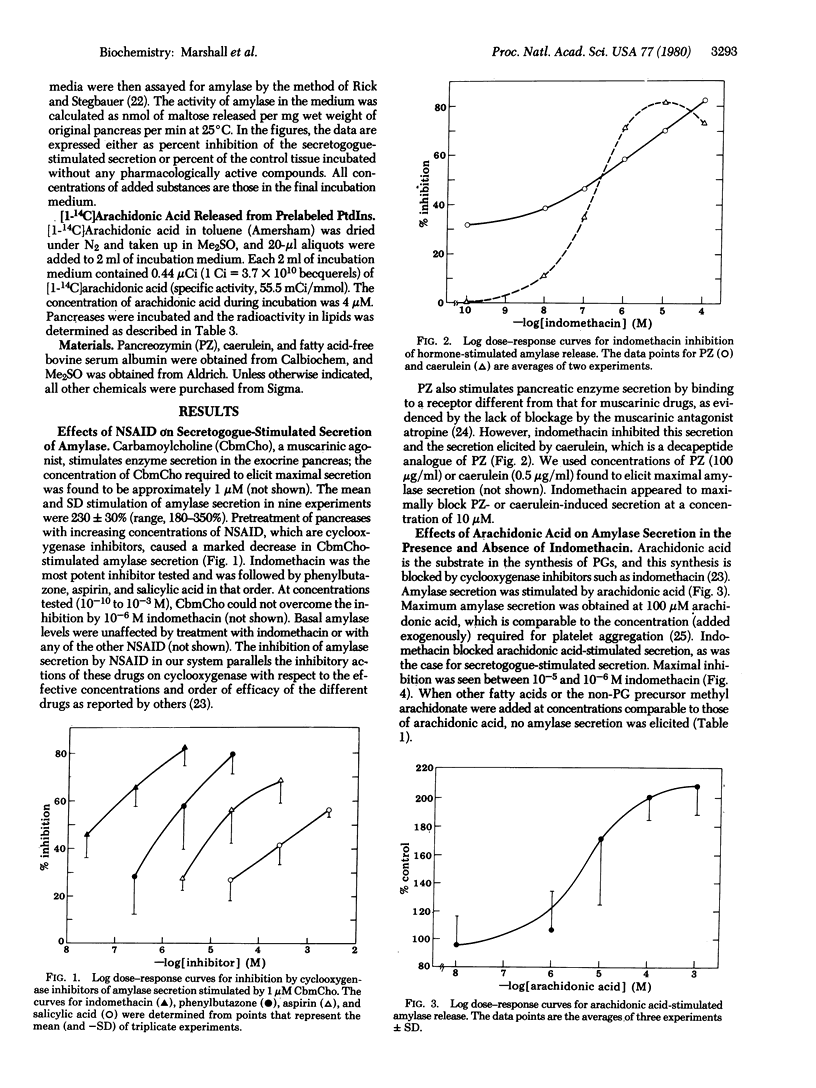
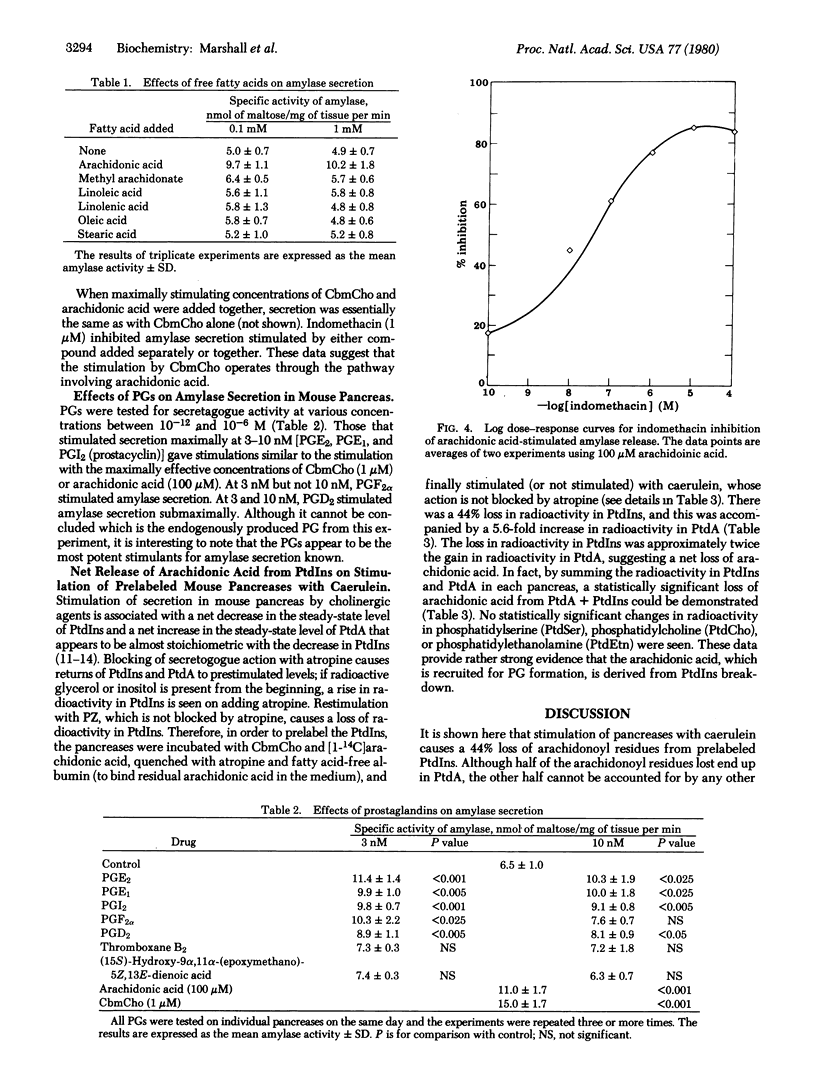
Abstract
That stimulation of secretion in exocrine and endocrine glands is associated with increased turnover of phosphatidylinositol and phosphatidic acid has been known for many years. In the present work, mouse pancreases were prelabeled with [14C]arachidonic acid in the presence of the secretogogue carbamoylcholine. They were then incubated in media containing atropine and 1% albumin. The atropine causes the tissue to revert to the resting state, and the albumin binds free [14C]arachidonic acid. The tissues were finally incubated in media containing no stimulant or the stimulant caerulein, which is not blocked by atropine. Stimulation with caerulein, which is not blocked by atropine. Stimulation with caerulein led to a 44% loss of [1-14C]arachidonic acid from phosphatidylinositol. About half of this released arachidonic acid ended up in phosphatidic acid. The remainder of the loss could not be accounted for in any other lipid. No other phospholipids showed statistically significant changes on stimulation. Several lines of evidence indicated that the missing arachidonic acid was converted to prostaglandins, which play a role in stimulus--secretion coupling. Four nonsteroidal anti-inflammatory drugs inhibited secretogogue-induced amylase secretion from pancreases, and their potencies paralleled their potencies in inhibiting cyclooxygenase, which converts arachidonic acid to prostaglandins. Amylase secretion was stimulated by arachidonic acid, and this stimulation was blocked by the nonsteroidal anti-inflammatory drug indomethacin. Other fatty acids failed to elicit amylase secretion. At concentrations of 3--10 nM, prostaglandins I2, E1, E2, D2, and F2 alpha gave statistically significant stimulations of secretion. Other prostaglandins tested gave no significant stimulation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Baker R. R., Thompson W. Positional distribution and turnover of fatty acids in phosphatidic acid, phosphinositides, phosphatidylcholine and phosphatidylethanolamine in rat brain in vivo. Biochim Biophys Acta. 1972 Aug 11;270(4):489–503. doi: 10.1016/0005-2760(72)90114-2. [DOI] [PubMed] [Google Scholar]
