Abstract
Recent data demonstrate that extracellular signals are transmitted through a network of proteins rather than hierarchical signaling pathways suggesting why inhibition of a single component of a canonical pathway is insufficient for the treatment of cancer. The biological outcome of signaling through a network is inherently more robust and resistant to inhibition of a single network component. In this study, we performed a functional chemical genetic screen to identify novel interactions between signaling inhibitors that would not be predicted based on our current understanding of signaling networks. We screened over 300 drug combinations in nine melanoma cell lines and have identified pairs of compounds that show synergistic cytotoxicity. The synergistic cytotoxicities identified did not correlate with the known RAS and BRAF mutational status of the melanoma cell lines. Among the most robust results was synergy between sorafenib, a multi-kinase inhibitor with activity against RAF, and diclofenac, a non-steroidal anti-inflammatory drug (NSAID). Drug substitution experiments using the NSAIDs celecoxib and ibuprofen or the MEK inhibitor PD325901 and the RAF inhibitor RAF265 suggest that inhibition of cyclooxygenase (COX) and MAP kinase signaling are targets for the synergistic cytotoxicity of sorafenib and diclofenac. Co-treatment with sorafenib and diclofenac interrupts a positive feedback signaling loop involving ERK, cPLA2, and COX. Genome-wide expression profiling demonstrates synergy-specific down-regulation of survival-related genes. This study has uncovered novel functional drug combinations and suggests that the underlying signaling networks that control responses to targeted agents can vary substantially depending on unexplored components of the cell genotype.
Keywords: Melanoma, RAF, MEK, cyclooxygenase, sorafenib, diclofenac, synthetic lethal, drug combination
Introduction
The promise of targeted therapy is that the identification of genetic changes that underlie malignancy, and the rational development of drugs that interdict those changes, will yield effective treatments with minimal toxicity (1). This approach has been spectacularly successful in the treatment of CML with inhibitors of Bcr-Abl (2), but this success has not been widely replicated (3). Many oncogenic “drivers” are difficult to target directly (e.g., P53, RAS). Moreover, even in cases where a primary driver is targeted effectively, such as inhibition of mutated EGFR or BRAF, responses to single-agent therapies are usually partial or do not endure (4,5). In these cases, resistance emerges due to the selection of cells that express mutant forms of the target protein that no longer are sensitive to the drug or by the expression of signaling activities that compensate for the inhibition of the primary target (6,7).
There is increasing interest in identifying combinations of therapies that will enhance the efficacy of targeted therapies. Thus, numerous attempts have been made to combine a targeted drug with a cytotoxic drug or with a molecule that would enhance the likelihood of apoptosis (8). However, it has become clear that the targeted therapy itself can induce the expression of compensatory pro-growth and pro-survival activities. This implies that inhibition of one or more of these compensatory pathways could enhance the efficacy of the primary targeted therapy. There are early indications to support this. For example, in our own work, we observed that treatment of prostate cancer xenografts with the MEK inhibitor, PD325901, induced the expression of genes in the MAP kinase, PI3Kinase, and Hedgehog pathways and also enhanced activation of members of those pathways as well as NFκB(9). Co-treatment of prostate cancer cell lines with the MEK inhibitor and any one of the alternative compensatory pathways led to synergistically enhanced cytotoxicity.
Combinations of targeted therapies have potential utility both in cases where one of the drugs directly inhibits a mutated driver and where the mutated driver cannot be directly targeted. For example, RAS-driven tumors could, in theory, be treated with RAF inhibitors, which block the downstream pathway. However, resistance mechanisms analogous to those that arise in RAF-driven tumors are likely also to appear in RAS-driven cancers. Thus, understanding the ways signaling pathways interact to form a robust network could aid construction of rational drug combinations in a broad variety of contexts.
To systematically identify pathways that would be appropriate for co-targeting, we assembled a library of signal transduction inhibitors focused on small molecules that were either FDA-approved anti-cancer drugs, potential drugs in early phase development, or tool compounds that inhibit pathways and molecules of known targets for anti-cancer drug development. We have screened this library, singly and in pairwise combinations, against a panel of melanoma cell lines that represent its major known genotypes (Supplemental Table 1). We searched for combinations that would cause synergistic cytotoxicity (based on Bliss independence (10,11)) to focus on those pathways that were functionally linked. We found that only a limited set of combinations were synergistic, and none of the combinations were synergistic in all cell lines, supporting the hypothesis that this methodology selects for specific pathways rather than general cytotoxic responses. Among the most robust and surprising of these synergistic combinations was the multi-kinase inhibitor sorafenib combined with the anti-inflammatory drug diclofenac. This combination was synergistic in melanomas with mutations in BRAF, NRAS or neither mutation, suggesting the existence of a subset of melanomas that share commonalities in the organization of their signaling networks, regardless of primary driver mutation. Drug substitution studies indicated that the MAP kinase pathway and the cyclooxygenase pathway were important components of this synergy. Genome-wide expression studies further demonstrated both common and distinct aspects of synergy-specific down-regulation of survival-related genes. Thus, this approach has identified cyclooxygenase (COX) as a potential survival mechanism for cells undergoing receptor tyrosine kinase – MAP kinase blockade. Moreover, it provides proof of principle that synthetic lethal screening with small molecules can be used to identify novel functional drug combinations.
Materials and Methods
Cell cultures, antibodies, and reagents
MeWo, SkMel2, SkMel28 cells (American Type Culture Collection; ATCC; Rockville, MD), A375, VMM5A, VMM39, SLM2, DM122, DM331 (kind gift from Dr. Craig Slingluff, University of Virginia (12)) and SLM2 (kind gift from Dr. Angela Zarling) were propagated in RPMI Medium 1640 (Invitrogen, Grand Island, NY) supplemented with 5% or 10% fetal bovine serum (FBS; Gemini Bio-Products, West Sacramento, CA). All cultures were maintained in a humidified chamber at 37°C with 5% CO2. An OncoMap analysis was performed at the Broad Institute to identify the mutational status of over 30 known oncogenes and tumor suppressor genes (13). The cell lines were authenticated by comparing the tumor mutation profile determined by OncoMap to published reports.
Antibodies were obtained from the following sources: anti-phosphoERK (Sigma-Aldrich, St. Louis, MO), anti-tubulin (Calbiochem, Gibbstown, NJ), anti-ERK (B3B9) from the UVa hybridoma facility, anti-cPLA2 (Cell Signaling Technology, Beverly, MA), and anti-phospho-cPLA2 (Santa Cruz Biotechnology, Santa Cruz, CA).
