Abstract
The epidermal growth factor receptor (EGFR)-mediated signaling pathways are important in a variety of cellular processes, including cell migration and wound re-epithelialization. Intracellular trafficking of EGFR is critical for maintaining EGFR surface expression. Galectin-3, a member of an animal lectin family, has been implicated in a number of physiological and pathological processes. Through studies of galectin-3-deficient mice and cells isolated from these mice, we demonstrated that absence of galectin-3 impairs keratinocyte migration and skin wound re-epithelialization. We have linked this pro-migratory function to a crucial role of cytosolic galectin-3 in controlling intracellular trafficking and cell surface expression of EGFR after EGF stimulation. Without galectin-3, the surface levels of EGFR are dramatically reduced and the receptor accumulates diffusely in the cytoplasm. This is associated with reduced rates of both endocytosis and recycling of the receptor. We have provided evidence that this novel function of galectin-3 may be mediated through interaction with its binding partner Alix, which is a protein component of the endosomal sorting complex required for transport (ESCRT) machinery. Our results suggest that galectin-3 is potentially a critical regulator of a number of important cellular responses through its intracellular control of trafficking of cell surface receptors.
Introduction
Galectins are a family of animal lectins containing conserved protein domains and preferential beta-galactoside-binding activity (Cummings and Liu, 2009). Fifteen members have been identified in mammals and described to participate in various physiological and pathological processes, including the immune response (Liu, 2005), inflammation (Liu and Rabinovich, 2010; Rabinovich and Toscano, 2009), as well as cancer progression and metastasis (Liu and Rabinovich, 2005), by modulating cell proliferation (Scott and Weinberg, 2004), cell adhesion (Taylor and Drickamer, 2007), and apoptosis (Hsu et al., 2006; Nakahara et al., 2005).
Galectin-3 is a chimeric protein and is one of the most extensively studied members. Its C-terminal region constitutes the carbohydrate-binding domain, and the N-terminal region contains tandem repeats that are rich in proline and glycine residues. Like other galectins, galectin-3 does not have a classical signal sequence, but can be released into the extracellular space through a non-classical secretory pathway (Hughes, 1999). Extracellular and intracellular functions have been demonstrated for this protein (Dumic et al., 2006). Galectin-3 can interact extracellularly with cell surface glycoproteins and influence a variety of cellular responses as well as modulate the properties of cell surface receptors (Demetriou et al., 2001; Partridge et al., 2004). Intracellularly, galectin-3 is anti-apoptotic in the human Jurkat T cell line (Yang et al., 1996) and a variety of normal and tumor cells (reviewed in (Nakahara et al., 2005)). Intracellular galectin-3 also contributes to apical non-raft domain protein sorting (Delacour et al., 2006), glycoprotein clustering (Delacour et al., 2007), and apical membrane organization (Delacour et al., 2008). Studies on galectin-3-deficient (gal3-/-) mice have demonstrated that galectin-3 regulates mast cell activation (Chen et al., 2006), macrophage phagocytosis (Sano H, 2003), alternative macrophage activation (MacKinnon et al., 2008), and TCR-mediated CD4+ T cell activation (Chen et al., 2009). Galectin-3 has also been demonstrated to play a role in pre-mRNA splicing (Dagher et al., 1995).
Diverse functions of galectin-3 suggest that it may have a role in global processes, such as wound healing. Keratinocyte migration is a crucial step required for skin wound re-epithelialization (Santoro and Gaudino, 2005) and the EGF-stimulated EGFR-ERK signaling pathway plays a central role in this process (McCawley et al., 1998; Shirakata et al., 2005). Upon EGF stimulation, EGFR is phosphorylated, which is followed by activation of the downstream Src-Ras-ERK signaling pathway. The receptor is ubiquitinated and down-regulated through either clathrin-mediated endocytosis (Haigler et al., 1979) or caveolae-mediated pinocytosis (Sigismund et al., 2005). After endocytosis, ubiquitinated EGFR is transported to early endosomes that can undergo membrane invagination to form multivesicular bodies (MVBs). These intracellular structures are crucial for receptor down-regulation (von Zastrow and Sorkin, 2007; Zwang and Yarden, 2009). EGFR can be sorted to different compartments inside the cell through MVBs (Katzmann et al., 2002), recycled back to the plasma membrane, or degraded when the MVBs are fused with lysosomes. Importantly, an identified intracellular binding partner of galectin-3 is Alix (Chen et al., 2009), a protein component of the endosomal sorting complex required for transport (ESCRT) machinery (Katoh et al., 2003). Alix has been demonstrated to attenuate EGFR endocytosis (Schmidt et al., 2004) and shown to regulate membrane invagination in early endosomes and formation of MVBs in vitro (Falguieres et al., 2008).
Galectin-3 has been reported to influence cell motility in different cell types, but its effect can be either positive (Kim et al., 2010) or negative (Debray et al., 2004). In gastric cancer cells, silencing galectin-3 expression decreased cell motility (Kim et al., 2010); however, stable transfection of a human glioblastoma cell line with an galectin-3 antisense plasmid resulted in increased cell motility (Debray et al., 2004). Exogenous galectin-3 has been reported to promote human keratinocyte migration at low concentrations through laminin-332 binding, but inhibits migration at high concentrations (Kariya et al.). Defective corneal wound re-epithelialization was observed in galectin-3-deficient (gal3-/-) mice (Cao et al., 2002), but the mechanism by which endogenous galectin-3 contributes to this process is unknown.
We employed gal3-/- mice and keratinocytes isolated from these mice to investigate the role of endogenous galectin-3 in skin wound re-epithelialization and keratinocyte migration and found that keratinocyte migration is impaired in the absence of galectin-3. Moreover, galectin-3 functions by regulating intracellular trafficking of EGFR. We provide evidence that galectin-3 does so by interacting with Alix and modulates the association of Alix with EGFR.
Results
Gal3-/- keratinocytes are defective in migration both in vitro and in vivo
To determine the effect of endogenous galectin-3 on keratinocyte migration, we compared the migratory rates of gal3+/+ and gal3-/- mouse keratinocytes by using a single cell migration assay. The migratory rate of the gal3-/- cells was significantly lower than gal3+/+ counterparts (Fig. 1a). We also examined keratinocyte migration by scratch assay with confluent keratinocyte monolayers. The scratched areas in gal3+/+ monolayers were repaired by 58%±4.1 within 48 h, while those in gal3-/- cultures were repaired by 36%±2.85 (P=0.006) in the same period (Fig. 1b,c).
Figure 1. Galectin-3-deficient keratinocytes exhibit defective migration in vitro and in vivo.
