Abstract
Objective
A better understanding of the response of the spinal cord blood supply to segmental artery (SA) sacrifice should help minimize the risk of paraplegia following both open and endovascular repair of thoracoabdominal aortic aneurysms (TAAA).
Methods
12 female juvenile Yorkshire pigs were randomized into three groups and perfused with a barium-latex solution. Pigs in group 1 (control) had infusion without previous intervention. Pigs in group 2 were infused 48 hours after ligation of all SAs (T4-L5), and those in group 3 at 120 hours after ligation. Post-mortem CT scanning of the entire pig enabled overall comparisons and measurement of vessel diameters in the spinal cord circulation.
Results
14.5±0.8 SAs were ligated: all filled retrograde to the ligature. Paraplegia occurred in 38% of operated pigs. A significant increase in the mean diameter of the anterior spinal artery (ASA) was evident after SA sacrifice (p=<0.0001 for 48h and 120h). The internal thoracic and intercostal arteries also increased in diameter. Quantitative assessment showed an increase in vessel density 48h after ligation of SAs, reflected by an obvious increase in small collateral vessels seen on 3-D reconstructions of CT scans at 120h.
Conclusions
Remodeling of the spinal cord blood supply—including dilatation of the ASA and proliferation of small collateral vessels—is evident at 48 and 120 hours after extensive SA sacrifice. It is likely that exploitation of this process will prove valuable in the quest to eliminate paraplegia after TAAA repair.
Introduction
Although its incidence is gradually diminishing, paraplegia following surgery or endovascular treatment of thoracoabdominal aortic aneurysms is still a devastating complication. The causes of spinal cord injury (SCI) are multifactorial, and different strategies for spinal cord protection have evolved over time (1). Despite this, when longer segments of the aorta are replaced—with more segmental arteries (SAs) sacrificed—the risk of SCI is still high. An understanding of the anatomical characteristics of the spinal cord vasculature is fundamental to knowing how to augment blood flow when surgery has compromised the usual sources of perfusion, and thus to minimize the risk of paraplegia.
The initial detailed description of the spinal cord circulation was carried out by A. Adamkiewicz many years ago (2-5). His studies depict the presence of a distinctive SA in the lower thoracic/upper abdominal region which he identified as being more important in supplying the anterior spinal artery (ASA) than other, smaller segmental arteries. This artery has been referred to as the artery of Adamkiewcz ever since. Unfortunately, however, its identification is difficult and not always possible, and its reimplantation has been disappointing in its efficacy in reducing the incidence of SCI following aortic aneurysm repair. Likewise, a strategy involving reimplantation of multiple intercostal and lumbar arteries is not always successful in averting SCI; it entails risks such as subsequent patch aneurysms (6), and is not feasible with an endovascular approach.
Lazorthes et al. were among the first to describe the important contribution of multiple other vessels to the spinal cord circulation, and their anastomoses with one another (2-4). In addition to input from multiple intercostal and lumbar arteries, the vertebral arteries and branches of the subclavian and hypogastric arteries have now been recognized as part of the system nourishing the cord (5). Over time, experimental studies have enabled visualization in detail of the complex collateral network involved in spinal cord perfusion (7-9).
We previously investigated the arterial network which supplies the spinal cord by infusing acrylic resin into the circulation: after tissue digestion, a cast of the collateral network was revealed, including a dense matrix of arteries in the paraspinous musculature. This technique generated remarkably detailed images of vessel orientation, dimensions and interconnections both in the native state, and after sacrifice of segmental vessels (7). Our understanding of spinal cord protection has changed with the revelation of the extensive, dense collateral arterial system surrounding the cord; the major shortcoming of these studies involved the loss of casts of small vessels adjacent to ligatures, owing to lack of secondary support following tissue digestion, but they did allow visualization of a significant increase in the diameter of the ASA already 24 hours after sacrifice of SAs. Based on these findings, we hypothesize that the collateral network feeding the spinal cord quite rapidly increases its capacity in response to diminished input by means both of dilatation and development of new vessels.
We undertook the current study to further investigate the anatomical and physiological response of the spinal cord vasculature to the sacrifice of all SAs. In order to study the vascular network more comprehensively and in greater detail, and avoid loss secondary to tissue digestion, we used a latex-barium mixture that can be visualized in situ using 3-D CT scanning.
Methods
Prior to the experiment, a pilot study was perfomed to determine the correct barium/latex mixture and optimal perfusion pressure to achieve filling of all vessels. All animals received humane care in compliance with the guidelines of ‘Principles of Laboratory Animal Care’ formulated by the National Society for Medical Research and the ‘Guide for the Care and Use of Laboratory Animals’ published by the National Institute of Health (NIH Publication No. 88-23, revised 1996). The study was approved by the Mount Sinai Institutional Research Committee.
