Abstract
Background
The primary aim of this study was to compare the sensitivity and rapidity of AKI detection by cystatin C relative to creatinine following cardiac surgery.
Study Design
Prospective cohort study
Settings and Participants
1,150 high-risk, adult cardiac surgery patients in the TRIBE-AKI (Translational Research Investigating Biomarker Endpoints for Acute Kidney Injury) Consortium.
Predictor
Changes in serum creatinine and cystatin C
Outcome
Post-surgical incidence of AKI
Measurements
Serum creatinine and cystatin C were measured at the preoperative visit and daily on postoperative days 1–5. To allow comparisons between changes in creatinine and cystatin C, AKI endpoints were defined by the relative increases in each marker from baseline (25, 50 and 100%) and the incidence of AKI was compared based upon each marker. Secondary aims were to compare clinical outcomes among patients defined as having AKI by cystatin C and/or creatinine.
Results
Overall, serum creatinine detected more cases of AKI than cystatin C: 35% developed a ≥25% increase in serum creatinine, whereas only 23% had ≥25% increase in cystatin C (p < 0.001). Creatinine also had higher proportions meeting the 50% (14% and 8%, p<0.001) and 100% (4% and 2%, p=0.005) thresholds for AKI diagnosis. Clinical outcomes were generally not statistically different for AKI cases detected by creatinine or cystatin C. However, for each AKI threshold, patients with AKI confirmed by both markers had significantly higher risk of the combined mortality/dialysis outcome compared with patients with AKI detected by creatinine alone (p=0.002).
Limitations
There were few adverse clinical outcomes, limiting our ability to detect differences in outcomes between subgroups of patients based upon their definitions of AKI.
Conclusion
In this large multicenter study, we found that cystatin C was less sensitive for AKI detection compared with creatinine. However, confirmation by cystatin C appeared to identify a subset of AKI patients with substantially higher risk of adverse outcomes.
Acute kidney injury (AKI) is an independent predictor of mortality in cardiac surgery patients.(1–4) Mortality rates of 15% have been reported for patients with mild AKI, and as high as over 60% for those patients needing dialysis.(4–6) Early detection of AKI in the post-operative period could allow for timely therapeutic intervention to prevent progression and potentially to improve post-surgical outcomes.(7–9) Although serum creatinine concentration remains the clinical standard for AKI diagnosis, it may not be ideal as creatinine is an imprecise measure of glomerular filtration. A small proportion of creatinine is secreted in the urine without being filtered and serum creatinine levels are dependent on its generation from muscle mass, which may be reduced in the post-operative setting.(10)
Serum cystatin C has emerged as an easily measurable marker of kidney function that is less influenced by non-GFR determinants, such as muscle mass, and it is eliminated solely by glomerular filtration.(11, 12) Cystatin C has been a stronger predictor for cardiovascular events, mortality and other adverse outcomes in community-based studies.(13–18) Prior studies for detection of AKI by cystatin C have shown mixed results. Cystatin C was reported to be superior to creatinine in predicting contrast induced kidney injury.(19) Cystatin C was also shown to detect AKI one to two days earlier than creatinine in critically ill patients,(20) and postoperative cystatin C was more effective at predicting AKI in pediatric cardiac surgery patients.(21) Two prior studies did not demonstrate a clear advantage for serum cystatin C in predicting AKI following adult cardiac surgery.(22, 23)
In the large prospective observational adult TRIBE-AKI cohort of patients undergoing cardiac surgery, we demonstrated that the presurgical cystatin C level was a stronger predictor of AKI than the presurgical creatinine or eGFRCr (glomerular filtration rate estimated from creatinine).(24) The primary aim of this study was to compare the sensitivity and rapidity of AKI detection by cystatin C relative to creatinine during post-operative follow-up of adult TRIBE-AKI patients. Secondary aims were to compare hospital outcomes among patients defined as having AKI by cystatin C and/or creatinine.
METHODS
Study Population
The TRIBE-AKI cohort has been previously described.(24, 25) Participants were recruited prior to their cardiac surgery (coronary artery bypass grafting [CABG], surgery for valve disease, or both) at six academic medical centers in North America between July 2007 and December 2009. We included 1150 participants who had both preoperative and at least one postoperative value for cystatin C and creatinine. All patients were at high risk for AKI, defined as the presence of one or more of the following criteria: pre-existing decreased kidney function (baseline serum creatinine >2 mg/dL [>177 µmol/L]), ejection fraction <35% or grade 3 or 4 left ventricular function, age older than 70 years, diabetes mellitus, concomitant CABG and valve surgery, urgent surgery or repeat revascularization surgery. Patients were excluded if they had evidence of AKI before surgery, prior kidney transplant, advanced chronic kidney disease (CKD), or chronic kidney failure. All participants provided written informed consent, and the study was approved by each institution’s research ethics board. Baseline and clinical characteristics were obtained from the patients and definitions using the Society of Thoracic Surgeons data collection tool (www.sts.org).
