Abstract
Beside their genomic mode of action, estrogens also activate a variety of cellular signaling pathways through non-genomic mechanisms. Until recently, little was known regarding the functional significance of such actions in males and the mechanism that control local estrogen concentration with a spatial and time resolution compatible with these non-genomic actions had rarely been examined. Here, we review evidence that estrogens rapidly modulate a variety of behaviors in male vertebrates. Then, we present in vitro work supporting the existence of a control mechanism of local brain estrogen synthesis by aromatase along with in vivo evidence that rapid changes in aromatase activity also occur in a region-specific manner in response to changes in the social or environmental context. Finally, we suggest that the brain estrogen provision may also play a significant role in females. Together these data bolster the hypothesis that brain-derived estrogens should be considered as neuromodulators.
Keywords: aromatase, estrogen receptors, non-genomic effects, male behavior
1. Introduction
Estrogens, such as 17β-estradiol (E2), profoundly alter a wide array of physiological and behavioral processes including gonadotropin secretion, social behaviors, nociception and cognition [164]. The effects of estrogens are often mediated via binding to their cognate nuclear receptors, the estrogen receptors alpha and beta (ERα and ERβ), that activate a chain of intracellular events leading to transcriptional regulation of target genes [42; 274]. To our knowledge, the fastest changes in protein concentration are observed for immediate early genes. New messenger RNA and protein product of these genes are detected within 5 min and 1 hour respectively [48]. To produce functional responses, most proteins have to undergo post-translational modifications and translocate to their site of action (e.g. acquire enzymatic activity, be integrated in functional complexes at the membrane or synapse). As a result these processes requiring protein synthesis usually develop slowly (hours-days) and produce enduring effects associated with the changes in circulating concentrations of gonadal steroids typical of specific physiological states (e.g. stages of the estrus cycle or the breeding cycle) [83; 278]. Genomic effects of estrogens on behavior are often ultimately mediated by changes in the production or action of neurotransmitters in specific brain circuits resulting for a modification of the transcription and translation of enzymes that synthesize or catabolize the transmitters or of their receptors [80].
Besides these genomic effects, estrogens can also act on membrane-associated receptors to activate multiple cellular processes that do not depend on the synthesis of new proteins. These non-genomic cellular actions occur in a much shorter time scale than previously anticipated (seconds/minutes) [55; 157]. Such effects have been documented in numerous cell types including tumor cells ([10; 26; 41; 107; 132; 181; 210]; For review see, [146; 182; 268]). Considering neuronal preparations specifically, acute changes in estrogens’ concentration alter numerous intracellular events in vitro including the modulation of intracellular calcium concentrations [1; 28; 168] or of cyclic AMP [105; 174] and protein phosphorylation [179; 281; 283]. In turn, these activated signaling cascades can lead to modulations of the coupling state of a neurotransmitter receptor with its effector system [139; 144; 175; 176; 177], of enzymatic activities [198; 205], of neurophysiological activity [124; 126; 179; 292] or finally to indirect transcriptional activation through the phosphorylation of transcription factors such as cAMP response element binding protein (CREB) [2; 3; 33; 295] in specific brain regions. It is thus not surprising that evidence is emerging in support of a role for estrogens in the acute modulation of physiological and behavioral responses in vivo [59; 224].
Although prolonged exposure to estrogens can induce non-genomic signaling, the recent demonstration that membrane initiated effects in neurons desensitize relatively rapidly ([32; 78; 79; 101]; reviewed in [170; 172]) suggests that mechanisms must exist to regulate estrogen concentration on much shorter time scales. Unfortunately, the question of the source of these rapid changes in estrogen concentration is rarely considered. Acute effects of estrogens on cellular, physiological or behavioral processes have been described in both sexes. The ovaries constitute the major source of estrogens in females which fluctuate relatively rapidly during the estrus cycle and could thus trigger rapid non-genomic actions of this steroid ([8; 35; 117; 254; 262; 285]; for review see [172; 278]; but see [55] for a critical discussion of the time course of fluctuations in gonadal secretions).
Although they circulate at much lower concentrations, estrogens also control many physiological and behavioral responses in males, sometimes in an acute manner. These estrogenic effects depend on the neuronal aromatization of androgens into estrogens [12; 87; 158; 235; 241; 269]. Androgen aromatization is a complex enzymatic reaction that plays a key limiting role for the organization (sexual differentiation) and/or activation of many neural or behavioral processes in vertebrates. Research on the control of local estrogen synthesis by brain aromatase has largely focused on changes in enzyme availability taking place over relatively long time periods following developmental or seasonal changes in circulating steroid concentrations [50; 109; 115; 201; 236; 245]. However, studies taking advantage of the high concentrations of the aromatase protein in the avian brain have recently uncovered a novel mechanism acutely regulating brain estrogen synthesis and thus offering a time resolution for changes in local concentration compatible with the rapid and transient effects of estrogens described in vitro and in vivo. Here, we will first review evidence that estrogens rapidly and reversibly modulate behavior in male vertebrates. Then, we will discuss the mechanisms that control local brain estrogen concentration and their functional significance in vivo. Finally, we will suggest that the local tissue-specific provision of estrogens might not be a male specific characteristic but may also play a significant role in the physiology of females.
2. Rapid behavioral effects of estrogens in males
Rapid effects of estrogens suggesting an action that is independent of the transcriptional activity of liganded nuclear estrogen receptors have now been reported in a variety of animal species in relation with the control of multiple aspects of behavior. The short latency of these effects has originally led to the assumption that they relied on non-genomic mechanisms. However, independent evidence has in many cases been collected in support of this interpretation. Many of these effects indeed appear to be initiated at the cell membrane and/or are blocked by inhibitors of intracellular signaling pathways. In the following, we shall thus qualify the presumptive non-genomic effects of estrogens as rapid if this is the only criterion that has been formally demonstrated. The qualification of non-genomic will be limited to effects for which additional information is available.
Studies of the rapid effects of estrogens can be subdivided in 5 main classes according to the dependent behavioral variable that is measured (see table 1).
Table 1. Summary table of the behaviors acutely modulated by estrogens.
The latency reflects when the effect is detected with regard to the timing of injection, while the duration reflects how long the effect lasts from initiation to disappearance. Note that, since this review focuses on males, this table summarizes mostly the results of studies conducted on males. However, when no data were available on males, work on females was added when providing useful information in the present context.
| Reference | Behavior | Species | Sex | Injection mode (brain region) |
Agonist (doses) | Effect | Latency | Duration |
|---|---|---|---|---|---|---|---|---|
| Hayden-Hixson & Ferris, 1991 [113] |
Agonistic | Hamster | M (GDI) | Central -AH | E2 - 27pg | ↑ flank marking | 15 min | - |
| Trainor et al., 2007 [270] | Aggressive | Beach mice | M (CX+T+ FAD) |
Peripheral (SC) | E2 (75µg/kg) | ↑ attacks | 15 min | - |
| Trainor et al., 2008 [271] | Aggressive | California mice | M (CX+T+ FAD) |
Peripheral (SC) | E2 (100µg/kg) | ↑ bites ↓ attack lat. |
15 min 15 min |
- - |
| Cross & Roselli, 1999 [63] | Sexual | Rat (Sprague Dawley) |
M (CX) | Peripheral (IP) | T � 2–10 mg/kg* E2 -100µg/kg* |
No effect ↑ AGI ↑ mouting |
- 15 min 15 min |
- - - |
| Huddleston et al. 2007 [119] | Sexual | Rat (Sprague Dawley) |
M (CX+DHT) |
Central -MPOA | E2-implant E2-BSA -implant |
↑ copulation ↑ copulation |
48h 48h |
- - |
| Cornil et al., 2006 [56] | Sexual | Quail | M (CX+low T) |
Peripheral (IP) |
E2-500 µg/kg* | ↑ copulation | 15 min | 15 min |
| Cornil et al., 2006 [57] | Sexual | Quail | M (GDI or CX+T) |
Peripheral (IP) | VOR -30 mg/kg* ATD -150 mg/kg* |
↓ motivation ↓ Copulation ↓ motivation ↓ Copulation |
30 min 30 min 30 min 30 min |
30 min 30 min 30 min 30 min |
| Taziaux et al., 2007 [264] | Sexual | Mouse (C57BL/6J) |
M (GDI) | Peripheral (IP) | VOR -1mg/subject VOR + E2500µg ATD -4 mg/subject ATD + E2500µg |
↓ copulation restore behav. ↓ AGI ↓ copulation restore behav |
10 min 10 min 10 min 10 min 10 min |
- - - - - |
| Seredynski et al., 2012 [248] | Sexual | Quail | M (CX+T) CX+T+VOR |
Central -ICV | VOR 50µg* VOR + E2 50µg* VOR + E2-biotin 50µg ATD 50µg ATD + E250µg E2 - 50–100µg* E2-BSA 50µg |
↓ copulation restores behav restores behav ↓ copulation restores behav ↑ motivation ↑ motivation |
30 min 15 min 15 min 30 min 15 min 15 min 15 min |
- - - - - - - |
| Lord et al., 2009 [153] | Sexual | Goldfish | M (GDI) | Peripheral -IP | E2 -100µg/kg T -100µg/kg FAD -12mg/kg |
↑ approach ↑ approach blocks T effect |
10 min 30 min 30 min |
35 min- - - |
| Mangiamele and Thompson, 2012 [13] |
Sperm release | Goldfish | M (GDI) | Peripheral -IP | DES -5µg/subject T -3µg/subject FAD -480 µg/subject |
↑ milt volume | 1 hour | - |
| Remage-Healey and Bass 2004 [214] |
Fictive vocaliz. | Midshipman fish | M (type I †; GDI) |
Peripheral -IM | E2 -1.0 mg/kg | ↑ duration | 5 min | 20 min |
| Remage-Healey and Bass 2007 [215] |
Fictive vocaliz. | Midshipman fish | M (type II †; GDI) & F |
Peripheral -IM | E2 - 0.02 & 0.002 mg/kg* |
↑ duration | 5 min | 40 min |
| Tremere et al. 2009 [272] | Song-evoked spiking activity |
Zebra finches (awake and restraint) |
M&F (GDI) | intracerebral-NCM | E2 -200 ng TMX -5.6 ng ATD -2.8 ng |
↑ firing rate ↓ firing rate ↓ firing rate |
5 min 5 min 5 min |
- - - |
| Tremere et al. 2011 [273] | Song-evoked activity Auditory coding Behavioral pref. |
Zebra finches (awake and restraint) Freely moving |
M (GDI) | intracerebral-NCM intracerebral-NCM intracerebral-NCM |
E2 -3 ng ICI -6 ng TMX -5.4 ng FAD -2.6 ng ATD -2.8 ng E2 -3 ng ICI -6 ng TMX -5.4 ng FAD -2.6 ng ATD -2.8 ng ICI -30 ng TMX -26.8 ng FAD -13 ng ATD -14 ng |
↑ firing rate ↓ firing rate ↓ firing rate ↓ firing rate ↓ firing rate ↑ discrim. ↓ discrim. ↓ discrim. ↓ discrim. ↓ discrim. X pref. for TUT X pref. for TUT X pref. for TUT X pref. for TUT |
5 min 5 min 5 min 30 min 30 min 5 min 5 min 5 min 30 min 30 min 30 min 30 min 30 min 30 min |
2h 2h 2h 2h 2h 2h 2h 2h 2h 2h - - - - |
| Remage-Healey et al. 2010 [219] | Behavioral pref. Auditory processing |
ZB (free moving) ZB anesthetized |
M (GDI) M (GDI) |
Retrodialysis - NCM Retrodialysis - NCM |
FAD .100µM E2 - 30µg/ml (30min) FAD - 100 µM (30min) |
↓ pref for BOS ↑ pref for CON ↓ pref for CON |
30 min 30 min 30 min |
- 30 min 30 min |
| Remage-Healey et al., 2012 [221] | Auditory processing | Zebra finches | F (GDI) | Retrodialysis - NCM | E2 30µg/ml E2 -biotin 60 µg/ml FAD 100µM |
↑ discrim. ↑ discrim. ↓ discrim. |
<30min <30min <30min |
<30min |
| Van Hartesveldt et al., 1989 [275] | Dorsal immobility | Rat (Long evans hooded) |
M&F (GNX) | Central - Striatum | E2-implant 17a- E2-implant |
↑ response ↑ response |
4 h 4 h |
- - |
| Van Hartesveldt et al., 1990 [276] | Dorsal immobility | Rat (Long evans hooded) |
M (GNX) | Central -Striatum | E2 -implant T -implant |
↑ response no effect |
4 h 4 h |
- - |
| Evrard and Balthazart, 2004 [85] | Nociception | Quail | M (CX, CX+T, CX+ E2) (GNX) |
Peripheral (IP) Central (IT) |
VOR -4.8 mg/kg E2 -4.4 mg/kg VOR -15µg ATD -15µg VOR+ E2 -4µg |
↑ FWL ↓ FWL ↑ FWL ↑ FWL ↓ FWL |
20 min 20 min 1 min 1 min 1 min |
- - <25min <25min <25min |
| Liu et al. 2011 [150] | Nociception | Rat (Sprague Dawley) |
F | Central (IT) | FAD -2pg ICI .1.8 pg |
↑ tail-flick lat. ↑ tail-flick lat. |
15 min 30 min |
45 min 30 min |
| Kuhn et al., 2008 [138] | Nociception | Rat (Sprague Dawley) |
M (GDI) | Peripheral (SC) -hind paw |
G1 -100 ng ICI -100 ng |
paw removal paw removal |
30 min 30 min |
- - |
| Packard et al. 1996 [194] | Learning | Rat (Long evans) | M (GDI) | Central -Hp | E2 -1µg | ↑ SM | few min | < 2h |
| Packard and Teather, 2007 [195] | Learning | Rat (Long evans) | F (OVX) | Peripheral - IP | E2 - .0.2 mg/kg | ↑ SM | few min | < 2h |
| Packard and Teather, 2007 [196] | Learning | Rat (Long evans) | F (OVX) | Central - Hp | E2 - 5µg | ↑ SM | few min | < 2h |
| Liu et al., 2008 [148] | Spatial Memory | Mice (C57/Bl6N)/rat (Long evans) |
F (OVX) M (GDI) |
Peripheral (SC) | E2 - 5µg WAY-200070 -10mg/kg WAY-202779 -10mg/kg PPT -10mg/kg |
↑ pCREB in Hp ↑ learning ↑ pCREB in Hp ↑ learning ↑ pCREB in Hp No effect |
15 min > 1day 15 min > 1day 15 min - |
2h - 2h - 2h - |
| Luine et al 2003 [155] | Learning | Rat (Sprague Dawley) |
F (OVX) | Peripheral (SC) | 17bE2 - 15 µg/kg 17a-E2 - 15 µg/kg DES - 15 µg/kg 16a-iodo-E2- 15µg/kg |
↑ OR ↑ OP ↑ OR ↑ OP ↑ OR ↑ OP ↑ OR OP no effect |
30 min <3 min 30 min 30 min <3 min 30 min <3 min |
- <2h - - <2h - - |
| Inagaki et al 2010 [121] | Learning | Rat (Sprague dawley) |
F (GNX) | Peripheral (SC) | 17bE2 - 15–20 µg/kg* 17bE2 - 5–10 µg/kg* 17a-E2 - 5 µg/kg* 17a-E2 - 1–2 µg/kg* |
↑ OP ↑ OR ↑ OP ↑ OR |
<3 min <3 min <3 min <3 min |
<45 min - - - |
| Fernandez et al., 2008 [88] | Learning | Mice (C57BL/6J) | F (OVX) | Peripheral - IP Central - Hp |
E2 - 0.2 mg/kg E2 (IP) + U0126 1µg E2 - 5µg E2-BSA - 5µg E2-BSA + U0126 1µg |
↑ OR X E2 effect ↑ OR ↑ OR X E2-BSA effect |
- - <3h - - |
- - <3h - - |
| Phan et al., 2011 [204] | Learning | Mice (CD1) | F (OVX) | Peripheral - SC | PPT - 50–75 µg* DPN - 30–150 µg* |
↑ SR, OR, OP No effect |
<40min | - |
AEA, auditory evoked activity; AGI, anogenital investigations; AH: anterior hypothalamus; ATD, androstatrienediol (aromatase inhibitor); BOS, bird’s own song; CON, conspecific song; CX, castrated; DES, diethylstilbestrol; DHT, dihydrotestosterone; Discrimi., stimulus discrimination; DPN, 2,3-bis(4-Hydroxyphenyl)-propionitrile (ERβ specific agonist); E2, 17β-estradiol; E2-BSA, estradiol conjugated to bovine serum albumine; FAD, fadrozole (aromatase inhibitor); Fictive vocaliz., fictive vocalizations; FWL, foot withdrawal latency (from a hot water bath); GDI, gonadally intact; GNX, gonadectomized; Hp, hippocampus; ICI, ICI 182,780 (fulvestrant; general estrogen receptor antagonist); ICV, intracerebroventricular; IM, intramuscular injection; IP; intraperitoneal injection; IT, intrathecal; Lat., latency; MPOA, medial preoptic area; NCM, caudomedial nidopallium; OP, object placement; OR, object recognition; OVX, ovariectomizd; PPt, 4,4’,4”-(4-Propyl-[1H]-pyrazole-1,3,5-triyl) trisphenol (ERα specific agonist); Pref., preference; REV, reversed conspecific song; SC, subcutaneous injection; SM, spatial memory; SR, social recognition; T, testosterone; TMX, tamoxifen (selective estrogen receptor modulator); TUT, tutor song; U0126, MEK inhibitor; Vocaliz., Vocalization; Vor, vorozole (aromatase inhibitor); WN, White noise; X, “blockade of”; ZB, zebra finches.
Plain midshipman fish have three adult morphotypes: females, type I (courting and vocalizing) and type II males (sneaking and satellite spawning at the nest).
these results represent the active doses obtained from studies where 2 or more doses were compared.
2.1. Aggression
In 1991, Hayden-Hixson and Ferris reported that a single injection of 17β-estradiol (E2) in the anterior-hypothalamus facilitated agonistic behaviors within 20 min in male hamsters [113]. In beach mice (Peromyscus polionotus) and California mice (Peromyscus californicus), males are more aggressive during short days mimicking winter conditions than in long days. In these two species, aggressive behavior E2 acutely (within 15 min) increases the number of bites while reducing the latency to attack in short but not in long days [270; 271]. Interestingly, a recent study conducted in song sparrows (Melospiza melodia morphna) found that E2 injections induce changes in intracellular signaling within 15 min, some of which are season-dependent [114] but unexpectedly these neurochemical changes were not associated with changes in behavior (aggression, singing, locomotion, maintenance behaviors).
2.2. Sexual behavior
Cross and Roselli initially found that an intraperitoneal injection of E2, but not testosterone, increases within 35 min the frequency of anogenital investigations and mounting behavior in castrated male rats (Rattus norvegicus; [63]). Subsequent studies conducted in castrated quail (Coturnix japonica) and mice (Mus musculus) treated with a sub-threshold concentration of testosterone demonstrated that peripheral administration of E2 acutely stimulates appetitive (a category of variable behaviors that serve to bring individuals into contact with their mates, often used as a measure of sexual motivation, e.g. courtship [13; 203]) and consummatory (highly stereotyped behaviors resulting in the termination of the behavioral sequence, e.g. actual copulation [13; 203]) aspects of male sexual behavior. These effects occur even faster than previously thought (within 10–15 min) and are transient; they disappear within 30–45 min of E2 administration [56; 264]. Conversely, blockade of estrogen synthesis with non-steroidal or steroidal aromatase inhibitors, Vorozole™ and androstatrienedione (ATD) respectively, rapidly and transiently reduces both behavioral components of male-typical sexual behavior [57; 264]. Several of these experiments were performed on castrates treated with exogenous testosterone. These observations thus suggested that the estrogens responsible for this rapid control of male sexual behavior are synthesized locally in the brain. In the absence of testes, the most likely site of aromatization was indeed the brain itself.
Direct evidence of the key role of local brain aromatization was recently provided by studies employing intracerebroventricular (ICV) injections of E2, ER antagonists or aromatase inhibitors in the third ventricle of Japanese quail [248]. A first experiment investigated the effect of acute E2 injections in castrated males chronically treated with testosterone and the aromatase inhibitor Vorozore™ in order to test the effect of a rapid increase in brain estrogen concentration. Chronic testosterone treatment allows the expression of all androgenic effects, while preventing estrogenic effects, and is known to reduce the display of appetitive sexual behaviors and profoundly inhibit copulation [263]. The ICV injection of E2 in these subjects chronically deprived from estrogens enhanced appetitive sexual behavior within 15 min [248]. No consummatory sexual behavior was exhibited by most subjects regardless of the dose and timing of the acute treatment with E2 suggesting that a prolonged exposure to E2 is required to prime this behavioral component by the transcriptional activity of estrogens, as indicated by earlier work in female rodents [133; 171; 172; 277].