Twelve female juvenile Yorkshire pigs (Animal Biotech Industries, Allentown, NJ) weighing 25± 2 kg (range 23-28) were randomized into three groups prior to perfusion with a mixture of latex and barium. Two groups underwent sacrifice of all SAs T4-L5 prior to perfusion with latex-barium, and one group had no intervention before perfusion with the radiopaque mixture (control). The casting procedure was undertaken 48 hours after SA ligation in Group 2, and was carried out 120 hours after SA ligation in Group 3; Group 1 was the control. Functional outcome was evaluated using the modified Tarlov score, as described in detail subsequently.
Segmental Artery (SA) ligation
After premedication with intramuscular ketamine (15mg/kg) and atropine (0.03 mg/kg), an endotracheal tube is placed. The animals are then transferred to the operating room and are mechanically ventilated with a FiO2 of 0.5, and a minute volume adequate for maintenance of a normal pCO2 (35-40mmHg). Anesthesia is induced via the bolus intravenous administration of propofol (1mg/kg) and fentanyl (50μg/kg). It is maintained with infusions of ketamine (15mg/kg/hr), propofol (7mg/kg/hr) and fentanyl (5μg/kg/hr). The pig is then cooled to 32°C using a cooling blanket. The chest is opened through a thoracotomy in the seventh intercostal space. The abdominal aorta is exposed through a left flank incision. The aorta is mobilized from the arch to the bifurcation with careful identification of the SAs as they arise from the aorta. The SAs are sacrificed serially in a cephalo-caudal sequence. Once the procedure is completed, the incisions are closed and the animal is gradually rewarmed and weaned from anesthesia. After signs of awakening are evident, ventilatory support is gradually decreased until the animal is breathing spontaneously. Regular analgesic medication is administered: intramuscular buphrenorphine (0.03 mg/kg bid) and a fentanyl patch (50 mcg/hr for 72 hours). During this procedure, a small arterial catheter is placed in the aorta and secured to the pig’s back. This enables us to measure the blood pressure postoperatively and adjust intravenous fluids accordingly.
Barium/latex infusion
After heparinization (300 IU/kg), a 26F single-stage cannula is inserted into the right atrium, and a 16F arterial cannula into the aortic arch. Blood washout is accomplished with 4000 mL 0.9% saline solution and 7500 IU heparin. The reservoir of the pump is then loaded with one liter high-viscosity latex (Blue Latex Solution, Ward’s Natural Science, Rochester, NY, USA) mixed with 360 ml barium (Barium Sulfate Suspension, E-Z-EM Canada Inc., Lake Success, NY, USA). Whole-body perfusion is started at physiologic pressures while exsanguination is achieved through the venous cannula. The animal is anesthetized throughout the procedure and is euthanized before being perfused with the carrier/barium-sulfate mixture. Perfusion pressures are monitored through a right axillary and a femoral artery catheter. Peak pressure for saline perfusion is 100 mmHg: it is 200 mmHg during latex perfusion to achieve complete filling of vascular structures. In 9 of 12 pigs, the latex was allowed to harden for 48 hours at 4°C before evisceration was carried out because it was found to be technically easier than carrying out evisceration after scanning. The pigs were then frozen at −80°C.
Computed tomography angiography
CT scans of whole pigs were performed using a 256-slice MDCT scanner. The high-resolution scans were carried out at 140kV and 250 mAS. Slice thickness is 0.67 mm, with an increment of 0.3 mm. 3D reconstructions were undertaken to evaluate the data and measure vessel diameters.
Measurements of vessels and calibration
CT images were processed and analyzed using OsiriX© v3.9 software. In 3 pigs that were not eviscerated before they were frozen and scanned, the intestines were extracted from the images using the OsiriX© software.
A frame of 568×356 pixels around the internal thoracic artery-- with the beginning of the bifurcation acting as the midpoint of the left side of the rectangle-- was excised. A 442×227 frame around the external iliac artery-- beginning just above the external iliac artery, with the right corner meeting the right common iliac artery-- was cropped to compare vessel density (see Figures 3 and 4). The GIMP© histogram feature was used to analyze the regions of interest using gray values.