Specimen Collection
Study coordinators collected blood preoperatively and daily for up to 5 postoperative days as previously described.(25) Median time between the preoperative visit and cardiac surgery was 4.6 (25th–75th percentile, 1.6–11.7) days. The first blood sample was collected soon after admission to the intensive care unit on day one. The remaining daily blood samples were obtained at the time of routine morning blood collection. Specimen collection was stopped on postoperative day 3 in participants without an increase in serum creatinine. Blood was collected in EDTA tubes and was centrifuged to separate plasma. Plasma was aliquoted into bar-coded cryovials and the samples were stored at −80°C until biomarker measurement.
Measurements
Creatinine was measured at the preoperative visit and daily on postoperative days 1–5 as part of clinical practice; 84% of the participants had at least 3 of the 5 daily creatinine values. Presurgical and postsurgical serum creatinine levels were measured in the same clinical laboratory for each patient at all sites. Sites used isotope-dilution mass spectrometry-calibrated or the Jaffe method to measure serum creatinine. Estimated GFR (eGFR) was calculated using the serum creatinine-based Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.(26)
Cystatin C was measured from specimens collected at the preoperative visit and daily on postoperative days 1–5, using samples that had not undergone additional freeze-thaw cycles; 89% of the participants had at least 3 of the 5 possible follow-up cystatin C values.
Both preoperative and postoperative cystatin C measurements were conducted in a large batch using a BN II nephelometer (Siemens AG, www.siemens.com), which has an approximate coefficient of variation of 2%.(27) Study technicians running the assays were blinded to the participant’s clinical information.
Outcomes
The primary outcome was the post-surgical incidence of AKI, as detected by elevations in creatinine and cystatin C, and the time (in days) to reach AKI, based on each marker. To allow comparisons between changes in creatinine and cystatin C, we compared AKI endpoints based on the relative increases in each marker from baseline (25, 50 and 100%). Comparisons based on absolute changes from baseline were impossible due to the different units of these filtration markers. However, the number of participants with a ≥ 25% increase in creatinine (N=400) was very similar to the number with AKI as defined by Acute Kidney Injury Network (AKIN) stage I or higher (n=406). Of these, 361 had both ≥ 25% increase in creatinine and a ≥0.3mg/dL increase in creatinine (90%).
To evaluate whether postsurgical changes in kidney function measured by each marker were clinically important, we compared associations of each AKI definition, detected by cystatin C versus serum creatinine, with subsequent clinical endpoints. These outcomes included length of ICU and hospital stay, in-hospital mortality, and the combined incidence of in-hospital mortality and dialysis.
Statistical Analysis
Incidence of AKI was calculated for creatinine and cystatin C using the 25%, 50% and 100% threshold increase from pre-operative baseline to peak values for each marker over 5 days. We compared the proportions of participants defined as having AKI by creatinine and cystatin C using the chi-squared test. Among AKI cases defined by each marker, we also assessed the rapidity of AKI detection by comparing the hospital day at which the AKI threshold was crossed. These comparisons were repeated for the 25%, 50% and 100% endpoints for AKI, and the distributions of time to AKI were compared using the Wilcoxon rank-sum test.
We next categorized participants into 4 mutually exclusive groups as follows: no AKI, AKI detected by creatinine only, AKI detected by cystatin only, and AKI detected by both markers. These categorizations were repeated using the 25%, 50%, and 100% thresholds of AKI, but limiting AKI cases to those detected within 3 days. An alternative analysis evaluated AKI definitions for creatinine and cystatin C separately.
Clinical outcomes for each AKI definition were then compared across these four groups. Hospital length of stay and ICU length of stay were presented by median (25th–75th percentile). Mortality and dialysis risks were described as proportions. We compared the length of stay outcomes between the groups with AKI detected by creatinine only versus AKI detected by cystatin C only using the Wilcoxon rank-sum test. Incidences of in-hospital mortality and combined incidence of in-hospital mortality and dialysis were compared between the same two groups using the Fisher’s exact test.
Finally, we analyzed only participants that met each AKI threshold based upon creatinine; in this group, we evaluated the risk-stratifying ability of cystatin C by comparing outcomes for participants with AKI detected by creatinine only versus AKI detected by both creatinine and cystatin C.
RESULTS
Baseline characteristics
Among the 1150 participants in this study, the average age was 71 ± 10 years, 68% were men and 93% were white. The mean preoperative serum creatinine was 1.1 ± 0.3 mg/dL and mean preoperative cystatin C was 0.93 ± 0.32 mg/L. Baseline characteristics were compared across 4 groups (no AKI, AKI detected by creatinine only, AKI detected by cystatin only, and AKI detected by both markers) for the 25% threshold. As expected, the group with AKI by creatinine had a higher elevation in creatinine and the group with AKI by cystatin C had a higher elevation in cystatin C. The group with AKI by both markers had the largest elevation from baseline values of both creatinine and cystatin C (Table 1).
Table 1.