A second set of experiments conducted in castrated male quail chronically treated with testosterone and displaying the full range of male sexual behaviors also showed that the aromatase inhibitors, Vorozole™ and ATD, profoundly reduce both the appetitive and consummatory components of male sexual behavior within 30 min. Furthermore, E2 injected 15 min after Vorozole™ counteracted the effect of aromatase inhibition thus demonstrating that this effect does not result from a non- specific effect of the blocker [248]. These data indicate that a local brain estrogen production is clearly implicated in the rapid activation of male sexual behavior in quail.
Finally, brain-derived estrogens also rapidly enhance visually guided sexual approaches in fish [153]. A single systemic testosterone injection to male goldfish (Carassius auratus) stimulates their approach toward the visual cues of a female within 30–45 min. This effect is mimicked by E2 within 10 min and blocked by an aromatase inhibitor administered along with testosterone thus indicating that the behavioral effect of testosterone requires its aromatization. In the same species, testosterone has recently been shown to rapidly increase through aromatization milt volume and sperm concentration [159].
2.3. Social communication (vocal production & perception)
Estrogens also rapidly influence social communication by altering vocal communication and/or the perception of species-typical auditory signals by conspecifics. To our knowledge, the only demonstration of rapid modulations of vocalizations by estrogens was reported in the plain midshipman fish (Porichthys notatus). In an anesthetized preparation of this species, systemic administration of E2 induces a rapid (5min) and transient (25–40 min) increase in the duration of fictive vocalizations reflecting the rhythmic activity of the vocal generator pattern in response trains of stimuli delivered to the midbrain [214; 215].
Evidence supporting an acute neuromodulatory role of brain-derived estrogens in auditory processing was recently provided by two independent groups working on songbirds (for reviews, see [158; 219]. Using simultaneous single unit extracellular recordings from neurons of the left and right caudomedial nidopallium (NCM, a telencephalic auditory region thought to be analogous to secondary auditory cortex in mammals) of awake zebra finches (Taeniopygia guttata), Tremere and colleagues first demonstrated that E2 delivered in the vicinity of the recording site increases within 5 min the firing activity evoked by playback of conspecific songs as compared to recordings made in the contralateral site where the vehicle was injected. Estrogen receptors (ER) antagonists (Tamoxifen and ICI 182,780 [ICI]) prevented this response and even reduced song-evoked spiking activity below control levels suggesting that endogenous estrogens may be implicated in this process. Furthermore, similar effects were obtained following acute blockade of local estrogen synthesis (Table 1), confirming that these effects rely on local estrogen provision. These effects are sustained for at least 2 hours but are no longer present 4 hours after the injection [272; 273]. Whole-cell patch-clamp recordings in slices revealed that E2 action in NCM is mediated through changes in GABA transmission probably involving a modulation of GABA release [272].
Using multi-unit recording coupled with E2 retrodialysis, Remage-Healey and colleagues subsequently showed that a 30 min provision of E2 in the NCM of anesthetized male zebra finches selectively increases the firing rate and bursting activity in response to playback of stimuli containing elements of conspecific songs (bird own song, novel conspecific song or reversed conspecific song). This effect is no longer detected after a wash out period of 30 min. By contrast, aromatase blockade produces either no change or a decrease in this selectively depending on the neurophysiological parameter studied [218].
Similarly, Tremere and Pinaud showed that locally produced E2 enhances neuronal discrimination through the modulation of the information content of auditory signals. These processes occur within 5 min, are prolonged for at least 2 hours past the initial injection but vanish within 4 hours [273]. Thus, although there are a few discrepancies in the characteristics of these effects (see Table 1) most likely due to protocol and technical differences between studies, together these data provide evidence supporting a role for locally produced estrogens in the control of auditory processing and more specifically in the ability of NCM neurons to discriminate between species-specific auditory cues. Interestingly, similar processes have recently been described in females [221]; see also below).
Importantly, both the Remage-Healey and the Pinaud and Tremere groups demonstrated that brain-derived estrogens acutely strengthen the bird’s preference for conspecific auditory cues. When given a choice between their tutor's or their own song (bird's own song or BOS) and the song of another conspecific, male zebra finches prefer their tutor's song or BOS. In 2010, Remage-Healey and collaborators first showed that fadrozole retrodialysis in the left, but not the right, NCM acutely disrupts this preference in awake and freely moving male zebra finches [218]. Tremere and Pinaud further demonstrated that the bilateral injection of ER antagonists or aromatase inhibitors in NCM impairs the preference for the tutor's sone relative to a novel conspecific song. This behavioral preference seems specific for learned songs since E2 did not affect the birds’ ability to discriminate between female and male calls [273]. Therefore, these data support the notion that locally synthesized estrogens acutely control the auditory-evoked spiking neuronal activity, which translates equally rapidly into changes in behavioral song discrimination. Interestingly, the inner ear of zebra finches also expresses aromatase and ERα [186] suggesting that the control by estrogens of auditory performance could take place both centrally and peripherally.
2.4. Locomotion and nociception
In the striatum, estrogens are known to affect in both sexes motor responses controlled by striatal dopamine [25]. Some of these responses have been shown to occur relatively quickly. For example, 17β- and 17β-estradiol administered in the striatum potentiate within 4 hours dorsal immobility in both males and females, although males respond better than females [275]. In males, this response is not mimicked by testosterone [276]. Faster effects of striatal administration of E2 have been detected in other motor responses (e.g. E2 enhanced sensorimotor performance [24], increased amphetamine-induced rotational behavior [246]), but these have been investigated in females only (for a review see, [25; 59]). These effects of estrogens appear to rely on the acute and non-genomic potentiation of dopaminergic activity that in turn enhances sensorimotor processing.
In both sexes of a variety of species including humans, estrogens are involved in the control of nociception and analgesia namely through their genomic action on the opioid and adrenergic systems both at the central and peripheral level [9; 71; 84; 86; 92; 149]. Locally produced estrogens also rapidly impair the perception of noxious stimuli. Indeed, in quail, acute aromatase blockade obtained by intrathecal injection of an aromatase inhibitor increases within 1–5 min the latency for foot withdrawal from a noxious stimulus. This effect is by-passed by a concomitant injection of E2. This change in pain threshold is transient as it is no longer detected 30 min post-injection. It is also anatomically restricted given that intrathecal spinal but not intracerebroventricular injections mimicked the effects obtained after systemic injections [85].
2.5. Learning and cognition
Estrogens can also influence by non-genomic mechanisms the firing rate, synaptic transmission and synaptic plasticity in the hippocampus and cortex in mammals ([96; 104; 134; 156; 180; 253; 258; 288, 289; 291]; For review see, [259; 290]). These observations suggested that acute actions of estrogens might play an important role in learning and memory processes that are known to be mediated by these structures. This hypothesis was confirmed by studies from several laboratories that employed a variety of cognitive tests. In females, systemic or intra-hippocampal administration of estrogens (17β- but, sometimes also, 17α-estradiol) immediately prior to or after (but not several hours after), the training session were shown to enhance memory acquisition or consolidation ([22; 88; 102; 110; 121; 147; 148; 155; 195; 196; 204]; for review see: [46; 47; 97; 154; 197]). Although the hippocampus of males also synthesizes estrogens [116; 240], far less is known about their acute impact on cognitive processes. In 1996, Packard and colleagues showed that rats that received intra-hippocampal injections of E2 immediately post-training, but not 2 hours later, displayed a lower latency to escape in the Morris water maze, a result indicative of enhanced spatial memory [194]. Other reports support a role for estrogens in learning processes in males but (1) these studies involve long-term exposures to the steroid precluding any statement about the temporal resolution of the observed effects and (2) they sometimes provide contradicting results such that there does not seem to be a consensus supporting a role of locally synthesized estrogens in males at the moment [99; 143; 161; 178; 187].
3. Identification of membrane estrogen receptors
Because estrogen receptors do not possess any intrinsic trans-membrane domain and are most often found in the nucleus, it had been originally thought that non-genomic actions of estrogens were mediated through a novel receptor. Over the years, several such novel receptors have been discovered that exhibit specific pharmacological properties and are associated with the neuronal membrane. Functional studies have also indicated that these new membrane receptors could mediate rapid effects of estrogens. In addition, it has turned out that, following post-translational modification and protein-protein interactions, a fraction of the classical nuclear receptors, ERα and ERβ, are translocated to the membrane where upon binding to estrogens they can activate multiple intracellular signaling cascades. All these membrane receptors seem to play an important role in the control of rapid behavioral responses to estrogens and therefore need also to be considered in detail.
The presence of classical nuclear estrogen receptors at the membrane in brain cells had been first described in the early 1990s [29]. It has been confirmed by more recent studies [112; 173], but, the first indication of ER trafficking to the membrane originated from studies that employed cancer cell lines and endothelial cells. These experiments showed that ERs interact with complexes of membrane-associated proteins including caveolin-1 or the adapter protein shc [199; 210; 211; 212; 255]. It was also demonstrated that membrane-associated ERs must undergo palmitoylation, a post-translational modification resulting in the addition of a lipid-moiety that permits intracellular trafficking and association with specific subcellular domains such as the caveolar rafts of the plasma membrane [5; 199]. In these cellular models, the membrane-associated ERs act as G-protein coupled-receptors, interact with growth factor receptors such as the epidermal growth factor (EGF) receptor and insulin-like growth factor (IGF) receptor and trigger the phosphorylation of intracellular messengers ([213; 256]; For review see, [145; 146]). Similarly, recent work demonstrated that a brief exposure to E2 induces in both sexes the trafficking of ERα and ERβ to the membrane of neurons and astrocytes involving an interaction with caveolins ([32; 79; 101; 250]; for review, see [171]). At the membrane, ERs can interact with metabotropic glutamate receptors (mGluRs) whose trafficking to the membrane is influenced by estrogens in parallel with ERs [32; 67; 79]. E2 action on these membrane-associated ERs results in the transactivation of mGluRs, that in turn attenuates L-type calcium channel currents and calcium-dependent phosphorylation of CREB or increases the mitogen activated protein kinase (MAPK)-dependent CREB phosphorylation [33; 38; 67; 103]. The type of mGluR and response activated depends on the brain region and the cell type considered (For review, see [165]). It has to be noted that the function of this interaction has only been studied in females. Finally, it has also been shown that ERα and ERβ interact with IGF-I signaling (For review, [82; 167]). Both estrogens and IGF-I share similar signaling pathways, among which the phosphatidylinositol phosphate-3-kinase (PI3K) and MAPK signaling pathways are the best characterized. Co-localization between IGF-I receptors (IGF- IR) and ERα has been demonstrated in the rat brain [36] and a one-hour exposure to E2 induces a transient association between ERα and IGF-IR in the hypothalamus of ovariectomized females [166]. This interaction has been proposed to regulate estrogen-dependent neuroplasticity [89], the LH surge [209] and lordosis behavior [81; 209]. To our knowledge, the existence of this interaction has never been investigated in males. Note that, although rapid behavioral effects of estrogens have also been identified in birds and fishes (table 1), there is to this date no evidence demonstrating the presence of nucleus ER at the level of the cell membrane in these species. Moreover, two isoforms of ERβ have been identified in fishes [200].
Three novel membrane estrogen receptors (mER) have been described to date. ER-X is the least characterized of them. It has been described in the developing neocortex and uterus of ERα-knock out (KO) mice. This putative receptor is enriched in caveolar-like microdomains and activates MAP kinases. Its pharmacological profile differs from ERα and ERβ in that it is activated by both 17β–and 17α–estradiol whose action is not affected by the ER receptor antagonist ICI 182,780. It is recognized by antibodies raised against ERα, but its apparent molecular weight is different from the molecular weight of the main isoforms of the classical nuclear ERs. The expression of ER-X in the neocortex and uterus is maximal at post-natal day 7 to 10 (P7–10), then gradually decrease until P21 and is dramatically decreased in adulthood. ER-X is also up-regulated following brain injury. However, its molecular identity is still unknown [267].
GPR30 (or GPER) is a G-protein coupled-receptor that works in concert with the EGF receptor ([90; 222; 265]; for review see [91; 266]). It is found in numerous tissues including the brain of birds and mammals [6; 34; 108; 122; 238]. In fishes, this receptor has so far only been reported in the gonads [266]. Questions have been raised about its cellular location, some have described it at the cell membrane [265] while others have observed it in the endoplasmic reticulum [192; 222]. Furthermore whether GPR30 actually binds and is activated by E2 is still subject of debate ([192; 193]; for review, see [140; 190]). Interestingly, the general ER antagonists ICI 182,780 and tamoxifen act as full agonists on GPR30 [222; 265]. Unlike ER-X, GPR30 appears stereospecific as it only binds 17β-estradiol. Recently, an agonist (G1) and an antagonist (G15) that do not cross-react with classical ERs have become available [31; 66; 185] and have facilitated the demonstration that this receptor is implicated in several neurophysiological processes [141; 151; 185; 261]. As these studies were conducted in females, less is still known on the function(s) of this receptor in males.
Gq-mER is another G-protein coupled-receptor originally identified in the hypothalamus. Its pharmacological profile is closer to ERα and ERβ than the other two mERs. Indeed, it is activated by 17β-, but not 17α-estradiol, and is antagonized by ICI 182,780. E2 effects mediated by Gq-mER are mimicked by the specific synthetic agonist STX, which activates a PLC-PKC-PKA pathway resulting in the uncoupling of mu-opioid and GABAB receptors from their effector (G-protein coupled inward rectifying potassium channel, GIRK) in POMC neurons of the arcuate nucleus [207]. This receptor also reduces the excitability of GnRH neurons [293] and increases the frequency of their calcium oscillations [127]. Finally, it was recently implicated in the control of energy homeostasis and thermoregulation [208; 223] as well as hippocampal neuroprotection [142]. Some of the physiological responses that are characteristic of Gq-mER and mimicked by STX have been reported in double ERαβ-KO mice [208] suggesting that it is not a product of the genes coding for ERα or ERβ. Unfortunately, as for ER-X, its molecular structure still remains to be uncovered.
4. Membrane estrogen receptors and male physiology and behavior
With this information in mind, we can now start discussing the nature of the receptors that might be involved in the regulation of the physiological and behavioral processes described above. Male sexual behavior is severely impaired in ERαKO [188; 284]; but much less so in ERβKO mice [30; 189]. Male sexual behavior is also profoundly inhibited in non-classical ER knock-in (or NERKI) mice with a mutated ERα knock-in that cannot bind to ERE and consequently signals only through membrane-initiated or ERE-independent genomic pathways [162]. Combined with the idea that some genomic priming is required to observe rapid effects of estrogens on male sexual behavior [56; 264], these results suggest that the acute control of male sexual behavior by estrogens likely involves an integration of non-genomic and genomic processes depending on ERα, at least in part. Interestingly, copulatory behavior was facilitated within 48h after the bilateral implantation of E2-BSA in the medial preoptic area (MPOA) of castrated male rats chronically treated with the androgenic metabolite of testosterone, dihydrotestosterone (DHT; [119]). This observation first suggested that estrogen membrane signaling was involved in the control of male sexual behavior. This has recently been confirmed by studies conducted in quail. Indeed, in castrated males chronically treated with testosterone and the aromatase inhibitor Vorozole™, the membrane impermeant analog E2-BSA rapidly restores appetitive sexual behavior. Moreover, DPN, a specific ERβ agonist and, to a lower extent, PPT, a specific ERα agonist, produce a similar behavioral activation as estradiol. Conversely, acute treatment with MPP, a specific ERα antagonist without behavioral activity when administered alone, completely abrogates the facilitating effect of estradiol on behavior [249]. Likewise, in castrated males chronically treated with testosterone, the acute inhibition of appetitive and consummatory sexual behavior resulting from aromatase blockade is prevented by the administration of E2, its membrane- impermeant analog E2-biotin and DPN but not PPT [249]. Together, these results suggest that the rapid action of brain-derived estrogens on male sexual behavior is initiated at the cell membrane and likely involves both classical ERs. The determination of the relative contribution of each receptor to this response will require further study. The role of GPR30 and STX in this experimental model is under investigation.
Much less is known about the receptor types involved in the acute estrogen effects on the other social behaviors. No information is available regarding the receptor underlying the rapid action of estrogens on aggression. However, long-term exposure to both PPT and DPN significantly alters different aspects of aggressive behavior suggesting that both classical receptors might be involved [270]. As for communication, it was recently demonstrated that the membrane-impermeant E2- biotin produces the same effect as E2 on auditory processing in songbirds [221] thus indicating that these acute effects are initiated at the membrane. The auditory cortex expresses both types of nuclear estrogen receptors [27; 123; 169] as well as GPR30 [6] suggesting that the three receptors might contribute to this effect. There is however no evidence so far that ERα and/or ERβ associate with the cell membrane in this brain area of songbirds.
Recent evidence also supports an involvement of these three receptors in the control of nociception and analgesia. First, in females, E2-BSA mimics the inhibition of the ATP-induced increase in intracellular calcium concentrations induced by E2 within 5 min in the dorsal root ganglia (DRG) that contains a subset of nociceptive neurons [39]. This inhibition is detected in mice lacking ERβ but not in those lacking ERα indicating that this non-genomic modulation of DRG signaling is mediated through ERα [40]. Moreover, it is attenuated by blockade of group II mGluRs [37] suggesting that this response might be mediated through the transactivation of mGluRs. Secondly, morphine-induced anti-nociception in females is acutely blocked by the general ER antagonist ICI 182,780, the selective ERα and ERβ antagonist, MPP and PHTPP respectively, and the specific GPR30 antagonist G15 [150] suggesting that several receptor types are involved in the control of this response. Finally, the acute mechanical hyperalgesia induced by E2 injection into the hind paw of male rats is mimicked by the GPR30 antagonist and by the general ER antagonist ICI 182,780 indicating a role for GPR30 [138].
Finally, analyses of the intracellular events involved in the effects of E2 on hippocampus-dependent tasks in females suggest that they might be mediated by ERβ activation, initiated at the cell membrane and depend on the activation of the extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK/MAPK) pathway [88; 148]. In addition, it might be inferred from the absence of stereoselectivity observed in some studies [155; 156] that ER-X might be at play, yet more work is needed before a firm conclusion can be drawn. A similar conclusion can be drawn from the potentiation of tonic immobility induced by both 17α- and 17β-estradiol [275; 276].
5. Interaction with genomic effects
The mechanisms controlling behavior involve chains of molecular and neurophysiological events precisely organized in time and space (from the intracellular space to neuronal networks controlling motivation or motor patterns). One can thus assume that, in vivo, the non-genomic effects of estrogens should produce observable effects on behavior after slightly longer latencies than the intracellular events (triggered within a few seconds to minutes [55]) that underlie them. As illustrated in preceding sections, multiple experiments have identified physiological or behavioral responses triggered by estrogens that occur after a few minutes to an hour. These effects should be regarded as extremely rapid when compared to genomic effects of estrogens on behavior that are typically detected following several days of treatment. Given this very short time course and their activation by membrane impermeant estrogen analogs, it is thus very likely that they rely on a non-genomic action rather than on a regulation of gene transcription.
Although the cellular events initially triggered by E2 acting at the cell membrane do not involve protein synthesis, the intracellular cascades activated by these events very often lead to indirect transcriptional activation [3; 114; 272] and both types of events might be required for the expression of a given behavior. One of the best-studied examples of such cooperation between non-genomic and genomic effects of estrogens concerns the control of female sexual receptivity (reviewed in [59; 172; 278]. In brief, numerous studies conducted by independent research groups indicate that, early events activated by E2 at the cell membrane affect an intracellular chain of events that ultimately facilitate lordosis but also trigger long lasting effects that likely result in the indirect modulation of protein synthesis (indirect genomic mechanisms involving pCREB binding to cAMP response elements (CRE) sites on the DNA; [3; 33]). These membrane-initiated effects of E2 also interact with classical genomic actions involving the interaction with estrogen-response elements (ERE; e.g. activation of progesterone receptor transcription required for progesterone action) or ERE-independent sequences (Reviewed in [163]).
As far as we know, the existence of membrane-initiated indirect genomic activation by E2 and its role in the control of physiological and behavioral processes has never been investigated in males. The existence of a major sex difference in the rapid intracellular signaling activated by estrogens has been suggested in gonadectomized mice: E2 action was in this study enhancing CREB phosphorylation within an hour in females but not in males [4]. Yet, recent studies showed that acute administration of estrogens also results in CREB phosphorylation in males of avian or rodent species [114; 148]. One explanation for the apparent lack of effect of estrogens previously reported in male rodents [4; 33] is that these effects might require long- term exposure (priming) by androgens. Indeed, the studies that failed to identify an E2-induced CREB phosphorylation in males were carried out on gonadectomized males while others used gonadally intact subjects with or without chronic treatment with an aromatase inhibitor to deprive them of estrogens while maintaining normal concentrations of circulating androgens.
