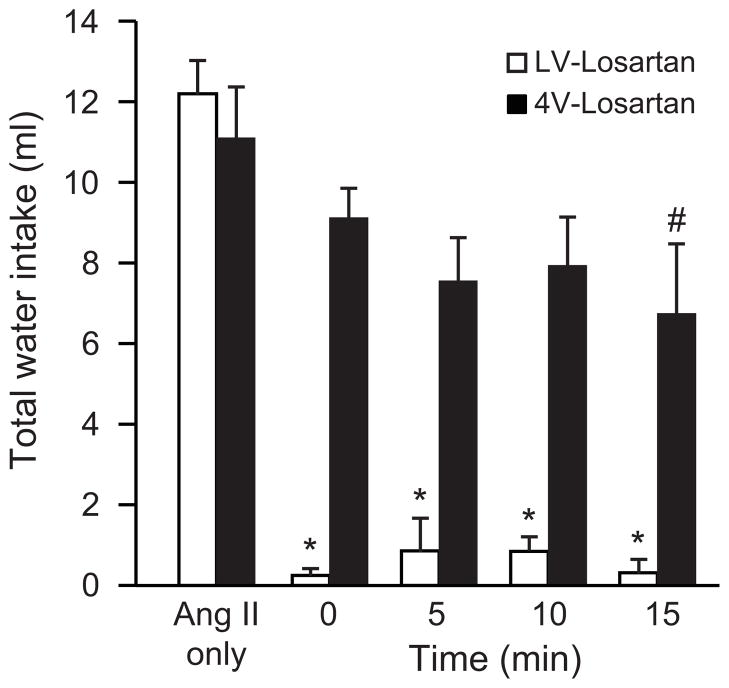
The experiments described above evaluate injections of losartan into the LV or 4V, but all used a 5 min interval between the antagonist and agonist injections. To evaluate the possibility that more or less time would increase the ability of the antagonist to block AngII-induced drinking, we used two separate groups of rats and varied the time between injection of losartan into the 4V or LV and AngII in the LV by 0, 5, 10, or 15 min. Experiments giving injections of losartan into the 4V or LV were conducted and analyzed separately and each included a group of rats injected with AngII alone for comparison. As shown in Figure 3, LV injection of 25 μg of losartan (the highest dose used in Experiment 2 above) virtually abolished all drinking when the losartan was given at the same time, 5 min, 10 min, or 15 min before AngII was injected into the LV. This was confirmed by a one-way ANOVA (F4,29=89.28, p<0.05) and subsequent SNK post hoc tests. We found no reliable effect of losartan, however, when it was injected into the 4V regardless of the time between the 4V injection of losartan and the LV injection of AngII (F4,29=1.71, p=0.18). Although the experiments were conducted separately, we ran a final analysis on all of the data from these two experiments, using a two-way ANOVA to test for effects of Route and Time. This analysis revealed a main effect of Route (F1,58=83.97, p<0.05), a main effect of Time (F4,58=22.17, p<0.05), and a significant Route x Time interaction (F4,58=7.38, p<0.05). Post hoc tests on the interaction confirmed the findings from the individual one-way ANOVAs above, but also detected a difference between the rats given injections of losartan into the 4V 15 min before AngII was injected into the LV and rats in either Route given no losartan. All other comparisons were exactly as would have been expected based on the individual one-way ANOVAs.
Figure 3.
Effect of time between losartan and AngII on drinking. In separate experiments, rats were injected with losartan into the 4V or LV before being injected with AngII into the LV. The time between the injections varied from 0 to 15 min and an additional group of rats that received only AngII was included in both experiments. Data from the two experiments were analyzed separately and collectively (see text for details). *p<0.05 compared with AngII only. #p<0.05 compared to AngII only, but only when data were analyzed collectively. Data from the entire 30-min intake tests are shown as mean + SEM.
