Abstract
Organic compounds such as sterols and hormones have been detected in surface waters at ecologically relevant concentrations with sources including effluent discharged from publicly owned treatment works (POTWs) as well as leachate and runoff from land amended with municipal sludge (biosolids). Greater than 20% of regulated effluents discharged into U.S. surface waters experience in-stream dilution of <10 fold and potential impacts are particularly likely in receiving waters dominated by POTW effluents. The increasing use of biosolids on agricultural land exerts additional stress, thereby necessitating environmental monitoring for ecological and human health. Alternatively or in addition to monitoring efforts, screening for potentially hazardous chemicals can be performed using empirical models that are scalable and can deliver results rapidly. The present study makes use of data from U.S. EPA's Targeted National Sewage Sludge Survey (TNSSS) to predict the aqueous-phase concentrations and removal efficiencies of 10 sterols (campesterol, β-sitosterol, stigmasterol, β-stigmastanol, cholesterol, desmosterol, cholestanol, coprostanol, epicoprostanol, ergosterol) as well as the putative toxicity posed by four specific hormones based on their reported biosolids concentrations using published empirical models. Model predictions indicated that removal efficiencies for sterols are uniformly high (∼sim;99%) and closely match removal rates calculated from chemical monitoring at POTWs (paired t-test; p = 0.01). Results from toxicity modeling indicated that the hormones estrone, estradiol and estriol had the highest leaching potentials amongst the compounds considered here and that 17 β-ethinylestradiol was found to pose a potentially significant threat to fathead minnows (P. promelas) via run-off or leaching from biosolids-amended fields. This study exemplifies the use of in silico analysis to (i) identify potentially problematic organic compounds in biosolids, (ii) predict influent and effluent levels for hydrophobic organic compounds (HOCs) of emerging concern, and (iii) provide initial estimates of runoff concentrations, in this case four prominent hormones known to act as endocrine disruptors.
Keywords: Biosolids, sterols, hormones, endocrine disruption, modeling
1. Introduction
The National Stream Reconnaissance conducted during the years 1999 and 2000 by the U.S Geological Survey (USGS) served to identify and quantify a wide range of emerging contaminants in U.S surface waters (Kolpin et al., 2002). Follow-up work on the behavior of organic compounds (OCs) during wastewater treatment firmly established a range of theoretically biodegradable compounds that are only removed incompletely and remain detectable in both treated effluent and biosolids, i.e., treated sewage sludge deemed fit for application on land (Ingrand et al., 2003; Lorenzen et al., 2004; Ying and Kookana, 2005; Cicek et al., 2007). When treated effluent and biosolids are used for beneficial purposes such as irrigation and soil amendment, there exists a threat of releasing the contained chemicals into the environment (Xia et al., 2005; Kinney et al., 2006; Wu et al., 2010).
The behavior of organic compounds during wastewater treatment is difficult to characterize due to the diversity of chemical structures and the complexity of the processes involved. Within the publicly owned treatment works (POTWs), the influent gets segregated and transformed into two distinct process flows, organic-rich biosolids and the liquid effluent. Compounds undergo preferential partitioning between the aqueous phase and the sludge primarily based on their hydrophobicity, which typically is gauged by examining their n-octanol-water partition coefficient (KOW) (Kinney et al., 2006; Heidler and Halden, 2008). Whereas hydrophobic compounds having large KOW values (>10,000) partition almost completely into biosolids, compounds having lower KOW values (<10,000) typically are removed by a combination of biodegradation and sorption processes. Since one of these processes results in the transformation of a chemical's mass (biodegradation) and the other merely implies a transfer of chemicals from the aqueous phase into the sorbed, solid phase, it is important to distinguish among the two when evaluating the fate of a chemical during municipal wastewater treatment.
The risk of adverse effects from exposure to chemicals contained in wastewater effluent increases for aquatic biota in surface waters with decreasing instream dilution. Depending on the season, some streams can be partially or fully dominated by discharged POTW effluent (Brooks et al., 2003). Such a scenario occurs in perennial and ephemeral streams in arid and semi-arid regions around the world and especially the southwestern U.S. (Mladenov et al., 2005; Brooks et al., 2006). Aquatic ecosystems that are supported in part or completely by effluent flow are quickly gaining interest in ecotoxicology owing to their unique and challenging water quality characteristics (Taylor, 2002; Brooks et al., 2005). One class of compounds that have long been scrutinized with regards to their occurrence in surface waters are phytosterols. Phytosterols occur naturally in the environment and also are major constituents of pulp and paper mill effluents. Sterol mixtures are known to be capable of inducing sexual and morphological changes in aquatic organisms (Nakari et al., 2003; Honkanen et al., 2004; Lopez et al., 2011), and a large volume of literature shows that the products of microbially mediated breakdown of phytosterols, such as androstenedione and steroid hormones, possess endocrine disrupting properties at the ng/L range (Jenkins et al., 2003; Orrego et al., 2009).