The following small molecule inhibitors were obtained from EMD Chemicals (Gibbstown, NJ): 5-Aza-2-Deoxycytidine, AACOCF3, AG490, AKT Inhibitor IX, AMPK Inhibitor, Anacardic Acid, Celecoxib, Cyclopamine-KAAD, D4476, Diclofenac Na, DMAT, DNA Dependent Protein Kinase Inhibitor, Geldanamycin, GM6001, H-89, Indirubin-3′-Monoxime, IP3 Kinase Inhibitor, Jak I Inhibitor, K-252c, ML-7, NDGA, Okadaic Acid, Olomoucine, PD173074, S3I-201, SANT-1, SB203580, SC-514, Sphingosine Kinase Inhibitor, STO-609, SU6656, TGFβ Receptor II Inhibitor, Trichostatin A, TX-1918, U0126, Withaferin A, Wortmannin, and WP1066. Bortezomib, Dasitinib, Erlotinib, Gefitinib, Imatinib, Lapatinib, Lestaurtinib, Nilotinib, Rapamycin, Sorafenib, Sunitinib, Temsirolimus, and Vandetanib were acquired from LC Laboratories (Woburn, MA). 5-AIQ-hydrochloride, Bevacizumab, D609 Pro-drug, GF109203X, GW441756, Picropodophyllotoxin (PPP) and SP600125 were obtained from Sigma-Alrich (St. Louis, MO). Debromohymeniadlisine (DBH) was purchased from Enzo Life Sciences (Farmingdale, NY). OSU-03012 was obtained from Cayman Chemical (Ann Arbor, MI). Y27632 dihydrochloride was acquired from Tocris Bioscience (Ellisville, MO). PD325901 was a gift from Pfizer (New York, NY). Slo-101 was a gift from Dr. Deborah Lannigan (University of Virginia). Compounds were diluted in vehicle as specified by the manufacturer. Interferon (IFN) alpha and was a gift from Dr. Craig Slingluff (University of Virginia) and SAHA was a gift from Dr. David Jones (University of Virginia).
Synthetic Lethal Pathway Screen
Cell lines were grown in their normal growth media to 80% confluence and then washed with 1x PBS, trypsinized, collected, counted (via hemacytometer), and re-suspended in phenol-red free RPMI 1640 + 5% FBS at concentrations that would result in 100% confluence of the vehicle-treated control wells after 3 days of growth. Plating of the cells was carried out using the BioMek NX (Beckman Coulter, Indianapolis, IN) workstation. 90 μL of cell suspension was added per well in 96-well format. Small molecular inhibitors were diluted to 10x concentration and plated by hand into master drug plates. The BioMek NX workstation was used to add 10 μL of drug from the master plates to each well. The cells were then incubated for 3 days at 37°C and 5% CO2. Following this incubation, the BioMek NX workstation was used to add 10 μL of alamarBlue (Invitrogen, Grand Island, NY) to each well. The plates were incubated for 4 hours and fluorescence was measured at 560 nm excitation/590 nm emission on a Synergy 2 plate reader (BioTek Instruments, Winooski, VT). Mean results and standard error were calculated for triplicate samples.
Cytotoxicity assays
alamarBlue: Four hours after being plated in 96-well plates, cells were treated with inhibitors or vehicle control in phenol red-free RPMI Medium 1640 (Invitrogen, Grand Island, NY) without fetal bovine serum and incubated for 3 days at 37°C. alamarBlue (Invitrogen, Grand Island, NY) was added to wells and incubated for 4 hours at 37°C. Fluorescence was measured as described above.
Crystal Violet: Cells were allowed to adhere to 12-well plates overnight before being treated with inhibitors or vehicle control in phenol red-free RPMI Medium 1640 (Invitrogen, Grand Island, NY) without fetal bovine serum for 7 days at 37°C. Plates were placed on ice and media was aspirated off cells. Cells were rinsed twice with cold 1x PBS (Invitrogen, Grand Island, NY) and fixed with cold 100% methanol for 10 minutes. Monolayers were stained with 0.5% crystal violet in 25% methanol:water for 10 minutes at room temperature and rinsed with distilled water until excess crystal violet solution has been removed. Plates were inverted to allow cells to air-dry overnight. Micrographs were taken with a Nikon SMZ1000 microscope equipped with a Nikon Coolpix 4300 digital camera. Digital images were processed with Adobe Photoshop CS2 version 9.0.2.
Immunoblot and Immunoprecipitation analysis
Cells were allowed to adhere to plates overnight before being treated with inhibitors or vehicle control in phenol red-free RPMI Medium 1640 (Invitrogen, Grand Island, NY) without fetal bovine serum for 1 hour at 37°C. Cells were rinsed with cold 1x PBS and lysed in Triton lysis buffer [10% Triton X-100, 5% 1M Tris (pH 7.5), 2.5% 4M NaCl, 0.5% 0.5M NaF, 0.01% 0.5M EDTA, 80% water] plus the following protease and phosphatase inhibitors: 1 μg/ml pepstatin, 1 μg/ml leupeptin, 0.4 TIU/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride, 200 μM orthovanadate, 50 mM β-glycerophosphate, and 0.4 μM Microcystin. Lysates were centrifuged at 13000 rpm at 4°C for 10 minutes and supernatants were collected. Protein concentrations were determined using BCA protein assay (Thermo Scientific, Waltham, MA). Protein extracts were prepared with Triton lysis buffer and LSB/beta-mercaptoethanol, boiled, loaded into acrylamide gels, and then run at 75V for 30 minutes then at 125V for 1 hour. Gels were transferred onto nitrocellulose membranes at 90V for 60 minutes at 4°C. Membranes were stained with ponceau blue and then were blocked with 5% bovine serum albumin (BSA) in PBS-T for one hour. Blots were incubated overnight at 4°C in primary antibodies, washed with PBS-T, incubated in a secondary antibody (LI-COR, Lincoln, NE) for 1 hour at room temperature, and imaged using the Odyssey Infrared Imaging System (LI-COR, Lincoln, NE). Quantification was done using the Odyssey software and mean results and standard error were calculated for triplicate samples.
For immunoprecipitation analysis, protein extracts were incubated with antibody at 4°C overnight. Agarose beads (Roche) were washed with Triton lysis buffer with protease inhibitors and were added to the sample-antibody solution and incubated for 4 hours at 4°C. The beads were then washed with Triton lysis buffer with protease and phosphatase inhibitors, re-suspended in LSB/beta-mercaptoethanol, and loaded into acrylamide gels. Gels were run, transferred and immunoblotted as describe above with the following exception: membranes were washed with TBS-T.