Baseline Characteristics of Adult Participants
| No AKI (n=666) |
SCr-only AKIa (n=223) |
SCysC-only AKIb (n=84) |
SCr + SCysC AKIc (n=177) |
P for Trend |
|
|---|---|---|---|---|---|
| Age at the time of surgery | 0.6 | ||||
| <65 y | 138 (21%) | 62 (28%) | 17 (20%) | 30 (17%) | |
| 65–75 y | 257 (39%) | 79 (35%) | 32 (38%) | 70 (40%) | |
| 75–85 y | 238 (36%) | 73 (33%) | 29 (35%) | 66 (37%) | |
| >85 y | 33 (5%) | 9 (4%) | 6 (7%) | 11 (6%) | |
| Male Sex | 456 (68%) | 147 (66%) | 50 (60%) | 130 (73%) | 0.6 |
| White Race | 625 (94%) | 207 (93%) | 77 (92%) | 166 (94%) | 0.8 |
| Diabetes | 250 (38%) | 116 (52%) | 34 (40%) | 72 (41%) | 0.2 |
| Hypertension | 511 (77%) | 181 (81%) | 64 (76%) | 150 (85%) | 0.03 |
| Congestive Heart Failure | 137 (21%) | 72 (32%) | 29 (35%) | 58 (33%) | <0.001 |
| Operative Characteristics | |||||
| Prior Cardiac Surgery | 86 (13%) | 31 (14%) | 10 (12%) | 22 (12%) | 0.9 |
| Elective | 559 (84%) | 169 (76%) | 63 (75%) | 121 (68%) | <0.001 |
| Cardiac Catheterization in Last 72 h | 52 (8%) | 17 (8%) | 17 (20%) | 21 (12%) | 0.01 |
| Surgery | <0.001 | ||||
| CABG | 340 (51%) | 104 (47%) | 40 (48%) | 70 (40%) | |
| Valve | 183 (27%) | 60 (27%) | 33 (39%) | 58 (33%) | |
| CABG and Valve | 143 (21%) | 59 (26%) | 11 (13%) | 49 (28%) | |
| Kidney Function | |||||
| Pre-op SCr, mg/dL | 1.08 ± 0.32 | 1.02 ± 0.33 | 1.17 ± 0.42 | 1.16 ± 0.37 | 0.1 |
| Pre-op eGFR, mL/min/1.73m2 | 67.6 ± 18.5 | 71.7 ± 20.0 | 62.1 ± 20.5 | 64.1 ± 19.9 | 0.2 |
| Pre-op SCysC, mg/L | 0.90 ± 0.28 | 1.01 ± 0.37 | 0.85 ± 0.34 | 0.99 ± 0.35 | 0.003 |
| Absolute Change in SCr | 0.05 ± 0.13 | 0.44 ± 0.25 | 0.11 ± 0.14 | 0.88 ± 0.70 | <0.001 |
| % Change in SCr | 5.1 ± 11.6 | 43.9 ± 23.1 | 9.3 ± 12.0 | 77.1 ± 60.9 | <0.001 |
| Absolute Change in SCysC | −0.015 ± 0.125 | 0.065 ± 0.136 | 0.337 ± 0.178 | 0.582 ± 0.424 | <0.001 |
| % Change in SCysC | −0.7 ± 12.9 | 6.6 ± 11.6 | 41.1 ± 21.3 | 59.4 ± 42.0 | <0.001 |
| Pre-op Medications | |||||
| Beta-Blockers | 498 (75%) | 168 (75%) | 57 (68%) | 126 (72%) | 0.2 |
| ACEi | 303 (46%) | 105 (47%) | 33 (39%) | 97 (55%) | 0.1 |
| ARBs | 131 (20%) | 51 (23%) | 16 (19%) | 41 (23%) | 0.3 |
| Aspirin | 508 (77%) | 158 (71%) | 52 (62%) | 139 (79%) | 0.6 |
| Statins | 497 (75%) | 171 (77%) | 54 (64%) | 125 (71%) | 0.1 |
Note: Continuous variables are shown as mean ± standard deviation; categorical variables, as number (percentage). Conversion factors for units: Serum Creatinine in mg/dL to mmol/L, multiply by 88.4.
Abbreviations: CABG, coronary artery bypass grafting; eGFR, Estimated Glomerular Filtration Rate calculated using the SCr-based CKD-EPI equation; ARB, Angiotensin Receptor Blockers; ACEi, Angiotensin Converting Enzyme inhibitors; pre-op, pre-operative; SCr, serum creatinine; SCysC, serum cystatin C
defined as ≥25% Increase in SCr level
defined ≥25% Increase in SCysC level
defined as ≥25% Increase in SCr and SCysC levels
Detection of AKI cases
Overall, serum creatinine detected more cases of AKI than cystatin C. Of 1150 participants, 35% developed AKI defined by a ≥25% increase in serum creatinine and 23% developed AKI defined by a ≥25% increase in cystatin C (p < 0.001). When using the ≥50% cutoff, the incidences of AKI were 14% and 8% by serum creatinine and cystatin C, respectively (p < 0.001), whereas the ≥100% cutoff resulted in an incidence of AKI of 4% and 2% by serum creatinine and cystatin C (p = 0.005).