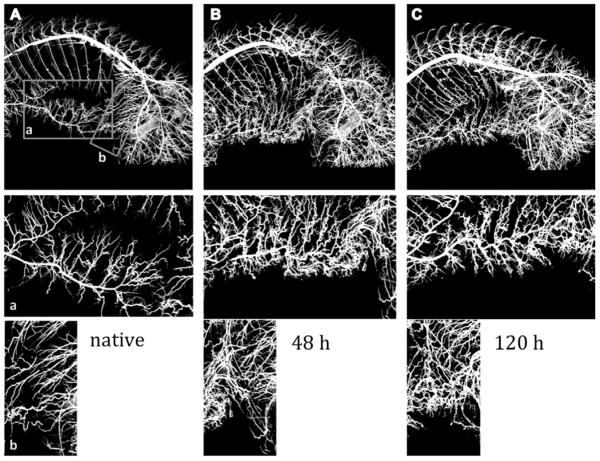
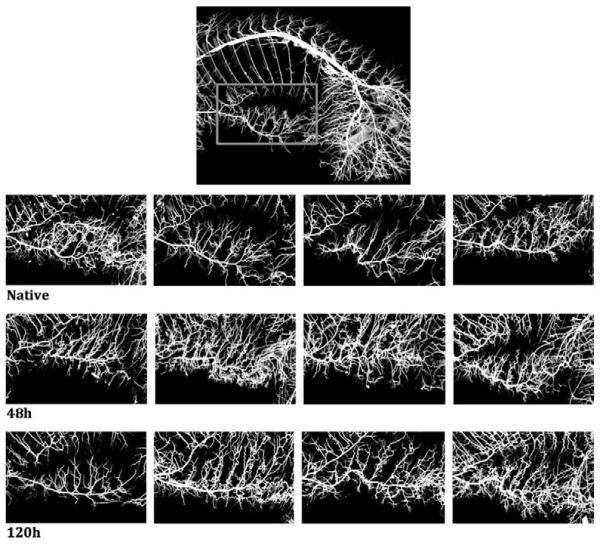
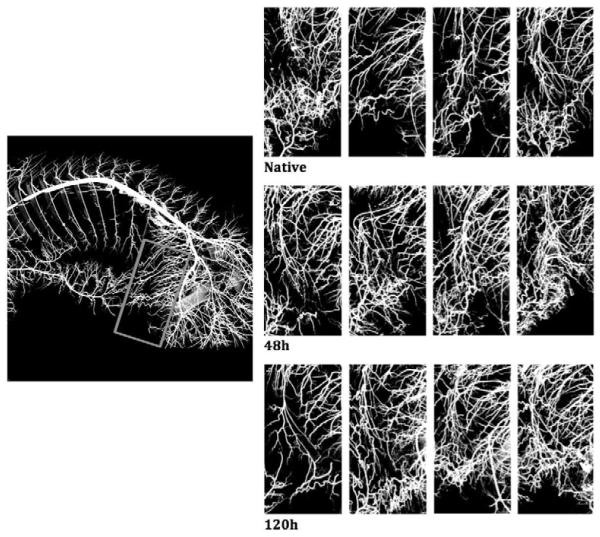
Figure 3.
CT image of the arterial vessel system of the entire pig. Bones, intestines and other tissues were extracted from the image. A, B, and C show examples of vessel casting for a native pig, at 48 hours, and at 120 hours after segmental artery ligation, respectively. A frame around the internal thoracic artery (a) and around the iliolumbar vessel (b) was cropped, and vessel density compared between the groups. We can see an impressive increase in density of small vessels, but no statistically significant differences could be demonstrated with the small number of pigs examined at each time point.
Figure 4.
a. Analyzing the area around the right internal thoracic artery with the beginning of the bifurcation acting as the midpoint of the left side of the 568 × 365 rectangle.
b. Analyzing the area around the right external iliac artery with the 442 × 227 rectangular frame being just above the external iliac artery and the right corner meeting the right common iliac artery.
The images from all four pigs in each group are shown in one horizontal row. There is one pig in the group of native pigs, and one pig in the 120h pigs that did not show results consistent with the others, pointing to likely interindividual variability in the response of the collateral network to SA sacrifice.
To achieve accuracy within our measurements, a calibration was performed and all measurements were converted. Tubes of different known diameters were filled with the barium/latex mixture, inserted in the pig intraoperatively, and measured using the same filter we applied to measure vessels. Measured diameter was then multiplied by a factor that was calculated to achieve the actual diameter. A smooth curve was created to obtain appropriate multiplication factors for all vessel sizes.
Behavioral evaluation
To evaluate functional outcome, we used a modified Tarlov scale, in which 0 indicates no voluntary movements; 1, perceptible movements at joints; 2, good movements at joints but inability to stand; 3, ability to get up and stand with assistance < 1 min; 4, ability to get up with assistance and stand unassisted < 1 min; 5, ability to get up with assistance and stand unassisted > 1 min; 6, ability to get up and stand unassisted > 1min; 7, ability to walk < 1 min; 8, ability to walk >1 min; and 9 indicates full recovery.
Statistical analysis
Data were entered in an excel spreadsheet (Microsoft Corp, Redmond, WA) and transferred to an SAS file (SAS Institute Inc, Cary, NC) for data description and analysis. Descriptive data of the pigs are presented as percentages, medians with range, or means ± standard deviations. A mixed model, with groups as the fixed effect and pigs within each group as the random effect, was used to compare the differences among the three groups. Least squares means (SE) were reported.