Finally, it should be noted that the changes in cognitive behavior described previously develop over the course of several days following repeated injections with estrogens [155; 156; 194]. It could thus be hypothesized that these effects reflect transcriptional activation rather than non-genomic actions. However, the timing of E2 injections relative to cue exposure and testing was shown to be critical: estrogens facilitate learning and memory when administered before or immediately after but not two hours after training. It can thus be concluded that the resulting cognitive changes depend, at least in part, on non-genomic mechanisms for which a short-term exposure to the active hormone is sufficient. Of course, any exposure (short, prolonged or repeated) to estrogens also activates genomic effects that likely participate in the long- term modifications of synaptic plasticity and cognitive abilities arising in these learning paradigms [148].
6. General features of non-genomic behavioral effects of E2 with a special emphasis on active doses
In summary, as illustrated in table 1, collectively, these studies demonstrate the implication of membrane-initiated effects of estrogens in multiple physiological and behavioral endpoints in a variety of vertebrate species. Most of these effects are detected within 5 min to an hour of the injection and last for a limited period of time. Such latencies can be considered to be extremely short when compared to the typical latencies required to detect behavioral effects resulting from genomic activation. There seems to be no rule relating the latency of the effects to the species analyzed nor to the type of behavioral response under investigation. It is likely that variations in the latency of estrogen action relates to the diversity of the underlying signaling pathways. More studies would obviously be needed to confirm this hypothesis.
The transient nature of these non-genomic behavioral actions of estrogens also contrasts with the enduring effects typical of genomic actions. Importantly, some of these effects are clearly region specific. Finally, these effects do not occur independently from genomic actions. In some cases, membrane-initiated actions potentiate genomic actions [277], while in others some genomic priming seems required to allow expression of non-genomic effects [248; 249]. These effects thus seem to share important properties: they occur across a wide range of species from fishes to mammals, they are rapid, often transient, anatomically confined and integrated with genomic action.
The active dose of estrogens mediating acute regulation of behavior requires a more elaborate discussion. It was originally assumed that non-genomic effects of estrogens would only be observed after injections of very large doses of the steroids. The reasoning was that non-genomic effects of estrogens are largely, if not exclusively, mediated by actions at the synapse of steroids produced at the pre- synaptic level and thus present in extremely high concentrations locally at the synapse but that were impossible to measure by available technologies. It was therefore necessary to inject very large doses that were obviously non-physiological in the periphery to mimic at the synaptic level a concentration that was actually physiological. The first studies investigating effects of exogenous estrogens on male sexual behavior accordingly employed fairly high doses of steroids (e.g., [54; 63; 214]) and thus seemed to support this interpretation. Some of these papers (e.g., [54]) additionally included dose-response studies indicating that the high doses (e.g. 500 µg/kg in quail) were actually required to obtain a fast behavioral response.
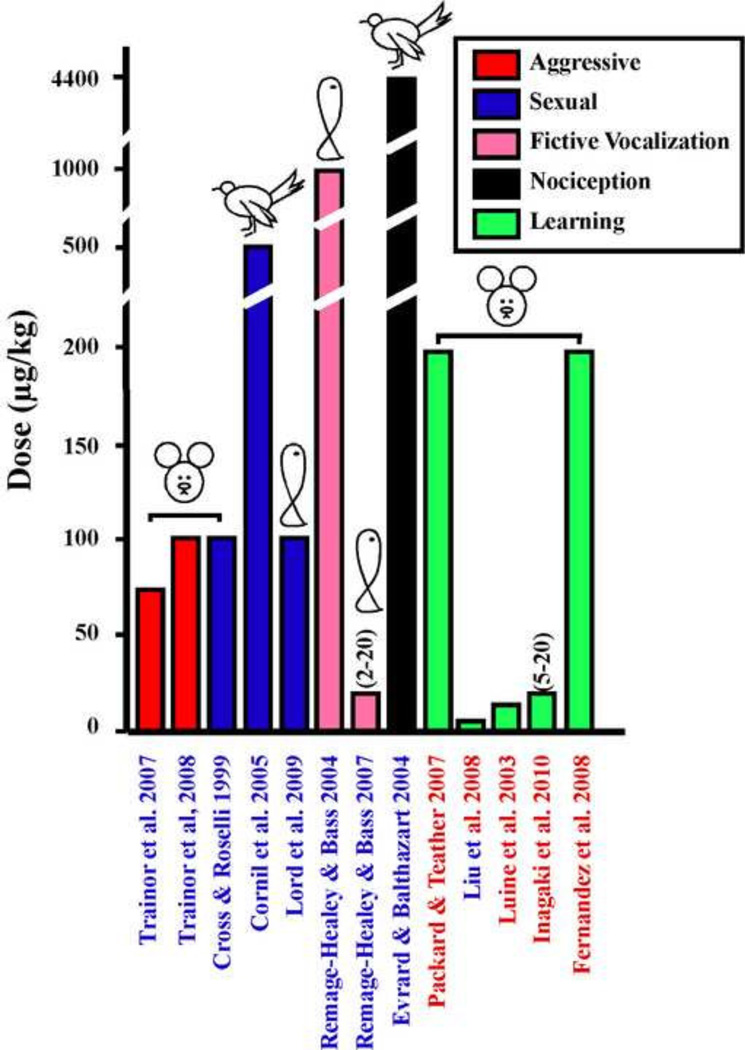
Since that time, fast behavioral effects of estrogens obtained with much lower doses (2–20 µg/kg) have been reported in both sexes in at least two different classes of vertebrates. As can be seen in figure 1, the range of behaviorally active doses is extremely wide and no general rule seems to emerge from the comparison of these high and low doses. They are not obviously associated with the sex of the subjects, nor with the type of behavior under consideration. Higher doses have usually been used in birds, which might be related to their higher body temperature and thus metabolism, but there are also studies in mammals (homeotherms with a lower body temperature) or fish (poikilotherms) that use doses in the same range.
Figure 1.
Comparison of the doses of estradiol expressed in µg/Kg that were shown to modulate after short latencies (15 to 60 min) the expression of behavior. The different bars are color coded to reflect the type of behavior under study as explained in the insert. References to the relevant work (X axis) are in blue for studies performed in males and in red for studies performed in females. One study carried out on both sexes is indicated by both colors. The class of vertebrates in which the study was performed (mammals, birds or fishes) is indicated at the top of the bars by a schematic drawing as well as the range of doses when multiple doses were used in a given study.
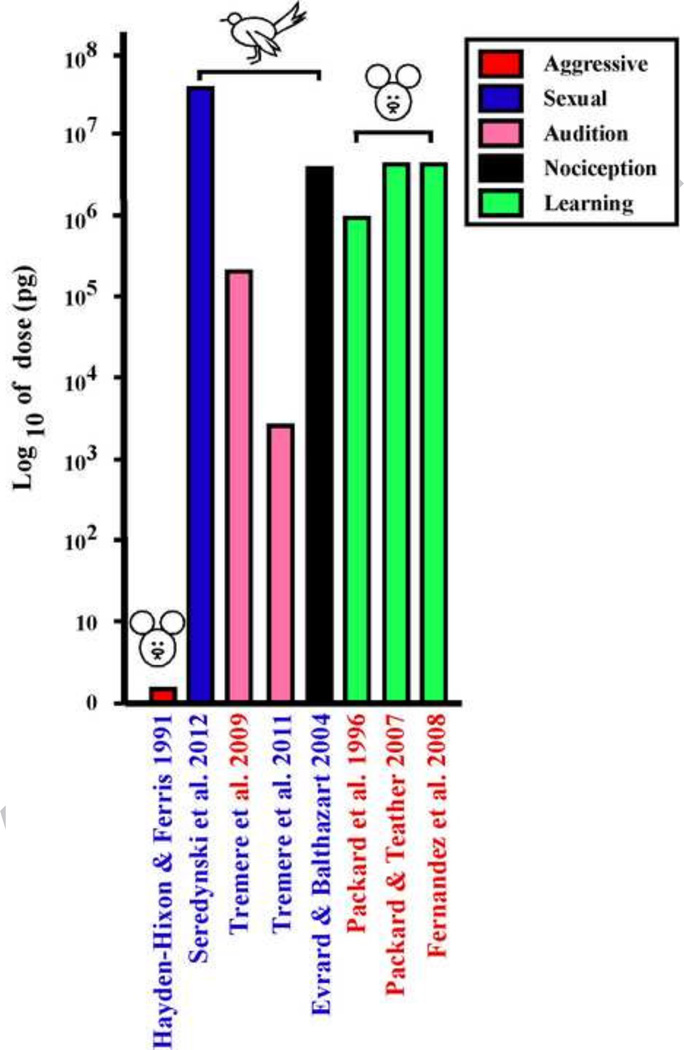
The comparison of doses used in experiments based on central injections show a similar or even a broader degree of diversity (see Figure 2). It makes no sense in this case to relate these doses to the body mass of the animals that are treated and absolute values of amounts injected are directly presented. As can bee seen, these doses range from 27 pg to 50 µg that is more than a million (106) fold difference. Here again, no rule seems to govern these differences: high and low doses have been used successfully in birds and in mammals irrespective of the behavioral response that was investigated.
Figure 2.
Comparison of the doses of estradiol injected centrally (ICV) in pg/subject that were shown to affect after short latencies (15 to 60 min) the expression of behavior. Note the logarithmic scale on the Y axis. The different bars are color coded to reflect the type of behavior under study as explained in the insert. References to the relevant work (X axis) are in blue for studies performed in males and in red for studies performed in females. One study carried out on both sexes is indicated by both colors. The class of vertebrates in which the study was performed (mammals, birds or fishes) is indicated by a schematic drawing at the top of the bars as well as the range of doses when multiple doses were used in a given study.
In conclusion, while the existence of rapid behavioral effects of estrogens can no longer be denied today given the large number of experiments that have successfully identified them, a major question remaining is to determine what are the active doses and why they differ so much from one experiment to another. It seems likely that these differences will relate to the type of membrane receptor and/or to the intracellular signaling cascade mediating the response. However, to this date, the available data do not permit a test of these hypotheses.
7. What is the source of estrogens?
Males are not exposed to the dramatic changes in circulating concentrations of estrogens experienced by females across the estrus cycle. Although prolonged exposure to estrogens can induce non-genomic signaling, membrane-initiated effects in neurons desensitize relatively rapidly ([32; 78; 79; 101]; reviewed in [170; 172]). Moreover, estrogen receptors are likely to be present in a fairly stable manner in the brain. Together, these observations suggests that if estrogens induce rapid and transient behavioral effects in males, mechanisms must exist to regulate local estrogen concentrations on much shorter time scales. Brain aromatase, which is expressed and active especially in brain regions controlling aggressive and sexual behaviors [14; 49; 93; 137; 226; 227; 229; 233; 234; 280; 282] but also in the hippocampus [14; 226; 229; 233; 239; 240; 260; 282], was thus considered as a potential local source of estrogens. However, due to their lipophilic nature, estrogens cannot be stored in synaptic vesicles before rapid release like classical neurotransmitters or neuropeptides. Therefore, in order to achieve rapid changes in local concentrations, it becomes necessary to invoke the existence of regulatory mechanisms that are able to change the local synthesis and bioavailability of estrogens in a dynamic manner, very much as is the case for gaseous neurotransmitters [19].
Estrogens are synthesized from androgens by aromatase, a P450 enzyme traditionally associated with microsomes. The enzyme is mainly expressed in the ovaries but is also found in many other tissues including the brain. In reptiles, birds and mammals, brain aromatase expression is restricted to specific neuronal populations mainly located in the hypothalamic/preoptic area (HPOA) including the medial preoptic nucleus and in the limbic system [11; 49; 60; 93; 136; 137; 169; 226; 227; 228; 229; 233; 234; 240; 260; 280; 282]. In songbird species, aromatase is found in another neuronal population located in the caudomedial nidopallium NCM, a pallial area (homologous to secondary auditory cortex in mammals) adjacent to the song control nucleus HVC (acronym used as proper name [14; 240; 251]). In teleost fishes, aromatase is abundantly expressed but specifically in glia ([73; 94]; For review, see [72; 95]). Finally, both the aromatase protein and activity are also detected in pre- synaptic terminals of various species [61; 183; 202; 225; 230; 244], suggesting that E2 synthesis may be achieved with a spatial subcellular specificity similar to neurotransmitters and neuromodulators [19; 242]. The presence of active aromatase in synapses of discrete brain regions indicates that estrogen provision can be modulated with a high spatial resolution independently from changes occurring in the periphery and/or surrounding brain regions.
As alluded to in the introduction, brain aromatase is known to play a key role in the control of a variety of physiological and behavioral endpoints including sexual behavior, neuroprotection and synaptic plasticity. However, until recently most studies investigated relatively slow, presumably genomic, effects of estrogens and, correlatively, most research on the regulation of aromatase activity focused on the genomic regulation of the enzyme concentration in parallel with relatively slow and enduring changes in reproductive states (seasonal changes). In the following sections, we will provide evidence gathered both in vitro and in vivo that aromatase activity can vary in a much shorter time scale that could produce variations in estrogen availability compatible with their rapid non-genomic actions. Rapid fluctuations in local estrogen concentrations can theoretically arise from variations in the enzymatic substrate concentration (testosterone) or in the catalytic ability of the enzyme (its efficiency), two mechanisms that are not mutually exclusive. The resulting changes in local estrogen provision could simultaneously provide answers to the issue of the source of rapid changes in their bioavailability and offer the spatial resolution that fits with the behavioral results described above.
8. Rapid changes in gonadal secretions
One way through which brain estrogens concentrations could be rapidly modulated involves rapid changes in peripheral androgen concentrations that would be available for local aromatization. Thus, short-term (min to hours) fluctuations in testosterone secretion from the testes in response to the pulsatile release of the luteinizing hormone from the pituitary may theoretically result in changes in local estrogen concentration in brain region expressing aromatase. Moreover, a sharp increase in plasma testosterone has been reported in response to social encounters such as territorial intrusion or sexual interactions (for review see [111; 191; 287]. Although most of these changes in gonadal secretions occur relatively slowly (for extensive review, see [55; 100; 286]), the fastest effects occur within 5–15 min [7; 23; 206; 237]. Interestingly, social stimulations, such as aggressive encounters, can also rapidly up- regulate the local synthesis of androgens in the brain [205]. Such rapid increases in the concentration of aromatizable androgens could translate into rapid and transient changes in local estrogen production by neural aromatization (reviewed in [55; 59]). This is for example the case in goldfish in which we have seen that an acute injection of testosterone, mimicking the rapid increase in androgen secretion occuring in the context of reproduction, facilitates visually guided sexual behavior and induces a rapid increase in milt volume and sperm concentration through aromatization [153; 159]. By contrast, in quail, two independent studies recently showed that the plasma testosterone concentration drops after sexual encounters but these changes do not seem dynamic enough to drive the rapid behavioral effects of estrogens [58; 65].
9. Rapid regulation of aromatase activity
In vitro studies taking advantage of the high concentrations of the aromatase protein present in the avian brain have recently demonstrated that aromatase activity (AA) can be rapidly modulated. In preoptic area/hypothalamus (HPOA) explants from Japanese quail, an acute increase in extracellular K+ concentration results in a rapid (5 min) and reversible reduction of AA (Figure 3A) [16]. A similar effect is obtained after incubation with thapsigargin, a sesquiterpene lactone that increases the intracellular pool of Ca2+. Several experiments subsequently tried to determine whether the K+-induced enzymatic inhibition is mediated by Ca2+ from extracellular origin or derived from intracellular stores (e.g. by incubating the hypothalamic blocks in a Ca2+-free medium or by producing a K+ depolarization in the presence of Ca2+ channel blockers) but additional work would be needed to draw final conclusions on this question [16]. It remains that collectively, these results show that a transient depolarization induced by elevated K+ extracellular concentration markedly and reversibly inhibits aromatase activity presumably by modulating intracellular Ca2+ concentrations.
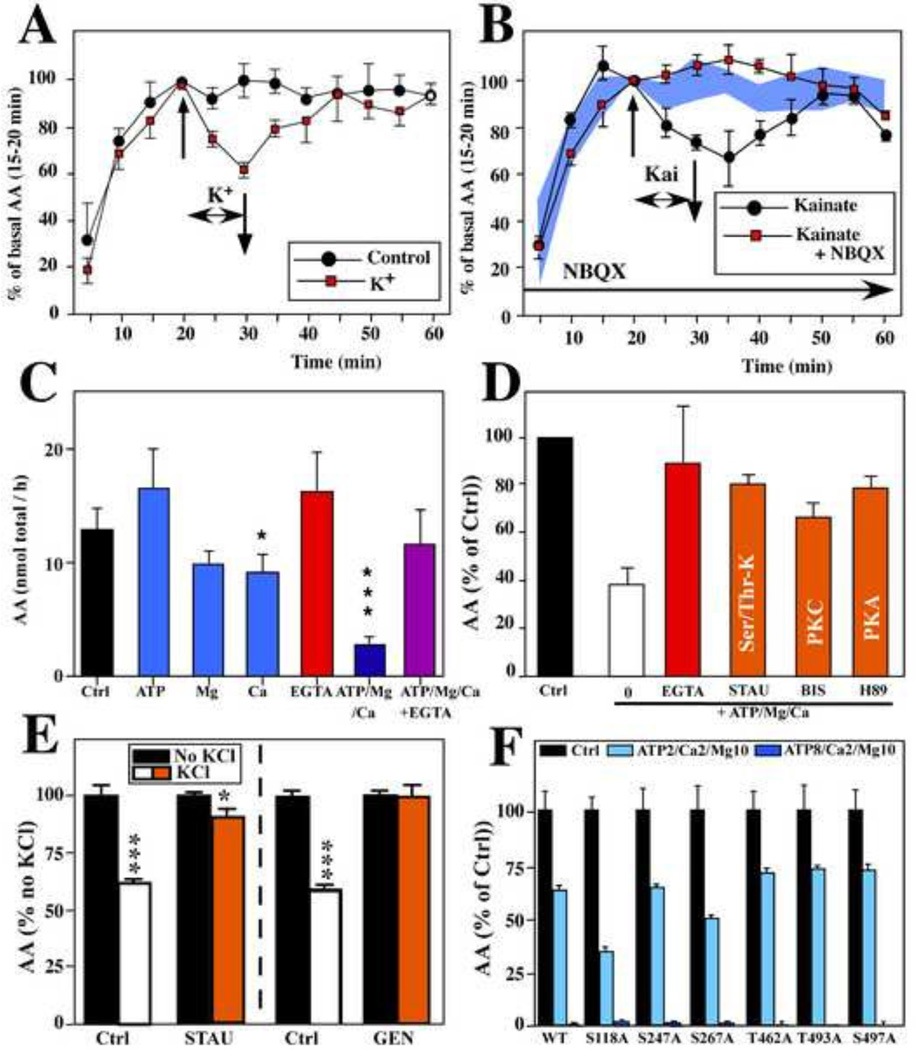
Figure 3.
Rapid changes in aromatase activity (AA) observed in hypothalamic- preoptic (HPOA) explants cultured in vitro (A-B), in HPOA homogenates (C-D) and in HEK293 cells transfected with human aromatase (E-F). All values are presented as mean ± SEM.
In explants, AA is inhibited within 5–10 min by a K+-induced depolarization (A) or by exposure to the glutamate receptor agonist kainate (B). This latter effect is blocked by co-exposure to the glutamate antagonist NBQX. The values observed in control conditions in the absence of any pharmacological treatment are indicated by the blue area. C. A 15 min pre-incubation of HPOA homogenates in the presence of ATP, Mg2+ and Ca2+ markedly inhibits AA, but these compounds alone have little or no effect. This inhibition is blocked by EGTA, a compound that chelates divalent ions such as Ca2+. D. The inhibition of AA produced in HPOA homogenates by the addition of ATP/Mg/Ca is largely blocked in the presence of EGTA or of the kinase inhibitors staurosporine (STAU, a Serine Threonine kinase inhibitor), Bisindolylmaleimide (BIS, a protein kinase C [PKC] inhibitor) or H89 (a protein kinase A [PKA] inhibitor. E. AA expressed by HEK293 cells is inhibited by a K+- induced depolarization and this effect is largely blocked by exposure to the protein kinase inhibitors staurosporine (STAU) or genistein (GEN). F. Effects of single amino acid mutations in the human aromatase expressed by HEK293 cells on the AA expressed in control conditions (black bars) and after pre-incubation with 2 mM Ca2+10 mM Mg2+, and 2 mM (pink bars) or 8 mM (red bars) ATP. AA in phosphorylating conditions is expressed as percentage of the mean activity compared in control conditions. No significant effect of the mutations is observed on the response to phosphorylating conditions (S=serine; A= alanine). * p < 0.05 vs CTL in (C), * and *** p <0.05 and 0.001 respectively vs No KCl conditions, same treatment in (E). Redrawn from data in [16] (A, C), [18](B), [17] (D) and [43](E,F)
Similar enzymatic down-regulations are also triggered by glutamate [16]. More specifically, the ionotropic glutamate receptor agonists AMPA, kainate and, to a lesser extent, NMDA rapidly (within 5 min) and reversibly decreased AA [18]. The effect of kainate was antagonized by the glutamate receptor antagonists CNQX and NBQX (Figure 3B) [18]. Calcium removal from the medium had no effect on the decrease in AA induced by glutamate suggesting that the effect of the activation of glutamate receptors on AA is not mediated by extracellular calcium influx into the neurons but by another signaling mechanism possibly including the release of calcium from intracellular stores [16]. Electrophysiological recordings demonstrated that aromatase-expressing neurons are sensitive to glutamate, a finding consistent with the view that glutamatergic inputs on aromatase cells acutely regulate AA [52; 53].
