SUMMARY
Trp63, a transcription factor related to the tumor suppressor p53, is activated by diverse stimuli and can initiate a range of cellular responses. TAp63 is the predominant Trp53 family member in primordial follicle oocytes and essential for their apoptosis triggered by DNA damage in vivo. Following γ-irradiation, induction of the pro-apoptotic BH3-only members Puma and Noxa was observed in primordial follicle oocytes from wt and Trp53−/− mice but not in those from TAp63 deficient mice. Primordial follicle oocytes from mice lacking Puma or both Puma and Noxa were protected from γ-irradiation-induced apoptosis and, remarkably, could produce healthy offspring. Hence, PUMA and NOXA are critical for DNA damage-induced, TAp63-mediated primordial follicle oocyte apoptosis. Thus, blockade of PUMA may protect fertility during cancer therapy and prevent premature menopause, improving women’s health.
Keywords: Trp63, PUMA, NOXA, γ-irradiation-induced apoptosis, oocytes, fertility, BH3-only proteins
INTRODUCTION
Trp63 (also called p63), a transcription factor related to Trp53 (also called p53), the “guardian of the genome” (Lane, 1992), is essential for craniofacial, skin and limb development (Mills et al., 1999; Yang et al., 1999). Defects in Trp63 function have been implicated in tumorigenesis and failure of tumor cells to respond to DNA damage-inducing anti-cancer therapeutics (Leong et al., 2007). Enforced expression of TAp63, the major Trp63 isoform with transactivating activity, was reported to activate multiple cellular responses that are also induced by Trp53, including cell cycle arrest and apoptotic cell death (Yang et al., 1998). However, the physiological relevance of some of these processes remains unclear and the effectors of the signaling pathways that activate these processes have not been identified. TAp63 is the predominant Trp53 family member in primordial follicle oocytes and is essential for apoptosis of primordial oocytes following DNA damage in vivo (Livera et al., 2008; Suh et al., 2006).
The Trp63 gene encodes two major isoform subsets: TAp63-α,β,γ, which have trans-activating activity, and deltaNp63-α,β,γ (N-terminal truncated), which have distinct transcriptional regulatory functions, possibly including “dominant negative” action (Yang et al., 1999). Trp53, the so-called “guardian of the genome” (Lane, 1992), exerts its tumor suppressor function in part (in at least certain contexts, such as Myc over-expression) through activation of apoptosis, and this requires transcriptional induction of the BH3-only pro-apoptotic Bcl-2 family members, PUMA (also known as BBC3) and, albeit to a lesser extent, NOXA (also known as PMAIP1 (Garrison et al., 2008; Jeffers et al., 2003; Michalak et al., 2009; Michalak et al., 2008; Shibue et al., 2003; Villunger et al., 2003). PUMA and NOXA, like all BH3-only proteins, initiate apoptosis by activating the multi-BH domain pro-apoptotic BCL-2 family members, BAX and BAK, either directly or indirectly by binding to pro-survival BCL-2 family members thereby unleashing BAX and BAK (Chipuk and Green, 2008; Strasser et al., 2011; Youle and Strasser, 2008). PUMA is the more potent apoptosis inducer, because it can bind with high affinity to all pro-survival BCL-2 family members, whereas NOXA only binds to MCL-1 and A1 (also known as BCL2A1A or BFL-1) (Chipuk and Green, 2008; Strasser et al., 2011; Youle and Strasser, 2008).
The pool of primordial follicles, established within the ovary around the time of birth, is crucial for fertility because it provides all of the oocytes for post-pubertal ovulations (Beaumont, 1962). Indeed, the importance of apoptosis in fertility has been demonstrated by inactivation of the pro-apoptotic Bax gene, which results in elevated follicle numbers and significantly prolonged fertility into advanced age (Perez et al., 1999). The extended ovarian lifespan afforded by loss of Bax was associated with health benefits, including ameliorating bone and muscle loss and was not accompanied by increased tumor incidence (Perez et al., 2007). Of particular importance, other than one primary report (Suh et al., 2006), previous literature does not address the mechanism of loss of primordial follicle oocytes, those oocytes responsible for long-term protection of the female germline, either spontaneously or after DNA damage. We focus on the mechanism by which DNA damage causes loss of primordial follicle oocytes with consequent infertility in female mice.
Longevity combined with the suspended diplotene state of their chromosomes render the oocytes in primordial follicles vulnerable to DNA damage (Hanoux et al., 2007). To protect against the introduction of germ-line mutations, it is critical that genomic integrity of oocytes is subject to stringent surveillance, with detection and repair of DNA damage or elimination through apoptosis of those oocytes with compromised genomic fidelity (Ashwood-Smith and Edwards, 1996; Tilly, 2001). Expression of Trp63, in particular the TAp63α isoform, is abundant in primordial follicle oocyte nuclei (Livera et al., 2008) and is essential for their death following genotoxic stress (Suh et al., 2006). In contrast, its close relative Trp53 is dispensable for this process (Livera et al., 2008; Suh et al., 2006). The predominance of TAp63α in DNA damage induced apoptosis of primordial follicle oocytes provides an opportunity to identify the mechanism by which TAp63α triggers cell killing.
Accordingly, we took advantage of mice lacking either a member of the p53 family or one or more pro-apoptotic BH3-only members of the BCL-2 family to reveal the molecular mechanisms of DNA damage induced apoptosis in primordial follicle oocytes. Indeed, following γ-irradiation, we observed induction of expression of pro-apoptotic Puma and Noxa in oocytes from wt and Trp53−/− mice but not from mice deficient for TAp63. Primordial follicle oocytes were protected from γ-irradiation induced apoptosis in Puma−/− mice and even more profoundly in Puma−/−Noxa−/− mice. Remarkably, γ-irradiated Puma−/− and Puma−/−Noxa−/− females produced healthy offspring. These data demonstrate that PUMA and NOXA are essential for DNA damage-induced, TAp63-mediated apoptosis of primordial follicle oocytes and that folliculogenesis and fertility may be preserved during anti-cancer therapy by blocking PUMA alone or PUMA and NOXA. As protection of primordial follicles in women undergoing DNA-damaging anti-cancer treatment is currently poorly understood (Xu et al., 2011), this work makes an important contribution by demonstrating that the immature murine oocyte can survive DNA damage and produce live offspring when protected from death by loss of PUMA.
