Abstract
Estradiol plays a pivotal role in the control of GnRH neuronal function, hence female reproduction. A series of recent studies in our laboratory indicate that rapid excitatory actions of estradiol directly modify GnRH neuronal activity in primate GnRH neurons through GPR30 and STX-sensitive receptors. Similar rapid direct actions of estradiol through estrogen receptor beta are also described in mouse GnRH neurons. In this review, we propose two novel hypotheses as a possible physiological role of estradiol in primates. First, while ovarian estradiol initiates the preovulatory GnRH surge through interneurons expressing estrogen receptor alpha, rapid direct membrane-initiated action of estradiol may play a role in sustaining GnRH surge release for many hours. Second, locally produced neuroestrogens may contribute to pulsatile GnRH release. Either way, estradiol synthesized in interneurons in the hypothalamus may play a significant role in the control of the GnRH surge and/or pulsatility of GnRH release.
Keywords: GnRH neurons, rapid action of estradiol, GPR30, GnRH surge, GnRH pulses, neuroestrogen, membrane estrogen receptors
Introduction
The classical endocrine effects of estradiol (E2) have a long history of study (141). A gradual secretion of E2 from the ovary into the general circulation reaches a variety of cells, binds to nuclear estrogen receptors (ERs), and causes genomic changes over the time course of several hours to days. One typical example is modification of the activity of neurons in the hypothalamus and gonadotrophs in the pituitary gland forming the negative and positive feedback loops of the reproductive cycle. In addition to these classical long-lasting effects of E2 in feedback mechanisms, investigation of rapid (or acute) actions of E2 on the uterus, vasculature, and neurons also has a relatively long history (65,72,140,155).
As early as 1971, E2 production in the hypothalamus by aromatization of androstendione (100) has been reported. However, despite over 40 years of research on acute/rapid E2 action and its synthesis in the brain, the progress of research in this area has been slow. Just recently, exciting features on the local synthesis of E2 and the role of rapid action of E2 in neuronal function have emerged: Locally synthesized E2 may serve as a neurotransmitter in the bird brain and rat hippocampus (7,57) and E2 appears to prevent cell death due to acute hypoxia in rodent stroke models (83,95,157).
E2 action in the brain is quite complex, as E2 is synthesized and released from the ovary as well as neurons in the brain. E2 released from the ovary is transported into the brain through the general circulation and causes a long-lasting genomic action in the brain, such as generation of the preovulatory GnRH surge and lordosis behavior. E2 released from the ovary also induces rapid action in the brain, as a single E2 injection results in phospho-CREB expression in GnRH neurons within 15 min (3). In contrast, as discussed in section 2, locally synthesized E2 appears to play a role in neurotransmission. Theoretically, locally synthesized estradiol in the brain may cause genomic action, but presently little direct evidence is available to support this possibility.
Since the discovery of the GnRH molecule (128) and the subsequent identification of GnRH neurons in the preoptic area (POA) and hypothalamus (11,74,131), mechanisms of E2 action on GnRH neurons have remained elusive. It has been believed for many years that E2 modifies the activity of GnRH neurons indirectly through interneurons. This is due to an initial study showing that GnRH neurons do not express estrogen receptors (ERs) using combined autoradiography and immunocytochemistry (133), whereas interneurons that synthesize norepinephrine, dopamine, glutamate, GABA, neuropeptide Y (NPY), and kisspeptin do (38,43,81,82,94,127, also reviewed by 49). However, the availability of more sensitive techniques for detection of ERs and the discovery of ERβ (77) have led to additional avenues for the rapid direct action of E2 in GnRH neurons. Co-localization of ERβ and GnRH has been reported in the POA-hypothalamus of several species (51,58-60,132,134) and novel membrane bound ERs, including the G-protein coupled ER (GPR30, 40), ER-X (149), and a membrane ER sensitive to the diphenylacrylamide compound STX (STX-R, 115) have been described. In fact, direct rapid E2 action on GnRH neurons has now been reported by several groups including our own (1-3,19,22,103,123,139,142). In this article, we will 1) briefly review recent progress on neurosynthesis of steroids in the brain focusing on neuroestrogen and their potential role as a neurotransmitter, 2) present data of rapid E2 action on the GnRH neuronal system, highlighting our findings in the nonhuman primate, and 3) discuss potential roles for locally synthesized E2 in GnRH neuronal function, including the preovulatory surge and pulsatile GnRH release.
1. Synthesis of estradiol in the brain
Discovery of steroid synthesis in the hypothalamus began in the early 1970s. Conversion of progesterone to 5-pregnane-3,20-dione in rat hypothalamic tissue (18,63) and aromatization of androstendione to E2 in the hypothalamus (101) were both reported within similar time frames. However, despite consistent reports on the presence of neuroestrogens (35,92,130,138), the role of neuroestrogens in brain function has not been studied until recently. This is quite a contrast to that of 3-hydroxy-D5-compounds, such as pregnenolone (PREG) and dehydroepiandrosterone (DHEA), their sulfates, and reduced metabolites such as the tetrahydroderivative of progesterone 3a-hydroxy-5a-pregnane-20-one (3a,5a-TH PROG). The 3-hydroxy-D5-compounds are one of the most prominent allosteric modulators of chloride channels in GABAA receptors (12,96) and therefore, they became synonymous with the term “Neurosteroids”.
Entering the 21st century, a hypothesis that locally produced E2 in the brain modulates neuronal function as a neurotransmitter or neuromodulator has been proposed (7,126). This hypothesis is based on the rapid timing of E2 synthesis in the brain, the rapid action of E2 inducing sex behavior in quails and rats (27-30), and the presence of aromatase in the presynaptic boutons in song birds (109).
Aromatase is expressed in many different brain regions, including the hypothalamus, in most vertebrate species (14,100,124). Not only is aromatase expressed in various regions of the brain in multiple species, but all of the enzymes necessary to synthesize E2 de novo are also expressed in specific brain regions (54,55,84,87-90,152). Aromatase is expressed not only as a cluster of neurons but also as scattered individual neurons in the hypothalamus (62,100,129), high vocal centers, and the caudomedial nidopallium of the bird brain (109), and in the rat hippocampus (55,122). Importantly, aromatase is found in neuronal cell bodies and neuroterminals (100,109,122,129). In zebra finches, 50% of aromatase expressed in the brain is at neuroterminals and the expression and activity of aromatase at the nerve terminal is differentially regulated from that in other cellular compartments (122).