These rapid changes of aromatase activity are most likely mediated by post- translational modifications of the enzymatic protein rather than transcription- dependent modulations of its concentration. Post-translational modifications such as phosphorylations are a common mechanism of control of protein activity in the brain [184]. The transfer of the terminal phosphate group from ATP to specific amino acid residues (tyrosine, threonine, serine) of the protein (i.e. phosphorylations) is catalyzed by specific kinases and often critically depends on divalent cations such as Mg2+ and Ca2+. Pre-incubation of male quail HPOA homogenates with high but physiological concentrations of ATP, Ca2+ and Mg2+ significantly inhibited AA. This effect was prevented by EGTA, a Ca2+ chelator, (Figure 3C; [16]) and by different kinase inhibitors (Figure 3D) thus strongly suggesting that this rapid regulation of aromatase catalytic ability is regulated by calcium-dependent phosphorylation processes [16; 17]. Direct evidence that aromatase itself is targeted by phosphorylation(s) rather than another co-existing protein that could secondarily regulate aromatase was provided in cultured cells transfected with human aromatase in which phosphorylated residues were detected on the immunoprecipitated protein after incubation with ATP/Mg/Ca [17; 43]. Importantly, AA is also rapidly and reversibly inhibited by KCl-induced depolarization. This response is blocked by kinase inhibitors thus demonstrating that the depolarization-induced changes in AA, described in HPOA explants, are likely mediated by phosphorylation processes (Figure 3E). The quantification of the enzymatic protein confirmed that this rapid enzymatic down-regulation does not result from protein degradation thus confirming the hypothesis that aromatase is regulated by conformational changes that do not involve new transcription [43]. Together these data strongly suggest that these phosphorylations directly cause the rapid decrease of enzymatic activity.
A bioinformatic analysis of the quail and human aromatase coding sequences identified several potential phosphorylation sites [17; 43]. Targeted mutagenesis of selected residues failed to identify one residue specifically involved in the acute regulation of aromatase activity (Figure 3F) [43] suggesting that another residue or a combination of several phosphorylated residues is likely required to control AA (for further discussion of these data see [43; 45]).
Interestingly, the phosphorylation-dependent inhibition of AA is observed in both male and female quail HPOA and in the songbird telencephalon [61; 130]. In addition, the rapid inhibition of AA by calcium-dependent phosphorylations is not specific to neuronal aromatase as similar effects were recently described in the ovary [43] and in neuronal or non-neuronal cell lines stably expressing human aromatase [43]. These data show that the regulation of aromatase by phosphorylations is a general process, found not only in birds, but also presumably in humans and other mammals. Finally, recent evidence suggests that these phosphorylating conditions preferentially affect synaptic aromatase compared to the enzyme isolated from microsomes, leading to the provocative idea that rapid changes in estrogen synthesis would mainly occur at the synapse allowing a precise spatial resolution for estrogen provision ([61], see also below).
In conclusion, these studies demonstrate that AA is rapidly controlled via post- translational modifications. This basic effect is observed in several tissues that express aromatase as well as in a variety of species, including humans and thus seems to be robust. Although much remains to be discovered with respect to the molecular mechanisms that mediate these effects, these rapid enzymatic changes must result in fluctuations of estrogen availability with a time and spatial resolution consistent with the non-genomic behavioral effects described earlier.
10. Aromatase activity is rapidly modulated in vivo
As demonstrated in the first part of this review, acute blockade of aromatase activity rapidly alters numerous physiological and behavioral processes (Table 1). This observation suggests that rapid modulations of estrogen synthesis similar to these described in vitro must also occur in vivo in functionally relevant contexts. The first evidence supporting this hypothesis came from work carried out in Japanese quail [54], that has recently been confirmed and expanded ([65; 68; 69; 70]; for review see also [62]). An important body of work supporting this idea is also provided by work on songbirds primarily in the context of social communication [43; 216; 217; 220; 221] for review see [219; 242]).
10.1. Effect of sexual interactions
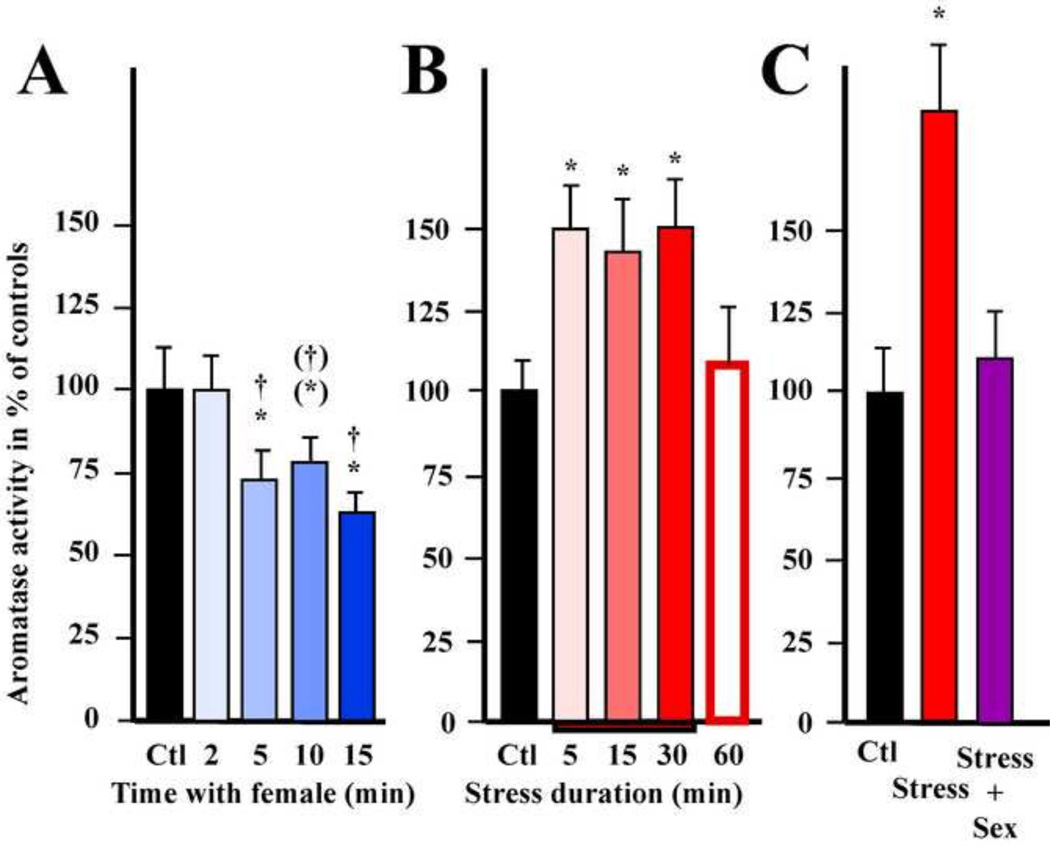
A first study demonstrated that the enzymatic activity measured in HPOA homogenates is modulated in a time-dependent fashion following both visual access to or copulation with a female. However, against all expectations, these sexual interactions resulted in a decrease in AA that was detectable after 1 min and reached its maximum after 5 min. The amplitude of this effect (20% change) is much less dramatic than the enzymatic changes observed in vitro (nearly complete inhibition). This discrepancy can be explained by the fact that the HPOA contains several populations of aromatase-containing cells that may be differentially controlled by behavioral interactions. This might result in a dilution of large effects located in discrete regions or in changes occurring in opposite directions that might partially cancel each other out. This former hypothesis was confirmed by a follow-up experiment investigating the time course of the effect of copulation on AA measured specifically in the different populations expressing high levels of aromatase activity that were in this case dissected by the Palkovits punch method adapted for quail [60; 247]. This second experiment identified rapid enzymatic inhibitions in the medial preoptic nucleus (POM) and the tuberal hypothalamus [65]. The fastest change was detected in the tuberal hypothalamus after only 2 min of interaction (-25%) and was followed at 5 min by a similar drop (−28%) in the POM (Figure 4A). Both effects developed progressively for at least 15 min at which point the maximal inhibition of activity reached about 40%. These data thus confirm that sexual interactions result in a rapid reduction in AA and demonstrate that these enzymatic changes occur in specific brain regions.
Figure 4.
Rapid changes in aromatase activity (AA) observed in the medial preoptic nucleus of male quail that have been exposed for various durations (2 to 15 min) to a female and allowed to copulate with her (A)or submitted to an acute restraint stress for 5 to 30 min, indicated by the red bar on the X axis (B) or exposed to stress for 5 min and then immediately allowed to copulate with a female (Stress+Sex) for 5 min (C). In panel B, birds exposed to restraint stress for 30 min were also sampled 30 min after the cessation of stress (time point marked 60 min). (*) and * p < 0.1 and 0.05 respectively vs Ctl (control), (†) and † p < 0.1 and 0.05 respectively vs 2 min. Redrawn from data in [65](A), [68](B) and [70](C).
The comparison of the time course of these enzymatic changes with the pattern of sexual activity displayed by these males revealed that sexual activity terminates before the drop in AA becomes detectable [65] implying that this enzymatic response is a consequence rather than the cause of changes in behavior. Moreover, submitting all subjects to 2 min long interactions (during which most of the sexual activity takes place) and collecting brains after different latencies post- copulation did not reveal any change in AA suggesting that this enzymatic down- regulation is not related to sexual performance but requires the presence of the female for a more extended period of time [65]. This conclusion is supported by the observation that simply seeing the female induces the same enzymatic response in the POM (but not in the tuberal hypothalamus) as copulation, providing further support to the idea that rapid changes in AA do not depend on behavior display but on the female's presence. Although the functional significance of such a reduction in brain aromatase activity is still debated, these data provide evidence that aromatase activity is rapidly modulated in behaviorally relevant situations in specific brain areas and in a time scale compatible with non-genomic effects of estrogens.
It must be stressed that these enzymatic changes cannot reflect local changes in testosterone or co-factor availability since all samples were incubated with the same concentration of enzymatic substrate (3H-androstenedione) and co-factor (NADPH). Therefore, such changes demonstrate either a reduction in the concentration of the enzyme due to degradation or a reduction in its catalytic ability due to post-translational modifications that are presumably similar to those described in vitro. It was recently shown that the enzymatic inhibition induced by a 5 min interaction is no longer detected 2 hours after this interaction [65] indicating that the enzymatic down-regulation unlikely relies on protein degradation (re-synthesis would take more time) but rather depends on post-translational modifications.
In male rats, sexual interactions are accompanied by a rise in extracellular glutamate concentration in the preoptic area [77]. As alluded to earlier, in rodents and birds, preoptic neurons, including aromatase neurons, are sensitive to glutamate [52; 53; 125]. It could thus be hypothesized that a female-induced rise in preoptic glutamate could result in a transient down-regulation of aromatase activity following copulation in quail. Although it is not yet known whether a similar glutamate release occurs in the avian POA, it is worth noting that in rats, glutamate controls the release of dopamine in the POA [75; 76]. Similar changes in extracellular dopamine concentration have been reported in male quail POA during copulation or visual interaction with a female [128; 129]. Only males that eventually engaged in behavior showed elevated dopamine levels but, in males who copulated, no difference in dopamine was observed between sampling periods during which copulation did or did not occur suggesting that the dopamine response is driven by the male's motivational state not by its copulatory activity [129]. Based on what is known in rodents [120], it is conceivable that these changes in extracellular dopamine concentrations in quail also reflect an increased glutamatergic release. Since glutamate was shown to inhibit AA within minutes in hypothalamic brain explants [18], these postulated changes in glutamate release would perfectly match and could explain the variations in enzymatic activity observed after sexual interactions in the quail brain.
10.2. Effect of stress
Evidence is also accumulating in support of a role for stress in the acute modulation of AA. Indeed, exposing male quail to acute restraint stress for 15 min or an acute injection of corticosterone 30 min prior to brain collection results in a significant increase in AA measured in HPOA homogenates [20]. As observed in the case of sexual interactions, the effect of stress is region-specific as the enzymatic up- regulation is detected only in the POM and to a lesser extent in the ventromedial nucleus of the hypothalamus [68]. In the POM, the increase in AA reaches 150% of control values within 5 min of stress exposure (Figure 4B). This elevated activity persists as long as the stressor is present and returns to control levels within 30 min after stress cessation. Interestingly, females also exhibit a moderate increase in AA in the POM and profound enzymatic reduction in the tuberal hypothalamus (60% of controls). In contrast to males, female responses are not as fast and are sustained beyond stressor cessation [68].
Although stress induced a significant increase in circulating corticosterone (CORT) levels, no correlation was found between individual CORT concentrations and AA measured in any brain region considered [68]. Since circulating corticosterone cannot explain by itself the sex and regional specificity of the enzymatic responses, other candidates were also considered. Experiments looking at the role of different components of the hypothalamic-pituitary-adrenal axis recently suggested that CORT, arginine-vasotocin (AVT) and corticosterone-releasing factor (CRF) all play a role in the control of stress-induced changes in AA in the POM but not in other regions [69]. More work is thus warranted to unravel the mechanisms of regulation of aromatase by stress.
Interestingly, the rapid changes in AA induced by stress occur in brain nuclei that play a critical role in the control of reproduction. Although little is known about the functional implications of local changes in estrogen synthesis in brain regions other than the POA, these results suggested that rapid changes in aromatase activity might mediate acute effects of stress on behavior and fertility. Surprisingly, male or female sexual behavior was not affected by 15 min of acute restraint stress, but fertilization was slightly reduced in stressed females [70]. Stress (15 min) or copulation (5 min) alone induced the same effects on AA as previously described. Yet, when presented in sequence, sexual behavior reversed the enzymatic up- regulation induced by stress in the male POM (Figure 4C). Likewise, the stress- induced decrease in AA in the female tuberal hypothalamus (presumably homologous to the arcuate nucleus of mammals) was reversed following pairing with a male. Strikingly, a clear anatomical specificity emerged when considering the profile of response to stress and mating. In some regions, such as the male POM or the female Tuber, aromatase seems responsive to both stimuli, while the enzyme appears exclusively sensitive to one stimulus in other regions [70].
Although the functional consequences of these region-specific enzymatic changes induced by stress remain unclear (for further discussion of this issue, see [62; 68; 70]), together these data provide compelling evidence that changes in the social and environmental context induce rapid changes in AA in specific brain regions in vivo. Importantly, the fast time course and reversibility of most of these effects strongly suggest that the underlying mechanism(s) relies on post-translational modifications of the enzyme in vivo. This idea is strongly supported by the reversal within 5 min of stress-induced changes by copulation.
10.3 Social communication
10.3.1 Mechanisms of control of aromatase in the caudo-medial nidopallium (NCM)
As mentioned earlier, the caudo-medial nidopallium (NCM, analogous to the mammalian auditory cortex) of songbirds such as zebra finches contains a dense population of aromatase-expressing cells [240; 251] and is accordingly one of the brain regions with the highest aromatase activity measured in any vertebrate species [44; 252]. This region, thought to play a role in the recognition of species-specific auditory information [98], thus constitutes an excellent locus to study the regulation of brain estrogen synthesis in vivo. To this end, Remage-Healey and colleagues developed an in vivo microdialysis system to quantify the extracellular concentration of estrogens in the NCM of awake zebra finches [216]. Using this approach, they first showed that local estrogen concentration and, thus probably local synthesis as well, is inhibited in vivo by the retrodialysis of glutamate in NCM [216] thus bolstering the conclusions drawn based on in vitro experiments in quail [16; 18]. Together these data suggest that the acute control of aromatase by glutamate is not limited to the HPOA but likely extends to other brain regions where the enzyme is expressed.
Additionally, in agreement with the effect of KCl-induced depolarization on AA measured in vitro [16; 43], estrogen concentration in NCM is rapidly reduced by KCl retrodialysis [220]. This effect is blocked by ω-conotoxin, a specific inhibitor of N-type Ca2+ channels predominantly expressed in presynaptic terminals [220]. Together with the recent identification of a preferential sensitivity of synaptic as compared to microsomal aromatase to phosphorylating conditions in zebra finch telencephalic homogenates [61], this finding strongly suggests that the rapid regulation of estrogen synthesis specifically occurs at the synaptic level in vivo.
10.3.2 Social cues involved in aromatase regulation in NCM
Investigations of the control and functional significance of these rapid fluctuations of brain-derived estrogens first demonstrated that playback of conspecific songs induces within 30 min a rise in extracellular E2 concentration compared to white noise when the microdialysis probe is implanted within but not outside of NCM. E2 returns to control concentrations within 30 min of female removal indicating that this effect is also rapidly reversible. No such fluctuations in estrogen concentrations are elicited by playback of female chirps or sounds from the colony suggesting that locally produced estrogens are specifically controlled by and potentially implicated in the auditory processing of specific social cues [216]. NCM estrogen synthesis is affected by songs of conspecific males in females as well [221].
Interestingly, a similar rapid and reversible elevation of E2 is induced by visual presentation of a female [216]. The absence of a correlation between the number of songs produced by males exposed to a female and the increase in E2 retrieved from the dialysate suggested that this female-induced increase in brain- derived estrogens is not a result of singing behavior but simply of seeing the female [216]. This possibility is in apparent contradiction with the higher enzymatic activity measured in the posterior telencephalon of males that sang when presented to a female compared to males that did not [217]. However, it could be argued that this effect does not reflect a rapid up-regulation of the enzymatic activity but a pre- existing condition (i.e. a higher expression of the enzymatic protein), which may have a facilitating role for singing behavior. This being said, the enhancement of local estrogen concentration and probably synthesis by visual stimuli was recently confirmed following E2 microdialysis in males presented with videotapes of behaving males or females. Importantly, elevated E2 was only detected in the NCM of males exposed to female conspecifics but not male conspecifics or heterospecific females (Bengalese finches) reinforcing the idea that this increased E2 synthesis is specific to visual cues from conspecific females [221]. In females, no such increase in E2 production is induced in NCM upon visual presentation of a male video.
10.4. Aggressive behavior
Rapid changes in AA and E2 content have also been examined in microdissected brain regions of the “social behavior network” of male white-crowned sparrows that experienced a simulated territorial intrusion for 30 min [44]. This stimulation leading to an intense aggressive response resulted in reduced E2 concentrations in the hippocampus, the ventromedial nucleus of the hypothalamus and the bed nucleus of the stria terminalis (BST), but surprisingly no change in aromatase activity was detected. Furthermore, no correlation was found between aromatase activity and local estradiol concentrations. Together, these data provide another example of the existence of rapid changes in estrogen availability in vivo but also highlight the complexity of the regulatory mechanisms of local estrogen concentration taking place within the 30 min interval between behavioral events and brain collection (See [44] for an in-depth discussion of the potential explanations of these results).
Overall, these studies that employed a variety of approaches confirm that the rapid changes in estrogen synthesis first described in vitro also take place in vivo in biologically relevant contexts and relate to changes in neuronal activity (K+ induced depolarization or glutamate release). They are also probably regulated by biochemical mechanisms identified in vitro although more work is still needed to firmly establish this last proposition. They also provide clear evidence that these processes are highly dynamic, occur in a region specific manner and might even be restricted to the synaptic terminals.
11. Role of brain aromatase in females?
Because females exhibit high circulating concentrations of estrogens, originating mostly from the ovaries, both genomic and non-genomic effects of estrogens are usually attributed to ovarian steroids in this sex. However, the female brain expresses a substantial amount of enzymatically active aromatase, even if it is less active than in males [15; 51; 60; 106; 131; 202; 225; 226; 229; 231; 232; 243]. Although the role of brain-derived estrogens has rarely been examined in females, a few studies suggest that it also contributes to normal ongoing physiological processes.
