Summary
Resveratrol has been reported to improve metabolic function in metabolically-abnormal rodents and humans, but has not been studied in non-obese people with normal glucose tolerance. We conducted a randomized, double-blind, placebo-controlled trial to evaluate the metabolic effects of 12 weeks of resveratrol supplementation (75 mg/day) in non-obese, postmenopausal women with normal glucose tolerance. Although resveratrol supplementation increased plasma resveratrol concentration, it did not change body composition, resting metabolic rate, plasma lipids, or inflammatory markers. A two-stage hyperinsulinemic-euglycemic clamp procedure, in conjunction with stable isotopically-labeled tracer infusions, demonstrated that resveratrol did not increase liver, skeletal muscle, or adipose tissue insulin sensitivity. Consistent with the absence of in vivo metabolic effects, resveratrol did not affect its putative molecular targets, including AMPK, Sirt1, Nampt, and Pgc-1α, in either skeletal muscle or adipose tissue. These findings demonstrate that resveratrol supplementation does not have beneficial metabolic effects in non-obese, postmenopausal women with normal glucose tolerance.
INTRODUCTION
Resveratrol, a naturally occurring polyphenol that is found primarily in the skin of grapes, is purported to mimic the health benefits of calorie restriction (CR) by improving metabolic function, reducing cancer risk, and ameliorating other age-related pathology (Baur and Sinclair, 2006; Mercken et al., 2011). These potential benefits have led to a marked growth in the purchase of resveratrol supplements, with annual sales of $30 million in the United States alone (http://newhope360.com/ingredients/what-will-be-superstar-ingredients-2010). Data from a series of studies conducted in rodent models of diet-induced obesity have shown that resveratrol increases insulin sensitivity, improves glucose tolerance and plasma lipids, prevents the development of fatty liver, enhances mitochondrial biogenesis, suppresses inflammation and oxidative stress and extends life span (Baur et al., 2006; Lagouge et al., 2006; Sun et al., 2007; Um et al., 2010). In contrast, resveratrol did not improve glucose tolerance, insulin sensitivity, plasma lipid profile, or lifespan in normal rodents (Jeon et al., 2012; Juan et al., 2002; Miller et al., 2011; Strong et al., 2012; Turrens et al., 1997), but did mimic transcriptional changes induced by calorie restriction (Barger et al., 2008a; Barger et al., 2008b; Pearson et al., 2008) and improved several age-associated abnormalities in different organ systems (Pearson et al., 2008).
Recently, it was reported that resveratrol improves insulin sensitivity, postprandial plasma glucose concentration, and mitochondrial function, and decreases inflammation in adults who are obese, have type 2 diabetes, or impaired glucose tolerance (Brasnyo et al., 2011; Crandall et al., 2012; Timmers et al., 2011). However, it is not known whether resveratrol supplementation has similar benefits in non-obese people who have normal oral glucose tolerance, which has important implications for the general population.
The purpose of the present study was to conduct a randomized, double-blind, placebo-controlled trial to evaluate the metabolic effects of resveratrol supplementation (75 mg/day, 99% pure trans-resveratrol [resVida™ from DSM Nutritional Products, Ltd.] for 12 weeks) in lean and overweight women. To this end, we determined the effect of resveratrol on insulin sensitivity in vivo, by using a two-stage hyperinsulinemic-euglycemic clamp procedure, in conjunction with stable isotopically labeled tracer infusions, and investigated global gene expression and the key molecular events induced by resveratrol in adipose tissue and skeletal muscle.
RESULTS
Resveratrol supplementation was well-tolerated
Subjects randomized to the resveratrol supplementation (n=15, age: 58.2±4.0 years) or placebo (n=14, age: 59.8±4.3 years) had similar baseline characteristics (Table 1 and 2). No adverse effects of resveratrol on standard blood tests or electrocardiogram were detected (Table S1 and Supplemental Experimental Procedures). Based on the assessment of pill counts, all subjects took at least 80% of the capsules with an average compliance of 92% in the placebo group and 94% in the resveratrol group. To further ensure that subjects were compliant with resveratrol supplementation, plasma resveratrol and dihydroresveratrol concentrations were measured before and after 12 weeks of treatment. Total resveratrol and dihydroresveratrol (free and conjugated forms) were not detected in plasma in the resveratrol or placebo groups at baseline, but were present in plasma after intervention in the resveratrol group only (Table 2). Total plasma resveratrol concentration increased to a maximal concentration of 992 ± 258 ng/mL at ~2 h after dosing and did not reach baseline levels after 6 h; the estimated half-life of elimination was ~6.5 h (range: 3.5 h to 11 h).
Table 1.