E2 synthesis in some brain regions is independent of E2 synthesis in the gonads, whereas other brain regions are dependent upon E2 from a gonadal origin (see further discussion on this issue in section 4). For example, aromatase activity in the male monkey ventromedial hypothalamic nucleus (VMH), cortical amygdala, and basal nucleus of the stria terminalis (BNST) are unaffected by castration, while aromatase activity in the POA, anterior hypothalamus, and medial amygdala are abrogated (124). In addition to genomic seasonal regulation of aromatase activity in birds and fish (45,48,86,108,111,137,150), evidence suggests that aromatase activity is also rapidly regulated within a time frame of a couple of minutes (13,26,28). In the quail hypothalamus, aromatase activity is inhibited by depolarization with high K+ as well as by treatment with the glutamate receptor agonists NMDA, AMPA, and kainate within 5 minutes and this rapid inhibition appears to require phosphorylation of the aromatase enzyme (7-10,17). Additionally, in response to acute stress, aromatase activity is rapidly increased within 15 min in the male quail hypothalamus (32). Collectively, these aromatase studies indicate possible rapid changes in de novo synthesis of E2 in the brain.
Indeed, in the past 10 years, the concentration of E2 in the brain has been directly assessed by RIA, ELISA, and liquid chromatography with single or tandem mass spectrometry (LS/MS or LC/MS/MS analysis). As summarized in Table 1, to date, five groups have reported E2 levels of homogenized rat brain tissue samples, spent/conditioned culture media from organotypic or dispersed cell cultures of the rat hippocampus, and microdialysate samples obtained from the bird cerebral cortex. Reviewing the published data, we have first noticed that there are distinct differences in E2 concentration due to differences in species, sampling method, or detection methodology. In fact, Hojo et al. (56) highlights methodological differences between LC/MS/MS and RIA for assessment of E2 measurements, showing that there are ~10 fold differences in the levels of E2 detected in brain tissues (LC/MS/MS values are higher than RIA values). However, within the data obtained from individual labs, E2 concentration differs among sex, age, and brain region studied (4,73): E2 concentrations in the hypothalamus and hippocampus are highest at late embryonic ages and decrease after birth on postnatal day 4 (P4) to adulthood levels in rats of both sexes (4,54,73). Importantly, E2 concentrations in the homogenized samples from various brain regions are in the high picomolar (pM) to low nanomolar (nM) range (4,55,64,73), about 10 to 100-fold higher than E2 concentrations in plasma of male rats (56,64). Apparently, E2 concentrations equivalent to the levels at the proestrous morning to afternoon are found in spent media of cultured hippocampal neurons (39,76), indicating that locally synthesized E2 is released into extracellular space. Strikingly, E2 release from cultured hippocampal neurons is enhanced by GnRH challenge (112).
Table 1.
Estradiol (E2) levels in the brain and plasma
| Publications | Specie s |
Brain Regions |
Age | Conditions or Challenges | Male E2 (nM)* |
Femal e E2 |
Sex unknow n E2 |
Sample Collection | E2 Assay |
|---|---|---|---|---|---|---|---|---|---|
|
Kawato et al., 2002 Group A |
Rat | Hippocampus Hippocampus |
Adult | Baseline NMDA (100 μM) |
0.67 1.35 |
- - |
- - |
Brain slices treated then homogenized tiss | sRIA |
| Plasma | Adult | Plasma | 0.10 | - | - | ||||
|
Prange-Kiel et al., 2003 Group B |
Rat | Hippocampus Hippocampus |
Adult, 8 div | Control Letrozole (1 nM) |
- - |
0.11 0.02 |
- - |
Conditioned medium from primary culture | RIA |
|
Kretz et al., 2004 Group B |
Rat | Hippocampus Hippocampus Hippocampus Hippocampus |
P4-7, 8 div | Control Letrozole (10 pM) Letrozole (1 nM) Letrozole (100 nM) |
- - - - - |
- - - - - |
0.09 0.06 0.03 0.03 |
Conditioned medium from brain slice cultur | RIA |
| Hippocampus Hippocampus |
P4-7, 8 div | Control Letrozole (100 nM) |
- - |
- - |
0.07 0.06 |
Conditioned medium from primary culture | |||
|
Hojo et al., 2004 Group A |
Rat | Hippocampus Hippocampus |
Adult | Baseline NMDA (100 μM) |
0.59 1.27 |
- - |
- - |
Brain slices treated then homogenized tissues | RIA |
|
Amateau et al., 2004 Group C |
Rat | Frontal cortex Hippocampus Hypothalamus POA Cerebellum Brainstem |
P 2h | Intact | 6.55 6.26 5.79 5.40 4.39 3.35 |
4.25 6.37 3.20 3.74 3.60 3.09 |
- - - - - - |
Homogenized tissues | RIA |
| Frontal cortex Hippocampus Hypothalamus POA Cerebellum Brainstem |
P 32h | Intact | 4.71 3.74 5.11 2.84 2.91 2.77 |
4.07 5.25 2.09 1.73 2.59 2.77 |
- - - - - - |
||||
|
Fester et al., 2006 Group B |
Rat | Hippocampus Hippocampus |
P5, 7 div | Control Letrozole (100 nM) |
- - |
- - |
0.32 0.21 |
Conditioned medium from primary culture | RIA |
| Hippocampus Hippocampus |
P5, 7 div | Control siRNA StAR siRNA |
- - |
- - |
0.26 0.20 |
||||
| Remage-Healey et al., Group D |
Bird | NCM NCM NCM NCM |
Adult | Baseline Female present Male song Glutamate (10 mM) |
1.34** 2.57** 2.20** 0.73** |
- - - - |
- - - - |
Microdialysis | ELISA |
| Plasma Plasma Plasma |
Adult | Baseline Female present Male song |
0.16 0.21 0.15 |
- - - |
- - - |
||||
|
Prange-Kiel et al., 2008 Group B |
Rat | Hippocampus Hippocampus |
P5, 8 div | Control GnRH (10 nM) |
- - |
- - |
0.73 0.88 |
Conditioned medium from brain slice culture | RIA |
| Hippocampus Hippocampus Hippocampus |
P5, 8 div | Control GnRH (10 nM) GnRH (100 nM) |
- - - |
- - - |
0.73 1.69 2.50 |
Conditioned medium from primary culture | |||
|
Fester et al., 2009 Group B |
Rat | Hippocampus Hippocampus Hippocampus Hippocampus Hippocampus |
P5, 7 div | Control Letrozole (100 nM) Testosterone (100 nM) Cholesterol (100 nM) Chol (100nM) +Letr (100 nM) |
- - - - - |
- - - - - |
0.07 0.04 0.31 0.64 0.51 |
Conditioned medium from primary culture | RIA |
|
Munetsuna et al., 2009 Group A |
Rat | Hippocampus Hippocampus |
Adult | Pair housed Single housed |
8.27 12.38 |
- - |
- - |
Homogenized tissues | EIA |
|
Hojo et al., 2009 Group A |
Rat | Hippocampus Hippocampus |
Adult | Intact Castrated |
8.44 6.98 |
- - |
- - |
Brain slices treated then homogenized tissu | LeCs/MS/MS |
| Plasma Plasma |
Adult | Intact Castrated |
0.01 0.01 |
- - |
- - |
||||
|
Konkle and McCarthy, Group C |
Rat | Hypothalamus Hypothalamus Hypothalamus |
E19 E21 P2-4 P60 |
Intact | 14.39 5.40 7.20 3.60 |
19.79 1.08 7.20 1.80 |
- - - - |