Gene array
SLM2, VMM39, and DM331 cells were plated and incubated overnight before being treated, in duplicate, with inhibitors or vehicle control in phenol red-free RPMI Medium 1640 without fetal bovine serum for 8 hours at 37°C. Cells were placed on ice and rinsed with cold 1x PBS. Cells were collected and RNA was isolated using the Qiashredder (Qiagen, Valencia, CA) and RNeasy Mini Kit (Qiagen, Valencia, CA). The gene array was performed using Illumina 3′IVT human HT-12 BeadChip arrays by Gene Analysis (Durham, NC). Principal component analysis and clustering QC analyses of the results indicated that two of the inhibitor-treated DM331 samples exhibited irregular quantities unseen in the majority of samples, leading us to conservatively exclude all DM331 sample results from further analyses. Differential expression analyses of quantile-normalized, log2-transformed expression values were performed using moderated ANOVA hypothesis testing (14), as provided by the R/BioConductor (15) limma package (16), with Benjamini-Hochberg corrections for multiple hypothesis testing to control false discovery rate. All microarray data have been deposited with the NCBI Gene Expression Omnibus (GEO) under accession GSE39192.
Results
Screening for synergistic signaling interactions
We assembled a library of 60 small molecule inhibitors that target proteins in diverse signaling pathways. In selecting these inhibitors, we focused on chemicals that were either FDA-approved, in clinical trial, or that targeted pathways for which there are FDA-approved drugs. The library included inhibitors of receptor tyrosine kinases (EGFR, IGFR, VEGFR), and intracellular kinases (MEK, SRC, PKC, PI3K, CDKs, mTOR, PKA). We also utilized inhibitors of other cellular enzyme classes (HDACs, lipoxygenases, cyclooxygenases, topoisomerases) as well as chemotherapy agents. Using this library, we probed cell signaling networks to discover functional relationships within this diverse set of compounds.
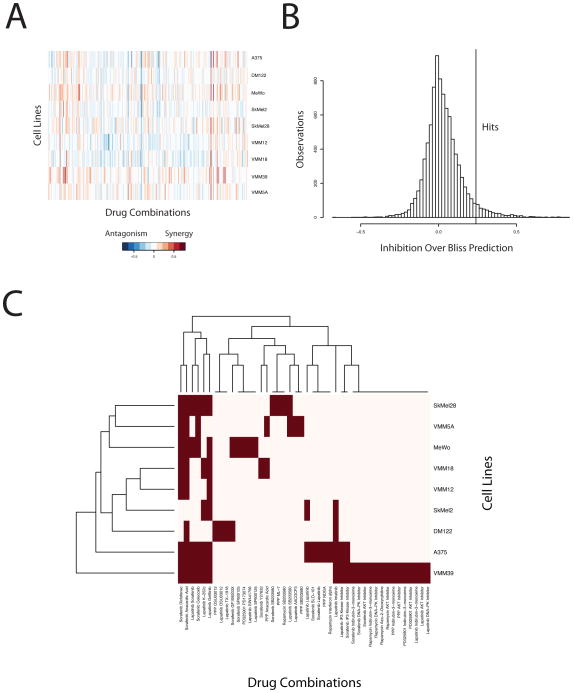
In a 96-well monolayer format using the Biomek NX high-throughput workstation, we performed a series of optimization experiments to determine the appropriate cell number and growth conditions (data not shown) for each of 9 melanoma cell lines that differ in genetic profile and are representative of the major known genetic alterations in melanoma (BRAF, NRAS, wild-type). The cell plating and drug addition were performed robotically using the Biomek and growth inhibition was measured with alamarBlue. Five drugs with primary activity against EGFR, IGFR, RAF, MEK, and mTOR were designated as primary drugs and screened against 60 compounds in our library of small molecule inhibitors. The results of the screen, summarized by synergy or antagonism compared to a Bliss prediction of independence is shown in Figure 1A. The columns denote different drug combinations, the rows the different melanoma cell lines, and the color the degree of synergy or antagonism according to a model of Bliss independence (10,11). The Bliss model of independence estimates the combined effect of two drugs as the multiplicative effect of each drug measured individually (1 – Effect Drug 1 x Effect Drug 2). We scored the top 5% of drug combinations with the greatest synergy as hits (Figure 1B). This 5% cutoff selected drug combinations with growth inhibition of 24% or greater than that predicted by Bliss independence. This is described in detail for one drug combination below and in Figure 2.
Figure 1. Synthetic lethality screen identifies synergistic and antagonistic drug pairs in melanoma cell lines.
(A) Screen results shown as inhibition beyond Bliss independence prediction. Positive values indicate synergy and negative values indicate antagonism. Columns denote drug combinations; rows denote cell lines; color denotes the difference between measured inhibition and bliss prediction. (B) Distribution of bliss differences for all drug combinations in screen (n = 8862). Hits are defined as the top 5% of combinations with the highest additivity over Bliss independence prediction. (C) Clustergram of drug combinations defined as hits (red). Cell lines and drug combinations are clustered by complete linkage.
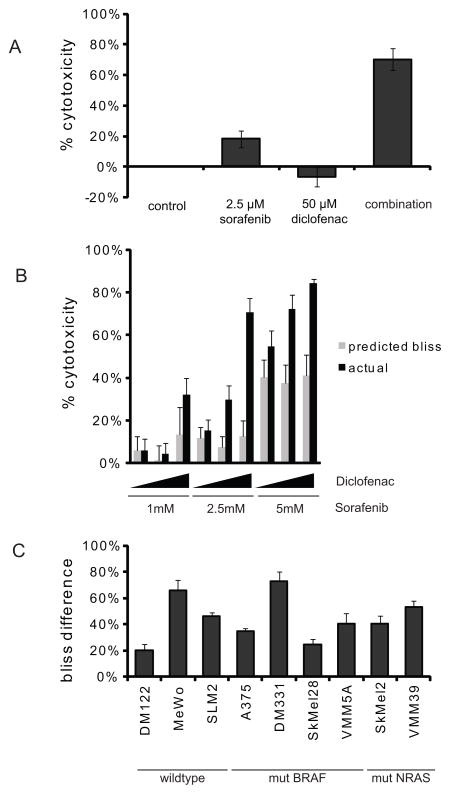
Figure 2. Sorafenib and diclofenac cause synergistic cytotoxicity.
(A) B-raf mutant cells DM331 were treated with vehicle control, 2.5 μM sorafenib, 50 μM diclofenac, or concurrent treatment of 2.5 μM sorafenib and 50 μM diclofenac for 3 days. Metabolic activity was read out using alamarBlue (n=9). (B) DM331 cells were concurrently treated with sorafenib (1 μM, 2.5 μM, or 5 μM) and diclofenac (10 μM, 25 μM, or 50 μM) for 3 days. AlamarBlue was used to read out metabolic activity. Predicted bliss (gray bars) and actual additivity are displayed for each dose combination (n=10). (C) Wild-type for both NRAS and BRAF cells: DM122, MeWo, and SLM2; BRAF mutant cells: A375, DM331, SKMel28, and VMM5A; and NRAS mutant cells: SKMel2 and VMM39 were treated with combinations of sorafenib and diclofenac for 3 days. AlamarBlue was used to read out metabolic activity and the bliss difference was calculated by subtracting the calculated bliss from the actual additivity (n≥3).
