Abstract
Over the years, our ideas about estrogen signaling have greatly expanded. In addition to estradiol having direct nuclear actions that mediate transcription and translation, more recent experiments have demonstrated membrane-initiated signaling. Both direct nuclear and estradiol membrane signaling can be mediated by the classical estrogen receptors, ERα and ERβ, which are two of the numerous putative membrane estrogen receptors. Thus far, however, only ERα has been shown to play a prominent role in regulating female reproduction and sexual behavior. Because ERα is a ligand-gated transcription factor and not a typical membrane receptor, trafficking to the cell membrane requires post-translational modifications. Two necessary modifications are palmitoylation and association with caveolins, a family of scaffolding proteins. In addition to their role in trafficking, caveolin proteins also serve to determine ERα interactions with metabotropic glutamate receptors (mGluRs). It is through these complexes that ERα, which cannot by itself activate G proteins, is able to initiate intracellular signaling. Various combinations of ERα-mGluR interactions have been demonstrated throughout the nervous system from hippocampus to striatum to hypothalamus to dorsal root ganglion (DRG) in both neurons and astrocytes. These combinations of ER and mGluR allow estradiol to have both facilitative and inhibitory actions in neurons. In hypothalamic astrocytes, the estradiol-mediated release of intracellular calcium stores regulating neurosteroid synthesis requires ERα-mGluR1a interaction. In terms of estradiol regulation of female sexual receptivity, activation of ERα-mGluR1a signaling complex leads to the release of neurotransmitters and alteration of neuronal morphology. This review will examine estradiol membrane signaling (EMS) activating a limbic-hypothalamic lordosis regulating circuit, which involves ERα trafficking, internalization, and modifications of neuronal morphology in a circuit that underlies female sexual receptivity.
Keywords: Estradiol receptor, mGluR, caveolin, spinogenesis, lordosis behavior, dendritic spines
INTRODUCTION
How does estradiol affect CNS circuits that control reproduction and sexual receptivity? Over the years there has been a subtle debate about whether estradiol is permissive or whether it is inductive. According to the “permissive hypothesis,” estradiol acts to allow certain inputs to activate reproductive circuits, like a person allowing another admission by opening a gate. The “inductive hypothesis” stresses that estradiol restructures reproductive circuits. In essence, this was a debate about whether estradiol altered the neurochemistry, transmitters, peptides and receptors; or whether estradiol altered the morphology of the brain, changing projections and synaptic contacts of reproductive circuits. Of course these hypotheses were not mutually exclusive and a great deal of evidence was generated to bolster each. Over the years, investigators demonstrated the estradiol regulation of gene transcription and the expression of a cornucopia of neuropeptides, transmitters, enzymes and their cognate receptors [89]. Altered ratios of transmitters and neuropeptides modified circuits in which the flow of information was different when estradiol was present compared to when estradiol was not. In parallel, the inductive hypothesis was supported by findings that estradiol changed the structure of the brain. Initially, this was thought to be restricted to development, during so-called “critical periods” [59, 78, 116, 151]. Some 20 years ago, it was revealed that estradiol also altered dendritic morphology in the adult brain. Initially, this was observed in the mediobasal hypothalamus, including the arcuate (ARH) and ventromedial nucleus (VMH; [45, 47, 79, 80]) and then the hippocampus, where it has been studied most extensively ([161, 162]; reviewed in [29]). Thus, estradiol is both permissive and inductive, altering both the neurochemistry and the morphology of the brain.
Concentrations of sex steroids in the brain are the result of gonadal steroidogenesis and brain synthesis [6, 64, 88, 127, 149]. These neurosteroids may be members of yet another class of transmitters, messengers that are controlled at the level of their synthesis. These transmitters, nitric oxide, carbon monoxide, endocannabinoids and steroids, are not stored but are rapidly released as soon as they are synthesized [88]. Additionally, estradiol has actions that resemble transmitter molecules that modify neuronal excitation and influence second messenger signaling. Such actions are mediated by estrogen receptors (ERs) that appear to function as G protein-coupled receptors (GPCRs) and mediate “rapid”, “membrane”, or “non-nuclear” actions. Membrane actions span the gamut from association with receptor tyrosine kinases to direct gating of channels to activating G proteins to transactivating other membrane receptors [105, 106]. “Membrane and non-nuclear” are terms that refer to the initial site of estradiol interaction with its receptor and do not exclude the possibility that transcription will eventually result. Indeed, one characteristic of membrane-initiated signaling has been activation of cAMP response element binding (CREB) protein [1, 16]. We refer to relatively rapid actions, those that are initiated within seconds to minutes at the membrane, as estradiol membrane signaling (EMS). Several putative receptors have been implicated in EMS and we will review the experimental evidence that this signaling is important in reproduction in terms of regulating sexual receptivity. The role of EMS in estrogen positive feedback regulating the LH surge has recently been reviewed and will only briefly be touched here [68, 90, 92, 123, 125]
Regulation of sexual receptivity
Estradiol is now known to affect almost all parts of the CNS and to influence a wide variety of functions from nociception to energy regulation to cognition. In addition, estradiol has been implicated in neuroprotection in degenerative diseases such as Parkinson's disease and Alzheimer's disease [30, 121], as well as acute neurotrauma and ischemia ([160]; reviewed in [144]). Arguably the best studied and most robust actions of estradiol in the brain are on neural circuits controlling female sexual receptivity and the hypothalamo-pituitary-gonadal (HPG) axis that regulates reproduction (reviewed in [10, 44, 68, 87, 90, 92, 115]).
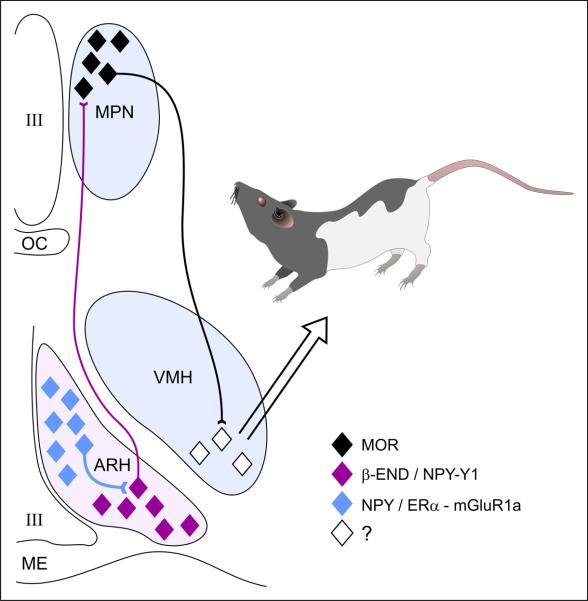
Estradiol acting on a highly distributed circuit that receives olfactory inputs from the accessory olfactory system and tactile sensory inputs from the flanks and perineum induces sexual receptivity (reviewed in [115]). This information is integrated in a limbic-hypothalamic lordosis-regulating circuit, including posterodorsal medial amygdaloid nucleus, bed nucleus of the stria terminalis, medial preoptic nucleus (MPN) and the ventromedial nucleus of the hypothalamus (VMH; reviewed in [91]). The final common outflow of this circuit is through the VMH, which projects to nuclei in the periaqueductal grey (PAG), vestibular complex and spinal cord motoneurons. More recently, it has been appreciated that the hypothalamic arcuate nucleus (ARH) through its β-endorphin (β-END) projection to the MPN is an important component of the circuitry regulating sexual receptivity ([87, 95]; Fig 1).
Figure 1.
The estradiol induction of sexual receptivity in the female rat is indicated by lordosis behavior. The CNS regulation of this global response to hormonal and sensory input is regulated by a diffuse circuit that extends from the limbic system to the spinal cord. Within this lordosis regulating circuit, estradiol acts rapidly through estradiol membrane signaling (EMS) to release neuropeptide Y (NPY) in the arcuate nucleus of the hypothalamus (ARH), which activates β-endorphin (β-END) projection neurons that extend to the medial preoptic nucleus (MPN). The MPN is an important integrative node receiving accessory olfactory and limbic input. β-END activates MOR, producing a transient inhibition of the MPN which is relieved by progesterone in the cycling female. The MPN MOR neurons in turn project to the ventromedial nucleus of the hypothalamus (VMH), the final common output of the hypothalamus. The integrated hypothalamic output is modified by inputs from the periaquaductal gray, and the vestibular complex on its way to the motoneurons mediating lordosis behavior. The EMS that mediates this activation of the circuit requires the transactivation on metabotropic glutamate receptor-1a (mGluR1a), which leads to the phosphorylation of PKCθ and the release of NPY and activation of the Y1 receptor on β-END projection cells. The EMS and resulting transient inhibition is necessary for the full expression of lordosis behavior in the rat.
Although estradiol eventually induces the capacity for lordosis behavior, immediately after systemic estradiol treatment females are not sexually receptive [8]. The delayed response is at least partially dependent on inhibitory mechanisms activated by estradiol [134, 137, 138]. Initially, systemic estradiol treatment activates μ-opioid receptors (MOR) in the MPN [40]. The time course of MOR activation is such that nuclear estradiol action was thought not to be involved; MOR is activated within 30 minutes of estradiol treatment. A membrane constrained estradiol construct, E-biotin, microinjected into the ARH internalized MOR [35], a circuit-activating event tied to the full display of sexual receptivity [138, 152, 153]. These results suggested that EMS preceded a nuclear action of estradiol. Indeed, such cooperativity between EMS and nuclear signaling was demonstrated by substituting stimulation by free estradiol with a membrane-constrained form of estradiol, E-BSA [62]. When E-BSA was applied first, followed by free estradiol, lordosis behavior was facilitated to the same level as with two injections of estradiol. These results are consistent with our results showing an estradiol activation of MOR and the electrophysiological observation of an estradiol inhibition of MPN neurons [18].