Body composition before and after placebo and resveratrol treatment
| Placebo |
Resveratrol |
|||
|---|---|---|---|---|
| Before | After | Before | After | |
| Body mass index (kg/m2) | 24.3 ± 2.7 | 24.3 ± 2.7 | 24.2 ± 2.8 | 24.2 ± 2.9 |
| Fat-free mass (kg) | 40.6 ± 3.1 | 40.8 ± 2.9 | 42.4 ± 4.2 | 42.6 ± 3.9 |
| Fat mass (% body weight) | 36.0 ± 5.6 | 35.6 ± 5.8 | 35.7 ± 6.1 | 35.3 ± 6.6 |
| Subcutaneous abdominal fat volume (cm3) | 2080 ± 794 | 2065 ± 785 | 2269 ± 785 | 2287 ± 811 |
| Intra-abdominal fat volume (cm3) | 822 ± 526 | 811 ± 517 | 1031 ± 550 | 1077 ± 569 |
| Intrahepatic triglyceride content (%) | 2.85 ± 4.55 | 2.50 ± 3.39 | 2.61 ± 1.47 | 3.17 ± 2.41 |
Values are means ± SD.
Table 2.
Plasma resveratrol and metabolic variables before and after placebo and resveratrol treatment
| Placebo |
Resveratrol |
|||
|---|---|---|---|---|
| Before | After | Before | After | |
| Total resveratrol (ng/ml) | ND | ND | ND | 109.2 ± 185.0 |
| Total dihydroresveratrol (ng/ml) | ND | ND | ND | 168.9 ± 106.0 |
| Glucose (mg/dl) | 94.2 ± 6.7 | 91.5 ± 6.2 | 94.8 ± 5.7 | 93.1 ± 5.5 |
| Insulin (mU/L) | 4.9 ± 2.7 | 4.2 ± 2.0 | 5.9 ± 3.2 | 5.7 ± 3.1 |
| HOMA-IR | 1.17 ± 0.69 | 0.96 ± 0.48 | 1.41 ± 0.80 | 1.32 ± 0.75 |
| Free fatty acids (mmol/L) | 0.62 ± 0.15 | 0.58 ± 0.10 | 0.67 ± 0.14 | 0.58 ± 0.15 |
| Total cholesterol (mg/dl) | 187 ± 30 | 186 ± 37 | 210 ± 35 | 197 ± 32 |
| LDL-cholesterol (mg/dl) | 108 ± 27 | 109 ± 32 | 131 ± 33 | 120 ± 33 |
| Triglyceride (mg/dl) | 97 ± 58 | 93 ± 57 | 118 ± 42 | 118 ± 52 |
| HDL-cholesterol (mg/dl) | 60 ± 13 | 58 ± 13 | 56 ± 10 | 54 ± 12 |
| Adiponectin (μg/ml) | 13.3 ± 4.8 | 13.4 ± 4.6 | 12.5 ± 5.6 | 12.0 ± 5.0 |
| Leptin (ng/ml) | 24.5 ± 17.4 | 22.4 ± 16.5 | 21.1 ± 13.1 | 20.4 ± 11.8 |
| IL-6 (pg/ml) | 2.08 ± 2.39 | 1.84 ± 1.68 | 1.58 ± 0.60 | 2.01 ± 1.98 |
| CRP (ng/ml) | 1.68 ± 1.64 | 1.51 ± 1.72 | 2.10 ± 2.33 | 2.78 ± 3.01 |
| Resting metabolic rate (kcal/kg/day) | 19.5 ± 2.5 | 19.0 ± 2.6 | 18.7 ± 2.5 | 17.7 ± 1.9 |
| HISI (1000/μmol/min × μU/ml) | 0.41 ± 0.24 | 0.47 ± 0.23 | 0.37 ± 0.25 | 0.38 ± 0.24 |
| Basal glucose Ra (μmol/kg FFM/min) | 17.1 ± 2.9 | 15.8 ± 1.9 | 15.9 ± 2.0 | 15.5 ± 1.5 |
| Basal palmitate Ra (μmol/kg FFM/min) | 1.78 ± 0.50 | 1.75 ± 0.42 | 1.82 ± 0.41 | 1.64 ± 0.62 |
| Systolic blood pressure (mm Hg) | 123 ± 15 | 121 ± 14 | 118 ± 16 | 119 ± 16 |
| Diastolic blood pressure (mm Hg) | 65 ± 10 | 63 ± 9 | 67 ± 11 | 72 ± 10 |
| Heart rate (beats/min) | 68 ± 8 | 65 ± 10 | 66 ± 8 | 65 ± 7 |
Values are means ± SD. ND, Not detectable; HOMA-IR, homeostasis model assessment of insulin resistance; LDL, low-density lipoprotein; HDL, high-density lipoprotein; HISI, hepatic insulin sensitivity index; FFM, fat-free mass.