A secondary objective of this paper was to also consider the potential for runoff and leaching of chemicals following application of biosolids on land for inexpensive disposal and for soil conditioning and fertilization. In the present study a validated empirical model leveraging mass balance approaches and partitioning theory (Deo and Halden, 2009) was used to model the aqueous concentrations of 10 sterols based on their reported biosolids concentrations. The model was originally introduced as a screening tool to identify potentially problematic sewage constituents and to predict the behavior and extent of partitioning a given compound is likely to undergo during treatment in a real-world POTW (Heidler and Halden, 2008; Deo and Halden, 2010). The sole parameters required for operation of the model are the compound's concentration in biosolids (Cbiosolids), its pH-adjusted n-octanol/water partitioning coefficient (DOW) and a dimensionless curve-fitting parameter (pfit) (Deo and Halden, 2009; Weir et al., 2010). Thus far, the model has been applied only to compounds within a limited range of log DOW values of 4.9 – 6.4. The present study intended to expand the applicability of the model and to validate its performance using data available in the peer-reviewed literature.
There have been several lab-scale and field-scale studies conducted that evaluate the leaching potential of compounds from land-applied biosolids to surface waters and groundwater. Although largely confined to pharmaceuticals and personal care products (PPCPs), studies conducted by Lapen et al., (2008), Topp et al., (2008) and Edwards et al., (2009) confirmed that biosolids can act as a non-point sources of water contamination when sorbed compounds migrate from the site of application to tile drainage systems and eventually to surface waters. Key parameters that were identified to influence the transport of compounds from biosolids-amended fields were macroporous structures in the soil, soil texture, composition and moisture of the biosolids, and the method of biosolids application (e.g., surface spreading of solids vs. injection of the materials as a slurry). A recent field study confirmed that hormones in land-applied biosolids are mobilized following strong rainfall events and that the total estrogen concentration in runoff exceeded thresholds for biological effects for time periods of >30 days post application (Yang et al., 2012). The authors also stated that 35 days after application, biosolids-borne hormones were still detectable in the soil and did not complete degrade. Langdon et al. (2010) further utilized another model to estimate the toxicity posed by natural and synthetic hormones contained in biosolids using pore-water concentrations (Cporewater) as model input. This model adopted an equilibrium-based partitioning approach that required Cbiosolids and KOW as primary input parameters in addition to general parameters discussed hereafter. In this study, the DOW value was used in place of the tradition KOW value to correlate each hormone's pH-dependent hydrophobicity profile with its respective migration potential. Both models were combined to estimate the concentration in influent and effluent (Cinf and Ceff, respectively) for 10 sterols, and the run-off potential for four hormones that have been frequently reported in literature, estrone (E1), estradiol (E2), estriol (E3) and 17-β-ethinylestradiol (EE2).
2. Materials and Methods
Aqueous phase concentrations were calculated for 14 hydrophobic organic compounds (HOCs) based on their Cbiosolids and log DOW values using two independent models.
2.1 Empirical model I
The fraction of the total mass of a given compound entering a POTW (fbiosolids) was calculated using an established empirical model (Eq. 1) that previously had been validated for application in the log DOW range of 4.9 – 6.4 (Deo and Halden, 2010). Prior studies also had identified a value of 1.76 x 10-6 as appropriate for the dimensionless fitting parameter, pfit.
| (Eq. 1) |
Using mass-balance approaches, the concentration of a chemical in biosolids, Cbiosolids, can be determined as a function of the total concentration of the chemical entering the POTW, Cinfluent, the fraction amenable to sequestration in biosolids (fbiosolids) and the yield of biosolids per volume of raw wastewater treated (Y).
| (Eq. 2) |
The value of Y was taken from the literature, 2.4 × 10-4 kg/L (Kinney et al., 2006). Eq. 2 was rearranged as shown in Eq. 3 to yield influent concentrations.
| (Eq. 3) |
Weir et al. (2010) showed that the concentration of a given sorption-contolled compound leaving a POTW, Ceffluent, can be estimated with reasonable accuracy using Eq. (4) as follows:
| (Eq. 4) |
The modeled values are conservative estimates that represent the maximum concentration of sterols that can be expected to occur in the influent and effluent streams. The removal efficiency of individual compounds and the model's accuracy was validated using the most recent results obtained from a study conducted on the removal of sterols in POTWs in Canada (Furtula et al., 2011). Removal efficiencies (Predicted vs. Observed) were matched for each compound by a paired t-test at the confidence level of p = 0.01.
2.2 Empirical Model II
The leaching potential of hormones was predicted based on Cbiosolids values reported in the recent Targeted National Sewage Sludge Survey (TNSSS) conducted by the U.S. EPA (USEPA, 2009a). Model II was developed previously by another research group (Langdon et al., 2010). It was specifically used for analyzing hormones, since these compounds are not as hydrophobic as the sterols, and previous models had a restrictive use with regards to compounds of low hydrophobicity.
The concentration of a given hormone in homogenized mixtures of soil and biosolids was calculated according to Eq. 5
| (Eq. 5) |
, where Cbiosolids and Csoil represent the mass of biosolids and soil, respectively, that were mixed together during application. For moist soils, the partitioning of a given compound between the solid and aqueous phases was estimated using Eq. 6,
| (Eq. 6) |
, where Mb is the mass in μg of the compound associated with the solid phase at equilibrium, and Msoln in units of μg is the mass of the compound present in the dissolved state in the aqueous phase at equilibrium. Representative values for soil density, Ms, of 1.3 g cm3 and for soil moisture content, Vo, of 0.22% were taken from the literature (De Lannoy et al., 2006). The soil:biosolids mixing ratio was assumed to equal 25:1 based on recommendations by the U.S. EPA (McClellan and Halden, 2010).