A circuit-level explanation of the initial inhibition of lordosis by estradiol is that estradiol acting in the ARH, releases NPY activating NPY-Y1 receptors on β-END neurons [95]. Subsequently, it was demonstrated that EMS in the ARH required ERα transactivation of mGluR1a to stimulate MOR in the MPN [35, 94]. First, ERα is colocalized with mGluR1a in a population of ARH neurons; second, ERα co-immunoprecipitates with mGluR1a in a membrane preparation from ARH tissue; and third, antagonism of mGluR1a attenuates estradiol-induced MOR activation and lordosis. Finally, in terms of activation, mGluR1a antagonism in the ARH blocks estradiol induction of lordosis behavior only at the time of estradiol treatment. Metabotropic GluR1a antagonism hours after estradiol treatment or microinjection of the mGluR1a antagonist LY367,385 into the MPN do not prevent lordosis behavior reinforcing the idea of a rapid activation of ER [35]. These results suggest that during the estrus cycle when estradiol levels are low (estrus and diestrus), the ARH-MPN projection is quiescent, indicated by the non-activated MOR (localized on the membrane) in the MPN, and the animal is not sexually receptive [93]. On proestrus, as systemic estradiol levels spike, β-END is released in the MPN and transiently activates/internalizes MOR, which is required for the full display of sexual behavior. Thirty to forty eight hours after the peak of estradiol, this MOR-mediated inhibition of lordosis wears off or is turned off by progesterone ([136]; Sinchak and Wagner, this volume), and estradiol activates full sexual receptivity (reviewed in [89]). The initial inhibition of lordosis through the rapid MOR activation likely allows time for the activation of transcription and translation by the nuclear ER. By inhibiting sexual behavior until later time points, the membrane ER works in concert with the nuclear receptor to allow for maximal receptivity. The MOR cells in the MPN project to the VMH [135], which is a critical integrator of information related to sexual behavior. It is likely that it is through this connection that lordosis is inhibited. Membrane ERα can be pharmacologically bypassed by directly stimulating mGluR1a under low estradiol conditions, resulting in MOR internalization and facilitation of lordosis [35]. Conversely, antagonizing mGluR1a when estradiol levels are high blocks MOR internalization and attenuates lordosis behavior. These data are consistent with the in vitro demonstration of ERα-mGluR1a signaling in hippocampal neurons and provided the first in vivo evidence that EMS requires the transactivation of mGluR1a. Further evidence of this rapid estradiol signaling is that estradiol in vivo stimulates the phosphorylation of a novel calcium independent PKCθ in the arcuate nucleus [36]. Pharmacological stimulation of PKC can overcome both ER and mGluR1a antagonism and stimulate lordosis behavior. This set of experiments demonstrates that lordosis behavior, a classical assay of estradiol action, has a membrane-initiated estradiol signaling component and underscores the importance of ER-mGluR interactions in neural function. To be clear, EMS by itself does not induce sexual receptivity. The function of EMS is to augment nuclear estradiol signaling, which is a necessary and important component of the induction of female sexual receptivity, as discussed below.
Morphological plasticity
A parallel series of experiments demonstrated a sex steroid modulation of dendritic structure throughout the CNS (reviewed in [29]). Estradiol and testosterone were shown to regulate dendritic length and the density of spines in the spinal cord nucleus of the bulbocavernosus (SNB), VMH, ARH, medial amygdala, striatum, cortex and hippocampus ([5, 17, 26, 34, 45, 48, 50, 69, 70, 79–81, 115, 147, 151]; see Luine et al and MacLusky et al., this volume). The most dramatic effects of estradiol on neuronal number and morphology occur during development, but significant steroid regulation of dendritic structure also occurs in adulthood. In the CA1 region of the hippocampus, the density of spines and synapses fluctuates during the estrus cycle due to the actions of estradiol and progesterone [161]. The greatest spine density occurred during proestrus when estradiol levels are high. A significant decrease was noted on estrus, when estradiol and progesterone levels had waned after peaking. Other experiments with gonadectomized rats utilized an estradiol treatment for several days, which restored the ovariectomy-induced loss of spines [163]. Interestingly, progesterone decreases estradiol induced spines [103].
In the VMH, a nucleus intimately associated with sexual receptivity, estradiol increased spine density and dendritic branching that mimicked the changes during the estrous cycle ([20, 21, 45, 74]; reviewed recently in [51]). Estradiol treatment of ovariectomized rats dramatically increased the number of spines on neurons that did not appear to express ERα [20, 21, 74]. Interestingly, estradiol also reduced the length of a class of long primary dendrites that extend laterally out of the VMH. The time course of this two-phased estradiol action suggests that retracting the extra-VMH dendrites while increasing spines on intra-VMH dendrites leads to an increase in sexual receptivity. The increase in intra-VMH spines being tied to lordosis behavior is congruent with observation that afferents to the VMH are initially inhibited by MOR activation [135]. In the context of a circuit, as MOR inhibition wears off or is blocked with progesterone, excitatory afferents contact newly formed dendritic spines, exciting the VMH.
More recently, we have examined the estradiol-induced morphological plasticity in the ARH [26]. As in the hippocampus and VMH, estradiol treatment induced dendritic spines. In these experiments, 4 hours of estradiol treatment was sufficient to increase ARH spine density, which remained at that level for the duration of the experiment, 48 hours. However, their morphology evolved. The newly formed spines were thin with a filapodial appearance. Spines with no or small heads are considered immature, unstable and not functional [60]. Filapodial spines are highly labile rapidly appearing and disappearing during intense neural activity until they are stabilized by contacting an appropriate signaling partner [53, 110, 154]. Spines with large heads (e.g., mushroom-shaped) are thought to be mature, stable and functional. These larger heads contain an extensive protein rich structure, known as the postsynaptic density, which is composed of receptors and anchoring proteins that allow for efficient synaptic transmission. The stabilization involves receptors being recruited into the spine membrane and anchored at the postsynaptic specialization by scaffold proteins [56, 61, 82, 165]. In the ARH, mushroom-shaped spines did not appear until 20 hours after estradiol treatment, the first time point after estradiol treatment that lordosis behavior can be elicited, suggesting that both spinogenesis and maturation are critical for regulating sexual receptivity.
Spinogenesis requires the rearrangement of β-actin to deform the membrane and induce a filapodial outgrowth. This increase in β-actin immunoreactivity is correlated with direct observation of spines with Golgi staining [26]. In the ARH, pharmacological inhibition of β-actin polymerization with cytochalasin D prevented spinogenesis and attenuated sexual receptivity [26]. Since actin remodeling is highly regulated by cell signaling cascades that are initiated at the cell membrane, we examined whether EMS was involved in spinogenesis. Group I mGluRs play an important role in the elongation of spines [33, 156]. The activation of these receptors leads to the release of calcium from internal stores as well as an increase in transcription, both of which are required for mGluR-mediated spine elongation [156]. The release of calcium leads to the activation of calmodulin and the eventual activation of CREB [122]. The role of estradiol activation of ERα-mGluR1a is underscored by the observation that intracellular calcium is increased and the MAPK pathway leading to phosphorylation of CREB is activated [16, 35, 66].
Thus, EMS which involves mGluR1a may modulate actin dynamics through phosphorylation of several molecules important for spine formation including cofilin, an actin depolymerizing factor (for review see [128]). To allow the formation of filamentous actin, cofilin must be deactivated, which occurs when it is phosphorylated. Within an hour of estradiol treatment, increased levels of phosphorylated cofilin (p-cofilin) were observed in the ARH, strongly suggesting a rapid activation of intracellular signaling ([26]; Fig 2). A strong indication of the involvement of EMS is that the estradiol-induced phosphorylation of cofilin was attenuated by antagonism of mGluR1a indicating that the mERα-mGluR1a complex initiated cell signaling. Our data are consistent with the idea that EMS activates LIM kinase-1, which in turn phosphorylates cofilin. The deactivation of cofilin allows the establishment of new spines [7, 84]. Regulation of the actin cytoskeleton is a mechanism by which estradiol induces the formation of filapodial dendritic spines in the ARH (Fig 3). Additionally, p-cofilin has been associated with the stabilization of long term potentiation (LTP) in the hippocampus synapses through the expansion of synaptic contacts [41]. Therefore, the estradiol regulation of cofilin may explain the generation and maturation of dendritic spines associated with lordosis behavior: initially increasing spine density and subsequently assisting in spine maturation. One hypothesis is that estradiol increases the expression of genes involved in spine maturation. Another idea for the mechanism is encapsulated in the “two-step wiring” hypothesis ([146]; reviewed in [145]). Estradiol rapidly induces spinogenesis, but these spines are very labile unless another stimulus stabilizes them. In the hippocampus, estradiol paired with a LTP protocol results in an increase in connectivity [63]. In the ARH and the VMH, estradiol provides the initial spinogenesis, but the salient reproductive sensory input (olfactory and tactile) occurs too slowly to be the second input required for two-step wiring. Sexually receptive females respond to males immediately, so the sensory information from this interaction cannot be the driving force for spine maturation and circuit rewiring. It is not clear whether there is sufficient time to stabilize the synapses. Thus, another input may be required for two-step wiring. Ultimately, this will need to be tested directly. In the ARH, disruption of spinogenesis at the time of estradiol administration with the infusion of an actindepolymerizing agent, cytochalasin D (CD), demonstrated that morphological plasticity in the ARH is necessary for female sexual receptivity.
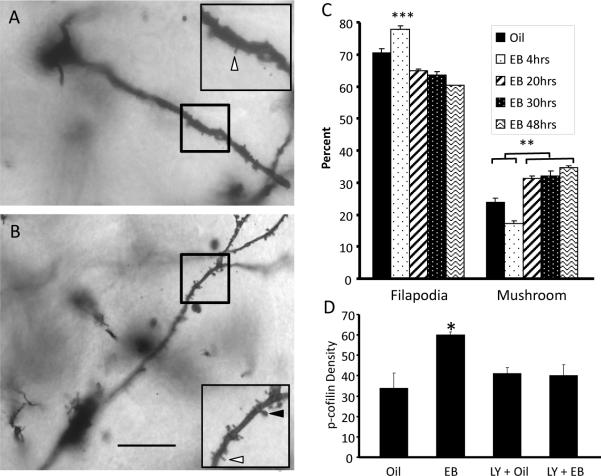
Figure 2.
Estradiol mediates spinogenesis in the ARH. Animals treated with (A) oil show many fewer dendritic spines than those treated with (B) estradiol for 48 hrs. (C) The estradiol mediated-spinogenesis in the ARH begins with the growth of immature, filapodial spines which mature to more mature mushroom spines at later time points. Filapodial spines are indicated by white arrowheads in (A) and (B) and mushroom spines are indicated by black arrowheads. (D) Estradiol was able to increase the phosphorylation of the actin depolymerizing factor, cofilin. The phosphorylation of cofilin is required for spinogenesis and was blocked by the mGluR1 antagonist, LY 367,385, supporting the idea that estradiol-mediated spine induction requires EMS. Scale bar indicates 50 μm. ** indicates P < 0.001. *** indicates P < 0.0001. (Modified from [26])
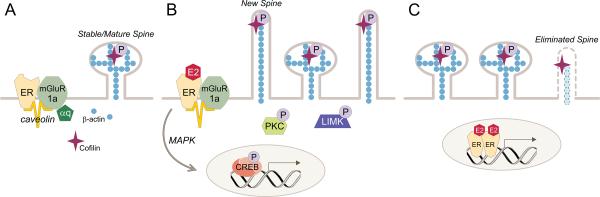
Figure 3.