For the ≥25% cutoff point, only 15% (n=177) were defined as AKI by both markers; 19% (n=223) had AKI by creatinine alone, whereas 7% (n=84) by cystatin C alone. The incidence of AKI was much lower for the 50% and 100% definitions, but similar patterns were observed. For all three thresholds of AKI detected by serum creatinine, less than half of the participants had a concomitant rise in cystatin C.
Time to AKI
Detection of AKI cases occurred earlier by serum creatinine than by cystatin C for each AKI definition: ≥25% (p = < 0.001), ≥50% (p = < 0.001), and ≥100% (p = 0.04) rise in each marker. Median time to AKI detection by creatinine was 2, 2, and 3 days for AKI definitions of ≥25%, ≥50% and ≥100% rise from baseline in creatinine values, respectively. Median time to AKI by cystatin C criteria was day 3 for all AKI definitions.
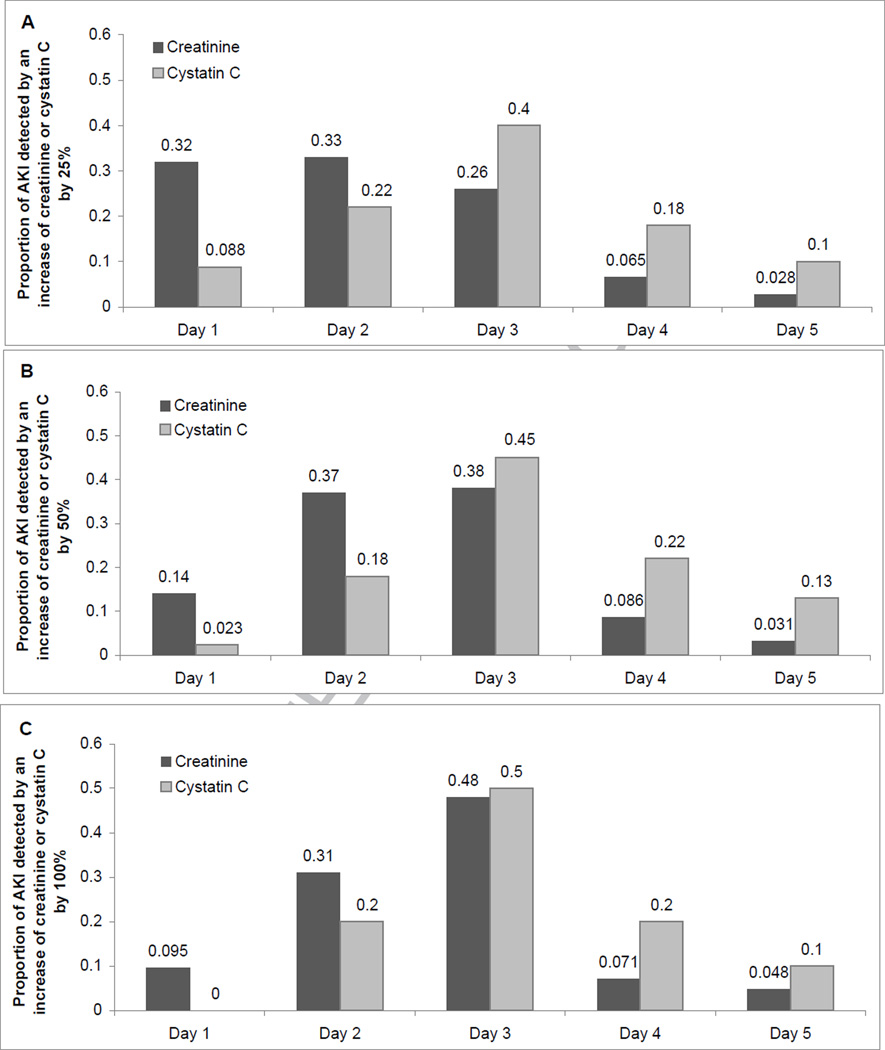
By day one, upon ICU arrival, 32% of all AKI cases defined by a 25% creatinine increase had been detected, compared with only 9% of AKI cases detected by a ≥25% increase in cystatin C (Figure 1A). Similar patterns of earlier detection were observed for the 50% and 100% AKI definitions (Figure 1B, C). There were no differences in the time to elevation of either creatinine or cystatin C when the cases of AKI were limited to persons with both markers elevated beyond each threshold.
Figure 1. Proportion of AKI Cases Detectable on Days 1–5, Defined by an Increase of Creatinine and Cystatin C by ≥25% (A), ≥50% (B), and ≥100% (C).
Creatinine-based AKI cases include those detected by creatinine and by both creatinine and cystatin C. Cystatin C-based AKI cases include those detected by cystatin C and by both creatinine and cystatin C.
Clinical Outcomes
We compared clinical outcomes among the four groups defined by presence or absence of AKI by creatinine and cystatin C. Those with AKI defined by both markers had the longest hospital and ICU length of stay, and had the highest risk for death and the combined death and dialysis outcomes (Table 2). This pattern was generally consistent throughout all AKI thresholds.
Table 2.