Results
The mean weight of the pigs was 25±2 kg (range 23-28). The average number of SAs ligated was 14.5±0.8: all ligated SAs remained patent, filling retrograde to the ligature site. Paraplegia occurred in 37.5% of operated pigs (3/8), as anticipated from previous studies in the same animal model. The latex/barium mixture was infused with a mean pressure of 177±20 mmHg; 4242±448 ml of saline was used to wash the blood out, and 798±130 ml of the barium/latex mixture was infused.
Group comparisons
Although comparisons were made between a control group of 4 pigs, and two groups of 4 pigs each perfused at 48 and at 120 hours after SA sacrifice, the results and discussion are phrased in physiological terms, as though measurements were occurring in the same animal over time: before intervention, and then twice thereafter.
Vessel diameter
The mean diameter of the ASA in controls was 0.29±0.02 mm, Table 1. The ASA showed an increase of 59% 48 hours after SA sacrifice, to 0.46±0.02 mm (p<0.0001), but no further increase at 120 hours (0.43±0.02, p<0.0001 compared with controls). We also analyzed diameters of the ASA in the thorax (T4-T13) and the lumbar area (L1-L5) separately. The diameter of the thoracic ASA increased 49% -- from 0.33±0.05 mm in controls to 0.49±0.05 mm after 48 hours (p=0.02)-- and by 36%-- to 0.45±0.05 mm-- after 120 hours (p=0.07). In the lumbar ASA, we found an increased diameter of 0.43±0.03 after 48 hours (p=0.0002) and 0.40±0.03 after 120 hours (p=0.002) compared with 0.25±0.03 mm in controls. This represents an increase of 72% within 48 hours and 60% within 120 hours.
Table 1.
Least squares means (SE) of vessel diameters (mm)
| Group 1 (control) | Group 2 | Group 3 | p-value | |
|---|---|---|---|---|
| Anterior spinal artery (ASA) | 0.29±0.02 | 0.46±0.02 | 0.43±0.02 | <0.0001 (1 vs 2) <0.0001 (1 vs 3) |
| ASA thoracic | 0.33±0.05 | 0.49±0.05 | 0.45±0.05 | 0.02 (1 vs 2) 0.07 [1 vs3) |
| ASA lumbar | 0.25±0.03 | 0.43±0.03 | 0.40±0.03 | 0.0002 (1 vs 2) 0.0020 (1 vs 3) |
| Internal thoracic artery (ITA) | 1.50±0.11 | 1.53±0.11 | 1.81±0.11 | 0.083 |
| Intercostal arteries (T4-T13) |
0.81±0.04 | 0.87±0.04 | 0.92±0.05 | 0.273 |
| IIiolumbar artery (IL) | 1.34±0.14 | 1.35±0.14 | 1.40±0.14 | 0.951 |
The diameter of the internal thoracic artery was 1.50±0.11 mm in controls, 1.53±0.11 mm after 48 hours and 1.81±0.11 mm after 120 hours (p=0.083). The intercostal arteries arising from ligated SAs measured 0.81±0.04 in controls and increased with time, measuring 0.87±0.04 mm at 48 hours, and 0.92±0.05 mm at 120 hours, (p=0.27). The diameter of the iliolumbar arteries remained quite constant: 1.34±0.14 mm in controls, 1.35±0.14 mm in the 48 hour group, and 1.40±0.14 mm at 120 hours (p=0.95).
Vessel density
A visually impressive increase in density of smaller collateral vessels was evident on CT scans by 48 and 120 hours postoperatively. When analyzing vessel density according to gray values on CT scans, we found that in the thoracic window (frame cropped around the internal thoracic artery) the percentage of gray value was higher at 48 hours than in the controls: 49±3% vs 37±4%, Figure 4a. The percentage of gray value was 42±6% after 120 hours (p=0.09). In the lumbar window, there was also an increase in vessel density, Figure 4b, albeit not as significant:. 48±4% of pixels in this window were counted as vessels in the unoperated group, with an increase to 56±2% after 48 hours, and 58±7% after 120 hours (p=0.23).
Discussion
Previous studies of vascular casts in our institution have already allowed a detailed description of the spinal cord and paraspinous muscle arterial network. Small arteries and arterioles were visualized with scanning electron microscopy. These studies provided insight into the mass of interconnected arteries, their orientation, and the importance of the SAs in providing nutrient blood not only to the spinal cord, but also—and predominantly—to paraspinous muscles. The current study of CT scanning of juvenile pigs infused with a latex-barium mixture has allowed further imaging of the collateral network and its response to sacrifice of SA input similar to what occurs with repair of descending aortic and thoracoabdominal aneurysms either during open surgery or endovascular repair. The high-viscosity of the latex/barium mixture, which does not shrink after infusion, allows us to measure and calculate actual dimensions of the ASA with greater accuracy. Multidetector high resolution CT scanning and 3 D reconstruction of the entire pig enabled us to also include additional vessels—such as a branches of the external iliac artery, the internal thoracic artery, and the intercostal arteries—in our analysis and discover significant changes in vessel diameter as well as vessel density triggered by SA sacrifice. Small interconnecting vessels up to a minimum diameter of 0.13 mm could be measured with accuracy, and computer-based definition of certain areas of interest with further analysis of pixel count enabled us to quantify vessel density.