Estradiol membrane signaling induces spinogenesis in the ARH. (A) During low estradiol conditions, as during diestrus, ARH neurons have a population of mature spines. The ERα-mGluR1a signaling complex is not stimulated. (B) Estradiol induces the formation of thin, filapodial spines by stimulating the ERα-mGluR1a signaling complex leading to activation of PKC and LIM kinase and the phosphorylation of cofilin. The deactivation of cofilin allows new spines to grow. EMS stimulates gene expression through the activation of the MAPK pathway leading to CREB-mediated transcription. (C) With time, mushroom-shaped spines appear in the ARH. These spines are thought to be functional and stable. The time course of their appearance coincides with the display of lordosis behavior in the female rat, starting at 20 hours after estradiol treatment. Whether the necessary gene transcription for spine maturation is regulated by membrane to nucleus signaling (B) or the result of direct nuclear action (C) is being investigated. Some immature spines are eliminated (C). In the ARH, the mechanism of this degeneration is not clear, but is probably due to a failure to associate with a presynaptic element, which stabilizes the spine.
Whether estradiol acts directly on neurons undergoing spinogenesis or transsynaptically remains an open question. Observations suggest that the type of estradiol action may be specific for a particular region and neuron. For example, rather than the positive action in the ARH and VMH, estradiol decreased spine density in the nucleus accumbens core [147]. In this nucleus, estradiol was seen to shift the population of spines from a more mature to a less mature morphology, suggesting a decrease in synaptic excitability. These results are diametrically opposed to the ARH, but can be accounted for by the observation that in the striatum, ERα interacts with mGluR3 leading to an inhibition of adenylyl cyclase and inhibition of L-type voltage-gated calcium channels, as also seen in DRG neurons [52, 85].
Do these findings indicate a direct estradiol action on neurons that increases spine density? Such a direct effect requires ER expression in spinogenic neurons. On its face, the lack of nuclear ERα expression in CA1 pyramidal neurons supports an indirect mechanism [55, 158]. However, ERα immunoreactivity has been localized to dendritic structures including spines [96, 97]. At present how ERα, a nuclear receptor, is targeted to dendritic spines is only beginning to be worked out. As mentioned below, alternatively spliced ERα products are preferentially targeted to the cell membrane. An indirect site of estradiol action is bolstered by the estradiol-induced decrease of GABA tone and estradiol-induced spinogenesis in some brain areas also dependent on glutamate ([4, 102, 104, 126, 132, 162]; for review see [141]). Another proposed transsynaptic mechanism involves the estradiol-induced activation of BDNF [130, 142]. In midbrain, a direct action of estradiol is suggested. Estradiol rapidly activates PKA and the PI3 kinase/ Akt signaling cascade both of which have been implicated in neurite outgrowth in these neurons [11, 58, 120]. In the ARH, the site of action has not been resolved. It is not known if the same neurons that express ERα have estradiol-induced spines. Further studies will be needed to answer this question, just as further studies will sort out how spinogenesis fits into the time course of estradiol activation of MOR in the MPN and the lagging lordosis behavior. Estradiol stimulates the circuit such that within 5 mins, MORs are internalized. We do not know how quickly estradiol induces spinogenesis, and whether these early, immature spines are capable of synaptic transmission [140]. Estradiol actions on dendritic morphology have a relatively rapid, EMS component and slower, transcriptional component – much like the process of sexual receptivity. It may be that the inhibitory action of estradiol is independent of new spinogenesis and only the facilitative circuits require newly formed, mature spines.
Estradiol membrane signaling (EMS)
Sexual receptivity and ARH spinogenesis both have an EMS component involving ERα. The classical ERα and ERβ are nuclear ligand-gated transcription factors. These receptors have a high sequence homology and in the nucleus they bind the major circulating estrogen, 17β-estradiol (estradiol). A sequence of homo- or heterodimerizations, associations with the estrogen-response-element (ERE) within the promoter region of a gene, and attraction of transcriptional machinery (including cofactors) leads to the regulation of gene expression [25]. Although a significant amount of evidence indicates that the same nuclear receptors can also mediate EMS throughout the brain [92, 93, 112], another three putative mER have been proposed: ER-X, GPR30, and Gq-ER. ER-X is the least defined, but has been proposed as a novel mER located in microdomains associated with caveolin proteins in a variety of tissues including the neocortex. ER-X appears only during development and after injury [150]. Unlike most ERs, ER-X is not antagonized by ICI 182,780 and is not stereospecific. Indeed, ER-X preferentially binds 17α-estradiol rather than the endogenously more plentiful and bioactive 17β-estradiol. Because of its distribution, pharmacology and developmental profile, ER-X does not appear to regulate reproductive behaviors.
Another candidate GPR30, also referred to as the GPER, is a membrane-associated protein expressed throughout the body and in cancer cells [22, 42, 43, 98]. GPR30 is an integral membrane protein with seven transmembrane domains - a classic GPCR with sequence homology to angiotensin II 1A, interleukin 8A, and chemokine type 1 receptors [118]). GPR30 has been difficult to localize to the cell membrane [13, 49, 65], but has been localized to the Golgi apparatus and endoplasmic reticulum [109, 117]. The idea of GPR30 as a mER has expanded our concepts of membrane-initiated signaling. In particular, a receptor for estradiol is not required to be localized in the cell membrane. Estradiol is lipophilic and can readily diffuse into cells activating GRP30 on the smooth endoplasmic reticulum. In astrocytes, which have the same ERα-mGluR1a EMS as neurons, stimulation of GPR30 with the selective GRP30 agonist, G-1, released endogenous stores of calcium [65]. However, the pharmacological profile of G-1 was not similar to the selective ERα agonist, PPT (4,4′,4″-(4-propyl-[1H]-pyrazole-1,3,5-triyl) trisphenol [148], or the Gq-mER agonist, STX. In fact, the G-1 dose-response relationship was closest to the ERβ agonist, DPN (diarylpropionitrile) that does not mediate estradiol facilitated progesterone synthesis [65]. Moreover, the function of GPR30 in the regulation of reproduction is not well understood [108]. In vivo, G-1 activation of GRP30 did not modulate lordosis [35]. GPR30 may be like mGluR1a, mediating estradiol signaling through association with ERα, an idea that is supported by results that G-1 has estrogenic actions in cells expressing ERα or ERβ ([108, 124]; but see [155]).
Finally, an analogue of tamoxifen, STX, activates Gq mediated signaling in ERα−/−/ERβ−/− mice, [119]. This Gq-mER that has been characterized pharmacologically in both neurons and astrocytes, is stereospecific, and is antagonized with ICI 182,780 (reviewed in [68, 92]). Also, STX has been reported to mediate energy balance and gonadotropin releasing hormone (GnRH) secretion (reviewed in [92]; see Kelly & Ronnekleiv in this issue). In terms of reproduction, STX activated the lordosis circuit. STX increased MOR internalization in the MPN and facilitated lordosis behavior 30 hours later in rats treated with sub-behavioral doses of estradiol [27]. In astrocytes, where estradiol induces progesterone synthesis via a membrane ERα mechanism, STX has a similar pharmacology and increased intracellular calcium and progesterone synthesis [65]. While the Gq-mER has a very tempting pharmacology, it has been difficult to reconcile the results with observations that ERα is critical for reproduction, and that global ERα knock out (ERKO) or neuron-specific ERα−/− mice do not display lordosis behavior or have a physiological luteinizing hormone (LH) surge [31, 32, 159].
ERα at the membrane
Immunohistochemical techniques identified ERα and ERβ associated with the membrane and in the membrane fraction [11, 24, 75]. While it has been suggested that membrane ERs (mERs) are attached to the inner leaflet of the cell membrane [143], surface biotinylation, a method of labeling membrane proteins, demonstrated that membrane-associated ERα has an exposed extracellular portion [13, 39, 49]. Like ERα, ERβ has also been identified on the cell membrane [24] and is trafficked to the cell membrane within 5–60 min of hormone estradiol exposure [133]. Palmitoylation explains how these nuclear ligand-gated transcription factors can be trafficked to the cell membrane, since these receptors have neither membrane targeting sequences nor stretches of hydrophobic residues normally associated with membrane proteins [111, 113, 143].
Palmitoylation is the covalent attachment of fatty acids to cysteine residues of proteins to increase the hydrophobicity of proteins and their membrane association. Palmitoylation helps localize ERα to lipid rafts, specialized areas of the cell membrane [72]. Indeed, a conserved nine amino acid membrane targeting sequence (including Cys447) has been identified in several steroid receptors including ERα, ERβ, androgen receptor and progesterone receptors A and B [113]. When ERα Cys447 is mutated to Ala, palmitoylation is prevented and ERα trafficking is abrogated [2, 76]. In addition to palmitoylation, ER trafficking required the association with a family of scaffolding proteins, the caveolins [14, 15, 131]. Caveolins traffic proteins to specific regions of the cell membrane involved in signal transduction, the lipid rafts [3, 77]. Specificity of ERα downstream signaling is specified by interactions with different caveolin proteins (reviewed by [86, 93]). This diversity ER interactions with caveolin is region-specific: in hippocampal neurons, ERα interacts with caveolin-3 (CAV3) or CAV1; in striatal and hypothalamic neurons, with CAV1; and in DRG neurons probably with CAV2 [15, 23, 46, 52, 83].
More recently, site-specific microinjections of CAV1 siRNA into the ARH, reduced CAV1 levels by ~60% [28]. CAV1 siRNA did not affect intracellular ERα levels, but reduced ERα levels on the cell membrane. These results suggest that a reduction of CAV1 interrupts trafficking of ERα to the cell membrane. The loss of EMS due to reduced membrane ERα diminished MOR activation/internalization in the MPN and attenuated lordosis behavior. This highlights the importance of membrane ERα and signaling for regulating female sexual receptivity.
Estradiol regulation of membrane ERα levels
Membrane ER levels are dynamic, changing with activity and local environment. As discussed, an important mechanism is trafficking of the receptor to the cell membrane. Does exposure to estradiol alter membrane levels of ERα? Surface biotinylation was used to label membrane proteins after different durations of estradiol exposure [13, 38, 39]. In neurons and astrocytes, estradiol transiently and in parallel increased the membrane levels of ERα and an alternatively spliced ERα, the ERαΔ4 [13, 38, 39]. Within 5 mins of estradiol treatment, membrane levels of each receptor were significantly increased, leading to peak levels at 30 mins. Two hours of estradiol treatment decreased membrane levels to those in unstimulated cells. Since ERα is complexed with mGluR1a, as expected, the time course of mGluR1a trafficking paralleled that of ERα. An indication that EMS is needed to regulate ERα trafficking was provided when ER antagonism with ICI 182,780 or mGluR1a antagonism with LY367,385 abrogated trafficking. In immortalized hypothalamic neurons (N-38 neurons), estradiol stimulation activated PKCθ and increased [Ca2+]i [38]. Blocking PKC phosphorylation prevented estradiol-induced ERα trafficking. Significantly, the estradiol increase of pPKCθ in ARH was necessary for the MOR activation and facilitation of lordosis behavior suggesting that increased membrane ERα-mGluR1a levels increase circuit activation ([36]; Fig 4).