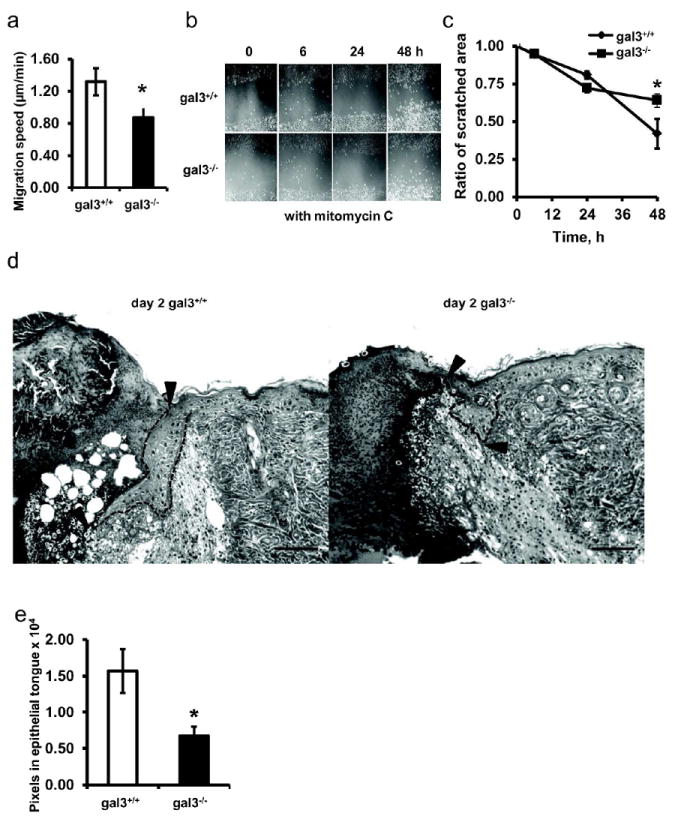
(a) Single cell migration rates were compared between gal3+/+ and gal3-/- keratinocytes. The speeds are presented as averages during a one-h period. Data shown are the mean speeds±s.e.m. of four independent experiments. *, P=0.025. (b, c), Gal3+/+ and gal3-/- keratinocyte monolayers were scratched and images at time 0, 6 h, 24 h, and 48 h after scratch are presented. Representative images from 9 non-overlapping regions from one of three independent experiments are shown. Fig. 1c compare gal3+/+ and gal3-/- wounds as a percentage of area at time t relative to time 0. Values are mean percentages ± s.e.m. n=9.* P=0.006. Scale bar =20μm. (d) Representative images from gal3+/+ and gal3-/- tissues of partially healed wounds and peri-wounded regions are shown. Arrowheads point to outlines of advancing epithelial tongues at one edge of the wound. Scale bar = 100 μm. (e) Areas of re-epithelialized regions in healing wounds from gal3+/+ and gal3-/- mice, as shown in Fig. 1d, were digitally measured from micrographs. Data represent the means±s.e.m from two wounds per mouse and six mice per genotype. * P=0.017.
The migratory defect in gal3-/- keratinocytes in vitro suggested that gal3-/- mice might exhibit impaired skin wound healing. We performed a dorsal wound assay. To minimize the contribution of dermal contraction, wound healing at an early time point post wounding (day 2) was compared; and the areas of epithelial tongues were measured. Gal3-/- mice demonstrated significantly impaired re-epithelialization compared to gal3+/+ mice (Fig. 1e), as determined by the out-growth of epithelial tongues of the regenerating epidermis (Fig. 1d). No difference was found in the epidermal thicknesses between the two genotypes of mice (data not shown).
We addressed whether extracellular galectin-3 plays a role in keratinocyte migration. First, no surface galectin-3 was detected by immunofluorescence microscopy. Galectin-3 is positively stained only when another intracellular cytoskeleton protein tubulin is also positive resulting from permeabilization and loss of cell membrane integrity (supplementary Fig. 1 a). Galectin-3 was also not detected on the cell surface of gal3+/+ keratinocytes by flow cytometry (supplementary Fig. 1 b and c). We tested the effect of recombinant galectin-3 on keratinocyte migration. In both mouse and human keratinocytes, recombinant galectin-3 stimulated cell migration in a lactose-inhibitable manner (supplementary Fig.2a, b). However, lactose at concentrations that effectively inhibits the pro-migratory effect of exogenous galectin-3 did not influence the intrinsic ability of keratinocytes to migrate (supplementary Fig. 2c). This suggests that higher motility of wild-type keratinocytes over gal3-/- cells is not due to secreted galectin-3 acting in an autocrine or paracrine manner.
Also, galectin-3 was found to translocate to the leading edge of keratinocytes prior to active cell migration 30 min after an in vitro scratch wound, and was co-localized with phosphotyrosine (supplementary Fig.3a, b), suggesting that galectin-3 may be involved in the signal transduction process prior to active migration.
Gal3-/- keratinocytes exhibit significantly reduced surface EGFR expression and downstream ERK signaling response
Since the EGFR-ERK signaling pathway plays a central role in keratinocyte migration (Fang et al., 1999), we compared phosphorylation of EGFR and ERK between gal3+/+ and gal3-/- mouse keratinocytes stimulated with mouse EGF at 50 ng/ml. Fig. 2a and b show that at 5 and 10 min after EGF stimulation, gal3+/+ keratinocytes exhibited higher phosphorylation of both EGFR (at Tyrosine 1068 residue) and ERK compared to gal3-/- keratinocytes. We demonstrated that the migratory defects in gal3-/- keratinocytes were due to a reduced EGFR-ERK response by using an EGFR tyrosine kinase specific inhibitor PD15035 in a single cell migration assay. The migratory rate of gal3+/+ keratinocytes was significantly reduced by PD15035 (Fig. 2c), but gal3-/- keratinocytes were unaffected. In the presence of this inhibitor the migratory rates of two genotypes of cells were equalized. The expression of galectin-3 did not change after PD15035 treatment (data not shown). These data suggest that galectin-3 modulates keratinocyte migration through its influence on EGFR signal transduction in response to EGF.
Figure 2. Aberrant EGFR expression by gal3-/- cells is the cause for defective migration.
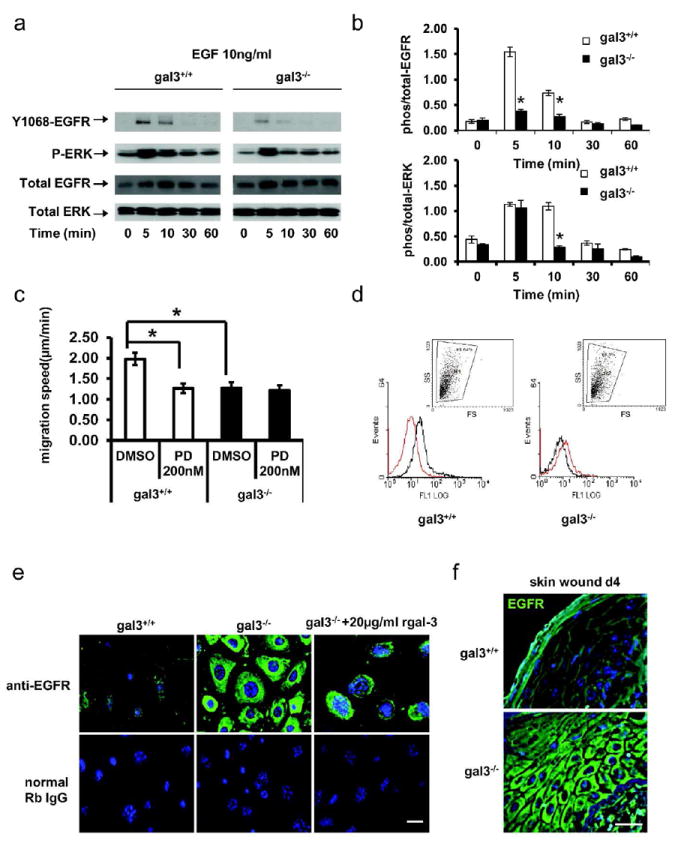
(a, b) Immunoblots were probed for phospho-EGFR (Y1068), phospho-ERK, and total EGFR and ERK. * P<0.01. (c) Migration of gal3+/+ and gal3-/- keratinocytes treated with 200 nM PD15035 or DMSO control was compared. Rates of cell migration are presented as mean±s.e.m μm/min. n=4 *, P =0.0004. (d) Cell surface expression of EGFR was measured by flow cytometry and is presented as MFI (mean fluorescence intensity compared with control rat-IgG antibody, red). P =0.0006. The inset (dot plots) shows 98.6% gal3+/+ and 98.3% gal3-/- keratinocytes were gated for EGFR expression analysis. (e) EGFR was visualized with rabbit anti-EGFR and Alexa488-conjugated secondary antibody (green). Scale bar = 20 μm. (f) Gal3+/+ and -/- mice skin wound samples were fixed at day 4 and sections were prepared for immunofluorescence detection of EGFR. Scale bar = 20μm.