3. Discussion
AngII is a powerful dipsogen when injected into the forebrain ventricles (Daniels-Severs et al., 1971; Epstein et al., 1969; Epstein et al., 1970) or even when injected into the brain parenchyma with a cannula that penetrates the ventricle en route to the injection target (Johnson and Epstein, 1975). A number of studies, however, indicate that the sensitive locations for AngII stimulation of water intake reside near the forebrain ventricles (Buggy et al., 1975; Buggy and Fisher, 1976; Mangiapane and Simpson, 1980; Simpson and Routtenberg, 1973). Accordingly, icv injections of AngII offer a powerful tool to study the potential for substances injected into the 4V to access sites near the forebrain ventricles. Using this approach, the present studies found little or no evidence supporting the hypothesis that losartan injected into the 4V can diffuse in the rostral direction and affect drinking after LV injections of AngII. In these experiments, we compared injections of losartan into the LV and the 4V and found that LV injections were very effective at blocking AngII-induced drinking, but 4V injections had no effect. We manipulated the dose of the losartan and found that losartan injected into the 4V failed to affect drinking, even when we injected doses as high as 25 times those needed to block drinking when injected into the LV. Lastly, we manipulated the time between injections of losartan and AngII and still failed to reliably affect drinking when the losartan was given into the 4V, even though the timing of the antagonist injection did not matter at all when it was injected into the LV. As such, these data add to the growing evidence that rostral diffusion in the cerebral ventricles is unlikely.
Although we believe that the data strongly support our conclusion that losartan injected into the 4V was not able to reach relevant forebrain sites and disrupt AngII-induced drinking, it is important to consider the results of the final experiment that manipulated the timing of the injections. Specifically, when we combined the two separate analyses in that experiment, we were able to detect a statistically significant difference in drinking between rats given AngII alone and rats given AngII in the LV 15 min after injection of a large amount (25 μg) of losartan. There are several important considerations when interpreting these data. First, the dose of losartan was 25 times greater than what was needed to affect drinking when injected into the LV. Second, any decrease in drinking, even if statistically significant, was a small fraction of the decrease observed after injection of a much lower dose of losartan into the LV. Third, the only statistically significant comparison within the 4V rats was the comparison with the AngII-only group. This group did not receive any injection, not even vehicle, into the 4V, so it is impossible for us to determine if the marginal change in drinking had more to do with the 4V injection itself than it did with the injection of losartan. In this respect, it is meaningful that there was no difference between the 15-min group and any other group that received losartan into the 4V. Last, and perhaps most important, the one-way ANOVA on 4V-injected rats that did not combine data from separate experiments was unable to detect any difference in drinking. Accordingly, even though we have included the omnibus analysis for the sake of completeness, we do not believe that this is the most appropriate statistical test, nor do we believe that the evidence supports the conclusion that losartan was able to diffuse in the rostral direction to affect forebrain sites involved in the drinking response to AngII.
The data reported here may appear in contrast to those from an earlier study that injected leptin into the 4V (Ruiter et al., 2010). More specifically, that study found that leptin injected into the 4V activated the Janus kinase/signal transducer and activator of transcription 3 (JAK/STAT3) pathway in forebrain tissues. Because STAT3 phosphorylation is widely considered a marker of leptin receptor binding and activation (Mercer et al., 1998; Myers, 2004; Peters et al., 2007; Tartaglia et al., 1995), this finding could be explained by leptin reaching structures near the forebrain ventricles. As discussed in more depth by Ruiter et al (2010), a more likely possibility is that leptin was able to access forebrain sites by entering the subarachnoid space with the flow of CSF from the 4V. Although this is one explanation, there may be different ways to explain the STAT3 phosphorylation in those studies. For instance, central injections of leptin can increase levels of circulating leptin by stimulating release from peripheral sites and this leptin, not the centrally applied leptin, could have cause the STAT3 phosphorylation (Maness et al., 1998; Penn et al., 2006). It is also possible that the specificity of the activation of STAT3 has been overstated in the literature and that the activation of STAT3 in the forebrain after hindbrain injection of leptin was the result of indirect, rather than direct, activation of these cells. Indeed, STAT3 phosphorylation is not unique to leptin receptor activation, but occurs after agonist binding of many other receptors found in brain including 5-HT receptors (Muma et al., 2007; Singh et al., 2007), adrenergic receptors (Zhong et al., 2000), and even AngII receptors (Marrero et al., 1995). As such, it remains unclear if the actions of leptin in that study occurred by the direct actions of leptin on the cells with activated STAT3 or indirectly through synaptic connections with those cells. In this respect, a previous study using ventricular injections of urocortin 1 (Ucn1) may be particularly informative (Daniels et al., 2004). These experiments demonstrated that 4V injections of Ucn1 activate neurons in forebrain areas of neurologically intact rats, but not in the forebrain of chronically maintained decerebrate (CD) rats that lack synaptic connections between hindbrain and forebrain structures. These studies suggest that at least some of the activation of forebrain neurons occurred indirectly, through ascending projections from hindbrain to forebrain. Whether this is also true for the response to leptin requires additional studies, but the finding that Ucn1 did not activate forebrain neurons in CD rats and the present finding that 4V losartan did not affect drinking after LV injections of AngII, at the very least, reveals a potentially important difference between the abilities of these peptides to access forebrain sites after hindbrain application. Nevertheless, the present results provide evidence that substances injected into the 4V, especially when proper doses are used, cannot access forebrain substrates either by rostral diffusion in the cerebral ventricles or by access through the subarachnoid space.
