Abstract
Recently, the R-spondin (RSPO) family of proteins has emerged as important regulators of WNT signaling. Considering the wide spectrum of WNT signaling functions in normal biological processes and disease conditions, there has been a significantly growing interest in understanding the functional roles of RSPOs in multiple biological processes and determining the molecular mechanisms by which RSPOs regulate the WNT signaling pathway. Recent advances in the RSPO research field revealed some of the in vivo functions of RSPOs and provided new information regarding the mechanistic roles of RSPO activity in regulation of WNT signaling. Herein, we review recent progress in RSPO research with an emphasis on signaling mechanisms and biological functions.
1. Introduction
Since hPWTSR (later renamed hRSPO3), the first member of the Rspo gene family, was identified in a high throughput sequencing study of human fetal brain cDNA library (Chen et al., 2002), other Rspo genes from different species were subsequently discovered (Kamata et al., 2004, Kazanskaya et al., 2004, Nam et al., 2006). The formal name of the Rspo (roof plate-specific spondin) gene was adopted from the mouse Rspo1 gene that is expressed in the roof plate of the neural tube of developing embryos (Kamata et al., 2004). In mammals, the RSPO protein family consists of four members, namely RSPO1 through RSPO4. They share approximately 40–60% amino acid sequence identity and substantial structural homologies within human and mouse members (Kazanskaya et al., 2004, Nam et al., 2006, Kim et al., 2006). A major breakthrough in RSPO research occurred when Rspo2 was identified by a functional screening study in Xenopus as a gene encoding a novel canonical WNT/β-catenin signaling activator (Kazanskaya et al., 2004). Later studies further confirmed that the other RSPO proteins from different species have a similar capacity to activate WNT/β-catenin signaling (Kim et al., 2006, Nam et al., 2006, Binnerts et al., 2007, Wei et al., 2007, Kim et al., 2008). Gene disruption studies in mice and genetic studies in humans began to uncover the in vivo functions of the Rspo genes in a wide array of developmental and physiological processes (Aoki et al., 2008, Aoki et al., 2007, Parma et al., 2006, Tomaselli et al., 2008, Blaydon et al., 2006, Tomizuka et al., 2008, Nam et al., 2007a, Chassot et al., 2008). During the last few years, there have been significant advances in identifying multiple RSPO receptors and understanding the regulatory function of RSPO in the WNT signaling pathway. In this review, we summarize recent findings in RSPO signaling and biological functions.
2. Structural and biochemical properties of the RSPO proteins
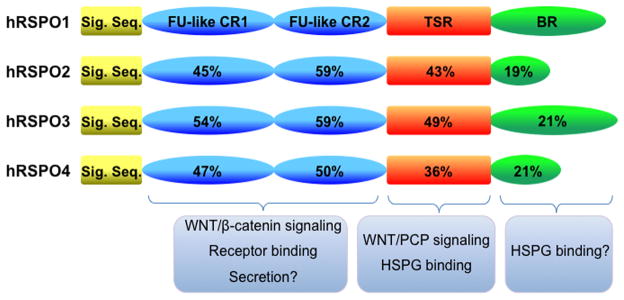
The human RSPO1-RSPO4 proteins range from 234 to 272 amino acids (aa) in length and feature the following: (i) a hydrophobic, putative signal peptide sequence at the N-terminus for secretion; (ii) two adjacent cysteine-rich furin-like (CR) domains (92–94 aa in length); (iii) a thrombospondin type I repeat (TSR) domain (55–57aa in length); and (iv) a basic amino acid-rich (BR) domain with varying length at the C-terminus (Figure 1). There are significant sequence similarities detected within the CR and TSR domains of the four hRSPO members, which are also apparent in the RSPO members of other mammalian species, thereby suggesting their conserved protein functions (Kazanskaya et al., 2004, Nam et al., 2006, Kim et al., 2008).
Figure 1. The human RSPO family of proteins.
Schematic representation is shown for four human RSPO proteins. The RSPO1, 2, 3 and 4 proteins are 263, 243, 292 and 234 amino acids in length, respectively. Four different domains are illustrated: signal sequence, two cysteine-rich furin-like repeats (FU-like CR), a single thrombospondin type1 repeat (TSR) domain, and a basic amino-acid-rich (BR) domain with variable lengths. The percentages of identical amino acids within these domains show relative protein sequence conservation. The known and suggested functions of each domain are described.
Consistent with the presence of signal sequences at the N-terminus, the RSPO proteins are secreted proteins (Nam et al., 2006, Kazanskaya et al., 2004). The subcellular localization of the RSPO proteins in the endoplasmic reticulum and the Golgi apparatus indicates that RSPOs are processed through the canonical secretory pathway (Nam et al., 2006). Generally, poor detection of the RSPO proteins in conditioned medium obtained from cell culture in which the RSPO proteins are ectopically expressed suggests that secretion of the RSPO proteins is tightly regulated or that the RSPO proteins can bind to the cell surface and extracellular matrix (Kazanskaya et al., 2004, Nam et al., 2006). Interestingly, substitutions of cysteine residues 78 and 113 with tyrosine and arginine within the CR domain of the mouse RSPO2 protein lead to diminished secretion of the mutant RSPO2 protein, suggesting that the CR domain is involved in the regulation of RSPO secretion (Li et al., 2009).
Because the TSR domain of the thrombospondin protein engages in binding to heparin sulfate proteoglycans (HSPGs) (Chen et al., 1996), it is predicted that HSPGs affect cell surface binding of RSPO. Indeed, the addition of soluble heparin (a sugar moiety similar to the one found on HSPGs) or sodium chlorate (an inhibitor of sulfatases mediating sulfation of HSPGs) to the medium during culture of cells overexpressing Rspos significantly enhanced the detection of secreted RSPO proteins in the conditioned medium (Nam et al., 2006), suggesting that secreted RSPOs are associated with HSPGs on the cell surface. Furthermore, deletion of the TSR domain from RSPOs resulted in an increase of soluble RSPO proteins in conditioned medium (Nam et al., 2006). Recently, the TSR domain of RSPO3 was found to bind syndecans and glypicans, thereby confirming a direct interaction between the RSPO proteins and HSPGs (Ohkawara et al., 2011). RSPO2 and RSPO3 proteins bind syndecans with high affinity via their TSR domain (e.g., a Kd of RSPO3 binding to syndecan4 is 0.88 nM). Interestingly, the RSPO1 and RSPO4 proteins do not show any significant interaction with syndecans despite their high amino acid sequence homology to that of RSPO2 and RSPO3 within the TSR domain (Ohkawara et al., 2011). Alternatively spliced isoforms of human RSPO1 lacking the TSR domain and human RSPO4 lacking both the TSR and BR domains have been identified from the NCBI protein database. Although the precise functions of these isoforms are unknown, they may exist as more diffusible forms of RSPOs.
3. Regulation of WNT signaling activation by RSPOs
3. 1. Overview of the WNT signaling pathway
WNT signaling regulates cell proliferation, cell survival, cell polarity, and cell fate determination during embryonic development and tissue homeostasis. Aberrant regulation of WNT signaling often results in pathological conditions including birth defects, cancer, and other diseases in humans (Grigoryan et al., 2008, Clevers, 2006). The WNT ligands activate two major intracellular pathways known as the canonical (or β-catenin-dependent) and non-canonical (β-catenin-independent) pathways (He et al., 2004, Kohn and Moon, 2005, Angers and Moon, 2009). In the canonical WNT pathway, two cell membrane proteins, Frizzled (FZD) and LDL-receptor related protein 5/6 (LRP5/6), function together as receptors for the WNT ligands. In both humans and mice, the FZD receptor family has ten members that belong to the seven-pass transmembrane G-protein coupled receptor superfamily (Schulte and Bryja, 2007). The LRP5/6 receptors are single-pass transmembrane proteins with an extracellular portion containing four EGF-repeats (He et al., 2004). WNT ligand binding to both FZD and LRP5/6 results in ternary complex formation and induces cytoplasmic accumulation and subsequent nuclear translocation of the β-catenin protein. In the nucleus, β-catenin forms a complex with TCF/LEF transcription factors that regulate target gene transcription (He et al., 2004, Angers and Moon, 2009).
In the non-canonical WNT pathway, the WNT signal is transduced via the FZD receptors independently from LRP5/6, activating two downstream signaling branches (Angers and Moon, 2009, Kohn and Moon, 2005). WNT induces cytoskeletal changes via activation of the small GTPases RHOA and RAC1 in the regulation of cell polarity, a pathway called the WNT/planar cell polarity (PCP) pathway. WNT also modulates cell adhesion and motility and transcription by nuclear factor of activated T-cells (NFAT) via activation of the heterotrimeric G-proteins, Ca++/calmodulin-dependent kinase II, and protein kinase C cascade in the WNT/Ca++ pathway.
Endocytosis of the LRP and FZD receptors through different plasma membrane compartments after WNT ligand binding is essential for WNT signaling and its specificity (Kikuchi et al., 2009). After stimulation with WNT3A, LRP6 is phosphorylated and internalized into a lipid raft-dependent caveolin-positive vesicular compartment, where it stabilizes β-catenin and activates downstream events (Bilic et al., 2007, Yamamoto et al., 2006). LRP6 internalization and LRP6 phosphorylation are not dependent upon each other, yet both are required for signal propagation (Yamamoto et al., 2008). In contrast, the FZD receptors (e.g., FZD5) undergo clathrin-dependent endocytosis upon WNT ligand stimulation (Yamamoto et al., 2006). Interestingly, with coexpression of the LRP6 and FZD5 receptors, the tertiary complex formed by WNT3A, LRP6, and FZD5 is internalized in a caveolin-dependent and clathrin-independent manner (Yamamoto et al., 2006). However, mice lacking both the caveolin1 and caveolin3 genes in which no caveolin expression was detected in most tissues did not show an overt disruption or modification of canonical WNT signaling (Park et al., 2002), thus the precise role of caveolin-mediated receptor endocytosis in canonical WNT signaling remains unclear.