Homogenized tissues | RIA |
| Hippocampus Hippocampus Hippocampus |
E19 E21 P2-4 P60 |
Intact | 1.73 0.40 0.54 0.05 |
1.44 0.36 0.45 0.05 |
- - - - |
||||
| POA | P 2h | Intact | 9.90 | 1.13 | - | ||||
| Hypothalamus Hypothalamus |
P3 P3 |
Intact ADX/GDX |
9.00 11.69 |
8.64 7.20 |
- - |
||||
| Hippocampus Hippocampus |
P3 P3 |
Intact ADX/GDX |
0.40 0.40 |
0.36 0.36 |
- - |
||||
| Plasma Plasma |
P3 P3 |
Intact ADX/GDX |
0.09 0.12 |
0.07 0.09 |
- - |
||||
|
Remage-Healey et al., Group D |
Bird | NCM NCM NCM NCM |
Adult | Baseline Male song Female visual stimuli Male visual stimuli |
1.70** - 3.06** 1.59** |
1.50* 2.32* 1.71* 1.47* |
- - - - |
Microdialysis | ELISA |
| Plasma Plasma |
Adult | Baseline Male Song |
- - |
0.12 0.11 |
- - |
Group A=Kawato lab; Group B=Rune lab; Group C=McCarthy lab; Group D=Schlinger and his former student’s lab
POA: preoptic area, NCM: caudomedial nidopallium
E: embryonic day, P: postnatal day, div: days in vitro
ADX/GDX: adrenalectomized/gonadectomized
RIA: radio immuno assay, ELISA: enzyme-linked immunosorbent assay, EIA: enzyme immunoassay, LC/MS/MS: liquid chromatagraphy/ tandem mass spectrometry
E2 concentrations converted to nM using the estimation that 1 ml≈ 1 g wet weight and 1 nM≈ 0.1 fmol/mg wet weight≈ 10 fmol/mg protein (see Hojo et al., 2004 sup
In an earlier study, aromatase activity in some brain regions, such the VMH, amygdala, and BNST, is not affected by castration (124). A recent direct E2 measurement study in neonatal rats further indicates that E2 levels in the hypothalamus are independent of circulating gonadal steroids, as E2 levels in the hypothalamus are approximately 100-fold higher than circulating E2 and developmental changes in E2 concentrations (reduction in E2 occurs between P0 and P2) are unaffected by neonatal gonadectomy/adrenalectomy in both sexes (73). However, it is also possible that a small portion of E2 synthesis in these brain areas is attributable to the conversion of circulating testosterone. In fact, in the neonatal rat hippocampus castration reduced E2 levels by 17% (56), although this small decrease by castration again suggests that the majority of E2 synthesis (nM concentrations) in the hippocampus is independent of circulating gonadal steroids. Collectively, locally synthesized E2 in the brain at high pM to low nM levels appears to be maintained regardless of circulating steroid levels, although there may be subtle modifications by peripheral steroids depending on brain region, sex, or age. Nonetheless, presently, we do not have any information regarding 1) whether the total E2 concentration in the hypothalamus is important or 2) whether the source of the E2 (peripheral vs. central) is important for control of GnRH release. While to date abundant data show that peripheral E2 modifies neuronal activity in the hypothalamus, the concept of neuroestrogen is newly born. Therefore, coordinated interactions caused between central and peripheral E2 remain to be investigated.
An important question arises as to whether E2 synthesized in the brain influences circulating E2 levels. The result that neonatal gonadectomy/adrenalectomy in male and female rats does not alter plasma E2 levels (73) support this possibility, at least during an early developmental stage. However, the answer to this question requires further investigations, as the authors of that study (73) did not measure the effects of gonadectomy/adrenalectomy on plasma testosterone and neonatal castration of male rats is well known to eliminate sexual differentiation of the brain,
Emerging evidence suggests that synthesis of neuroestrogens is also regulated by neurotransmitters and neuromodulators. In rat hippocampal slices, application of NMDA results in an increase in the production of E2 within 30 min in vitro (55,64). E2 concentration in the rat hippocampus also increases in response to stress (99). Exposure of male songbirds to females increases E2 levels in the forebrain cortex within 30 min in vivo without changes in testosterone levels (120). Infusion of glutamate inhibits E2 neurosynthesis, whereas infusion of GABA stimulates testosterone neurosynthesis in the forebrain cortex of birds within 30 min in vivo (120). These time resolutions can be refined, whenever a more sensitive method for detecting E2 becomes available. Nonetheless, these results indicate that E2 synthesis occurs rapidly when neurons receive excitatory and inhibitory signals from other neurons, such as glutamatergic and/or GABAergic input.
2. E2 induces a rapid excitatory action on primate GnRH neurons
There are several models to examine the effects of E2 on GnRH neurons (145). Among them we have been using cultured GnRH neurons derived from the nasal placode of monkeys at embryonic age (E) 35-37, which are obtained from time-mated pregnancies. Because transgenic monkeys with GFP-labeled GnRH neurons are not yet available, this approach is quite useful for studying the cellular physiology of GnRH neurons in primates. Earlier, we have demonstrated that 1) cultured GnRH neurons from monkeys contain almost no non-GnRH neurons or glia, as they are not present in the nasal placode at this specific developmental stage (146), 2) cultured GnRH neurons undergo maturational changes in vitro (79,146), and 3) placode derived GnRH neurons are functional, as transplantation of fetal placode into the infundibular recess of the third ventricle of adult female monkeys, in which the GnRH final common pathway has been lesioned, restores cyclic ovulation (125).
We first examined the effects of E2 on firing activity. Using cell-attached patch clamp recording we found that application of E2 (1 nM) to cultured primate GnRH neurons induces a ~250% increase in action potential firing frequency within a minute (Figure 1A). E2 also increases the number of action potentials per burst and burst duration. However, E2 does not change the timing of bursts (interburst interval) nor the cluster pattern in primate GnRH neurons (2). Similar rapid stimulatory E2 effects on action potential firing rate have also been reported in GFP-labeled GnRH neurons of ovariectomized mice: E2 (100 pM-100 nM) directly enhances the firing rate of GnRH neurons in a dose responsive manner, whereas 10 pM E2 indirectly reduces firing activity of GnRH neurons via suppressing the excitatory GABA neurotransmission (22). The authors of that study state that the E2 effects started within 5 min and were completed between 10 and 15 min after the initiation of E2 treatment (22).