We compared the cell surface EGFR expression between gal3+/+ and gal3-/- mouse keratinocytes by flow cytometry (Fig. 2d, e). The mean fluorescence intensity (MFI) of EGFR in gal3+/+ mouse keratinocytes was significantly higher than that in gal3-/- cells (5.5 ± 0.9 vs 0.8 ± 0.04, P<0.05). Remarkably, while immunofluorescence staining revealed that EGFR was distributed on the plasma membrane in gal3+/+ cells, as expected, it was found to be largely localized in the cytoplasm in gal3-/- keratinocytes. To test whether exogenously added galectin-3 can correct the aberrant distribution of EGFR in gal3-/- keratinocytes, we added recombinant galectin-3 to gal3-/- keratinocytes. We found this did not alter the cytoplasmic accumulation of EGFR (Fig. 2e).
This differential localization was also observed in wounded skin sections (Fig. 2f). It is known that after skin wounding, EGFR is up-regulated in peri-wounded epidermis. In gal3+/+ mice, EGFR was localized at the basolateral domain in regions of cell-to-cell contact in the epidermis, four days after full-thickness skin wounding. In significant contrast, EGFR accumulated in the cytoplasm of keratinocytes and was not detectable on the plasma membrane in gal3-/- mice (Fig. 2f).
Galectin-3’s regulation of EGFR surface expression is dependent on EGF stimulation
We then tested the consequence of EGF stimulation based on the dramatic differences we observed in the surface expression patterns of EGFR in gal3+/+ and gal3-/- mouse keratinocytes. After growth factor deprivation, EGFR was localized on the plasma membrane in both genotypes (Fig. 3a 0h). Six h after EGF stimulation, the receptor was present on the plasma membrane in gal3+/+ keratinocytes, but was accumulated in the cytoplasm of gal3-/- cells (Fig. 3a 6h). Expression levels of EGFR were comparable between gal3+/+ and gal-/- keratinocytes in immunoblots after growth factor deprivation (Fig. 3b lanes 3&4). However, after culturing in medium containing growth factor, the levels of the receptor in gal3-/- keratinocytes were higher compared to those in gal3+/+ counterparts (Fig. 3b lanes 1&2).
Figure 3. Regulation of EGFR surface expression by galectin-3 is dependent on EGF stimulation.
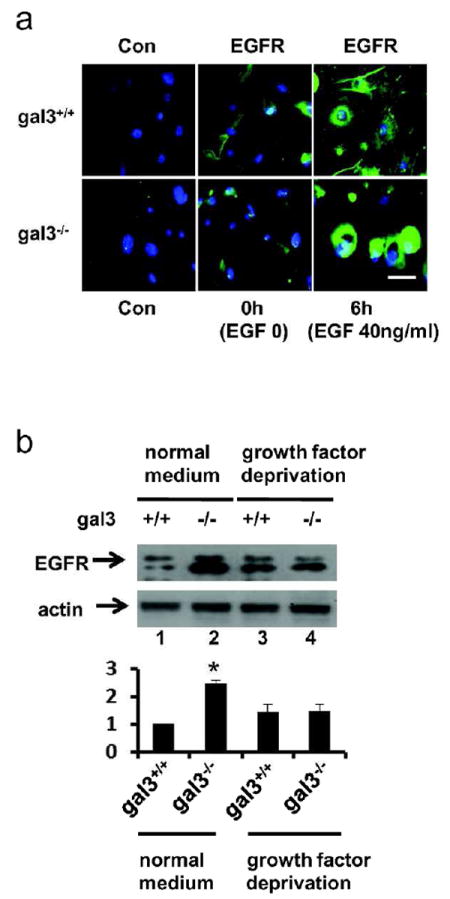
(a) Cells were grown on collagen I-coated glass coverslips in mouse keratinocyte growth factor-deficient medium (EGF 0) or growth factor-containing medium (EGF 40 ng/ml) for 6h. EGFR (green) was visualized by immunofluorescence microscopy. Scale bar = 20 μm. (b) EGFR in cells cultured in mouse keratinocyte growth medium (EGF 40 ng/ml) or medium deprived of growth factors for 16 h were analyzed by immunoblotting. Relative levels of EGFR by densitometric analyses of blots appear in the lower panel. * P=0.002
Intracellular trafficking of EGFR is impaired in gal3-/- keratinocytes
Different pathways are engaged for endocytosis and recycling of EGFR in response to different concentrations of its ligand. Under physiological condition (EGF 1-2ng/ml), EGFR is endocytosed mainly through a clathrin-mediated pathway. However, under pathological conditions such as during a wound healing process, when the concentrations of growth factors increase significantly in the affected area, caveolae-mediated endocytosis is also involved. So in this study, various concentrations of EGF stimulation were used. The observed differences in EGF-induced redistribution of the EGFR in gal3+/+ and gal3-/- keratinocytes suggested the presence of a defect in receptor intracellular trafficking in gal3-/- keratinocytes. We observed the rate of EGF-induced endocytosis of EGFR was lower in gal3-/- keratinocytes relative to gal3+/+ cells (Fig. 4a, b). At low concentrations of EGF (1-2 ng/ml), the rate of EGFR endocytosis in gal3-/- keratinocytes was lower than that in wild type cells (Fig. 4a), and at high concentrations of EGF (40-100 ng/ml), EGFR endocytosis in gal3-/- keratinocytes was undetectable during the time frame when the receptor clearly underwent endocytosis in wild-type cells (Fig. 4b). EGFR recycling was also deficient in gal3-/- keratinocytes. Following stimulation with low concentrations of EGF, EGFR recycled to the cell surface shortly after its internalization in both genotypes, but at a lower rate in gal3-/- keratinocytes (Fig. 4c). At high concentrations of EGF stimulation, EGFR was able to recycle to the cell surface in wild type cells within 30 min, whereas no EGFR recycling was detected in gal3-/- keratinocytes within this period (Fig. 4d). In order to monitor endocytosis and recycling in gal3-/- keratinocytes treated with high concentrations of EGF for a longer period, we cultured the cells in the presence of 40 ng/ml of EGF for 6 h, a condition under which EGFR is known to accumulate in the cytosol in gal3-/- keratinocytes. We then cultured the cells in the presence of a protein synthesis inhibitor, cycloheximide, and monitored recycling of the receptor. We did not find significant recycling during the 6 hr of incubation (Fig. 4e).
Figure 4. Intracellular trafficking of EGFR is disrupted in gal3-/- keratinocytes.