3. 2. RSPO in canonical WNT/β-catenin signaling
The functional connection of RSPOs to the WNT signaling pathway was established by the discovery of Rspo2 in screening for canonical WNT signaling activators in Xenopus (Kazanskaya et al., 2004). Subsequently, several studies have confirmed that RSPOs from different vertebrate species also possess properties of canonical WNT signaling activators (Kim et al., 2006, Nam et al., 2006, Binnerts et al., 2007, Wei et al., 2007, Kim et al., 2008). The CR domain of the RSPO proteins is primarily responsible for activation of WNT/β-catenin signaling (Figure 1). Deletion of either one of two furin-like motifs within the CR domain of RSPO abolishes its ability to activate canonical WNT signaling (Kazanskaya et al., 2004, Nam et al., 2006, Kim et al., 2008). The Q70R mutation in the CR domains of RSPO2 dramatically reduced the WNT signaling activity of RSPO2 without affecting its secretion (Li et al., 2009). The TSR and BR domains are also presumed to regulate the strength of RSPO activity on canonical WNT signaling because the RSPO proteins lacking the TSR and BR domains activate canonical WNT signaling less effectively (Kim et al., 2008). Interestingly, the RSPO proteins show strong synergistic or potentiating activity in canonical WNT signaling activated by WNT ligands (Kazanskaya et al., 2004, Nam et al., 2006, Binnerts et al., 2007, Wei et al., 2007, Kim et al., 2008). However, the precise molecular mechanism of this potentiation is not completely understood.
Although the RSPO proteins act as positive WNT signaling regulators and exhibit ligand properties, there are conflicting results regarding the cell surface receptors interacting with the RSPO proteins and the molecular mechanisms of RSPO action in WNT signaling. The requirement of the LRP5/6 receptors to transmit RSPO activation of canonical WNT signaling pathway is well established (Nam et al., 2006, Binnerts et al., 2007, Wei et al., 2007). However, whether or not the LRP5/6 receptors function as a genuine receptor for the RSPO protein still remains controversial. Several laboratories independently demonstrated that the RSPO proteins bind to the LRP6 receptor in cell-free binding and co-immunoprecipitation assays (Nam et al., 2006, Wei et al., 2007, Li et al., 2009). Human RSPO1 protein binds to the LRP6 receptor with a Kd of 1.2 nM and this interaction occurs between the CR domain of RSPO1 and multiple β-propeller repeat domains of LRP6 (Wei et al., 2007). In contrast, several laboratories reported conflicting results. These studies failed to detect effective binding of the RSPO proteins to LRP6 in cell surface binding, co-immunoprecipitation and mass spectrometric assays (Kazanskaya et al., 2004, Binnerts et al., 2007, Carmon et al., 2011, de Lau et al., 2011, Glinka et al., 2011). The reason for these conflicting data may be due to the different experimental conditions and approaches employed in different laboratories.
In several WNT-dependent stem cell compartments such as the small intestine, colon, stomach, and hair follicles, the leucine-rich repeat containing G protein-coupled receptor 5 (LGR5) was identified as a WNT signaling target and validated as a marker for stem cells (van der Flier and Clevers, 2009, Jaks et al., 2008, Sato et al., 2009). Interestingly, the RSPO1 protein showed a strong mitogenic activity on LGR5+ cells of the intestinal crypts and hair follicles (Sato et al., 2009, Jaks et al., 2008). Several subsequent studies conclusively determined that the RSPO proteins are the ligands for the LGR4/5/6 receptors (Carmon et al., 2011, de Lau et al., 2011, Glinka et al., 2011, Carmon et al., 2012, Ruffner et al., 2012). The direct interaction of RSPO to LGR4/5/6 was determined by cell surface binding and cell-free co-immunoprecipitation assays, a tandem affinity purification mass spectrometry, and surface plasmon resonance array-imaging approach (Carmon et al., 2011, de Lau et al., 2011, Glinka et al., 2011). The Kds of binding between different RSPOs and LGRs are within nM ranges (e.g., hRSPO1 binding to LGR5 is 3.1.nM) (de Lau et al., 2011, Glinka et al., 2011). The first leucine-rich repeat domain at the N-terminus of the LGR5 receptor is required for RSPO1 binding (Glinka et al., 2011, de Lau et al., 2011). While the CR domain of the RSPO protein is involved in the interaction with LGR4 and LGR5 (Glinka et al., 2011, de Lau et al., 2011), the TSR domain of the RSPO proteins is not involved in binding to the LGR receptors (Glinka et al., 2011). Activation of WNT/β-catenin signaling by RSPOs is severely compromised when the LGR4 and LGR5 gene expression is suppressed by siRNA-mediated gene knockdown approach (Carmon et al., 2011, de Lau et al., 2011, Glinka et al., 2011). However, under the same conditions, WNT3A-mediated β-catenin signaling activation did not seem to be significantly affected in these cells (Carmon et al., 2011, de Lau et al., 2011, Glinka et al., 2011). Intriguingly, the level of β-catenin in LGR5 overexpressing cells is higher immediately after RSPO stimulation, but declines faster than control cells (Carmon et al., 2011). Moreover, enhanced WNT/β-catenin signaling is observed in the intestine of Lgr5 mutant mice (Garcia et al., 2009). Considering that the Lgr5 gene is a WNT/β-catenin signaling target, it would be interesting to determine if LGR5 acts as a negative feedback inhibitor in the absence of RSPOs, even though it engages in activation of WNT/β-catenin signaling by RSPO binding. In addition, unlike the other LGR family members (McDonald et al., 1998, Hsu et al., 1998), the LGR 4/5/6 receptors do not induce typical GPCR signaling activities (Carmon et al., 2011, de Lau et al., 2011, Glinka et al., 2011, Carmon et al., 2012, Gong et al., 2012).
It is generally thought that RSPOs do not directly bind to the FZD receptors (Wei et al., 2007). Because FZD are major receptor components in WNT/β-catenin signaling, it will be important to determine how the FZD receptors are involved in RSPO-induced WNT/β-catenin activation. Interestingly, RSPO1-stimulated WNT signaling is enhanced by the overexpression of the specific FZD members, such as FZD1, FZD4, FZD5, and FZD8, but not by some other FZD proteins (Wei et al., 2007). Furthermore, in co-immunoprecipitation/mass spectrometric analysis using the LGR4 or LGR5 receptor as bait, interactions between LGR4 and FZD5/FZD7, and LGR5 and FZD5/FZD6 were detected (de Lau et al., 2011), suggesting that the FZD receptors are likely a critical component of RSPO-induced WNT/β-catenin signaling, although the precise molecular mechanism remains to be determined.
3.3. Potentiation of WNT/β-catenin signaling by RSPOs and WNTs
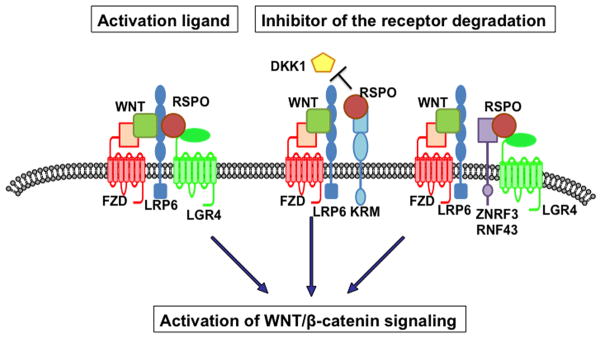
Synergistic activation of WNT/β-catenin signaling observed in cells treated with both RSPO and WNT ligands suggests that this synergism is likely a crucial regulatory mechanism governing the activation of WNT/β-catenin signaling. Significant overlap or close proximity of Wnt and Rspo gene expression in mouse embryos suggest that WNT and RSPO proteins co-exist in many cellular contexts (Nam et al., 2007b, Kamata et al., 2004, Kazanskaya et al., 2004). Currently available data imply two models (Figure 2).
Figure 2. Models of RSPO-induced WNT/β-catenin signaling activation.
In the left side, a model illustrates that RSPO acts as an activation ligand on LRP6 and LGR4 to form a multiple ligands-receptors-cluster with WNT and FZD. In a model presented in the middle and right side, RSPO inhibits degradation of the LRP6 and FZD receptors by antagonizing either DKK1-Kremen or ZNRF3.
In the first model, RSPO and WNT together act on the LRP5/6 and LGR4/5/6 receptors directly and immediately activate WNT/β-catenin signaling. Phosphorylation of serine residue at 1490 in the LRP6 receptor, which is the earliest molecular event occurring during activation of WNT/β-catenin signaling, is detected within 30 minutes after RSPO stimulation (Wei et al., 2007, Carmon et al., 2011), and simultaneously, endocytosis of the ligand-receptor complex occurs (Carmon et al., 2012). It was previously demonstrated that the endocytosis of the FZD and LRP6 receptors is a critical step in WNT/β-catenin signaling (Yamamoto et al., 2006, Kikuchi et al., 2009). In contrast to caveolin-dependent LRP6 endocytosis after WNT stimulation (Yamamoto et al., 2006), the endocytosis of LRP6 and LGR4/5 induced by WNT and RSPO co-treatment appears to be mediated by clathrin (Glinka et al., 2011, Carmon et al., 2012). However, whether endocytosis of the LRP6-LGR4 receptor complex is a critical step for WNT/β-catenin signaling activation remains unknown. While blocking endocytosis of the LRP6 and LGR5 receptors induced by the WNT and RSPO proteins showed no effect on WNT/β-catenin signaling activation in one study (Carmon et al., 2012), in another study, endocytosis of the receptor complex appeared to be critical for WNT signaling activation (Glinka et al., 2011).
