Abstract
Recombinant human Apo2L/TRAIL (dulanermin) is based on the ligand for death receptors (DR4 and DR5), which promotes apoptosis. We report a patient with refractory chondrosarcoma who demonstrated a prolonged response to dulanermin, and explore mechanisms of response and resistance. This heavily pretreated patient had progressive metastatic chondrosarcoma to the lung. On dulanermin (8 mg/kg IV on days 1 through 5 in a 21-day cycle) the patient achieved a sustained partial response with only sub-centimeter nodules remaining. After 62 months of dulanermin treatment, progressive disease in the lungs was noted, and the patient underwent a resection that confirmed chondrosarcoma. DR4 was detected (immunohistochemistry) in the patient’s tumor, which may have enabled the response. However, up-regulation of pro-survival proteins, namely, phosphorylated (p)-NF-kappaBp65 (Ser 536), p-STAT3 (Tyr 705), pERK 1/2 (Thr 202/Tyr 204), p-mTOR (Ser 2448), FASN and Bcl-2, was also detected, which may have provided the underlying mechanisms for acquired dulanermin resistance. The patient was restarted on dulanermin and has continued on this treatment for an additional 16 months since surgery (78 months since initiation of treatment), with his most recent CT scans showing no evidence of disease.
Keywords: Chondrosarcoma, Targeted therapy, Phase 1 trials, TRAIL, rhuApo2L/TRAIL, Apoptosis, Death receptor, Sarcoma
INTRODUCTION
Chondrosarcomas are the second most common primary bone tumors in adults, with histology ranging from low-grade to very high-grade tumors(1–3). Complete surgical resection remains the mainstay of treatment with a role for radiation in tumors with positive margins. This tumor is notorious for resistance to conventional types of chemotherapy that are effective against other bone sarcomas, and clinical outcomes have not changed during the past 30 years(2).
Apoptosis (type 1 programmed cell death) is a means by which excessive and unnecessary cells are eradicated by multicellular organisms. This mechanism could augment innate immunity against cancer. However, the process is aberrant in some tumor cells, and deregulation of apoptosis is a key hallmark of cancer(4–6). Apoptosis can occur either through the intrinsic pathway (moderated by the Bcl-2 protein family) or the extrinsic pathway (controlled by cell surface pro-apoptotic death receptors). Advances in targeted drug development have led to the development of pro-apoptotic receptor agonists (PARAs), which include the recombinant human protein apoptosis ligand 2/TNF-related apoptosis-inducing ligand (rhuApo2L/TRAIL or dulanermin); agonistic monoclonal antibodies directed against DR4 as well as the ligand-based molecule dulanermin trigger apoptosis via its cognate receptors DR4 and/or DR5(4–6).
Binding of dulanermin to DR4 and DR5 has been shown to trigger programmed cell death by activating a highly conserved signaling cascade in various cancer cell lines(4–7). This pre-clinically significant activity has led to the experimental clinical development of several pro-apoptotic receptor agonists(4). These new pro-apoptotic agents may hold great promise in overcoming key resistance pathways, especially when combined with other targeted or chemotherapeutic agents(4). Herein, we report substantial and sustained tumor regression in response to dulanermin in a patient with refractory chondrosarcoma, and explore the mechanisms of response and resistance.
MATERIALS AND METHODS
We reviewed the medical record of a patient with chondrosarcoma who was seen in the Phase I Clinical Trials Program at The University of Texas MD Anderson Cancer Center.
Patient Selection, Treatment and Clinical Assessments
Treatment on investigational trials, data collection and morphoproteomic analysis were performed in accordance with the guidelines of the University of Texas MD Anderson Cancer Center Institutional Review Board and with the patient’s consent. After initiation of an investigational therapy, the patient was evaluated clinically at approximately 3- to 4-week intervals. Tumor response was determined using Response Evaluation Criteria in Solid Tumors (RECIST) by CT scans or PET/CT scans obtained about every 6 to 8 weeks. A section of recurrent tumor was available for analysis.