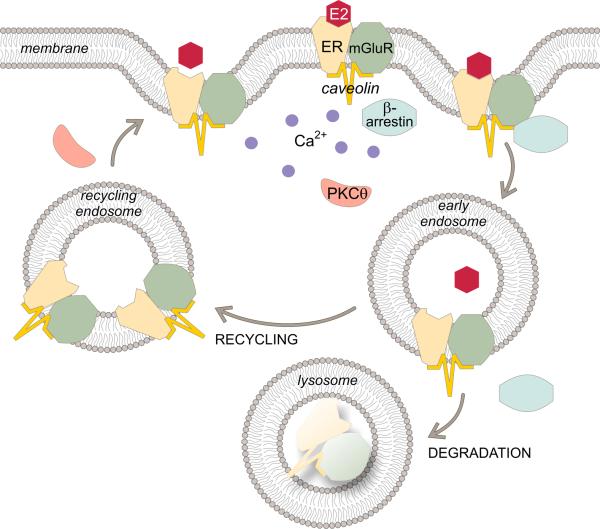
Figure 4.
Estradiol regulates membrane ERα levels. ERα is trafficked to the membrane in a complex with mGluR1a and caveolin. Estradiol activates this complex, and indices the association of β-arrestin leading to the internalization of the ERα-mGluR1a complex into early endosomes. The acidic environment in this compartment allows the estradiol to dissociate from ERα. The ERα-mGluR1a complex can recycle back to the membrane and be activated again. EMS induces the activation of PKCθ and release of intracellular stores of calcium (Ca2+). While the phosphorylation of PKCθ is necessary for ERα trafficking, we speculate that the release of Ca2+ is also required to induce recycling endosomes to fuse with the membrane. Prolonged estradiol stimulation shifts pathway from recycling to degradation leading to receptor down regulation and cessation of EMS.
The other mechanism that regulates membrane receptor levels is removal from the membrane. Receptor internalization is a well-characterized mechanism of desensitization that follows receptor activation. Thus, internalization is used as a measure of receptor activation. Endocytic vesicles with the internalized receptors fuse with endosomes allowing receptors to release their ligands. Receptors are then sorted into those destined for recycling back to the membrane and those targeted for degradation in lysosomes. The cycle of endosomal trafficking is a hallmark of membrane receptors [73, 101, 129, 166].
Estradiol internalizes membrane ERα [13, 37, 39] responding to agonist stimulation like other membrane receptors in the CNS. Several approaches have been utilized to demonstrate this. Fluorescently labeled membrane-impermeable estradiol constructs (e.g., E-BSA, estradiol coupled to bovine serum albumin) were incubated with neurons for increasing lengths of time. Initially, the fluorescent E-BSA was excluded from the neuron and visualized outlining the cell, but within minutes of treatment, the fluorescent conjugates were visualized in early endosome-like structures, an indication of internalization of the ER/E-BSA complex [9, 37, 99, 100]. Another approach has been the use of estradiol dendrimers [54]. Several estradiol molecules are linked to macromolecules which prevent the activation of nuclear ERs. Estradiol dendrimers activate membrane-initiated cell signaling in cells and can be found in the cytoplasm, but not the nucleus, after activation of signaling, suggesting internalization of the estradiol dendrimer [164]. Moreover, using surface biotinylation to label membrane ERα in primary cultures of neurons, adult astrocytes and immortalized hypothalamic N-38 neurons treatment with estradiol increased internalization of ERα and ERαΔ4. In the presence of estradiol, ERα trafficking was increased and at the same time the receptors were activated and internalized - leading to both an increase in membrane and internalized ERs. As expected, the receptor antagonist ICI 182,789, prevented internalization, reinforcing the idea that ERα activation is necessary for intracellular signaling. ERα behaved like other membrane receptors in response to ligand stimulation with internalization, and antagonism blocked the internalization (Fig 4). In a physiological context, these results suggest that when estradiol levels are sufficiently high, mERα are activated, increasing the rate of internalization (desensitization). In response to constant stimulation, estradiol eventually down regulates ERα (levels on the membrane and internalized are below pre-stimulation levels). This regulation of ERα levels is a mechanism through which estradiol limits the duration of EMS.
Interestingly, by some estimates there are over 12 different splice variants of the ESR1 gene in the brain [57, 71, 114, 157]. Resulting proteins have varied abilities to bind estradiol and regulate translation. Changes in relative ratios have been associated with pathologies from schizophrenia to Alzheimer's disease. In surface biotinylation studies, both a full length ERα and a ~52 kDa variant have been identified [13, 38, 39, 49]. In fact, the 52 kDa form was more abundant than the 66 kDa ERα in primary cultures of hypothalamic embryonic and immortalized neurons, and astrocytes from adult hypothalamus [13, 39]. Two alternatively spliced ERα mRNA could potentially code for a protein of the 52 kDa size. These variants, one missing the fourth exon, called ERαΔ4, and the other ERαΔ7, missing exon 7 and the COOH terminus, had previously been described in the brain [57, 139]. Using RT-PCR and primers spanning exons 3 to 5, and other primers spanning exon 5 to 8, we found mRNA for ERαΔ4 in both cultures of primary hypothalamic neurons and in N-38 neurons. The same 52 kDa and 66 kDa ERα immunoreactive proteins are expressed in rat and wild type mouse astrocytes. Both are missing in ERαKO mice, indicating that they are derived from the ESR1 gene. Thus, in astrocytes, we assume that like in neurons the 52 kDa ERα is also derived from the ERαΔ4 mRNA [13]. One explanation for why there is a greater abundance of ERαΔ4 on the membrane is that the loss of exon 4 impacts the nuclear localization sequence and hinge region. Site specific mutations in the hinge region suggest that this region alters nuclear translocation, but in the H2-NES mutated ERα, EMS was retained [19]. However, deletion of exon 4 may also impact the ligand-binding domain, calling into question the ability of ERαΔ4 to bind estradiol [12].
Does ERαΔ4 participate in EMS? In neurons and astrocytes, activation of EMS leads to increased DAG and IP3 dependent signaling. In hypothalamic astrocytes, EMS facilitates progesterone synthesis needed for estrogen positive feedback (reviewed in [68, 90]). Like in neurons, mGluR1 is transactivated by ERα so a protein-protein interaction is expected. Co-immunoprecipitation demonstrated an interaction between the receptors. In all cases only the full length (66 kDa) ERα co-immunoprecipitated with mGluR1a. We did not detect an interaction of ERαΔ4 with mGluR1a suggesting that EMS mediating reproduction involves the full length ERα and not ERαΔ4 [28, 35, 66]. Moreover, the CAV1 knockdown studies did not significantly reduce membrane levels of ERαΔ4. The function of ERαΔ4, which is targeted to the cell membrane in greater abundance than ERα in vitro, remains elusive.
Another interesting facet of EMS is the potential for integrating estradiol and glutamate signaling. In astrocytes, activation of the mGluR1a without estradiol induced a robust [Ca2+]i flux and progesterone synthesis. When estradiol and DHPG were applied together, the [Ca2+]i flux and consequent progesterone synthesis were greatly amplified suggesting that an estrogenic response can be augmented by neuronal activity [66]. This intriguing hypothesis requires further testing in vivo.
SUMMARY
EMS represents a new way of thinking about steroid signaling in the nervous system. These discoveries need to be coupled with those that point to the production of steroids by the nervous system. Together neurosteroids and membrane-initiated actions indicate that these are yet another class of neural signaling molecules. In astrocytes, peaking levels of estradiol from the ovaries augments progesterone synthesis, helping to trigger the LH surge [88, 90]. The estradiol affects the steroidogenic pathway by transactivating mGluR1a. While mGluR1a stimulation is not needed for estradiol signaling, in vitro experiments indicate that the estradiol effect can be facilitated when estradiol and an mGluR1a agonist, DHPG, are applied together. The estradiol-induced increase in intracellular calcium and the resulting progesterone synthesis were greatly amplified suggesting that neural activity can augment EMS [66, 86, 93]. At this point it is unclear whether such an integration of signals is the rule in EMS or an astrocyte exception.
An emerging concept of ERα in the membrane is that while ERα is not a typical GPCR, it shares many of the same functional traits. First, like other membrane receptors it is trafficked to regions of the membrane that segregate signaling molecules, lipid rafts, and interacts with scaffolding proteins. Second, based on surface biotinylation experiments, ERα has an extracellular portion. Third, following activation, ERα interacts with β-arrestin and is internalized [37]. Fourth, ERα interacts with mGluRs to initiate cell signaling. Similar interactions have been demonstrated for other receptors (oxytocin, [67]; serotonin, [107]). Internalization reveals a level of regulation not previously appreciated for these receptors. Estrogen receptors are not a stable population, but are trafficked to and then sequestered from the cell membrane following activation, as needed. The fact that estradiol increases the insertion of ERα and ERαΔ4 into the membrane, where estradiol activation leads to desensitization and down regulation, suggests that continuing exposure to estradiol does not continually activate membrane ERα. If activity can be based on receptor levels in the membrane, it may peak within minutes and then, as ERα is removed, estradiol may no longer be able to signal through this membrane-initiated signaling mechanism. This was supported by the significantly larger [Ca2+]i at the time when membrane ERα levels peaked, 30 mins of estradiol exposure compared with earlier or later time points. Currently, we do not know how the levels of Gq-mER on the membrane or GPR30 on smooth endoplasmic reticulum respond. As previously stated, estradiol can access/activate receptors that are not on the surface so the localization of ERα is not because of the restrictions of the ligand, but because of the position of signaling machinery that is activated. In terms of modulating reproduction, both EMS and direct nuclear action is needed. Since EMS can lead to CREB activation, transcriptional events also involve membrane to nucleus signaling mediating long term consequences. This is well demonstrated in terms of the rapid action of estradiol on spinogenesis, which requires ERα-mGluR1a signaling, but whether EMS has any role in spine maturation remains to be elucidated. Similarly, estradiol stimulation of NPY to β-END signaling needed to activate MOR in the MPN depends on EMS, but blocking membrane signaling with a mGluR1a antagonist dramatically attenuates lordosis behavior - in spite of the necessity of transcription and translation. But again, is this due to ERE activation or membrane to nuclear signaling? How all this is integrated into a unified theory of estradiol signaling in the brain is only now beginning to be unraveled. We speculate that the newly formed estradiol-induced spines are necessary for the signaling between the ARH and MPN. The spines begin to form on β-END cells in the ARH when there is only a small amount of estradiol present. When levels of estradiol peak, NPY cells signal to β-END to release this neuropeptide into the MPN to activate the MOR cells and temporarily inhibit lordosis behavior. Progesterone then has the dual role of releasing the inhibition induced by MOR activation and removing the newly formed spines, so the cycle can begin anew.