In ovariectomized musk shrews, receptive behavior is activated by the implantation of estradiol benzoate in the MPOA or VMN [279]. Effects are mimicked by implantation of testosterone, but not dihydrotestosterone or cholesterol, suggesting that local aromatization of testosterone contributes to the activation of female receptivity. Accordingly, in ovariectomized hamsters, aromatase blockade specifically in the VMN prevents the activation of lordosis by testosterone [118]. It has also been suggested that slices of female hippocampus secrete estrogens in vitro and this local estrogen synthesis is involved in synaptogenesis [135; 294]. Acute intrathecal injections of the estrogen receptor antagonist ICI 182,780 or the aromatase inhibitor fadrozole were recently shown to rapidly modulate morphine-induced anti- nociception in female rats thus demonstrating the involvement of spinal estrogen synthesis in nociception in female (Table 1; [150]). Finally, a recent study revealed that long-term systemic aromatase blockade results in increased depressive-like behavior in ovariectomized females as measured in the forced swim test [64]. These behavioral effects are likely due to a blockade of brain aromatase since subjects of this experiment had no ovaries. They also raise the interesting possibility that the substrate for aromatization (testosterone or another aromatizable androgens) supporting the behavior might be produced directly in the brain, which we know possesses all the enzymatic machinery to synthesize steroids de novo [73; 74; 152].
In parallel, it was recently reported that brain estrogen synthesis can be rapidly modulated by phosphorylating conditions in vitro in the female quail HPOA or zebra finch telencephalon as it is in males [61; 130]. Aromatase activity in the female zebra finch brain is also modulated by biologically relevant stimuli in vivo [68; 70; 221].
Together, these data thus bolster the notion that in females local estrogen provision impacts brain function and behavior independently from ovarian steroids. Substrates for aromatization are clearly present in females since they have substantial concentrations of circulating androgens produced either by their ovary or adrenal gland and can additionally produce androgens directly in their brain. However, the evidence remains scarce and the relative importance of peripheral vs. central- produced estrogens still needs to be determined.
12. Conclusions
Besides their slow and enduring effects, mounting evidence strongly indicates that estrogens also exert rapid, probably non-genomic, effects on various physiological and behavioral endpoints including social behavior, sensory processing and cognition. These non-genomic of effects of estrogens are generally characterized by their short latency of occurrence, their reversibility and some degree of regional specificity. In males, these effects are triggered by dynamic fluctuations in estrogen concentrations resulting from the local aromatization of testosterone in specific brain regions. Estrogen synthesis is indeed rapidly modulated by processes that depend on neuronal activity both in vitro and in vivo in a time scale compatible with their rapid behavioral actions. This acute control of estrogens provision seems to prevail in the presynaptic terminals putting estrogens in a perfect position to regulate synaptic transmission with great spatial specificity. It is argued that acute fluctuations of local estrogen concentrations also participate in the regulation of brain function in females independently from peripherally derived steroids. Together with the observation that the non-genomic effects of estrogens rapidly desensitize [170; 172] and the existence of mechanisms for the rapid elimination of estrogens ([21; 160; 257; 296] for further discussion see [55]), the data reviewed here provide further evidence supporting the hypothesis that brain derived estrogens should be considered as neuromodulators [19; 242].
Although there is clearly an increasing interest in the study of the behavioral effects of brain-derived estrogens over the past few years, the cellular mechanisms regulating the synthesis and action of estrogens on specific brain circuits remain largely unknown. Understanding these cellular mechanisms and how the non-genomic and genomic effects of these brain-derived estrogens interact with those produced by steroids circulating in the blood to activate complex behaviors at present constitutes a major challenge for behavioral neuroendocrinology.
Table 2. Summary table of the conditions leading to rapid changes in AA or E2 concentration in vivo.
The latency reflects when the effect is detected with regard to the timing of the manipulation, while the duration reflects how long the effect lasts from initiation to disappearance. Abbreviations: [E2], estradiol concentration; STI: stimulated territorial intrusions.
| Study | Methods | Species | Stimulus | Sex | Brain region | Effect | Latency | Duration |
|---|---|---|---|---|---|---|---|---|
| Cornil et al., 2005 [54] | Enzymatic assay | Quail | Copulation View of a female |
M | HPOA HPOA |
↓ ↓ |
5 min 5 min |
< 10min - |
| de Bournonville et al., 2012 [65] | Enzymatic assay | Quail | Copulation View of a female |
M | POM Tub POM BST |
↓ ↓ ↓ ↓ |
5 min 2 min 5 min 5 min |
< 2h < 2h - - |
| Dickens et al., 2011 [68] | Enzymatic assay | Quail | Acute Restraint stress | M F |
POM VMN VMN Tub |
↑ ↑ ↓ ↓ |
5 min 15 min 15 min 15 min |
< 30min 15 min >1h >1h |
| Dickens et al., Accepted [70] | Enzymatic assay | Quail | Acute Restraint stress Copulation Acute Restraint stress Copulation Acute Restraint stress Copulation Acute Restraint stress Copulation Acute Restraint stress Copulation Acute Restraint stress Copulation |
M F |
POM VMN Tub POM VMN Tub |
↑ - - ↓ - ↓ ↑ - ↓ - ↓ - |
15 min 5 min 15 min 5 min 15 min 5 min 15 min 5 min 15 min 5 min 15 min 5 min |
- - - - - - - - - - - - |
| Charlier et al., 2011 [43] | Enzymatic assay Tissue [E2] |
White crowned sparrow |
STI | M | MPOA Hp VMN BST NCM TnA MPOA |
- - - - - - - |
30min 30min 30min 30min 30min 30min 30min |
- - - - - - - |
| (Radioimmunoassay) | Hp VMN BST NCM TnA |
↓ ↓ ↓ - - |
30min 30min 30min 30min 30min |
- - - - - |
||||
| Remage-Healey et al., 2008 [216] | E2 microdialysis | Zebra finch | Glutamate retrodialysis Conspecific song playback Exposure to a female |
M | NCM NCM Outside NCM NCM Outside NCM |
↓ ↑ - ↑ - |
30 min 30 min 30 min |
30 min 30 min - 30 min |
| Remage-Healey et al., 2009 [217] | Enzymatic assay | Zebra finch | Singing while looking at females Conspecific song playback |
M | Anterior Tel Posterior Tel TnA Anterior Hyp Anterior Tel Posterior Tel |
- ↑ - - - - |
30 min 30 min 30 min 30 min 30 min 30 min |
- - - - - - |
| Remage-Healey et al., 2011 [220] | E2 microdialysis | Zebra finch | KCl retrodialysis KCl + ω-conotoxin retrodialysis |
M | NCM | ↓ X KCl↓ |
30 min 30 min |
30 min 30 min |
| Remage-Healey et al., 2012 [221] | E2 microdialysis | Zebra finch | Conspecific male song playback Heterospecific male song playback Videotape of conspecific male Videotape of conspecific female Videotape of conspecific male |
F M |
NCM | ↑ - - ↑ - |
30 min 30 min |
30 min 30 min |
FiN – Highlights.
-
-
estrogens rapidly affect a variety of behavioral processes in several vertebrates
-
-
aromatase activity is rapidly regulated by Ca2+-dependent phosphorylations in vitro
-
-
aromatase activity rapidly varies in vivo in behaviorally relevant contexts
-
-
brain estrogens’ provision may also play a significant role in females
-
-
brain-derived estrogens should be considered as neuromodulators
Acknowledgements
The work of the authors described in this review was supported by grants from the NIMH (R01 MH50388) and the NINDS (RO1 NS35467) to GFB and JB and by grants from the Belgian FRFC (Nbr. 2.4537.9) and from the University of Liège (Crédits spéciaux) to JB and CAC. CAC is F.R.S-FNRS Research Associate. We thank all members of our laboratories, past and present, who contributed to the data summarized here and are too numerous to be listed individually.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abe H, Keen KL, Terasawa E. Rapid action of estrogens on intracellular calcium oscillations in primate luteinizing hormone-releasing hormone-1 neurons. Endocrinology. 2008;149:1155–1162. doi: 10.1210/en.2007-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham IM, Han S-K, Todman MG, Korach KS, Herbison AE. Estrogen receptor Beta mediates rapid estrogen actions on gonadotropin- releasing hormone neurons in vivo. Journal of Neuroscience. 2003;23:5771–5777. doi: 10.1523/JNEUROSCI.23-13-05771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abraham IM, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signalling in mouse brain. Endocrinology. 2004;145:3055–3061. doi: 10.1210/en.2003-1676. [DOI] [PubMed] [Google Scholar]
- 4.Abraham IM, Herbison AE. Major sex differences in non-genomic estrogen actions on intracellular signaling in mouse brain in vivo. Neuroscience. 2005;131:945–951. doi: 10.1016/j.neuroscience.2004.10.046. [DOI] [PubMed] [Google Scholar]
- 5.Acconcia F, Ascenzi P, Bocedi A, Spisni E, Tomasi V, Trentalance A, Visca P, Marino M. Palmitoylation-dependent estrogen receptor alpha membrane localization: regulation by 17beta-estradiol. Mol Biol Cell. 2005;16:231–237. doi: 10.1091/mbc.E04-07-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Acharya KD, Veney SL. Characterization of the G-protein coupled membrane bound estrogen receptor GPR30 in the zebra finch brain reveals a sex difference in gene and protein expression. Dev Neurobiol. doi: 10.1002/dneu.22004. (In press) [DOI] [PubMed] [Google Scholar]
- 7.Addis EA, Busch DS, Clark AD, Wingfield JC. Seasonal and social modulation of testosterone in Costa Rican rufous-collared sparrows (Zonotrichia capensis costaricensis) Gen Comp Endocrinol. 2010;166:581–589. doi: 10.1016/j.ygcen.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Ahdieh HB, Siegel HI, Wade GN. The role of the uterus in regulation of heat duration in cycling rats. Horm Behav. 1985;19:292–303. doi: 10.1016/0018-506x(85)90028-5. [DOI] [PubMed] [Google Scholar]
- 9.Amandusson A, Hallbeck M, Hallbeck AL, Hermanson O, Blomqvist A. Estrogen-induced alterations of spinal cord enkephalin gene expression. Pain. 1999;83:243–248. doi: 10.1016/s0304-3959(99)00109-8. [DOI] [PubMed] [Google Scholar]
- 10.Aronica SM, Kraus WL, Katzenellenbogen BS. Estrogen action via the cAMP signaling pathway: stimulation of adenylate cyclase and cAMP- regulated gene transcription. PNAS. 1994;91:8517–8521. doi: 10.1073/pnas.91.18.8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azcoitia I, Yague JG, Garcia-Segura LM. Estradiol synthesis within the human brain. Neuroscience. 2011;191:139–147. doi: 10.1016/j.neuroscience.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Bakker J, Honda S, Harada N, Balthazart J. The aromatase knockout (ArKO) mouse provides new evidence that estrogens are required for the development of the female brain. Ann N Y Acad Sci. 2003;1007:251–262. doi: 10.1196/annals.1286.024. [DOI] [PubMed] [Google Scholar]
- 13.Ball GF, Balthazart J. How useful is the appetitive and consummatory distinction for our understanding of the neuroendocrine control of sexual behavior? Horm Behav. 2008;53:307–311. doi: 10.1016/j.yhbeh.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balthazart J, Absil P, Foidart A, Houbart M, Harada N, Ball GF. Distribution of aromatase-immunoreactive cells in the forebrain of zebra finches (Taeniopygia guttata): implications for the neural action of steroids and nuclear definition in the avian hypothalamus. Journal of Neurobiology. 1996;31:129–148. doi: 10.1002/(SICI)1097-4695(199610)31:2<129::AID-NEU1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 15.Balthazart J, Tlemcani O, Harada N. Localization of testosterone- sensitive and sexually dimorphic aromatase-immunoreactive cells in the quail preoptic area. J Chem Neuroanat. 1996;11:147–71. doi: 10.1016/0891-0618(96)00149-4. [DOI] [PubMed] [Google Scholar]
- 16.Balthazart J, Baillien M, Ball GF. Rapid and reversible inhibition of brain aromatase activity. J.Neuroendocrinol. 2001;13:63–73. doi: 10.1046/j.1365-2826.2001.00598.x. [DOI] [PubMed] [Google Scholar]
- 17.Balthazart J, Baillien M, Charlier TD, Ball GF. Calcium-dependent phosphorylation processes control brain aromatase in quail. European Journal of Neuroscience. 2003;17:1591–1606. doi: 10.1046/j.1460-9568.2003.02598.x. [DOI] [PubMed] [Google Scholar]
- 18.Balthazart J, Baillien M, Ball GF. Rapid control of brain aromatase activity by glutamatergic inputs. Endocrinology. 2006;147:359–366. doi: 10.1210/en.2005-0845. [DOI] [PubMed] [Google Scholar]
- 19.Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29:241–249. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Balthazart J, Cornil CA, Charlier TD, Taziaux M, Ball GF. Estradiol, a key endocrine signal in the sexual differentiation and activation of reproductive behavior in quail. J Exp Zool A Ecol Genet Physiol. 2009;311:323–345. doi: 10.1002/jez.464. [DOI] [PubMed] [Google Scholar]
- 21.Barbier O, Bélanger A. The cynomolgus monkey (Macaca fascicularis) is the best animal model for the study of steroid glucuronidation. Journal of Steroid Biochemistry & Molecular Biology. 2003;85:235–245. doi: 10.1016/s0960-0760(03)00235-8. [DOI] [PubMed] [Google Scholar]
- 22.Barha CK, Dalton GL, Galea LA. Low doses of 17alpha-estradiol and 17beta-estradiol facilitate, whereas higher doses of estrone and 17alpha- and 17beta-estradiol impair, contextual fear conditioning in adult female rats. Neuropsychopharmacology. 2010;35:547–559. doi: 10.1038/npp.2009.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Batty J. Acute changes in plasma testosterone levels and their relation to measures of sexual behaviour in the male house mouse (Mus musculus) Anim Behav. 1978;26:349–357. doi: 10.1016/0003-3472(78)90053-2. [DOI] [PubMed] [Google Scholar]
- 24.Becker JB, Snyder PJ, Miller MM, Westgate SA, Jenuwine MJ. The influence of estrous cycle and intrastriatal estradiol on sensorimotor performance in the female rat. Pharmacol Biochem Behav. 1987;27:53–59. doi: 10.1016/0091-3057(87)90476-x. [DOI] [PubMed] [Google Scholar]
- 25.Becker JB. Oestrogen effects on dopaminergic function in striatum. Novartis Found Symp. 2000;230:134–45. discussion 145-54. [PubMed] [Google Scholar]
- 26.Benten WPM, Stephan C, Lieberherr M, Wunderlich F. Estradiol signaling via sequestrable surface receptors. Endocrinology. 2001;142:1669–1677. doi: 10.1210/endo.142.4.8094. [DOI] [PubMed] [Google Scholar]
- 27.Bernard DJ, Bentley GE, Balthazart J, Turek FW, Ball GF. Androgen receptor, estrogen receptor alpha, and estrogen receptor beta show distinct patterns of expression in forebrain song control nuclei of European starlings. Endocrinology. 1999;140:4633–4643. doi: 10.1210/endo.140.10.7024. [DOI] [PubMed] [Google Scholar]
- 28.Beyer C, Raab H. Nongenomic effects of oestrogen: embryonic mouse midbrain neurones respond with a rapid release of calcium from intracellular stores. Eur.J.neurosc. 2000;10:255–262. doi: 10.1046/j.1460-9568.1998.00045.x. [DOI] [PubMed] [Google Scholar]
- 29.Blaustein JD, Lehman MN, Turcotte JC, Greene G. Estrogen receptors in dendrites and axon terminals in the guinea pig hypothalamus. Endocrinology. 1992;131:281–290. doi: 10.1210/endo.131.1.1612006. [DOI] [PubMed] [Google Scholar]
- 30.Bodo C, Rissman EF. New roles for estrogen receptor beta in behavior and neuroendocrinology. Front Neuroendocrinol. 2006;27:217–232. doi: 10.1016/j.yfrne.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2:207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- 32.Bondar G, Kuo J, Hamid N, Micevych P. Estradiol-induced estrogen receptor-alpha trafficking. J Neurosci. 2009;29:15323–15330. doi: 10.1523/JNEUROSCI.2107-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol. 2007;193:311–321. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- 35.Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94:1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- 36.Cardona-Gomez GP, Chowen JA, Garcia-Segura LM. Estradiol and progesterone regulate the expression of insulin-like growth factor-I receptor and insulin-like growth factor binding protein-2 in the hypothalamus of adult female rats. J Neurobiol. 2000;43:269–281. doi: 10.1002/(sici)1097-4695(20000605)43:3<269::aid-neu5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 37.Chaban V, Li J, McDonald JS, Rapkin A, Micevych P. Estradiol attenuates the adenosine triphosphate-induced increase of intracellular calcium through group II metabotropic glutamate receptors in rat dorsal root ganglion neurons. Journal of Neuroscience Research. 2011;89:1707–1710. doi: 10.1002/jnr.22718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaban V, Li J, McDonald JS, Rapkin A, Micevych P. Estradiol attenuates the adenosine triphosphate-induced increase of intracellular calcium through group II metabotropic glutamate receptors in rat dorsal root ganglion neurons. J Neurosci Res. 2011;89:1707–1710. doi: 10.1002/jnr.22718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaban VV, Mayer EA, Ennes HS, Micevych PE. Estradiol inhibits ATP-induced intracellular calcium concentration increase in dorsal root ganglia neurons. Neuroscience. 2003;118:941–948. doi: 10.1016/s0306-4522(02)00915-6. [DOI] [PubMed] [Google Scholar]
- 40.Chaban VV, Micevych PE. Estrogen receptor-alpha mediates estradiol attenuation of ATP-induced Ca2+ signaling in mouse dorsal root ganglion neurons. J Neurosci Res. 2005;81:31–37. doi: 10.1002/jnr.20524. [DOI] [PubMed] [Google Scholar]
- 41.Chambliss KL, Shaul PW. Estrogen modulation of endothelial nitric oxide synthase. Endocrine reviews. 2002;23:665–686. doi: 10.1210/er.2001-0045. [DOI] [PubMed] [Google Scholar]
- 42.Charlier TD, Cornil CA, Ball GF, Balthazart J. Diversity of mechanisms involved in aromatase regulation and estrogen action in the brain. Biochim Biophys Acta. 2010;1800:1094–1005. doi: 10.1016/j.bbagen.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Charlier TD, Harada N, Balthazart J, Cornil CA. Human and Quail Aromatase Activity Is Rapidly and Reversibly Inhibited by Phosphorylating Conditions. Endocrinology. 2011;152:4199–4210. doi: 10.1210/en.2011-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Charlier TD, Po KW, Newman AE, Shah AH, Saldanha CJ, Soma KK. 17beta-Estradiol levels in male zebra finch brain: combining Palkovits punch and an ultrasensitive radioimmunoassay. Gen Comp Endocrinol. 2011;167:18–26. doi: 10.1016/j.ygcen.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Charlier TD, Cornil CA, Ball GF, J B. Cellular mechanisms controlling rapid changes in brain aromatase activity. In: Balthazart J, Ball GF, editors. Brain aromatase, estrogens and behavior. Oxford University Press; In press. [Google Scholar]
- 46.Choleris E, Clipperton-Allen AE, Phan A, Kavaliers M. Neuroendocrinology of social information processing in rats and mice. Front Neuroendocrinol. 2009;30:442–459. doi: 10.1016/j.yfrne.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 47.Choleris E, Clipperton-Allen AE, Phan A, Valsecchi P, Kavaliers M. Estrogenic involvement in social learning, social recognition and pathogen avoidance. Front Neuroendocrinol. doi: 10.1016/j.yfrne.2012.02.001. (In press) [DOI] [PubMed] [Google Scholar]
- 48.Clayton DF. The genomic action potential. Neurobiol Learn Mem. 2000;74:185–216. doi: 10.1006/nlme.2000.3967. [DOI] [PubMed] [Google Scholar]
- 49.Cohen RE, Wade J. Aromatase mRNA in the brain of adult green anole lizards: effects of sex and season. J Neuroendocrinol. 2011;23:254–260. doi: 10.1111/j.1365-2826.2010.02102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Conley A, Orr VN, Trainor BC, Berger T, Hughes A. Brain aromatase, estrogens and behavior. Oxford University Press; Multiple forms of aromatase in suiforms: tissue distribution, regulation and functional significance. In press. [Google Scholar]