Resveratrol does not affect body composition, basal metabolic variables, or insulin sensitivity
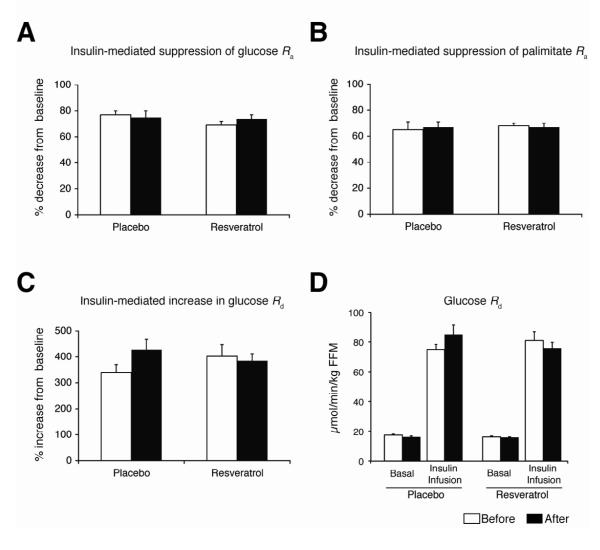
After 12 weeks of resveratrol supplementation, body weight and body composition (fat mass, fat-free mass [FFM], intra-abdominal fat volume and intrahepatic triglyceride content) did not change (Table 1). Plasma substrates and hormones (glucose, insulin, and plasma lipids), adipokines (adiponectin and leptin), markers of inflammation (c-reactive protein [CRP] and interleukin-6 [IL-6]), the homeostasis model assessment of insulin resistance (HOMA-IR) score, blood pressure, heart rate, and resting metabolic rate did not change after resveratrol supplementation (Table 2). A hyperinsulinemic-euglycemic clamp procedure was performed to more carefully assess multi-organ insulin action. No effect of resveratrol supplementation was detected in basal glucose or fatty acid kinetics (Table 2), or insulin sensitivity in liver (hepatic insulin sensitivity index and insulin-mediated suppression of glucose rate of appearance [Ra] into plasma), adipose tissue (insulin-mediated suppression of palmitate Ra), and skeletal muscle (insulin-mediated stimulation of glucose rate of disappearance [Rd]) (Figure 1).
Figure 1. Liver, adipose tissue, and skeletal muscle insulin sensitivity.
Insulin-mediated suppression of glucose rate of appearance (Ra) (A), insulin-mediated suppression of palmitate Ra (B), insulin-mediated increase in glucose rate of disappearance (Rd) (C), and absolute glucose Rd values (D) before (white bars) and after (black bars) placebo (n=14) or resveratrol (n=15) supplementation. Values are means ± SE.
Resveratrol supplementation does not induce beneficial molecular adaptations
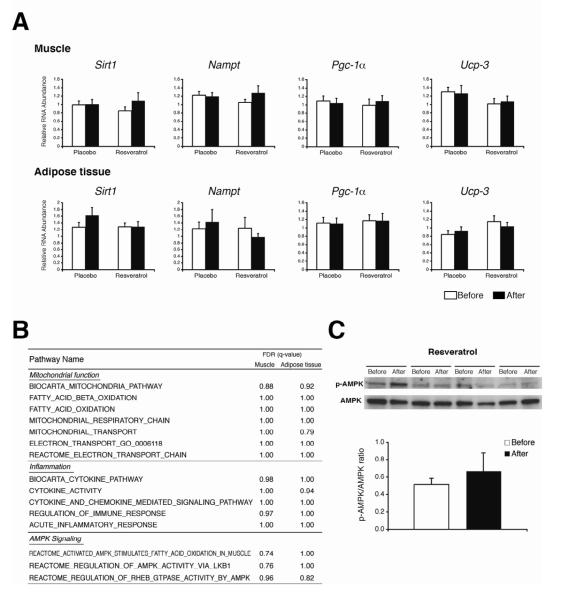
Data from studies conducted in animal models suggest the beneficial effects of resveratrol are mediated by the pathways involving AMP-activated protein kinase (AMPK), NAD+ biosynthesis, NAD+-dependent protein deacetylase SIRT1, and peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α), which stimulate mitochondrial biogenesis by increasing key regulators such as uncoupling protein-3 (Ucp-3)(Baur et al., 2006; Lagouge et al., 2006; Park et al., 2012; Um et al., 2010). Moreover, gene expression of Sirt1, nicotinamide phosphoribosyltransferase (Nampt; a key NAD+ biosynthetic enzyme), Pgc-1α, and Ucp-3 are up-regulated by resveratrol (Ajmo et al., 2008; Lagouge et al., 2006; Mukherjee et al., 2009; Um et al., 2010). Therefore, we measured gene expression of these putative resveratrol targets in skeletal muscle and adipose tissue in a subset of subjects in the placebo and resveratrol groups, and found the expression of these genes in both skeletal muscle and adipose tissue were not affected by resveratrol supplementation (Figure 2A). To further examine global transcriptional changes caused by resveratrol, we performed microarray analyses of both skeletal muscle and adipose tissue samples and conducted a gene set enrichment analysis (GSEA) to identify the biological pathways that might be affected by resveratrol. Resveratrol supplementation was associated with a significant effect on only two pathways (KINESIN_COMPLEX: False discovery rate [FDR] = 0.015 and UBIQUITIN_LIGASE_COMPLEX: FDR = 0.216) in skeletal muscle, and did not have any significant effects in adipose tissue. In contrast with data from previous reports that found resveratrol affected the biological pathways linked to mitochondrial function and inflammation in obese humans (Timmers et al., 2011) and rodents (Lagouge et al., 2006), we did not detect any effect of resveratrol on these pathways in either skeletal muscle or adipose tissue (Figure 2B). Furthermore, resveratrol had no effect on the biological pathways related to AMPK (Figure 2B), and did not alter the phosphorylation levels of AMPKα (Thr172) in skeletal muscle (Figure 2C).