The pore-water concentration of a given compound at equilibrium was calculated using Eq. 7,
| (Eq. 7) |
, where Cporewater is the concentration of a compound in saturated soil (μg/L) and Mo is the mass of compound in 1 cm3 of soil after equilibration (μg/1.3gm).
3. Results
3.1 Validation of empirical model I
The present study served to expand the applicability of an existing empirical model and validate its output by comparing the predicted versus actual removal efficiencies (expressed in %) for the sterol compounds shown in Table 1. A paired t-test was performed for nine of the ten sterols considered here; ergosterol could not be included because the dataset by Furtula et al, (2012) lacked information on this compound. Predicted values were found to match observed ones very closely, as indicated by a factor of 1.04 ± 0.04 that was very close to the ideal value of unity. Results from the t-test analysis confirmed the two datasets to be statistically indistinguishable at the 99% confidence interval.
Table 1.
List of sterols that were analyzed in this study. Cbiosolids (μg kg1) values were taken from the TNSSS 2009 reports (U.S. EPA, 2009) and compound structures, log Dow values at pH 7.5, and CAS numbers were taken from RSC online database and the literature (Ying et al., 2002). Half-life values reported are for aquatic environments and were estimated using the PBT Profiler of the U.S. EPA.
| Desmosterol |
|
Stigmasterol |
|
| log Dow: 9.27 | log Dow: 10.07 | ||
| CAS: 313-04-2 | CAS: 83-48-7 | ||
| Cbiosolids: 2230 (min) | Cbiosolids: 455 (min) | ||
| 806,000 (max) | |||
| 94,400 (max) | 120,671 (mean) | ||
| 14,816 (mean) | t1/2: 60 days | ||
| t1/2: 60 days |
|
||
|
β-Stigmastanol | ||
| Ergosterol | log Dow: 10.07 | ||
| log Dow: 9.3 | CAS: 83-45-4 | ||
| CAS: 57-87-4 | Cbiosolids: 3440 (min) | ||
| Cbiosolids: 2180(min) | 1,330,000 (max) | ||
| 152,834 (mean) | |||
| 91,900 (max) | t1/2: 60 days | ||
| 20,080 (mean) |
|
||
| t1/2: 60 days | β-Sitosterol | ||
|
log Dow: 10.73 | ||
| Campesterol | CAS: 83-46-5 | ||
| log Dow: 9.97 | Cbiosolids: 1210 (min) | ||
| CAS: 474-62-4 | 1,640,000 (max) | ||
| Cbiosolids: 2840(min) | 302,123 (mean) | ||
| t1/2: 60 days | |||
| 524,000 (max) |
|
||
| 97,298 (mean) | Estrone (E1) | ||
| t1/2: 60 days | log Dow: 3.62 | ||
|
CAS: 53-16-7 | ||
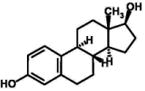
| Coprostanol | Cbiosolids: 19.70 (min) | ||
| log Dow: 10.06 | 965 (max) | ||
| CAS: 360-68-9 | 109.34 (mean) | ||
| Cbiosolids: 7720(min) | t1/2: 38 days | ||
|
|||
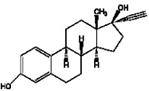
| 43,700,000 (max) | 17-β-Estradiol (E2) | ||
| 2,795,254 (mean) | log Dow: 4.15 | ||
| t1/2: 60 days | CAS: 50-28-2 | ||

|
Cbiosolids: 16.20 (min) | ||
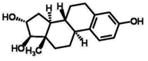
| Cholestanol | 355 (max) | ||
| log Dow: 10.07 | 35.57 (mean) | ||
| CAS: 80-97-7 | t1/2: 38 days | ||
| Cbiosolids: 3860(min) |
|
||
| Estriol (E3) | |||
| 4,590,000 (max) | log Dow: 2.53 | ||
| 473,067 (mean) | CAS: 50-27-1 | ||
| t1/2: 60 days | Cbiosolids: 5.80 (min) | ||
|
232 (max) | ||
| 38.85 (mean) | |||
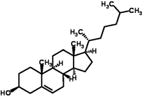
| Cholesterol | t1/2: 38 days | ||
| log Dow: 9.62 |
|
||
| CAS: 57-88-5 | 17-β-Ethinylestradiol(EE2) | ||
| Cbiosolids: 2,340(min) | |||
| log Dow: 4.15 | |||
| 5,390,000 (max) | CAS: 57-91-0 | ||
| 727,338 (mean) | Cbiosolids: 12.80 (min) | ||
| t1/2: 60 days | 61.90 (max) | ||
|
22.53 (mean) | ||
| t1/2: 38 days | |||
| Epicoprostanol |
|
||
| log Dow: 10.06 | Androstenedione | ||
| CAS: 516-92-7 | log Dow: 2.72 | ||
| Cbiosolids: 868 (min) | CAS: 63-05-8 | ||
| 6,030,000 (max) | Cbiosolids: 57.70 (min) | ||
| 818,673 (mean) | 1520 (max) | ||
| t1/2: 60 days | 328.44 (mean) | ||
| t1/2: 60 days |
3.2 Modeled Cinf and Ceff values
Modeling results for aqueous phase concentrations are shown in Fig. 1. For a comparative analysis, the concentrations were plotted as logarithmic equivalents of the obtained values. The top panel shows concentrations estimated for raw sewage entering the POTWs. The middle panel shows the model input values, i.e., the concentrations of analytes in biosolids on a dry weight basis. The bottom panel of Fig. 1 shows the range of effluent concentrations returned by the model. An examination of the top and bottom panels of Fig.1 and the information presented in Table 2 reveals that the predicted removal efficiencies for all sterols were uniformly high at 99.9% and thus were in good agreement with those (86.4 to 99.1%) determined experimentally by Furtula et al. (2011). Similarly, values reported by the U.S. EPA for Cinf and Ceff detected at POTWs (USEPA, 2009b) were of the same magnitude as those predicted here and depicted in Fig. 1.