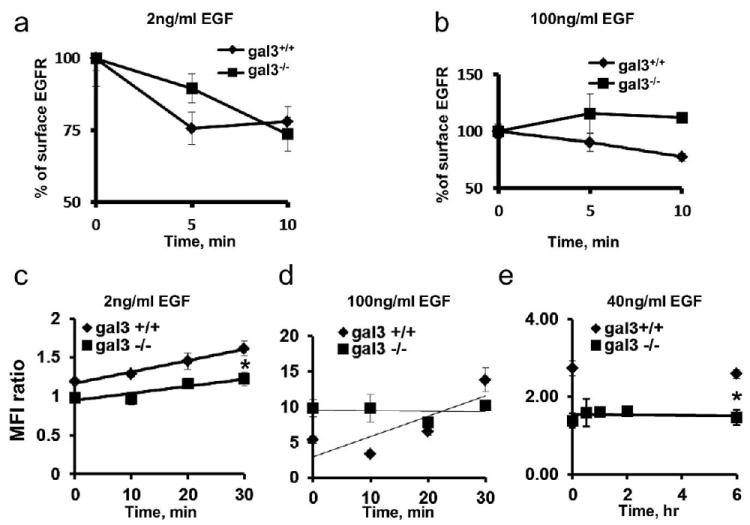
(a, b), EGFR endocytosis rates were measured by flow cytometry. Starved cells were stimulated with 2 ng/ml or 100 ng/ml EGF for indicated periods. Surface EGFR levels were detected by flow cytometry. The percentage of cell surface EGFR = MFI (time t)/(time 0) * 100. (c) Comparison of EGFR recycling in gal3+/+ and gal3-/- keratinocytes. Starved cell suspensions were incubated with 2 ng/ml EGF at 4°C for 1 h and at 37°C for 15 min. After acidic stripping, cells were chased at 37°C for indicated periods. (d) Cell suspensions were incubated with 100 ng/ml EGF. (e) Cells were cultured without starvation. EGFR recycling rates were measured in the presence of 50 μg/ml cycloheximide.
Galectin-3 regulates intracellular trafficking of EGFR through Alix
By yeast two-hybrid screening of a cDNA library generated from the human Jurkat T cell line, we previously identified Alix as a binding partner of galectin-3 (Chen et al., 2009). Alix is known to attenuate EGFR endocytosis (Schmidt et al., 2004) and regulate membrane invagination in early endosomes and formation of MVBs in vitro (Falguieres et al., 2008). Here we demonstrate that after chemical crosslinking with DSS, galectin-3 could be co-immunoprecipitated with Alix from cultured keratinocytes without EGF stimulation (time 0); and there was increased interaction between galectin-3 and Alix 30 min after EGF stimulation (Fig. 5a). In addition, galectin-3 was colocalized with Alix at 30 min after scratch in neonatal human keratinocyte (NHK) monolayers at the leading edge of the migrating cells (Fig. 5c).
Figure 5. Galectin-3 regulates EGFR-Alix association through binding to Alix.
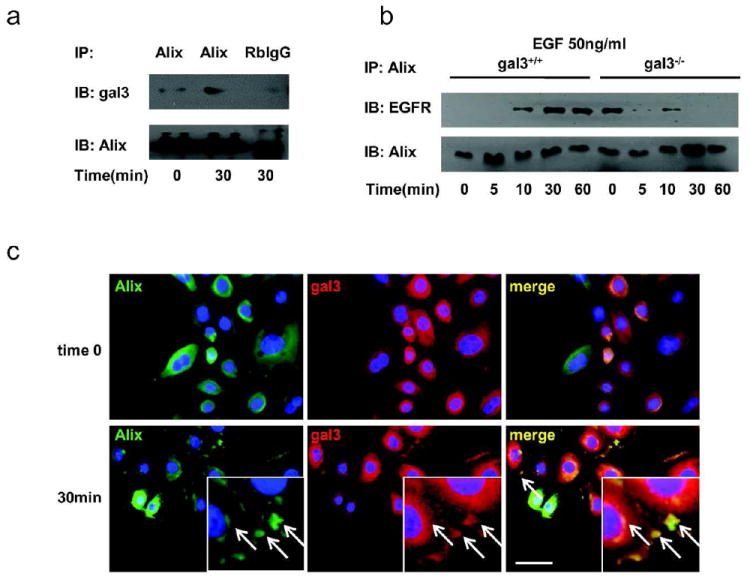
(a) Immunoprecipitation of Alix and galectin-3 in mouse keratinocytes. Cells were chemical crosslinked with DSS, then cell lysates adsorbed with mouse anti-Alix antibody or control mouse IgG1 on protein A-conjugated beads were processed for immunoblot detection for galectin-3 and Alix on the beads (pull down) or in the supernatant (flow through). (b) EGFR-Alix interaction is attenuated in gal3-/- keratinocytes after EGF stimulation. After crosslinking with DSS, cell lysates were immunoprecipitated with anti-Alix antibody. EGFR and Alix were detected by immunoblotting. (c) Galectin-3 is colocalized with Alix at the leading edge and peri-nuclear region in NHKs. Galectin-3 (red) and Alix (green) were visualized by immunofluorescence microscopy. The inset represents a higher magnification of the area where these two proteins co-localize. Colocalization of galectin-3 and Alix is represented in yellow in merged images, as indicated by arrows. Scale bar = 20 μm.
EGFR is known to be indirectly associated with Alix (Schmidt et al., 2004). To address whether intracellular galectin-3 affects the association between EGFR and Alix, we compared the amounts of EGFR co-immunoprecipitated with Alix in gal3+/+ and gal3-/- keratinocytes that was treated with a membrane-permeable chemical crosslinker (Fig. 5b). EGFR released from the plasma membrane during cell lysis can potentially bind to galectin-3 through lectin-carbohydrate interactions and appear as being associated with Alix, if the galectin-3 protein is complexed with Alix and its carbohydrate-binding site is unoccupied. To exclude this possibility, we included 5 mM lactose in the lysis buffer to block galectin-3-carbohydrate binding. In gal3+/+ keratinocytes, EGFR was not associated with Alix at time 0, but increased association was noted 10 min after EGF stimulation. In contrast, there was a significant amount of EGFR associated with Alix at time 0 in gal3-/- keratinocytes, but the association was reduced 10 min after EGF stimulation, and the amount of EGFR associated with Alix was significantly lower compared to that in gal3+/+ cells (Fig. 5b). The results suggest that galectin-3 is critical for modulating Alix-EGFR association after EGF stimulation by binding to Alix.
Discussion
We have established that endogenous galectin-3 positively regulates keratinocyte migration and skin wound re-epithelialization in a mouse model. This effect is mediated by the regulation of cell surface EGFR expression by cytosolic galectin-3 and it does so by modulating intracellular trafficking of the receptor, including endocytosis and recycling. We also show here that intracellular galectin-3 functions by modulating the properties of its binding partner, Alix.
The most striking findings in our study are dramatic over expression and redistribution of the EGFR protein in cultured gal3-/- keratinocytes, and during skin wound healing in gal3-/- mice (Fig. 2e and 2f). This is induced by ligand re-stimulation. Under normal cell culture conditions or during skin wounding, where keratinocytes are exposed to various growth factors, including EGF, galectin-3 is critical for intracellular EGFR trafficking and surface expression. However, when keratinocytes are cultured in growth factor-deficient medium (although ligands of EGFR secreted by keratinocytes through autocrine mechanism may still be operating (Kansra et al., 2004)) or under low concentrations of EGF stimulation, galectin-3 does not significantly affect the cell surface level of EGFR. Therefore, the presence of abundant growth factor and growth factor-induced receptor internalization are the pre-requisites for galectin-3’s regulation of EGFR trafficking and surface expression.