In the second model, RSPOs act to prevent the degradation or removal of LRP6 and possibly FZD from the plasma membrane. In the presence of RSPO, more LRP6 and FZD receptors are present on the membrane, a condition allowing the WNT ligands to generate much stronger signals (Binnerts et al., 2007, Hao et al., 2012). Two proteins are proposed to be involved in this process. First, Binnerts et al. showed that RSPO binds to the Kremen family of transmembrane proteins that negatively regulates the LRP6 receptor by DKK1-mediated endocytosis and antagonizes the Kremen/DKK1-mediated LRP6 receptor downregulation on the plasma membrane (Binnerts et al., 2007). However, this model remains unsubstantiated because RSPO induces WNT/β-catenin signaling activation in Krm1/2 double mutant fibroblast cells as effectively as wild type fibroblasts (Ellwanger et al., 2008). Second, the cell-surface transmembrane E3 ubiquitin ligases, zinc and ring finger 3 (ZNRF3) and its homologue ring finger 43 (RNF43), emerged as negative feedback regulators of WNT signaling by promoting the turnover of the FZD and LRP6 receptors on the cell surface (Hao et al., 2012, Koo et al., 2012). Through its interactions with the extracellular domains of ZNRF3 and LGR4, RSPO1 was demonstrated to play an antagonistic role against ZNRF3 by inducing the removal of the ZNRF3 protein (Hao et al., 2012). However, further study is required to delineate how these two seemingly different models of RSPO action can be integrated, and under what biological systems they function.
3.4. RSPO in the non-canonical WNT/PCP pathway
In addition to their regulatory role in WNT/β-catenin signaling, RSPOs also regulate non-canonical WNT signaling (Ohkawara et al., 2011). During gastrulation and head cartilage morphogenesis in Xenopus embryos, two processes regulated by the non-canonical WNT/PCP pathway, Xenopus RSPO3 activates WNT/PCP signaling in cooperation with WNT5A in a syndecan4-dependent manner. RSPO3 promotes syndecan4-mediated FZD7/WNT5A complex internalization (Ohkawara et al., 2011). This RSPO3-induced endocytosis of FZD7/WNT5A complex is attenuated by clathrin-specific inhibitors, indicating that RSPO3 is associated with clathrin-dependent endocytosis (Ohkawara et al., 2011). Because direct binding between RSPO3 and FZD7 may not exist, it remains unclear how the RSPO protein imposes its activity on FZD7 without direct binding. It also remains to be determined whether syndecans and other HSPGs are similarly involved in the activation of non-canonical WNT signaling by RSPO in mammalian cell models. In addition, there is little information on the regulatory roles for HSPGs in RSPO-induced activation of canonical WNT signaling, even though the roles of HSPGs in the regulation of WNT signaling have been investigated.
4. In vivo functions of the RSPO genes
WNT signaling is broadly involved in regulating cell proliferation, differentiation, migration, survival and polarity during embryonic development and tissue homeostasis (Clevers, 2006, Grigoryan et al., 2008). Interest in RSPO’s ability to function as canonical and non-canonical WNT signaling regulators has initiated a wide range of in vivo and in vitro investigations including human genetic studies and gene targeting approaches in mice (Table 1). These studies have begun to reveal the in vivo functions of the Rspo genes and also suggest the potential of the RSPO proteins and their signaling as novel therapeutic targets.
Table 1.
Loss-of-function (LOF) and gain-of-function (GOF) mutations of the R-spondin genes in mammals
| Gene | Species | Mutation type | Phenotypes | References |
|---|---|---|---|---|
| R-spondin1 | Human | LOF | Female to male (XX) sex conversion XX true hermaphroditism Palmoplantar hyperkeratosis Predisposition to skin squamous cell carcinoma |
(Parma et al., 2006, Tomaselli et al., 2008) |
| Mouse | LOF | Pseudohermaphroditism Masculinized ovaries Abnormal development of the mammary glands |
(Auguste et al., 2011, Chadi et al., 2009, Chassot et al., 2011, Tomizuka et al., 2008) | |
| R-spondin2 | Human | GOF | APC-negative colon cancers | (Seshagiri et al., 2012) |
| Mouse | LOF | Distal limb defects Craniofacial and laryngeal-tracheal malformation Lung hypoplasia Kidney defect Polycystic ovaries in heterozygous mice |
(Aoki et al., 2008, Bell et al., 2003, Bell et al., 2008, Jin et al., 2011, Nam et al., 2007, Yamada et al., 2009) | |
| Dog | GOF | Dog coat type change | (Cadieu et al., 2009) | |
| R-spondin3 | Human | GOF | APC-negative colon cancers | (Seshagiri et al., 2012) |
| Mouse | LOF | Impaired placental development Vascular defects in the yolk sac and embryo, Enhanced limb defect under Rspo2 homozygous background |
(Aoki et al., 2007, Kazanskaya et al., 2008, Neufeld et al., 2012) | |
| R-spondin4 | Human | LOF | Anonychia/hyponychia | (Bergmann et al., 2006, Blaydon et al., 2006, Bruchle et al., 2008, Ishii et al., 2008, Seitz et al., 2007) |
4.1. RSPO1
Recently, individuals with complete female-to-male sex reversal or XX true hermaphroditism from two families were identified as homozygous for RSPO1 gene mutations (Parma et al., 2006, Tomaselli et al., 2008). The identified mutations of the RSPO1 gene resulted in either no functional protein production (Parma et al., 2006) or deletion of the second half of the CR domain of the RSPO1 protein (Tomaselli et al., 2008), thereby causing severe disruption of the RSPO1-dependent WNT signaling activation. Additionally, these individuals also displayed palmoplantar hyperkeratosis and predisposition to squamous cell carcinoma of the skin.
The function of mouse Rspo1 gene was assessed by a gene-targeting strategy (Chassot et al., 2008, Tomizuka et al., 2008). Unlikely in humans, loss of the mouse Rspo1 gene only caused masculinized ovaries (ovotestis) with epididymis and vas deferens-like structures rather than a complete phenotypic male conversion in XX mice. The Rspo1 null ovaries also showed fetal oocyte depletion, XY-specific vascularization, and steroidogenesis relevant to extremely poor fertility of Rspo1 null female mice. Molecular analyses demonstrated that the compromised WNT/β-catenin signaling and an absence of female-specific activation of Wnt4 might account for the XY-like vascularization and steroidogenesis. Germ cells of XX knockout embryos showed changes in cellular adhesions and a failure to enter XX specific meiosis. Interestingly, loss of Rspo1 and Foxl2, a transcription factor required for follicular formation, amplifies female-to-male sex reversal in XX mice (Auguste et al., 2011).
In mammals, the sex of the individual is determined by the male or female genetic program-directed testicular or ovarian organogenesis. While the Y chromosome-located sex-determining region Y (SRY) gene is absolutely required for testis development, ovary development is regarded as a passive process due to the absence of SRY expression in the female gonad (Liu et al., 2009). RSPO1/Rspo1 genetic studies provided the first evidence that RSPO1-induced WNT/β-catenin signaling positively regulates female differentiation by suppressing male differentiation in females. Consistent to this notion, RSPO1/β-catenin signaling is demonstrated to be involved in meiosis in fetal germ cells and contributes to the cellular decision of germ cells to differentiate into oocyte or sperm (Chassot et al., 2011). In addition, Rspo1 showed a female-specific expression profile in vertebrates including chicken and turtle (Smith et al., 2008), indicating a conserved role of Rspo1 in the vertebrate female-sex determination pathway.
Additionally, some Rspo1 null XX mice remain sub-fertile and are able to produce offspring; however, these mice are unable to feed their pups (Chadi et al., 2009). Transplantation of mammary tissues from Rspo1−/− virgin mice into nude mice revealed that the lack of Rspo1 expression resulted in the absence of duct side-branching development and subsequent alveolar formation, indicating that a local epithelial RSPO1 signal is required for normal development of the mammary gland.
4.2. RSPO2
Recently, recurrent RSPO2 gene fusion events were identified in human colon tumors (Seshagiri et al., 2012). The fusion between the non-coding exon 1 of the eukaryotic translation initiation factor 3 subunit E (EIF3E) gene and exon2 of the RSPO2 gene resulted in elevated expression of full-length RSPO2 protein driven by the E1F3E promoter. Additionally, a genome-wide association study initially suggested that the human RSPO2 gene locus is linked to genetic susceptibility in Dupuytren’s disease, a benign fibromatosis (Dolmans et al., 2011).
Four independent mouse lines bearing Rspo2 mutant alleles have been generated by both transgene insertion and gene targeting approaches (Bell et al., 2003, Nam et al., 2007a, Yamada et al., 2009, Aoki et al., 2008). Footless mice are homozygous for an inserted transgene of the rat H+K+-ATPase gene promoter driving chloramphenicol acetyl-transferase, and showed an asymmetric pattern of limb malformations (Bell et al., 2003). Positional cloning revealed that this transgene is inserted into the 5′-upstream region proximal to the Rspo2 gene; footless is a strong hypomorphic allele of the Rspo2 gene (Bell et al., 2008). Footless and Rspo2 homozygous mutant mice (Nam et al., 2007a, Aoki et al., 2008, Yamada et al., 2009) die immediately after birth and display multiple defects including limb defects, craniofacial and laryngeal-tracheal malformation, lung hypoplasia, and kidney malformations (Nam et al., 2007a, Aoki et al., 2008, Bell et al., 2008, Yamada et al., 2009). Interestingly, female mice heterozygous for these Rspo2 mutations showed partially penetrant, age-dependent sterility accompanied by development of polycystic ovaries (Bell et al., 2003, Nam et al., 2007a).