Figure 1.
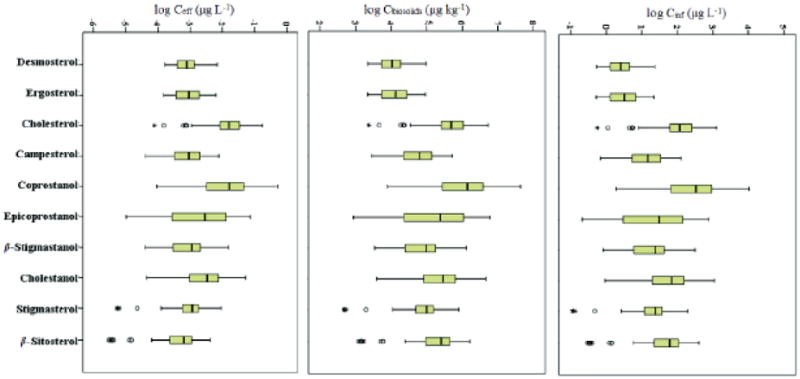
Predicted concentration ranges of 10 steroids, plotted on a logarithmic scale, in POTW influe (Cinf) and effluent (Ceff), calculated based on concentrations in biosolids (Cbiosolids) reported in the peer-reviewed literature. Values that exceed or fall below the upper and lower quartiles by 1.5x appear as outlier symbols marked with the asterisk symbol (*). The greatest and smallest values excluding the outliers are denoted by the whiskers, whereas the box is delineated by the upper quartile (top of box), lower quartile (bottom of box) and the median value (center line).
Table 2.
Modeled influent, effluent concentrations and removal efficiency for 10 sterols. Removals calculated at POTWs were obtained from Furtula et al. (2011).
| Compound | Concentration in influent (ng L-1) | Concentration in effluent (ng L-1) | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Cinfluent (Min) | Cinfluent (Max) | Cinfluent (Mean) | Ceff (Min) | Ceff (Max) | Ceff (Mean) | % Removal Predicted | % Removl Observed | |
| Desmosterol | 5.35E+02 | 2.27E+04 | 3.56E+03 | 0.163 | 6.9 | 1.1 | 99.9 | 93.1 |
| Ergosterol | 5.23E+02 | 2.21E+04 | 4.82E+03 | 0.149 | 6.3 | 1.4 | 99.9 | - |
| Cholesterol | 5.62E+02 | 1.29E+06 | 1.75E+05 | 0.077 | 176.3 | 23.8 | 99.9 | 98.7 |
| Campesterol | 6.82E+02 | 1.26E+05 | 2.34E+04 | 0.041 | 7.7 | 1.4 | 99.9 | 98 |
| Coprostanol | 1.85E+03 | 1.05E+07 | 6.71E+05 | 0.092 | 519 | 33.2 | 99.9 | 99.1 |
| Epicoprostanol | 2.08E+02 | 7.82E+05 | 1.06E+05 | 0.01 | 71.6 | 9.7 | 99.9 | 94.3 |
| β-Stigmastanol | 8.26E+02 | 3.19E+05 | 3.67E+04 | 0.04 | 15.4 | 1.8 | 99.9 | 96.7 |
| Cholestanol | 9.26E+02 | 1.10E+06 | 1.14E+05 | 0.045 | 53.3 | 5.5 | 99.9 | 98.7 |
| Stigmasterol | 1.09E+02 | 1.93E+05 | 2.90E+04 | 0.005 | 9.4 | 1.4 | 99.9 | 86.4 |
| β-Sitosterol | 2.90E+02 | 3.94E+05 | 7.25E+04 | 0.003 | 4.2 | 0.8 | 99.9 | 97.7 |
3.3 Modeled Cporewater values
Porewater concentrations (shown in Table 3) indicate the migration potential of compounds applied on soils in biosolids. Chemicals present in porewater are available for leaching into ground and surface water during rainfall events. Modeled Cporewater values for hormones, shown in Table 3, were in the parts-per-trillion range, owing to the low Cbiosolids values.
Table 3.
Modeled porewater concentrations for four hormones.
| Cporewater(ng L-1) | Cporewater(ng L-1) | Cporewater(ng L-1) | Aquatic toxicity(ng L-1) | |
|---|---|---|---|---|
| Compound | Minimum | Maximum | Mean | |
| Estrone (E1) | 0.00 | 43.47 | 4.57 | 10d, 15a |
| 17-β-Estradiol (E2) | 0.38 | 8.23 | 0.82 | 5b |
| Estriol (E3) | 0.00 | 40.91 | 6.52 | 10d |
| 17-α-Ethinylestradiol (EE2) | 0.00 | 0.70 | 0.55 | 0.32c |
| Androstenedione* | 0.00 | 211.30 | 42.40 | - |
Androstenedione is an androgen that was selected for modeling purposes only.