Thus, sexual receptive behavior requires both the inductive and permissive actions of estradiol, and the details of this interaction are being elucidated. EMS stimulates existing circuits and initiates changes in morphology of neurons within the circuit controlling sexual behavior by influencing spinogenesis and dendrite length. Acting more slowly, estradiol must also increase the expression of neuropeptides, transmitter enzymes and receptors that regulate the circuit. The combined actions of EMS and transcription by nuclear ERs are what allow for the full activation of the circuit. The synergy of these signaling mechanisms allows for the regulation of a complex physiological response – sexual receptivity.
-
-
Estradiol membrane signaling is required for female sexual receptivity.
-
-
Estradiol-mediated spinogenesis is required for sexual behavior.
-
-
Trafficking of the estrogen receptor to the membrane is regulated by estradiol.
ACKNOWLEDGEMENTS
This work was supported by DA013185, HD042635, and HD007228
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Abraham IM, Han SK, Todman MG, Korach KS, Herbison AE. Estrogen receptor beta mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J Neurosci. 2003;23:5771–5777. doi: 10.1523/JNEUROSCI.23-13-05771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Acconcia F, Ascenzi P, Bocedi A, Spisni E, Tomasi V, Trentalance A, Visca P, Marino M. Palmitoylation-dependent estrogen receptor alpha membrane localization: regulation by 17beta-estradiol. Mol Biol Cell. 2005;16:231–237. doi: 10.1091/mbc.E04-07-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Acconcia F, Bocedi A, Ascenzi P, Marino M. Does palmitoylation target estrogen receptors to plasma membrane caveolae? IUBMB Life. 2003;55:33–35. doi: 10.1080/1521654031000081256. [DOI] [PubMed] [Google Scholar]
- [4].Adams MM, Fink SE, Shah RA, Janssen WG, Hayashi S, Milner TA, McEwen BS, Morrison JH. Estrogen and aging affect the subcellular distribution of estrogen receptor-alpha in the hippocampus of female rats. J Neurosci. 2002;22:3608–3614. doi: 10.1523/JNEUROSCI.22-09-03608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bailey ME, Wang AC, Hao J, Janssen WG, Hara Y, Dumitriu D, Hof PR, Morrison JH. Interactive effects of age and estrogen on cortical neurons: implications for cognitive aging. Neuroscience. 2011;191:148–158. doi: 10.1016/j.neuroscience.2011.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29:241–249. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- [7].Bamburg JR. Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu Rev Cell Dev Biol. 1999;15:185–230. doi: 10.1146/annurev.cellbio.15.1.185. [DOI] [PubMed] [Google Scholar]
- [8].Beach F. Hormones and Behavior. Hoeber; New York: 1948. [Google Scholar]
- [9].Benten WP, Stephan C, Lieberherr M, Wunderlich F. Estradiol signaling via sequestrable surface receptors. Endocrinology. 2001;142:1669–1677. doi: 10.1210/endo.142.4.8094. [DOI] [PubMed] [Google Scholar]
- [10].Beyer C, Hoffman KL, Gonzalez-Flores O. Neuroendocrine regulation of estrous behavior in the rabbit: similarities and differences with the rat. Horm Behav. 2007;52:2–11. doi: 10.1016/j.yhbeh.2007.03.027. [DOI] [PubMed] [Google Scholar]
- [11].Beyer C, Karolczak M. Estrogenic stimulation of neurite growth in midbrain dopaminergic neurons depends on cAMP/protein kinase A signalling. J Neurosci Res. 2000;59:107–116. [PubMed] [Google Scholar]
- [12].Bollig A, Miksicek RJ. An estrogen receptor-alpha splicing variant mediates both positive and negative effects on gene transcription. Mol Endocrinol. 2000;14:634–649. doi: 10.1210/mend.14.5.0460. [DOI] [PubMed] [Google Scholar]
- [13].Bondar G, Kuo J, Hamid N, Micevych P. Estradiol-induced estrogen receptor-alpha trafficking. J Neurosci. 2009;29:15323–15330. doi: 10.1523/JNEUROSCI.2107-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Boonyaratanakornkit V. Scaffolding proteins mediating membrane-initiated extra-nuclear actions of estrogen receptor. Steroids. 2011;76:877–884. doi: 10.1016/j.steroids.2011.02.017. [DOI] [PubMed] [Google Scholar]
- [15].Boulware MI, Kordasiewicz H, Mermelstein PG. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J Neurosci. 2007;27:9941–9950. doi: 10.1523/JNEUROSCI.1647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Breedlove SM, Arnold AP. Sexually dimorphic motor nucleus in the rat lumbar spinal cord: response to adult hormone manipulation, absence in androgen-insensitive rats. Brain Res. 1981;225:297–307. doi: 10.1016/0006-8993(81)90837-4. [DOI] [PubMed] [Google Scholar]
- [18].Bueno J, Pfaff DW. Single unit recording in hypothalamus and preoptic area of estrogen-treated and untreated ovariectomized female rats. Brain Res. 1976;101:67–78. doi: 10.1016/0006-8993(76)90988-4. [DOI] [PubMed] [Google Scholar]
- [19].Burns KA, Li Y, Arao Y, Petrovich RM, Korach KS. Selective mutations in estrogen receptor alpha D-domain alters nuclear translocation and non-estrogen response element gene regulatory mechanisms. J Biol Chem. 2011;286:12640–12649. doi: 10.1074/jbc.M110.187773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Calizo LH, Flanagan-Cato LM. Estrogen-induced dendritic spine elimination on female rat ventromedial hypothalamic neurons that project to the periaqueductal gray. J Comp Neurol. 2002;447:234–248. doi: 10.1002/cne.10223. [DOI] [PubMed] [Google Scholar]
- [21].Calizo LH, Flanagan-Cato LM. Estrogen selectively regulates spine density within the dendritic arbor of rat ventromedial hypothalamic neurons. J Neurosci. 2000;20:1589–1596. doi: 10.1523/JNEUROSCI.20-04-01589.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Carmeci C, Thompson DA, Ring HZ, Francke U, Weigel RJ. Identification of a gene (GPR30) with homology to the G-protein-coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics. 1997;45:607–617. doi: 10.1006/geno.1997.4972. [DOI] [PubMed] [Google Scholar]
- [23].Chaban V, Li J, McDonald JS, Rapkin A, Micevych P. Estradiol attenuates the adenosine triphosphate-induced increase of intracellular calcium through group II metabotropic glutamate receptors in rat dorsal root ganglion neurons. J Neurosci Res. 2011;89:1707–1710. doi: 10.1002/jnr.22718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chaban VV, Lakhter AJ, Micevych P. A membrane estrogen receptor mediates intracellular calcium release in astrocytes. Endocrinology. 2004;145:3788–3795. doi: 10.1210/en.2004-0149. [DOI] [PubMed] [Google Scholar]
- [25].Cheskis BJ, Greger JG, Nagpal S, Freedman LP. Signaling by estrogens. J Cell Physiol. 2007;213:610–617. doi: 10.1002/jcp.21253. [DOI] [PubMed] [Google Scholar]
- [26].Christensen A, Dewing P, Micevych P. Membrane-initiated estradiol signaling induces spinogenesis required for female sexual receptivity. J Neurosci. 2011;31:17583–17589. doi: 10.1523/JNEUROSCI.3030-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Christensen A, Dewing P, Micevych P. The STX-binding estrogen receptor regulates female sexual receptivity through interaction with the metabotropic glutamate receptor Society for Neuroscience. Washington, D.C.: 2011. [Google Scholar]
- [28].Christensen A, Micevych P. CAV1 siRNA reduces membrane ERα levels and attenuates sexual receptivity. Endocrinology. doi: 10.1210/en.2012-1312. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cooke BM, Woolley CS. Gonadal hormone modulation of dendrites in the mammalian CNS. J Neurobiol. 2005;64:34–46. doi: 10.1002/neu.20143. [DOI] [PubMed] [Google Scholar]
- [30].Correia SC, Santos RX, Cardoso S, Carvalho C, Santos MS, Oliveira CR, Moreira PI. Effects of estrogen in the brain: is it a neuroprotective agent in Alzheimer's disease? Curr Aging Sci. 2010;3:113–126. doi: 10.2174/1874609811003020113. [DOI] [PubMed] [Google Scholar]
- [31].Couse JF, Bunch DO, Lindzey J, Schomberg DW, Korach KS. Prevention of the polycystic ovarian phenotype and characterization of ovulatory capacity in the estrogen receptor-alpha knockout mouse. Endocrinology. 1999;140:5855–5865. doi: 10.1210/endo.140.12.7222. [DOI] [PubMed] [Google Scholar]
- [32].Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- [33].Cruz-Martin A, Crespo M, Portera-Cailliau C. Glutamate Induces the Elongation of Early Dendritic Protrusions via mGluRs in Wild Type Mice, but Not in Fragile X Mice. PLoS One. 2011;7:e32446. doi: 10.1371/journal.pone.0032446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Davis EC, Popper P, Gorski RA. The role of apoptosis in sexual differentiation of the rat sexually dimorphic nucleus of the preoptic area. Brain Res. 1996;734:10–18. [PubMed] [Google Scholar]