Disruption of distal skeletal structures, especially in the hindlimbs, occurs in Rspo2 homozygous mutant and footless mice (Bell et al., 2003, Nam et al., 2007a). Reciprocal FGF signaling between limb mesenchyme and overlaying epithelium (or AER at later stages) is crucial to control limb outgrowth and patterning. Fgf gene expression in Rspo2 mutant mice suggests that maturation of the AER is either delayed or fails. This defect results from compromised WNT/β-catenin signaling as measured by reduced expression of the transcriptional target Axin2 and the in vivo WNT/β-catenin signaling reporter TopGAL in the AER of Rspo2 null mutant embryos (Yamada et al., 2009, Nam et al., 2007a). The existence of genetic epistasis between the Rspo2 and Lrp6 genes further confirmed that Rspo2 function is associated with WNT/β-catenin signaling (Bell et al., 2008). A redundant function of Rspo2 and Rspo3 during hind limb development was recently determined. Although limb mesenchyme-specific Rspo3 gene ablation does not cause any noticeable hindlimb defect, hindlimb truncation defects detected in Rspo3 and footless double mutant mice are more severe than those observed in footless mutant mice (Neufeld et al., 2012).
Craniofacial defects including cleft of the secondary palate with or without cleft lip and abnormalities of the skeletal structures derived from branchial arch 1 (BA1) occur in Rspo2 null mice (Yamada et al., 2009, Jin et al., 2011). Gene expression of several signaling cytokines and transcription factors including Fgf8, Endothelin1, Dlx5/6, and Msx1/2, was significantly disrupted in BA1 mesenchyme of Rspo2 null mice. Additionally, laryngeal, tracheal and bronchial cartilage derived from BA2 to BA6 was malformed (Bell et al., 2008), suggesting multiple roles for Rspo2 in BA development. Similar to the AER of Rspo2 null mice, reduced canonical WNT/β-catenin signaling was detected within BA1, and genetic epistasis between Rspo2 and Lrp6 was also present in BA1-derived skeletal development (Jin et al., 2011).
Hypoplasia and branching defects within the lungs were also observed in Rspo2 mutant mice (Bell et al., 2008, Yamada et al., 2009). The fetal lungs isolated from Rspo2 mutant mice were approximately half the size of the lungs of wild type littermates but generally retained normal structure. The hypoplasia of the Rspo2 mutant lung was not associated with differentiation defects of specific types of cells. Rspo2 mutant lung explants showed severely impaired growth and branching in ex vivo culture, when compared to wild type explants (Bell et al., 2008). The branching defect was effectively rescued by the addition of RSPO2 conditional medium into the culture (Bell et al., 2008). In agreement with AER and BA1, Rspo2 acts through the LRP6-mediated WNT/β-catenin signaling during lung development as Rspo2 mutant lungs showed reduced WNT/β-catenin signaling and Rspo2 and Lrp6 compound mutant mice exhibited synergistic hypoplasia phenotype (Bell et al., 2008).
The Rspo2 gene was also identified as a key genetic variant for dog coat types in a genome-wide association study (Cadieu et al., 2009). Wire-haired breeds such as the Portuguese water dog are homozygous for the Rspo2 gene mutation containing a 167 bp insertion in the 3′-untranslated region of Rspo2 mRNA (Parker et al., 2010). This insertion affects Rspo2 mRNA stability without causing any change in RSPO2 protein coding sequence, thus leading to increased steady-state Rspo2 mRNA in the skin.
In Xenopus, injection of Rspo2 morpholino anti-sense oligonucleotides in four-cell stage embryos impaired the expression of the myogenic marker genes, Myf5 and MyoD, and later muscle development. Inhibition of Myf5 expression by Rspo2 morpholino was reversed by ectopic β-catenin expression, confirming that Rspo2 function is mediated by the WNT/β-catenin pathway (Kazanskaya et al., 2004). In mice, loss of the Rspo2 gene causes significant reduction of Myf5 expression in developing limbs, suggesting the role of Rspo2 in myogenesis is conserved in vertebrate species (Han et al., 2011).
4.3. RSPO3
Similar to the RSPO2 gene, recurrent RSPO3 gene fusions were identified in human colon tumors (Seshagiri et al., 2012). The coding exon 1 of the Protein tyrosine phosphatase receptor type K (PTPRK) gene was fused to the exon 2 of the RSPO3 gene, resulting in expression of a fusion protein that exhibited a full capacity of RSPO3 activity in WNT signaling. Interestingly, both RSPO2 and RSPO3 fusions occurred in an exclusive manner to APC mutations, indicating that these mutations represent a unique subclass of human colon tumors.
Rspo3 gene knockout mutants die around E10 due to angiogenesis defects in yolk sac and placenta (Kazanskaya et al., 2008, Aoki et al., 2007). Consistent with specific expression of Rspo3 in the allantoic component of the labyrinth, the fetal blood vessels did not penetrate into the chorion in the placenta of Rspo3 homozygous mutant, suggesting a critical role for Rspo3 in the interaction between chorion and allantois in labyrinthine development (Aoki et al., 2007). PECAM staining and histological analysis also showed that the primary capillary plexus in the yolk sac of mutant embryos formed but failed to remodel, resulting in an underdeveloped vasculature (Aoki et al., 2007, Kazanskaya et al., 2008). A compromised VEGF expression, which is under the regulation by RSPO3-mediated WNT/β-catenin signaling, is likely the major cause of the angiogenesis defect (Kazanskaya et al., 2008). The embryonic lethality around E10 prevents the assessment of Rspo3 function in the embryo beyond this developmental stage. With available conditional Rspo3 gene knockout alleles (Kazanskaya et al., 2008, Neufeld et al., 2012), additional functions of the Rspo3 gene during development and postnatal stages will be uncovered. Most recently, it is reported that Rspo3 gene function may be partly redundant to that of the Rspo2 gene in hindlimb development as Rspo2 and Rspo3 double gene deletion in limb mesenchymal cells caused more severe hindlimb defects than those of Rspo2 mutant mice (Neufeld et al., 2012).
In Xenopus embryos, inhibition of the Rspo3 gene by Rspo3-specific morpholino injection resulted in three major developmental defects. The first defect caused by Rspo3 inhibition is ventral edema and vascular defects in tadpoles and imbalance of endothelial and blood differentiation towards increased blood cell specification. These defects seem to be WNT/β-catenin-dependent (Kazanskaya et al., 2008). The second and third defects caused by Rspo3 inhibition are disruption of gastrulation and malformation of head cartilage (Ohkawara et al., 2011). Because the WNT/PCP pathway regulates both of these developmental processes, it is reasonable to speculate that Rspo3 may play critical roles in these processes by regulating WNT/PCP signaling.
4.4. RSPO4
Anonychia/hyponychia is a condition defined by total or partial absence of fingernails and toenails without significant bone anomalies in human (Baran and Kechijian, 2001). The link of human RSPO4 gene mutations to anonychia was discovered in several genome-wide mapping studies (Bergmann et al., 2006, Blaydon et al., 2006, Blaydon et al., 2007, Seitz et al., 2007, Ishii et al., 2008, Bruchle et al., 2008, Wasif and Ahmad, 2012). Individuals homozygous for these RSPO4 mutations exhibit anonychia. Most identified mutations are clustered within the CR domain of the RSPO4 protein (Blaydon et al., 2006, Ishii et al., 2008), resulting in production of RSPO4 protein lacking an ability to activate canonical WNT signaling. Rspo4 is expressed in the primordia of the claw during mouse nail development (Bergmann et al., 2006, Blaydon et al., 2006, Ishii et al., 2008). In addition, expression of RPSO4 transcript was detected in human primary fibroblasts but not in keratinocytes (Blaydon et al., 2007). Therefore, RSPO4 functions as a key regulator of nail development and potentially modulates WNT/β-catenin signaling activation at the dermal–epidermal boundary. Furthermore, considering that the nail bed in patients with RSPO4 mutations is fully formed, RSPO4 is involved in advanced stages of nail development rather than in early stages (Seitz et al., 2007). Since the claws were also missing in Rspo2 null mutant mice (Nam et al., 2007a, Bell et al., 2003), it remains to be investigated whether human RSPO2 gene mutations are linked to RSPO4-independent anonychia/hyponychia (Nam et al., 2007a, Nakamura and Miyachi, 2008).
5. Therapeutics potentials of RSPOs
5.1. Skeletal diseases
WNT signaling plays a crucial role in normal bone development and remodeling. Aberrant WNT signaling, exemplified by null, hypo- and hypermorphic mutations in two WNT receptor genes, LRP5 and LRP6, is strongly associated with bone diseases such as osteoporosis and osteoarthritis in humans (Kerkhof et al., 2008, van Meurs et al., 2008). Recent studies imply a therapeutic potential of RSPO and RSPO signaling in bone diseases. In mouse multipotential myogenic progenitor C2C12 cells and primary mouse osteoblast cells, RSPO1 treatment induces expression of the osteoblast differentiation marker genes, osteocalcin and osteoprotegerin, and increased alkaline phosphatase level through the WNT/β-catenin signaling pathway (Lu et al., 2008). In MC3T3-E1 pre-osteoblast cells pretreated with BMP2, the non-canonical WNT11 ligand enhanced expression of osteoblast-associated genes and osteoblast maturation and mineralization (Friedman et al., 2009). In this study, the Rspo2 gene was identified as a potential target to mediate WNT11 activity, and Rspo2 overexpression promoted osteogenic differentiation in MC3T3-E1 cells pretreated with BMP2, but not in naive MC3T3-E1 cells. Interestingly, Rspo2 activity to promote osteogenic differentiation was not WNT/β-catenin-dependent. Considering the ability of RSPO to activate WNT/PCP signaling in Xenopus (Ohkawara et al., 2011), it is plausible that RSPO2 transmits its activity through the non-canonical WNT pathway in MC3T3-E1 cells.