Figure 2. Assessment of putative resveratrol molecular targets.
Relative gene expressions of Sirt1, nicotinamide phosphoribosyltransferase (Nampt), Pgc-1α, and Ucp-3 (means ± SE) determined by quantitative-PCR in skeletal muscle (upper panel) and adipose tissue (lower panel) before (white bars) and after (black bars) placebo or resveratrol supplementation (n = 8-12 per group) (A). Microarray analyses were performed using skeletal muscle and adipose tissue biopsy samples. Gene set enrichment analysis (GSEA) was used to identify potential biological pathways affected by resveratrol. Representative resveratrol target pathways related to mitochondrial function, inflammation, and AMPK are shown (B). The levels of phosphorylated AMPKα (Thr172) and total AMPKα in skeletal muscle from 4 subjects before (white bars) and after (black bars) resveratrol supplementation were determined by using western blotting (C). Values are means ± SE.
Moderate weight loss induced by short-term CR changes body composition and tissue gene expression
Moderate weight loss induced by short-term CR decreased total body fat mass, intra-abdominal adipose tissue volume, and intrahepatic triglyceride content, but did not result in significant improvements in in vivo metabolic outcomes (Table S2), which is consistent with the results obtained in previous weight loss studies conducted in metabolically healthy obese people and in postmenopausal women (Janiszewski and Ross, 2010; Joseph et al., 2001; Karelis et al., 2008; Shin et al., 2006). Nonetheless, CR-induced moderate weight loss altered adipose tissue gene expression profiles in several CR targets identified previously in human subjects, including up-regulation of Ctep (Johansson et al., 2012) and down-regulation of Leptin (Viguerie et al., 2005), Aldoc (Capel et al., 2008; Johansson et al., 2012; Ong et al., 2009), Abcc6 (Ong et al., 2009), and Ccnd2 (Kolehmainen et al., 2008) (Figure S1).
DISCUSSION
The use of resveratrol supplements to promote health has become increasingly popular (Mercken et al., 2011). Data from a series of studies conducted in obese, metabolically-abnormal rodent models have demonstrated that resveratrol improves metabolic outcomes, particularly insulin sensitivity, glucose tolerance, and plasma lipids (Baur et al., 2006; Lagouge et al., 2006; Sun et al., 2007; Um et al., 2010). Furthermore, it was recently reported that resveratrol improves metabolic outcomes in people who were either obese, had impaired glucose tolerance or had type 2 diabetes (Brasnyo et al., 2011; Crandall et al., 2012; Timmers et al., 2011). The present study is the first evaluation of the use of resveratrol in non-obese women with normal glucose tolerance. Our findings demonstrate that 12 weeks of resveratrol supplementation (75 mg/day) increased plasma total resveratrol and total dihydroresveratrol concentrations, but did not alter liver, skeletal muscle, or adipose tissue insulin sensitivity and did not have any effects on other key metabolic variables, such as body composition, plasma lipids, plasma markers of inflammation, or resting metabolic rate. Furthermore, resveratrol supplementation did not affect its major putative molecular targets in either adipose tissue or skeletal muscle, including Nampt, Sirt1, Pgc-1α, and Ucp-3 expression, AMPK phosphorylation, and biological pathways linked to mitochondrial function or inflammation. These data show that resveratrol supplementation (equivalent to the amount of resveratrol ingested by consuming ~8 L of red wine per day (Stark et al., 2011)) in non-obese women with normal glucose tolerance does not affect cellular signaling or result in metabolic benefits.