A cluster analysis of all of the hits from the screen (Figure 1C) shows that many drug combinations were synergistic in a single cell line, although many combinations were synergistic across multiple cell lines. The cells showing synergy did not cluster according to RAS or RAF mutational status, which was determined by OncoMap (13). For example, two V600E mutant lines, VMM12 and VMM18 clustered together, however, the next closest cell lines were the NRAS mutant SkMel2 and the double wild-type DM122 where double wild-type denotes wild-type for both RAF and RAS. Furthermore, no drug combination generated synthetic lethality selectively in the RAF or RAS mutant cell lines. These results suggest the importance of the global genetic background in determining drug synergy.
One intriguing and surprising example that demonstrates synergy is the combination of sorafenib, a multi-kinase inhibitor with RAF activity, and the anti-inflamatory cyclooxygenase (COX) inhibitor diclofenac. We also observed similar, though less robust, results in our screen with celecoxib in combination with sorafenib, suggesting that diclofenac was acting as a COX inhibitor. The sorafenib and diclofenac combination showed synergy in most cell lines tested at physiologically relevant doses (17,18). Both drugs are well tolerated and FDA approved (19,20). Synergy with sorafenib and diclofenac did not correlate with RAS or RAF mutational status of the cell lines. We selected this drug combination for further analysis because of its robustness and wide utility.
In subsequent experiments, we confirmed Bliss synergy and the level of cytotoxicity for sorafenib and diclofenac. The greater effect of the drug combination over the individual drugs is illustrated in Figure 2A. In the V600E RAF mutant cell line, DM331, 2.5μM sorafenib generated less than 20% cytotoxicity and 50μM diclofenac had no effect on growth. However, the combination treatment with those same doses generated over 75% cytotoxicity in DM331 cells. For 2.5μM sorafenib and 50μM diclofenac in DM331 cells, the predicted Bliss model of independence is 20% (1 - 0.80×1). The actual observed effect of this drug combination’s dose was 75%, indicative of synergy. Synergy was observed for multiple sorafenib and diclofenac doses (Figure 2B). We define the Bliss Difference as the numerical difference between the actual observed percent cytotoxicity and the percent cytotoxicity predicted by the Bliss model of independence. We observed synergy with sorafenib and diclofenac in all melanoma cell lines tested, although the magnitude varied (Figure 2C).
Since alamarBlue is a measure of cell metabolism, we wanted to use a more direct measure to confirm that sorafenib and diclofenac induced synergistic cell death. Melanoma cells were treated with sorafenib and diclofenac, singly or in combination, and stained three days later with crystal violet as a surrogate for cell number (Supplemental Figure 1). Diclofenac alone had minimal to no effect on the cells and sorafenib decreased the number of cells. Combinatorial treatment with sorafenib and diclofenac led to a dramatic loss of cells.
Mechanisms of synergy for sorafenib and diclofenac
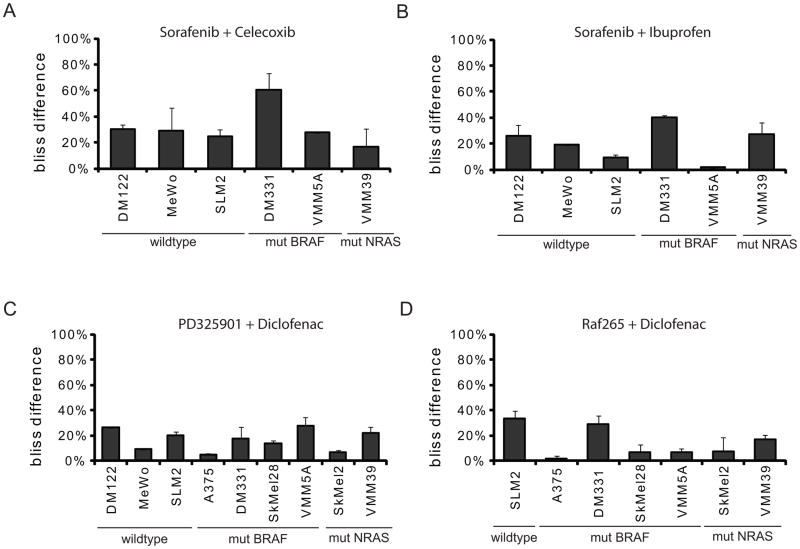
While most of the inhibitors used in our synthetic lethal screen are marketed as specific for a single cellular target, it is likely that many of the compounds have off-target effects where the drug inhibits more than one cellular target (21–23). Sorafenib is known to inhibit multiple kinases and diclofenac may inhibit more than just COX1 and COX2. To determine whether synergistic growth inhibition was due to off-target effects, we substituted drugs of different chemical structure, but similar pathway specificity. Both celecoxib and ibuprofen qualitatively substituted for diclofenac in combination with sorafenib, showing a Bliss Difference across multiple melanoma cell lines with different genetic backgrounds for BRAF and NRAS mutational status (Figure 3A and 3B). The magnitude of Bliss Difference was greatest with diclofenac, followed by celecoxib and then ibuprofen (compare Figures 2C, 3A, and 3B). This quantitative difference may be due to the relative selectivity of these drugs for COX1 and COX2, where ibuprofen is the least selective for COX2. These experiments suggest that the synergy displayed by sorafenib and diclofenac is due, at least in part, to diclofenac inhibition of COX activity.
Figure 3. Inhibition of COX and MEK signaling are involved in the synergistic cytotoxicity observed with sorafenib and diclofenac.
NRAS and BRAF wild-type cells: DM122, MeWo, and SLM2; BRAF mutant cells: DM331 and VMM5A; and NRAS mutant cells: VMM39 were concurrently treated with (A) sorafenib and celecoxib or (B) sorafenib and ibuprofen. (C) NRAS and BRAF cells wild-type cells: DM122, MeWo, and SLM2; BRAF mutant cells: A375, DM331, SkMel28, and VMM5A; and NRAS mutant cells: SkMel2 and VMM39 were concurrently treated with PD325901 and diclofenac. (D) NRAS and BRAF cells wild-type cells: SLM2; BRAF mutant cells: A375, DM331, SkMel28, and VMM5A; and NRAS mutant cells: SkMel2 and VMM39 were concurrently treated with RAF265 and diclofenac. Cells were treated for 3 days. AlamarBlue was used to read out metabolic activity and the bliss difference was calculated as in Figure 2 (n≥3).