Immunohistochemical and Morphoproteomic Analysis
Immunohistochemical (IHC) probes were used to detect the following phosphorylated (p) antigens, as published previously (8–10): p-mTOR (Ser 2448); p-Akt (Ser 473); (p)-NF-kappaBp65-(Ser 536) and p-extracellular signal-regulated kinase (ERK) 1/2 (Thr 202/Tyr204) [Cell Signaling Technology, Beverly, MA]; and TRAIL R1(DR4), p-signal transducer and activator of transcription (STAT)3 (Tyr 705) [Santa Cruz Biotechnology, Santa Cruz, CA]. Chromogenic signals were evaluated by brightfield microscopy and semi-quantified with regard to percentage of cells stained (0–100%) and the staining intensity (0: non-staining, 1+: weak staining, 2 +: moderate staining, and 3 +: strong staining)(8–10). Subcellular compartmentalizations were assessed as plasmalemmal, cytoplasmic, and/or nuclear. Concurrently run positive and negative immunohistochemical (IHC) controls reacted appropriately. The methods have been published previously(10) and were performed in a laboratory that is certified under the Clinical Laboratory Improvement Amendments of 1988 (“CLIA”) as qualified to perform high-complexity clinical testing.
Mutation analysis
Mutation analysis for IDH1 and IDH2 genes were performed by PCR-based DNA primer extension analysis in the CLIA–certified Molecular Diagnostic Laboratory in the Division of Pathology and Laboratory Medicine at MD Anderson. The analysis was limited to codon 132 of the IDH1 gene and codon 172 of the IDH2 gene.
RESULTS
Patient presentation and treatment
A Caucasian man (age: 58 years in 2005) developed left elbow lesions that were resected in the 1960s and were diagnosed as “synovial chondromas”. In 1990, he developed a local recurrence and was referred to our institution where a repeat resection showed pathologic confirmation of synovial chondroma. In 1993, he again developed a localized recurrence and underwent arthrodesis of the elbow; pathology review revealed grade I chondrosarcoma in the synovium. After two years, an above-the-elbow amputation of the left upper extremity was performed following malignant transformation in a site that would not permit limb-salvage surgery. The pathology review revealed sarcomatous transformation of chondromatosis. In 2000, the patient had a left axillary recurrence, underwent wide local excision of tumor with pathology showing metastatic chondrosarcoma. Radiation therapy (60 Gy) was given for microscopic residual disease. Chest x-ray and ultrasound follow up identified recurrence in the left chest wall close to the left scapular tip that was recognized by needle biopsy as recurrent chondrosarcoma. In 2003, a chest CT revealed bilateral pulmonary metastases that were treated with six cycles of irinotecan, after which progression led to discontinuation of this agent. In 2004, the patient underwent a wide local excision of metastatic chondrosarcoma on the anterior left chest wall. Pathologic review of tissue at MD Anderson Cancer Center confirmed the diagnosis.
In 2005, progressive disease (Figure 1A) and the absence of effective treatments led to the patient’s referral to the phase I clinic at MD Anderson Cancer Center. The patient enrolled in the clinical study, “Phase I dose-escalation study of dulanermin, recombinant human Apo2L/TRAIL, a dual proapoptotic receptor agonist, in patients with advanced cancer(11)”. rhuApo2L/TRAIL (dulanermin)(6) is the ligand for the death receptors DR4 and DR5, which upon ligation promote apoptotic cell death.
Figure 1. CT scan of the chest of the patient with chondrosarcoma showing lung metastasis of chondrosarcoma.
A: Before Apo2L/TRAIL therapy
B: Three years after Apo2L/TRAIL. The patient achieved a sustained partial response and ultimately a complete response by RECIST.
C and E: Image showing resistant tumor that emerged during Apo2L/TRAIL therapy before surgical resection
D and F: Image showing scan after 75 months after Apo2L/TRAIL therapy demonstrating a complete response
At the time of study initiation he had multiple large lung nodules as well as a 4 cm axillary node. Dulanermin treatment was given as 8 mg/kg IV on days 1 through 5 in a 21-day cycle (11) with cycle 1, day 1 commencing in August 2005. The patient achieved a sustained partial response by RECIST, with only residual sub-centimeter lung nodules remaining, which were not FDG avid on PET scan (Figure 1B)(11). Moreover, he has tolerated the investigational therapy without significant side effects and maintained a performance status of 100%.