Clinical Outcomes Among Cases of AKI Over Five Days
| No. | Length of Hospital Stay* |
Length of ICU Stay* |
In-Hospital Mortality/ Dialysis |
In-Hospital Mortality |
|
|---|---|---|---|---|---|
| AKI defined by ≥25% Increase | |||||
| No AKI | 666 | 6 [5–7] | 2 [1–2] | 3 (0.5%) | 2 (0.3%) |
| SCr-only AKI | 223 | 7 [6–10] | 2 [1–4] | 7 (3.0%) | 5 (2.2%) |
| SCysC-only AKI | 84 | 6 [5–8] | 2 [1–3] | 0 (0%) | 0 (0%) |
| SCr and SCysC AKI | 177 | 7 [6–11] | 2 [1–4.5] | 20 (11.3%) | 12 (6.8%) |
| P Value for Trend | <0.001 | <0.001 | <0.001 | <0.001 | |
| AKI defined by ≥50% Increase | |||||
| No AKI | 962 | 6 [5–8] | 2 [1–3] | 7 (0.7%) | 4 (0.4%) |
| SCr only AKI | 101 | 8 [6–11] | 3 [1–4.5] | 6 (20.0%) | 6 (5.9%) |
| SCysC only AKI | 26 | 7 [6–9] | 2.5 [1–4] | 3 (11.5%) | 1 (3.9%) |
| SCr and SCysC AKI | 61 | 8 [6–15] | 3 [2–7] | 14 (23.0%) | 8 (13.0%) |
| P Value for Trend | <0.001 | <0.001 | <0.001 | <0.001 | |
| AKI defined by ≥100% Increase | |||||
| No AKI | 1101 | 6 [5–8] | 2 [1–3] | 17 (1.5%) | 11 (1%) |
| SCr only AKI | 29 | 7 [5–10] | 3 [1–5] | 4 (13.8%) | 3 (10%) |
| SCysC only AKI | 7 | 8 [6–11] | 2 [1–7] | 1 (14.3%) | 1 (14%) |
| SCr and SCysC AKI | 13 | 17 [13–32] | 14 [4–21] | 8 (61.5%) | 4 (31%) |
| P Value for Trend | <0.001 | <0.001 | <0.001 | <0.001 |
AKI, acute kidney injury; SCr, serum creatinine; SCysC, serum cystatin C; ICU, intensive care unit
Results displayed as Median [25th–75th percentile]
When we compared the participants with AKI detected by a ≥25% rise in creatinine only or cystatin C only over 5 days, we found that the creatinine only group had a statistically significant longer hospital stay (p <0.001) but there were no significant differences in ICU length of stay (p = 0.3), in-hospital mortality (p = 0.3) and composite of mortality and dialysis (p = 0.2). There were no statistically significant differences in hospital length of stay, ICU length of stay, in-hospital mortality and composite of mortality and dialysis rates between the groups with creatinine only and cystatin only elevation at either the 50% or 100% thresholds (Table 2). Similar findings were observed when the AKI threshold was reached in the first 3 days (Table 3).
Table 3.
Clinical Outcomes Among Cases of AKI Over the First Three Days
| No. | Length of Hospital Stay* |
Length of ICU Stay* |
In-Hospital Mortality/ Dialysis |
In-Hospital Mortality |
|
|---|---|---|---|---|---|
| AKI defined by ≥25% Increase | |||||
| No AKI | 726 | 6 [5–8] | 2 [1,3] | 3 (0.4%) | 2 (0.3%) |
| SCr Only AKI | 238 | 7 [6–10] | 2 [1–4] | 8 (3.4%) | 5 (2.1%) |
| SCysC Only AKI | 61 | 6 [5–8] | 2 [1–3] | 1 (1.6%) | 1 (1.6%) |
| SCr and SCysC AKI | 125 | 7 [6–11] | 2 [1–4] | 18 (14%) | 11 (8.8%) |
| P Value for Trend | <0.001 | <0.001 | <0.001 | <0.001 | |
| AKI defined by ≥50% Increase | |||||
| No AKI | 988 | 6 [5–8] | 2 [1–3] | 8 (0.8%) | 4 (0.4%) |
| SCr Only AKI | 105 | 8 [6–11] | 3 [2–5] | 10 (9.5%) | 8 (7.6%) |
| SCysC Only AKI | 19 | 7 [6–9] | 2 [1–4] | 2 (11%) | 1 (5.3%) |
| SCr and SCysC AKI | 38 | 7 [6–14] | 3 [2–7] | 10 (26%) | 6 (16%) |
| P Value for Trend | <0.001 | <0.001 | <0.001 | <0.001 | |
| AKI defined by ≥100% Increase | |||||
| No AKI | 1107 | 6 [5–8] | 2 [1–3] | 18 (1.6%) | 12 (1.1%) |
| SCr Only AKI | 29 | 8 [6–13] | 3 [1–6] | 5 (17%) | 3 (10%) |
| SCysC Only AKI | 6 | 9.5 [6–13] | 1.5 [1–7] | 1 (17%) | 1 (17%) |
| SCr and SCysC AKI | 8 | 17 [5–24] | 10 [3–19] | 6 (75%) | 3 (38%) |
| P Value for Trend | <0.001 | <0.001 | <0.001 | <0.001 |
AKI, acute kidney injury; SCr, serum creatinine; SCysC, serum cystatin C; ICU, intensive care unit
Results displayed as Median [25th, 75th percentile]
We then compared clinical outcomes when AKI was defined by creatinine or cystatin C separately. There were 50% more cases of AKI detected by creatinine (n=400) versus cystatin C (n=261) for the 25% AKI threshold. The incidence of creatinine detected AKI was nearly two-fold that of the cystatin C AKI definition by the 50% (n=162 versus n=87) and 100% (n=42 versus n=20) thresholds. The associated clinical outcomes were similar for the 25% and 50% thresholds but the group with AKI by 100% elevation in cystatin C appeared to have worse prognosis (Table S1, available as online supplementary material).