We performed similar drug substitution experiments for sorafenib, substituting the highly selective MEK inhibitor, PD325901, or the more selective RAF inhibitor derived from sorafenib, RAF265, in combination with diclofenac (Figure 3C and 3D). Qualitatively, these drugs substituted for sorafenib although, again, the Bliss Difference was quantitatively less than that achieved with sorafenib plus diclofenac. PD325901 plus diclofenac yielded greater Bliss Differences across more melanoma cell lines than RAF265 plus diclofenac. These data suggest that sorafenib inhibition of the MAP kinase pathway contributes to the synergy observed with the sorafenib plus diclofenac combination. However, other sorafenib targets likely contribute to the observed synergy as well.
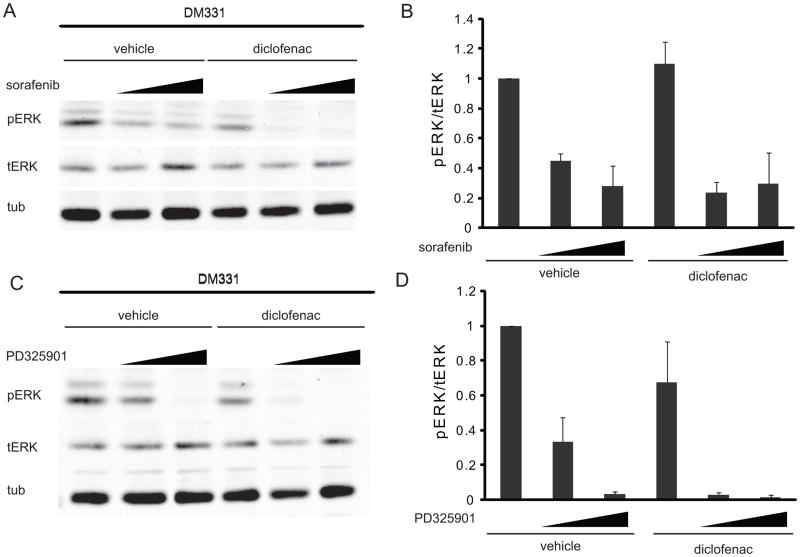
To determine whether the synergy was caused by increased inhibition of the MAP kinase pathway, or by enhanced sensitivity of cells to MAP kinase inhibition, we measured the effect of diclofenac on the ability of sorafenib to inhibit levels of phospho-ERK. Short-term treatment of melanoma cells with sorafenib or PD325901 decreased ERK phosphorylation in a dose-dependent manner in all cell lines examined. Surprisingly, the ID50 for phospho-ERK was reduced by diclofenac in the BRAF mutant cell lines, but not in the mutant RAS or wild-type cell lines (Figure 4 and data not shown). Thus, the mechanism of synergy differed in BRAF mutant melanoma lines from the other lines tested.
Figure 4. The effect of drug combinations on ERK activity.
Total protein was isolated and immunoblot analyses for pERK, tERK, and tubulin were performed from cells treated as follows: (A) DM331 were incubated with vehicle, 2.5 μM sorafenib, 5 μM sorafenib, 50 μM diclofenac, or sorafenib and diclofenac for 1 h. (B) Quantification of Western blot analysis (n≥4). (C) DM331 cells incubated with vehicle, 1 nM PD325901, 5 nM PD325901, 50 μM diclofenac, or PD325901 and diclofenac for 1 h. (D) Quantification of Western blot analysis (n=3).
In DM331 cells harboring a mutant BRAF, 2.5μM sorafenib alone decreased pERK by 56% while 50μM diclofenac alone did not inhibit pERK activity (Figure 4A and 4B). The combination of sorafenib and diclofenac at these doses decreased pERK signaling by 76%. Bliss Independence predicts 50% inhibition of pERK at 2.5μM sorafenib and 50uM diclofenac. Thus, the observed inhibition of pERK exceeds what is predicted by Bliss Independence indicating synergistic inhibition (Figure 4B, compare 2nd and 5th bars). Increasing the dose of sorafenib to 5μM results in additive inhibition of pERK when combined with diclofenac (Figure 4B). A parallel effect occurred when using PD325901 in place of sorafenib (Figure 4C and 4D); 1nM PD325901 inhibited pERK by 67% and when combined with 50μM diclofenac pERK was inhibited by 97% (Figure 3D, compare 2nd and 5th bars). This observed inhibition of pERK exceeded that predicted by Bliss Independence denoting synergy. Using a higher dose of PD325901 (2.5nM) resulted in additive inhibition of pERK when combined with diclofenac. Collectively these data suggest that in the BRAF mutant DM331 cell line, pERK activity is synergistically inhibited by the combination of sorafenib and diclofenac in a dose dependent manner.
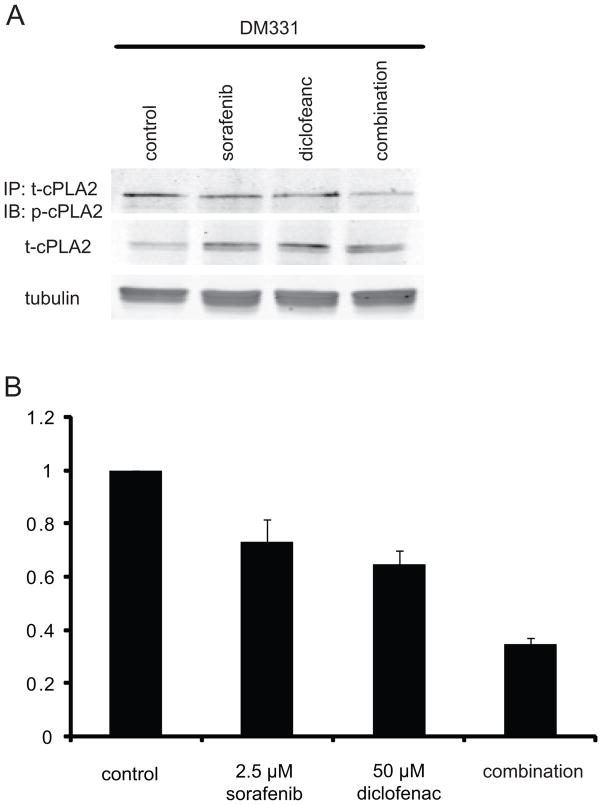
A positive feedback relationship exists between ERK and COX signaling. It is known that Prostaglandin E2 (PGE2) is able to activate the RAS - MAP kinase pathway (24,25). Conversely, ERK can activate cellular phospholipase A2 (cPLA2) by phosphorylation at S505, enhancing the hydrolysis of membrane phospholipids to release arachidonic acid (26). COX then metabolizes arachidonic acid into eicosanoids such as PGE2 (27). We therefore determined whether sorafenib and diclofenac altered cPLA2 phosphorylation on S505 (Figure 5). The addition of 2.5μM sorafenib or 50μM diclofenac each slightly reduced phospho-cPLA2 levels, 27% and 36% respectively. The combination of sorafenib and diclofenac further reduced phospho-cPLA2 levels, inhibiting phospho-cPLA2 by 65%. This exceeds the level of inhibition predicted by Bliss Independence (52%) and indicates synergistic inhibition of phospho-cPLA2 and is consistent with ERK and COX feed forward signaling.