Consistent with this, the defects in EGFR endocytosis and recycling are more prominent at high concentrations of EGF stimulation in gal3-/- keratinocytes. We conclude that while the absence of galectin-3 results in inefficiency in both endocytosis and recycling of EGFR under conditions of abundant growth factor, the defect in the latter is the primary cause of reduced surface expression of EGFR and cytoplasmic accumulation of this protein under these conditions in gal3-/- keratinocytes (i.e., the receptor is highly ineffective in recycling to the cell surface once internalized).
We previously identified Alix as a binding partner galectin-3. We also observed that galectin-3 is colocalized with Alix both in the leading edge of migrating cells and in the cytoplasm (Fig. 5c). Moreover, Alix is known to be indirectly associate with EGFR and suppress its endocytosis (Schmidt et al., 2004) and we observed that galectin-3 regulates the association of Alix and EGFR after EGF stimulation, as this association is diminished in the absence of galectin-3 (Fig. 5b). Alix has been identified as a component of ESCRT protein complex and is involved in MVB formation. Through binding to Alix, galectin-3 may have a broader impact on membrane protein intracellular trafficking in general.
Galectin-3 is known to bind to EGFR through lectin-carbohydrate interactions (Dennis et al., 2002; Lau et al., 2007; Ramasamy et al., 2007). Partridge et al. found that highly branched glycans on EGFR, resulting from modification by 1,6 N-acetylglucosaminyltransferase V (Mgat5/GnT-Va), are recognized by galectin-3 (Partridge et al., 2004). Further, they found in Mgat5-/- cells containing limited glycan branching that: 1) cell surface expression levels of both EGFR and galectin-3 were constitutively reduced; 2) the levels of galectin-3 associated with EGFR were reduced; and 3) EGFR endocytosis was increased in the absence of ligand stimulation, as detected by the amount of EGFR colocalized with an early endosome marker, EEA1. In addition, removal of galectin-3 from the cell surface of wild-type cells by lactose resulted in a phenotype similar to that of Mgat5-/- cells. Thus, they proposed a model in which galectin-3 interacts with Mgat5-modified EGFR on the plasma membrane to form lattices, thereby retarding receptor endocytosis. Although this model can account for the reduced surface expression of EGFR in gal3-/- keratinocytes, it does not fully explain our findings. We observed that the ligand-induced rate of EGFR endocytosis was actually lower in gal3-/- mouse keratinocytes compared to gal3+/+ counterparts (Fig. 4a, b), in contrast to higher rates of endocytosis, as the galectin-3 lattice model would predict. It should be mentioned that, another study found that knockdown of GnT-Va (or Mgat5a) by siRNA treatment (which would reduce branching of EGFR glycans) in MDA-MB231 cells resulted in lower EGF-induced rates of EGFR endocytosis. However, extracellular galectin-3 did not appear to play a role in receptor endocytosis resulting from knocking down the enzyme (Guo et al., 2009).
We were not able to detect galectin-3 on the cell surface of primary human keratinocytes by immunofluorescence microscopy. Galectin-3 is positively stained only when another intracellular cytoskeleton protein tubulin is also positive, upon loss of cell membrane integrity by permeabilization (supplementary Fig. 1). It should also be mentioned that, although we observed the ability of recombinant galectin-3 to stimulate keratinocyte migration in a lactose-inhibitable manner, lactose at concentrations that effectively inhibits the pro-migratory effect of exogenous galectin-3 did not inhibit the intrinsic ability of keratinocytes to migrate (supplementary Fig. 2a, b). This suggests that higher motility of wild-type keratinocytes over gal3-/- cells is not due to the presence of secreted galectin-3 acting in an autocrine or paracrine manner.
Galectin-3 has been reported to function in trans-Golgi network vesicles and to regulate sorting of non-raft proteins to the apical domain in MDCK cells (Delacour et al., 2006). When galectin-3 is down-regulated by siRNA, these proteins were found to be mis-sorted to the basolateral domain. However, EGFR is not an apical protein, but normally located in the basolateral domain in keratinocytes (Fang et al., 1999), and it has also been shown to be present in lipid rafts (Yamabhai and Anderson, 2002). Gal3-/- keratinocytes showed no defect in the transport of EGFR to the plasma membrane; but only under growth factor stimulation or in vivo conditions of wound repair was EGFR found to be accumulated in the cytoplasm, rather than mis-sorted to different domains of the plasma membrane. Importantly, we were unable to demonstrate EGFR and endogenous galectin-3 interaction by chemical crosslinking.
Cao et al. (Cao et al, 2002) found that gal3-/- mice were defective in cornea wound healing and recombinant galectin-3 could promote wound re-epithelialization only in wild type mice and not in gal3-/- mice. This intriguing finding can be explained by our discovery of the intracellular function of galectin-3 in regulating EGFR trafficking. In wild type mice, where EGFR intracellular trafficking pathways are intact, exogenously added galectin-3 can engage the receptor and promote cell migration. However, in the absence of endogenous galectin-3, EGFR intracellular trafficking is defective and, as a consequence, the cell surface levels of EGFR are reduced and exogenous galectin-3 is not able to promote cell migration. Indeed, we showed exogenously added galectin-3 did not change the cytoplasmic distribution of EGFR in gal3-/- cells (Fig.2 e).
A large number of extracellular functions of galectin-3 have been described, which involves its binding to cell surface glycoproteins (reviewed in (Ochieng et al., 2004)). Our studies revealed a novel intracellular function of this protein in the regulation of EGFR expression. EGFR is well established as a master regulator of cell survival, cell cycle progression, and neoplastic transformation and is implicated in a number of pathological conditions, including cancers (Hynes and Lane, 2005). Thus, galectin-3 may play an important role in these processes through regulation of this receptor and the potential exists for galectin-3 to be considered as a therapeutic target in EGFR-dependent pathologies. Our studies have uncovered a new paradigm for intracellular regulation of multiple cellular processes by galectin-3. This has a significant impact on our understanding of the roles of intracellular galectins. Finally, our studies also suggest a role for galectin-3 in skin wound re-epithelialization and this information may be exploited for development of a novel approach for treatment of chronic skin wounds.
Methods
Mice
Gal3+/+ and gal3-/- C57BL6 mice were generated as described previously (Hsu et al., 2000) and backcrossed to C57BL/6 for over nine generations (Sano H, 2003). All animal experiments were approved by the University of California Davis Institutional Animal Care and Use Committee (IACUC) and followed the guidelines of the Animal Welfare Act and the Health Research Extension Act. Experiments using human tissues were approved by University of California Davis Institutional Review Board.
Human primary keratinocyte culture and mouse primary keratinocyte culture
Human keratinocytes were isolated from neonatal foreskins as described (Isseroff et al., 1987) and cultured using a modified method of Rheinwald and Green (Rheinwatd and Green, 1975). Mouse epidermal keratinocytes were isolated from the epidermis of the newborn pups from galectin-3 heterozygous breeders as described (Isseroff et al., 1985). Purities were determined to be 99-100%. Human keratinocytes were cultured with Epilife basal medium with the addition of 0.06 mM CaCl2 and 1% HKGS (human keratinocyte growth supplement) from Invitrogen Inc. HKGS contains 0.2 ng/ml human EGF, 5 μg/mL insulin, 5 μg/mL transferrin, 0.18 μg/mL hydrocortisone, and 0.2% bovine pituitary extract.