Finally, we compared clinical outcomes between the creatinine only group and the group with AKI detected by both creatinine and cystatin C in order to assess the ability of cystatin C to distinguish AKI cases associated with high risk. Hospital and ICU length of stay was greater for patients with both markers elevated only for the highest AKI threshold. The group with AKI by ≥25% rise in both markers had a statistically significant doubling of mortality and combined mortality/dialysis incidence compared to the creatinine only (p = 0.04 and 0.002, respectively). The groups with AKI by ≥50% and 100% rise in both markers had a statistically significant increase of combined mortality/dialysis incidence compared to the group with AKI by creatinine only (p = 0.001 and 0.002 respectively) (Table 2).
DISCUSSION
Acute kidney injury is associated with significant morbidity and mortality. This has driven current efforts to explore novel biomarkers for earlier and improved detection of AKI. The purpose of this study was to compare cystatin C with the clinical standard, creatinine, for detecting AKI after cardiac surgery. Cystatin C is a promising biomarker with physiologic characteristics that suggest it is likely a better estimate of glomerular filtration, at least in the ambulatory setting. Cystatin C is eliminated mainly by glomerular filtration and has a short half-life that allows rapid attainment of steady state levels. Based on these properties, we hypothesized that cystatin C would be a more sensitive and rapid marker of AKI than creatinine.
Prior smaller studies of AKI in cardiac surgery patients have focused on the discriminatory ability of early post cardiac surgery plasma cystatin C measurements to predict AKI defined by creatinine criteria. In the study by Wald et al, a positive association between cystatin C levels and AKI development was noted; however, 2 hour post cardiopulmonary bypass cystatin C values failed to predict AKI in patients without baseline CKD.(22) Similarly, in a single center study of adult cardiac surgery patients, there was no significant difference between plasma cystatin C values of those with and without AKI 6 hours after surgery. In addition, cystatin C rose later than creatinine in those who developed AKI.(23)
Our study was different as it attempted to provide a head-to-head comparison between the abilities of cystatin C and creatinine to diagnose AKI, and the population studied was selected for high AKI risk. Given the larger number of measured clinical outcomes, it also attempted to answer whether AKI cases detected by cystatin C versus creatinine had different clinical significance. In line with these prior results, in this large multicenter study, we found that cystatin C was less sensitive and detected AKI later than creatinine. The only potential advantage noted for cystatin C in our study was its potential to distinguish the high-risk subset among patients with clinical AKI detected by creatinine.
The discrepancies between our results and those of prior studies favoring cystatin C may lie in the study design and populations studied. Zappitelli et al. found that the 6-hour post-operative cystatin C was a better predictor of AKI stage II than creatinine in pediatric cardiac surgery patients.(21) This study evaluated a pediatric cardiac cohort, and used creatinine as the ultimate determinant of AKI. Briguori et al. also used creatinine criteria to determine sensitivity and specificity of relative changes in cystatin C for contrast nephrotoxicity-induced AKI.(19) Herget-Rosenthal et al. demonstrated an earlier rise in cystatin C than creatinine in cases of AKI in ICU patients, but this study included patients with a higher acuity of illness.(20) In patients with more prolonged hospitalization, creatinine generation may be impaired and thus delay its rise in response to changes in kidney function. Other factors related to cardiac surgery are hemodilution due to fluid administration(22, 23, 28) and use of drugs that may affect the two filtration markers differently. For example, abnormalities of thyroid function, steroid therapy or systemic sepsis may affect serum cystatin C.(29, 30) Another possible reason for the discrepancy between this study and prior studies is the potential selection bias introduced by the preoperative creatinine level and its potential impact on the decision to proceed with surgery. Potential participants whose pre-operative creatinine was higher than expected may have had their surgery postponed or cancelled. Therefore, the participants may have thus been selected for having lower than usual creatinines at the baseline visit. This could have inflated the proportion meeting each AKI threshold. Cystatin C measurements on the other hand were unknown and did not affect the decision to undergo surgery. This may have contributed somewhat to the apparent superior sensitivity of creatinine in detection of AKI relative to cystatin C.