Increased aggressiveness of fathead minnows (P. promelas) as seen during a 21-day study by Dammann et al. (2011).
Caused vitellogenin induction in adult male Zebra fish (D. rerio) as reported by Brion et al. (2004).
Decreased male sex characteristics and reduced egg fertilization success observed in adult male P. promelas were reported by Parrott and Blunt (2005).
Altered sex ratio seen in Japanese Medaka fish (O. latipes) as reported by Metcalfe et al. (2001).
In order to assess the hazard posed to aquatic organisms by the flushable mass fraction of compounds present in the aqueous phase (i.e., porewater), toxicity values taken from the literature were plotted alongside modeled values. As can be seen in Figure 2, the expected range of porewater concentrations of all 4 analytes considered bracketed the toxicity values for sensitive aquatic species including fathead minnows (P. promelas) (Dammann et al. 2011), Zebra fish (D. rerio) (Brion et al. 2004) and Japanese Medaka fish (O. latipes) (Metcalfe et al. 2001). Additional information on calculated minima, means and maxima is presented in Table 3 along with specific information on observed adverse outcomes (Table 3, footnotes).
Figure 2.
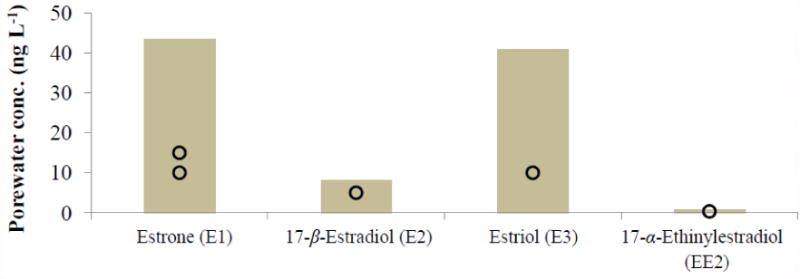
Predicted Cporewater range based on Cbiosolids values for 4 hormones. Circles represent aquatic toxicity threshold values reported in the literature.
4. Discussion
4.1 Sterols
As shown in Table 2, the predicted removal efficiencies for all sterols were >99% which resulted in the extremely low concentrations in the effluent (Figure 1). These predictions matched closely the empirical removal efficiencies observed at treatment plants, as reported by Furtula et al., (2012). Of the compounds that were analyzed, four were grouped as phytosterols (campesterol, β-sitosterol, stigmasterol and β-stigmastanol), five were associated with cholesterol as either precursors or breakdown products (cholesterol, desmosterol, cholestanol, coprostanol and epicoprostanol), and one was a fungal cell-wall component (ergosterol).
Phytosterols are naturally occurring compounds that are known to be present in high concentrations in paper mill effluents and have been detected at the parts-per-billion range downstream of rivers receiving effluent from mills. These compounds have been linked to androgenic effects in aquatic organisms caused either directly or as breakdown products of the parental sterols (Ellis et al., 2003; Jenkins et al., 2003; Orrego et al., 2009). Similar to phytosterols, cholesterol and related compounds have long been suspected of causing androgenic effects or giving rise to compounds that have androgenic effects (Ellis et al., 2003; Jenkins et al., 2003). More specifically, androstenedione, a microbial transformation product of phytosterols and cholesterol, is a known androgen (Jenkins et al., 2004). In the present study, androstenedione (not presented in Fig. 2) was found to have highest runoff potential among the hormones analyzed, owing to its low hydrophobicity (log DOW = 2.72) (See Table 1, Table 3). The Cporewater value calculated for androstenedione was 211 ng L-1 and nearly five times greater than that of the hormone estrone (E1), which had the highest migration potential amongst the four hormones analyzed. Considering that both phytosterols and cholesterol related compounds were detected in mg kg−1 range in biosolids, the concentration of androstenedione that could migrate/leach into the aqueous phase could potentially be higher than the values reported here. EPA's PBT Profiler software suggests aquatic half-life values for the sterols considered here on the order of 60 days (Table 1).
4.2 Hormones
A recent study ranked hormones among the most studied compounds with regards to their presence in the environment, and the compounds estrone (E1) and 17-β-estradiol (E2) being the most thoroughly investigated (Miège et al., 2009). The list of hormones analyzed in this study comprises both naturally occurring (E1, E2, E3) and synthetic (EE2) ones. While E1, E2 and E3 are excreted by humans naturally, EE2 is excreted only by women taking contraceptive pills. All of these hormones have been detected in the natural environment in the part-per-trillion range and above, especially in surface waters and all are known to cause a variety of secondary effects in aquatic organisms, including reversal of sex-ratios and delayed fertilization (Brion et al., 2004; Parrott and Blunt, 2004; Schäfers et al., 2007; Dammann et al., 2011).