It was found recently that elevated TGF-β1 downregulated RSPO2 expression, resulting in a reduction of WNT/β-catenin signaling in primary cultured human osteoblasts from tibia plateaus of osteoarthritis patients (Abed et al., 2011). Importantly, while siRNA-mediated RSPO2 gene knockdown decreased mineralization of normal osteoblasts, treatment with recombinant RSPO2 protein during bone differentiation enhanced mineralization of osteoblasts isolated from osteoarthritis patients (Abed et al., 2011).
Additional proof of the therapeutic potential of the RSPO1 protein on joint disorders was demonstrated. When the recombinant RSPO1 protein was locally injected into the joints of TNFα overexpressing transgenic mice (TNFtg), a mouse model of arthritis, it effectively inhibited osteoclast development and bone resorption (Kronke et al., 2010). Consequently, RSPO1 injection prevented bone erosion and improved cartilage integrity in the joints of TNFtg mice. Reduced proteoglycan loss and chondrocyte death was observed in RSPO1-injected TNFtg mice with less damage in the cartilage manifested by decreased expression level of VDIPEN, a neoepitope generated by matrix metalloproteinase-mediated proteoglycan damage (Kronke et al., 2010). In addition, the Pgia8 gene locus is associated with proteoglycan-induced arthritis in mice and rheumatoid arthritis in humans with a gender disparity (Kudryavtseva et al., 2011). The mouse Rspo2 gene is one of four genes correlated with arthritis severity in Pgia8 congenic mice, which illustrates the strong connection between Rspo2 (and RSPO2 signaling) and inflammatory arthritis. These studies show the therapeutic potential of the recombinant RSPO protein in treatment of some skeletal diseases.
5.2. Inflammatory bowel disease and chemotherapy-induced mucositis
Human RSPO1 protein effectively supports survival and proliferation of LGR5-positive intestinal stem cells in vitro through activation of WNT/β-catenin signaling (Sato et al., 2009). Ectopic expression of human RSPO1 in the B cell lineage in mice causes severe abdominal distention by 8 weeks of age, which is characterized by a substantial increase in the diameter, length, and weight of the small intestine (Kim et al., 2005). Consistently, 3 days of administration of human RSPO1 protein into normal mice fully recapitulated the intestinal phenotypes (Kim et al., 2005). This mitogenic activity of RSPO1 on intestinal stem cells may be useful to treat two specific diseases. First, RSPO1 may be clinically useful in the therapeutic treatment of inflammatory bowel disease because of its stimulating effect on crypt cell growth to accelerate mucosal regeneration. In both acute and chronic phases of colitis in mouse models, administration of the RSPO1 protein preserves mucosal integrity in both small and large bowels by stimulating crypt epithelial cell mitosis (Zhao et al., 2007). This results in the regeneration of both crypt and villus compartments in the mouse intestinal mucosa. Importantly, RSPO1 treatment suppresses intestinal inflammation, leukocyte infiltration, and overproduction of proinflammatory cytokines observed during colitis (Zhao et al., 2007). Second, RSPO1 can be used as a supplement to prevent architectural damage of the small intestine and colon arising from cytotoxicity of anti-cancer drug for cancer patients undergoing chemotherapy. Significant reverse of the intestinal epithelial cell damage caused by 5-FU was observed in a mouse model carrying a tumor (Kim et al., 2005).
5.3. Cancer
Aberrant canonical WNT signaling is strongly associated with many forms of human cancer (Clevers, 2006). The Rspo2 and Rspo3 genes are identified for their oncogenic potential in mouse mammary tumor virus associated with mammary tumorigenesis in mice (Theodorou et al., 2007, Klauzinska et al., 2012, Lowther et al., 2005). The oncogenic potential of the Rspo3 gene was further verified by the enhanced tumor forming capability of Rspo3 overexpressing p53-deficient mammary epithelial cells when they are subcutaneously injected into nude mice (Theodorou et al., 2007). Additionally, alteration of the Rspo2 gene is linked to colorectal cancer in a transposon-based genetic screening in mice (Starr et al., 2009). Recent discovery of recurrent gene fusion of RSPO2 and RSPO3 in human colon tumors with no APC mutations illustrates the critical role of RSPOs and their activation of WNT signaling in some type of human colon cancer (Seshagiri et al., 2012). Antagonizing RSPO activity can be an effective therapeutic strategy against APC-negative colon cancers.
In contrast to oncogenic function of Rspo2 and Rspo3 in breast and colon cancers in mouse models, RSPO1 is suggested as a potential tumor suppressor gene in both acute and chronic lymphocytic leukemia in humans (Kuang et al., 2008, Tong et al., 2010). Humans carrying RSPO1 null mutations are also predisposed to the skin carcinoma (Kuang et al., 2008, Tong et al., 2010, Parma et al., 2006). The RSPO4 gene also showed aberrant DNA methylation in esophageal squamous cells and in human esophageal squamous carcinoma specimens (Oka et al., 2009). Interestingly, recent studies suggest that LGR5 functions as a tumor suppressor, while increased expression of LGR5 in colon cancer and basal cell carcinoma may be potentially enriched in cancer stem cells (Tanese et al., 2008, McClanahan et al., 2006, Merlos-Suarez et al., 2011). In addition, the LGR6 gene is hypermethylated in its promoter region in approximately 20% of colon cancer (Schuebel et al., 2007), thereby suggesting that its transcription is repressed. A loss-of-function LGR6 mutation was also found in colon cancer (Gong et al., 2012). It is likely that RSPOs and their signaling can function as both oncogenic or tumor suppressive, depending on the types of cancer. Therefore, carefully designed inhibition or activation of RSPO signaling activity may be a promising anti-cancer strategy in various types of human cancers.
6. Conclusion
The importance of the RSPO family of proteins, which act as positive regulators of canonical and non-canonical WNT signaling pathways, has been established in several in vitro and in vivo studies in various animal models and human genetic studies. Although there has been a significant increase in our understanding of how RSPO regulates the WNT signaling pathway at the molecular level, many questions still remain unanswered. A fundamental question is whether the RSPO proteins themselves act as activating ligands for WNT signaling or simply function as potentiators of the WNT ligands. Additionally, as noted above, integration of the different models of RSPO action is needed for comprehensive understanding of the molecular mechanism by which RSPO regulates WNT signaling, especially canonical WNT signaling. Furthermore, additional conditional alleles of the Rspo and Lgr4/5/6 genes in mice are necessary to identify functions of RSPO signaling during postnatal development and disease conditions. Finally, our growing knowledge of the functions and signaling mechanisms of the RSPO proteins will facilitate development of therapeutic applications based on RSPO signaling for human diseases in which modulation of WNT signaling is critical.
Highlights.
R-spondins are a family of cysteine-rich, thrombospondin type I repeat containing proteins that activate both the WNT/β-catenin and WNT/PCP pathways.
The LGR4/5/6 proteins act as receptors for R-spondins.
R-spondins potentiate WNT ligand activity as either coactivators or inhibitors of WNT signaling receptor degradation.
The R-spondin gene mutations are associated with human genetic diseases and cancer.
The R-spondin genes are essential for various developmental processes in mice.
Acknowledgments
We thank Drs Lucy Liaw and Doug Spicer and the members of our laboratory for comments and suggestions. This publication was made possible by 8P20 GM103465 (NIGMS/NIH, Program Director: D. Wojchowski) and 5R01 AR055278 (NIAMS/NIH) grants to JKY.