- 51.Corbin CJ, Berger T, Ford JJ, Roselli CE, Sienkiewicz W, Trainor BC, Roser JF, Vidal JD, Harada N, Conley AJ. Porcine hypothalamic aromatase cytochrome P450: isoform characterization, sex-dependent activity, regional expression, and regulation by enzyme inhibition in neonatal boars. Biol Reprod. 2009;81:388–395. doi: 10.1095/biolreprod.109.076331. [DOI] [PubMed] [Google Scholar]
- 52.Cornil C, Foidart A, Minet A, Balthazart J. Immunocytochemical localization of ionotropic glutamate receptors subunits in the adult quail forebrain. Journal of Comparative Neurology. 2000;428:577–608. [PubMed] [Google Scholar]
- 53.Cornil CA, Seutin V, Motte P, Balthazart J. Electrophysiological and neurochemical characterization of neurons of the medial preoptic area in Japanese quail (Coturnix japonica) Brain Research. 2004;1029:224–240. doi: 10.1016/j.brainres.2004.09.047. [DOI] [PubMed] [Google Scholar]
- 54.Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Dejace C, Balthazart J. Sexual behavior affects preoptic aromatase activity and brain monoamines’ levels. Endocrinology. 2005;146:3809–3820. doi: 10.1210/en.2005-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cornil CA, Ball GF, Balthazart J. Functional significance of the rapid regulation of brain estrogen action: Where do the estrogens come from? Brain Res. 2006;1126:2–26. doi: 10.1016/j.brainres.2006.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Balthazart J. Estradiol rapidly activates male sexual behavior and affects brain monoamine levels in the quail brain. Behavioural Brain Research. 2006;66:110–123. doi: 10.1016/j.bbr.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 57.Cornil CA, Taziaux M, Baillien M, Ball GF, Balthazart J. Rapid effects of aromatase inhibition on male reproductive behaviors in Japanese quail. Horm Behav. 2006;49:45–67. doi: 10.1016/j.yhbeh.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cornil CA, Stevenson TJ, Ball GF. Are rapid changes in gonadal testosterone release involved in the fast modulation of brain estrogen effects? Gen Comp Endocrinol. 2009;163:298–305. doi: 10.1016/j.ygcen.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cornil CA, Charlier TD. Rapid behavioural effects of oestrogens and fast regulation of their local synthesis by brain aromatase. J Neuroendocrinol. 2010;22:664–673. doi: 10.1111/j.1365-2826.2010.02023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cornil CA, Ball GF, Balthazart J, Charlier TD. Organizing effects of sex steroids on brain aromatase activity in quail. PLoS One. 2011;6:e19196. doi: 10.1371/journal.pone.0019196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cornil CA, Leung CH, Pletcher ER, Naranjo KC, Blauman SJ, Saldanha CJ. Acute and Specific Modulation of Presynaptic Aromatization in the Vertebrate Brain. Endocrinology. 2012;153:2562–2567. doi: 10.1210/en.2011-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cornil CA, Dickens MJ, Ball GF, J B. Rapid modulation of aromatase activity by social and environmental stimuli in quail. In: Balthazart J, Ball GF, editors. Brain aromatase, estrogens and behavior. Oxford University Press; In press. [Google Scholar]
- 63.Cross E, Roselli CE. 17β-estradiol rapidly facilitates chemoinvestigation and mounting in castrated male rats. American Journal of Physiology. 1999;276:R1346–R1350. doi: 10.1152/ajpregu.1999.276.5.R1346. [DOI] [PubMed] [Google Scholar]
- 64.Dalla C, Kokras N, Pastromas N, Kafetzopoulos V, Tzouveka E, Balthazart J, Cornil CA, Papadopoulou-Daifoti Z. Sex differences in the forced swim test of antidepressant activity: effects of gonadal- and brain- derived estrogens. Soc.Neurosci.Abstr. 2012;37 [Google Scholar]
- 65.de Bournonville C, Dickens MJ, Ball GF, Balthazart J, Cornil CA. Region- and context-specific rapid changes in brain aromatase activity following social interactions. Soc. Behav. Neuroendocrinol. Abstr. 2012;16 [Google Scholar]
- 66.Dennis MK, Burai R, Ramesh C, Petrie WK, Alcon SN, Nayak TK, Bologa CG, Leitao A, Brailoiu E, Deliu E, Dun NJ, Sklar LA, Hathaway HJ, Arterburn JB, Oprea TI, Prossnitz ER. In vivo effects of a GPR30 antagonist. Nat Chem Biol. 2009;5:421–427. doi: 10.1038/nchembio.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P. Membrane estrogen receptor-alpha interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J Neurosci. 2007;27:9294–9300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dickens MJ, Cornil CA, Balthazart J. Acute Stress Differentially Affects Aromatase Activity in Specific Brain Nuclei of Adult Male and Female Quail. Endocrinology. 2011;152:4242–4251. doi: 10.1210/en.2011-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dickens MJ, Cornil CA, Balthazart J. Rapid stress-induced changes in brain aromatase activity: the underlying neurochemical mechanisms and correlation to local estradiol concentrations. Soc.Neurosci.Abstr. 2012;37 [Google Scholar]
- 70.Dickens MJ, Balthazart J, Cornil CA. Brain aromatase and circulating corticosterone are rapidly regulated by combined acute stress and sexual interaction in a sex specific manner. Journal of Neuroendocrinolgy. doi: 10.1111/j.1365-2826.2012.02340.x. (Accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dina OA, Aley KO, Isenberg W, Messing RO, Levine JD. Sex hormones regulate the contribution of PKCepsilon and PKA signalling in inflammatory pain in the rat. Eur J Neurosci. 2001;13:2227–2233. doi: 10.1046/j.0953-816x.2001.01614.x. [DOI] [PubMed] [Google Scholar]
- 72.Diotel N, Le Page Y, Mouriec K, Tong SK, Pellegrini E, Vaillant C, Anglade I, Brion F, Pakdel F, Chung BC, Kah O. Aromatase in the brain of teleost fish: expression, regulation and putative functions. Front Neuroendocrinol. 2010;31:172–192. doi: 10.1016/j.yfrne.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 73.Diotel N, Do Rego JL, Anglade I, Vaillant C, Pellegrini E, Gueguen MM, Mironov S, Vaudry H, Kah O. Activity and expression of steroidogenic enzymes in the brain of adult zebrafish. Eur J Neurosci. 2011;34:45–56. doi: 10.1111/j.1460-9568.2011.07731.x. [DOI] [PubMed] [Google Scholar]
- 74.Do Rego JL, Seong JY, Burel D, Leprince J, Luu-The V, Tsutsui K, Tonon MC, Pelletier G, Vaudry H. Neurosteroid biosynthesis: enzymatic pathways and neuroendocrine regulation by neurotransmitters and neuropeptides. Front Neuroendocrinol. 2009;30:259–301. doi: 10.1016/j.yfrne.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 75.Dominguez J, Riolo JV, Xu Z, Hull EM. Regulation by the medial amygdala of copulation and medial preoptic dopamine release. J Neurosci. 2001;21:349–355. doi: 10.1523/JNEUROSCI.21-01-00349.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dominguez JM, Muschamp JW, Schmich JM, Hull EM. Nitric oxide mediates glutamate-evoked dopamine release in the medial preoptic area. Neuroscience. 2004;125:203–210. doi: 10.1016/j.neuroscience.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 77.Dominguez JM, Gil M, Hull EM. Preoptic glutamate facilitates male sexual behavior. J Neurosci. 2006;26:1699–1703. doi: 10.1523/JNEUROSCI.4176-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dominguez R, Hu E, Zhou M, Baudry M. 17beta-estradiol-mediated neuroprotection and ERK activation require a pertussis toxin-sensitive mechanism involving GRK2 and beta-arrestin-1. J Neurosci. 2009;29:4228–4238. doi: 10.1523/JNEUROSCI.0550-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dominguez R, Micevych P. Estradiol rapidly regulates membrane estrogen receptor alpha levels in hypothalamic neurons. J Neurosci. 2010;30:12589–12596. doi: 10.1523/JNEUROSCI.1038-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Etgen AM. Estrogen regulation of neurotransmitter and growth factor signaling in the brain. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. San Diego, CA: Academic Press; 2002. [Google Scholar]
- 81.Etgen AM, Acosta-Martinez M. Participation of growth factor signal transduction pathways in estradiol facilitation of female reproductive behavior. Endocrinology. 2003;144:3828–3835. doi: 10.1210/en.2003-0157. [DOI] [PubMed] [Google Scholar]
- 82.Etgen AM, Gonzalez-Flores O, Todd BJ. The role of insulin-like growth factor-I and growth factor-associated signal transduction pathways in estradiol and progesterone facilitation of female reproductive behaviors. Front Neuroendocrinol. 2006;27:363–375. doi: 10.1016/j.yfrne.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 83.Etgen AM, Pfaff DW. Molecular mechanisms of hormone action on behavior. Academic press; 2009. [Google Scholar]
- 84.Evrard H, Baillien M, Foidart A, Absil P, Harada N, Balthazart J. Localization and controls of aromatase in the quail spinal cord. Journal of Comparative Neurology. 2000:552–564. doi: 10.1002/1096-9861(20000807)423:4<552::aid-cne2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 85.Evrard HC, Balthazart J. Rapid regulation of pain by estrogens synthesized in spinal dorsal horn neurons. Journal of Neuroscience. 2004;24:9225–9229. doi: 10.1523/JNEUROSCI.1638-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Evrard HC, Balthazart J. Aromatization of androgens into estrogens reduces response latency to a noxious thermal stimulus in male quail. Hormones and Behavior. 2004;45:181–189. doi: 10.1016/j.yhbeh.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 87.Evrard HC. Estrogen synthesis in the spinal dorsal horn: a new central mechanism for the hormonal regulation of pain. Am J Physiol Regul Integr Comp Physiol. 2006;291:R291–R299. doi: 10.1152/ajpregu.00930.2005. [DOI] [PubMed] [Google Scholar]
- 88.Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal- regulated kinase activation and membrane-bound estrogen receptors. J Neurosci. 2008;28:8660–8667. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fernandez-Galaz MC, Naftolin F, Garcia-Segura LM. Phasic synaptic remodeling of the rat arcuate nucleus during the estrous cycle depends on insulin-like growth factor-I receptor activation. J Neurosci Res. 1999;55:286–292. doi: 10.1002/(SICI)1097-4547(19990201)55:3<286::AID-JNR3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 90.Filardo E, Quinn J, Pang Y, Graeber C, Shaw S, Dong J, Thomas P. Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology. 2007;148:3236–3245. doi: 10.1210/en.2006-1605. [DOI] [PubMed] [Google Scholar]
- 91.Filardo EJ, Thomas P. GPR30 : a seven-transmembrane-spanning estrogen receptor that triggers EGF release. Trends Endocrinol Metab. 2005;16:362–367. doi: 10.1016/j.tem.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 92.Fillingim RB, Ness TJ. Sex-related hormonal influences on pain and analgesic responses. Neuroscience and Biobehavioral Reviews. 2000;24:485–501. doi: 10.1016/s0149-7634(00)00017-8. [DOI] [PubMed] [Google Scholar]
- 93.Foidart A, Reid J, Absil P, Yoshimura N, Harada N, Balthazart J. Critical re-examination of the distribution of aromatase-immunoreactive cells in the quail forebrain using antibodies raised against human placental aromatase and against the recombinant quail, mouse or human enzyme. J.Chem.Neuroanat. 1995;8:267–282. doi: 10.1016/0891-0618(95)00054-b. [DOI] [PubMed] [Google Scholar]
- 94.Forlano PM, Deitcher DL, Myers DA, Bass AH. Anatomical distribution and cellular basis for high levels of aromatase activity in the brain of teleost fish: aromatase enzyme and mRNA expression identify glia as source. J.Neurosci. 2001;21:8943–8955. doi: 10.1523/JNEUROSCI.21-22-08943.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Forlano PM, Schlinger BA, Bass AH. Brain aromatase: new lessons from non-mammalian model systems. Front Neuroendocrinol. 2006;27:247–274. doi: 10.1016/j.yfrne.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 96.Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. 17β - estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J.Neurophysiol. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- 97.Frick KM, Fernandez SM, Harburger LL. A new approach to understanding the molecular mechanisms through which estrogens affect cognition. Biochim Biophys Acta. 2010;1800:1045–1055. doi: 10.1016/j.bbagen.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gentner TQ. Neural systems for individual song recognition in adult birds. Ann N Y Acad Sci. 2004;1016:282–302. doi: 10.1196/annals.1298.008. [DOI] [PubMed] [Google Scholar]
- 99.Gibbs RB. Testosterone and estradiol produce different effects on cognitive performance in male rats. Horm Behav. 2005;48:268–277. doi: 10.1016/j.yhbeh.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gleason ED, Fuxjager MJ, Oyegbile TO, Marler CA. Testosterone release and social context: when it occurs and why. Front Neuroendocrinol. 2009;30:460–469. doi: 10.1016/j.yfrne.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 101.Gorosito SV, Lorenzo AG, Cambiasso MJ. Estrogen receptor alpha is expressed on the cell-surface of embryonic hypothalamic neurons. Neuroscience. 2008;154:1173–1177. doi: 10.1016/j.neuroscience.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 102.Gresack JE, Frick KM. Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacol Biochem Behav. 2006;84:112–119. doi: 10.1016/j.pbb.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 103.Grove-Strawser D, Boulware MI, Mermelstein PG. Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience. 2010;170:1045–1055. doi: 10.1016/j.neuroscience.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gu Q, Moss RL. Novel mechanism for non-genomic action of 17β- oestradiol on kainate-induced in isolated rat CA1 hippocampal neurones. J.Physiol. 1998;506:745–754. doi: 10.1111/j.1469-7793.1998.745bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gu Q, Korach KS, Moss RL. Rapid action of 17β-estradiol on kainate- induced currents in hippocampal neurons lacking intracellular estrogen receptor. Endocrinology. 1999;140:660–666. doi: 10.1210/endo.140.2.6500. [DOI] [PubMed] [Google Scholar]
- 106.Guerriero G, Roselli CE, Paolucci M, Botte V, Ciarcia G. Estrogen receptors and aromatase activity in the hypothalamus of the female frog, Rana esculenta. Fluctuations throughout the reproductive cycle. Brain Research. 2000;880:92–101. doi: 10.1016/s0006-8993(00)02798-0. [DOI] [PubMed] [Google Scholar]
- 107.Guo X, Razandi M, Pedram A, Kassab G, Levin ER. Estrogen induces vascular wall dilation: mediation through kinase signaling to nitric oxide and estrogen receptors alpha and beta. J Biol Chem. 2005;280:19704–19710. doi: 10.1074/jbc.M501244200. [DOI] [PubMed] [Google Scholar]
- 108.Hammond R, Nelson D, Gibbs RB. GPR30 co-localizes with cholinergic neurons in the basal forebrain and enhances potassium- stimulated acetylcholine release in the hippocampus. Psychoneuroendocrinology. 2011;36:182–192. doi: 10.1016/j.psyneuen.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Harada N. Molecular mechanisms controlling brain aromatase expression. In: Balthazart J, Ball GF, editors. Brain aromatase, estrogens and behavior. Oxford University Press; In press. [Google Scholar]
- 110.Harburger LL, Saadi A, Frick KM. Dose-dependent effects of post- training estradiol plus progesterone treatment on object memory consolidation and hippocampal extracellular signal-regulated kinase activation in young ovariectomized mice. Neuroscience. 2009;160:6–12. doi: 10.1016/j.neuroscience.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Harding CF. Social modulation of circulating hormone levels in the male. Amer.Zool. 1981;21:223–231. [Google Scholar]
- 112.Hart SA, Snyder MA, Smejkalova T, Woolley CS. Estrogen mobilizes a subset of estrogen receptor-alpha-immunoreactive vesicles in inhibitory presynaptic boutons in hippocampal CA1. J Neurosci. 2007;27:2102–2111. doi: 10.1523/JNEUROSCI.5436-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hayden-Hixson DM, Ferris CF. Steroid-specific regulation of agonistic responding in the anterior hypothalamus of male hamsters. Physiology & Behavior. 1991;50:793–799. doi: 10.1016/0031-9384(91)90020-o. [DOI] [PubMed] [Google Scholar]
- 114.Heimovics SA, Prior NH, Maddison CJ, Soma KK. Rapid and widespread effects of 17beta-estradiol on intracellular signaling in the male songbird brain: a seasonal comparison. Endocrinology. 2012;153:1364–1376. doi: 10.1210/en.2011-1525. [DOI] [PubMed] [Google Scholar]
- 115.Heimovics SA, Fokidis HB, Soma KK. Balthazart J, Ball GF. Brain aromatase, estrogens and behavior. Oxford University Press; Brain Aromatase and Territorial Aggression Across the Seasons in Male Song Sparrows. In press. [Google Scholar]
- 116.Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, Harada N, Kimoto T, Kawato S. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc Natl Acad Sci U S A. 2004;101:865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hotchkiss J, Knobil E. The menstrual cycle and its neuroendocrine control. In: Knobil E, Neill JD, Greenwald GS, Markert CL, Pfaff DW, editors. The physiology of reproduction. New York: Raven Press; 1994. pp. 711–750. [Google Scholar]
- 118.Hsu C-H. Blockade of lordosis by androst-1,4,6-triene-3,17-dione (ATD) and tamoxifen in female hamsters primed with testosterone propionate. Horm.Behav. 1990;24:14–19. doi: 10.1016/0018-506x(90)90023-q. [DOI] [PubMed] [Google Scholar]
- 119.Huddleston GG, Paisley JC, Graham S, Grober MS, Clancy AN. Implants of estradiol conjugated to bovine serum albumin in the male rat medial preoptic area promote copulatory behavior. Neuroendocrinology. 2007;86:249–59. doi: 10.1159/000107695. [DOI] [PubMed] [Google Scholar]
- 120.Hull EM, Dominguez JM. Getting his act together: Roles of glutamate, nitric oxide, dopamine in the medial preoptic area. Brain Res. 2006;1126:66–75. doi: 10.1016/j.brainres.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 121.Inagaki T, Gautreaux C, Luine V. Acute estrogen treatment facilitates recognition memory consolidation and alters monoamine levels in memory-related brain areas. Horm Behav. 2010;58:415–426. doi: 10.1016/j.yhbeh.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Isensee J, Meoli L, Zazzu V, Nabzdyk C, Witt H, Soewarto D, Effertz K, Fuchs H, Gailus-Durner V, Busch D, Adler T, de Angelis MH, Irgang M, Otto C, Noppinger PR. Expression pattern of G protein-coupled receptor 30 in LacZ reporter mice. Endocrinology. 2009;150:1722–1730. doi: 10.1210/en.2008-1488. [DOI] [PubMed] [Google Scholar]
- 123.Jeong JK, Burrows K, Tremere LA, Pinaud R. Neurochemical organization and experience-dependent activation of estrogen-associated circuits in the songbird auditory forebrain. Eur J Neurosci. 2011;34:283–291. doi: 10.1111/j.1460-9568.2011.07743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Joels M. Steroid hormones and excitability in the mammalian brain. Front.Neuroendocrinol. 1997;18:2–48. doi: 10.1006/frne.1996.0144. [DOI] [PubMed] [Google Scholar]
- 125.Karlsson U, Sundgren AK, Näsström J, Johansson S. Glutamate- evoked currents in acutely dissociated neurons from the rat medial preoptic nucleus. Brain Research. 1997;759:270–276. doi: 10.1016/s0006-8993(97)00262-x. [DOI] [PubMed] [Google Scholar]
- 126.Kelly MJ, Ronnekleiv OK. Control of CNS neuronal excitability by estrogens via membrane-initiated signaling. Mol Cell Endocrinol. 2009;308:17–25. doi: 10.1016/j.mce.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kenealy BP, Keen KL, Ronnekleiv OK, Terasawa E. STX, a novel nonsteroidal estrogenic compound, induces rapid action in primate GnRH neuronal calcium dynamics and peptide release. Endocrinology. 2011;152:3182–3191. doi: 10.1210/en.2011-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kleitz-Nelson HK, Dominguez JM, Ball GF. Dopamine release in the medial preoptic area is related to hormonal action and sexual motivation. Behav Neurosci. 2010;124:773–779. doi: 10.1037/a0021490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kleitz-Nelson HK, Dominguez JM, Cornil CA, Ball GF. Is sexual motivational state linked to dopamine release in the medial preoptic area? Behav Neurosci. 2010;124:300–304. doi: 10.1037/a0018767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Konkle AT, Balthazart J. Sex differences in the rapid control of aromatase activity in the quail preoptic area. J Neuroendocrinol. 2011;23:424–434. doi: 10.1111/j.1365-2826.2011.02121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Konkle AT, McCarthy MM. Developmental time course of estradiol, testosterone, and dihydrotestosterone levels in discrete regions of male and female rat brain. Endocrinology. 2011;152:223–235. doi: 10.1210/en.2010-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kousteni S, Bellido T, Plotkin LI, O'Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104:719–730. [PubMed] [Google Scholar]