While adequate details on the presence of E3 in the environment could not be gathered, the hazard posed by the remaining four hormones was assessed. The range of Cporewater predicted for E1, E2, E3 and EE2 was noted to encompass the values at which these compounds exert adverse effects on fathead minnows and zebra fish (see Table 3 and Figure 2). EE2 was found to have the smallest Lowest Observed Effect Concentration (LOEC) value amongst the hormones and was determined to possess the highest toxicity, whereas E1 was found to have the highest leaching potential, due to its relatively high concentration in biosolids and comparatively low hydrophobicity (log DOW 3.62 at pH 7.5). Yang et al. (2012) suggested that both increased rainfall and increased hormone load applied in biosolids on land can trigger elevated runoff concentrations. The mass of hormones contained in runoff and leachate also is expected to vary based on the temporal precipitation profile, with intermittent rains potentially leading to the largest concentrations released due to the extended equilibration time. However, the present model does not take into consideration variations in rainfall and assumes constant soil moisture content.
4.3 Environmental implications
The present study served to predict influent and effluent concentration ranges for 10 sterols and investigate the implications of four hormones detected in biosolids samples as reported in the 2009 TNSSS study. The results presented in this study are representative of effluent discharges that receive little or no dilution via surface waters, and the here presented empirical model serves to function as a screening tool that can be used to calculate the theoretical removal rates and the aqueous phase concentrations based on reported Cbiosolids values for compounds with log DOW values in the range of 4.9 to 10 at pH 7.5, i.e., in conditions typical of POTWs (Deo and Halden, 2009).
Also calculated in this study were the migration potentials of four hormones included in the TNSSS. Modeled Cporewater values indicated that the migration potential of the hormones were in the descending order of E1>E3>E2>EE2; EE2 was found to have a problematic hazard potential since the compound had the smallest LOEC value. The run-off predictions presented here and the order of migration potentials were found to agree with those presented by Yang et al. (2012). In conjunction with these results, a medaka assay conducted by Metcalfe et al. (2001) revealed the relative estrogenic potentials of these four hormones were EE2>E2≈ E1>E3. Conventional POTWs are faced with the challenge of treating a continuous load of organic compounds, including phytosterols and hormones that possess endocrine disrupting properties. Findings from this study suggest that although sterols are removed from wastewaters at high efficiencies they tend to accumulate in biosolids, where they may be subject to microbial conversion to metabolites that possess relatively greater endocrine disrupting potential than the parent compounds. Compounds sequestered in biosolids and subsequently applied on land potentially can migrate into groundwater and surface waters and thus can pose a significant hazard to aquatic organisms, as shown by the results of this study. However, it is important to note that the mass fraction of compounds present in porewater is very small when compared to the amount of chemical sorbed onto particles, and that equilibrium concentrations present in porewater will be diluted significantly during rainfall events. Thus, a hazard assessment based on equilibrium porewater concentrations has to be interpreted as a worst-case scenario. In reality, flushed porwater volumes will be diluted significantly by rainwater, thereby lowering due to dilution the risk of harmful exposures. Results of this modeling study suggest that although hydrophobic compounds readily partition into biosolids, they also are expected to be bioavailable at levels sufficiently high to cause endocrine disruption in sensitive aquatic species upon leaching from field soils amended with biosolids.
5. Conclusions
The expansion, validation and application of mathematical modeling performed in this work provided new estimates on the concentration range of ten sterols and four hormones in environmental compartments. A hazard assessment revealed that the hormones E1, E2 and E3 feature high migratory potentials in soil, with E1 and E3 projected to yield the highest porewater concentrations, and EE2 posing a potential threat to fathead minnows (P. promelas). This work underscored the utility of modeling for assessing the potential impact of endocrine disrupting compounds sequestered in biosolids during wastewater treatment.
Highlights.
We model the concentrations of endocrine disruptors in raw and treated wastewater
We obtain additional info on levels of endocrine disruptors leaching from soils
We estimate the risk associated with hormone release to the environment
We show how modeling can inform environmental monitoring needs
Acknowledgments
The authors would like to thank Arjun K. Venkatesan and Kristin McClellan of The Biodesign Institute for their assistance and feedback. This project was supported in part by Award Number R01ES015445 from the National Institute of Environmental Health Sciences (NIEHS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS or the National Institutes of Health (NIH).