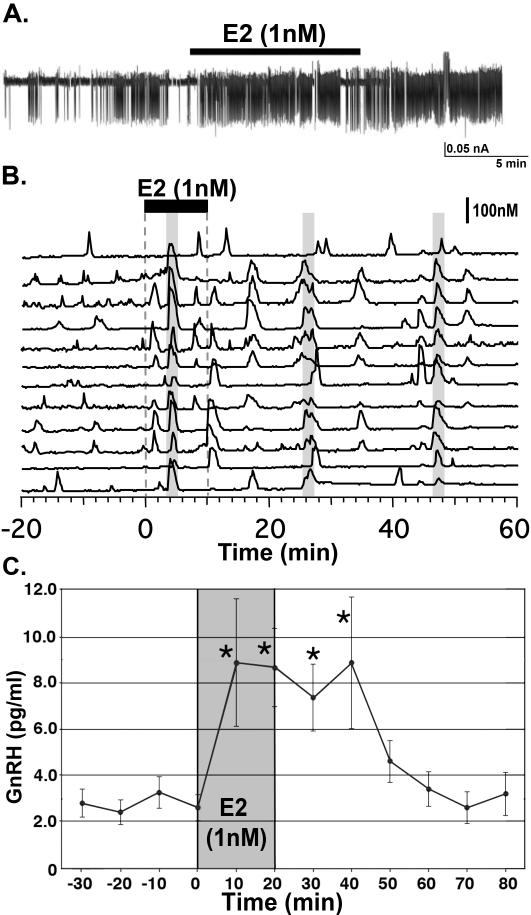
Figure 1.
Application of E2 induces excitatory firing activity (A), intracellular calcium, [Ca2+]i, oscillations (B) and GnRH release (C) in cultured primate GnRH neurons. E2 (1nM) was applied to GnRH neurons for 10 or 20 min, as indicated by the black bar (A) and (B) and gray bar (C). Note that in all cases, excitatory effects are induced within 10 min. Gray bars (B) indicate synchronization induced by the E2 treatment. Modified from 1,2,103, permission pending.
Next, we examined the effects of E2 on [Ca2+]i oscillations (1,67,69,103). Exposure of GnRH neurons to 1 nM E2 for 10 min causes an increase in the frequency of [Ca2+]i oscillations starting during the E2 application and lasting for 40-50 min (Figure 1B). E2 also increases the number of activated GnRH neurons. The E2 effects on [Ca2+]i oscillations are dose dependent, as 10 nM E2 induces [Ca2+]i oscillations with a higher frequency and a longer period as well as a higher percentage of activated cells (69). In addition, E2 increases the number of synchronized [Ca2+]i oscillations/hour from ~1 event/hour to ~2.7 events/hour after the initiation of E2 treatment (1), although the frequency of synchronized [Ca2+]i oscillations is not dose dependent (69). Importantly, the E2-induced increase in [Ca2+]i oscillations is not blocked by TTX (1), indicating that E2 causes rapid action directly on GnRH neurons (see more discussion in section 4). Additionally, treatment with the membrane impermeable E2, E2-BSA, and the nuclear impermeable estrogen dendrimer conjugate (EDC) also increases the frequency of [Ca2+]i oscillations, suggesting that E2-induced [Ca2+]i oscillations are a membrane initiated event (1,103). Similar stimulatory effects of E2, including E2-BSA conjugates, on [Ca2+]i oscillations have been reported in cultured mouse GnRH neurons. However, because these neurons are exposed to E2 for 30 min before recording (142,143) the latency of the response is unclear. Another study of [Ca2+]i changes in Pericam expressing mouse GnRH neurons shows that at minimum 15 min is required for direct stimulatory E2 action as well as indirect inhibitory E2 action transsynaptically mediated through GABA neurons (123). The data in Pericam expressing mouse GnRH neurons indicate that the latency in murine GnRH neurons appears to be longer than that in primate GnRH neurons.
Henceforth, as E2 induces a rapid excitatory action in primate GnRH neurons, E2 should also stimulate GnRH release. Indeed, exposure of cultures to 1 nM E2 for 20 min results in a rapid increase of GnRH peptide release (103). Again, the increase occurs within 10 min of E2 application and lasts for 40 min (Figure 1C). Moreover, the plasma membrane impermeable E2-BSA and the nuclear membrane impermeable form of E2, EDC, both stimulated GnRH release within 10 min, although the duration and amplitude is smaller than E2 alone (103). A rapid release of GnRH from mouse cultured or GFP-labeled GnRH neurons, comparable to ours, has not been reported. Although the suppressed frequency of GnRH release with a 4-hour treatment of E2 (17 pM) in GT1-7 cells (102) has been reported, a 4-hour treatment period is difficult to categorize as a “rapid” E2 action, because most “rapid” E2 actions occur within minutes. In addition, E2 application to rat median eminence explants rapidly stimulates GnRH release (33), suggesting that E2 can cause excitatory action at the GnRH neuroterminals and this observation from nearly 30 years ago is consistent with our observation. Importantly, we recently found that this rapid stimulatory E2 action on GnRH release was seen in vivo, when we directly applied E2 to the MBH in both ovarian intact or ovariectomized adult female monkeys (66).
3. Rapid E2 action is mediated through multiple membrane receptors
The consequence of E2 action is dependent upon several factors including dosage, timing, spatial aspects, and receptor subtypes or signaling molecules involved. A fundamental question to understanding rapid direct E2 action on GnRH neurons is which ER(s) mediate(s) E2 effects. The most commonly studied ERs are ERα and ERβ. Initially, we too expected to find a role of ERα and/or ERβ in the rapid E2 action in primate GnRH neurons. Surprisingly, however, the ER inhibitor, ICI182,780, fails to block the E2-induced increase in [Ca2+]i oscillations and GnRH release (1,103), indicating that neither ERα, nor ERβ, is involved. In a follow-up study, we transfected primate GnRH neurons with siRNA specific to human ERα or ERβ and tested the effects of E2. Again, exposure to siRNA for ERα or ERβ fails to block the E2-induced [Ca2+]i oscillations (67), confirming our previous results. By contrast, in mouse GnRH neurons ERβ appears to mediate the majority of rapid direct E2 effects (3,19,22,139,142) or alternatively, indirect E2 effects are mediated through ERα involving presynaptic GABA inputs (19,22,123). Although Sun et al. (139) report that in mouse GnRH neurons E2 causes a minor direct effect through GPR30, in general, receptors involved in E2 action in mouse GnRH neurons are significantly different from those in primate GnRH neurons.