RESULTS
Expression of Puma and Noxa in Primordial Follicle Oocytes Following γ-Irradiation-Induced DNA Damage
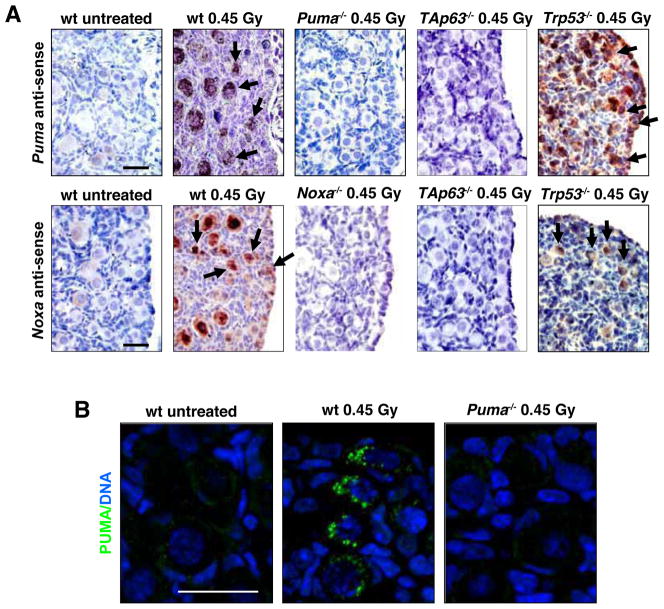
To begin to examine whether PUMA and NOXA might be critical for TAp63α-mediated apoptosis in oocytes that have sustained DNA damage, we studied expression of BH3-only genes following γ-irradiation. Exposure to 0.45 or 4.5 Gy γ-irradiation induced Puma as well as Noxa mRNA and PUMA protein expression in primordial follicle oocytes from postnatal day 5 (PN5) C57BL/6 (wt) mice (Figure 1 and Figure S1A, D). Induction of Puma and Noxa was also seen in primordial follicle oocytes from γ-irradiated Trp53−/− mice (Figure 1 and Figure S1C, F) but not in those deficient for TAp63 (Figure 1 and Figure S1C, F). As previously reported (Suh et al., 2006), TAp63 protein was readily detected in primordial follicles of both untreated as well as γ-irradiated (4.5 Gy) wt mice, but this was, as expected (Suh et al., 2006), not seen in TAp63 mutant mice (Figure S1G). These results show that TAp63 but not Trp53 is essential for DNA damage triggered transcriptional induction of Puma and Noxa in primordial follicle oocytes.
Figure 1. Expression of Puma mRNA, Noxa mRNA and PUMA Protein in Primordial Follicle Oocytes Following γ-irradiation-induced DNA damage.
(A) Ovaries were harvested from PN5 wt, Puma−/− (negative control), Noxa−/− (negative control), Trp53−/− and TAp63 mutant mice at 0 (untreated control) and 3 h post whole-body γ-irradiation (0.45 Gy). In situ hybridization (top panel) was performed using anti-sense probes for Puma and Noxa. Puma and Noxa mRNAs were detected in primordial follicle oocytes from wt and Trp53−/− mice but not in those from TAp63 mutant mice, 3 h post γ-irradiation. Arrows indicate positively stained primordial follicle oocytes (dark purple/brown staining). Control experiments, including in situ hybridization of sections from Puma−/− ovaries with Puma anti-sense probes and in situ hybridization of sections from Noxa−/− ovaries with Noxa anti-sense probes are shown in Figure S1. (B) PUMA antibody immunofluorescent staining (bottom panel; green) in wt and Puma−/− (negative control) primordial follicle oocytes at 6 h post γ-irradiation. Scale bar: 20 μm.
Loss of PUMA and even more so Combined Loss of PUMA and NOXA Rescues Primordial Follicle Oocytes from DNA Damage-Induced Apoptosis
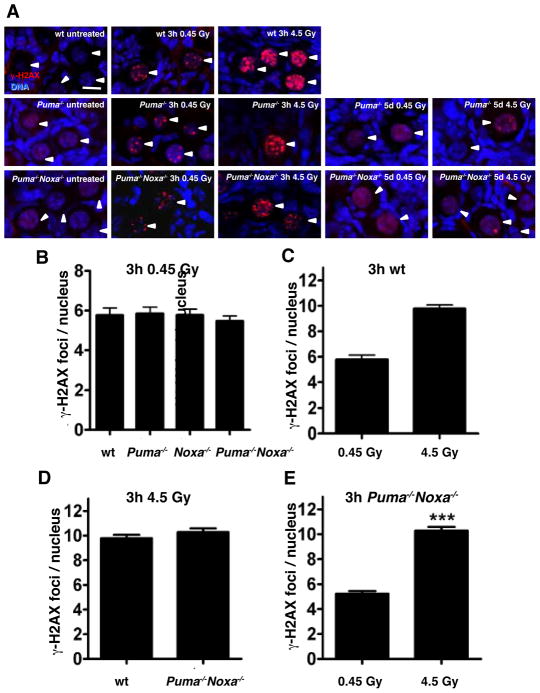
To examine whether PUMA and/or NOXA constitute the essential pro-apoptotic effectors in the death mediated by TAp63α in response to DNA damage, we exposed PN5 Puma−/−, Noxa−/−, Puma−/−Noxa−/− and, as controls, PN5 wt as well as Trp53−/− mice to 0.45 or 4.5 Gy γ-irradiation (or left them untreated) and counted follicle numbers in PN10 ovaries. Immunostaining for a marker of double-strand DNA breaks (γ-H2AX) at the early time-points (3–6 h), revealed that γ-irradiation induced comparable levels of DNA damage in oocytes from wt, Puma−/− and Puma−/− Noxa−/− mice, with increased DNA damage evident at the higher dose (Figure 2A–E). Immunostaining for phosphorylated (i.e. activated) ATM (Ataxia Telangiectasia Mutated) kinase revealed that the DNA damage response was activated within 3 h in primordial follicle oocytes of all genotypes (Figure S2A). Importantly oocytes from Puma−/− and Puma−/−Noxa−/− mice, which were protected from death (see below), were capable of resolving the DNA damage within 5 days, as evidence of prior DNA damage could no longer be detected after 5 days with only a few Puma−/− and Puma−/−Noxa−/− oocytes exhibiting 1 or 2 γ-H2AX foci (Figure 2A) and high levels of phosphorylated ATM were no longer evident (Figure S2A). Consistent with previous reports TUNEL staining confirmed that γ-irradiation induced extensive apoptosis in primordial follicle oocytes of wt mice (Figure S2B) (Livera et al., 2008; Suh et al., 2006).
Figure 2. Detection of DNA Damage and Apoptosis in Primordial Follicle Oocytes Following γ-Irradiation.
(A) DNA double-strand breaks were detected in oocytes from untreated wt primordial follicles and 3 h after exposure to whole-body γ-irradiation at the lower (0.45 Gy) and higher (4.5 Gy) doses by immunofluorescent staining with antibodies to γH2AX (red). Ovaries from Puma−/−, Noxa−/− and Puma−/−Noxa−/− mice were also analyzed 3 h after low dose (0.45 Gy) γ-irradiation. γ-H2AX foci were resolved in Puma−/− and Puma−/−Noxa−/− primordial follicle oocytes within 5 days of γ-irradiation induced DNA damage (no primordial follicles survive at this timepoint). Female Puma−/− and Puma−/−Noxa−/− mice (PN5) were left untreated or exposed to whole body γ-irradiation (0.45 and 4.5 Gy) and then 5 days later (PN10) ovaries were analyzed for double-strand DNA breaks by immunofluorescent staining with antibodies to γ-H2AX (red). γ-H2AX foci could no longer be detected in oocytes 5 days following γ-irradiation for either genotype at 0.45 Gy. Even at the higher dose of γ-irradiation (4.5 Gy), evidence of prior DNA damage could no longer be detected after 5 days in most oocytes, with only a few Puma−/− and Puma−/−Noxa−/− oocytes exhibiting 1 or 2 γ-H2AX foci. Arrowheads indicate the nuclei of primordial follicle oocytes. (B) γ-irradiation-induced DNA damage was quantified by counting the numbers of γ-H2AX foci within the nuclei of wt, Puma−/−, Noxa−/− and Puma−/−Noxa−/− primordial follicles 3 h after exposure to whole-body γ-irradiation (0.45 Gy). No significant differences in the numbers of foci were observed between females of the different genotypes (p>0.05). (C) The numbers of γ-H2AX foci within the nuclei of wt primordial follicles 3 h after exposure to whole-body γ-irradiation at the lower (0.45 Gy) and higher (4.5 Gy) dose were quantified. ***p<0.001. (D) The numbers of γ-H2AX foci within the nuclei of wt and Puma−/−Noxa−/− primordial follicles 3 h after exposure to the higher dose of whole-body γ-irradiation (4.5 Gy) were quantified. No significant difference was observed in the numbers of foci between the different genotypes (p>0.05). (E) The numbers of γ-H2AX foci within the nuclei of Puma−/−Noxa−/− primordial follicles were quantified as in (C), i.e. 3 h after exposure to whole-body γ-irradiation at the lower (0.45 Gy) and higher (4.5 Gy) dose. EB represent means ± SEM, n=3–5 mice. ***p<0.001. n=50–100 primordial follicles for all. Scale bar: 20 μm.