After 62 months (~5 years) of treatment, the patient was noted to have progressive lung disease (Figure 1C) and underwent a resection that confirmed chondrosarcoma. He was restarted on dulanermin at the same dose and has continued on this treatment for an additional 16 months (78 months since initiation of treatment) (Figure 1D). At his re-staging in October 2011, CT scans showed no evidence of disease (Figure 1D).
Immunohistochemistry and morphoproteomic analysis on resistant tumor tissue
We performed morphoproteomic analysis of the patient’s resistant tumor (resected following disease progression after 65 months of dulanermin therapy) to elucidate mechanisms of response and resistance(9). Quantification by morphoproteomics allows for the following with respect to protein analytes in tumor and companionate cells: (a) their immunohistochemical detection and an assessment of their relative expression levels in the tumor cells vis-à-vis the cells in the microenvironment of the tumor; (b) their subcellular compartmentalization (nuclear versus cytoplasmic versus plasmalemmal), which has implications in signal transduction; and (c) an assessment of their state of activation, including phosphorylation on putative sites of activation, as determined by phosphospecific probes, compartmental translocation and functional grouping with correlative expression of upstream transducers and downstream effectors in the signal transduction pathways. (8, 9)
Bcl-2 is expressed in the resistant tissue
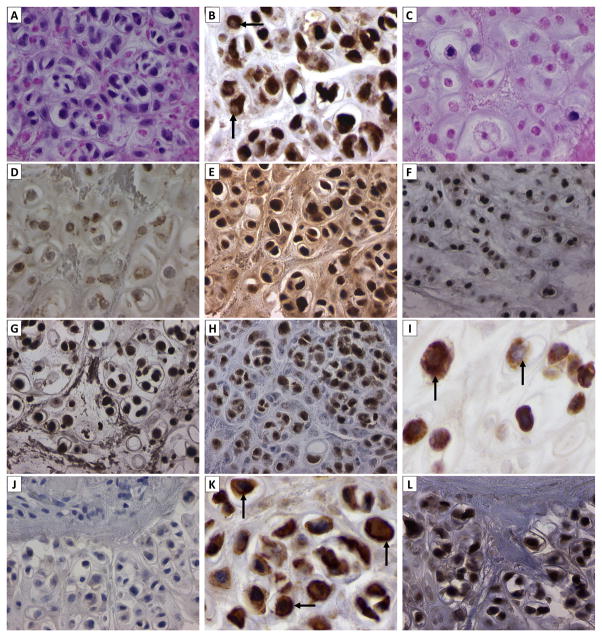
Hematoxylin and eosin stained tumor sections revealed necrosis in > 50% of the patient’s residual tumor (Figure 2A and C). Considered against the background of comparable digital images of the anti-apoptotic protein Bcl-2 expressed in such regions, the relative overexpression of Bcl-2 in tumor cells that appear to be viable compared to necrotic cells is noteworthy (Figure 2B and D). The Bcl-2 family of anti-apoptotic proteins has been implicated in resistance to Apo2L/TRAIL-mediated apoptosis(12–14), as has been inactivation of the pro-apoptotic Bcl-2 protein family member Bax (15). Therefore, strong Bcl-2 expression in residual, viable tumor cells was possibly a mechanism for resistance to dulanermin of this patient’s tumor. As such, it may provide a target for further therapeutic intervention. DR4 is expressed in the plasmalemmal and cytoplasmic compartments of viable tumor cells (Figure 2K; vide infra). DR4 is a therapeutic target for dulanermin(11) and viable tumor cells undergo apoptosis unless anti-apoptotic factors such as Bcl-2 counteract the efficacy of the targeted treatment (Figure 2B and C). That Bcl-2, the anti-apoptotic protein, plays a key role in chemotherapy resistance in chondrosarcomas and that this mechanism is a late event in central chondrosarcoma has been described. (16–18)
Figure 2. Immuhistochemical/morphoproteomic analysis of resistant tumor that recurred and was resected after 65 months of dulanermin therapy.
Frames A and C show largely viable and largely necrotic portions of tumor, respectively (hematoxylin-eosin, original magnification x600). Frames B and D reveal corresponding expressions of anti-apoptotoic, Bcl-2 protein in the largely viable and largely necrotic tumor, respectively (original magnification x600). Note relatively strong intensity in the viable tumor cells. (Arrows in Frame B indicate pale nuclear compartment devoid of Bcl-2.) The Bcl-2 family of anti-apoptotic proteins has been implicated in resistance to TRAIL-mediated apoptosis, as has inactivation of the Bcl-2 counteracting protein Bax. Therefore, strong Bcl-2 expression in residual, viable tumor cells was likely a mechanism for resistance to Apo2L/TRAIL of this patient’s tumor.