The anterior spinal artery (ASA) is dilated 48 hours following sacrifice of all SAs, and the number of small interconnecting vessels—not only in the paraspinous region but also in areas around the internal thoracic artery and in the lumbar region—seems to increase over time. Overall vessel density increases impressively, likely in response to local ischemia. Evidence from this and earlier studies confirm that this process is already significant 48 hours after SA ligation, and tends to become more robust by 5 days. This explains earlier physiological observations, which showed that significantly lower values for spinal cord blood flow at 5 hours after segmental artery sacrifice were seen in pigs with subsequent spinal cord injury than in those who recovered normal function (10). This suggests that critical spinal cord ischemia after complete sacrifice of all thoracoabdominal SA’s does not occur immediately (intraoperatively), but is delayed 1—5 h or longer after clamping, and represents failure to mount a hyperemic response to rewarming and awakening. Immediate injury can be kept minimal or prevented with good intraoperative management such as hypothermia, spinal cord drainage, spinal cord monitoring, distal perfusion etc. The reason for delayed paraplegia is usually an episode of hypotension which occurs before postoperative adaption to a loss of input into the collateral network which supplies the spinal cord has had a chance to occur.
Taking together microsphere studies of blood flow, direct measurements of collateral network pressure, previous casting studies, and the observations from the current study, we can construct an approximate scenario of how the collateral network responds to extensive SA sacrifice. We know that blood flow takes 72 hours to recover reliably to preoperative levels, and that the pressure in the collateral network takes up to five days to return to baseline values. In the previous casting studies, we saw an increase in the diameter of the ASA already at 24 hours, and further enlargement at 5 days: in the current study, we see significant dilatation at 48 hours, with no significant further increase at 5 days. Thus it seems that the immediate response of the collateral network is to increase flow by means of dilatation of existing named vessels: the ASA, the epidural arcades, the internal thoracic artery and the intercostal arteries.
Between 48 and 120 hours, the augmentation of blood flow seems to be a combination of generation of new small vessels—angiogenesis—and transformation of existing very small vessels into larger ones—arteriogenesis. Both of these mechanisms likely contribute to the increase in vascular density seen in the current study, which confirms the observation of increased vascular density made by morphometric analysis in the earlier study. The earlier study also showed a re-orientation of these small vessels within the musculature to allow them to feed into the cranial and caudal collateral vessels providing flow to the lower thoracic ASA.
The increase of vessel density after SA sacrifice can clearly be appreciated in the CT images (see Figures 3 and 4). However, it is also apparent that there is some variability. The failure of one pig in the 120-hour group to show marked vascular changes likely influenced the outcome in this small study, and did not allow us to demonstrate whether larger diameters and greater vessel densities are usually present at 120 hours as compared with 48 hours after SA sacrifice. The differences observed between individual pigs in this small study emphasize the likelihood that there is inter-individual variability in the response of the collateral network to SA sacrifice.
But all the studies combined leave no doubt that within 48 hours and certainly within five days, the collateral circulation has undergone profound changes to support spinal cord perfusion in the absence of antegrade flow through SAs to the ASA. Multiple vessels and their interconnections function in order to supply the spinal cord: not only does a dense network within the paraspinous muscles develop, but also other, larger vessels contribute to compensate for loss of direct inflow into the mid-thoracic region of the spinal cord. We were hoping to demonstrate significant increases in the diameters of several of these vessels, but inter-individual variability made it difficult to demonstrate this conclusively, although a trend was apparent.
We confirmed the observation made in the previous casting study that the diameter of the ASA increases more significantly in the lumbar than in the thoracic region. This may be due to anatomical circumstances: the lumbar region of the spinal cord has a small peripheral and a large central supply (11), the latter arising from the ASA and perfusing two-thirds of the spinal cord. In the thoracic region, central supply is less critical and the peripheral arterial supply is more significant. Mechanisms to increase peripheral blood flow—possibly an increase in size of the posterior spinal arteries (PSA) in the thoracic area—may be activated by SA ligation. The fact that the ASA contributes less to spinal cord perfusion in the thoracic part of the cord may be one of the reasons the increase in size of the ASA is more prominent in the lumbar region, where central supply is dominant.