Figure 5. The effect of drug combinations on cPLA2 activity.
Total protein was isolated and immunoprecipitation analysis was performed for tcPLA2. These samples were blotted for phosph-CPLA2. Immunoblot analyses for total-cPLA2 and tubulin were also performed from cells treated as follows: (A) DM331 were incubated with vehicle, 2.5 μM sorafenib, 5 μM sorafenib, 50 μM diclofenac, or sorafenib and diclofenac for 1 h. (B) Quantification of Western blot analysis (n=3).
Because ERK was not synergistically inhibited by sorafenib and diclofenac in the VMM39 or SLM2 cell lines, we turned to gene expression analysis to elucidate the underlying mechanism(s) leading to synergistic cytotoxicity in these cell lines. Both VMM39 and SLM2 displayed significantly different basal gene transcription profiles consistent with the different mutational status of RAS and BRAF in the cell lines; VMM39 cells are NRAS mutant and SLM2 cells are double wild-type. Treatment of either cell line with the individual drugs had little effect on gene expression, consistent with the low cytotoxicity of single drug administration. At a 1% FDR, there were no transcriptional changes in response to 50μM diclofenac in either SLM2 or VMM39; there were also no transcriptional changes in SLM2 in response to 5μM sorafenib at 24 hours. At a 1% FDR, the transcription of only 18 genes was altered in VMM39 cells in response to sorafenib. A heatmap of the significant genome-wide changes in VMM39 and SLM2 transcription in response to single drug and combination treatment is shown in Figure 6. The combination of sorafenib and diclofenac generated significant transcriptional changes; for each cell line (compare the last two columns in the heatmap (Figure 6) to the preceding six columns). Four different patterns of transcriptional responses emerge from the combination of sorafenib and diclofenac. Some genes are down-regulated by the combination in both VMM39 and SLM2 (Figure 6, group B), although with varying magnitude. There are also many genes that are up-regulated by the combination of sorafenib and diclofenac in both VMM39 and SLM2 or in either VMM39 or SLM2 (Figure 6, group D). Interestingly, there are genes that are strongly down-regulated by the drug combination in VMM39, but not in SLM2 (Figure 6, group A). Finally, there are genes with widely disparate basal expression levels between VMM39 and SLM2 that are similarly dysregulated by the sorafenib and diclofenac combination (Figure 6, group C). These different patterns of gene expression induced by the drug combination suggest that most transcriptional changes triggered by sorafenib and diclofenac are different in VMM39 and SLM2. We analyzed the annotated gene ontology (GO) terms associated with the transcriptional changes triggered by the sorafenib and diclofenac drug combination to determine if common biological processes were regulated by the drug combination in VMM39 and SLM2 cells: survival-related GO terms consistently emerge as a common response. Thus, while the different transcriptional response of the two cell lines suggests that different mechanisms of drug synergy may be active, there is convergence on common biological processes.
Figure 6. The effect of drug combinations on genome-wide gene expression.
Total RNA was isolated from cells (VM339 and SLM2) 24 hours following inhibitor treatment (DMSO vehicle, sorafenib and diclofenac, both alone and in combination), performed in duplicate. mRNA abundance was measured by Illumina HT-12 microarray. Genes exhibiting statistically significant (FDR < 0.1%) inhibitor-induced changes were clustered hierarchically by average linkage of scaled cosine-correlation similarity, delineating four major response patterns: A) genes strongly down-regulated by combination inhibition in VMM39, but weakly (or not at all) in SLM2; B) genes down-regulated by combination inhibition in both VMM39 and SLM2, with varying magnitude; C) genes with widely-disparate basal expression levels between VMM39 and SLM2 that are dysregulated by combination inhibition; and D) genes that are up-regulated by combination inhibition in either/both VMM39 and SLM2. Black boxes to the left of HUGO gene names denote those genes having annotated associations with survival-related GO terms.
Discussion
Resistance to targeted therapies can appear after prolonged remission and is often associated with the emergence of mutations that render the target kinase resistant to drug inhibition (6). However, in melanoma and most solid tumors, resistance to therapy often exists de novo, sometimes developing in a few weeks or months and often independent of mutations in the target kinase (28–30). It is becoming apparent that redundant, compensatory and/or survival signaling pathways can rapidly overcome single-point blockade of a signaling pathway (7). This implies that more effective therapies could be achieved by inhibiting a combination of targets: the primary target and a redundant or compensatory target. The classic combinatorial approach for cytotoxic anti-cancer agents has been to identify agents with different dose-limiting toxicities under the expectation that the combination will allow higher levels of cytotoxic activity in patients. For targeted therapies, however, we have an opportunity to identify biologically effective drug combinations based on the interactions of various signaling pathways (31). Such rational combinations would specifically target complementary signaling components to achieve synergistic outcomes. Some combinations are widely recognized and under development, such as co-targeting the MAP kinase and PI3 kinase pathways (32,33). However, this may not be the only or most efficacious combination in every cancer lineage and genetic background. The current understanding of signaling networks limits our ability to rationally explore the range of possibilities. Even for the MAP kinase pathway, which has been studied in depth for two decades, we do not fully understand the ways in which it is connected to other pathways that control cell proliferation and death. Furthermore, our ability to predict the relationships between oncogenic mutations, cell lineage, signaling networks, and therapeutic effectiveness is inadequate.
In this study, we have applied a chemical genetic approach analogous to synthetic lethal screening in yeast genetics to probe the cancer cell signaling network for novel functional interactions. The prediction is that identifying synergistic lethal effects through effective combinatorial targeting of cooperating oncogenic and survival pathways will achieve robust clinical responses in melanoma and other tumors. Synthetic lethal screens have been applied previously to cancer models and uncovered novel therapeutic targets or compounds (34–36). In these studies, investigators used RNAi or small molecule inhibitors to screen cell lines differing in a specific fixed genetic change, such as in an oncogene or tumor suppressor gene. This approach has led successfully to the discovery of unknown synthetic lethal interactions with numerous oncogenes and tumor suppressor genes including RAS, MYC, BRCA1, and PTEN (37). This application of synthetic lethality may account for the clinical effectiveness of some small molecule inhibitors. Cancers may respond to therapies targeting mutations that occur early in cancer development and progression in cases where subsequent mutations carry a selective advantage only in the context of the preceding transforming mutations.