Three previous studies, conducted in different cohorts of metabolically-abnormal subjects, found 4 weeks of resveratrol therapy, given at doses ranging from 10 mg to 2000 mg per day, resulted in several metabolic benefits, including an improvement in insulin sensitivity (Brasnyo et al., 2011; Crandall et al., 2012; Timmers et al., 2011), postprandial plasma glucose concentrations (Crandall et al., 2012), and plasma lipid profile (Timmers et al., 2011). However, the overall conclusions from these studies are limited because the beneficial effects were not consistent across studies and were not proportional to resveratrol dose. For example, both low and moderate doses (10 mg/day and 150 mg/day) (Brasnyo et al., 2011; Timmers et al., 2011), but not high doses (1000-2000 mg/day) (Crandall et al., 2012), of resveratrol reduced insulin resistance as measured by HOMA-IR score, and 150 mg/day (Timmers et al., 2011), but not 1000-2000 mg/day (Crandall et al., 2012), of resveratrol decreased plasma triglyceride concentration.
If resveratrol supplementation is beneficial as reported, why did we not detect any metabolic effects of resveratrol in our subjects? It is unlikely that the lack of metabolic benefits is simply due to differences in the dose or duration of resveratrol supplementation. The dose of resveratrol given to our subjects (75 mg/day for 12 weeks) was lower than the dose given in the previous study involving obese subjects (150 mg/day for 30 days) (Timmers et al., 2011) or older subjects with impaired glucose tolerance (1000-2000 mg/day for 4 weeks) (Crandall et al., 2012), but much higher than the dose given in the study involving subjects with diabetes (10 mg/day for 4 weeks) (Brasnyo et al., 2011). We are not able to determine the bioavailability of resveratrol in some of these studies because different resveratrol compounds were used and plasma concentrations were not reported (Brasnyo et al., 2011; Crandall et al., 2012). The plasma concentrations of total resveratrol and total dihydroresveratrol in our subjects were 40-50% lower than the plasma concentrations reported in the study conducted in obese subjects supplemented with 150 mg/day (Timmers et al., 2011), which provided the same resveratrol compound used in our study, but our plasma levels were likely higher than the concentrations achieved in the study conducted in diabetic subjects supplemented with 10 mg/day (Brasnyo et al., 2011). Furthermore, the duration of resveratrol supplementation in our subjects (12 weeks) was longer than the duration of supplementation in the previous three studies (4 weeks). Nonetheless, we cannot exclude the possibility that we were unable to detect modest metabolic benefits of resveratrol supplementation due to the number of subjects in our study. However, it seems unlikely that clinically meaningful effects were missed, because the values for the key metabolic outcomes after resveratrol supplementation were nearly identical to values obtained before supplementation.
We did not detect an effect of resveratrol supplementation on the gene expression profiles that are affected by resveratrol in normal mice (Barger et al., 2008a; Barger et al., 2008b; Pearson et al., 2008). In contrast, we found moderate weight loss induced significant changes in gene expression profiles in adipose tissue and skeletal muscle, particularly genes that are known as CR targets in adipose tissue, identified in previous microarray studies conducted in human subjects (Capel et al., 2008; Johansson et al., 2012; Kolehmainen et al., 2008; Ong et al., 2009; Viguerie et al., 2005). However, data from several studies conducted in rodent models have found many putative resveratrol targets, such as Sirt1, Nampt, and Pgc-1α, are affected by fasting (Hayashida et al., 2010; Yang et al., 2007; Yoon et al., 2001), so it is possible that collecting tissue samples from our subjects after they fasted overnight (~12 h) masked an effect induced by resveratrol on tissue gene expression.
An important difference between the present study and those conducted previously is that our subjects were non-obese women with normal glucose tolerance, whereas the subjects in the other studies had more severe pre-existing metabolic dysfunction, such as obesity, type 2 diabetes, and impaired glucose tolerance. Studies conducted in rodent models of diet-induced obesity have shown that resveratrol improves insulin sensitivity, lipids, and mitochondrial function (Baur et al., 2006; Lagouge et al., 2006; Sun et al., 2007; Um et al., 2010), but does not show beneficial metabolic effects in normal rodents (Jeon et al., 2012; Juan et al., 2002; Miller et al., 2011; Strong et al., 2012; Turrens et al., 1997). Therefore, it is possible that resveratrol only improves metabolic outcomes in obese and metabolically-abnormal people, but not in non-obese glucose tolerant women.