Based on the present data, we conclude that losartan injected into the 4V was unable to access forebrain sites required for AngII to stimulate drinking. These data suggest that concerns about diffusion from hindbrain ventricles to forebrain substrates are likely overstated and that these concerns can be completely circumvented with proper consideration of dose and timing. Moreover, these data provide additional support to the existing literature showing that forebrain structures are key sites in the stimulation of drinking behavior by AngII.
4. Experimental Procedures
4.1 Subjects
Adult, male Sprage Dawley rats weighing 325–349 g were purchased from Harlan Laboratories (Indianapolis, IN). Rats were allowed to habituate to the colony for 1 week before each was implanted with chronic indwelling cannulae aimed at the lateral and fourth ventricles. To this end, rats were anesthetized by IM injection of ketamine (70mg/kg) and xylazine (5mg/kg) and placed in a stereotaxic frame. Two burr holes were drilled in the skull and each rat was implanted with two 26 ga guide cannulae, one aimed at the LV and one aimed at the 4V using the following coordinates (LV, cannula was placed 1.4 mm lateral from midline, 0.9 mm posterior from bregma, 1.8mm ventral from dura; 4V, cannulae was placed 2.5 mm anterior from occipital structure, and 4.8mm ventral from skull). The cannulae were affixed to the skull with screws and dental cement and rats were given a single SC injection of carprofen (5mg/kg). After 1 week of recovery from surgery, cannula placement was verified using the drinking response after injection of 10 ng AngII (LV) and increased blood glucose after injection of 210 mg 5-thio-d-glucose (4V). Animals that failed to drink more than 5 ml after injection of AngII or double blood glucose after injection of 5-thio-d-glucose were excluded from further experimentation.
4.2 Drinking measures
Total water intake during a test was measured by subtracting the post-test bottle weight from the initial bottle weight. Water intake during discrete time bins within a test was determined using the number of licks as measured by a contact lickometer (designed and constructed by the Psychology Electronics Shop, University of Pennsylvania, Philadelphia, PA). The lickometer interfaced with a computer using an integrated USB digital I/O device (National Instruments, Austin, TX) and was processed in a MATLAB (MathWorks, Natick, MA) software environment before being ported to Excel (Microsoft, Redmond, WA) for further analysis. Water spouts were behind an electrically isolated metal plate with a 3.175 mm-wide opening, through which the rat needed to lick to reach the spout, minimizing the possibility of non-tongue contact with the spout.
4.3 Data analysis
Statistical testing was performed using Statistica (version 9.0, Statsoft, Tulsa, OK). ANOVA was used for total test intake and repeated measures ANOVA was used for binned intake. Statistically significant main or interaction effects (p<0.05) were further probed using Student Newman-Keuls (SNK) post-hoc tests.
4.4. Experimental designs
4.4.1. Experiment 1
Water bottles were removed and rats were given injections of losartan (10 μg) or vehicle (1 μl saline) into the LV (n=7) or 4V (n=9) 5 min before all rats were injected with 10 ng AngII into the LV. Immediately after the injection of AngII, water bottles were replaced and intake was measured for 30 min. Rats were used as their own controls with a randomized order of treatment within a given injection route of losartan. Experiments making losartan injections into the 4V and into the LV were conducted and analyzed separately.
4.4.2. Experiment 2
To test the dose-response profile of losartan, water bottles were removed and rats were given injections of different doses of losartan into the LV or 4V (all delivered in 1 μl saline) 5 min before injection of 10 ng AngII into the LV. Immediately after the injection of AngII, water was given back to the rats and intake was measured for 30 min. Each rat received only one dose of losartan in a between-subjects design. Injections of losartan into the LV were 0.01 μg (n=4), 0.1 μg (n=9), 1 μg (n=11), 10 μg (n=8), or 25 μg (n=6). Injections of losartan into the 4V were 0.1 μg (n=5), 1 μg (n=5), 10 μg (n=5), or 25 μg (n=6).