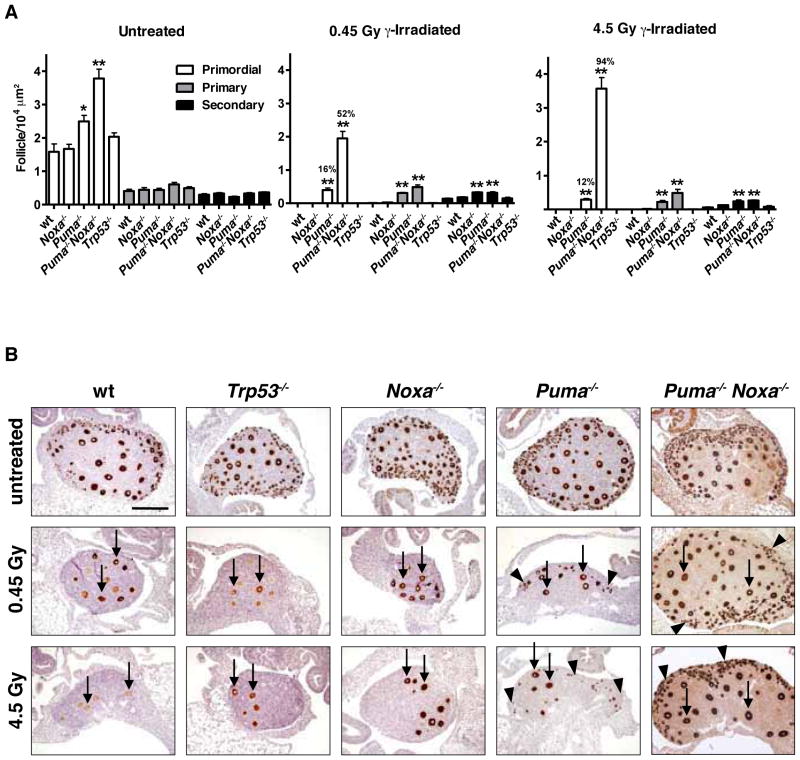
Untreated Puma−/− and Puma−/−Noxa−/− mice had 1.5- to 2.4-fold increased primordial follicle numbers compared to wt or Noxa−/− mice (p<0.05 or p<0.001, respectively; Figure 3A), revealing that PUMA-mediated apoptosis limits oocyte supply during the establishment of the primordial follicle pool in the developing ovary. As reported (Livera et al., 2008; Suh et al., 2006), 0.45 or 4.5 Gy whole-body γ-irradiation resulted in complete destruction of the primordial follicle pool in wt and Trp53−/− mice (Figure 3A). In striking contrast, after 0.45 Gy γ-irradiation 16±3% (mean ± SEM, range 9–27%) of primordial follicles were protected from apoptosis in Puma−/− mice (p<0.001) and 52±6% (range 25–71%) in Puma−/−Noxa−/− mice (Puma−/−Noxa−/− vs wt: p<0.001; Puma−/−Noxa−/− vs Puma−/−: p<0.001) (Figure 3A). Following 4.5 Gy γ-irradiation 12±1% (mean ± SEM, range 7–15%) of primordial follicles in Puma−/− mice (p<0.001) and a remarkable 94±8% in Puma−/−Noxa−/− mice (range 78–100%; Puma−/−Noxa−/− vs wt: p<0.001; Puma−/−Noxa−/− vs Puma−/−: p<0.001) were protected from apoptosis (Figure 3A). No protection was observed in mice lacking Noxa alone or even in mice lacking Noxa and one allele of Puma (Puma+/−Noxa−/−; Figure 3A and data not shown). Similarly, no protection was observed in mice lacking either Bim or Bmf (Figure S3), two pro-apoptotic BH3-only proteins that play critical roles in the programmed death of several other cell types (Bouillet et al., 1999; Labi et al., 2008).
Figure 3. Loss of PUMA Rescues Primordial Follicle Oocytes from DNA Damage-Induced Apoptosis.
(A) Quantification (means ± SEM) of follicles from mice of the indicated genotypes either exposed to whole body γ-irradiation (0.45 or 4.5 Gy) or untreated at PN5 and analyzed at PN10. Percent primordial follicle survival (compared to untreated) shown above each bar. n=3–8 animals/genotype. For comparison with wt: * p<0.05, ** p<0.001. (B) MSY2 (the germ cell specific Y-box cytoplasmic protein marker, MSY2, which stains all follicle types) antibody staining of ovaries of PN10 wt, Noxa−/−, Puma−/−, Puma−/−Noxa−/− and Trp53−/− mice 5 days after exposure to 0.45 or 4.5 Gy γ-irradiation or no treatment (control). Arrows indicate intrinsically DNA damage resistant growing (post-primordial) follicles. Black arrowheads indicate primordial follicles. Scale bar 200 μm.
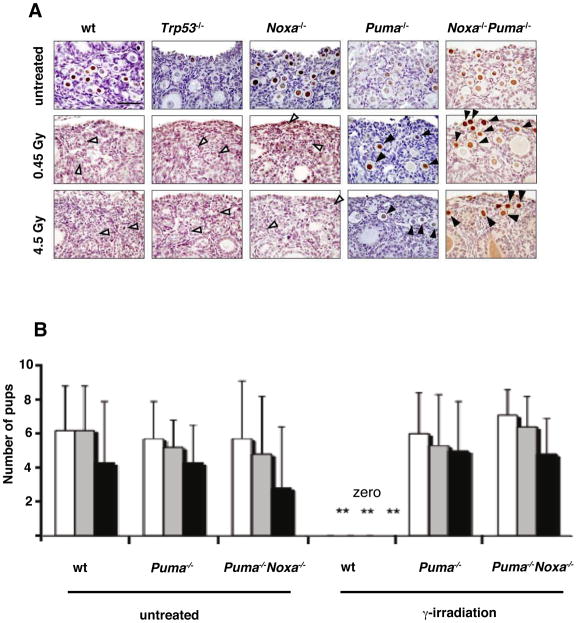
To support the histological enumeration of oocytes in particular follicle types, their identity in ovaries from untreated and γ-irradiated mice was confirmed by staining with antibodies to the germ cell specific Y-box cytoplasmic protein marker, MSY2, which stains all follicle types (Figure 3B) and the nuclear marker, germ cell nuclear antigen (GCNA), which importantly stains only primordial follicles, the focus of this study (Figure 4A). Growing follicles (primary and secondary), which show partial resistance to DNA damage induced apoptosis (Hanoux et al., 2007), were also present at higher numbers after γ-irradiation in Puma−/− and Puma−/−Noxa−/− mice (Figures 3 and 4A). In contrast, only remnants of empty primordial follicles in which no viable oocytes remained were evident in γ-irradiated wt or Trp53−/− mice (Figures 3B and 4A). Strikingly, primordial follicles that survived γ-irradiation were readily detectable in Puma−/− and considerably more so in Puma−/−Noxa−/− mice (Figures 3B and 4A).