Abbreviations and Acronyms
- BA
branchial arch
- BMP
bone morphogenetic protein
- BR
basic amino acid-rich
- CR
cysteine-rich
- DKK1
dickkopf homolog1
- Dlx
distal-less homeobox
- EIF3E
eukaryotic translation initiation factor 3 subunit E
- EGF
epidermal growth factor
- FGF
fibroblast growth factor
- 5-FU
5-fluorouracil
- Foxl
forkhead box L2
- FZD
Frizzled
- HSPGs
heparan sulfate proteoglycans
- KRM
kremen
- LEF1
lymphoid enhancer binding factor1
- LGR
leucine-rich repeat containing G protein-coupled receptor
- LRP
Low density lipoprotein-receptor related protein
- Msx
msh-like homeobox
- NFAT
nuclear factor of activated T-cells
- PCP
planar cell polarity
- PTPRK
protein tyrosine phosphatase receptor type K
- RAC1
Ras-related C3 botulinum substrate1
- RHOA
Ras homolog gene family, member A
- RNF43
ring finger protein43
- RSPO
roof plate-specific spondin
- hPWTSR
human protein with thrombospondin type I repeat domain
- WNT
wingless-type MMTV integration site family
- SRY
sex-determining region of chromosome Y
- TCF
T cell specific HMG-box factor
- TNFα
tumor necrosis factor α
- TSR
thrombospondin type I repeat
- VEGF
vascular endothelial growth factor
- ZNRF3
zinc and ring finger3
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abed E, Chan TF, Delalandre A, Martel-Pelletier J, Pelletier JP, Lajeunesse D. R-spondins are newly recognized players in osteoarthritis that regulate Wnt signaling in osteoblasts. Arthritis Rheum. 2011;63:3865–3875. doi: 10.1002/art.30625. [DOI] [PubMed] [Google Scholar]
- Angers S, Moon RT. Proximal events in Wnt signal transduction. Nature reviews Molecular cell biology. 2009;10:468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- Aoki M, Kiyonari H, Nakamura H, Okamoto H. R-spondin2 expression in the apical ectodermal ridge is essential for outgrowth and patterning in mouse limb development. Development, Growth & Differentiation. 2008;50:85–95. doi: 10.1111/j.1440-169X.2007.00978.x. [DOI] [PubMed] [Google Scholar]
- Aoki M, Mieda M, Ikeda T, Hamada Y, Nakamura H, Okamoto H. R-spondin3 is required for mouse placental development. Developmental Biology. 2007;301:218–226. doi: 10.1016/j.ydbio.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Auguste A, Chassot AA, Gregoire EP, Renault L, Pannetier M, Treier M, Pailhoux E, Chaboissier MC. Loss of R-Spondin1 and Foxl2 Amplifies Female-to-Male Sex Reversal in XX Mice. Sex Dev. 2011;5:304–317. doi: 10.1159/000334517. [DOI] [PubMed] [Google Scholar]
- Baran R, Kechijian P. Understanding nail disorders. Eur J Dermatol. 2001;11:159–162. [PubMed] [Google Scholar]
- Bell SM, Schreiner CM, Hess KA, Anderson KP, Scott WJ. Asymmetric limb malformations in a new transgene insertional mutant, footless. Mech Dev. 2003;120:597–605. doi: 10.1016/s0925-4773(03)00021-2. [DOI] [PubMed] [Google Scholar]
- Bell SM, Schreiner CM, Wert SE, Mucenski ML, Scott WJ, Whitsett JA. R-spondin 2 is required for normal laryngeal-tracheal, lung and limb morphogenesis. Development. 2008;135:1049–1058. doi: 10.1242/dev.013359. [DOI] [PubMed] [Google Scholar]
- Bergmann C, Senderek J, Anhuf D, Thiel CT, Ekici AB, Poblete-Gutierrez P, van Steensel M, Seelow D, Nurnberg G, Schild HH, Nurnberg P, Reis A, Frank J, Zerres K. Mutations in the gene encoding the Wnt-signaling component R-spondin 4 (RSPO4) cause autosomal recessive anonychia. Am J Hum Genet. 2006;79:1105–1109. doi: 10.1086/509789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, Niehrs C. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–1622. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- Binnerts ME, Kim KA, Bright JM, Patel SM, Tran K, Zhou M, Leung JM, Liu Y, Lomas WE, Dixon M, Hazell SA, Wagle M, Nie WS, Tomasevic N, Williams J, Zhan X, Levy MD, Funk WD, Abo A. R-Spondin1 regulates Wnt signaling by inhibiting internalization of LRP6. Proceedings of the National Academy of Sciences. 2007;104:14700–14705. doi: 10.1073/pnas.0702305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaydon DC, Ishii Y, O’Toole EA, Unsworth HC, Teh MT, Ruschendorf F, Sinclair C, Hopsu-Havu VK, Tidman N, Moss C, Watson R, de Berker D, Wajid M, Christiano AM, Kelsell DP. The gene encoding R-spondin 4 (RSPO4), a secreted protein implicated in Wnt signaling, is mutated in inherited anonychia. Nat Genet. 2006;38:1245–1247. doi: 10.1038/ng1883. [DOI] [PubMed] [Google Scholar]
- Blaydon DC, Philpott MP, Kelsell DP. R-spondins in cutaneous biology: nails and cancer. Cell Cycle. 2007;6:895–897. doi: 10.4161/cc.6.8.4136. [DOI] [PubMed] [Google Scholar]
- Bruchle NO, Frank J, Frank V, Senderek J, Akar A, Koc E, Rigopoulos D, van Steensel M, Zerres K, Bergmann C. RSPO4 is the major gene in autosomal-recessive anonychia and mutations cluster in the furin-like cysteine-rich domains of the Wnt signaling ligand R-spondin 4. J Invest Dermatol. 2008;128:791–796. doi: 10.1038/sj.jid.5701088. [DOI] [PubMed] [Google Scholar]
- Cadieu E, Neff MW, Quignon P, Walsh K, Chase K, Parker HG, VonHoldt BM, Rhue A, Boyko A, Byers A, Wong A, Mosher DS, Elkahloun AG, Spady TC, André C, Lark KG, Cargill M, Bustamante CD, Wayne RK, Ostrander EA. Coat Variation in the Domestic Dog Is Governed by Variants in Three Genes. Science. 2009;326:150–153. doi: 10.1126/science.1177808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmon KS, Gong X, Lin Q, Thomas A, Liu Q. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/{beta}-catenin signaling. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1106083108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmon KS, Lin Q, Gong X, Thomas A, Liu Q. LGR5 interacts and cointernalizes with Wnt receptors to modulate Wnt/beta-catenin signaling. Mol Cell Biol. 2012;32:2054–2064. doi: 10.1128/MCB.00272-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadi S, Buscara L, Pechoux C, Costa J, Laubier J, Chaboissier MC, Pailhoux E, Vilotte JL, Chanat E, Le Provost F. R-spondin1 is required for normal epithelial morphogenesis during mammary gland development. Biochem Biophys Res Commun. 2009;390:1040–1043. doi: 10.1016/j.bbrc.2009.10.104. [DOI] [PubMed] [Google Scholar]
- Chassot AA, Gregoire EP, Lavery R, Taketo MM, de Rooij DG, Adams IR, Chaboissier MC. RSPO1/beta-catenin signaling pathway regulates oogonia differentiation and entry into meiosis in the mouse fetal ovary. PLoS ONE. 2011;6:e25641. doi: 10.1371/journal.pone.0025641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassot AA, Ranc F, Gregoire EP, Roepers-Gajadien HL, Taketo MM, Camerino G, de Rooij DG, Schedl A, Chaboissier MC. Activation of beta-catenin signaling by Rspo1 controls differentiation of the mammalian ovary. Human molecular genetics. 2008;17:1264–1277. doi: 10.1093/hmg/ddn016. [DOI] [PubMed] [Google Scholar]
- Chen H, Sottile J, Strickland DK, Mosher DF. Binding and degradation of thrombospondin-1 mediated through heparan sulphate proteoglycans and low-density-lipoprotein receptor-related protein: localization of the functional activity to the trimeric N-terminal heparin-binding region of thrombospondin-1. Biochem J. 1996;318(Pt 3):959–963. doi: 10.1042/bj3180959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JZ, Wang S, Tang R, Yang QS, Zhao E, Chao Y, Ying K, Xie Y, Mao YM. Cloning and identification of a cDNA that encodes a novel human protein with thrombospondin type I repeat domain, hPWTSR. Mol Biol Rep. 2002;29:287–292. doi: 10.1023/a:1020479301379. [DOI] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- de Lau W, Barker N, Low TY, Koo BK, Li VS, Teunissen H, Kujala P, Haegebarth A, Peters PJ, van de Wetering M, Stange DE, van Es J, Guardavaccaro D, Schasfoort RB, Mohri Y, Nishimori K, Mohammed S, Heck AJ, Clevers H. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011 doi: 10.1038/nature10337. [DOI] [PubMed] [Google Scholar]
- Dolmans GH, Werker PM, Hennies HC, Furniss D, Festen EA, Franke L, Becker K, van der Vlies P, Wolffenbuttel BH, Tinschert S, Toliat MR, Nothnagel M, Franke A, Klopp N, Wichmann HE, Nurnberg P, Giele H, Ophoff RA, Wijmenga C. Wnt Signaling and Dupuytren’s Disease. N Engl J Med. 2011 doi: 10.1056/NEJMoa1101029. [DOI] [PubMed] [Google Scholar]