Here we show that a synthetic lethal chemical genetic screen can be used to identify novel synergistic drug combinations. We focused on the combination of sorafenib, a Raf inhibitor with multi-kinase activity, and the anti-inflammatory cyclooxygenase inhibitor diclofenac. Sorafenib and diclofenac combined synergistically to inhibit the growth of multiple melanoma cell lines. A highly selective MEK inhibitor and a derivative of sorafenib, RAF265, both substituted in part for sorafenib. Similarly, the COX inhibitors celecoxib and ibuprofen both partially substituted for diclofenac. Together, this suggests that the synergistic effect of sorafenib and diclofenac are due, at least in part, to inhibition of RAF and COX. While the drug substitution experiments with different NSAIDs support COX as the relevant molecular target of diclofenac, these experiments are not definitive. We have assessed COX1 and COX2 activity by ELISA but found the basal COX activity in the melanoma lines too low to robustly assess inhibition with NSAIDs (data not shown). Using qPCR we did see COX1 mRNA increase (2x) in VMM39 cells and IL-1beta mRNA increase (5x) in DM331 cells with diclofenac consistent with inhibition of COX activity (data not shown) (38,39). However, we found only a small number of gene expression changes to occur following diclofenac treatment for 8 hours and no changes consistent across both cell lines (Figure 6). Thus, it is possible that other molecular targets of diclofenac contribute, either in part or whole, to the synergy with sorafenib.
The melanoma cell lines tested were sensitive to the combination of sorafenib and diclofenac independent of the RAS and RAF mutational status of the melanoma lines. This contrasts with the correlation of sensitivity to single agent RAF and MEK inhibitors in BRAF mutant melanomas (40). However, sorafenib is a multi-kinase inhibitor that targets many kinases in addition to RAF, complicating this interpretation. Further work is required to evaluate fully if drug combinations with agents that target RAF or MEK correlate with RAS and RAF mutational status in a way similar to the effect of single-agent RAF and MEK inhibitors.
Of the three cell lines studied in detail, only DM331, a RAF V600E mutant line, showed synergistic inhibition of ERK and cPLA2 signals consistent with interrupting a feed forward signaling loop involving ERK and COX. ERK phosphorylates cPLA2 on Serine 505, facilitating cPLA2 activation, which results in the release of arachidonic acid and lysophospholipid from the sn-2 position on phospholipids (26). COX catalyzes arachidonic acid to prostaglandins that then activate the RAS-RAF-ERK signaling cascade (24,25,27). We suggest that, in DM331 melanoma cells, mutationally activated RAF drives ERK activation and subsequent cPLA2 phosphorylation, leading to enhanced arachidonic acid release which feeds forward through prostaglandin production to ERK. The drug combination of sorafenib and diclofenac interrupts this feed forward loop by inhibiting RAF and COX. Consistent with this, the V600E mutant cell lines VMM5A, A375, and SkMel28 also showed synergistic inhibition of ERK activity with the sorafenib and diclofenac combination (data not shown).
The VMM39 cells carrying mutant RAS and the SLM2 cells that are wild-type for RAS and RAF did not show synergistic inhibition of ERK activity in response to the combination of sorafenib and diclofenac, suggesting a different mechanism of synergistic cytotoxicity for this drug combination. It is reasonable that, even when the biological outcome is the same (i.e., synergistic cytotoxicity), a drug such as sorafenib with multi-kinase activity would have different mechanisms of action depending on the genetic background of the tumor. Both VMM39 and SLM2 showed little transcriptional change in response to sorafenib or diclofenac alone. Only when these melanoma cell lines were treated with the combination of sorafenib and diclofenac were profound statistically significant transcriptional changes observed. These selective transcriptional effects suggest that the cytotoxic effect of the sorafenib and diclofenac drug combination is due to changes in the transcriptional program. A GO term analysis of the transcriptional changes induced by the drug combination indicated that genes involved in cell survival were changing in a drug combination selective manner. Additional studies are necessary to determine the specific role of these transcriptional changes in the cytotoxic response to sorafenib and diclofenac in the VMM39 and SLM2 melanoma cell lines. Nevertheless our gene array studies suggest that the underlying signaling networks that control responses to targeted agents can vary substantially depending on cell genotype. This is likely to complicate attempts to construct combinatorial therapies without a detailed understanding of the mutational status of the cancer cells. Identifying the mechanism of synergy will entail overlaying drug target networks with genomics as well as transcriptomic and proteomic biological network changes (41,42). Such a systems approach has been applied with some success (41,43). As we increase our understanding of cell biological networks our ability to rationally select effective drug combinations will continue to improve.
Supplementary Material
Acknowledgments
This work was supported by an award from the Melanoma Research Alliance to MJ Weber, the Timothy Aycock Melanoma Research Foundation to the UVA Cancer Center, and National Institute of Health Grant R01 CA124706 to D Gioeli.
We would like to thank Drs. Kevin Janes and Jason Papin, along with doctoral candidate Paul Jensen for their insight and helpful discussions on this project.
Footnotes
There are no conflicts of interest to report.
References
- 1.Bild AH, Yao G, Chang JT, Wang Q, Potti A, Chasse D, et al. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature. 2005;6;439:353–7. doi: 10.1038/nature04296. [DOI] [PubMed] [Google Scholar]
- 2.Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–7. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 3.Quigley D, Balmain A. Systems genetics analysis of cancer susceptibility: from mouse models to humans. Nat Rev Genet. 2009;10:651–7. doi: 10.1038/nrg2617. [DOI] [PubMed] [Google Scholar]
- 4.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;30;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roumiantsev S, Shah NP, Gorre ME, Nicoll J, Brasher BB, Sawyers CL, et al. Clinical resistance to the kinase inhibitor STI-571 in chronic myeloid leukemia by mutation of Tyr-253 in the Abl kinase domain P-loop. Proc Natl Acad Sci US A. 2002;99:10700–5. doi: 10.1073/pnas.162140299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cortot AB, Janne PA. Resistance to targeted therapies as a result of mutation(s) in the target. In: Gioeli D, editor. Targeted Therapies: Mechanisms of Resistance. Humana Press; 2011. pp. 1–31. [Google Scholar]
- 7.Gioeli D. The dynamics of the cell signaling network; implications for targeted therapies. In: Gioeli D, editor. Targeted Therapies: Mechanisms of Resistance. Humana Press; 2011. pp. 33–53. [Google Scholar]