In conclusion, we found that 12 weeks of resveratrol supplementation (75 mg/day) does not affect its putative molecular targets in skeletal muscle and adipose tissue or improve metabolic function, including insulin sensitivity and plasma lipids, in non-obese women with normal glucose tolerance. These findings are consistent with data from studies conducted in lean metabolically-normal rodents. Additional randomized controlled studies are still needed to assess the potential benefits of resveratrol supplementation in metabolically abnormal individuals.
EXPERIMENTAL PROCEDURES
Study Subjects
A total of 45 lean and overweight, Caucasian, postmenopausal women were randomly assigned to one of three groups: 1) placebo treatment for 12 weeks (n=15); 2) resveratrol supplementation (75 mg/day) for 12 weeks (n=15), or 3) calorie restriction targeted to achieve a 5% weight loss within 12 weeks (n=15). One subject who was randomized to the placebo group was dropped from the study because of self-dieting and an 8.7% weight loss. All subjects completed a comprehensive medical evaluation, including a detailed history, physical examination, blood tests, a 12-lead electrocardiogram, and a 2-hour oral glucose tolerance test. No subject had any history or evidence of type 2 diabetes or cardiovascular disease and none had a diagnosis or were being treated for abnormal plasma lipids or hypertension. However, 10 subjects within the placebo and resveratrol groups had ≥1 feature of the metabolic syndrome (HDL-cholesterol <50 mg/dl, triglyceride >150 mg/dl, or increased blood pressure [systolic blood pressure ≥135 mmHg or diastolic blood pressure ≥85 mmHg]). Subjects provided written informed consent before participating in this study (ClinicalTrials.gov Identifier NCT00823381), which was approved by the Institutional Review Board of Washington University in St. Louis, MO.
Study Protocol
Body composition
Body fat mass and FFM were determined by dual-energy X-ray absorptiometry, intra-abdominal and subcutaneous adipose tissue volumes were quantified by using magnetic resonance imaging, and intrahepatic triglyceride content was determined by using magnetic resonance spectroscopy (Frimel et al., 2007).
Hyperinsulinemic-euglycemic clamp procedure and tissue biopsies
Subjects were admitted to the Clinical Research Unit at Washington University School of Medicine in the afternoon on the day before the clamp procedure. After subjects fasted for 12 h overnight, a 9.5 h, two-stage hyperinsulinemic-euglycemic clamp procedure, in conjunction with stable isotopically-labeled tracer infusion, was performed, as previously described (Fabbrini et al., 2009). Subcutaneous abdominal adipose tissue and skeletal muscle (vastus lateralis) biopsies were obtained during the basal period of the clamp procedure to investigate the molecular events induced by resveratrol treatment. Resting metabolic rate was measured, by using indirect calorimetry, during the basal period of the clamp procedure.
Intervention and post-intervention studies
After the baseline studies were completed, each subject was randomized to 12 weeks of treatment with either resveratrol (75 mg/day; resVida 99.7% trans-resveratrol, provided by DSM Nutritional Products Ltd, Kaiseraugst, Switzerland), placebo, or calorie restriction, by using a three block computer-generated randomization scheme with a stratification of subjects based on a BMI value <25 kg/m2 and ≥25 kg/m2. Subjects were instructed to take one capsule (75 mg resveratrol or placebo) in the morning with breakfast. After 12 weeks of supplementation, all studies performed at baseline were repeated. On the day of the final clamp procedure, resveratrol was given in the morning and blood samples were obtained before and at 30, 60, 90, 120, 240, 360 min after resveratrol administration (before insulin infusion) to evaluate resveratrol pharmacokinetics.
Sample processing and analyses
Details of analyses and calculations used to evaluate metabolic variables, substrate kinetics, real-time PCR, microarray analyses, and western blot are available in the Supplemental Experimental Procedure.
Statistical Analyses
The statistical significance of differences in post-intervention outcome measures between resveratrol and placebo treatment were evaluated by using analysis of covariance (ANCOVA) with the pre-treatment values as the covariates. Adding the CR weight loss group to the analysis of outcome measures did not change the comparisons between the placebo and resveratrol groups. Results are presented as means ± SD, except in the figures, which report data as means ± SE.
Supplementary Material
Highlights.
Resveratrol supplementation does not improve plasma lipids in non-obese women.
Resveratrol does not improve insulin sensitivity in non-obese women.
Resveratrol does not affect its putative targets in fat or muscle in non-obese women.
ACKNOWLEDGEMENTS
The authors thank Emily Lake, Janine Kampelman, Melisa Moore, Dr. Adewole Okunade, Freida Custodio, Jennifer Shew, Anna Moseley, Ruteja Barve, and the DSM Application Laboratory and Analytical Research Center for technical assistance, and the study subjects for their participation.