- Bell R. L., Kennerly D. A., Stanford N., Majerus P. W. Diglyceride lipase: a pathway for arachidonate release from human platelets. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3238–3241. doi: 10.1073/pnas.76.7.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bills T. K., Smith J. B., Silver M. J. Selective release of archidonic acid from the phospholipids of human platelets in response to thrombin. J Clin Invest. 1977 Jul;60(1):1–6. doi: 10.1172/JCI108745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flower R. J. Drugs which inhibit prostaglandin biosynthesis. Pharmacol Rev. 1974 Mar;26(1):33–67. [PubMed] [Google Scholar]
- Geison R. L., Banschbach M. W., Sadeghian K., Hokin-Neaverson M. Acetylcholine stimulation of selective increases in stearic and arachidonic acids in phosphatidic acid in mouse pancreas. Biochem Biophys Res Commun. 1976 Jan 26;68(2):343–349. doi: 10.1016/0006-291x(76)91149-9. [DOI] [PubMed] [Google Scholar]
- HOKIN L. E., HOKIN M. R. Effects of acetylcholine on the turnover of phosphoryl units in individual phospholipids of pancreas slices and brain cortex slices. Biochim Biophys Acta. 1955 Sep;18(1):102–110. doi: 10.1016/0006-3002(55)90013-5. [DOI] [PubMed] [Google Scholar]
- HOKIN L. E., HOKIN M. R. Phosphoinositides and protein secretion in pancreas slices. J Biol Chem. 1958 Oct;233(4):805–810. [PubMed] [Google Scholar]
- HOKIN M. R., HOKIN L. E. Enzyme secretion and the incorporation of P32 into phospholipides of pancreas slices. J Biol Chem. 1953 Aug;203(2):967–977. [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Samuelsson B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper A. A., Raper H. S. Pancreozymin, a stimulant of the secretion of pancreatic enzymes in extracts of the small intestine. J Physiol. 1943 Jun 30;102(1):115–125. doi: 10.1113/jphysiol.1943.sp004021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokin-Neaverson M. Acetylcholine causes a net decrease in phosphatidylinositol and a net increase in phosphatidic acid in mouse pancreas. Biochem Biophys Res Commun. 1974 Jun 4;58(3):763–768. doi: 10.1016/s0006-291x(74)80483-3. [DOI] [PubMed] [Google Scholar]