Previous studies have shown a significant increase in all regions of the ASA, although the increase in the caudal and cranial ends was more pronounced (7, 8). The intercostal arteries that were ligated (Th4-Th13/L1; rib 6-15/16) during operation also showed an increase in size. This increase was most impressive in vessels along the mid-to-lower thoracic ribs. Given the observations of our studies and evidence from multiple previous studies (2-5) that the most cervical and caudal parts of the cord are connected to the subclavian and internal iliac arteries, the changes in the collateral network which we observed in response to SA sacrifice are likely due to a sequence beginning with dilatation, and then involving arteriogenesis and angiogenesis as well as vessel reorientation. In areas far from major collaterals, adaptation to relative ischemia may involve development of new vessels rather than an increase in the size of existing ones.
No single adjustment in operative technique can as yet prevent paraplegia following extensive thoracoabdominal aneurysm repair. Knowing that an impressive network of collaterals builds up within five days after ligating SAs—by means of dilatation, angiogenesis and arteriogenesis—is essential to exploring the efficacy of various strategies for improving postoperative spinal cord perfusion. Stimulating vascular remodeling as well as augmenting cardiac output to increase flow through the collateral network are possible strategies. A multifaceted approach may be more effective than the current strategy, which is largely limited to short-term efforts to increase mean arterial pressure postoperatively. An adequate pressure is of unquestioned importance in increasing perfusion within the collateral network, especially in conjunction with spinal cord drainage, but may not be sufficient or sustained enough to prevent delayed paraplegia. In addition, precise control and maintenance of sufficiently high blood pressure is sometimes impractical in patients who are chronically hypertensive, bleeding, or hemodynamically unstable.
Although replacement of the entire thoracoabdominal aorta during a single procedure still carries a significant risk of SCI, results from experimental studies as well as clinical experience at our institution suggest that paraplegia and paraparesis are almost never seen— even with an equivalently extensive total resection—if aneurysm repair is carried out in stages, with abdominal replacement following thoracic replacement (or vice versa) after an interval of weeks, months or years (12-14). Various existing reports identify patients undergoing endovascular TA aneurysm repair following previous or concomitant AA repair to be at higher risk for spinal cord injury (15-19). However, the largest and most recent single-center study in which a hybrid procedure was performed in 47 patients – 33 simultaneous AA and TA repairs, and 14 staged procedures-found the staged approach to be superior. Their overall paraplegia rate was 4.3%, comparable to results after open TAA repair. Both of the subsequently paraplegic patients had undergone simultaneous repair (20).
Causes for paraplegia are multifactorial; optimal intraoperative as well as postoperative care is needed to prevent this devastating complication. The extent of aneurysm repair is nevertheless the most important factor: not only does the number of segmental arteries sacrificed matter, but also the additional occlusion or compromise of vessels feeding into the collateral network, such as the subclavian and hypogastric arteries influences recovery after segmental artery sacrifice. Patients undergoing combined open and endovascular procedures may have had these major sources of collateral flow impinged upon during earlier operations. At present, patients undergoing hybrid repairs tend also to have more comorbidities than patients having open surgical repair, and therefore perioperative management may be more difficult, leading to complications including paraplegia. We speculate that reduced paraplegia rate clearly seen after staged procedures separated by several days to weeks or months is due to development of additional collateral vessels in the time between the two operations, which compensates for the loss of the SAs. The questions now being explored include the minimum interval required for adequate stable enhancement of the collateral network after the first stage of a two-stage procedure; the minimum stimulus required for such enhancement to be initiated, and how one can ascertain that the necessary augmentation of the collateral system has been induced and/or achieved in any individual instance.
At present, an effective strategy for minimizing the occurrence of postoperative paraplegia is the deliberate staging of extensive thoracoabdominal resections where clinically feasible. Possible future approaches include induction of a collateral network response prior to the definitive operation using pharmacological, minimally invasive, or endovascular strategies. An understanding of the anatomy and pathophysiology of the collateral network response to SA sacrifice will help in the development of such strategies, which we expect will ultimately eliminate the problem of postoperative paraplegia after extensive thoracoabdominal aorta resection.
Conclusion
In this experimental simulation of TAAA repair, remodeling of the blood supply to the spinal cord is evident at 48-120 hours, and is characterized by recruitment and dilatation of small unnamed vessels, and by dilatation of some major axial vessels. Exploitation of our understanding of the collateral network response to loss of SA input is likely to prove invaluable in the quest to eliminate postoperative paraplegia following treatment of extensive thoracoabdominal aneurysms.
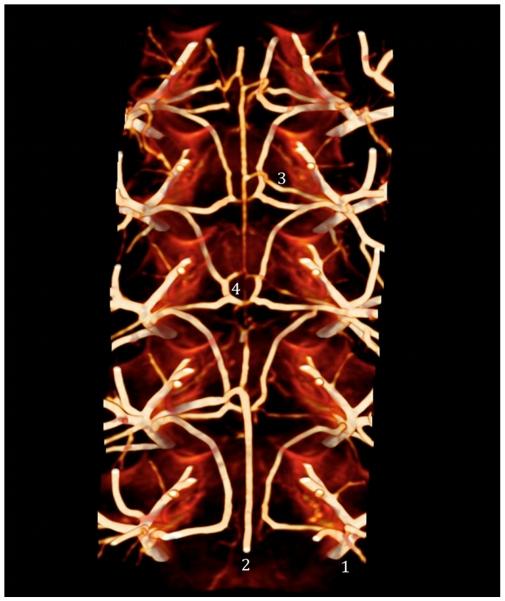
Figure 1.