To date three nonclassical membrane ERs have been proposed: ER-X (149), GPR30 (148), and STX-R (115). We first investigated the role of GPR30 in rapid E2 action in primate GnRH neurons. To our surprise, treatment of the GPR30 agonist G1 at 10 nM, but not 1 nM, stimulates [Ca2+]i oscillations similar to E2 (103). Additionally, GPR30 knockdown with siRNA blocks both E2- and EDC-induced [Ca2+]i oscillations (103). Interestingly, a high dose of ICI182,780 (1 μM) alone elicits an increase in [Ca2+]i oscillations (103). It has been shown that a high dose (1 μM) of ICI182,780 is an agonist for GPR30 in cancer cells (40). Finally GPR30 is expressed in a subset of adult GnRH neurons in the monkey hypothalamus (~30%, 103).
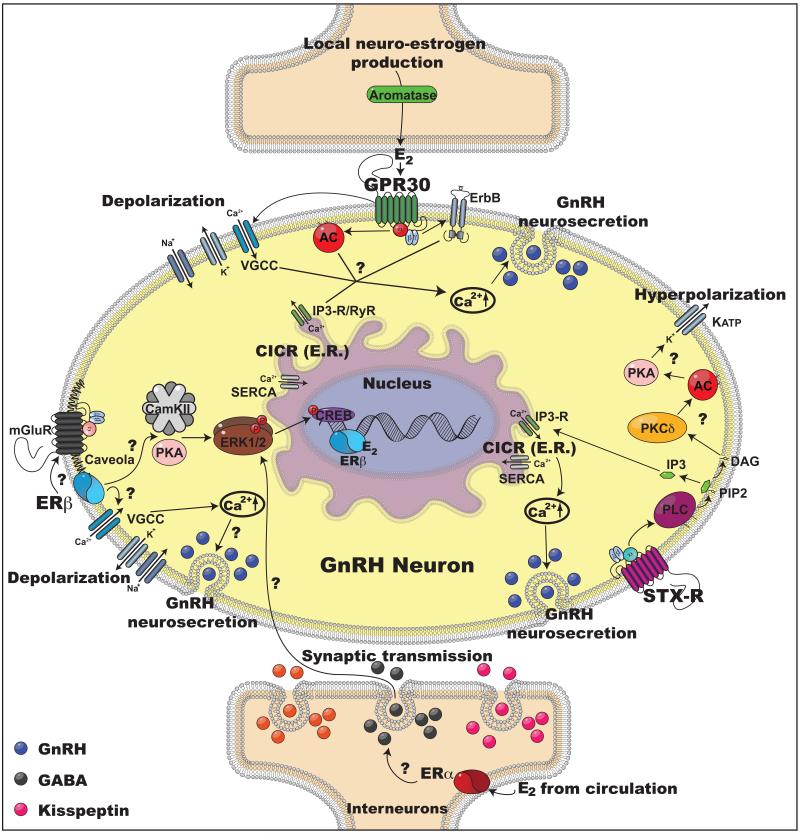
There are two reasons to believe that the rapid excitatory E2 action in primate GnRH neurons is mediated by more than one receptor subtype. First, 10 nM E2 effects on [Ca2+]i oscillations are larger than those of 1 nM E2 and GPR30 siRNA reduces but does not completely block 10 nM E 2+ 2 effects on [Ca ]i oscillations (69). Second, a higher dose of G1 is required to elicit a response similar to E2 (103). Because STX has been shown to elicit changes in hypothalamic neurons through a phospholipase C (PLC) mechanism in mutant mice lacking ERα, ERβ, ERα/ERβ, and GPR30 (115-117), we examined the role of STX-R in 10 nM E2 action in primate GnRH neurons. To our surprise, STX (10 nM) treatment of primate GnRH neurons elicits changes in [Ca2+ ]i oscillations, similar to those with 1 nM E2 and STX (10 or 100 nM) treatment also stimulates GnRH release in a dose dependent manner (69). Moreover, GPR30 siRNA transfection of GnRH neurons fails to block effects of STX, whereas treatment with ICI182,780, an antagonist for STX-R (115), blocks STX-induced [Ca2+]i oscillations, suggesting that E2 action through STX-R in primate GnRH neurons is independent of GPR30 (69). Therefore, multiple receptor mechanisms are clearly involved in mediating rapid E2 action on primate GnRH neurons (Figure 2).
Figure 2.
Schematic illustration showing two modes of rapid E2 action in GnRH neurons (middle) with two synapses (Top and Bottom). Assuming E2 is released at the synaptic cleft, there are direct (top) and indirect (bottom) rapid E2 actions. Top: E2 released at the synaptic cleft directly activates GPR30 and STX-R in the primate hypothalamus (and ERβ in the rodent hypothalamus) and modulates activity of GnRH neurons. Exposure of primate GnRH neurons to E2 rapidly induces [Ca2+]i oscillations and GnRH peptide release (vesicles in blue color) within 10 min (69,103). Two possible mechanisms for the rapid E2 action through GPR30 and STX-R are discussed in this article, although many details are yet to be clarified, which are noted by question marks in the scheme. First, E2 binding to GPR30 may induce activation of two intracellular pathways: 1) E2 activation through GPR30 depolarizes the GnRH neuronal membrane via VGCCs (139), which allows [Ca2+]e entry, resulting in CICR (68) and 2) E2 transactivates AC and/or ErbB pathways (41), which also results in CICR. Second, E2 binding to STX-R appears to cause 1) activation of CICR through a PLC and IP3-R mechanism leading to a [Ca2+]i increase (69) and 2) activation of a PKCδ-AC-PKA mechanism resulting in hyperpolarization of the GnRH neuronal membrane through KATP channels (159), which are essential for burst firing of GnRH neurons, hence neurosecretion. Direct E2 action through ERß has also been reported in mice GnRH neurons (19,139). Bottom: Rapid action of E2 may be indirect, transsynaptically mediated by other neural input, as shown by Romano et al. (123). In this case, E2 rapidly stimulates GABA release (vesicles in black color), which causes IPSPs in mouse GnRH neurons, resulting in suppression of GnRH release. Alternatively, it is possible that E2 rapidly stimulates excitatory interneurons (vesicles in magenta color), which would cause EPSPs in GnRH neurons, resulting in stimulation of GnRH release. Abbreviations: AC: adenylyl cyclase; Ca2+: calcium; [Ca2+]e: extracellular Ca2+; [Ca2+]i: intracellular Ca2+; CamKII: Ca2+ calmodulin kinase II; CICR: calcium induced calcium release; DAG: diacylglycerol; E2: estradiol; E.R.: endoplasmic reticulum; ErbB: epidermal growth factor receptor; ERK1/2: Extracellular signal regulated kinase 1 and 2; GnRH: gonadotropin-releasing hormone neuron; GPR30: G protein coupled estrogen receptor; IP3: inositol triphosphate; IP3-R: inositol triphosphate receptor; KATP: ATP sensitive potassium channel; mGluR: metabotropic glutamate receptor; PIP2: phosphatidylinositol biphosphate; PKA: protein kinase A; PKCδ: Protein kinase C delta; PLC: phospholipase C; RyR: ryanodine receptor; SERCA: Sarco/endoplasmic reticulum Ca2+ ATPase; STX-R: membrane estrogen receptor sensitive to STX; VGCC: voltage gated calcium channel.