- 133.Kow L-M, Pfaff DW. The membrane actions of estrogens can potentiate their lordosis behavior-facilitating genomic actions. PNAS. 2004;101:12354–12357. doi: 10.1073/pnas.0404889101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kramar EA, Chen LY, Brandon NJ, Rex CS, Liu F, Gall CM, Lynch G. Cytoskeletal Changes Underlie Estrogen's Acute Effects on Synaptic Transmission and Plasticity. Journal of Neuroscience. 2009;29:12982–12993. doi: 10.1523/JNEUROSCI.3059-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kretz O, Fester L, Wehrenberg U, Zhou L, Brauckmann S, Zhao S, Prange-Kiel J, Naumann T, Jarry H, Frotscher M, Rune GM. Hippocampal synapses depend on hippocampal estrogen synthesis. Journal of Neuroscience. 2004;24:5913–5921. doi: 10.1523/JNEUROSCI.5186-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Krohmer RW, Bieganski GJ, Baleckaitis DD, Harada N, Balthazart J. Distribution of aromatase immunoreactivity in the forebrain of red-sided garter snakes at the beginning of the winter dormancy. J.Chem.Neuroanat. 2002;23:59–71. doi: 10.1016/s0891-0618(01)00145-4. [DOI] [PubMed] [Google Scholar]
- 137.Krohmer RW, Boyle MH, Lutterschmidt DI, Mason RT. Seasonal aromatase activity in the brain of the male red-sided garter snake. Horm Behav. 2010;58:485–492. doi: 10.1016/j.yhbeh.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 138.Kuhn J, Dina OA, Goswami C, Suckow V, Levine JD, Hucho T. GPR30 estrogen receptor agonists induce mechanical hyperalgesia in the rat. Eur J Neurosci. 2008;27:1700–1709. doi: 10.1111/j.1460-9568.2008.06131.x. [DOI] [PubMed] [Google Scholar]
- 139.Lagrange AH, Ronnekleiv OK, Kelly MJ. Modulation of G protein- coupled receptors by an estrogen receptor that activates protein kinase A. Mol.Pharmacol. 1997;51:605–612. doi: 10.1124/mol.51.4.605. [DOI] [PubMed] [Google Scholar]
- 140.Langer G, Bader B, Meoli L, Isensee J, Delbeck M, Noppinger PR, Otto C. A critical review of fundamental controversies in the field of GPR30 research. Steroids. 2010;75:603–610. doi: 10.1016/j.steroids.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 141.Lebesgue D, Reyna-Neyra A, Huang X, Etgen AM. GPR30 differentially regulates short latency responses of luteinising hormone and prolactin secretion to oestradiol. J Neuroendocrinol. 2009;21:743–752. doi: 10.1111/j.1365-2826.2009.01893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lebesgue D, Traub M, De Butte-Smith M, Chen C, Zukin RS, Kelly MJ, Etgen AM. Acute administration of non-classical estrogen receptor agonists attenuates ischemia-induced hippocampal neuron loss in middle- aged female rats. PLoS One. 2010;5:e8642. doi: 10.1371/journal.pone.0008642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Leonard ST, Moerschbaecher JM, Winsauer PJ. Estradiol replacement in gonadectomized male rats alters scopolamine-induced disruptions of nonspatial learning. Exp Clin Psychopharmacol. 2008;16:532–546. doi: 10.1037/a0013718. [DOI] [PubMed] [Google Scholar]
- 144.Levesque D, Di Paolo T. Rapid conversion of high into low striatal D2- dopamine receptor agonist binding states after an acute physiological dose of 17 beta-estradiol. Neuroscience Letters. 1988;88:113–118. doi: 10.1016/0304-3940(88)90324-2. [DOI] [PubMed] [Google Scholar]
- 145.Levin ER. Membrane oestrogen receptor alpha signalling to cell functions. J Physiol. 2009;587:5019–5023. doi: 10.1113/jphysiol.2009.177097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Levin ER. Minireview: Extranuclear steroid receptors: roles in modulation of cell functions. Mol Endocrinol. 2011;25:377–384. doi: 10.1210/me.2010-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lewis MC, Kerr KM, Orr PT, Frick KM. Estradiol-induced enhancement of object memory consolidation involves NMDA receptors and protein kinase A in the dorsal hippocampus of female C57BL/6 mice. Behav Neurosci. 2008;122:716–721. doi: 10.1037/0735-7044.122.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liu F, Day M, Muniz LC, Bitran D, Arias R, Revilla-Sanchez R, Grauer S, Zhang G, Kelley C, Pulito V, Sung A, Mervis RF, Navarra R, Hirst WD, Reinhart PH, Marquis KL, Moss SJ, Pangalos MN, Brandon NJ. Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11:334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- 149.Liu NJ, Gintzler AR. Prolonged ovarian sex steroid treatment of male rats produces antinociception: identification of sex-based divergent analgesic mechanisms. Pain. 2000;85:273–281. doi: 10.1016/s0304-3959(99)00278-x. [DOI] [PubMed] [Google Scholar]
- 150.Liu NJ, Chakrabarti S, Schnell S, Wessendorf M, Gintzler AR. Spinal synthesis of estrogen and concomitant signaling by membrane estrogen receptors regulate spinal kappa- and micro-opioid receptor heterodimerization and female-specific spinal morphine antinociception. J Neurosci. 2011;31:11836–11845. doi: 10.1523/JNEUROSCI.1901-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Liu SB, Zhang N, Guo YY, Zhao R, Shi TY, Feng SF, Wang SQ, Yang Q, Li XQ, Wu YM, Ma L, Hou Y, Xiong LZ, Zhang W, Zhao MG. G- Protein-Coupled Receptor 30 Mediates Rapid Neuroprotective Effects of Estrogen via Depression of NR2B-Containing NMDA Receptors. J Neurosci. 2012;32:4887–4900. doi: 10.1523/JNEUROSCI.5828-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.London SE, Remage-Healey L, Schlinger BA. Neurosteroid production in the songbird brain: a re-evaluation of core principles. Front Neuroendocrinol. 2009;30:302–314. doi: 10.1016/j.yfrne.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lord LD, Bond J, Thompson RR. Rapid steroid influences on visually guided sexual behavior in male goldfish. Horm Behav. 2009;56:519–526. doi: 10.1016/j.yhbeh.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Luine V. (this issue) [Google Scholar]
- 155.Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- 156.MacLusky NJ, Luine VN, Hajszan T, Leranth C. The 17alpha and 17beta isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology. 2005;146:287–293. doi: 10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- 157.Maggi A, Ciana P, Belcredito S, Vegeto E. Estrogens in the nervous system : Mechanisms and nonreproductive functions. Annual Reviews of Physiology. 2004;66:291–313. doi: 10.1146/annurev.physiol.66.032802.154945. [DOI] [PubMed] [Google Scholar]
- 158.Maney DL, Pinaud R. Estradiol-dependent modulation of auditory processing and selectivity in songbirds. Front Neuroendocrinol. 2011;32:287–302. doi: 10.1016/j.yfrne.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mangiamele LA, Thompson RR. Testosterone rapidly increases ejaculate volume and sperm density in competitively breeding goldfish through an estrogenic membrane receptor mechanism. Horm Behav. 2012;62:107–112. doi: 10.1016/j.yhbeh.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 160.Männistö PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacity of the new selective COMT inhibitors. Pharmacological Reviews. 1999;51:593–628. [PubMed] [Google Scholar]
- 161.McConnell SE, Alla J, Wheat E, Romeo RD, McEwen B, Thornton JE. The role of testicular hormones and luteinizing hormone in spatial memory in adult male rats. Horm Behav. 2012;61:479–486. doi: 10.1016/j.yhbeh.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 162.McDevitt MA, Glidewell-Kenney C, Weiss J, Chambon P, Jameson JL, Levine JE. Estrogen response element-independent estrogen receptor (ER)-alpha signaling does not rescue sexual behavior but restores normal testosterone secretion in male ERalpha knockout mice. Endocrinology. 2007;148:5288–5294. doi: 10.1210/en.2007-0673. [DOI] [PubMed] [Google Scholar]
- 163.McDevitt MA, Glidewell-Kenney C, Jimenez MA, Ahearn PC, Weiss J, Jameson JL, Levine JE. New insights into the classical and non-classical actions of estrogen: evidence from estrogen receptor knock-out and knock-in mice. Mol Cell Endocrinol. 2008;290:24–30. doi: 10.1016/j.mce.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocrine reviews. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- 165.Meitzen J, Mermelstein PG. Estrogen receptors stimulate brain region specific metabotropic glutamate receptors to rapidly initiate signal transduction pathways. J Chem Neuroanat. 2011;42:236–241. doi: 10.1016/j.jchemneu.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mendez P, Azcoitia I, Garcia-Segura LM. Estrogen receptor alpha forms estrogen-dependent multimolecular complexes with insulin-like growth factor receptor and phosphatidylinositol 3-kinase in the adult rat brain. Molecular Brain Research. 2003;112:170–176. doi: 10.1016/s0169-328x(03)00088-3. [DOI] [PubMed] [Google Scholar]
- 167.Mendez P, Wandosell F, Garcia-Segura LM. Cross-talk between estrogen receptors and insulin-like growth factor-I receptor in the brain: cellular and molecular mechanisms. Front Neuroendocrinol. 2006;27:391–403. doi: 10.1016/j.yfrne.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 168.Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J.Neurosci. 1996;16:595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Metzdorf R, Gahr M, Fusani L. Distribution of aromatase, estrogen receptor, and androgen receptor mRNA in the forebrain of songbirds and nonsongbirds. J Comp Neurol. 1999;407:115–129. [PubMed] [Google Scholar]
- 170.Micevych P, Dominguez R. Membrane estradiol signaling in the brain. Front Neuroendocrinol. 2009;30:315–327. doi: 10.1016/j.yfrne.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Micevych P, Kuo J, Christensen A. Physiology of membrane oestrogen receptor signalling in reproduction. J Neuroendocrinol. 2009;21:249–256. doi: 10.1111/j.1365-2826.2009.01833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Micevych P. (this issue) [Google Scholar]
- 173.Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. Journal of Comparative Neurology. 2001;429:355–371. [PubMed] [Google Scholar]
- 174.Minami T, Oomura Y, Nabekura J, Fukuda A. 17β-estradiol depolarization of hypothalamic neurons is mediated by cyclic AMP. Brain Res. 1990;519:301–307. doi: 10.1016/0006-8993(90)90092-p. [DOI] [PubMed] [Google Scholar]
- 175.Mize AL, Alper RH. Acute and long-term effects of 17β-estradiol on Gi/o coupled neurotransmitter receptor function in the female rat brain as assessed by agonist-stimulated [35S]GTPγS binding. Brain Research. 2000;859:326–333. doi: 10.1016/s0006-8993(00)01998-3. [DOI] [PubMed] [Google Scholar]
- 176.Mize AL, Poisner AM, Alper RH. Estrogens act in rat hippocampus and frontal cortex to produce rapid, receptor-mediated decreases in serotonin 5-HT1A receptor function. Neuroendocrinology. 2001;73:166–174. doi: 10.1159/000054633. [DOI] [PubMed] [Google Scholar]
- 177.Mize AL, Alper RH. Rapid uncoupling of serotonin-1A receptors in rat hippocampus by 17β-estradiol in vitro requires protein kinases A and C. Neuroendocrinology. 2002;76:339–347. doi: 10.1159/000067583. [DOI] [PubMed] [Google Scholar]
- 178.Moradpour F, Naghdi N, Fathollahi Y. Anastrozole improved testosterone-induced impairment acquisition of spatial learning and memory in the hippocampal CA1 region in adult male rats. Behav Brain Res. 2006;175:223–232. doi: 10.1016/j.bbr.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 179.Moss RL, Gu Q, Wong M. Estrogen : nontranscriptional signaling pathway. Recent progress in hormone research. 1997;52:33–69. [PubMed] [Google Scholar]
- 180.Mukai H, Tsurugizawa T, Murakami G, Kominami S, Ishii H, Ogiue- Ikeda M, Takata N, Tanabe N, Furukawa A, Hojo Y, Ooishi Y, Morrison JH, Janssen WG, Rose JA, Chambon P, Kato S, Izumi S, Yamazaki T, Kimoto T, Kawato S. Rapid modulation of long-term depression and spinogenesis via synaptic estrogen receptors in hippocampal principal neurons. J Neurochem. 2007;100:950–967. doi: 10.1111/j.1471-4159.2006.04264.x. [DOI] [PubMed] [Google Scholar]
- 181.Nadal A, Ropero AB, Laribi M, Fuentes E, Soria B. Nongenomic actions of estrogens and xenoestrogens by binding at a plasma membrane receptor unrelated to estrogen receptor α and estrogen receptor β. PNAS. 2000;97:11603–11608. doi: 10.1073/pnas.97.21.11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nadal A, Diaz M, Valverde MA. The estrogen trinity: membrane, cytosolic, and nuclear effects. News in Physiological Sciences. 2001;16:251–255. doi: 10.1152/physiologyonline.2001.16.6.251. [DOI] [PubMed] [Google Scholar]
- 183.Naftolin F, Horvath TL, Jakab RL, Leranth C, Harada N, Balthazart J. Aromatase immunoreactivity in axon terminals of the vertebrate brain. Neuroendocrinol. 1996;63:149–155. doi: 10.1159/000126951. [DOI] [PubMed] [Google Scholar]
- 184.Nestler EJ, Greengard P. In: Protein phosporylation and the regulation of neuronal function Basic neurochemistry. Siegel GJ, Agranoff BW, Albers RW, Molinoff PB, editors. New York, NY: Raven Press; 1989. pp. 373-398-0. [Google Scholar]
- 185.Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E. Involvement of G Protein-Coupled Receptor 30 (GPR30) in Rapid Action of Estrogen in Primate LHRH Neurons. Mol Endocrinol. 2009;23:349–359. doi: 10.1210/me.2008-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Noirot IC, Adler HJ, Cornil CA, Harada N, Dooling RJ, Balthazart J, Ball GF. Presence of aromatase and estrogen receptor alpha in the inner ear of zebra finches. Hear Res. 2009;252:49–55. doi: 10.1016/j.heares.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 187.Oberlander JG, Schlinger BA, Clayton NS, Saldanha CJ. Neural aromatization accelerates the acquisition of spatial memory via an influence on the songbird hippocampus. Horm Behav. 2004;45:250–258. doi: 10.1016/j.yhbeh.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 188.Ogawa S, Washburn TF, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Modifications of testosterone-dependent behaviors by estrogen receptor alpha gene disruption in male mice. Endocrinology. 1998;139:5058–5069. doi: 10.1210/endo.139.12.6358. [DOI] [PubMed] [Google Scholar]
- 189.Ogawa S, Chan J, Chester AE, Gustafsson JÅ, Korach KS, Pfaff DW. Survival of reproductive behaviors in estrogen receptor beta gene- deficient (betaERKO) male and female mice. Proc.Natl.Acad.Sci.USA. 1999;96:12887–12892. doi: 10.1073/pnas.96.22.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Olde B, Leeb-Lundberg LM. GPR30/GPER1: searching for a role in estrogen physiology. Trends Endocrinol Metab. 2009;20:409–416. doi: 10.1016/j.tem.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 191.Oliveira RF, Hirschenhauser K, Carneiro LA, Canario AV. Social modulation of androgen levels in male teleost fish. Comp Biochem Physiol B Biochem Mol Biol. 2002;132:203–215. doi: 10.1016/s1096-4959(01)00523-1. [DOI] [PubMed] [Google Scholar]
- 192.Otto C, Rohde-Schulz B, Schwarz G, Fuchs I, Klewer M, Brittain D, Langer G, Bader B, Prelle K, Nubbemeyer R, Fritzemeier KH. G protein-coupled receptor 30 localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology. 2008;149:4846–4856. doi: 10.1210/en.2008-0269. [DOI] [PubMed] [Google Scholar]
- 193.Otto C, Fuchs I, Kauselmann G, Kern H, Zevnik B, Andreasen P, Schwarz G, Altmann H, Klewer M, Schoor M, Vonk R, Fritzemeier KH. GPR30 does not mediate estrogenic responses in reproductive organs in mice. Biol Reprod. 2009;80:34–41. doi: 10.1095/biolreprod.108.071175. [DOI] [PubMed] [Google Scholar]
- 194.Packard MG, Kohlmaier JR, Alexander GM. Posttraining intrahippocampal estradiol injections enhance spatial memory in male rats: interaction with cholinergic systems. Behav Neurosci. 1996;110:626–632. doi: 10.1037//0735-7044.110.3.626. [DOI] [PubMed] [Google Scholar]
- 195.Packard MG, Teather LA. Posttraining estradiol injections enhance memory in ovariectomized rats: cholinergic blockade and synergism. Neurobiol Learn Mem. 1997;68:172–188. doi: 10.1006/nlme.1997.3785. [DOI] [PubMed] [Google Scholar]
- 196.Packard MG, Teather LA. Intra-hippocampal estradiol infusion enhances memory in ovariectomized rats. Neuroreport. 1997;8:3009–3013. doi: 10.1097/00001756-199709290-00004. [DOI] [PubMed] [Google Scholar]
- 197.Packard MG. Posttraining estrogen and memory modulation. Horm Behav. 1998;34:126–139. doi: 10.1006/hbeh.1998.1464. [DOI] [PubMed] [Google Scholar]
- 198.Pasqualini C, Olivier V, Guibert B, Frain O, Leviel V. Acute stimulatory effect of estradiol on striatal dopamine synthesis. J.Neurochem. 1995;65:1651–1657. doi: 10.1046/j.1471-4159.1995.65041651.x. [DOI] [PubMed] [Google Scholar]
- 199.Pedram A, Razandi M, Sainson RC, Kim JK, Hughes CC, Levin ER. A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem. 2007;282:22278–22288. doi: 10.1074/jbc.M611877200. [DOI] [PubMed] [Google Scholar]
- 200.Pellegrini E, Menuet A, Lethimonier C, Adrio F, Gueguen MM, Tascon C, Anglade I, Pakdel F, Kah O. Relationships between aromatase and estrogen receptors in the brain of teleost fish. Gen Comp Endocrinol. 2005;142:60–66. doi: 10.1016/j.ygcen.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 201.Pellegrini E, Vaillant C, Diotel N, Benquet P, Brion F, Kah O. Expression, regulation and potential functions of aromatase in radial glial cells of the fish brain. In: Balthazart J, Ball GF, editors. Brain aromatase, estrogens and behavior. Oxford University Press; In press. [Google Scholar]
- 202.Peterson RS, Yarram L, Schlinger BA, Saldanha CJ. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proc Biol Sci. 2005;272:2089–2096. doi: 10.1098/rspb.2005.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Pfaus JG. Homologies of animal and human sexual behaviors. Hormones and Behavior. 1996;30:187–200. doi: 10.1006/hbeh.1996.0024. [DOI] [PubMed] [Google Scholar]
- 204.Phan A, Lancaster KE, Armstrong JN, MacLusky NJ, Choleris E. Rapid effects of estrogen receptor alpha and beta selective agonists on learning and dendritic spines in female mice. Endocrinology. 2011;152:1492–1502. doi: 10.1210/en.2010-1273. [DOI] [PubMed] [Google Scholar]
- 205.Pradhan DS, Lau LY, Schmidt KL, Soma KK. 3beta-HSD in songbird brain: subcellular localization and rapid regulation by estradiol. J Neurochem. 2010;115:667–675. doi: 10.1111/j.1471-4159.2010.06954.x. [DOI] [PubMed] [Google Scholar]
- 206.Purvis K, Haynes NB. Short-term effects of copulation, human chorionic gonadotrophin injection and non-tactile association with a female on testosterone levels in the male rat. Journal of Endocrinology. 1974;60:429–439. doi: 10.1677/joe.0.0600429. [DOI] [PubMed] [Google Scholar]
- 207.Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, Kelly MJ. Rapid signalling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. Journal of Neuroscience. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Qiu J, Bosch MA, Tobias SC, Krust A, Graham SM, Murphy SJ, Korach KS, Chambon P, Scanlan TS, Ronnekleiv OK, Kelly MJ. A G- protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci. 2006;26:5649–5655. doi: 10.1523/JNEUROSCI.0327-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Quesada A, Etgen AM. Functional interactions between estrogen and insulin-like growth factor-I in the regulation of alpha 1B-adrenoceptors and female reproductive function. J Neurosci. 2002;22:2401–2408. doi: 10.1523/JNEUROSCI.22-06-02401.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERα and ERβ expressed in chinese hamster ovary cells. Molecular Endocrinology. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- 211.Razandi M, Oh P, Pedram A, Schnitzer J, Levin ER. ERs associate with and regulate the production of caveolin: implications for signaling and cellular actions. Mol Endocrinol. 2002;16:100–115. doi: 10.1210/mend.16.1.0757. [DOI] [PubMed] [Google Scholar]