CT image showing the anatomy of the anterior spinal artery and epidural arcades. The main trunks of the segmental arteries (1) feed the anterior spinal artery (2) through the anterior radicular-medullary artery (ARMA) (3), and give off branches to the epidural arcades (4).
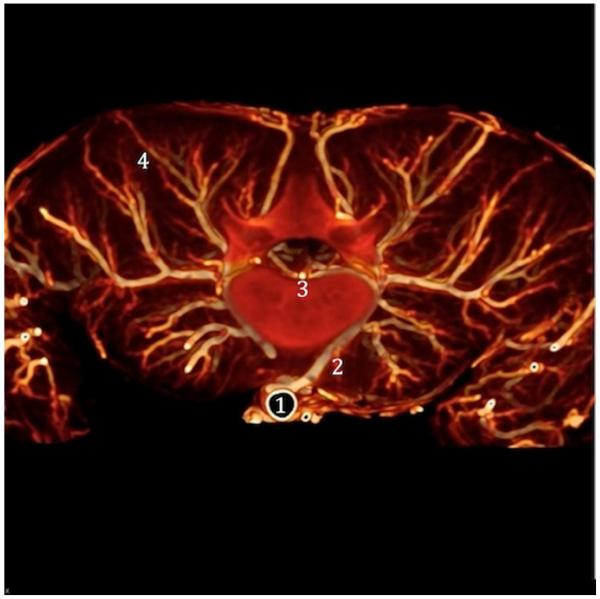
Figure 2.
This picture shows the vasculature of the spinal cord and the arterial network within the paraspinous muscles. The segmental artery (2) arises from the aorta (1) and runs around the vertebral body, connecting to the ASA (3) through the anterior radiculo-medullary arteries (ARMA). Note the extensive network within the paraspinous muscle (4) and its interconnections with the segmental arteries.
Acknowledgements
This study was supported by grant R01 HL 045636 from the National Heart, Lung and Blood Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shimizu H, Yozu R. Current strategies for spinal cord protection during thoracic and thoracoabdominal aortic aneurysm repair. Gen Thorac Cardiovasc Surg. 2011;59(3):155–63. doi: 10.1007/s11748-010-0705-9. [DOI] [PubMed] [Google Scholar]
- 2.Lazorthes G, Gouaze A, Zadeh JO, Santini JJ, Lazorthes Y, Burdin P. Arterial vascularization of the spinal cord. Recent studies of the anastomotic substitution pathways. J Neurosurg. 1971;35(3):253–62. doi: 10.3171/jns.1971.35.3.0253. [DOI] [PubMed] [Google Scholar]
- 3.Lazorthes G, Poulhes J, Bastide G, Roulleau J, Chancholle AR. Research on the arterial vascularization of the medulla; applications to medullary pathology. Bull Acad Natl Med. 1957;141(21-23):464–77. [PubMed] [Google Scholar]
- 4.Lazorthes G, Poulhes J, Bastide G, Roulleau J, Chancholle AR. Arterial vascularization of the spine; anatomic research and applications in pathology of the spinal cord and aorta. Neurochirurgie. 1958;4(1):3–19. [PubMed] [Google Scholar]
- 5.Adamkiewicz A. Die Blutgefaesse des menschlichen Rueckenmarks. S B Heidelberg Akad Wiss. 1882:101–30. [Google Scholar]
- 6.Kulik A, Castner CF, Kouchoukos NT. Outcomes after thoracoabdominal aortic aneurysm repair with hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 2011;141(4):953–60. doi: 10.1016/j.jtcvs.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Etz CD, Kari FA, Mueller CS, Silovitz D, Brenner RM, Lin HM, et al. The collateral network concept: a reassessment of the anatomy of spinal cord perfusion. J Thorac Cardiovasc Surg. 2011;141(4):1020–8. doi: 10.1016/j.jtcvs.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Etz CD, Kari FA, Mueller CS, Brenner RM, Lin HM, Griepp RB. The collateral network concept: remodeling of the arterial collateral network after experimental segmental artery sacrifice. J Thorac Cardiovasc Surg. 2011;141(4):1029–36. doi: 10.1016/j.jtcvs.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strauch JT, Lauten A, Zhang N, Wahlers T, Griepp RB. Anatomy of spinal cord blood supply in the pig. Ann Thorac Surg. 2007;83(6):2130–4. doi: 10.1016/j.athoracsur.2007.01.060. [DOI] [PubMed] [Google Scholar]
- 10.Etz CD, Homann TM, Luehr M, Kari FA, Weisz DJ, Kleinman G, et al. Spinal cord blood flow and ischemic injury after experimental sacrifice of thoracic and abdominal segmental arteries. Eur J Cardiothorac Surg. 2008;33(6):1030–8. doi: 10.1016/j.ejcts.2008.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martirosyan NL, Feuerstein JS, Theodore N, Cavalcanti DD, Spetzler RF, Preul MC. Blood supply and vascular reactivity of the spinal cord under normal and pathological conditions. J Neurosurg Spine. 2011;15(3):238–51. doi: 10.3171/2011.4.SPINE10543. [DOI] [PubMed] [Google Scholar]