Figure 4. Loss of PUMA Rescues GCNA-Positive Primordial Follicle Oocytes from DNA Damage Induced Apoptosis and Loss of PUMA or PUMA plus NOXA Rescue Fertility in γ-Irradiated Mice.
(A) Anti-GCNA (the nuclear marker, germ cell specific antigen which stains only primordial follicles) antibody staining of ovaries of PN10 wt, Trp53−/−, Noxa−/−, Puma−/− and Puma−/−Noxa−/− mice five days after exposure to 0.45 Gy or 4.5 Gy γ-irradiation or no treatment (control). Arrows indicate intrinsically resistant growing follicles. Black arrowheads indicate primordial follicles. White arrowheads indicate primordial follicle remnants. Scale bar: 20 μm. (B) Female wt, Puma−/− and Puma−/−Noxa−/− mice (PN5) were left untreated or exposed to whole-body γ-irradiation (0.45 Gy) and after at least 7 weeks of age (i.e. 45 days post γ-irradiation) breeding trials were commenced. The numbers of pups from first and second litters from matings to wt C57BL/6 proven male breeders are shown. ** p value <0.001 for Puma−/− γ-irradiated vs wt γ-irradiated or Puma−/−Noxa−/− γ-irradiated vs wt γ-irradiated, for all categories. All other comparisons NS. Second litters from Puma−/− and Puma−/−Noxa−/− females were included to show protection was not limited to the first litter. EB represent Standard Deviation, n=6–12 litters See also Table 1, 2 and Table S1.
Histological examination and immunohistochemical staining revealed a complete absence of normal primordial follicles in γ-irradiated wt, Trp53−/− and Noxa−/− ovaries (Figures 3B and 4A). The protection of histologically normal primordial follicles observed at the higher dose (4.5 Gy) of γ-irradiation in Puma−/−Noxa−/− mice (Figure 3A) was impressive, although slightly less than that reported for loss of TAp63 (Livera et al., 2008; Suh et al., 2006). Collectively, these results demonstrate that PUMA and, albeit to a lesser extent, NOXA are essential for DNA damage induced TAp63-mediated killing of primordial follicle oocytes.
Loss of Puma or Puma and Noxa Preserves Fertility in γ-Irradiated Females, Allowing Production of Healthy Offspring
Endogenous, environmental or anti-cancer therapy-induced DNA damage can cause oocyte depletion, resulting in female sterility (Jeruss and Woodruff, 2009). We investigated whether resistance to DNA damage-induced apoptosis afforded to primordial follicle oocytes by loss of PUMA or combined loss of PUMA and NOXA could allow survival of functionally significant numbers of primordial follicle oocytes and therefore preserve fertility. In the mouse, folliculogenesis from primordial follicle activation to ovulation requires ~20 days (Pedersen, 1970). We therefore surmised that through 45 days, all growing follicles (primary and more mature) present in the PN5 ovaries at the time of γ-irradiation would have either undergone atresia or completed folliculogenesis and ovulated prior to the initiation of fertility trials. Hence, ovulatory follicles present beyond 7 weeks after γ-irradiation at PN5 were considered to be derived from primordial follicles that had survived this insult. Remarkably, 13 out of 16 Puma−/− females and 9 out of 12 Puma−/−Noxa−/− females that had been γ-irradiated at PN5 (0.45 Gy) and mated from 7 weeks of age with non-irradiated wt or Puma−/− proven males produced viable offspring, whereas all (5 out of 5) γ-irradiated PN5 wt females tested were, as expected (Beaumont, 1962), infertile (Figure 4B). All thirteen fertile γ-irradiated Puma−/− females produced ≥2 litters (Table 1A). Eight of these females produced ≥4 litters; no difference was seen in the ability of Puma−/− or Puma−/−Noxa−/− females to produce fourth litters (Table 1B, p=0.16). Remarkably, of first and second litters from γ-irradiated Puma−/− females mated to wt males, the proportion of healthy offspring present at weaning was similar to that observed for litters from non-irradiated Puma−/− or wt females (p=0.62; Figure 4B), confirming that γ-irradiated primordial follicles could give rise to healthy pups. Notably, even five out of six Puma−/− females γ-irradiated (4.5 Gy) as adults (PN49 or 7 weeks of age), were able to bear healthy offspring, whereas none of the five γ-irradiated wt adult females regained fertility (Table 1C).
Table 1. Loss of PUMA or Loss of both PUMA and NOXA Preserves Fertility Following γ-Irradiation.
Loss of Puma or both Puma and Noxa protects fertility following γ-irradiation. Litters produced by females, which had themselves either been left untreated or γ-irradiated. (A) Offspring from 1st and 2nd litters (wt, Puma−/− or Puma−/−Noxa−/− females γ-irradiated (0.45 Gy) at PN5, included in Figure 6); (B) Offspring from 4th litters (Puma−/− or Puma−/−Noxa−/− females γ-irradiated (0.45 Gy) at PN5, included in Figure 6); (C) Females (wt or Puma−/−) which had been γ-irradiated (4.5 Gy) as adults (PN49) were tested for fertility. Adult mice (wt and Puma−/−) were γ-irradiated (4.5 Gy) at 7 weeks of age, mated to vasectomized males for 7 weeks and then mated with non-irradiated, proven C57BL/6 males; (D) Fourteen F1 female offspring (not γ-irradiated themselves) from γ-irradiated mothers were set up for breeding and 13 bred successfully, producing 514 F2 offspring, i.e. F2 offspring of P females γ-irradiated at PN5 (the 14th F1 female set up for breeding was culled due to birthing difficulties during first delivery).