Abbreviations
- POTW
POTW, Publicly owned treatment work
- EPA
Environmental Protection Agency
- HOCs
Hydrophobic Organic Compounds
- OCs
Organic Compounds
- TNSSS
Targeted National Sewage Sludge Survey
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brion F, Tyler CR, Palazzi X, et al. Impacts of 17h-estradiol, including environmentally relevant concentrations, on reproduction after exposure during embryolarval-Juvenile- and adult-life stages in zebrafish (Danio rerio) Aquat Toxicol. 2004;68:193–217. doi: 10.1016/j.aquatox.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Brooks BW, Chambliss CK, Stanley JK, et al. Determination of select antidepressants in fish from an effluent-dominated stream. Environ Toxicol Chem. 2005;24:464–9. doi: 10.1897/04-081r.1. [DOI] [PubMed] [Google Scholar]
- Brooks BW, Foran CM, Richards SM, et al. Aquatic ecotoxicology of fluoxetine. Toxicol Lett. 2003;142:169–83. doi: 10.1016/s0378-4274(03)00066-3. [DOI] [PubMed] [Google Scholar]
- Brooks WB, Riley TM, Taylor RD. Water quality of effluent-dominated ecosystems: ecotoxicological, hydrological, and management considerations. Hydrobiologia. 2006;556:365–79. [Google Scholar]
- Cicek N, Londry K, Oleszkiewicz JA, et al. Removal of Selected Natural and Synthetic Estrogenic Compounds in a Canadian Full-Scale Municipal Wastewater Treatment Plant. Water Environ Res. 2007;79:795–800. doi: 10.2175/106143007x175744. [DOI] [PubMed] [Google Scholar]
- Dammann AA, Shappell NW, Bartell SE, Schoenfuss HL. Comparing biological effects and potencies of estrone and 17β-estradiol in mature fathead minnows, Pimephales promelas. Aqua Toxic. 2011;105:559–568. doi: 10.1016/j.aquatox.2011.08.011. [DOI] [PubMed] [Google Scholar]
- De Lannoy GJM, Verhoest NEC, Houser PR, et al. Spatial and temporal characteristics of soil moisture in an intensively monitored agricultural field (OPE3) Journal of Hydrology. 2006;331:719–730. [Google Scholar]
- Deo RP, Halden RU. Empirical model for predicting concentrations of refractory hydrophobic organic compounds in digested sludge. Environ Chem. 2009;6:544–550. doi: 10.1071/EN09063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deo RP, Halden RU. In silico screening for unmonitored, potentially problematic high production volume (HPV) chemicals prone to sequestration in biosolids. J Environ Monit. 2010;12:1840–1845. doi: 10.1039/c001559h. [DOI] [PubMed] [Google Scholar]
- Edwards M, Topp E, Metcalfe CD, et al. Pharmaceutical and personal care products in tile drainage following surface spreading and injection of dewatered municipal biosolids to an agricultural field. Sci Tot Environ. 2009;407:4220–30. doi: 10.1016/j.scitotenv.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Van Den Heuvel MR, Bandelj E, et al. In vivo and in vitro assessment of the androgenic potential of a pulp and paper mill effluent. Environ Toxicol Chem. 2003;22:1448–1456. [PubMed] [Google Scholar]
- Furtula V, Liu J, Chambers P, et al. Sewage treatment plants efficiencies in removal of sterols and sterol ratios as indicators of fecal contamination sources. Water Air Soil Pollut. 2011 accepted for publication August 2011. [Google Scholar]
- Heidler J, Halden RU. Meta-analysis of mass balances examining chemical fate during wastewater treatment. Environ Sci Tech. 2008;42:6324–32. doi: 10.1021/es703008y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkanen JO, Kostamo A, Kukkonen VK. Toxicity of a Phytosterol Mixture to Grayling (Thymallus thymallus) during Early Developmental Stages. Arch Environ Contam Toxicol. 2005;48:391–396. doi: 10.1007/s00244-003-9238-x. [DOI] [PubMed] [Google Scholar]
- Ingrand V, Herry G, Beausse J, de Roubin M. Analysis of steroid hormones in effluents of wastewater treatment plants by liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2003;1020:99–104. doi: 10.1016/s0021-9673(03)00770-2. [DOI] [PubMed] [Google Scholar]
- Jenkins RL, Wilson EM, Angus RA, et al. Androstenedione and progesterone in the sediment of a river receiving paper mill effluent. Toxicol Sci. 2003;73:53–9. doi: 10.1093/toxsci/kfg042. [DOI] [PubMed] [Google Scholar]
- Jenkins RL, Wilson EM, Angus RA, et al. Production of Androgens by Microbial Transformation of Progesterone in Vitro: A Model for Androgen Production in Rivers Receiving Paper Mill Effluent. Environ Health Perspect. 2004;112:1508–1511. doi: 10.1289/ehp.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney C, Furlong E, Zaugg S, et al. Survey of organic wastewater contaminants in biosolids destined for land application. ES&T. 2006;40:7207–15. doi: 10.1021/es0603406. [DOI] [PubMed] [Google Scholar]
- Kolpin DW, Furlong ET, Meyer MT, Thurman, et al. Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000: a national reconnaissance. Environ Sci Technol. 2002;36:1202–1211. doi: 10.1021/es011055j. [DOI] [PubMed] [Google Scholar]
- Langdon KA, Warne MStJ, Kookana RS. Aquatic hazard assessment for pharmaceuticals, personal care products and endocrine disrupting compounds from biosolidsamended land. Integr Environ Assess Manage. 2010;6:663–76. doi: 10.1002/ieam.74. [DOI] [PubMed] [Google Scholar]