4. The role of rapid E2 action in the mechanism of GnRH release
A “rapid” timing of E2 action is a membrane initiated phenomenon, rather than “long term” E2 action, which requires nuclear transcription after E2 binding to ERs. Then a question arises as to what is the physiological significance of rapid E2 action in the hypothalamus, and more specifically within the GnRH system? It has been proposed that locally synthesized E2 contributes to acute synaptic formation in the hippocampus, as E2 increases spine density and enhances long-term potentiation (LTP) and long-term depression (LTD) in hippocampal neurons within 30 min (42,91,98), whereas spine density decreases by treatment with an aromatase inhibitor (76). Whether there is a similar function of E2 in GnRH neurons remains unknown.
Is there any role of rapid E2 action in the negative and positive feedback control of GnRH release? In ovariectomized female rhesus monkeys, injection of E2 induces suppression of LH/GnRH release with a latency of 2-4 hours (the negative feedback phase, 20,53,97,156), followed by stimulation of LH/GnRH release with a latency of 24-36 hours, lasting for 36-48 hours in a positive feedback phase (53,80,156). Because in primate GnRH neurons E2 induces membrane initiated excitatory, not inhibitory, action within 10 min, it is unlikely that E2 is involved in the negative feedback mechanism. Is it then involved in the positive feedback mechanism? We will discuss this further in section 5.
Alternatively, is there any possible role in GnRH pulse generation? As discussed earlier, our observations with [Ca2+]i dynamics in vitro consistently show that E2 is a potent frequency modulator of GnRH neurons. Before we present our view, however, we need to discuss several issues.
Species differences
There are clear species differences in the preovulatory and E2-induced GnRH and gonadotropin surges. As discussed in a recent review by Plant (110), in rodents the preovulatory GnRH surge is controlled by the AVPV and circadian signals, whereas in highly evolved primates (old world monkeys and humans) the medial basal hypothalamus is sufficient for cyclic ovulations and the preovulatory LH surge is independent of circadian signals. Moreover, the male rodent brain is sterilized by the perinatal elevation of estrogens aromatized from androgens and thus E2 is not able to induce a surge in castrated males. In contrast, the capacity of the male primate hypothalamus for the preovulatory LH surge remains, as the GnRH neuronal system is not sterilized by prenatal/perinatal gonadal steroids (104). Finally, while a neuronal signal for GnRH/LH surges in rodents is limited to the pentobarbital sensitive critical period of 2h (36,37) and surges last for 4-6 hours (136), in primates there is no critical period for the GnRH/LH surges and surges last for over 36-48 hours (53,80,153,156). Importantly, as discussed above, direct rapid excitatory E2 effects on GnRH neurons in non-human primates are mediated by GPR30 and STX-R, whereas in mice ERβ appears to be responsible (19). It is also important to point out the fact that the promoter region of the GnRH gene in humans contain an ERE, whereas the rodent GnRH gene does not (118), indicating that genomic E2 actions through classical ER in rodent GnRH neurons are likely through interneurons. In primates, however, genomic E2 actions through classical ERs in GnRH neurons can occur both directly or indirectly.
Concentration
Concern has been cast regarding the doses of 1-100 nM E2 used in in vitro studies examining the effects of E2 on GnRH neurons (50). In our studies in non-human primates the doses at 1-10 nM E2 were routinely used (1,2,67,69,103) and in electrophysiological studies with acute brain slices or embryonic GnRH neurons in rodents the doses of E2 as high as 100-1000 nM were used (22,139,142). Certainly, these doses are higher than circulating E2 levels during the ovulatory cycle, when E2 levels fluctuate from pM to low nM (0.1-0.2 nM in the follicular phase and 0.7-1.4 nM at the preovulatory surge) in female monkeys (106,107) and 20 pM in diestrus and 200 pM at the preovulatory surge in female mice and rats (136, also see 25). However, as discussed in section 1 and Table 1, the adult rat hypothalamus, hippocampus, and other brain regions of both sexes contain E2 at high pM to low nM levels and our preliminary data indicate similar E2 levels in the female rhesus monkey hypothalamus as well as in microdialysate samples obtained from the stalk-median eminence in vivo (B.P. Kenealy and E. Terasawa, unpublished data). Therefore, E2 at 1-10 nM in the hypothalamus is not likely “supraphysiological”. How about 100-1000 nM used in rodent electrophysiology and culture studies? Peak concentration of E2 in the synaptic cleft could reach 100 nM or even higher, when E2 is released as a neurotransmitter. In fact, it has been shown that calculated peak concentrations of the neurotransmitters glutamate and GABA are much higher than those in tissue concentrations measured by a microdialysis method. That is, in the synaptic cleft, glutamate and GABA concentrations reach 1.1 mM (24) and 30-100 mM (47,61), respectively, whereas concentrations of glutamate and GABA in the hippocampus and striatum measured with in vivo microdialysis methods are 1-4 μM (6,85,105) and 25 nM in hippocampal brain slice preparation (52), respectively.
Receptors
The classical positive and negative feedback effects of E2 are mediated by mechanisms through ERα, requiring nuclear transcription. First, E2 fails to induce LH surges in ERα knockout mice as well as in mutant mice lacking estrogen response element (ERE)-dependent ERα signaling (21,46). By contrast, in ERβ knockout mice E2 induces LH surges (154), although these mice had ERβ splice variants (75), therefore, reexamination of the role of ERβ in positive feedback in mice with complete elimination of ERβ splice variants (5) is still needed. Second, whereas convincing evidence for the presence of ERα in GnRH neurons has not been shown, the presence of ERα in interneurons that innervate GnRH neurons, such as kisspeptin, have been consistently reported (43,94,135). Taken together, while it is possible that membrane ERα signaling may be, in part, responsible for negative feedback effects of E2 on LH release, as E2 can suppress LH levels in mice lacking ERE-dependent ERα signaling (46), ERα expression in interneurons, such as kisspeptin neurons (94) that directly innervate GnRH neurons, is indispensable for LH/GnRH surges. Little is known about ERs involved in the feedback actions of E2 in primates. Nonetheless, this does not preclude the role of rapid E2 action in the positive feedback mechanism, as discussed in section 5.