While most of our findings did not support that cystatin C is better than creatinine in detection of AKI, they suggest that cystatin C could be important in risk stratification of AKI cases with more severe complications. Less than half of the AKI cases detected by creatinine in our study had concomitant elevation of cystatin C. However, the participants with AKI confirmed by cystatin C had substantially worse in-hospital outcomes, including a doubling of mortality and combined mortality/dialysis risk. Previously, in the setting of ambulatory CKD patients, Peralta et al. demonstrated that cystatin C had a useful role for confirming cases of CKD that were defined by an creatinine-based eGFR <60ml/min/1.73m2. CKD cases confirmed by cystatin C had substantially higher risk for cardiovascular and renal complications.(18) Our study has somewhat parallel findings in patients with potential AKI; as in CKD diagnosis, patients with abnormal values by two filtration markers had much higher risk than patients with only an abnormal creatinine results. If replicated, cystatin C could potentially have a role in clinical practice related to AKI diagnosis by signaling patients with highest risk of in-hospital adverse outcomes. Cystatin C could also predict those AKI patients that later develop CKD. Greater application requires further validation of these findings in different patient populations and for longer duration of follow-up. To our knowledge, current AKI guidelines do not include definitions based upon cystatin C as the filtration marker.
The major strengths of this study are its large, multicenter design with rigorous data collection undertaken to examine the role of cystatin C in AKI detection after cardiac surgery. It is the largest AKI study to date to compare cystatin C and creatinine in the detection of AKI. This study is however limited by the lack of a gold-standard definition of AKI in clinical practice or clinical research. Furthermore, while the studied population was a diverse representation of adult cardiac surgery patients from 6 different institutions, most participants were male and white. A potential source of variability could be the lack of standardization of serum creatinine methods across the clinical sites of this multi-center study, whereas all cystatin C measures were conducted at a single laboratory within a short period of time. However, within each individual participant, all creatinine measures were conducted in the same hospital laboratory within a 5-day period, minimizing the impact of this potential bias. Lastly, the number of adverse clinical outcomes was small in our study, limiting our ability to detect differences in outcomes between subgroups of patients based upon their definitions of AKI.
In conclusion, cystatin C detected fewer cases of AKI compared to creatinine and those cases that were detected were discovered at later time points. However, among patients with clinical AKI detected by creatinine, the subgroup with AKI that was confirmed by cystatin C had a much higher incidence of adverse outcomes. If the latter findings are replicated in future studies, cystatin C may have potential utility for risk stratification of AKI.
Supplementary Material
Acknowledgements
The Collaborators in the TRIBE-AKI Consortium (www.yale.edu/tribeaki) are Steven G. Coca, Amit X. Garg, Michael Zappitelli, Prasad Devarajan, Catherine Krawczeski and Simon Li.
Support: The research reported in this article was supported by the grant R01HL-085757 from the National Heart, Lung, and Blood Institute. The study was also supported by a Clinical and Translational Science Award grant (UL1 RR024139) from the National Center for Research Resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
References
- 1.Chertow GM, Lazarus JM, Christiansen CL, et al. Preoperative renal risk stratification. Circulation. 1997;95:878–884. doi: 10.1161/01.cir.95.4.878. [DOI] [PubMed] [Google Scholar]
- 2.Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol. 2005;16:162–168. doi: 10.1681/ASN.2004040331. [DOI] [PubMed] [Google Scholar]
- 3.Brown JR, Cochran RP, Dacey LJ, et al. Perioperative increases in serum creatinine are predictive of increased 90-day mortality after coronary artery bypass graft surgery. Circulation. 2006;114:I409–I413. doi: 10.1161/CIRCULATIONAHA.105.000596. [DOI] [PubMed] [Google Scholar]
- 4.Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med. 1998;104:343–348. doi: 10.1016/s0002-9343(98)00058-8. [DOI] [PubMed] [Google Scholar]
- 5.Loef BG, Epema AH, Smilde TD, et al. Immediate postoperative renal function deterioration in cardiac surgical patients predicts in-hospital mortality and long-term survival. J Am Soc Nephrol. 2005;16:195–200. doi: 10.1681/ASN.2003100875. [DOI] [PubMed] [Google Scholar]
- 6.Ostermann ME, Taube D, Morgan CJ, Evans TW. Acute renal failure following cardiopulmonary bypass: a changing picture. Intensive Care Med. 2000;26:565–571. doi: 10.1007/s001340051205. [DOI] [PubMed] [Google Scholar]