| Table 1A. 1st and 2nd litters from females ± γ-irradiated (0.45 Gy) as pups (PN5) | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Genotype of females | Untreated | γ-irradiated | ||||
|
| ||||||
| Number of females tested | Total number of litters | Number of live offspring at weaning | Number of females tested | Total number of litters | Number of live offspring at weaning | |
| wt | 6 | 6 | 26 | 5 | 0 | 0 |
| Puma−/− | 4 | 6 | 26 | 8 | 13 | 65 |
| Puma−/− Noxa−/− | 6 | 12 | 31 | 7 | 11 | 38 |
| Table 1B. 4th litters from females ± γ-irradiated (0.45 Gy) as pups (PN5) | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Genotype of females | Untreated | γ-irradiated | ||||
|
| ||||||
| Number of females tested | Total number of pups born/litter | Number of live offspring at birth/litter | Number of females tested | Total number of pups born/litter | Number of live offspring at birth/litter | |
| Puma−/− | 8 (8 litters) | 7.8 ± 2.8 | 6.9 ± 2.6 | 9 (9 litters) | 5.7 ± 2.4 | 4.1 ± 2.5 |
| Puma−/− Noxa−/− | 3 (3 litters) | 7.0 ± 1.7 | 5.7 ± 2.6 | 3 (3 litters) | 7.0 ± 3.0 | 6.7 ± 2.5 |
| Table 1C. Females γ-irradiated (4.5 Gy) as adults (PN48) | |||
|---|---|---|---|
| Genotype of females | γ-irradiated | ||
| Number of females tested/litters obtained | Total number of pups born /litter | Number of live offspring at birth/litter | |
| wt | 5 (0 litters) | 0 | 0 |
| Puma−/− | 6 (5 litters) | 4.2 ± 3.2 | 3.7 ± 3.4 |
| Table 1D. Females are F1 offspring of γ-irradiated females | |||
|---|---|---|---|
| Genotype of females | Untreated | ||
| Number of females tested | Number of females producing litters | Number of litters produced | |
| Puma−/− (PN5, 0.45 Gy) | 4 | 4 | 1–4 litters each |
| Puma−/− (PN49, 4.5 Gy) | 2 | 2 | 7,8 litters |
| Puma−/− Noxa−/− (PN5, 0.45 Gy) | 6 | 5 (6th had birth difficulties) | 1,4,6,7,9 litters |
| Puma−/− Noxa−/− (PN49, 4.5 Gy) | 2 | 2 | 6,6 litters |
In total, 438 F1 offspring of γ-irradiated females were generated in these studies (from females irradiated at either PN5 (0.45 Gy) or PN49 (4.5 Gy)). The majority of these offspring were culled at weaning at PN 28–30 and a representative selection of outcomes prior to, or at weaning, is described above (Table 1A–C). Fourteen F1 female offspring from γ-irradiated mothers were set up for breeding and 13 bred successfully (Table 1D). Within the 241 F1 and F2 offspring followed beyond weaning, no deformities were noted, above those observed at low incidence (~1–2%) for this strain of mice in our colony (O’Reilly et al., 2009) (Table 2 and Table S1). Four F1 offspring from Puma−/− mothers γ-irradiated at PN5 (0.45 Gy) were followed for >400 days, as were six F1 offspring from γ-irradiated Puma−/−Noxa−/− mothers. For offspring from mothers γ-irradiated in adult life (PN49, 4.5 Gy), five F1 offspring from Puma−/− mothers were followed for >400 days. None of these animals showed obvious abnormalities or developed diseases, such as cancer. Collectively, these results demonstrate that loss of PUMA or combined loss of PUMA and NOXA preserves fertility in γ-irradiated female mice, including those γ-irradiated as adults, allowing production of healthy offspring.
Table 2.
No Deformities Observed in F1 Offspring of γ-Irradiated Females Lacking PUMA or both PUMA and NOXA
Following γ-irradiation of female mice lacking either PUMA or both PUMA and NOXA, at either PN5 (0.45 Gy) or PN49 (4.5 Gy), 438 F1 offspring were generated. Of the 208 F1 offspring followed beyond weaning, no deformities were noted, above those observed for this strain of mice in our colony (O’Reilly et al., 2009). Mice were inspected twice weekly and more frequently if any problem was noted. Mice were observed for variable lengths of time as indicated.
| Table 2. F1 offspring from γ-irradiated females | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 0.45 Gy as pups (PN5) | 4.5 Gy as adults (PN49) | ||||||
|
| |||||||
| Genotype of γ-irradiated females | Number of F1 offspring | Followed post weaning | Followed 100–200 d | Followed > 200 d | Number of F1 offspring | Followed post weaning | Followed > 200 d |
| Puma−/− | 169 | 104 | 11 | 22 | 38 | 29 | 11 |
| Puma−/− Noxa−/− | 203 | 64 | 17 | 24 | 28 | 11 | 8 |
| Genetic deformities | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
|
| |||||||
| Total 438 | 372 | 168 | 28 | 46 | 66 | 40 | 19 |
Discussion
In order to address the fundamental issue of how the p53 family member, Trp63, induces apoptosis, we chose to examine a physiological system in which TAp63 is the predominant p53-family isoform. Most importantly, the oocyte system represents arguably one of the most important functions of Trp63, that of protection of the female germline. Indeed, we have combined the use of relevant BH3-only gene deleted mouse strains and our knowledge of apoptosis, oocyte biology and fertility to demonstrate that Trp63 acts via transcriptional induction of the BH3-only proteins, PUMA and NOXA to cause apoptosis of DNA-damaged primordial follicle oocytes. Strikingly, if PUMA or both PUMA and NOXA are deleted, then the oocyte is capable of highly efficient DNA repair and subsequent normal function, producing healthy offspring. Thus, PUMA and NOXA are critical for DNA damage-induced, TAp63-mediated oocyte apoptosis.
We have shown that γ-irradiation (0.45 or 4.5 Gy) induced Puma as well as Noxa mRNA and PUMA protein expression in primordial follicle oocytes from postnatal day 5 (PN5) wt mice. Induction of Puma and Noxa was also seen in oocytes from γ-irradiated Trp53−/− mice but not in those deficient for TAp63. The observed TAp63-dependent induction of Puma mRNA synthesis post DNA damage in oocytes is supported by data from over-expression studies that revealed TAp63 binding to the Puma promoter with consequent Puma induction (Bergamaschi et al., 2004; Khokhar et al., 2008; Patel et al., 2008). Our data are also in keeping with the fact that the BH3-only proteins, PUMA and NOXA are the predominant pro-apoptotic effectors activated by p53 (Jeffers et al., 2003; Shibue et al., 2003; Villunger et al., 2003). Moreover, when over-expressed, the DNA binding domain of TAp63 has been shown to bind to the Puma promoter in association with the transcriptional co-factors, apoptosis stimulating p53 proteins (ASPPs), the first recognized common co-activators of the p53 protein family (Bergamaschi et al., 2004; Patel et al., 2008). In addition, in human cancer derived cell lines, TAp63γ over-expression was reported to induce Puma expression (Khokhar et al., 2008). Conversely, in keeping with its proposed dominant negative action and transcriptional repressive function, RNAi-mediated knock-down of the deltaNp63 isoforms de-repressed TAp63 target genes, causing Trp53-independent induction of Puma with consequent reduction in clonogenicity of primary human keratinocytes, diminished proliferation of human cancer derived cell lines and induction of BCL-2-inhibitable apoptosis (Rocco et al., 2006).
The fertility implications of this study inform an intense debate about whether or not immature ovarian follicles can be protected in women undergoing DNA-damaging anti-cancer treatment (Xu et al., 2011). It is unclear whether it is possible to rescue oocytes at the time women are treated with DNA-damaging anti-cancer therapy, let alone whether such “rescued” oocytes would be capable of appropriate DNA repair to allow generation of live offspring (Xu et al., 2011). Answering these questions has profound implications for the infertility, which develops in women undergoing such treatment, as well as for the timing of premature menopause in young women and perhaps even natural menopause. We have uncovered the molecular mechanisms by which DNA damage kills primordial follicle oocytes and have thereby revealed that, at least in the mouse, oocytes can be rescued from apoptosis by blocking pro-apoptotic PUMA alone or both PUMA and NOXA, indeed facilitating DNA repair to occur, allowing the production of live, healthy offspring.
Primordial follicles are structures, which house immature oocytes containing the female germline, arrested in meiotic prophase I. In mammals, the population of primordial follicles is established during fetal or early neonatal development and it is from this original pool of germ cells that all eggs for ovulation are eventually drawn. In the case of women, a primordial follicle may exist in this unique stasis for a period of 40 years or more, before being activated to enter the folliculogenesis developmental pathway that ultimately culminates in the ovulation of a mature egg. Extreme longevity, combined with the unusual diplotene state of their nucleus, may make primordial follicle oocytes especially vulnerable to DNA damage. Therefore, to ensure that mutations are not introduced into the germ line, it is critical that oocyte genomic integrity be under constant surveillance. DNA damage must be detected and the decision made to either initiate/continue repair or to eliminate, through apoptosis, those primordial follicle oocytes with compromised genomic fidelity. Female fertility relies heavily on the ability of oocytes to undertake repair, together with tight regulation of the life/death decision, because although tolerance of DNA damage by oocytes is a source of potential mutations, which may lead to failed pregnancies or birth defects, the uncontrolled apoptotic elimination of oocytes results in a shortened reproductive lifespan.