4.4.3. Experiment 3
To determine the time-course of the effect of losartan on AngII-induced water intake, water was removed from the cages and rats were injected with 25 mg of losartan into the LV or 4V 0, 5, 10, or 15 min before 10 ng AngII was injected into the LV (6–7 rats per group). Immediately after the injection of AngII, water was given back to the rats and intake was measured for 30 min. Each rat was used in only one group in a between-subjects design.
Highlights.
AngII injected into the fourth ventricle did not stimulate drinking at any dose.
Lateral ventricle injection of losartan blocked AngII-induced drinking.
Fourth ventricle injection of losartan did not block AngII-induced drinking.
Acknowledgments
Naomi McKay, Kimberly Plyler, Jessica Santollo, and Peter Vento provided helpful comments on the manuscript. A preliminary version of these data was presented in abstract form at the 2012 meeting of the Society for the Study of Ingestive Behavior. Support provided by NIH award HL-91911.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aja S, Sahandy S, Ladenheim EE, Schwartz GJ, Moran TH. Intracerebroventricular CART peptide reduces food intake and alters motor behavior at a hindbrain site. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1862–7. doi: 10.1152/ajpregu.2001.281.6.R1862. [DOI] [PubMed] [Google Scholar]
- Buggy J, Fisher AE, Hoffman WE, Johnson AL, Phillips MI. Ventricular obstruction: effect on drinking induced by intracranial injection of angiotensin. Science. 1975;190:72–4. doi: 10.1126/science.1166302. [DOI] [PubMed] [Google Scholar]
- Buggy J, Fisher AE. Anteroventral third ventricle site of action for angiotensin induced thirst. Pharmacol Biochem Behav. 1976;4:651–60. doi: 10.1016/0091-3057(76)90216-1. [DOI] [PubMed] [Google Scholar]
- Daniels-Severs A, Ogden E, Vernikos-Danellis J. Centrally mediated effects of angiotensin II in the unanesthetized rat. Physiol Behav. 1971;7:785–7. doi: 10.1016/0031-9384(71)90150-8. [DOI] [PubMed] [Google Scholar]
- Daniels D, Markison S, Grill HJ, Kaplan JM. Central structures necessary and sufficient for ingestive and glycemic responses to Urocortin I administration. J Neurosci. 2004;24:11457–62. doi: 10.1523/JNEUROSCI.2702-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein AN, Fitzsimons JT, Simons BJ. Drinking caused by the intracranial injection of angiotensin into the rat. J Physiol. 1969;200:98P–100P. [PubMed] [Google Scholar]
- Epstein AN, Fitzsimons JT, Rolls BJ. Drinking induced by injection of angiotensin into the brain of the rat. J Physiol. 1970;210:457–74. doi: 10.1113/jphysiol.1970.sp009220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulconbridge LF, Cummings DE, Kaplan JM, Grill HJ. Hyperphagic effects of brainstem ghrelin administration. Diabetes. 2003;52:2260–5. doi: 10.2337/diabetes.52.9.2260. [DOI] [PubMed] [Google Scholar]
- Faulconbridge LF, Grill HJ, Kaplan JM. Distinct forebrain and caudal brainstem contributions to the neuropeptide Y mediation of ghrelin hyperphagia. Diabetes. 2005;54:1985–93. doi: 10.2337/diabetes.54.7.1985. [DOI] [PubMed] [Google Scholar]
- Fitts DA, Masson DB. Forebrain sites of action for drinking and salt appetite to angiotensin or captopril. Behav Neurosci. 1989;103:865–72. doi: 10.1037/h0092457. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Ginsberg AB, Seeley RJ, Kaplan JM. Brainstem application of melanocortin receptor ligands produces long-lasting effects on feeding and body weight. J Neurosci. 1998;18:10128–35. doi: 10.1523/JNEUROSCI.18-23-10128.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill HJ, Markison S, Ginsberg A, Kaplan JM. Long-term effects on feeding and body weight after stimulation of forebrain or hindbrain CRH receptors with urocortin. Brain Res. 2000;867:19–28. doi: 10.1016/s0006-8993(00)02193-4. [DOI] [PubMed] [Google Scholar]
- Johnson AK, Epstein AN. The cerebral ventricles as the avenue for the dipsogenic action of intracranial angiotensin. Brain Res. 1975;86:399–418. doi: 10.1016/0006-8993(75)90891-4. [DOI] [PubMed] [Google Scholar]
- Joy MD, Lowe RD. The site of cardiovascular action of angiotensin II in the brain. Clin Sci. 1970;39:327–36. doi: 10.1042/cs0390327. [DOI] [PubMed] [Google Scholar]