Phosphospecific immunohistochemical probes for the detection of activation sites of p-NF-kappaBp65 (Ser 536), p-STAT3 (Tyr 705), p-ERK 1/2 (Thr 202/Tyr204) and p-mTOR (Ser 2448) reveal nuclear translocation of each of these analytes in viable tumor, consistent with their constitutive activation (Frames E-H, respectively). Cytoplasmic FASN expression is noted in viable appearing chondrosarcoma cells (Frame I).
The overnight negative control is provided as a reference for comparison (Frame J). Original magnifications x400 for frames E-H and J, and x600 for Frame I).
Immunohistochemical probes applied to viable tumor for the detection of TRAIL-DR4, PPARgamma demonstrate: strong chromogenic signal for TRAIL-DR4 on the plasmalemmal aspect of some tumor cells (Frame K, arrows); nuclear signal for PPARgamma (Frame L); (Original magnifications, x600 for Frame K and x400 for Frame L.)
Constitutive activation of pro-survival pathways in resistant tumor tissue
Pro-survival protein analytes that might also have contributed to resistance to dulanermin-mediated apoptosis in this patient’s viable, residual chondrosarcoma include constitutively activated nuclear factor (NF)-kappaB and signal transducer and activator of transcription (STAT)3 pathways(19), the extracellular signal-regulated kinase (ERK) pathway, the mammalian target of rapamycin (mTOR) pathway (specifically, mTOR complex 2 signaling), and fatty acid synthase (FASN)-mediated signaling. The expression in viable tumor cells can be characterized as nuclear translocation of phosphorylated (p)-NF-kappaBp65-(Ser 536) and p-STAT3-(Tyr 705), of p-ERK-½-(Thr 202/Tyr 204), of p-mTOR-(Ser 2448) with nuclear translocation (mTORC2)(20), and of cytoplasmic FASN (Figure 2). Immunohistochemical analysis of p-NF-kappaBp65(Ser 536), p-STAT3(Tyr 705), p-ERK-1/2(Thr 202/Tyr204) and p-mTOR(Ser 2448) revealed nuclear translocation of each of these analytes in viable tumor, consistent with their constitutive activation [E–H]. Cytoplasmic fatty acid synthase N (FASN) expression is seen in viable appearing chondrosarcoma cells [I] and negative control (without primary antibody) [J]. In addition to DR4 expression on the plasmalemmal and cytoplasmic compartments, as previously noted, peroxisome proliferator-activated receptor (PPAR) gamma is expressed in the nuclei of the viable tumor cells in this patient’s tumor (Figure 2, K and L). In preclinical studies, PPAR gamma ligands induce apoptosis in renal cell carcinoma cells and in chondrosarcoma by decreasing the levels of antiapoptotic proteins, Bcl-2 and Bcl-xL, and upregulating proapoptotic Bax(21–23). A review of morphoproteomic findings demonstrated that the patient’s tumor was relatively indolent, as shown by the mitotic index in the tissue of zero (0) mitotic figures per ten (10) high power fields. The mitotic index was calculated from the number of mitotic figures per 10 high power fields (the field diameter of the high power field is 0.5 mm and the area is 0.196 mm2).
Mutation analysis for IDH1 and IDH2
Recently, IDH1 and IDH2 mutations have been reported in central chondrosarcomas (24). We performed IDH1 mutation analysis by PCR-based DNA primer extension analysis and no mutation detected in codon 132 of the IDH1 gene. Similarly, there was no mutation of codon 172 of the IDH2 gene. It would be interesting to assess IDH1 and IDH2 mutations in future patients with chondrosarcoma for any clinical and prognostic implications(24).