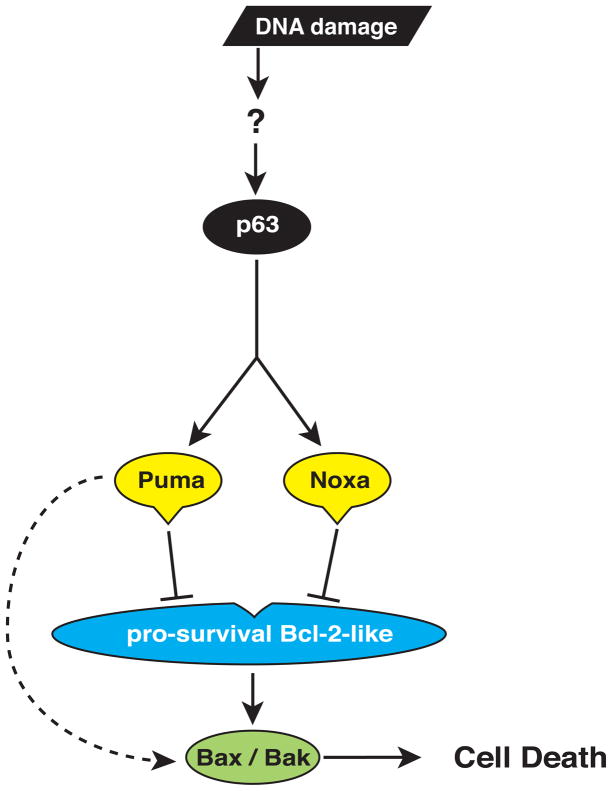
As reported (Livera et al., 2008; Suh et al., 2006), 0.45 or 4.5 Gy whole-body γ-irradiation resulted in complete destruction of the primordial follicle pool in wt and Trp53−/− mice. In striking contrast, after 0.45 Gy γ-irradiation ~16% of primordial follicles were protected from apoptosis in Puma−/− mice (p<0.001) and an even greater proportion ~52%, in mice lacking both PUMA and NOXA. Immunostaining for a marker of double-strand DNA breaks (γ-H2AX) or phosphorylated (i.e. activated ATM, an indicator of detection of DNA damage and initiation of DNA repair (Derheimer and Kastan, 2010; Marangos and Carroll, 2012) at the early time-points (3–6 h), revealed that γ-irradiation induced comparable levels of DNA damage and initiation of DNA repair processes in oocytes from wt, Puma−/− and Puma−/−Noxa−/− mice, with increased DNA damage being observed, at least initially, at the higher dose. Although counter-intuitive, it is possible that the higher dose of γ-irradiation might trigger more effective but as yet undefined DNA repair processes in PN5 oocytes that may be related to more efficient activation of the Rad51 protein (Kujjo et al., 2010) or spatial/cluster arrangements (Paap et al., 2008). Of note, oocytes from young mice, which were abnormally resistant to apoptosis because they lacked the pro-apoptotic protein BAX, were shown to have efficient DNA repair, associated with maintenance of activity of RAD51 (Kujjo et al., 2010). Collectively, these results establish that DNA damage-induced apoptosis of primordial follicle oocytes is dependent on TAp63-mediated transcriptional induction of Puma and, to a lesser extent, Noxa (Figure 5).
Figure 5. PUMA and NOXA Are Critical for TAp63-Induced Apoptosis.
DNA damage leads to activation of TAp63, resulting in transcriptional induction of Puma and Noxa (which may be direct or indirect), with subsequent binding of PUMA and NOXA proteins to pro-survival BCL-2-family members (Bcl-2, Bcl-xL, Bcl-w, Mcl-1 or A1) and possibly also direct binding of PUMA to BAX/BAK. This causes activation of BAX and BAK, resulting in apoptotic cell death.
Remarkably, females lacking PUMA or both PUMA and NOXA that had been γ-irradiated at PN5 (0.45 Gy) and mated from 7 weeks of age with non-irradiated wt or Puma−/− proven males produced viable offspring, whereas none of the γ-irradiated PN5 wt females were fertile, as reported (Beaumont, 1962). These data indicate that the fraction of Puma−/− oocytes rescued from γ-irradiation induced apoptosis (16% on average following 0.45 Gy) are functionally robust and are capable of giving rise to normal numbers of healthy offspring. The lack of deformities observed in F1 and F2 offspring of γ-irradiated females followed beyond weaning, as well as the repair of DNA DSB in primordial follicle oocytes by 5 d following γ-irradiation, support the conclusion that primordial follicle oocytes lacking PUMA or both PUMA and NOXA, which survive γ-irradiation can repair their DNA to the extent of being able to produce viable offspring with normal appearance, including being fertile themselves (although a longer term study of health is imperative). Of relevance, other cell types lacking Puma are known to be highly proficient at repairing damaged DNA to prevent accumulation of oncogenic mutations. For example, Puma−/− mice survive a dose of γ-irradiation that kills control (wt) mice, as Puma−/− hematopoietic stem/progenitor cells can produce sufficient numbers of all hematopoietic cell subsets for normal health over an extended period (Yu et al., 2010). In addition, repeated exposure to low dose (1.5 Gy non-lethal) γ-irradiation, which causes induction of thymic lymphoma in wt mice through mobilization of hematopoietic stem/progenitor cells that had sustained oncogenic lesions, fails to cause lymphoma in Puma−/− and Puma−/−Noxa−/− mice because their stem/progenitor cells are not mobilized (due to increased survival of mature blood cells) (Labi et al., 2010; Michalak et al., 2010). Our results indicate that when apoptosis is blocked and sufficient time available, DNA repair processes appear to be capable of rescuing oocyte quality, suggesting that at least for some types of insults, apoptosis may not be a critical mechanism for protecting germ line integrity.
These data demonstrate that PUMA and NOXA are critical for DNA damage-induced, TAp63-mediated apoptosis of primordial follicle oocytes and they indicate that folliculogenesis and fertility may be preserved during anti-cancer therapy by blocking PUMA alone or PUMA and NOXA. Therefore, reduction in Puma levels in oocytes may prevent oocyte apoptosis, allowing DNA repair and oocyte survival. This is unlikely to reduce the efficacy of concomitant anticancer therapy, if reduction in Puma levels could be targeted to the oocyte. In addition, blockade of Puma would not be expected to promote tumorigenesis as neither Puma−/− nor Puma−/−Noxa−/− mice are abnormally cancer prone (Michalak et al., 2010; Michalak et al., 2008). Moreover, we have shown that DNA damaging chemotherapeutic drugs kill lymphoma cells and most likely also other cancer cell types, by activation of a broad range of pro-apoptotic BH3-only proteins. PUMA is only one of the BH3-only proteins activated following treatment with DNA damaging drugs and is not solely responsible for cancer cell killing; BIM and NOXA and other BH3-only proteins can also promote cancer cell killing (Happo et al., 2010). It is therefore imperative to explore the potential of reduction in Puma levels in the protection of oocyte survival.
We have also found that mice lacking PUMA have an increased supply of primordial follicle oocytes at baseline. Most importantly, as increased oocyte endowment has been associated with reproductive longevity (Perez et al., 2007), prevention of oocyte apoptosis also has the potential to prevent premature menopause, which has profound implications for women’s health. These data constitute a significant advance, providing an exciting direction for future research.