- 212.Razandi M, Alton G, Pedram A, Ghonshani S, Webb P, Levin ER. Identification of a structural determinant necessary for the localization and function of estrogen receptor alpha at the plasma membrane. Mol Cell Biol. 2003;23:1633–1646. doi: 10.1128/MCB.23.5.1633-1646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Razandi M, Pedram A, Park ST, Levin ER. Proximal events in signaling by plasma membrane estrogen receptors. J Biol Chem. 2003;278:2701–2712. doi: 10.1074/jbc.M205692200. [DOI] [PubMed] [Google Scholar]
- 214.Remage-Healey L, Bass AH. Rapid, hierarchical modulation of vocal patterning by steroid hormones. Journal of Neuroscience. 2004;24:5892–5900. doi: 10.1523/JNEUROSCI.1220-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Remage-Healey L, Bass AH. Plasticity in brain sexuality is revealed by the rapid actions of steroid hormones. J Neurosci. 2007;27:1114–1122. doi: 10.1523/JNEUROSCI.4282-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci. 2008;11:1327–1334. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Remage-Healey L, Oyama RK, Schlinger BA. Elevated aromatase activity in forebrain synaptic terminals during song. J Neuroendocrinol. 2009;21:191–199. doi: 10.1111/j.1365-2826.2009.01820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Remage-Healey L, Coleman MJ, Oyama RK, Schlinger BA. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc Natl Acad Sci U S A. 2010;107:3852–3857. doi: 10.1073/pnas.0906572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Remage-Healey L, London SE, Schlinger BA. Birdsong and the neural production of steroids. J Chem Neuroanat. 2010;39:72–81. doi: 10.1016/j.jchemneu.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Remage-Healey L, Dong S, Maidment NT, Schlinger BA. Presynaptic control of rapid estrogen fluctuations in the songbird auditory forebrain. Journal of Neuroscience. 2011;31:10034–10038. doi: 10.1523/JNEUROSCI.0566-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Remage-Healey L, Dong SM, Chao A, Schlinger BA. Sex-specific, rapid neuroestrogen fluctuations and neurophysiological actions in the songbird auditory forebrain. J Neurophysiol. 2012;107:1621–1631. doi: 10.1152/jn.00749.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signalling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 223.Roepke TA, Bosch MA, Rick EA, Lee B, Wagner EJ, Seidlova-Wuttke D, Wuttke W, Scanlan TS, Ronnekleiv OK, Kelly MJ. Contribution of a membrane estrogen receptor to the estrogenic regulation of body temperature and energy homeostasis. Endocrinology. 2010;151:4926–4937. doi: 10.1210/en.2010-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Roepke TA, Ronnekleiv OK, Kelly MJ. Physiological consequences of membrane-initiated estrogen signaling in the brain. Front Biosci. 2011;16:1560–1573. doi: 10.2741/3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Rohmann KN, Schlinger BA, Saldanha CJ. Subcellular compartmentalization of aromatase is sexually dimorphic in the adult zebra finch brain. Dev Neurobiol. 2007;67:1–9. doi: 10.1002/dneu.20303. [DOI] [PubMed] [Google Scholar]
- 226.Roselli CE, Ellinwood WE, Resko JA. Regulation of brain aromatase activity in rats. Endocrinology. 1984;114:192–200. doi: 10.1210/endo-114-1-192. [DOI] [PubMed] [Google Scholar]
- 227.Roselli CE, Horton LE, Resko JA. Distribution and regulation of aromatase activity in the rat hypothalamus and limbic system. Endocrinology. 1985;117:2471–2477. doi: 10.1210/endo-117-6-2471. [DOI] [PubMed] [Google Scholar]
- 228.Roselli CE, Salisbury RL, Resko JA. Genetic evidence for androgen- dependent and independent control of aromatase activity in the rat brain. Endocrinology. 1987;121:2205–2210. doi: 10.1210/endo-121-6-2205. [DOI] [PubMed] [Google Scholar]
- 229.Roselli CE. Sex differences in androgen receptors and aromatase activity in microdissected regions of the rat brain. Endocrinology. 1991;128:1310–1316. doi: 10.1210/endo-128-3-1310. [DOI] [PubMed] [Google Scholar]
- 230.Roselli CE. Subcellular localization and kinetic properties of aromatase activity in rat brain. J.Steroid Biochem.Mol.Biol. 1995;52:469–477. doi: 10.1016/0960-0760(94)00192-o. [DOI] [PubMed] [Google Scholar]
- 231.Roselli CE, Abdelgadir SE, Jorgensen E, Resko JA. Sex differences in androgen-regulated cytochrome P450 aromatase mRNA in the rat brain. Endocrine. 1996;5:59–65. doi: 10.1007/BF02738657. [DOI] [PubMed] [Google Scholar]
- 232.Roselli CE, Klosterman SA, Fasasi TA. Sex differences in androgen responsiveness in the rat brain: Regional differences in the induction of aromatase activity. Neuroendocrinol. 1996;64:139–145. doi: 10.1159/000127111. [DOI] [PubMed] [Google Scholar]
- 233.Roselli CE, Abdelgadir SE, Ronnekleiv OK, Klosterman SA. Anatomic distribution and regulation of aromatase gene expression in the rat brain. Biol.Reprod. 1998;58:79–87. doi: 10.1095/biolreprod58.1.79. [DOI] [PubMed] [Google Scholar]
- 234.Roselli CE, Klosterman SA, Resko JA. Anatomic relationships between aromatase and androgen receptor mRNA expression in the hypothalamus and amygdala of adult male cynomolgus monkeys. J.Comp.Neurol. 2001;439:208–223. doi: 10.1002/cne.1343. [DOI] [PubMed] [Google Scholar]
- 235.Roselli CE, Liu M, Hurn PD. Brain aromatization: classic roles and new perspectives. Semin Reprod Med. 2009;27:207–217. doi: 10.1055/s-0029-1216274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Roselli CE. Distribution and regulation of aromatase in the mammalian brain: from mice to monkeys. In: Balthazart J, Ball GF, editors. Brain aromatase, estrogens and behavior. Oxford University Press; In press. [Google Scholar]
- 237.Saginor M, Horton R. Reflex Release of Gonadotropin and Increased Plasma Testosterone Concentration in Male Rabbits during Copulation. Endocrinology. 1968;82 doi: 10.1210/endo-82-3-627. 627-&. [DOI] [PubMed] [Google Scholar]
- 238.Sakamoto H, Matsuda K, Hosokawa K, Nishi M, Morris JF, Prossnitz ER, Kawata M. Expression of g protein-coupled receptor-30, a g protein- coupled membrane estrogen receptor, in oxytocin neurons of the rat paraventricular and supraoptic nuclei. Endocrinology. 2007;148:5842–5850. doi: 10.1210/en.2007-0436. [DOI] [PubMed] [Google Scholar]
- 239.Saldanha CJ, Popper P, Micevych PE, Schlinger BA. The passerine hippocampus is a site of high aromatase: inter- and intraspecies comparisons. Horm Behav. 1998;34:85–97. doi: 10.1006/hbeh.1998.1447. [DOI] [PubMed] [Google Scholar]
- 240.Saldanha CJ, Tuerk MJ, Kim YH, Fernandes AO, Arnold AP, Schlinger BA. Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J Comp Neurol. 2000;423:619–630. doi: 10.1002/1096-9861(20000807)423:4<619::aid-cne7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 241.Saldanha CJ, Duncan KA, Walters BJ. Neuroprotective actions of brain aromatase. Front Neuroendocrinol. 2009;30:106–118. doi: 10.1016/j.yfrne.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Saldanha CJ, Remage-Healey L, Schlinger BA. Synaptocrine Signaling: Steroid Synthesis and Action at the Synapse. Endocr Rev. 2011;32:532–549. doi: 10.1210/er.2011-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Schlinger BA, Callard GV. A comparison of aromatase, 5 alpha-, and 5 beta- reductase activities in the brain and pituitary of male and female quail (C. c. japonica) J Exp Zool. 1987;242:171–180. doi: 10.1002/jez.1402420208. [DOI] [PubMed] [Google Scholar]
- 244.Schlinger BA, Callard GV. Localization of aromatase in synaptosomal and microsomal subfractions of quail (Coturnix coturnix japonica) brain. Neuroendocrinol. 1989;49:434–441. doi: 10.1159/000125149. [DOI] [PubMed] [Google Scholar]
- 245.Schlinger BA, Balthazart J. Aromatase and behavior: concepts gained from studies of aromatase in the avian brain. In: Balthazart J, Ball GF, editors. Brain aromatase, estrogens and behavior. Oxford University Press; In press. [Google Scholar]
- 246.Schultz KN, von Esenwein SA, Hu M, Bennett AL, Kennedy RT, Musatov S, Toran-Allerand CD, Kaplitt MG, Young LJ, Becker JB. Viral vector-mediated overexpression of estrogen receptor-alpha in striatum enhances the estradiol-induced motor activity in female rats and estradiol-modulated GABA release. J Neurosci. 2009;29:1897–1903. doi: 10.1523/JNEUROSCI.4647-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Schumacher M, Balthazart J. Neuroanatomical distribution of testosterone metabolizing enzymes in the Japanese quail. Brain Research. 1987;422:137–148. doi: 10.1016/0006-8993(87)90548-8. [DOI] [PubMed] [Google Scholar]
- 248.Seredynski AL, Balthazart J, Ball GF, Cornil CA. Brain-derived estrogens acutely affect male sexual motivation but not performance in Japanese quail. Soc.Neurosci.Abstr. 2012;37 [Google Scholar]
- 249.Seredynski AL, Balthazart J, Ball GF, Cornil CA. Acute effects of estrogen nuclear receptor agonists on male sexual behavior in Japanese quail. Soc. Behav. Neuroendocrinol. Abstr. 2012;16 [Google Scholar]
- 250.Sheldahl LC, Shapiro RA, Bryant DN, Koerner IP, Dorsa DM. Estrogen induces rapid translocation of estrogen receptor beta, but not estrogen receptor alpha, to the neuronal plasma membrane. Neuroscience. 2008;153:751–761. doi: 10.1016/j.neuroscience.2008.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Shen P, Schlinger BA, Campagnoni AT, Arnold AP. An atlas of aromatase mRNA expression in the zebra finch brain. J.Comp.Neurol. 1995;360:172–184. doi: 10.1002/cne.903600113. [DOI] [PubMed] [Google Scholar]
- 252.Silverin B, Baillien M, Foidart A, Balthazart J. Distribution of aromatase activity in the brain and peripheral tissues of passerine and nonpasserine avian species. Gen Comp Endocrinol. 2000;117:34–53. doi: 10.1006/gcen.1999.7383. [DOI] [PubMed] [Google Scholar]
- 253.Smejkalova T, Woolley CS. Estradiol acutely potentiates hippocampal excitatory synaptic transmission through a presynaptic mechanism. J Neurosci. 2010;30:16137–16148. doi: 10.1523/JNEUROSCI.4161-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology. 1975;96:219–226. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
- 255.Song RX, Barnes CJ, Zhang Z, Bao Y, Kumar R, Santen RJ. The role of Shc and insulin-like growth factor 1 receptor in mediating the translocation of estrogen receptor alpha to the plasma membrane. Proc Natl Acad Sci U S A. 2004;101:2076–2081. doi: 10.1073/pnas.0308334100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Song RX, Zhang Z, Chen Y, Bao Y, Santen RJ. Estrogen signaling via a linear pathway involving insulin-like growth factor I receptor, matrix metalloproteinases, and epidermal growth factor receptor to activate mitogen-activated protein kinase in MCF-7 breast cancer cells. Endocrinology. 2007;148:4091–4101. doi: 10.1210/en.2007-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Song W-C, Melner MH. Steroid tranformation enzymes as critical regulators of steroid action in vivo. Endocrinology. 2000;141:1587–1589. doi: 10.1210/endo.141.5.7526. [DOI] [PubMed] [Google Scholar]
- 258.Srivastava DP, Woolfrey KM, Jones KA, Shum CY, Lash LL, Swanson GT, Penzes P. Rapid enhancement of two-step wiring plasticity by estrogen and NMDA receptor activity. Proc Natl Acad Sci U S A. 2008;105:14650–14655. doi: 10.1073/pnas.0801581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Srivastava DP, Waters EM, Mermelstein PG, Kramar EA, Shors TJ, Liu F. Rapid Estrogen Signaling in the Brain: Implications for the Fine-Tuning of Neuronal Circuitry. Journal of Neuroscience. 2011;34:16056–16063. doi: 10.1523/JNEUROSCI.4097-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Stoffel-Wagner B, Watzka M, Schramm J, Bidlingmaier F, Klingmuller D. Expression of CYP19 (aromatase) mRNA in different areas of the human brain. J Steroid Biochem Mol Biol. 1999;70:237–241. doi: 10.1016/s0960-0760(99)00114-4. [DOI] [PubMed] [Google Scholar]
- 261.Sun J, Chu Z, Moenter SM. Diurnal in vivo and rapid in vitro effects of estradiol on voltage-gated calcium channels in gonadotropin-releasing hormone neurons. J Neurosci. 2010;30:3912–3923. doi: 10.1523/JNEUROSCI.6256-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Takeo Y. Influence of continuous illumination on estrous cycle of rats: time course of changes in levels of gonadotropins and ovarian steroids until occurrence of persistent estrus. Neuroendocrinology. 1984;39:97–104. doi: 10.1159/000123964. [DOI] [PubMed] [Google Scholar]
- 263.Taziaux M, Cornil CA, Balthazart J. Aromatase inhibition blocks the expression of sexually-motivated cloacal gland movements in male quail. Behavioural Processes. 2004;67:461–469. doi: 10.1016/j.beproc.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 264.Taziaux M, Keller M, Bakker J, Balthazart J. Sexual behavior activity tracks rapid changes in brain estrogen concentrations. J Neurosci. 2007;27:6563–6572. doi: 10.1523/JNEUROSCI.1797-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 266.Thomas P, Alyea R, Pang Y, Peyton C, Dong J, Berg AH. Conserved estrogen binding and signaling functions of the G protein-coupled estrogen receptor 1 (GPER) in mammals and fish. Steroids. 2010;75:595–602. doi: 10.1016/j.steroids.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Sander Connolly EJ, Nethrapalli IS, Tinnikov AA. ER-X : a novel membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. Journal of Neuroscience. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Toran-Allerand CD. A plethora of estrogen receptors in the brain : where will it end? Endocrinology. 2004;145:1069–1074. doi: 10.1210/en.2003-1462. [DOI] [PubMed] [Google Scholar]
- 269.Trainor BC, Kyomen HH, Marler CA. Estrogenic encounters: How interactions between aromatase and the environment modulate aggression. Frontiers in Neuroendocrinology. 2006 doi: 10.1016/j.yfrne.2005.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Trainor BC, Lin S, Finy MS, Rowland MR, Nelson RJ. Photoperiod reverses the effects of estrogens on male aggression via genomic and nongenomic pathways. Proc Natl Acad Sci U S A. 2007;104:9840–9845. doi: 10.1073/pnas.0701819104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Trainor BC, Finy MS, Nelson RJ. Rapid effects of estradiol on male aggression depend on photoperiod in reproductively non-responsive mice. Horm Behav. 2008;53:192–199. doi: 10.1016/j.yhbeh.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Tremere LA, Jeong JK, Pinaud R. Estradiol shapes auditory processing in the adult brain by regulating inhibitory transmission and plasticity-associated gene expression. J Neurosci. 2009;29:5949–5963. doi: 10.1523/JNEUROSCI.0774-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Tremere LA, Pinaud R. Brain-generated estradiol drives long-term optimization of auditory coding to enhance the discrimination of communication signals. J Neurosci. 2011;31:3271–3289. doi: 10.1523/JNEUROSCI.4355-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Tsai M-J, O'Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamilly members. Annual Reviews of Biochemistry. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 275.Van Hartesveldt C, Cottrell GA, Meyer ME. The effects of intrastriatal hormones on the dorsal immobility response in gonadectomized male and female rats. Pharmacol Biochem Behav. 1989;34:459–463. doi: 10.1016/0091-3057(89)90541-8. [DOI] [PubMed] [Google Scholar]
- 276.Van Hartesveldt C, Cottrell GA, Meyer ME. Effects of intrastriatal hormones on the dorsal immobility response in male rats. Pharmacol Biochem Behav. 1990;35:307–310. doi: 10.1016/0091-3057(90)90160-j. [DOI] [PubMed] [Google Scholar]
- 277.Vasudevan N, Kow L-M, Pfaff DW. Early membrane estrogenic effects required for full expression of slower genomic actions in a nerve cell line. PNAS. 2001;98:12267–12271. doi: 10.1073/pnas.221449798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Vasudevan N, Pfaff DW. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front Neuroendocrinol. 2008;29:238–257. doi: 10.1016/j.yfrne.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 279.Veney SL, Rissman EF. Steroid implants in the medial preoptic area or ventromedial nucleus of the hypothalamus activate female sexual behaviour in the musk shrew. J Neuroendocrinol. 2000;12:1124–1132. doi: 10.1046/j.1365-2826.2000.00567.x. [DOI] [PubMed] [Google Scholar]
- 280.Voigt C, Ball GF, Balthazart J. Neuroanatomical specificity of sex differences in expression of aromatase mRNA in the quail brain. J Chem Neuroanat. 2007;33:75–86. doi: 10.1016/j.jchemneu.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 281.Wade CB, Dorsa DM. Estrogen activation of cyclic adenosine 5'- monophosphatase response element-mediated transcription requires the extracellularly regulated kinase/mitogen-activated protein kinase pathway. Endocrinology. 2003;144:832–838. doi: 10.1210/en.2002-220899. [DOI] [PubMed] [Google Scholar]
- 282.Wagner CK, Morrell JI. Neuroanatomical distribution of aromatase mRNA in the rat brain : indications of regional regulation. J.Steroid Biochem.Mol.Biol. 1997;61:307–314. [PubMed] [Google Scholar]
- 283.Watters JJ, campbell JS, Cunningham MJ, Krebs EG, Dorsa DM. Rapid membrane effects of steroids in neuroblastoma cells : effects of estrogen on mitogen activated protein kinase signalling cascade and c-fos immediate early gene transcription. Endocrinology. 1997;138:4030–4033. doi: 10.1210/endo.138.9.5489. [DOI] [PubMed] [Google Scholar]
- 284.Wersinger SR, Sannen K, Villalba C, Lubahn DB, Rissman EF, De Vries GJ. Masculine sexual behavior is disrupted in male and female mice lacking a functional estrogen receptor alpha gene. Horm Behav. 1997;32:176–183. doi: 10.1006/hbeh.1997.1419. [DOI] [PubMed] [Google Scholar]
- 285.Wildt L, Marshall G, Knobil E. Experimental induction of puberty in the infantile female rhesus monkey. Science. 1980;207:1373–1375. doi: 10.1126/science.6986658. [DOI] [PubMed] [Google Scholar]
- 286.Wingfield JC. Communication behaviors, hormone-behavioral interactions and reproduction in vertebrates. In: Neil JD, editor. Knobil and Neil's Physiology of Reproduction. San DIego, CA: Academic Press-Elsevier; 2006. pp. 1995–2040. [Google Scholar]
- 287.Wingfield JC, Silverin B. Ecophysiological studies of hormone- behavior relations in birds. In: Pfaff DW, Arnold AP, Etgen AM, Rubin RT, Fahrbach SE, editors. Hormones, Brain and Behavior. Academic press; 2009. [Google Scholar]
- 288.Wong M, Moss RL. Long-term and short-term electrophysiological effects of estrogen on the synaptic properties of hippocampal CA1 neurons. Journal of Neuroscience. 1992;12:3217–3225. doi: 10.1523/JNEUROSCI.12-08-03217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Wong M, Moss RL. Patch-clamp analysis of direct steroidal modulation of glutamate receptor-channels. J.Neuroendocrinol. 1994;6:347–355. doi: 10.1111/j.1365-2826.1994.tb00592.x. [DOI] [PubMed] [Google Scholar]
- 290.Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- 291.Zadran S, Qin Q, Bi X, Zadran H, Kim Y, Foy MR, Thompson R, Baudry M. 17-{beta}-Estradiol increases neuronal excitability through MAP kinase-induced calpain activation. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0912558106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Zakon HH. The effects of steroid hormones on electrical activity of excitable cells. TINS. 1998;21:202–207. doi: 10.1016/s0166-2236(97)01209-5. [DOI] [PubMed] [Google Scholar]
- 293.Zhang C, Kelly MJ, Ronnekleiv OK. 17Beta-estradiol rapidly increases K(ATP) activity in GnRH via a protein kinase signaling pathway. Endocrinology. 2010;151:4477–4484. doi: 10.1210/en.2010-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Zhou L, Fester L, von Blittersdorff B, Hassu B, Nogens H, Prange-Kiel J, Jarry H, Wegscheider K, Rune GM. Aromatase inhibitors induce spine synapse loss in the hippocampus of ovariectomized mice. Endocrinology. 2010;151:1153–1160. doi: 10.1210/en.2009-0254. [DOI] [PubMed] [Google Scholar]
- 295.Zhou Y, Watters JJ, Dorsa DM. Estrogen rapidly induces the phosphorylation of the cAMP response element binding protein in rat brain. Endocrinology. 1996;137:2163–2166. doi: 10.1210/endo.137.5.8612562. [DOI] [PubMed] [Google Scholar]
- 296.Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells : review and perspectives. Carcinogenesis. 1998;19:1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]