- Lapen DR, Topp E, Metcalfe CD, et al. Pharmaceutical and personal care products in tile drainage following land application of municipal biosolids. Sci Total Environ. 2008;399:50–65. doi: 10.1016/j.scitotenv.2008.02.025. [DOI] [PubMed] [Google Scholar]
- LÓpez D, Chamorro S, Silva J, Bay-Schmith E, Vidal G. Chronic Effects of Pinus radiata and Eucalyptus globulus Kraft Mill Effluents and Phytosterols on Daphnia magna. Bull Environ Contam Toxicol. 2011;87:633–637. doi: 10.1007/s00128-011-0409-6. [DOI] [PubMed] [Google Scholar]
- Lorenzen A, Hendel JG, Conn KL, et al. Survey of hormone activities in municipal biosolids and animal manures. Environ Toxicol. 2004;19:216–25. doi: 10.1002/tox.20014. [DOI] [PubMed] [Google Scholar]
- Metcalfe C, Metcalfe T, Kiparissis Y, Koenig B. Estrogenic potency of chemicals detected in sewage treatment plant effluents as determined by in vivo assays with Japanese medaka (Oryzias latipes) Environ Toxicol Chem. 2001;20:297–308. [PubMed] [Google Scholar]
- Miege C, Choubert JM, Ribeiro L, et al. Fate of pharmaceuticals and personal care products in wastewater treatment plants — conception of a database and first results. Environ Pollut. 2009;157:1721–6. doi: 10.1016/j.envpol.2008.11.045. [DOI] [PubMed] [Google Scholar]
- Mladenov N, Strzepek K, Serumola OM. Water quality assessment and modeling of an effluent-dominated stream, the Notwane River, Botswana. Environ Monit Assess. 2005;109(1–3):97–121. doi: 10.1007/s10661-005-5842-8. [DOI] [PubMed] [Google Scholar]
- Nakari T, Erkomaa K. Effects of phytosterols on zebrafish reproduction in multigeneration test. Environ Poll. 2003;123:267–273. doi: 10.1016/s0269-7491(02)00377-9. [DOI] [PubMed] [Google Scholar]
- Orrego R, Guchardi J, Hernandez V, et al. Pulp and paper mill effluent treatments have differential endocrine disrupting effects on rainbow trout. Environ Toxicol Chem. 2009;28:181–8. doi: 10.1897/08-191.1. [DOI] [PubMed] [Google Scholar]
- Parrott JL, Blunt BR. Life-cycle exposure of fathead minnows (Pimephales promelas) to an ethinylestradiol concentration below 1 ng/L reduces egg fertilization success and demasculinizes males. Environ Toxicol. 2005;20:131–41. doi: 10.1002/tox.20087. [DOI] [PubMed] [Google Scholar]
- Samir KK, Xie B, Thompson ML, Sung, et al. Fate, transport, and biodegradation of natural estrogens in the environment and engineered systems. Environ Sci Technol. 2006;40:6537–46. doi: 10.1021/es0607739. [DOI] [PubMed] [Google Scholar]
- Schäfers C, Teigeler M, Wenzel A, et al. Concentration- and time-dependent effects of the synthetic estrogen, 17 alpha-ethinylestradiol, on reproductive capabilities of the zebrafish, Danio rerio. J Toxicol Environ Health Part Curr Issues. 2007;70:768–79. doi: 10.1080/15287390701236470. [DOI] [PubMed] [Google Scholar]
- Taylor RD. Ph D Dissertation. University of North Texas, Denton, TX: 2002. Water Quality Aspects of an Intermittent Stream and Backwaters in an Urban North Texas Watershed. [Google Scholar]
- Topp E, Monteiro SC, Beck A, et al. Runoff of pharmaceuticals and personal care products following application of biosolids to an agricultural field. Sci Tot Environ. 2008;396:52–9. doi: 10.1016/j.scitotenv.2008.02.011. [DOI] [PubMed] [Google Scholar]
- USEPA. Office of Water. Washington, DC, U.S.: Environmental Protection Agency; 2009b. Occurrence of contaminants of emerging concern in wastewater from nine publicly owned treatment works. [Google Scholar]
- USEPA. EPA-822-R-08-018. Washington, DC: Office of Water, U.S. Environmental Protection Agency; 2009a. Targeted National Sewage Sludge Survey Statistical Analysis Report. [Google Scholar]
- Weir A, Moiles WE, Brockman B, et al. In: Concentrations of Hydrophobic Organic Pollutants in U S Wastewater Treatment Plants and in Receiving Surface Waters Modeled from EPA Biosolids Monitoring Data. Halden RU, editor. Oxford University Press; New York, NY: pp. 421–436. (ACS Book Series Vol 1048 Contaminants of Emerging Concern: Ecotoxicological and Human Health Considerations. American Chemical Society (ACS) Book Series). ISBN13: 9780841224964; eISBN: 9780841224971. [DOI] [Google Scholar]
- Wu C, Spongberg AL, Witter JD, et al. Dissipation and leaching potential of selected pharmaceuticall active compounds in soils amended with biosolids. Arch Environ Contam Toxicol. 2010;59:343–51. doi: 10.1007/s00244-010-9500-y. [DOI] [PubMed] [Google Scholar]
- Xia K, Bhandari A, Das K, Pillar G. Occurrence and fate of pharmaceuticals and personal care produc (PPCPs) in biosolids. J Environ Qual. 2005;34:91–104. doi: 10.2134/jeq2005.0091. [DOI] [PubMed] [Google Scholar]
- Yang YY, Gray JL, Furlong ET, et al. Steroid Hormone Runoff from Agricultural Test Plots Applied with Municipal Biosolids. Environ Sci Technol. 2012 Jan 30; doi: 10.1021/es203896t. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Ying GG, Kookana RS. Sorption and degradation of estrogen-like-endocrine disrupting chemicals in soil. Environ Toxicol Chem. 2005;24:2640–5. doi: 10.1897/05-074r.1. [DOI] [PubMed] [Google Scholar]