The medium used for culturing mouse keratinocytes, contained 20 ng mouse EGF, 10 ng/ml cholera toxin, and 0.5% FBS, in addition to 1% HKGS and the calcium concentration was reduced to 0.02 mM. In some experiments, growth factor-free basal Epilife medium containing 0.02 mM CaCl2 and 10 ng/ml cholera toxin was used.
Single cell migration
Single cell migration experiments were performed as described (Chen et al., 2002). Mouse keratinocytes were plated on glass coated with 60 μg/ml collagen I (Cohesion Technologies, Palo Alto, CA) for 3 h. Slides were put on a heated metal plate with negative feedback temperature control system set at 37 °C. Time-lapse images of the cell migration were captured every 10 min for one hour. ‘Speed’ is the average speed in μm/min that the cells travel in a 1 h period. In some experiments, gal3+/+ and gal3-/- keratinocytes were treated with 200 nM PD15035 (in 0.05% DMSO) or with 0.05% DMSO control. To exclude the competing effects by the growth factors in the medium, for experiments involving recombinant galectin-3, wild type mouse keratinocytes or human keratinocytes were starved and then incubated in the presence of recombinant galectin-3 (1μg/ml), galectin-3 plus 25 mM lactose or sucrose, or 25 mM lactose or sucrose in growth factor-deficient medium. Cells were cultured in an environmental chamber monitored under microscopy with the temperature maintained at 37 °C and 5% CO2.
Scratch assay
Scratch assay was performed as described (Haas et al., 1990). Cells were pretreated with 5 μg/ml mitomycin C for 1 h. A sterile 200 μl pipette tip was used to scratch a 1 mm-wide cell free zone on the monolayer. After removing the cell debris (time 0), nine demarcated areas of the wound in each treatment were photographed under an inverted Nikon Diaphot microscope at 20x magnification. The same areas were photographed at various times after wounding for 48 h.
Skin wounding experiment
Age- (8-12 w) and sex-matched gal3+/+ and gal3-/- C57BL/6 mice were anesthetized with 90 mg/kg ketamine/10 mg/kg xylazine i.p.. After hair removal and sterilization, full-thickness wounds 3 mm in diameter were made. Skin wound samples were collected at day 2 for skin wound re-epithelialization comparison, and day 4 for immunohistochemical staining of EGFR. Re-epithelialized areas of the wound were measured as we have previously described (Sivamani et al., 2009).
Immunofluorescence staining
3 × 104 NHKs were cultured on collagen I-coated glass coverslips. After fixation, cells were either permeabilized with 0.1% Triton X-100/PBS or not permeabilized, and standard immunofluorescence techniques were used to detect galectin-3 and tubulin. For gal3+/+ and gal3-/- mouse keratinocytes, cells were stained with rabbit anti-EGFR antibody (Cell Signaling, Danvers, MA) or rat anti-mouse EGFR antibody (R&D systems, Minneapolis, MN). Rabbit anti-Alix sera were generated and described previously (Chen et al., 2009), and further purified by using Protein A-conjugated agarose beads (Thermo Scientific, Rockford, IL). Rabbit IgG, rat IgG1, rat IgG2a, mouse IgG1, mouse IgG2b were used as isotype controls (eBioscience, San Diego, CA). The concentration of all the primary antibodies is 5μg/ml.
Flow cytometry
Mouse keratinocytes were freshly isolated as described above. Cells were blocked with 10% goat serum in PBS without fixation, and then incubated with rat anti-mouse EGFR antibody (R&D systems, MN, USA), followed by Alexa 488-conjugated goat-anti-rat antibody (Invitrogen, Inc., Carlsbad, CA, USA). Cell surface EGFR expression was detected by flow cytometry (Epics XL, Beckman-Coulter, Miami FL).
Detection of galectin-3 on the cell surface was also accomplished by flow cytometry. Cells were grown till 80% confluence and then after washing with 1x PBS, detached with Cellstripper for 30 min at 37 °C. Detached cells were resuspended with PBS on ice. After blocking with 10% BSA/PBS, cells were incubated with 5μg/ml biotinylated rabbit anti-rat galectin-3 antibody or biotinylated rabbit IgG, and, after washing with 1% BSA/1x PBS, then incubated with 5 μg/ml Alexa 488 conjugated streptavidin. Propidium iodide (PI, 1 μg/ml) was used to exclude dead cells.
Cell lysate and western blot
The whole cell lysates were prepared as previously described (Pullar et al., 2006). The standard Tris-glycine based western blot protocol was used for detection of EGFR and phospho-ERK.
Crosslinking and immunoprecipitation
Mouse keratinocytes were grown to 80% confluence. After starvation for 16 h, cells were stimulated with 50 ng/ml EGF for 30 min, and crosslinked with disuccinimidyl suberate (DSS, 5 mM (final), Thermo Scientific, Pittsburg, PA, USA). Cells were then lysed in buffer containing 5 mM lactose. Hundred μg proteins were immunoabsorbed with 10 μl protein G-conjugated agarose beads (from Thermo Scientific) and 5 μg mouse anti-Alix (AbD Serotec Inc. Raleigh, NC, USA) or control mouse IgG1 antibody at 4°C overnight. The samples were centrifuged at 5000 × g for 1 min, and the supernatants were saved as the unbound samples. The agarose beads in the pellets were washed 5 times with lysis buffer, and processed for immunoblotting.
EGFR endocytosis and recycling
Rates of EGFR endocytosis were measured by a flow cytometry-based method (Gostring et al., 2010; Roepstorff et al., 2009; Sigismund et al., 2008). Cells were deprived of growth factor for 4 h and detached with Cellstripper (Thermo Scientific, Pittsburg, PA, USA) at 37°C for 20 min. Cell viability was typically 90%. Cells were resuspended in medium containing mouse EGF at 37°C for various periods, and fixed with 3.7% formalin. Cells were stained with antibody, and cell surface EGFR levels were calculated as the ratio of Mean Fluorescence Intensity (MFI) of the sample/Rat IgG1.
EGFR recycling rates were measured by flow cytometry. Starved cells were detached with Cellstripper, as described above and incubated with 2 ng/ml or 10 ng/ml EGF at 4°C for 1 h, followed by incubation at 37°C for 15 min. After removal of bound EGF by mild acidic stripping (0.2 M acetic acid, 0.5 M NaCl, pH 4.0) for 1 min, cells were washed with PBS, resuspended in EGFR-free medium, and re-incubated for various periods at 37°C. Surface EGFR levels were measured by flow cytometry as described above.
Statistical analysis
Non-paired equal variance, two tailed Student t-test was used in this study, and P values <0.05 were considered significant.