- [35].Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P. Membrane estrogen receptor-alpha interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J Neurosci. 2007;27:9294–9300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Dewing P, Christensen A, Bondar G, Micevych P. Protein kinase C signaling in the hypothalamic arcuate nucleus regulates sexual receptivity in female rats. Endocrinology. 2008;149:5934–5942. doi: 10.1210/en.2008-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Dominguez R, Hu E, Zhou M, Baudry M. 17beta-estradiol-mediated neuroprotection and ERK activation require a pertussis toxin-sensitive mechanism involving GRK2 and beta-arrestin-1. J Neurosci. 2009;29:4228–4238. doi: 10.1523/JNEUROSCI.0550-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Dominguez R, Kuo J, Dewing P, Micevych P. Trafficking of membrane ERα involved membrane-initiated estrogen signaling in immortalized hypothalamic N-38 neurons. J Neuroendocrinol. doi: 10.1016/j.steroids.2012.12.008. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Dominguez R, Micevych P. Estradiol rapidly regulates membrane estrogen receptor alpha levels in hypothalamic neurons. J Neurosci. 2010;30:12589–12596. doi: 10.1523/JNEUROSCI.1038-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Eckersell CB, Popper P, Micevych PE. Estrogen-induced alteration of mu-opioid receptor immunoreactivity in the medial preoptic nucleus and medial amygdala. J Neurosci. 1998;18:3967–3976. doi: 10.1523/JNEUROSCI.18-10-03967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fedulov V, Rex CS, Simmons DA, Palmer L, Gall CM, Lynch G. Evidence that long-term potentiation occurs within individual hippocampal synapses during learning. J Neurosci. 2007;27:8031–8039. doi: 10.1523/JNEUROSCI.2003-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- [43].Filardo EJ, Quinn JA, Frackelton AR, Jr., Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol. 2002;16:70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- [44].Flanagan-Cato LM. Sex differences in the neural circuit that mediates female sexual receptivity. Front Neuroendocrinol. 2011;32:124–136. doi: 10.1016/j.yfrne.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Frankfurt M, Gould E, Woolley CS, McEwen BS. Gonadal steroids modify dendritic spine density in ventromedial hypothalamic neurons: a Golgi study in the adult rat. Neuroendocrinology. 1990;51:530–535. doi: 10.1159/000125387. [DOI] [PubMed] [Google Scholar]
- [46].Galbiati F, Volonte D, Gil O, Zanazzi G, Salzer JL, Sargiacomo M, Scherer PE, Engelman JA, Schlegel A, Parenti M, Okamoto T, Lisanti MP. Expression of caveolin-1 and -2 in differentiating PC12 cells and dorsal root ganglion neurons: caveolin-2 is up-regulated in response to cell injury. Proc Natl Acad Sci U S A. 1998;95:10257–10262. doi: 10.1073/pnas.95.17.10257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Garcia-Segura LM, Baetens D, Naftolin F. Synaptic remodelling in arcuate nucleus after injection of estradiol valerate in adult female rats. Brain Res. 1986;366:131–136. doi: 10.1016/0006-8993(86)91287-4. [DOI] [PubMed] [Google Scholar]
- [48].Gonzalez-Burgos I, Rivera-Cervantes MC, Velazquez-Zamora DA, Feria-Velasco A, Garcia-Segura LM. Selective estrogen receptor modulators regulate dendritic spine plasticity in the hippocampus of male rats. Neural Plast. 2012:309494. doi: 10.1155/2012/309494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Gorosito SV, Lorenzo AG, Cambiasso MJ. Estrogen receptor alpha is expressed on the cell-surface of embryonic hypothalamic neurons. Neuroscience. 2008;154:1173–1177. doi: 10.1016/j.neuroscience.2008.05.001. [DOI] [PubMed] [Google Scholar]
- [50].Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Griffin GD, Flanagan-Cato LM. Ovarian hormone action in the hypothalamic ventromedial nucleus: remodelling to regulate reproduction. J Neuroendocrinol. 2011;23:465–471. doi: 10.1111/j.1365-2826.2011.02143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Grove-Strawser D, Boulware MI, Mermelstein PG. Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience. 2010;170:1045–1055. doi: 10.1016/j.neuroscience.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Grutzendler J, Kasthuri N, Gan WB. Long-term dendritic spine stability in the adult cortex. Nature. 2002;420:812–816. doi: 10.1038/nature01276. [DOI] [PubMed] [Google Scholar]
- [54].Harrington WR, Kim SH, Funk CC, Madak-Erdogan Z, Schiff R, Katzenellenbogen JA, Katzenellenbogen BS. Estrogen dendrimer conjugates that preferentially activate extranuclear, nongenomic versus genomic pathways of estrogen action. Mol Endocrinol. 2006;20:491–502. doi: 10.1210/me.2005-0186. [DOI] [PubMed] [Google Scholar]
- [55].Hart SA, Patton JD, Woolley CS. Quantitative analysis of ER alpha and GAD colocalization in the hippocampus of the adult female rat. J Comp Neurol. 2001;440:144–155. doi: 10.1002/cne.1376. [DOI] [PubMed] [Google Scholar]
- [56].Holtmaat A, Wilbrecht L, Knott GW, Welker E, Svoboda K. Experience-dependent and cell-type-specific spine growth in the neocortex. Nature. 2006;441:979–983. doi: 10.1038/nature04783. [DOI] [PubMed] [Google Scholar]
- [57].Ishunina TA, Swaab DF, Fischer DF. Estrogen receptor-alpha splice variants in the medial mamillary nucleus of Alzheimer's disease patients: identification of a novel MB1 isoform. J Clin Endocrinol Metab. 2005;90:3757–3765. doi: 10.1210/jc.2004-1858. [DOI] [PubMed] [Google Scholar]
- [58].Ivanova T, Mendez P, Garcia-Segura LM, Beyer C. Rapid stimulation of the PI3-kinase/Akt signalling pathway in developing midbrain neurones by oestrogen. J Neuroendocrinol. 2002;14:73–79. doi: 10.1046/j.0007-1331.2001.00742.x. [DOI] [PubMed] [Google Scholar]
- [59].Jacobson CD, Csernus VJ, Shryne JE, Gorski RA. The influence of gonadectomy, androgen exposure, or a gonadal graft in the neonatal rat on the volume of the sexually dimorphic nucleus of the preoptic area. J Neurosci. 1981;1:1142–1147. doi: 10.1523/JNEUROSCI.01-10-01142.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. Structure-stability-function relationships of dendritic spines. Trends Neurosci. 2003;26:360–368. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- [61].Knott GW, Holtmaat A, Wilbrecht L, Welker E, Svoboda K. Spine growth precedes synapse formation in the adult neocortex in vivo. Nat Neurosci. 2006;9:1117–1124. doi: 10.1038/nn1747. [DOI] [PubMed] [Google Scholar]
- [62].Kow LM, Pfaff DW. The membrane actions of estrogens can potentiate their lordosis behavior-facilitating genomic actions. Proc Natl Acad Sci U S A. 2004;101:12354–12357. doi: 10.1073/pnas.0404889101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kramar EA, Chen LY, Brandon NJ, Rex CS, Liu F, Gall CM, Lynch G. Cytoskeletal changes underlie estrogen's acute effects on synaptic transmission and plasticity. J Neurosci. 2009;29:12982–12993. doi: 10.1523/JNEUROSCI.3059-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kretz O, Fester L, Wehrenberg U, Zhou L, Brauckmann S, Zhao S, Prange-Kiel J, Naumann T, Jarry H, Frotscher M, Rune GM. Hippocampal synapses depend on hippocampal estrogen synthesis. J Neurosci. 2004;24:5913–5921. doi: 10.1523/JNEUROSCI.5186-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kuo J, Hamid N, Bondar G, Prossnitz ER, Micevych P. Membrane estrogen receptors stimulate intracellular calcium release and progesterone synthesis in hypothalamic astrocytes. J Neurosci. 2010;30:12950–12957. doi: 10.1523/JNEUROSCI.1158-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kuo J, Hariri OR, Bondar G, Ogi J, Micevych P. Membrane estrogen receptor-alpha interacts with metabotropic glutamate receptor type 1a to mobilize intracellular calcium in hypothalamic astrocytes. Endocrinology. 2009;150:1369–1376. doi: 10.1210/en.2008-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Kuo J, Hariri OR, Micevych P. An interaction of oxytocin receptors with metabotropic glutamate receptors in hypothalamic astrocytes. J Neuroendocrinol. 2009;21:1001–1006. doi: 10.1111/j.1365-2826.2009.01922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kuo J, Micevych P. Neurosteroids, trigger of the LH surge. J Steroid Biochem Mol Biol. 2012 doi: 10.1016/j.jsbmb.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Leranth C, Shanabrough M, Horvath TL. Hormonal regulation of hippocampal spine synapse density involves subcortical mediation. Neuroscience. 2000;101:349–356. doi: 10.1016/s0306-4522(00)00369-9. [DOI] [PubMed] [Google Scholar]
- [70].Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, Magarinos AM, Allen PB, Greengard P, Luine V, McEwen BS. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc Natl Acad Sci U S A. 2004;101:2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Li L, Haynes MP, Bender JR. Plasma membrane localization and function of the estrogen receptor alpha variant (ER46) in human endothelial cells. Proc Natl Acad Sci U S A. 2003;100:4807–4812. doi: 10.1073/pnas.0831079100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Liu P, Rudick M, Anderson RG. Multiple functions of caveolin-1. J Biol Chem. 2002;277:41295–41298. doi: 10.1074/jbc.R200020200. [DOI] [PubMed] [Google Scholar]
- [73].Lohse MJ, Benovic JL, Caron MG, Lefkowitz RJ. Multiple pathways of rapid beta 2-adrenergic receptor desensitization. Delineation with specific inhibitors. J Biol Chem. 1990;265:3202–3211. [PubMed] [Google Scholar]
- [74].Madeira MD, Ferreira-Silva L, Paula-Barbosa MM. Influence of sex and estrus cycle on the sexual dimorphisms of the hypothalamic ventromedial nucleus: stereological evaluation and Golgi study. J Comp Neurol. 2001;432:329–345. doi: 10.1002/cne.1106. [DOI] [PubMed] [Google Scholar]
- [75].Marin R, Diaz M, Alonso R, Sanz A, Arevalo MA, Garcia-Segura LM. Role of estrogen receptor alpha in membrane-initiated signaling in neural cells: interaction with IGF-1 receptor. J Steroid Biochem Mol Biol. 2009;114:2–7. doi: 10.1016/j.jsbmb.2008.12.014. [DOI] [PubMed] [Google Scholar]
- [76].Marino M, Ascenzi P. Steroid hormone rapid signaling: the pivotal role of S-palmitoylation. IUBMB Life. 2006;58:716–719. doi: 10.1080/15216540601019485. [DOI] [PubMed] [Google Scholar]
- [77].Massimino ML, Griffoni C, Spisni E, Toni M, Tomasi V. Involvement of caveolae and caveolae-like domains in signalling, cell survival and angiogenesis. Cell Signal. 2002;14:93–98. doi: 10.1016/s0898-6568(01)00232-7. [DOI] [PubMed] [Google Scholar]
- [78].Matsumoto A, Arai Y. Effect of androgen on sexual differentiation of synaptic organization in the hypothalamic arcuate nucleus: an ontogenetic study. Neuroendocrinology. 1981;33:166–169. doi: 10.1159/000123223. [DOI] [PubMed] [Google Scholar]
- [79].Matsumoto A, Arai Y. Neuronal plasticity in the deafferented hypothalamic arcuate nucleus of adult female rats and its enhancement by treatment with estrogen. J Comp Neurol. 1981;197:197–205. doi: 10.1002/cne.901970203. [DOI] [PubMed] [Google Scholar]