- 8.Kwak EL, Clark JW, Chabner B. Targeted Agents: The Rules of Combination. Clin Cancer Res. 2007;13:5232–7. doi: 10.1158/1078-0432.CCR-07-1385. [DOI] [PubMed] [Google Scholar]
- 9.Gioeli D, Wunderlich W, Sebolt-Leopold J, Bekiranov S, Wulfkuhle JD, Petricoin EF, 3rd, et al. Compensatory pathways induced by MEK inhibition are effective drug targets for combination therapy against castration-resistant prostate cancer. Mol Cancer Ther. 2011;10:1581–90. doi: 10.1158/1535-7163.MCT-10-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bliss C. The toxicity of poisons applied jointly. Annals of Applied Biology. 1939;26:585–615. [Google Scholar]
- 11.Fitzgerald JB, Schoeberl B, Nielsen UB, Sorger PK. Systems biology and combination therapy in the quest for clinical efficacy. Nature Chemical Biology. 2006;2:458–66. doi: 10.1038/nchembio817. [DOI] [PubMed] [Google Scholar]
- 12.Molhoek KR, Shada AL, Smolkin M, Chowbina S, Papin J, Brautigan DL, et al. Comprehensive analysis of receptor tyrosine kinase activation in human melanomas reveals autocrine signaling through IGF-1R. Melanoma Res. 2011;21:274–84. doi: 10.1097/CMR.0b013e328343a1d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacConaill LE, Campbell CD, Kehoe SM, Bass AJ, Hatton C, Niu L, et al. Profiling critical cancer gene mutations in clinical tumor samples. PLoS ONE. 2009;4:e7887. doi: 10.1371/journal.pone.0007887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3:Article3. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 15.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smyth GK. Limma: linear models for microarray data. In: Gentleman R, Carey V, Dudoit S, Irizarry R, Huber W, editors. Bioinformatics and Computational Biology Solutions using R and Bioconductor. New York: Springer; 2005. pp. 397–420. [Google Scholar]
- 17.Clark JW, Eder JP, Ryan D, Lathia C, Lenz H-J. Safety and pharmacokinetics of the dual action Raf kinase and vascular endothelial growth factor receptor inhibitor, BAY 43–9006, in patients with advanced, refractory solid tumors. Clin Cancer Res. 2005;11:5472–80. doi: 10.1158/1078-0432.CCR-04-2658. [DOI] [PubMed] [Google Scholar]
- 18.Auler JO, Júnior, Espada EB, Crivelli E, Quintavalle TB, Kurata A, Stolf NA, et al. Diclofenac plasma protein binding: PK-PD modelling in cardiac patients submitted to cardiopulmonary bypass. Braz J Med Biol Res. 1997;30:369–74. doi: 10.1590/s0100-879x1997000300010. [DOI] [PubMed] [Google Scholar]
- 19.Kane RC, Farrell AT, Saber H, Tang S, Williams G, Jee JM, et al. Sorafenib for the Treatment of Advanced Renal Cell Carcinoma. Clin Cancer Res. 2006;12:7271–8. doi: 10.1158/1078-0432.CCR-06-1249. [DOI] [PubMed] [Google Scholar]
- 20.FDA approves marketing of diclofenac. Clin Pharm. 1988;7:785. [PubMed] [Google Scholar]
- 21.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, et al. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–32. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 23.Fabian MA, Biggs WH, Treiber DK, Atteridge CE, Azimioara MD, Benedetti MG, et al. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol. 2005;23:329–36. doi: 10.1038/nbt1068. [DOI] [PubMed] [Google Scholar]
- 24.Krysan K, Reckamp KL, Dalwadi H, Sharma S, Rozengurt E, Dohadwala M, et al. Prostaglandin E2 activates mitogen-activated protein kinase/Erk pathway signaling and cell proliferation in non-small cell lung cancer cells in an epidermal growth factor receptor-independent manner. Cancer Res. 2005;65:6275–81. doi: 10.1158/0008-5472.CAN-05-0216. [DOI] [PubMed] [Google Scholar]
- 25.Wang D, Buchanan FG, Wang H, Dey SK, DuBois RN. Prostaglandin E2 enhances intestinal adenoma growth via activation of the Ras-mitogen-activated protein kinase cascade. Cancer Res. 2005;65:1822–9. doi: 10.1158/0008-5472.CAN-04-3671. [DOI] [PubMed] [Google Scholar]
- 26.Lin LL, Wartmann M, Lin AY, Knopf JL, Seth A, Davis RJ. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993;72:269–78. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- 27.Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181–93. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga-Kalabis M, Cipolla AK, et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010;18:683–95. doi: 10.1016/j.ccr.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468:968–72. doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–7. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dancey JE, Chen HX. Strategies for optimizing combinations of molecularly targeted anticancer agents. Nat Rev Drug Discov. 2006;5:649–59. doi: 10.1038/nrd2089. [DOI] [PubMed] [Google Scholar]
- 32.Kinkade CW, Castillo-Martin M, Puzio-Kuter A, Yan J, Foster TH, Gao H, et al. Targeting AKT/mTOR and ERK MAPK signaling inhibits hormone-refractory prostate cancer in a preclinical mouse model. J Clin Invest. 2008;118:3051–64. doi: 10.1172/JCI34764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, Upadhyay R, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–6. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barbie DA, Tamayo P, Boehm JS, Kim SY, Moody SE, Dunn IF, et al. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature. 2009;462:108–12. doi: 10.1038/nature08460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scholl C, Fröhling S, Dunn IF, Schinzel AC, Barbie DA, Kim SY, et al. Synthetic lethal interaction between oncogenic KRAS dependency and STK33 suppression in human cancer cells. Cell. 2009;137:821–34. doi: 10.1016/j.cell.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 36.Luo J, Emanuele MJ, Li D, Creighton CJ, Schlabach MR, Westbrook TF, et al. A genome-wide RNAi screen identifies multiple synthetic lethal interactions with the Ras oncogene. Cell. 2009;137:835–48. doi: 10.1016/j.cell.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan DA, Giaccia AJ. Harnessing synthetic lethal interactions in anticancer drug discovery. Nat Rev Drug Discov. 2011;10:351–64. doi: 10.1038/nrd3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yano A, Higuchi S, Tsuneyama K, Fukami T, Nakajima M, Yokoi T. Involvement of immune-related factors in diclofenac-induced acute liver injury in mice. Toxicology. 2012;293:107–14. doi: 10.1016/j.tox.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 39.Lu X, Xie W, Reed D, Bradshaw WS, Simmons DL. Nonsteroidal antiinflammatory drugs cause apoptosis and induce cyclooxygenases in chicken embryo fibroblasts. Proc Natl Acad Sci US A. 1995;92:7961–5. doi: 10.1073/pnas.92.17.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439:358–62. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chua HN, Roth FP. Discovering the targets of drugs via computational systems biology. J Biol Chem. 2011;286:23653–8. doi: 10.1074/jbc.R110.174797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Azmi AS, Wang Z, Philip PA, Mohammad RM, Sarkar FH. Proof of concept: network and systems biology approaches aid in the discovery of potent anticancer drug combinations. Mol Cancer Ther. 2010;9:3137–44. doi: 10.1158/1535-7163.MCT-10-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cokol M, Chua HN, Tasan M, Mutlu B, Weinstein ZB, Suzuki Y, et al. Systematic exploration of synergistic drug pairs. Mol Syst Biol. 2011;7:544. doi: 10.1038/msb.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.