DISCUSSION
The response of chondrosarcoma to dulanermin is noteworthy because this represents a targeted molecular therapy capable of inducing a near- complete remission in chondrosarcoma –a tumor that is notorious for its resistance to conventional types of chemotherapy (1–3). Our patient has continued treatment for a total of 78 months. Of interest, we resumed treatment with dulanermin in this patient after surgical resection of recurrent tumor noted at 62 months. We chose this strategy since chemoradiation is generally ineffective at eradicating this type of cancer, perhaps because of its low mitotic index. The patient continued on treatment with the drug as he was deriving clinical benefit from the drug. Moreover, he had no side effects and protocol allowed treatment after complete response. Of interest is the fact that the patient continued to respond even after initial progression, albeit with intervening surgery to remove some of his lung nodules. This phenomenon suggests that even patients with progressive disease may have remaining residual responsive clones. Similar results have been shown in colorectal cancer, where retreatment after initial progression can occasionally re-induce response (25). Also, in breast cancer, continuation of trastuzumab (Herceptin) after progression may also result in superior outcome (26). This patient is now, at 78 months, in complete remission.
Based on clinical experience and biological experiments, it appears that multiple pathways can drive cancer cell survival. Better success in cancer treatment will likely be achieved through combination treatment strategies targeted at key moieties in these diverse pathways, especially since few patients with any type of advanced malignancy are cured with single-agent therapy. Analysis of the resistant tumor tissue that emerged in our patient during dulanermin therapy has provided several potential insights into the biology of response and resistance mechanisms that might help inform the design of optimal combinations. We detected DR4 in the patient’s tumor. It is conceivable that DR4 expression is important for the cognate ligand dulanermin to be effective. Indeed, epigenetic silencing of DR4 has been shown to contribute to Apo2L/TRAIL resistance (27). Although we were unable to analyze expression of DR5 (which also may mediate dulanermin activity), it is also plausible that deregulation of other oncogenic proteins contributes to resistance. For instance, in our patient’s resistant tumor tissue assessed after treatment with dulanermin, we detected constitutive activation of pro-survival proteins [phosphorylated (p)-NF-kappaBp65 (Ser 536), p-STAT3 (Tyr 705), p-ERK 1/2 (Thr 202/Tyr 204)], and p-mTOR (Ser 2448) with nuclear translocation (mTORC2), and correlative expression of cytoplasmic FASN and anti-apoptotic, Bcl-2 in the resistant tumor treated with dulanermin. These pro-survival and anti-apoptotic signals may provide the underlying mechanisms for dulanermin resistance. Several agents targeting these pathways are now available for use in the clinical setting, and studies combining them with PARAs such as dulanermin or DR4 and DR5 agonistic antibodies could be worthwhile. Finally, further exploration of PARAs in chondrosarcoma should be considered.
Acknowledgments
Financial Support: The University of Texas MD Anderson Cancer Center is supported in part by the National Institutes of Health through Cancer Center Support Grant no. CA 016672. The patient studied here was enrolled into a phase 1 study of dulanermin that was sponsored by Genentech (South San Francisco, CA) (Amgen, Thousand Oaks, CA).
Vivek Subbiah acknowledges “The Connie and Jim Walter Fellowship in Sarcoma Research”. We wish to thank Ms. Joann Aaron for her scientific editing of the manuscript. Disclosures are honoraria: Genentech (to RK) and Research Funding: Roche (to RK)
Grant Support: The University of Texas MD Anderson Cancer Center is supported in part by the National Institutes of Health through Cancer Center Support Grant no. CA 016672. The patient studied here was enrolled into a phase 1 study of dulanermin that was sponsored by Genentech (South San Francisco, CA) (Amgen, Thousand Oaks, CA).