Role of non-neuronal cells
Because our GnRH cultures contain non-neuronal cells (146) and they are involved in the propagation of the [Ca2+]i wave of the GnRH neuronal network (121), it is possible that E2 action to GnRH neurons are also mediated through non-neuronal cells. In fact, E2 rapidly induces a [Ca2+]i release from astrocytes, stimulates progesterone synthesis within 5 min in astrocytes of rat hypothalamus which are dependent upon both nonclassical and classical receptors (16,78), and modifies tanycyte morphology (70,113). E2 also rapidly alters function of epithelial cells in the median eminence (31,114). Moreover, E2 stimulates release of prostaglandin E2 (PGE2) from astroglia (107), which directly depolarizes the membrane of GnRH neurons (23). Although we have previously shown that TTX does not block the E2-induced rapid changes in [Ca2+]i oscillations (1), this observation does not exclude input from non-neuronal cells. Thus, we need to address this issue in the future.
Time domain
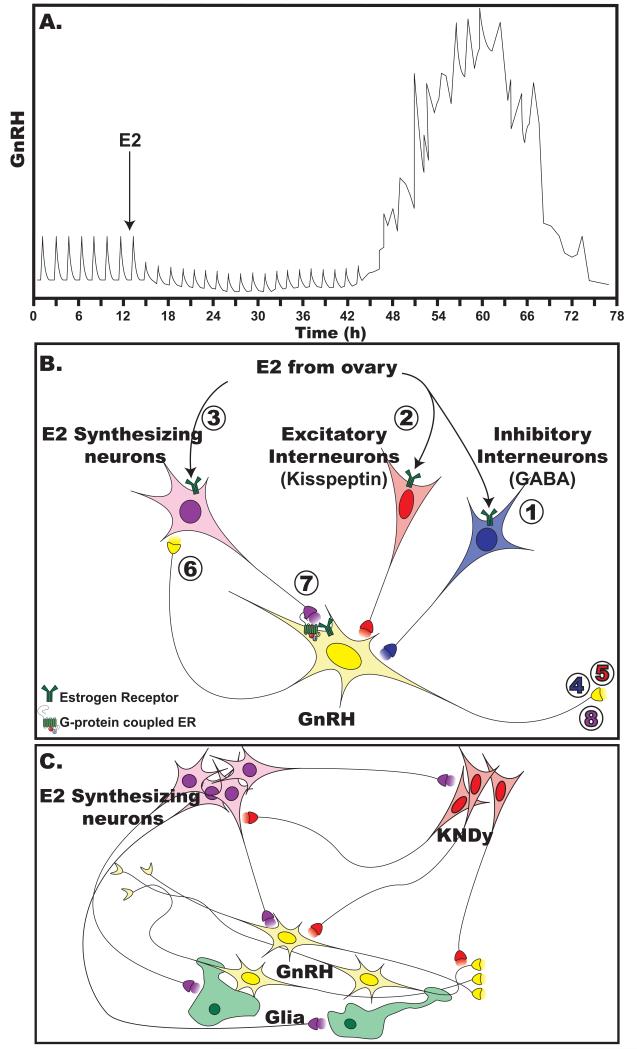
It is important to point out that the negative feedback effect of E2 on LH/GnRH release precedes its positive feedback effect (144,156). Also, as we have already discussed, the membrane-initiated E2 action occurs within min, whereas E2 induced genomic changes require at least several hours to days. Based on this major difference in the time domain of the two modes of E2 action, it is clear that the positive feedback effects of E2 are initiated by “genomic action” of E2. However, this does not preclude the possible role of the membrane-initiated rapid excitatory E2 action in augmenting the preovulatory GnRH surge. To extend this issue, while the rapid E2 action on GnRH release in both in vitro and in vivo are “brief” (5-20 min exposure of E2 inducing a brief GnRH increase), E2 increases from the ovary or by peripheral administration last several hours to days resulting in suppression (negative feedback) for at least several hours and stimulation (positive feedback) for 24-48 hours of GnRH release (Figure 3A).
Figure 3.
Schematic illustration showing the effect of ovarian estradiol (E2) on GnRH release (A) and possible roles of neuroestrogens in control of GnRH surge (B) and/or pulsatility of GnRH release (C) in primates. A. E2 from the ovary suppresses GnRH release (negative feedback phase) with a latency of a couple of hours lasting 24-48 hours. Subsequently, E2 facilitates GnRH release (positive feedback phase) with a latency of 24-48 hours lasting 36-48 hours. B. A possible role of E2 synthesized in the MBH in the preovulatory GnRH surge. An increase in preovulatory E2 from the ovary causes action in three types of interneurons to GnRH neurons in the hypothalamus: (1) inhibitory interneurons, such as GABA neurons, (2) excitatory interneurons, such as kisspeptin neurons, and (3) E2 synthesizing neurons. Exposure of inhibitory interneurons (1) to E2 inhibits GnRH release (4), initially through a membrane-initiated E2 mechanism and subsequently through a genomic E2 mechanism. Over 24 hours after E2 exposure, activity of excitatory interneurons is stimulated by a genomic action of E2 (2), leading to the initial increase in GnRH release into the portal circulation (5) as well as at the synaptic junction between GnRH neurons and E2-synthesizing interneurons (6). Although it is possible that neurotransmitter E2 released from E2-synthesizing interneurons starts to stimulate GnRH release via the membrane-initiated mechanism, synaptic GnRH release may also feedback to stimulate neurotransmitter E2 release from E2-synthesizing interneurons (7). This positive feedback loop between GnRH neurons and E2-synthesizing interneurons sustains GnRH release to the portal circulation (8). Although this figure illustrates input of interneurons (e.g., GABA and kisspeptin expressing neurons and E2 synthesizing neurons), to GnRH cell bodies, interaction between GnRH neurons and interneurons could also occur at the neuroterminals. C. A possible role of E2 synthesized in the MBH in pulsatility of GnRH release in primates. E2 synthesized in the MBH may modulate GnRH neurons directly or indirectly through KNDy (kisspeptin, neurokinin B, ß-dynorphine) neurons and/or glia.
5. Two possible hypotheses
As discussed in above, we propose two hypotheses for a physiological role of rapid E2 action: Hypothesis 1 is a potential role for local E2 that augments the preovulatory GnRH surge. Hypothesis 2 is that local E2 contribute to GnRH pulse frequency. At this time, evidence for both hypotheses is circumstantial and direct evidence needs to be shown.