- [80].Matsumoto A, Arai Y. Synaptogenic effect of estrogen on the hypothalamic arcuate nucleus of the adult female rat. Cell Tissue Res. 1979;198:427–433. doi: 10.1007/BF00234187. [DOI] [PubMed] [Google Scholar]
- [81].Matsumoto A, Murakami S, Arai Y. Neurotropic effects of estrogen on the neonatal preoptic area grafted into the adult rat brain. Cell Tissue Res. 1988;252:33–37. doi: 10.1007/BF00213823. [DOI] [PubMed] [Google Scholar]
- [82].Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y. M. Iino, and H. Kasai, Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Meitzen J, Mermelstein PG. Estrogen receptors stimulate brain region specific metabotropic glutamate receptors to rapidly initiate signal transduction pathways. J Chem Neuroanat. 2011 doi: 10.1016/j.jchemneu.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Meng Y, Zhang Y, Tregoubov V, Janus C, Cruz L, Jackson M, Lu WY, MacDonald JF, Wang JY, Falls DL, Jia Z. Abnormal spine morphology and enhanced LTP in LIMK-1 knockout mice. Neuron. 2002;35:121–133. doi: 10.1016/s0896-6273(02)00758-4. [DOI] [PubMed] [Google Scholar]
- [85].Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci. 1996;16:595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Mermelstein PG, Micevych PE. Nervous system physiology regulated by membrane estrogen receptors. Rev Neurosci. 2008;19:413–424. doi: 10.1515/revneuro.2008.19.6.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Micevych P, Dewing P. Membrane-initiated estradiol signaling regulating sexual receptivity. Front in Neuroendocrine Science. 2011;2:1–9. doi: 10.3389/fendo.2011.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Micevych P, Sinchak K. Estradiol regulation of progesterone synthesis in the brain. Mol Cell Endocrinol. 2008;290:44–50. doi: 10.1016/j.mce.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Micevych P, Sinchak K. The neurochemistry of limbic-hypothalamic circuits regulating sexual receptivity. In: Lajtha A, editor. Behavioral Neurochemistry and Neuroendocrinology. Springer; New York: 2006. [Google Scholar]
- [90].Micevych P, Sinchak K. The neurosteroid progesterone underlies estrogen positive feedback of the LH surge. Frontiers in Genomic Endocrinology. 2011;2:1–11. doi: 10.3389/fendo.2011.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Micevych P, Ulibarri C. Development of the limbic-hypothalamic cholecystokinin circuit: a model of sexual differentiation. Dev Neurosci. 1992;14:11–34. doi: 10.1159/000111643. [DOI] [PubMed] [Google Scholar]
- [92].Micevych PE, Kelly MJ. Membrane Estrogen Receptor Regulation of Hypothalamic Function. Neuroendocrinology. 2012 doi: 10.1159/000338400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Micevych PE, Mermelstein PG. Membrane estrogen receptors acting through metabotropic glutamate receptors: an emerging mechanism of estrogen action in brain. Mol Neurobiol. 2008;38:66–77. doi: 10.1007/s12035-008-8034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Micevych PE, Rissman EF, Gustafsson JA, Sinchak K. Estrogen receptor-alpha is required for estrogen-induced mu-opioid receptor internalization. J Neurosci Res. 2003;71:802–810. doi: 10.1002/jnr.10526. [DOI] [PubMed] [Google Scholar]
- [95].Mills RH, Sohn RK, Micevych PE. Estrogen-induced mu-opioid receptor internalization in the medial preoptic nucleus is mediated via neuropeptide Y-Y1 receptor activation in the arcuate nucleus of female rats. J Neurosci. 2004;24:947–955. doi: 10.1523/JNEUROSCI.1366-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J Comp Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- [97].Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429:355–371. [PubMed] [Google Scholar]
- [98].Mizukami Y. In vivo functions of GPR30/GPER-1, a membrane receptor for estrogen: from discovery to functions in vivo. Endocr J. 2010;57:101–107. doi: 10.1507/endocrj.k09e-332. [DOI] [PubMed] [Google Scholar]
- [99].Moats RK, 2nd, Ramirez VD. Electron microscopic visualization of membrane-mediated uptake, and translocation of estrogen-BSA:colloidal gold by hep G2 cells. J Endocrinol. 2000;166:631–647. doi: 10.1677/joe.0.1660631. [DOI] [PubMed] [Google Scholar]
- [100].Moats RK, 2nd, Ramirez VD. Rapid uptake and binding of estradiol-17beta-6-(O-carboxymethyl)oxime:125I-labeled BSA by female rat liver. Biol Reprod. 1998;58:531–538. doi: 10.1095/biolreprod58.2.531. [DOI] [PubMed] [Google Scholar]
- [101].Mundell SJ, Matharu AL, Pula G, Roberts PJ, Kelly E. Agonist-induced internalization of the metabotropic glutamate receptor 1a is arrestin- and dynamin-dependent. J Neurochem. 2001;78:546–551. doi: 10.1046/j.1471-4159.2001.00421.x. [DOI] [PubMed] [Google Scholar]
- [102].Murphy DD, Cole NB, Greenberger V, Segal M. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci. 1998;18:2550–2559. doi: 10.1523/JNEUROSCI.18-07-02550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Murphy DD, Segal M. Progesterone prevents estradiol-induced dendritic spine formation in cultured hippocampal neurons. Neuroendocrinology. 2000;72:133–143. doi: 10.1159/000054580. [DOI] [PubMed] [Google Scholar]
- [104].Murphy DD, Segal M. Regulation of dendritic spine density in cultured rat hippocampal neurons by steroid hormones. J Neurosci. 1996;16:4059–4068. doi: 10.1523/JNEUROSCI.16-13-04059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Nadal A, Diaz M, Valverde MA. The estrogen trinity: membrane, cytosolic, and nuclear effects. News Physiol Sci. 2001;16:251–255. doi: 10.1152/physiologyonline.2001.16.6.251. [DOI] [PubMed] [Google Scholar]
- [106].Nadal A, Ropero AB, Fuentes E, Soria B. The plasma membrane estrogen receptor: nuclear or unclear? Trends Pharmacol Sci. 2001;22:597–599. doi: 10.1016/s0165-6147(00)01846-0. [DOI] [PubMed] [Google Scholar]
- [107].Neverova NV, Saywell SA, Nashold LJ, Mitchell GS, Feldman JL. Episodic stimulation of alpha1-adrenoreceptors induces protein kinase C-dependent persistent changes in motoneuronal excitability. J Neurosci. 2007;27:4435–4442. doi: 10.1523/JNEUROSCI.2803-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Otto C, Fuchs I, Kauselmann G, Kern H, Zevnik B, Andreasen P, Schwarz G, Altmann H, Klewer M, Schoor M, Vonk R, Fritzemeier KH. GPR30 does not mediate estrogenic responses in reproductive organs in mice. Biol Reprod. 2009;80:34–41. doi: 10.1095/biolreprod.108.071175. [DOI] [PubMed] [Google Scholar]
- [109].Otto C, Rohde-Schulz B, Schwarz G, Fuchs I, Klewer M, Brittain D, Langer G, Bader B, Prelle K, Nubbemeyer R, Fritzemeier KH. G protein-coupled receptor 30 localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology. 2008;149:4846–4856. doi: 10.1210/en.2008-0269. [DOI] [PubMed] [Google Scholar]
- [110].Parnass Z, Tashiro A, Yuste R. Analysis of spine morphological plasticity in developing hippocampal pyramidal neurons. Hippocampus. 2000;10:561–568. doi: 10.1002/1098-1063(2000)10:5<561::AID-HIPO6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- [111].Pedram A, Razandi M, Deschenes RJ, Levin ER. DHHC-7 and -21 are palmitoylacyltransferases for sex steroid receptors. Mol Biol Cell. 2012;23:188–199. doi: 10.1091/mbc.E11-07-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol. 2006;20:1996–2009. doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- [113].Pedram A, Razandi M, Sainson RC, Kim JK, Hughes CC, Levin ER. A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem. 2007;282:22278–22288. doi: 10.1074/jbc.M611877200. [DOI] [PubMed] [Google Scholar]
- [114].Perlman WR, Matsumoto M, Beltaifa S, Hyde TM, Saunders RC, Webster MJ, Rubinow DR, Kleinman JE, Weickert CS. Expression of estrogen receptor alpha exon-deleted mRNA variants in the human and non-human primate frontal cortex. Neuroscience. 2005;134:81–95. doi: 10.1016/j.neuroscience.2005.03.055. [DOI] [PubMed] [Google Scholar]
- [115].Pfaff DW, Kow LM, Loose MD, Flanagan-Cato LM. Reverse engineering the lordosis behavior circuit. Horm Behav. 2008;54:347–354. doi: 10.1016/j.yhbeh.2008.03.012. [DOI] [PubMed] [Google Scholar]
- [116].Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the femaleguinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- [117].Prossnitz ER, Maggiolini M. Mechanisms of estrogen signaling and gene expression via GPR30. Mol Cell Endocrinol. 2009;308:32–38. doi: 10.1016/j.mce.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Prossnitz ER, Oprea TI, Sklar LA, Arterburn JB. The ins and outs of GPR30: a transmembrane estrogen receptor. J Steroid Biochem Mol Biol. 2008;109:350–353. doi: 10.1016/j.jsbmb.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Quesada A, Lee BY, Micevych PE. PI3 kinase/Akt activation mediates estrogen and IGF-1 nigral DA neuronal neuroprotection against a unilateral rat model of Parkinson's disease. Dev Neurobiol. 2008;68:632–644. doi: 10.1002/dneu.20609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Quesada A, Micevych PE. Estrogen interacts with the IGF-1 system to protect nigrostriatal dopamine, and maintain motoric behavior after 6-hydroxdopamine lesions. J Neurosci Res. 2004;75:107–116. doi: 10.1002/jnr.10833. [DOI] [PubMed] [Google Scholar]
- [122].Redmond L, Kashani AH, Ghosh A. Calcium regulation of dendritic growth via CaM kinase IV and CREB-mediated transcription. Neuron. 2002;34:999–1010. doi: 10.1016/s0896-6273(02)00737-7. [DOI] [PubMed] [Google Scholar]
- [123].Romano N, Herbison AE. Activity-dependent modulation of GnRH neuron activity by acute estradiol. J Neuroendocrinol. 2012 doi: 10.1111/j.1365-2826.2012.02342.x. [DOI] [PubMed] [Google Scholar]