- Ellwanger K, Saito H, Clement-Lacroix P, Maltry N, Niedermeyer J, Lee WK, Baron R, Rawadi G, Westphal H, Niehrs C. Targeted disruption of the Wnt regulator Kremen induces limb defects and high bone density. Mol Cell Biol. 2008;28:4875–4882. doi: 10.1128/MCB.00222-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman MS, Oyserman SM, Hankenson KD. Wnt11 promotes osteoblast maturation and mineralization through R-spondin 2. J Biol Chem. 2009;284:14117–14125. doi: 10.1074/jbc.M808337200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MI, Ghiani M, Lefort A, Libert F, Strollo S, Vassart G. LGR5 deficiency deregulates Wnt signaling and leads to precocious Paneth cell differentiation in the fetal intestine. Dev Biol. 2009;331:58–67. doi: 10.1016/j.ydbio.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Glinka A, Dolde C, Kirsch N, Huang YL, Kazanskaya O, Ingelfinger D, Boutros M, Cruciat CM, Niehrs C. LGR4 and LGR5 are R-spondin receptors mediating Wnt/beta-catenin and Wnt/PCP signalling. EMBO reports. 2011;12:1055–1061. doi: 10.1038/embor.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong X, Carmon KS, Lin Q, Thomas A, Yi J, Liu Q. LGR6 Is a High Affinity Receptor of R-Spondins and Potentially Functions as a Tumor Suppressor. PLoS ONE. 2012;7:e37137. doi: 10.1371/journal.pone.0037137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryan T, Wend P, Klaus A, Birchmeier W. Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes Dev. 2008;22:2308–2341. doi: 10.1101/gad.1686208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han XH, Jin YR, Seto M, Yoon JK. A WNT/{beta}-catenin signaling activator, R-spondin, plays positive regulatory roles during skeletal myogenesis. J Biol Chem. 2011 doi: 10.1074/jbc.M110.169391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao HX, Xie Y, Zhang Y, Charlat O, Oster E, Avello M, Lei H, Mickanin C, Liu D, Ruffner H, Mao X, Ma Q, Zamponi R, Bouwmeester T, Finan PM, Kirschner MW, Porter JA, Serluca FC, Cong F. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature. 2012;485:195–200. doi: 10.1038/nature11019. [DOI] [PubMed] [Google Scholar]
- He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004;131:1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- Hsu SY, Liang SG, Hsueh AJ. Characterization of two LGR genes homologous to gonadotropin and thyrotropin receptors with extracellular leucine-rich repeats and a G protein-coupled, seven-transmembrane region. Mol Endocrinol. 1998;12:1830–1845. doi: 10.1210/mend.12.12.0211. [DOI] [PubMed] [Google Scholar]
- Ishii Y, Wajid M, Bazzi H, Fantauzzo KA, Barber AG, Blaydon DC, Nam JS, Yoon JK, Kelsell DP, Christiano AM. Mutations in R-spondin 4 (RSPO4) underlie inherited anonychia. J Invest Dermatol. 2008;128:867–870. doi: 10.1038/sj.jid.5701078. [DOI] [PubMed] [Google Scholar]
- Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, Toftgard R. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nature genetics. 2008;40:1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- Jin YR, Turcotte TJ, Crocker AL, Han XH, Yoon JK. The canonical Wnt signaling activator, R-spondin2, regulates craniofacial patterning and morphogenesis within the branchial arch through ectodermal-mesenchymal interaction. Developmental Biology. 2011;352:1–13. doi: 10.1016/j.ydbio.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata T, Katsube K-i, Michikawa M, Yamada M, Takada S, Mizusawa H. R-spondin, a novel gene with thrombospondin type 1 domain, was expressed in the dorsal neural tube and affected in Wnts mutants. Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression. 2004;1676:51–62. doi: 10.1016/j.bbaexp.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Kazanskaya O, Glinka A, del Barco Barrantes I, Stannek P, Niehrs C, Wu W. R-Spondin2 Is a Secreted Activator of Wnt/[beta]-Catenin Signaling and Is Required for Xenopus Myogenesis. Developmental Cell. 2004;7:525–534. doi: 10.1016/j.devcel.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Kazanskaya O, Ohkawara B, Heroult M, Wu W, Maltry N, Augustin HG, Niehrs C. The Wnt signaling regulator R-spondin 3 promotes angioblast and vascular development. Development. 2008;135:3655–3664. doi: 10.1242/dev.027284. [DOI] [PubMed] [Google Scholar]
- Kerkhof JM, Uitterlinden AG, Valdes AM, Hart DJ, Rivadeneira F, Jhamai M, Hofman A, Pols HA, Bierma-Zeinstra SM, Spector TD, van Meurs JB. Radiographic osteoarthritis at three joint sites and FRZB, LRP5, and LRP6 polymorphisms in two population-based cohorts. Osteoarthritis Cartilage. 2008;16:1141–1149. doi: 10.1016/j.joca.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Kikuchi A, Yamamoto H, Sato A. Selective activation mechanisms of Wnt signaling pathways. Trends Cell Biol. 2009;19:119–129. doi: 10.1016/j.tcb.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Kim KA, Kakitani M, Zhao J, Oshima T, Tang T, Binnerts M, Liu Y, Boyle B, Park E, Emtage P, Funk WD, Tomizuka K. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science. 2005;309:1256–1259. doi: 10.1126/science.1112521. [DOI] [PubMed] [Google Scholar]
- Kim KA, Wagle M, Tran K, Zhan X, Dixon MA, Liu S, Gros D, Korver W, Yonkovich S, Tomasevic N, Binnerts M, Abo A. R-Spondin family members regulate the Wnt pathway by a common mechanism. Mol Biol Cell. 2008;19:2588–2596. doi: 10.1091/mbc.E08-02-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KA, Zhao J, Andarmani S, Kakitani M, Oshima T, Binnerts ME, Abo A, Tomizuka K, Funk WD. R-Spondin proteins: a novel link to beta-catenin activation. Cell Cycle. 2006;5:23–26. doi: 10.4161/cc.5.1.2305. [DOI] [PubMed] [Google Scholar]
- Klauzinska M, Baljinnyam B, Raafat A, Rodriguez-Canales J, Strizzi L, Greer YE, Rubin JS, Callahan R. Rspo2/Int7 regulates invasiveness and tumorigenic properties of mammary epithelial cells. J Cell Physiol. 2012;227:1960–1971. doi: 10.1002/jcp.22924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn AD, Moon RT. Wnt and calcium signaling: beta-catenin-independent pathways. Cell Calcium. 2005;38:439–446. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Koo BK, Spit M, Jordens I, Low TY, Stange DE, van de Wetering M, van Es JH, Mohammed S, Heck AJ, Maurice MM, Clevers H. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature. 2012 doi: 10.1038/nature11308. [DOI] [PubMed] [Google Scholar]
- Kronke G, Uderhardt S, Kim KA, Stock M, Scholtysek C, Zaiss MM, Surmann-Schmitt C, Luther J, Katzenbeisser J, David JP, Abdollahi-Roodsaz S, Tran K, Bright JM, Binnerts ME, Akhmetshina A, Bohm C, Distler JH, Joosten LA, Schett G, Abo A. R-spondin 1 protects against inflammatory bone damage during murine arthritis by modulating the Wnt pathway. Arthritis Rheum. 2010;62:2303–2312. doi: 10.1002/art.27496. [DOI] [PubMed] [Google Scholar]
- Kuang SQ, Tong WG, Yang H, Lin W, Lee MK, Fang ZH, Wei Y, Jelinek J, Issa JP, Garcia-Manero G. Genome-wide identification of aberrantly methylated promoter associated CpG islands in acute lymphocytic leukemia. Leukemia. 2008;22:1529–1538. doi: 10.1038/leu.2008.130. [DOI] [PubMed] [Google Scholar]
- Kudryavtseva E, Forde TS, Pucker AD, Adarichev VA. Wnt signaling genes of murine chromosome 15 are involved in gender-affected pathways of inflammatory arthritis. Arthritis Rheum. 2011 doi: 10.1002/art.33414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SJ, Yen TY, Endo Y, Klauzinska M, Baljinnyam B, Macher B, Callahan R, Rubin JS. Loss-of-function point mutations and two-furin domain derivatives provide insights about R-spondin2 structure and function. Cell Signal. 2009;21:916–925. doi: 10.1016/j.cellsig.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CF, Bingham N, Parker K, Yao HH. Sex-specific roles of beta-catenin in mouse gonadal development. Hum Mol Genet. 2009;18:405–417. doi: 10.1093/hmg/ddn362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowther W, Wiley K, Smith GH, Callahan R. A New Common Integration Site, Int7, for the Mouse Mammary Tumor Virus in Mouse Mammary Tumors Identifies a Gene Whose Product Has Furin-Like and Thrombospondin-Like Sequences. J Virol. 2005;79:10093–10096. doi: 10.1128/JVI.79.15.10093-10096.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Kim KA, Liu J, Abo A, Feng X, Cao X, Li Y. R-spondin1 synergizes with Wnt3A in inducing osteoblast differentiation and osteoprotegerin expression. FEBS Lett. 2008;582:643–650. doi: 10.1016/j.febslet.2008.01.035. [DOI] [PubMed] [Google Scholar]
- McClanahan T, Koseoglu S, Smith K, Grein J, Gustafson E, Black S, Kirschmeier P, Samatar AA. Identification of overexpression of orphan G protein-coupled receptor GPR49 in human colon and ovarian primary tumors. Cancer Biol Ther. 2006;5:419–426. doi: 10.4161/cbt.5.4.2521. [DOI] [PubMed] [Google Scholar]
- McDonald T, Wang R, Bailey W, Xie G, Chen F, Caskey CT, Liu Q. Identification and cloning of an orphan G protein-coupled receptor of the glycoprotein hormone receptor subfamily. Biochem Biophys Res Commun. 1998;247:266–270. doi: 10.1006/bbrc.1998.8774. [DOI] [PubMed] [Google Scholar]
- Merlos-Suarez A, Barriga FM, Jung P, Iglesias M, Cespedes MV, Rossell D, Sevillano M, Hernando-Momblona X, da Silva-Diz V, Munoz P, Clevers H, Sancho E, Mangues R, Batlle E. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell. 2011;8:511–524. doi: 10.1016/j.stem.2011.02.020. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Miyachi Y. Congenital hyponychia without RSPO4 mutation. Acta Derm Venereol. 2008;88:511–512. doi: 10.2340/00015555-0476. [DOI] [PubMed] [Google Scholar]