- 7.Brienza N, Giglio MT, Marucci M, Fiore T. Does perioperative hemodynamic optimization protect renal function in surgical patients? A meta-analytic study. Crit Care Med. 2009;37:2079–2090. doi: 10.1097/CCM.0b013e3181a00a43. [DOI] [PubMed] [Google Scholar]
- 8.Elahi M, Asopa S, Pflueger A, Hakim N, Matata B. Acute kidney injury following cardiac surgery: impact of early versus late haemofiltration on morbidity and mortality. Eur J Cardiothorac Surg. 2009;35:854–863. doi: 10.1016/j.ejcts.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 9.Vidal S, Richebe P, Barandon L, et al. Evaluation of continuous veno-venous hemofiltration for the treatment of cardiogenic shock in conjunction with acute renal failure after cardiac surgery. Eur J Cardiothorac Surg. 2009;36:572–579. doi: 10.1016/j.ejcts.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 11.Herget-Rosenthal S, Trabold S, Pietruck F, Holtmann M, Philipp T, Kribben A. Cystatin C: efficacy as screening test for reduced glomerular filtration rate. Am J Nephrol. 2000;20:97–102. doi: 10.1159/000013564. [DOI] [PubMed] [Google Scholar]
- 12.Newman DJ, Thakkar H, Edwards RG, et al. Serum cystatin C measured by automated immunoassay: a more sensitive marker of changes in GFR than serum creatinine. Kidney Int. 1995;47:312–318. doi: 10.1038/ki.1995.40. [DOI] [PubMed] [Google Scholar]
- 13.Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 14.Sarnak MJ, Katz R, Stehman-Breen CO, et al. Cystatin C concentration as a risk factor for heart failure in older adults. Ann Intern Med. 2005;142:497–505. doi: 10.7326/0003-4819-142-7-200504050-00008. [DOI] [PubMed] [Google Scholar]
- 15.Shlipak MG, Wassel Fyr CL, Chertow GM, et al. Cystatin C and mortality risk in the elderly: the health, aging, and body composition study. J Am Soc Nephrol. 2006;17:254–261. doi: 10.1681/ASN.2005050545. [DOI] [PubMed] [Google Scholar]
- 16.Ix JH, Shlipak MG, Chertow GM, Whooley MA. Association of cystatin C with mortality, cardiovascular events, and incident heart failure among persons with coronary heart disease: data from the Heart and Soul Study. Circulation. 2007;115:173–179. doi: 10.1161/CIRCULATIONAHA.106.644286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Astor BC, Levey AS, Stevens LA, Van Lente F, Selvin E, Coresh J. Method of glomerular filtration rate estimation affects prediction of mortality risk. J Am Soc Nephrol. 2009;20:2214–2222. doi: 10.1681/ASN.2008090980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peralta CA, Katz R, Sarnak MJ, et al. Cystatin C identifies chronic kidney disease patients at higher risk for complications. J Am Soc Nephrol. 2011;22:147–155. doi: 10.1681/ASN.2010050483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Briguori C, Visconti G, Rivera NV, et al. Cystatin C and contrast-induced acute kidney injury. Circulation. 2010;121:2117–2122. doi: 10.1161/CIRCULATIONAHA.109.919639. [DOI] [PubMed] [Google Scholar]
- 20.Herget-Rosenthal S, Marggraf G, Husing J, et al. Early detection of acute renal failure by serum cystatin C. Kidney Int. 2004;66:1115–1122. doi: 10.1111/j.1523-1755.2004.00861.x. [DOI] [PubMed] [Google Scholar]
- 21.Zappitelli M, Krawczeski CD, Devarajan P, et al. Early postoperative serum cystatin C predicts severe acute kidney injury following pediatric cardiac surgery. Kidney Int. 2011;80:655–662. doi: 10.1038/ki.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wald R, Liangos O, Perianayagam MC, et al. Plasma cystatin C and acute kidney injury after cardiopulmonary bypass. Clin J Am Soc Nephrol. 2010;5:1373–1379. doi: 10.2215/CJN.06350909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koyner JL, Bennett MR, Worcester EM, et al. Urinary cystatin C as an early biomarker of acute kidney injury following adult cardiothoracic surgery. Kidney Int. 2008;74:1059–1069. doi: 10.1038/ki.2008.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shlipak MG, Coca SG, Wang Z, et al. Presurgical serum cystatin C and risk of acute kidney injury after cardiac surgery. Am J Kidney Dis. 2011;58:366–373. doi: 10.1053/j.ajkd.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parikh CR, Devarajan P, Zappitelli M, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol. 2011;22:1748–1757. doi: 10.1681/ASN.2010121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erlandsen EJ, Randers E, Kristensen JH. Evaluation of the Dade Behring N Latex Cystatin C assay on the Dade Behring Nephelometer II System. Scand J Clin Lab Invest. 1999;59:1–8. doi: 10.1080/00365519950185940. [DOI] [PubMed] [Google Scholar]
- 28.Svenmarker S, Haggmark S, Holmgren A, Naslund U. Serum markers are not reliable measures of renal function in conjunction with cardiopulmonary bypass. Interact Cardiovasc Thorac Surg. 2011;12:713–717. doi: 10.1510/icvts.2010.259432. [DOI] [PubMed] [Google Scholar]
- 29.Fricker M, Wiesli P, Brandle M, Schwegler B, Schmid C. Impact of thyroid dysfunction on serum cystatin C. Kidney Int. 2003;63:1944–1947. doi: 10.1046/j.1523-1755.2003.00925.x. [DOI] [PubMed] [Google Scholar]
- 30.Knight EL, Verhave JC, Spiegelman D, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004;65:1416–1421. doi: 10.1111/j.1523-1755.2004.00517.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.