- Hokin-Neaverson M. Metabolism and role of phosphatidylinositol in acetylcholine-stimulated membrane function. Adv Exp Med Biol. 1977;83:429–446. doi: 10.1007/978-1-4684-3276-3_40. [DOI] [PubMed] [Google Scholar]
- Hokin L. E., Huebner D. Radioautographic localization of the increased synthesis of phosphatidylinositol in response to pancreozymin or acetylcholine in guinea pig pancreas slices. J Cell Biol. 1967 Jun;33(3):521–530. doi: 10.1083/jcb.33.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holub B. J., Kuksis A. Differential distribution of orthophosphate- 32 P and glycerol- 14 C among molecular species of phosphatidylinositols of rat liver in vivo. J Lipid Res. 1971 Nov;12(6):699–705. [PubMed] [Google Scholar]
- Isakson P. C., Raz A., Hsueh W., Needleman P. Lipases and prostaglandin biosynthesis. Adv Prostaglandin Thromboxane Res. 1978;3:113–120. [PubMed] [Google Scholar]
- Jones L. M., Michell R. H. Breakdown of phosphatidylinositol provoked by muscarinic cholinergic stimulation of rat parotid-gland fragments. Biochem J. 1974 Sep;142(3):583–590. doi: 10.1042/bj1420583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Poorthuis B. J., Yazaki P. J., Hostetler K. Y. An improved two dimensional thin-layer chromatography system for the separation of phosphatidylglycerol and its derivatives. J Lipid Res. 1976 Jul;17(4):433–437. [PubMed] [Google Scholar]
- REDMAN C. M., HOKIN L. E. Phospholipide turnover in microsomal membranes of the pancreas during enzyme secretion. J Biophys Biochem Cytol. 1959 Oct;6:207–214. doi: 10.1083/jcb.6.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenhouse-Simmons S. Production of diglyceride from phosphatidylinositol in activated human platelets. J Clin Invest. 1979 Apr;63(4):580–587. doi: 10.1172/JCI109339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHUCHER R., HOKIN L. E. The synthesis and secretion of lipase and ribonuclease by pigeon pancreas slices. J Biol Chem. 1954 Oct;210(2):551–557. [PubMed] [Google Scholar]
- Vapaatalo H., Parantainen J. Prostaglandins; their biological and pharmacological role. Med Biol. 1978 Aug;56(4):163–183. [PubMed] [Google Scholar]