- Nam JS, Park E, Turcotte TJ, Palencia S, Zhan X, Lee J, Yun K, Funk WD, Yoon JK. Mouse R-spondin2 is required for apical ectodermal ridge maintenance in the hindlimb. Developmental Biology. 2007a;311:124–135. doi: 10.1016/j.ydbio.2007.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam JS, Turcotte TJ, Yoon JK. Dynamic expression of R-spondin family genes in mouse development. Gene Expression Patterns. 2007b;7:306–312. doi: 10.1016/j.modgep.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Nam JS, Turcotte TJ, Smith PF, Choi S, Yoon JK. Mouse cristin/R-spondin family proteins are novel ligands for the Frizzled 8 and LRP6 receptors and activate beta-catenin-dependent gene expression. J Biol Chem. 2006;281:13247–13257. doi: 10.1074/jbc.M508324200. [DOI] [PubMed] [Google Scholar]
- Neufeld S, Rosin JM, Ambasta A, Hui K, Shaneman V, Crowder R, Vickerman L, Cobb J. A conditional allele of Rspo3 reveals redundant function of R-spondins during mouse limb development. Genesis. 2012 doi: 10.1002/dvg.22040. [DOI] [PubMed] [Google Scholar]
- Ohkawara B, Glinka A, Niehrs C. Rspo3 Binds Syndecan 4 and Induces Wnt/PCP Signaling via Clathrin-Mediated Endocytosis to Promote Morphogenesis. Developmental Cell. 2011;20:303–314. doi: 10.1016/j.devcel.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Oka D, Yamashita S, Tomioka T, Nakanishi Y, Kato H, Kaminishi M, Ushijima T. The presence of aberrant DNA methylation in noncancerous esophageal mucosae in association with smoking history. Cancer. 2009;115:3412–3426. doi: 10.1002/cncr.24394. [DOI] [PubMed] [Google Scholar]
- Park DS, Woodman SE, Schubert W, Cohen AW, Frank PG, Chandra M, Shirani J, Razani B, Tang B, Jelicks LA, Factor SM, Weiss LM, Tanowitz HB, Lisanti MP. Caveolin-1/3 double-knockout mice are viable, but lack both muscle and non-muscle caveolae, and develop a severe cardiomyopathic phenotype. Am J Pathol. 2002;160:2207–2217. doi: 10.1016/S0002-9440(10)61168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker HG, Chase K, Cadieu E, Lark KG, Ostrander EA. An insertion in the RSPO2 gene correlates with improper coat in the Portuguese water dog. The Journal of heredity. 2010;101:612–617. doi: 10.1093/jhered/esq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parma P, Radi O, Vidal V, Chaboissier MC, Dellambra E, Valentini S, Guerra L, Schedl A, Camerino G. R-spondin1 is essential in sex determination, skin differentiation and malignancy. Nat Genet. 2006;38:1304–1309. doi: 10.1038/ng1907. [DOI] [PubMed] [Google Scholar]
- Ruffner H, Sprunger J, Charlat O, Leighton-Davies J, Grosshans B, Salathe A, Zietzling S, Beck V, Therier M, Isken A, Xie Y, Zhang Y, Hao H, Shi X, Liu D, Song Q, Clay I, Hintzen G, Tchorz J, Bouchez LC, Michaud G, Finan P, Myer VE, Bouwmeester T, Porter J, Hild M, Bassilana F, Parker CN, Cong F. R-Spondin Potentiates Wnt/beta-Catenin Signaling through Orphan Receptors LGR4 and LGR5. PLoS ONE. 2012;7:e40976. doi: 10.1371/journal.pone.0040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Schuebel KE, Chen W, Cope L, Glockner SC, Suzuki H, Yi JM, Chan TA, Van Neste L, Van Criekinge W, van den Bosch S, van Engeland M, Ting AH, Jair K, Yu W, Toyota M, Imai K, Ahuja N, Herman JG, Baylin SB. Comparing the DNA hypermethylome with gene mutations in human colorectal cancer. PLoS Genet. 2007;3:1709–1723. doi: 10.1371/journal.pgen.0030157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte G, Bryja V. The Frizzled family of unconventional G-protein-coupled receptors. Trends Pharmacol Sci. 2007;28:518–525. doi: 10.1016/j.tips.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Seitz CS, van Steensel M, Frank J, Senderek J, Zerres K, Hamm H, Bergmann C. The Wnt signalling ligand RSPO4, causing inherited anonychia, is not mutated in a patient with congenital nail hypoplasia/aplasia with underlying skeletal defects. Br J Dermatol. 2007;157:801–802. doi: 10.1111/j.1365-2133.2007.08059.x. [DOI] [PubMed] [Google Scholar]
- Seshagiri S, Stawiski EW, Durinck S, Modrusan Z, Storm EE, Conboy CB, Chaudhuri S, Guan Y, Janakiraman V, Jaiswal BS, Guillory J, Ha C, Dijkgraaf GJ, Stinson J, Gnad F, Huntley MA, Degenhardt JD, Haverty PM, Bourgon R, Wang W, Koeppen H, Gentleman R, Starr TK, Zhang Z, Largaespada DA, Wu TD, de Sauvage FJ. Recurrent R-spondin fusions in colon cancer. Nature. 2012 doi: 10.1038/nature11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA, Shoemaker CM, Roeszler KN, Queen J, Crews D, Sinclair AH. Cloning and expression of R-Spondin1 in different vertebrates suggests a conserved role in ovarian development. BMC Dev Biol. 2008;8:72. doi: 10.1186/1471-213X-8-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr TK, Allaei R, Silverstein KA, Staggs RA, Sarver AL, Bergemann TL, Gupta M, O’Sullivan MG, Matise I, Dupuy AJ, Collier LS, Powers S, Oberg AL, Asmann YW, Thibodeau SN, Tessarollo L, Copeland NG, Jenkins NA, Cormier RT, Largaespada DA. A transposon-based genetic screen in mice identifies genes altered in colorectal cancer. Science. 2009;323:1747–1750. doi: 10.1126/science.1163040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanese K, Fukuma M, Yamada T, Mori T, Yoshikawa T, Watanabe W, Ishiko A, Amagai M, Nishikawa T, Sakamoto M. G-protein-coupled receptor GPR49 is up-regulated in basal cell carcinoma and promotes cell proliferation and tumor formation. Am J Pathol. 2008;173:835–843. doi: 10.2353/ajpath.2008.071091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodorou V, Kimm MA, Boer M, Wessels L, Theelen W, Jonkers J, Hilkens J. MMTV insertional mutagenesis identifies genes, gene families and pathways involved in mammary cancer. Nat Genet. 2007;39:759–769. doi: 10.1038/ng2034. [DOI] [PubMed] [Google Scholar]
- Tomaselli S, Megiorni F, De Bernardo C, Felici A, Marrocco G, Maggiulli G, Grammatico B, Remotti D, Saccucci P, Valentini F, Mazzilli MC, Majore S, Grammatico P. Syndromic true hermaphroditism due to an R-spondin1 (RSPO1) homozygous mutation. Hum Mutat. 2008;29:220–226. doi: 10.1002/humu.20665. [DOI] [PubMed] [Google Scholar]
- Tomizuka K, Horikoshi K, Kitada R, Sugawara Y, Iba Y, Kojima A, Yoshitome A, Yamawaki K, Amagai M, Inoue A, Oshima T, Kakitani M. R-spondin1 plays an essential role in ovarian development through positively regulating Wnt-4 signaling. Hum Mol Genet. 2008;17:1278–1291. doi: 10.1093/hmg/ddn036. [DOI] [PubMed] [Google Scholar]
- Tong WG, Wierda WG, Lin E, Kuang SQ, Bekele BN, Estrov Z, Wei Y, Yang H, Keating MJ, Garcia-Manero G. Genome-wide DNA methylation profiling of chronic lymphocytic leukemia allows identification of epigenetically repressed molecular pathways with clinical impact. Epigenetics. 2010;5:499–508. doi: 10.4161/epi.5.6.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- van Meurs JB, Trikalinos TA, Ralston SH, Balcells S, Brandi ML, Brixen K, Kiel DP, Langdahl BL, Lips P, Ljunggren O, Lorenc R, Obermayer-Pietsch B, Ohlsson C, Pettersson U, Reid DM, Rousseau F, Scollen S, Van Hul W, Agueda L, Akesson K, Benevolenskaya LI, Ferrari SL, Hallmans G, Hofman A, Husted LB, Kruk M, Kaptoge S, Karasik D, Karlsson MK, Lorentzon M, Masi L, McGuigan FE, Mellstrom D, Mosekilde L, Nogues X, Pols HA, Reeve J, Renner W, Rivadeneira F, van Schoor NM, Weber K, Ioannidis JP, Uitterlinden AG. Large-scale analysis of association between LRP5 and LRP6 variants and osteoporosis. JAMA. 2008;299:1277–1290. doi: 10.1001/jama.299.11.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasif N, Ahmad W. A Novel Nonsense Mutation in RSPO4 Gene Underlies Autosomal Recessive Congenital Anonychia in a Pakistani Family. Pediatric dermatology. 2012 doi: 10.1111/j.1525-1470.2011.01587.x. [DOI] [PubMed] [Google Scholar]
- Wei Q, Yokota C, Semenov MV, Doble B, Woodgett J, He X. R-spondin1 Is a High Affinity Ligand for LRP6 and Induces LRP6 Phosphorylation and β-Catenin Signaling. Journal of Biological Chemistry. 2007;282:15903–15911. doi: 10.1074/jbc.M701927200. [DOI] [PubMed] [Google Scholar]
- Yamada W, Nagao K, Horikoshi K, Fujikura A, Ikeda E, Inagaki Y, Kakitani M, Tomizuka K, Miyazaki H, Suda T, Takubo K. Craniofacial malformation in R-spondin2 knockout mice. Biochem Biophys Res Commun. 2009;381:453–458. doi: 10.1016/j.bbrc.2009.02.066. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Komekado H, Kikuchi A. Caveolin is necessary for Wnt-3a-dependent internalization of LRP6 and accumulation of beta-catenin. Dev Cell. 2006;11:213–223. doi: 10.1016/j.devcel.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Sakane H, Yamamoto H, Michiue T, Kikuchi A. Wnt3a and Dkk1 regulate distinct internalization pathways of LRP6 to tune the activation of beta-catenin signaling. Dev Cell. 2008;15:37–48. doi: 10.1016/j.devcel.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Zhao J, de Vera J, Narushima S, Beck EX, Palencia S, Shinkawa P, Kim KA, Liu Y, Levy MD, Berg DJ, Abo A, Funk WD. R-spondin1, A Novel Intestinotrophic Mitogen, Ameliorates Experimental Colitis in Mice. Gastroenterology. 2007;132:1331–1343. doi: 10.1053/j.gastro.2007.02.001. [DOI] [PubMed] [Google Scholar]