Abbreviations
- rhu Apo2L/TRAIL
Recombinant human protein apoptosis ligand 2/Tumor Necrosis Factor-related apoptosis-inducing ligand
- DR
death receptor
- PARA
pro-apoptotic receptor agonist
- RECIST
Response Evaluation Criteria in Solid Tumors
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- STAT 3
Signal transducer and activator of transcription 3
- ERK
Extracellular signal-regulated kinase
- mTOR
mammalian target of rapamycin
- FASN
fatty acid synthase
Footnotes
Conflicts of Interest and Disclosures: Disclosures are honoraria: Genentech (to RK) and Research Funding: Roche (to RK)
References
- 1.Riedel RF, Larrier N, Dodd L, Kirsch D, Martinez S, Brigman BE. The clinical management of chondrosarcoma. Curr Treat Options Oncol. 2009;10:94–106. doi: 10.1007/s11864-009-0088-2. [DOI] [PubMed] [Google Scholar]
- 2.Giuffrida AY, Burgueno JE, Koniaris LG, Gutierrez JC, Duncan R, Scully SP. Chondrosarcoma in the United States (1973 to 2003): an analysis of 2890 cases from the SEER database. J Bone Joint Surg Am. 2009;91:1063–72. doi: 10.2106/JBJS.H.00416. [DOI] [PubMed] [Google Scholar]
- 3.Gelderblom H, Hogendoorn PC, Dijkstra SD, van Rijswijk CS, Krol AD, Taminiau AH, et al. The clinical approach towards chondrosarcoma. Oncologist. 2008;13:320–9. doi: 10.1634/theoncologist.2007-0237. [DOI] [PubMed] [Google Scholar]
- 4.Ashkenazi A. Directing cancer cells to self-destruct with pro-apoptotic receptor agonists. Nat Rev Drug Discov. 2008;7:1001–12. doi: 10.1038/nrd2637. [DOI] [PubMed] [Google Scholar]
- 5.Ashkenazi A, Herbst RS. To kill a tumor cell: the potential of proapoptotic receptor agonists. J Clin Invest. 2008;118:1979–90. doi: 10.1172/JCI34359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–62. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gong J, Yang D, Kohanim S, Humphreys R, Broemeling L, Kurzrock R. Novel in vivo imaging shows up-regulation of death receptors by paclitaxel and correlates with enhanced antitumor effects of receptor agonist antibodies. Mol Cancer Ther. 2006;5:2991–3000. doi: 10.1158/1535-7163.MCT-06-0188. [DOI] [PubMed] [Google Scholar]
- 8.Subbiah V, Naing A, Brown RE, Chen H, Doyle L, Lorusso P, et al. Targeted Morphoproteomic Profiling of Ewing’s Sarcoma Treated with Insulin-Like Growth Factor 1 Receptor (IGF1R) Inhibitors: Response/Resistance Signatures. PLoS One. 2011;6:e18424. doi: 10.1371/journal.pone.0018424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown RE. Morphoproteomics: exposing protein circuitries in tumors to identify potential therapeutic targets in cancer patients. Expert Rev Proteomics. 2005;2:337–48. doi: 10.1586/14789450.2.3.337. [DOI] [PubMed] [Google Scholar]
- 10.Brown RE. Morphogenomics and morphoproteomics: a role for anatomic pathology in personalized medicine. Arch Pathol Lab Med. 2009;133:568–79. doi: 10.5858/133.4.568. [DOI] [PubMed] [Google Scholar]
- 11.Herbst RS, Eckhardt SG, Kurzrock R, Ebbinghaus S, O’Dwyer PJ, Gordon MS, et al. Phase I dose-escalation study of recombinant human Apo2L/TRAIL, a dual proapoptotic receptor agonist, in patients with advanced cancer. J Clin Oncol. 2010;28:2839–46. doi: 10.1200/JCO.2009.25.1991. [DOI] [PubMed] [Google Scholar]
- 12.Koehler BC, Urbanik T, Vick B, Boger RJ, Heeger S, Galle PR, et al. TRAIL-induced apoptosis of hepatocellular carcinoma cells is augmented by targeted therapies. World J Gastroenterol. 2009;15:5924–35. doi: 10.3748/wjg.15.5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin V, Garcia-Santos G, Rodriguez-Blanco J, Casado-Zapico S, Sanchez-Sanchez A, Antolin I, et al. Melatonin sensitizes human malignant glioma cells against TRAIL-induced cell death. Cancer Lett. 2010;287:216–23. doi: 10.1016/j.canlet.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 14.Lamothe B, Aggarwal BB. Ectopic expression of Bcl-2 and Bcl-xL inhibits apoptosis induced by TNF-related apoptosis-inducing ligand (TRAIL) through suppression of caspases-8, 7, and 3 and BID cleavage in human acute myelogenous leukemia cell line HL-60. J Interferon Cytokine Res. 2002;22:269–79. doi: 10.1089/107999002753536248. [DOI] [PubMed] [Google Scholar]