- 12.Bischoff MS, Scheumann J, Brenner RM, Ladage D, Bodian CA, Kleinman G, et al. Staged approach prevents spinal cord injury in hybrid surgical-endovascular thoracoabdominal aortic aneurysm repair: an experimental model. Ann Thorac Surg. 2011;92(1):138–46. doi: 10.1016/j.athoracsur.2011.03.098. [DOI] [PubMed] [Google Scholar]
- 13.Zoli S, Etz CD, Roder F, Brenner RM, Bodian CA, Kleinman G, et al. Experimental two-stage simulated repair of extensive thoracoabdominal aneurysms reduces paraplegia risk. Ann Thorac Surg. 2010;90(3):722–9. doi: 10.1016/j.athoracsur.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 14.Etz CD, Zoli S, Mueller CS, Bodian CA, Di Luozzo G, Lazala R, et al. Staged repair significantly reduces paraplegia rate after extensive thoracoabdominal aortic aneurysm repair. J Thorac Cardiovasc Surg. 2010;139(6):1464–72. doi: 10.1016/j.jtcvs.2010.02.037. [DOI] [PubMed] [Google Scholar]
- 15.Dake MD, Miller DC, Mitchell RS, Semba CP, Moore KA, Sakai T. The “first generation” of endovascular stent-grafts for patients with aneurysms of the descending thoracic aorta. The Journal of thoracic and cardiovascular surgery. 1998;116(5):689–703. doi: 10.1016/S0022-5223(98)00455-3. discussion -4. [DOI] [PubMed] [Google Scholar]
- 16.Gravereaux EC, Faries PL, Burks JA, Latessa V, Spielvogel D, Hollier LH, et al. Risk of spinal cord ischemia after endograft repair of thoracic aortic aneurysms. Journal of vascular surgery : official publication, the Society for Vascular Surgery [and] International Society for Cardiovascular Surgery, North American Chapter. 2001;34(6):997–1003. doi: 10.1067/mva.2001.119890. [DOI] [PubMed] [Google Scholar]
- 17.Makaroun MS, Dillavou ED, Kee ST, Sicard G, Chaikof E, Bavaria J, et al. Endovascular treatment of thoracic aortic aneurysms: results of the phase II multicenter trial of the GORE TAG thoracic endoprosthesis. Journal of vascular surgery : official publication, the Society for Vascular Surgery [and] International Society for Cardiovascular Surgery, North American Chapter. 2005;41(1):1–9. doi: 10.1016/j.jvs.2004.10.046. [DOI] [PubMed] [Google Scholar]
- 18.Schlosser FJ, Verhagen HJ, Lin PH, Verhoeven EL, van Herwaarden JA, Moll FL, et al. TEVAR following prior abdominal aortic aneurysm surgery: increased risk of neurological deficit. Journal of vascular surgery : official publication, the Society for Vascular Surgery [and] International Society for Cardiovascular Surgery, North American Chapter. 2009;49(2):308–14. doi: 10.1016/j.jvs.2008.07.093. discussion 14. [DOI] [PubMed] [Google Scholar]
- 19.Martin DJ, Martin TD, Hess PJ, Daniels MJ, Feezor RJ, Lee WA. Spinal cord ischemia after TEVAR in patients with abdominal aortic aneurysms. Journal of vascular surgery : official publication, the Society for Vascular Surgery [and] International Society for Cardiovascular Surgery, North American Chapter. 2009;49(2):302–6. doi: 10.1016/j.jvs.2008.08.119. discussion 6-7. [DOI] [PubMed] [Google Scholar]
- 20.Hughes GC, Barfield ME, Shah AA, Williams JB, Kuchibhatla M, Hanna JM, et al. Staged total abdominal debranching and thoracic endovascular aortic repair for thoracoabdominal aneurysm. Journal of vascular surgery : official publication, the Society for Vascular Surgery [and] International Society for Cardiovascular Surgery, North American Chapter. 2012 doi: 10.1016/j.jvs.2011.11.149. [DOI] [PMC free article] [PubMed] [Google Scholar]