- [124].Romano N, Lee K, Abraham IM, Jasoni CL, Herbison AE. Nonclassical estrogen modulation of presynaptic GABA terminals modulates calcium dynamics in gonadotropin-releasing hormone neurons. Endocrinology. 2008;149:5335–5344. doi: 10.1210/en.2008-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Ronnekleiv OK, Bosch MA, Zhang C. Regulation of endogenous conductances in GnRH neurons by estrogens. Brain Res. 2010;1364:25–34. doi: 10.1016/j.brainres.2010.08.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Rudick CN, Woolley CS. Estrogen regulates functional inhibition of hippocampal CA1 pyramidal cells in the adult female rat. J Neurosci. 2001;21:6532–6543. doi: 10.1523/JNEUROSCI.21-17-06532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Saldanha CJ, Remage-Healey L, Schlinger BA. Synaptocrine signaling: steroid synthesis and action at the synapse. Endocr Rev. 32:532–549. doi: 10.1210/er.2011-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Sanchez AM, Flamini MI, Polak K, Palla G, Spina S, Mannella P, Genazzani AD, Simoncini T. Actin cytoskeleton remodelling by sex steroids in neurones. J Neuroendocrinol. 2012;24:195–201. doi: 10.1111/j.1365-2826.2011.02258.x. [DOI] [PubMed] [Google Scholar]
- [129].Sandilands E, Frame MC. Endosomal trafficking of Src tyrosine kinase. Trends Cell Biol. 2008;18:322–329. doi: 10.1016/j.tcb.2008.05.004. [DOI] [PubMed] [Google Scholar]
- [130].Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, MacLusky NJ. Hippocampal excitability increases during the estrous cycle in the rat: a potential role for brain-derived neurotrophic factor. J Neurosci. 2003;23:11641–11652. doi: 10.1523/JNEUROSCI.23-37-11641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Schlegel A, Wang C, Pestell RG, Lisanti MP. Ligand-independent activation of oestrogen receptor alpha by caveolin-1. Biochem J. 2001;359:203–210. doi: 10.1042/0264-6021:3590203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Schwartz N, Schohl A, Ruthazer ES. Neural activity regulates synaptic properties, and dendritic structure in vivo through calcineurin/NFAT signaling. Neuron. 2009;62:655–669. doi: 10.1016/j.neuron.2009.05.007. [DOI] [PubMed] [Google Scholar]
- [133].Sheldahl LC, Shapiro RA, Bryant DN, Koerner IP, Dorsa DM. Estrogen induces rapid translocation of estrogen receptor beta, but not estrogen receptor alpha, to the neuronal plasma membrane. Neuroscience. 2008;153:751–761. doi: 10.1016/j.neuroscience.2008.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Sinchak K, Dewing P, Cook M, Micevych P. Release of orphanin FQ/nociceptin in the medial preoptic nucleus and ventromedial nucleus of the hypothalamus facilitates lordosis. Horm Behav. 2007;51:406–412. doi: 10.1016/j.yhbeh.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Sinchak K, Garcia B, Bowlby R, Charukulvanich, Garcia M, Sanathara N. Mu-opioid receptor neurons and opioid receptor-like receptor neurons in the medial preoptic nucleus project to the region of the ventromedial nucleus of the hypothalamus. Society for Neuroscience Annual Meeting; San Diego, CA. 2010. [Google Scholar]
- [136].Sinchak K, Micevych PE. Progesterone blockade of estrogen activation of mu-opioid receptors regulates reproductive behavior. J Neurosci. 2001;21:5723–5729. doi: 10.1523/JNEUROSCI.21-15-05723.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Sinchak K, Mills RH, Eckersell CB, Micevych PE. Medial preoptic area delta-opioid receptors inhibit lordosis. Behav Brain Res. 2004;155:301–306. doi: 10.1016/j.bbr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- [138].Sinchak K, Shahedi K, Dewing P, Micevych P. Sexual receptivity is reduced in the female mu-opioid receptor knockout mouse. Neuroreport. 2005;16:1697–1700. doi: 10.1097/01.wnr.0000181585.49130.93. [DOI] [PubMed] [Google Scholar]
- [139].Skipper JK, Young LJ, Bergeron JM, Tetzlaff MT, Osborn CT, Crews D. Identification of an isoform of the estrogen receptor messenger RNA lacking exon four and present in the brain. Proc Natl Acad Sci U S A. 1993;90:7172–7175. doi: 10.1073/pnas.90.15.7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Smejkalova T, Woolley CS. Estradiol acutely potentiates hippocampal excitatory synaptic transmission through a presynaptic mechanism. J Neurosci. 2010;30:16137–16148. doi: 10.1523/JNEUROSCI.4161-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Smith CC, Vedder LC, McMahon LL. Estradiol and the relationship between dendritic spines, NR2B containing NMDA receptors, and the magnitude of long-term potentiation at hippocampal CA3-CA1 synapses. Psychoneuroendocrinology. 2009;34(Suppl 1):S130–142. doi: 10.1016/j.psyneuen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Sohrabji F, Miranda RC, Toran-Allerand CD. Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92:11110–11114. doi: 10.1073/pnas.92.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Song RX, Fan P, Yue W, Chen Y, Santen RJ. Role of receptor complexes in the extranuclear actions of estrogen receptor alpha in breast cancer. Endocr Relat Cancer. 2006;13(Suppl 1):S3–13. doi: 10.1677/erc.1.01322. [DOI] [PubMed] [Google Scholar]
- [144].Sribnick EA, Ray SK, Banik NL. Estrogen as a multi-active neuroprotective agent in traumatic injuries. Neurochem Res. 2004;29:2007–2014. doi: 10.1007/s11064-004-6874-0. [DOI] [PubMed] [Google Scholar]
- [145].Srivastava DP, Waters EM, Mermelstein PG, Kramar EA, Shors TJ, Liu F. Rapid estrogen signaling in the brain: implications for the fine-tuning of neuronal circuitry. J Neurosci. 2011;31:16056–16063. doi: 10.1523/JNEUROSCI.4097-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Srivastava DP, Woolfrey KM, Jones KA, Shum CY, Lash LL, Swanson GT, Penzes P. Rapid enhancement of two-step wiring plasticity by estrogen and NMDA receptor activity. Proc Natl Acad Sci U S A. 2008;105:14650–14655. doi: 10.1073/pnas.0801581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Staffend NA, Loftus CM, Meisel RL. Estradiol reduces dendritic spine density in the ventral striatum of female Syrian hamsters. Brain Struct Funct. 2011;215:187–194. doi: 10.1007/s00429-010-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J Med Chem. 2000;43:4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- [149].Taves MD, Schmidt KL, Ruhr IM, Kapusta K, Prior NH, Soma KK. Steroid concentrations in plasma, whole blood and brain: effects of saline perfusion to remove blood contamination from brain. PLoS One. 2010;5:e15727. doi: 10.1371/journal.pone.0015727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES, Jr., Nethrapalli IS, Tinnikov AA. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Toran-Allerand CD, Hashimoto K, Greenough WT, Saltarelli M. Sex steroids and the development of the newborn mouse hypothalamus and preoptic area in vitro: III. Effects of estrogen on dendritic differentiation. Brain Res. 1983;283:97–101. doi: 10.1016/0165-3806(83)90085-8. [DOI] [PubMed] [Google Scholar]
- [152].Torii M, Kubo K, Sasaki T. Influence of opioid peptides on the priming action of estrogen on lordosis in ovariectomized rats. Neurosci Lett. 1996;212:68–70. doi: 10.1016/0304-3940(96)12763-4. [DOI] [PubMed] [Google Scholar]
- [153].Torii M, Kubo K, Sasaki T. Naloxone and initial estrogen action to induce lordosis in ovariectomized rats: the effect of a cut between the septum and preoptic area. Neurosci Lett. 1995;195:167–170. doi: 10.1016/0304-3940(95)11809-b. [DOI] [PubMed] [Google Scholar]
- [154].Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, Svoboda K. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- [155].Tsui KH, Wang PH, Chen CK, Chen YJ, Chiou SH, Sung YJ, Li HY. Non-classical estrogen receptors action on human dermal fibroblasts. Taiwan J Obstet Gynecol. 2011;50:474–478. doi: 10.1016/j.tjog.2011.10.013. [DOI] [PubMed] [Google Scholar]
- [156].Vanderklish PW, Edelman GM. Dendritic spines elongate after stimulation of group 1 metabotropic glutamate receptors in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 2002;99:1639–1644. doi: 10.1073/pnas.032681099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Weickert CS, Miranda-Angulo AL, Wong J, Perlman WR, Ward SE, Radhakrishna V, Straub RE, Weinberger DR, Kleinman JE. Variants in the estrogen receptor alpha gene and its mRNA contribute to risk for schizophrenia. Hum Mol Genet. 2008;17:2293–2309. doi: 10.1093/hmg/ddn130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Weiland NG, Orikasa C, Hayashi S, McEwen BS. Distribution and hormone regulation of estrogen receptor immunoreactive cells in the hippocampus of male and female rats. J Comp Neurol. 1997;388:603–612. doi: 10.1002/(sici)1096-9861(19971201)388:4<603::aid-cne8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- [159].Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Wise PM, Dubal DB, Wilson ME, Rau SW. Estradiol is a neuroprotective factor in in vivo and in vitro models of brain injury. J Neurocytol. 2000;29:401–410. doi: 10.1023/a:1007169408561. [DOI] [PubMed] [Google Scholar]
- [161].Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Woolley CS, McEwen BS. Estradiol regulates hippocampal dendritic spine density via an N-methyl-D-aspartate receptor-dependent mechanism. J Neurosci. 1994;14:7680–7687. doi: 10.1523/JNEUROSCI.14-12-07680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- [164].Yang LC, Zhang QG, Zhou CF, Yang F, Zhang YD, Wang RM, Brann DW. Extranuclear estrogen receptors mediate the neuroprotective effects of estrogen in the rat hippocampus. PLoS One. 2010;5 doi: 10.1371/journal.pone.0009851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Yoshihara Y, De Roo M, Muller D. Dendritic spine formation and stabilization. Curr Opin Neurobiol. 2009;19:146–153. doi: 10.1016/j.conb.2009.05.013. [DOI] [PubMed] [Google Scholar]
- [166].Zhang J, Ferguson SS, Barak LS, Aber MJ, Giros B, Lefkowitz RJ, Caron MG. Molecular mechanisms of G protein-coupled receptor signaling: role of G protein-coupled receptor kinases and arrestins in receptor desensitization and resensitization. Receptors Channels. 1997;5:193–199. [PubMed] [Google Scholar]