- 15.LeBlanc H, Lawrence D, Varfolomeev E, Totpal K, Morlan J, Schow P, et al. Tumor-cell resistance to death receptor--induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nat Med. 2002;8:274–81. doi: 10.1038/nm0302-274. [DOI] [PubMed] [Google Scholar]
- 16.Bovee JV, Hogendoorn PC, Wunder JS, Alman BA. Cartilage tumours and bone development: molecular pathology and possible therapeutic targets. Nat Rev Cancer. 2010;10:481–8. doi: 10.1038/nrc2869. [DOI] [PubMed] [Google Scholar]
- 17.Bovee JV, van den Broek LJ, Cleton-Jansen AM, Hogendoorn PC. Up-regulation of PTHrP and Bcl-2 expression characterizes the progression of osteochondroma towards peripheral chondrosarcoma and is a late event in central chondrosarcoma. Lab Invest. 2000;80:1925–34. doi: 10.1038/labinvest.3780202. [DOI] [PubMed] [Google Scholar]
- 18.Rozeman LB, Hameetman L, Cleton-Jansen AM, Taminiau AH, Hogendoorn PC, Bovee JV. Absence of IHH and retention of PTHrP signalling in enchondromas and central chondrosarcomas. J Pathol. 2005;205:476–82. doi: 10.1002/path.1723. [DOI] [PubMed] [Google Scholar]
- 19.Ivanov VN, Ghandhi SA, Zhou H, Huang SX, Chai Y, Amundson SA, et al. Radiation response and regulation of apoptosis induced by a combination of TRAIL and CHX in cells lacking mitochondrial DNA: A role for NF-kappaB-STAT3-directed gene expression. Exp Cell Res. 2011 doi: 10.1016/j.yexcr.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosner M, Hengstschlager M. Cytoplasmic and nuclear distribution of the protein complexes mTORC1 and mTORC2: rapamycin triggers dephosphorylation and delocalization of the mTORC2 components rictor and sin1. Hum Mol Genet. 2008;17:2934–48. doi: 10.1093/hmg/ddn192. [DOI] [PubMed] [Google Scholar]
- 21.Yang FG, Zhang ZW, Xin DQ, Shi CJ, Wu JP, Guo YL, et al. Peroxisome proliferator-activated receptor gamma ligands induce cell cycle arrest and apoptosis in human renal carcinoma cell lines. Acta Pharmacol Sin. 2005;26:753–61. doi: 10.1111/j.1745-7254.2005.00753.x. [DOI] [PubMed] [Google Scholar]
- 22.Nishida K, Kunisada T, Shen ZN, Kadota Y, Hashizume K, Ozaki T. Chondrosarcoma and peroxisome proliferator-activated receptor. PPAR Res. 2008;2008:250568. doi: 10.1155/2008/250568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen ZN, Nishida K, Doi H, Oohashi T, Hirohata S, Ozaki T, et al. Suppression of chondrosarcoma cells by 15-deoxy-Delta 12,14-prostaglandin J2 is associated with altered expression of Bax/Bcl-xL and p21. Biochem Biophys Res Commun. 2005;328:375–82. doi: 10.1016/j.bbrc.2004.12.186. [DOI] [PubMed] [Google Scholar]
- 24.Amary MF, Bacsi K, Maggiani F, Damato S, Halai D, Berisha F, et al. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J Pathol. 2011;224:334–43. doi: 10.1002/path.2913. [DOI] [PubMed] [Google Scholar]
- 25.Naing A, Kurzrock R. Chemotherapy resistance and retreatment: a dogma revisited. Clin Colorectal Cancer. 2010;9:E1–4. doi: 10.3816/CCC.2010.n.026. [DOI] [PubMed] [Google Scholar]
- 26.von Minckwitz G, du Bois A, Schmidt M, Maass N, Cufer T, de Jongh FE, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a german breast group 26/breast international group 03–05 study. J Clin Oncol. 2009;27:1999–2006. doi: 10.1200/JCO.2008.19.6618. [DOI] [PubMed] [Google Scholar]
- 27.Horak P, Pils D, Haller G, Pribill I, Roessler M, Tomek S, et al. Contribution of epigenetic silencing of tumor necrosis factor-related apoptosis inducing ligand receptor 1 (DR4) to TRAIL resistance and ovarian cancer. Mol Cancer Res. 2005;3:335–43. doi: 10.1158/1541-7786.MCR-04-0136. [DOI] [PubMed] [Google Scholar]