This study was supported by National Institutes of Health grants UL1 RR024992 (Clinical Translational Science Award), DK 56341 (Nutrition and Obesity Research Center), DK 37948, and grants from DSM Nutritional Products, Kaiseraugst, Switzerland and the Longer Life Foundation (A RGA/Washington University Partnership). J.Y. is supported by the Japanese Research Foundation for Clinical Pharmacology, the Manpei Suzuki Diabetes Foundation, and the Kanae Foundation for the Promotion of Medical Science. S.I. serves on a Scientific Advisory Board for Sirtris (Cambridge, MA). I.K. is employed by DSM Nutritional Products.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ajmo JM, Liang X, Rogers CQ, Pennock B, You M. Resveratrol alleviates alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G833–842. doi: 10.1152/ajpgi.90358.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger JL, Kayo T, Pugh TD, Prolla TA, Weindruch R. Short-term consumption of a resveratrol-containing nutraceutical mixture mimics gene expression of long-term caloric restriction in mouse heart. Experimental gerontology. 2008a;43:859–866. doi: 10.1016/j.exger.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, Wang Y, Raederstorff D, Morrow JD, Leeuwenburgh C, Allison DB, Saupe KW, Cartee GD, Weindruch R, Prolla TA. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PloS one. 2008b;3:e2264. doi: 10.1371/journal.pone.0002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Brasnyo P, Molnar GA, Mohas M, Marko L, Laczy B, Cseh J, Mikolas E, Szijarto IA, Merei A, Halmai R, Meszaros LG, Sumegi B, Wittmann I. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br J Nutr. 2011;106:383–389. doi: 10.1017/S0007114511000316. [DOI] [PubMed] [Google Scholar]
- Capel F, Viguerie N, Vega N, Dejean S, Arner P, Klimcakova E, Martinez JA, Saris WH, Holst C, Taylor M, Oppert JM, Sorensen TI, Clement K, Vidal H, Langin D. Contribution of energy restriction and macronutrient composition to changes in adipose tissue gene expression during dietary weight-loss programs in obese women. The Journal of clinical endocrinology and metabolism. 2008;93:4315–4322. doi: 10.1210/jc.2008-0814. [DOI] [PubMed] [Google Scholar]
- Crandall JP, Oram V, Trandafirescu G, Reid M, Kishore P, Hawkins M, Cohen HW, Barzilai N. Pilot Study of Resveratrol in Older Adults With Impaired Glucose Tolerance. The journals of gerontology. Series A, Biological sciences and medical sciences. 2012 doi: 10.1093/gerona/glr235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, Okunade A, Klein S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15430–15435. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frimel TN, Deivanayagam S, Bashir A, O’Connor R, Klein S. Assessment of intrahepatic triglyceride content using magnetic resonance spectroscopy. J Cardiometab Syndr. 2007;2:136–138. doi: 10.1111/j.1559-4564.2007.07168.x. [DOI] [PubMed] [Google Scholar]
- Hayashida S, Arimoto A, Kuramoto Y, Kozako T, Honda S, Shimeno H, Soeda S. Fasting promotes the expression of SIRT1, an NAD+ -dependent protein deacetylase, via activation of PPARalpha in mice. Molecular and cellular biochemistry. 2010;339:285–292. doi: 10.1007/s11010-010-0391-z. [DOI] [PubMed] [Google Scholar]
- Janiszewski PM, Ross R. Effects of weight loss among metabolically healthy obese men and women. Diabetes care. 2010;33:1957–1959. doi: 10.2337/dc10-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon BT, Jeong EA, Shin HJ, Lee Y, Lee DH, Kim HJ, Kang SS, Cho GJ, Choi WS, Roh GS. Resveratrol Attenuates Obesity-Associated Peripheral and Central Inflammation and Improves Memory Deficit in Mice Fed a High-Fat Diet. Diabetes. 2012 doi: 10.2337/db11-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson LE, Danielsson AP, Parikh H, Klintenberg M, Norstrom F, Groop L, Ridderstrale M. Differential gene expression in adipose tissue from obese human subjects during weight loss and weight maintenance. The American journal of clinical nutrition. 2012;96:196–207. doi: 10.3945/ajcn.111.020578. [DOI] [PubMed] [Google Scholar]
- Joseph LJ, Trappe TA, Farrell PA, Campbell WW, Yarasheski KE, Lambert CP, Evans WJ. Short-term moderate weight loss and resistance training do not affect insulin-stimulated glucose disposal in postmenopausal women. Diabetes care. 2001;24:1863–1869. doi: 10.2337/diacare.24.11.1863. [DOI] [PubMed] [Google Scholar]
- Juan ME, Vinardell MP, Planas JM. The daily oral administration of high doses of trans-resveratrol to rats for 28 days is not harmful. J Nutr. 2002;132:257–260. doi: 10.1093/jn/132.2.257. [DOI] [PubMed] [Google Scholar]