EXPERIMENTAL PROCEDURES
Generation and Genotyping of Mice
The generation and genotyping of Puma−/−, Noxa−/− (Villunger et al., 2003), Bmf−/− (Labi et al., 2008) (generated on an inbred C57BL/6 background using C57BL/6-derived ES cells), Puma−/− Noxa−/− (Michalak et al., 2008), Bim−/−(Bouillet et al., 1999), Trp53−/− (Jacks et al., 1994) and TAp63 mutant mice (Guo et al., 2009) (generated on a mixed C57BL/6x129SV background using 129SV-derived ES cells but backcrossed with C57BL/6 mice for >20 (Bim−/− and Trp53−/−) or 5 (TAp63 mutant mice) generations, respectively) have been described.
Follicle Quantification
Oocytes in representative primordial, primary and secondary follicles (mean ± SEM) were expressed per 104μm2 ovarian tissue area. Randomly selected left or right ovaries from γ-irradiated or control (untreated) mice were fixed for 2 h in Bouin’s fluid, processed into paraffin and 5 μm serial sections of each ovary were stained with hematoxylin and eosin. From each set of serial sections, the middle section and two to three other sections located at 100 μm intervals on either side of the middle section (to avoid counting follicles twice) were selected for semi-quantitative estimation of follicles using morphological criteria previously described (Kerr et al., 2012; Kerr et al., 2006; Myers et al., 2004). Follicles with a morphologically normal oocyte nucleus were counted in sections and section areas (in μm2) were measured with image analysis software.
In situ Hybridization
In situ hybridization was performed as previously described (Hutt et al., 2006). Positive staining was indicated by the development of a dark purple/brown colour. Sections were counterstained with hematoxylin.
Fertility Trials
Female mice (PN5) were exposed to 0.45 Gy of γ-irradiation and then allowed to mature until 7 weeks of age before commencing breeding trials with non-irradiated proven males (wt C57BL/6 and/or Puma−/−). Adult mice were γ-irradiated (4.5 Gy) at 7 weeks of age, mated to vasectomized males for 7 weeks and then mated with non-irradiated proven C57BL/6 males.
Supplementary Material
HIGHLIGHTS.
Puma and Noxa induction in primordial follicle oocytes post-γ-irradiation depends on TAp63 but not Trp53
Primordial follicle oocytes lacking Puma or both Puma and Noxa are protected from γ-irradiation-induced apoptosis
Unlike wt mice, mice lacking Puma or both Puma and Noxa, produce healthy offspring post γ-irradiation
PUMA and (to a lesser extent) NOXA are critical for DNA damage-induced, TAp63-mediated primordial follicle oocyte apoptosis and loss of fertility
Acknowledgments
We thank Profs JM Adams, S Cory and A Villunger for gifts of mice, G Tarulli and H Morgan for assistance with histology, E Jansen for technical assistance and Drs G Enders and R Schultz for gifts of antibodies. This work was supported by fellowships and grants from the National Health and Medical Research Council (NHMRC Australia; Project Grant #1006460, Program Grants #494802 and #257502, Fellowships JKF (#441101), KJH (#494836), CLS (#406675), AS (#461299)); the Cancer Council Victoria (EMM and Sir Edward Dunlop Cancer Research Fellow to CLS); the Leukemia and Lymphoma Society (New York; SCOR grant #7015), the National Cancer Institute (NIH, US; CA 43540), American Cancer Society (AAM), the Victorian Cancer Agency (Clinical Fellowship to CLS). This work was made possible through Victorian State Government Operational Infrastructure Support and Australian Government NHMRC IRIISS.
Footnotes
Author contributions
JBK, KJH and EMM performed and planned experiments, interpreted data and wrote the manuscript. MC, CV and SHL helped with experiments and contributed data. PB and AM contributed essential reagents and helped with interpretation of results and manuscript writing. AS, JKF and CLS conceived of the study, planned experiments, interpreted data and wrote the manuscript.
References
- Ashwood-Smith MJ, Edwards RG. DNA repair by oocytes. Mol Hum Reprod. 1996;2:46–51. doi: 10.1093/molehr/2.1.46. [DOI] [PubMed] [Google Scholar]
- Beaumont HM. The radiosensitivity of germ-cells at various stages of ovarian development. Int J Radiat Biol. 1962;4:581–590. doi: 10.1080/09553006214550391. [DOI] [PubMed] [Google Scholar]
- Bergamaschi D, Samuels Y, Jin B, Duraisingham S, Crook T, Lu X. ASPP1 and ASPP2: common activators of p53 family members. Mol Cell Biol. 2004;24:1341–1350. doi: 10.1128/MCB.24.3.1341-1350.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillet P, Metcalf D, Huang DCS, Tarlinton DM, Kay TWH, Köntgen F, Adams JM, Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18:157–164. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derheimer FA, Kastan MB. Multiple roles of ATM in monitoring and maintaining DNA integrity. FEBS letters. 2010;584:3675–3681. doi: 10.1016/j.febslet.2010.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison SP, Jeffers JR, Yang C, Nilsson JA, Hall MA, Rehg JE, Yue W, Yu J, Zhang L, Onciu M, et al. Selection against PUMA gene expression in Myc-driven B-cell lymphomagenesis. Mol Cell Biol. 2008;28:5391–5402. doi: 10.1128/MCB.00907-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Keyes WM, Papazoglu C, Zuber J, Li W, Lowe SW, Vogel H, Mills AA. TAp63 induces senescence and suppresses tumorigenesis in vivo. Nat Cell Biol. 2009;11:1451–1457. doi: 10.1038/ncb1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanoux V, Pairault C, Bakalska M, Habert R, Livera G. Caspase-2 involvement during ionizing radiation-induced oocyte death in the mouse ovary. Cell Death Differ. 2007;14:671–681. doi: 10.1038/sj.cdd.4402052. [DOI] [PubMed] [Google Scholar]
- Happo L, Cragg MS, Phipson B, Haga JM, Jansen ES, Herold MJ, Dewson G, Michalak EM, Vandenberg CJ, Smyth GK, et al. Maximal killing of lymphoma cells by DNA-damage inducing therapy requires not only the p53 targets Puma and Noxa but also Bim. Blood. 2010;116:5256–5267. doi: 10.1182/blood-2010-04-280818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutt KJ, McLaughlin EA, Holland MK. KIT/KIT ligand in mammalian oogenesis and folliculogenesis: roles in rabbit and murine ovarian follicle activation and oocyte growth. Biol Reprod. 2006;75:421–433. doi: 10.1095/biolreprod.106.051516. [DOI] [PubMed] [Google Scholar]
- Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, Weinberg RA. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- Jeffers JR, Parganas E, Lee Y, Yang C, Wang J, Brennan J, MacLean KH, Han J, Chittenden T, Ihle JN, et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell. 2003;4:321–328. doi: 10.1016/s1535-6108(03)00244-7. [DOI] [PubMed] [Google Scholar]
- Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N Engl J Med. 2009;360:902–911. doi: 10.1056/NEJMra0801454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JB, Brogan L, Myers M, Hutt KJ, Mladenovska T, Ricardo S, Hamza K, Scott CL, Strasser A, Findlay JK. The primordial follicle reserve is not renewed after chemical or gamma-irradiation mediated depletion. Reproduction. 2012;143:469–476. doi: 10.1530/REP-11-0430. [DOI] [PubMed] [Google Scholar]