Supplementary Material
References
- Cao Z, Said N, Amin S, Wu HK, Bruce A, Garate M, et al. Galectins-3 and -7, but not Galectin-1, Play a Role in Re-epithelialization of Wounds. J Biol Chem. 2002;277:42299–42305. doi: 10.1074/jbc.M200981200. [DOI] [PubMed] [Google Scholar]
- Chen H-Y, Sharma BB, Yu L, Zuberi R, Weng IC, Kawakami Y, et al. Role of Galectin-3 in Mast Cell Functions: Galectin-3-Deficient Mast Cells Exhibit Impaired Mediator Release and Defective JNK Expression. J Immunol. 2006;177:4991–4997. doi: 10.4049/jimmunol.177.8.4991. [DOI] [PubMed] [Google Scholar]
- Chen HY, Fermin A, Vardhana S, Weng IC, Lo KF, Chang EY, et al. Galectin-3 negatively regulates TCR-mediated CD4+ T-cell activation at the immunological synapse. Proc Natl Acad Sci U S A. 2009;106:14496–14501. doi: 10.1073/pnas.0903497106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Hoffman BB, Isseroff RR. Beta-adrenergic receptor activation inhibits keratinocyte migration via a cyclic adenosine monophosphate-independent mechanism. J Invest Dermatol. 2002;119:1261–1268. doi: 10.1046/j.1523-1747.2002.19611.x. [DOI] [PubMed] [Google Scholar]
- Cummings RD, Liu FT. Galectins. In: Varki RDC A, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. New York: Cold Spring Harbor Laboratory Press; 2009. pp. 475–488. [PubMed] [Google Scholar]
- Dagher SF, Wang JL, Patterson RJ. Identification of galectin-3 as a factor in pre-mRNA splicing. Proc Natl Acad Sci U S A. 1995;92:1213–1217. doi: 10.1073/pnas.92.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debray C, Vereecken P, Belot N, Teillard P, Brion JP, Pandolfo M, et al. Multifaceted role of galectin-3 on human glioblastoma cell motility. Biochem Biophys Res Commun. 2004;325:1393–1398. doi: 10.1016/j.bbrc.2004.10.181. [DOI] [PubMed] [Google Scholar]
- Delacour D, Cramm-Behrens CI, Drobecq H, Le Bivic A, Naim HY, Jacob R. Requirement for Galectin-3 in Apical Protein Sorting. Current Biology. 2006;16:408–414. doi: 10.1016/j.cub.2005.12.046. [DOI] [PubMed] [Google Scholar]
- Delacour D, Greb C, Koch A, Salomonsson E, Leffler H, Le Bivic A, et al. Apical sorting by galectin-3-dependent glycoprotein clustering. Traffic. 2007;8:379–388. doi: 10.1111/j.1600-0854.2007.00539.x. [DOI] [PubMed] [Google Scholar]
- Delacour D, Koch A, Ackermann W, Parco IE-L, Elsasser H-P, Poirier F, et al. Loss of galectin-3 impairs membrane polarisation of mouse enterocytes in vivo. J Cell Sci. 2008;121:458–465. doi: 10.1242/jcs.020800. [DOI] [PubMed] [Google Scholar]
- Demetriou M, Granovsky M, Quaggin S, Dennis JW. Negative regulation of T-cell activation and autoimmunity by Mgat5N-glycosylation. Nature. 2001;409:733–739. doi: 10.1038/35055582. [DOI] [PubMed] [Google Scholar]
- Dennis JW, Pawling J, Cheung P, Partridge E, Demetriou M. UDP-N-acetylglucosamine:[alpha]-6--mannoside [beta]1,6 N-acetylglucosaminyltransferase V (Mgat5) deficient mice. Biochimica et Biophysica Acta (BBA) - General Subjects. 2002;1573:414–422. doi: 10.1016/s0304-4165(02)00411-7. [DOI] [PubMed] [Google Scholar]
- Dumic J, Dabelic S, Flogel M. Galectin-3: an open-ended story. Biochim Biophys Acta. 2006;1760:616–635. doi: 10.1016/j.bbagen.2005.12.020. [DOI] [PubMed] [Google Scholar]
- Falguieres T, Luyet PP, Bissig C, Scott CC, Velluz MC, Gruenberg J. In vitro budding of intralumenal vesicles into late endosomes is regulated by Alix and Tsg101. Mol Biol Cell. 2008;19:4942–4955. doi: 10.1091/mbc.E08-03-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang KS, Ionides E, Oster G, Nuccitelli R, Isseroff RR. Epidermal growth factor receptor relocalization and kinase activity are necessary for directional migration of keratinocytes in DC electric fields. J Cell Sci. 1999;112(Pt 12):1967–1978. doi: 10.1242/jcs.112.12.1967. [DOI] [PubMed] [Google Scholar]
- Gostring L, Chew MT, Orlova A, Hoiden-Guthenberg I, Wennborg A, Carlsson J, et al. Quantification of internalization of EGFR-binding Affibody molecules: Methodological aspects. Int J Oncol. 2010;36:757–763. doi: 10.3892/ijo_00000551. [DOI] [PubMed] [Google Scholar]
- Guo HB, Johnson H, Randolph M, Lee I, Pierce M. Knockdown of GnT-Va expression inhibits ligand-induced downregulation of the epidermal growth factor receptor and intracellular signaling by inhibiting receptor endocytosis. Glycobiology. 2009;19:547–559. doi: 10.1093/glycob/cwp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas AF, Isseroff RR, Wheeland RG, Rood PA, Graves PJ. Low-energy heliumneon laser irradiation increases the motility of cultured human keratinocytes. J Invest Dermatol. 1990;94:822–826. doi: 10.1111/1523-1747.ep12874679. [DOI] [PubMed] [Google Scholar]
- Haigler HT, McKanna JA, Cohen S. Direct visualization of the binding and internalization of a ferritin conjugate of epidermal growth factor in human carcinoma cells A-431. J Cell Biol. 1979;81:382–395. doi: 10.1083/jcb.81.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DK, Yang R, Liu F. Methods in Enzymology. Vol. 417. Academic Press; 2006. Galectins in Apoptosis; pp. 256–273. [DOI] [PubMed] [Google Scholar]
- Hsu DK, Yang RY, Pan Z, Yu L, Salomon DR, Fung-Leung WP, et al. Targeted disruption of the galectin-3 gene results in attenuated peritoneal inflammatory responses. Am J Pathol. 2000;156:1073–1083. doi: 10.1016/S0002-9440(10)64975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RC. Secretion of the galectin family of mammalian carbohydrate-binding proteins. Biochimica et Biophysica Acta (BBA) - General Subjects. 1999;1473:172–185. doi: 10.1016/s0304-4165(99)00177-4. [DOI] [PubMed] [Google Scholar]
- Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- Isseroff RR, Martinez DT, Ziboh VA. Alterations in fatty acid composition of murine keratinocytes with in vitro cultivation. J Invest Dermatol. 1985;85:131–134. doi: 10.1111/1523-1747.ep12276536. [DOI] [PubMed] [Google Scholar]
- Isseroff RR, Ziboh VA, Chapkin RS, Martinez DT. Conversion of linoleic acid into arachidonic acid by cultured murine and human keratinocytes. J Lipid Res. 1987;28:1342–1349. [PubMed] [Google Scholar]
- Kansra S, Stoll SW, Johnson JL, Elder JT. Autocrine extracellular signal-regulated kinase (ERK) activation in normal human keratinocytes: metalloproteinase-mediated release of amphiregulin triggers signaling from ErbB1 to ERK. Mol Biol Cell. 2004;15:4299–4309. doi: 10.1091/mbc.E04-03-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariya Y, Kawamura C, Tabei T, Gu J. Bisecting GlcNAc residues on laminin-332 down-regulate galectin-3-dependent keratinocyte motility. J Biol Chem. 285:3330–3340. doi: 10.1074/jbc.M109.038836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Shibata H, Suzuki H, Nara A, Ishidoh K, Kominami E, et al. The ALG-2-interacting protein Alix associates with CHMP4b, a human homologue of yeast Snf7 that is involved in multivesicular body sorting. J Biol Chem. 2003;278:39104–39113. doi: 10.1074/jbc.M301604200. [DOI] [PubMed] [Google Scholar]