- Karelis AD, Messier V, Brochu M, Rabasa-Lhoret R. Metabolically healthy but obese women: effect of an energy-restricted diet. Diabetologia. 2008;51:1752–1754. doi: 10.1007/s00125-008-1038-4. [DOI] [PubMed] [Google Scholar]
- Kolehmainen M, Salopuro T, Schwab US, Kekalainen J, Kallio P, Laaksonen DE, Pulkkinen L, Lindi VI, Sivenius K, Mager U, Siitonen N, Niskanen L, Gylling H, Rauramaa R, Uusitupa M. Weight reduction modulates expression of genes involved in extracellular matrix and cell death: the GENOBIN study. Int J Obes (Lond) 2008;32:292–303. doi: 10.1038/sj.ijo.0803718. [DOI] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Mercken EM, Carboneau BA, Krzysik-Walker SM, de Cabo R. Of mice and men: The benefits of caloric restriction, exercise, and mimetics. Ageing Res Rev. 2011 doi: 10.1016/j.arr.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. The journals of gerontology. Series A, Biological sciences and medical sciences. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Lekli I, Gurusamy N, Bertelli AA, Das DK. Expression of the longevity proteins by both red and white wines and their cardioprotective components, resveratrol, tyrosol, and hydroxytyrosol. Free Radic Biol Med. 2009;46:573–578. doi: 10.1016/j.freeradbiomed.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Ong KR, Sims AH, Harvie M, Chapman M, Dunn WB, Broadhurst D, Goodacre R, Wilson M, Thomas N, Clarke RB, Howell A. Biomarkers of dietary energy restriction in women at increased risk of breast cancer. Cancer Prev Res (Phila) 2009;2:720–731. doi: 10.1158/1940-6207.CAPR-09-0008. [DOI] [PubMed] [Google Scholar]
- Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, Kim MK, Beaven MA, Burgin AB, Manganiello V, Chung JH. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148:421–433. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell metabolism. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin MJ, Hyun YJ, Kim OY, Kim JY, Jang Y, Lee JH. Weight loss effect on inflammation and LDL oxidation in metabolically healthy but obese (MHO) individuals: low inflammation and LDL oxidation in MHO women. Int J Obes (Lond) 2006;30:1529–1534. doi: 10.1038/sj.ijo.0803304. [DOI] [PubMed] [Google Scholar]
- Stark T, Wollmann N, Losch S, Hofmann T. Quantitation of resveratrol in red wines by means of stable isotope dilution analysis-ultra-performance liquid chromatography-Quan-time-of-flight mass spectrometry and cross validation. Anal Chem. 2011;83:3398–3405. doi: 10.1021/ac103305s. [DOI] [PubMed] [Google Scholar]
- Strong R, Miller RA, Astle CM, Baur JA, de Cabo R, Fernandez E, Guo W, Javors M, Kirkland JL, Nelson JF, Sinclair DA, Teter B, Williams D, Zaveri N, Nadon NL, Harrison DE. Evaluation of Resveratrol, Green Tea Extract, Curcumin, Oxaloacetic Acid, and Medium-Chain Triglyceride Oil on Life Span of Genetically Heterogeneous Mice. The journals of gerontology. Series A, Biological sciences and medical sciences. 2012 doi: 10.1093/gerona/gls070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007;6:307–319. doi: 10.1016/j.cmet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S, Moonen-Kornips E, Hesselink MK, Kunz I, Schrauwen-Hinderling VB, Blaak EE, Auwerx J, Schrauwen P. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell metabolism. 2011;14:612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrens JF, Lariccia J, Nair MG. Resveratrol has no effect on lipoprotein profile and does not prevent peroxidation of serum lipids in normal rats. Free Radic Res. 1997;27:557–562. doi: 10.3109/10715769709097859. [DOI] [PubMed] [Google Scholar]
- Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW, Kim MK, Viollet B, Chung JH. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59:554–563. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viguerie N, Vidal H, Arner P, Holst C, Verdich C, Avizou S, Astrup A, Saris WH, Macdonald IA, Klimcakova E, Clement K, Martinez A, Hoffstedt J, Sorensen TI, Langin D. Adipose tissue gene expression in obese subjects during low-fat and high-fat hypocaloric diets. Diabetologia. 2005;48:123–131. doi: 10.1007/s00125-004-1618-x. [DOI] [PubMed] [Google Scholar]
- Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A, de Cabo R, Sauve AA, Sinclair DA. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130:1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413:131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.