- Kerr JB, Duckett R, Myers M, Britt KL, Mladenovska T, Findlay JK. Quantification of healthy follicles in the neonatal and adult mouse ovary: evidence for maintenance of primordial follicle supply. Reproduction. 2006;132:95–109. doi: 10.1530/rep.1.01128. [DOI] [PubMed] [Google Scholar]
- Khokhar SK, Kommagani R, Kadakia MP. Differential effects of p63 mutants on transactivation of p53 and/or p63 responsive genes. Cell Res. 2008;18:1061–1073. doi: 10.1038/cr.2008.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujjo LL, Laine T, Pereira RJ, Kagawa W, Kurumizaka H, Yokoyama S, Perez GI. Enhancing survival of mouse oocytes following chemotherapy or aging by targeting Bax and Rad51. PLoS One. 2010;5:e9204. doi: 10.1371/journal.pone.0009204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labi V, Erlacher M, Kiessling S, Manzl C, Frenzel A, O’Reilly L, Strasser A, Villunger A. Loss of the BH3-only protein Bmf impairs B cell homeostasis and accelerates gamma irradiation-induced thymic lymphoma development. J Exp Med. 2008;205:641–655. doi: 10.1084/jem.20071658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labi V, Erlacher M, Krumschnabel G, Manzl C, Tzankov A, Pinon J, Egle A, Villunger A. Apoptosis of leukocytes triggered by acute DNA damage promotes lymphoma formation. Genes Dev. 2010;24:1602–1607. doi: 10.1101/gad.1940210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane DP. p53, guardian of the genome. Nature. 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- Leong CO, Vidnovic N, DeYoung MP, Sgroi D, Ellisen LW. The p63/p73 network mediates chemosensitivity to cisplatin in a biologically defined subset of primary breast cancers. J Clin Invest. 2007;117:1370–1380. doi: 10.1172/JCI30866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livera G, Petre-Lazar B, Guerquin MJ, Trautmann E, Coffigny H, Habert R. p63 null mutation protects mouse oocytes from radio-induced apoptosis. Reproduction. 2008;135:3–12. doi: 10.1530/REP-07-0054. [DOI] [PubMed] [Google Scholar]
- Marangos P, Carroll J. Oocytes progress beyond prophase in the presence of DNA damage. Current biology : CB. 2012;22:989–994. doi: 10.1016/j.cub.2012.03.063. [DOI] [PubMed] [Google Scholar]
- Michalak EM, Jansen ES, Happo L, Cragg MS, Tai L, Smyth GK, Strasser A, Adams JM, Scott CL. Puma and to a lesser extent Noxa are suppressors of Myc-induced lymphomagenesis. Cell Death Differ. 2009;16:684–696. doi: 10.1038/cdd.2008.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak EM, Vandenberg CJ, Delbridge ARD, Wu L, Scott CL, Adams JM, Strasser A. Apoptosis-promoted tumorigenesis: gamma-irradiation-induced thymic lymphomagenesis requires Puma-driven leukocyte death. Genes Dev. 2010;24:1608–1613. doi: 10.1101/gad.1940110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak EM, Villunger A, Adams JM, Strasser A. In several cell types the tumour suppressor p53 induces apoptosis largely via Puma but Noxa can contribute. Cell Death Differ. 2008;15:1019–1029. doi: 10.1038/cdd.2008.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- Myers M, Britt KL, Wreford NG, Ebling FJ, Kerr JB. Methods for quantifying follicular numbers within the mouse ovary. Reproduction. 2004;127:569–580. doi: 10.1530/rep.1.00095. [DOI] [PubMed] [Google Scholar]
- O’Reilly LA, Tai L, Lee L, Kruse EA, Grabow S, Fairlie WD, Haynes NM, Tarlinton DM, Zhang JG, Belz GT, et al. Membrane-bound Fas ligand only is essential for Fas-induced apoptosis. Nature. 2009;461:659–663. doi: 10.1038/nature08402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paap B, Wilson DM, 3rd, Sutherland BM. Human abasic endonuclease action on multilesion abasic clusters: implications for radiation-induced biological damage. Nucleic Acids Res. 2008;36:2717–2727. doi: 10.1093/nar/gkn118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, George R, Autore F, Fraternali F, Ladbury JE, Nikolova PV. Molecular interactions of ASPP1 and ASPP2 with the p53 protein family and the apoptotic promoters PUMA and Bax. Nucleic Acids Res. 2008;36:5139–5151. doi: 10.1093/nar/gkn490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen T. Follicle kinetics in the ovary of the cyclic mouse. Acta Endocrinol (Copenh) 1970;64:304–323. doi: 10.1530/acta.0.0640304. [DOI] [PubMed] [Google Scholar]
- Perez GI, Jurisicova A, Wise L, Lipina T, Kanisek M, Bechard A, Takai Y, Hunt P, Roder J, Grynpas M, et al. Absence of the proapoptotic Bax protein extends fertility and alleviates age-related health complications in female mice. Proc Natl Acad Sci U S A. 2007;104:5229–5234. doi: 10.1073/pnas.0608557104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez GI, Robles R, Knudson CM, Flaws JA, Korsmeyer SJ, Tilly JL. Prolongation of ovarian lifespan into advanced chronological age by Bax-deficiency. Nat Genet. 1999;21:200–203. doi: 10.1038/5985. [DOI] [PubMed] [Google Scholar]
- Rocco JW, Leong CO, Kuperwasser N, DeYoung MP, Ellisen LW. p63 mediates survival in squamous cell carcinoma by suppression of p73-dependent apoptosis. Cancer Cell. 2006;9:45–56. doi: 10.1016/j.ccr.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Shibue T, Takeda K, Oda E, Tanaka H, Murasawa H, Takaoka A, Morishita Y, Akira S, Taniguchi T, Tanaka N. Integral role of Noxa in p53-mediated apoptotic response. Genes Dev. 2003;17:2233–2238. doi: 10.1101/gad.1103603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A, Cory S, Adams JM. Deciphering the rules of programmed cell death to improve therapy of cancer and other diseases. EMBO J. 2011;30:3667–3683. doi: 10.1038/emboj.2011.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh EK, Yang A, Kettenbach A, Bamberger C, Michaelis AH, Zhu Z, Elvin JA, Bronson RT, Crum CP, McKeon F. p63 protects the female germ line during meiotic arrest. Nature. 2006;444:624–628. doi: 10.1038/nature05337. [DOI] [PubMed] [Google Scholar]
- Tilly JL. Commuting the death sentence: how oocytes strive to survive. Nat Rev Mol Cell Biol. 2001;2:838–848. doi: 10.1038/35099086. [DOI] [PubMed] [Google Scholar]
- Villunger A, Michalak EM, Coultas L, Müllauer F, Böck G, Ausserlechner MJ, Adams JM, Strasser A. p53- and drug-induced apoptotic responses mediated by BH3-only proteins Puma and Noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- Xu M, Pavone ME, Woodruff T. Fruitful progress to fertility: Preserving oocytes from chemodestruction. Nat Med. 2011;17:1562–1563. doi: 10.1038/nm.2595. [DOI] [PubMed] [Google Scholar]
- Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dötsch V, Andrews NC, Caput D, McKeon F. p63, a p53 homolog at 3q27–29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Molecular Cell. 1998;2:305–316. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, Tabin C, Sharpe A, Caput D, Crum C, et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- Yu H, Shen H, Yuan Y, Xufeng R, Hu X, Garrison SP, Zhang L, Yu J, Zambetti G, Cheng T. Deletion of Puma protects hematopoietic stem cells and confers long-term survival in response to high-dose {gamma}-irradiation. Blood. 2010;115:3472–3480. doi: 10.1182/blood-2009-10-248278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.