- Katzmann DJ, Odorizzi G, Emr SD. Receptor downregulation and multivesicular-body sorting. Nat Rev Mol Cell Biol. 2002;3:893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Choi IJ, Cheong TC, Lee SJ, Lotan R, Park SH, et al. Galectin-3 increases gastric cancer cell motility by up-regulating fascin-1 expression. Gastroenterology. 2010;138:1035–1045. e1031–1032. doi: 10.1053/j.gastro.2009.09.061. [DOI] [PubMed] [Google Scholar]
- Lau KS, Partridge EA, Grigorian A, Silvescu CI, Reinhold VN, Demetriou M, et al. Complex N-Glycan Number and Degree of Branching Cooperate to Regulate Cell Proliferation and Differentiation. Cell. 2007;129:123–134. doi: 10.1016/j.cell.2007.01.049. [DOI] [PubMed] [Google Scholar]
- Liu FT. regulatory roles of galectins in the immune response. international archives of allergy and immunology. 2005;136:385–400. doi: 10.1159/000084545. [DOI] [PubMed] [Google Scholar]
- Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- Liu FT, Rabinovich GA. Galectins: regulators of acute and chronic inflammation. Ann N Y Acad Sci. 2010;1183:158–182. [Google Scholar]
- MacKinnon AC, Farnworth SL, Hodkinson PS, Henderson NC, Atkinson KM, Leffler H, et al. Regulation of alternative macrophage activation by galectin-3. J Immunol. 2008;180:2650–2658. doi: 10.4049/jimmunol.180.4.2650. [DOI] [PubMed] [Google Scholar]
- McCawley LJ, O’Brien P, Hudson LG. Epidermal growth factor (EGF)- and scatter factor/hepatocyte growth factor (SF/HGF)- mediated keratinocyte migration is coincident with induction of matrix metalloproteinase (MMP)-9. J Cell Physiol. 1998;176:255–265. doi: 10.1002/(SICI)1097-4652(199808)176:2<255::AID-JCP4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Nakahara S, Oka N, Raz A. On the role of galectin-3 in cancer apoptosis. Apoptosis. 2005;10:267–275. doi: 10.1007/s10495-005-0801-y. [DOI] [PubMed] [Google Scholar]
- Ochieng J, Furtak V, Lukyanov P. Extracellular functions of galectin-3. Glycoconj J. 2004;19:527–535. doi: 10.1023/B:GLYC.0000014082.99675.2f. [DOI] [PubMed] [Google Scholar]
- Partridge EA, Le Roy C, Di Guglielmo GM, Pawling J, Cheung P, Granovsky M, et al. Regulation of Cytokine Receptors by Golgi N-Glycan Processing and Endocytosis. Science. 2004;306:120–124. doi: 10.1126/science.1102109. [DOI] [PubMed] [Google Scholar]
- Pullar CE, Grahn JC, Liu W, Isseroff RR. Beta2-adrenergic receptor activation delays wound healing. FASEB J. 2006;20:76–86. doi: 10.1096/fj.05-4188com. [DOI] [PubMed] [Google Scholar]
- Rabinovich GA, Toscano MA. Turning ‘sweet’ on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat Rev Immunol. 2009;9:338–352. doi: 10.1038/nri2536. [DOI] [PubMed] [Google Scholar]
- Ramasamy S, Duraisamy S, Barbashov S, Kawano T, Kharbanda S, Kufe D. The MUC1 and Galectin-3 Oncoproteins Function in a MicroRNA-Dependent Regulatory Loop. Molecular Cell. 2007;27:992–1004. doi: 10.1016/j.molcel.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinwatd JG, Green H. Seria cultivation of strains of human epidemal keratinocytes: the formation keratinizin colonies from single cell is. Cell. 1975;6:331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- Roepstorff K, Grandal MV, Henriksen L, Knudsen SL, Lerdrup M, Grovdal L, et al. Differential effects of EGFR ligands on endocytic sorting of the receptor. Traffic. 2009;10:1115–1127. doi: 10.1111/j.1600-0854.2009.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H, H D, Apgar JR, Yu L, Sharma BB, Kuwabara I, Izui S, Liu FT. critical role of galectin-3 in phagocytosis by macrophages. J Clin Invest. 2003;112:389–397. doi: 10.1172/JCI17592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro MM, Gaudino G. Cellular and molecular facets of keratinocyte reepithelization during wound healing. Exp Cell Res. 2005;304:274–286. doi: 10.1016/j.yexcr.2004.10.033. [DOI] [PubMed] [Google Scholar]
- Schmidt MH, Hoeller D, Yu J, Furnari FB, Cavenee WK, Dikic I, et al. Alix/AIP1 antagonizes epidermal growth factor receptor downregulation by the Cbl-SETA/CIN85 complex. Mol Cell Biol. 2004;24:8981–8993. doi: 10.1128/MCB.24.20.8981-8993.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott K, Weinberg C. Galectin-1: a bifunctional regulator of cellular proliferation. Glycoconj J. 2004;19:467–477. doi: 10.1023/B:GLYC.0000014076.43288.89. [DOI] [PubMed] [Google Scholar]
- Shirakata Y, Kimura R, Nanba D, Iwamoto R, Tokumaru S, Morimoto C, et al. Heparin-binding EGF-like growth factor accelerates keratinocyte migration and skin wound healing. J Cell Sci. 2005;118:2363–2370. doi: 10.1242/jcs.02346. [DOI] [PubMed] [Google Scholar]
- Sigismund S, Argenzio E, Tosoni D, Cavallaro E, Polo S, Di Fiore PP. Clathrin-mediated internalization is essential for sustained EGFR signaling but dispensable for degradation. Dev Cell. 2008;15:209–219. doi: 10.1016/j.devcel.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Sigismund S, Woelk T, Puri C, Maspero E, Tacchetti C, Transidico P, et al. Clathrin-independent endocytosis of ubiquitinated cargos. Proc Natl Acad Sci U S A. 2005;102:2760–2765. doi: 10.1073/pnas.0409817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivamani RK, Pullar CE, Manabat-Hidalgo CG, Rocke DM, Carlsen RC, Greenhalgh DG, et al. Stress-mediated increases in systemic and local epinephrine impair skin wound healing: potential new indication for beta blockers. PLoS Med. 2009;6:e12. doi: 10.1371/journal.pmed.1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor ME, Drickamer K. Paradigms for glycan-binding receptors in cell adhesion. Current Opinion in Cell Biolology. 2007;19:572–577. doi: 10.1016/j.ceb.2007.09.004. [DOI] [PubMed] [Google Scholar]
- von Zastrow M, Sorkin A. Signaling on the endocytic pathway. Curr Opin Cell Biol. 2007;19:436–445. doi: 10.1016/j.ceb.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamabhai M, Anderson RG. Second cysteine-rich region of epidermal growth factor receptor contains targeting information for caveolae/rafts. J Biol Chem. 2002;277:24843–24846. doi: 10.1074/jbc.C200277200. [DOI] [PubMed] [Google Scholar]
- Yang R-Y, Hsu DK, Liu F-T. Expression of galectin-3 modulates T-cell growth and apoptosis. Proceedings of the National Academy of Sciences. 1996;93:6737–6742. doi: 10.1073/pnas.93.13.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwang Y, Yarden Y. Systems biology of growth factor-induced receptor endocytosis. Traffic. 2009;10:349–363. doi: 10.1111/j.1600-0854.2008.00870.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