- Joy MD. The intramedullary connections of the area postrema involved in the central cardiovascular response to angiotensin II. Clin Sci. 1971;41:89–100. doi: 10.1042/cs0410089. [DOI] [PubMed] [Google Scholar]
- Kinzig KP, Scott KA, Hyun J, Bi S, Moran TH. Lateral ventricular ghrelin and fourth ventricular ghrelin induce similar increases in food intake and patterns of hypothalamic gene expression. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1565–9. doi: 10.1152/ajpregu.00785.2005. [DOI] [PubMed] [Google Scholar]
- Maness LM, Kastin AJ, Farrell CL, Banks WA. Fate of leptin after intracerebroventricular injection into the mouse brain. Endocrinology. 1998;139:4556–62. doi: 10.1210/endo.139.11.6319. [DOI] [PubMed] [Google Scholar]
- Mangiapane ML, Simpson JB. Subfornical organ: forebrain site of pressor and dipsogenic action of angiotensin II. Am J Physiol. 1980;239:R382–9. doi: 10.1152/ajpregu.1980.239.5.R382. [DOI] [PubMed] [Google Scholar]
- Marrero MB, Schieffer B, Paxton WG, Heerdt L, Berk BC, Delafontaine P, Bernstein KE. Direct stimulation of Jak/STAT pathway by the angiotensin II AT1 receptor. Nature. 1995;375:247–50. doi: 10.1038/375247a0. [DOI] [PubMed] [Google Scholar]
- McKay NJ, Kanoski SE, Hayes MR, Daniels D. Glucagon-like peptide-1 receptor agonists suppress water intake independent of effects on food intake. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1755–64. doi: 10.1152/ajpregu.00472.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer JG, Moar KM, Hoggard N. Localization of leptin receptor (Ob-R) messenger ribonucleic acid in the rodent hindbrain. Endocrinology. 1998;139:29–34. doi: 10.1210/endo.139.1.5685. [DOI] [PubMed] [Google Scholar]
- Muma NA, Singh RK, Vercillo MS, D’Souza DN, Zemaitaitis B, Garcia F, Damjanoska KJ, Zhang Y, Battaglia G, Van de Kar LD. Chronic olanzapine activates the Stat3 signal transduction pathway and alters expression of components of the 5-HT2A receptor signaling system in rat frontal cortex. Neuropharmacology. 2007;53:552–62. doi: 10.1016/j.neuropharm.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MG., Jr Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog Horm Res. 2004;59:287–304. doi: 10.1210/rp.59.1.287. [DOI] [PubMed] [Google Scholar]
- Penn DM, Jordan LC, Kelso EW, Davenport JE, Harris RB. Effects of central or peripheral leptin administration on norepinephrine turnover in defined fat depots. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1613–21. doi: 10.1152/ajpregu.00368.2006. [DOI] [PubMed] [Google Scholar]
- Peters JH, Simasko SM, Ritter RC. Leptin analog antagonizes leptin effects on food intake and body weight but mimics leptin-induced vagal afferent activation. Endocrinology. 2007;148:2878–85. doi: 10.1210/en.2006-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter RC, Slusser PG, Stone S. Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science. 1981;213:451–2. doi: 10.1126/science.6264602. [DOI] [PubMed] [Google Scholar]
- Ruiter M, Duffy P, Simasko S, Ritter RC. Increased hypothalamic signal transducer and activator of transcription 3 phosphorylation after hindbrain leptin injection. Endocrinology. 2010;151:1509–19. doi: 10.1210/en.2009-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JB, Routtenberg A. Subfornical organ: site of drinking elicitation by angiotensin II. Science. 1973;181:1172–5. doi: 10.1126/science.181.4105.1172. [DOI] [PubMed] [Google Scholar]
- Singh RK, Shi J, Zemaitaitis BW, Muma NA. Olanzapine increases RGS7 protein expression via stimulation of the Janus tyrosine kinase-signal transducer and activator of transcription signaling cascade. J Pharmacol Exp Ther. 2007;322:133–40. doi: 10.1124/jpet.107.120386. [DOI] [PubMed] [Google Scholar]
- Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Wool EA, Monroe CA, Tepper RI. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–71. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- Zhong H, Murphy TJ, Minneman KP. Activation of signal transducers and activators of transcription by alpha(1A)-adrenergic receptor stimulation in PC12 cells. Mol Pharmacol. 2000;57:961–7. [PubMed] [Google Scholar]