Augmentation of the preovulatory GnRH surge (Figure 3B)
During the preovulatory phase, ovarian E2 binds interneurons, such as kisspeptin neurons and GABA neurons, in the hypothalamus/ POA, which express ERα (and also ERβ to a certain extent). At the negative feedback phase, the suppression of GnRH release is caused by interneurons. In fact, involvement of both membrane initiated and genomic E2 action through interneurons during the negative E2 feedback phase has been reported (46,94). In the initial phase, inhibitory interneurons, such as GABA neurons, may rapidly reduce the activity of GnRH neurons via increasing inhibitory postsynaptic potentials (IPSP, 123) resulting in reduction of GnRH release. E2 activation of nuclear transcription of GABA neurons leads to an increase in GABAergic activity resulting in a subsequent decrease of GnRH release. At the positive feedback phase, excitatory interneurons, such as kisspeptin neurons, are stimulated by genomic action of ovarian E2 resulting in facilitation of GnRH neuronal activity and an increase in GnRH release.
Ovarian E2 may also stimulate E2 synthesis in interneurons, the phenotypes of which are yet to be determined. Evidence for this stimulation of synthesis is based on E2 effects on aromatase expression. For example, the promoter region of the aromatase gene contains an ERE and aromatase expression is up-regulated in an in vitro culture system (158). In addition, aromatase expression in the rat BST and medial amygdala decreases after castration with adrenalectomy, while treatment with E2 reverses this decrease (160). Assuming that an E2-induced increase in E2 production leads to elevated E2 release from the neuroterminal/ synaptic junction between an aromatase expressing interneuron and GnRH neuron, the released E2 could rapidly stimulate GnRH release through GPR30 and/or STX-R in primates and ERβ and/or STX-R in mice. Finally, if the GnRH neuron makes a synaptic connection with the aromatase expressing interneuron, GnRH release at synapses could stimulate E2 release. Because in the hippocampus GnRH stimulates E2 release (112), it is not difficult to speculate that a similar GnRH stimulation of E2 release occurs in the hypothalamus. This hypothetical perpetuating positive feedback loop within the hypothalamus would be quite advantageous in the primate brain, as the preovulatory GnRH surge needs to be sustained for 24-48 hours until the pituitary and ovary properly respond for successful ovulation. Importantly, the termination of the GnRH surge would also be prompt due to the rapid nature of E2 action. Currently, the mechanism of GnRH surge termination is unknown.
GnRH pulse frequency modulation (Figure 3C)
Considering our knowledge that the frequency modulation of GnRH pulses is essential for proper maintenance of the female reproductive cycle (71), we can extend our hypothesis that local E2 may modulate pulsatility of GnRH release in a subtle manner. This hypothesis is derived from our observations that E2 modulates frequency of [Ca2+]i oscillations and synchronization of [Ca2+]i oscillations in GnRH neurons in vitro (1,69,103). It is also known that E2 is a potent frequency modulator of LH/GnRH release. Although presently, the mechanism of GnRH pulse generation is unclear, coordinated periodical release of GnRH is regarded as synchronized activity among GnRH neurons. In fact, GnRH neurons appear to communicate through dendro-dendritic interactions (15) and recently, we have shown that GnRH is released from the cell body and dendrites (44). Local E2 could cause its effect through KNDy neurons, kisspeptin, neurokinin B, and ß-dynorphin co-expressing neurons, in the ARC (34,119), a concept that has been proposed as the source of GnRH pulse-generation (93,151). Either way (directly or indirectly), it is possible that locally synthesized E2 may contribute to the frequency modulation of pulsatile GnRH release in vivo.
Conclusion
Estradiol causes direct excitatory action within min in GnRH neurons. This rapid E2 action is a membrane-initiated phenomenon mediated by non-classical receptors, GPR30 and STX-sensitive receptors in primate GnRH neurons. Surprisingly, preliminary data suggest that the hypothalamus of female monkeys release high pM to low nM levels of E2, which is significantly higher than circulating E2 levels and infusion of E2 at a similar concentration into the medial basal hypothalamus results in stimulation of GnRH release in vivo (66). Based on the time course and stimulatory nature, this rapid action of E2 is not likely involved in the negative feedback phase of the ovulatory cycle. Consequently, we propose two hypotheses that the rapid membrane-initiated E2 action augments and sustains the positive feedback phase of GnRH release or pulsatility of GnRH release. Specifically, ovarian E2 initiates the preovulatory GnRH surge through indirect stimulation of interneurons, but neuroestrogens in the hypothalamus augment and sustain GnRH release for a prolonged period by a positive feedback loop between GnRH neurons and E2 synthesizing neurons in the hypothalamus. Alternatively, neuroestrogens may be profoundly involved in GnRH pulse generation. Certainly, these hypotheses are quite provocative. Nonetheless, if future studies validate these hypotheses, we can make a significant advancement in the field of Reproductive Neuroendocrinology.
Highlights.
* Estradiol induces a rapid excitatory action in primate GnRH neurons
* The rapid estradiol action is mediated by non-classical estrogen receptors
* Possible role of neuroestrogens in control of GnRH release is discussed
* Historical perspective of neuroestrogen in the hypothalamus is reviewed
Acknowledgments
This research was supported by NIH grants: R01HD15433 and R01HD11355 for ET and T32HD041921 for BPK, and was possible to perform by NIH support (OD011106) to the Wisconsin National Primate Research Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure summary: The authors have nothing to disclose.
References
- [1].Abe H, Keen KL, Terasawa E. Rapid action of estrogens on intracellular calcium oscillations in primate luteinizing hormone-releasing hormone-1 neurons. Endocrinology. 2008;149:1155–1162. doi: 10.1210/en.2007-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Abe H, Terasawa E. Firing pattern and rapid modulation of activity by estrogen in primate luteinizing hormone releasing hormone-1 neurons. Endocrinology. 2005;146:4312–4320. doi: 10.1210/en.2005-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Abrahám IM, Han SK, Todman MG, Korach KS, Herbison AE. Estrogen receptor beta mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J. Neurosci. 2003;23:5771–5777. doi: 10.1523/JNEUROSCI.23-13-05771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Amateau SK, Alt JJ, Stamps CL, McCarthy MM. Brain estradiol content in newborn rats: sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology. 2004;145:2906–2917. doi: 10.1210/en.2003-1363. [DOI] [PubMed] [Google Scholar]
- [5].Antal MC, Krust A, Chambon P, Mark M. Sterility and absence of histopathological defects in nonreproductive organs of a mouse ERbeta-null mutant. Proc. Natl. Acad. Sci. U.S.A. 2008;105:2433–2438. doi: 10.1073/pnas.0712029105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. J. Neurosci. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends in Neurosci. 2006;29:241–249. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- [8].Balthazart J, Baillien M, Ball GF. Phosphorylation processes mediate rapid changes of brain aromatase activity. J. Steroid Biochem. Mol. Biol. 2001;79:261–277. doi: 10.1016/s0960-0760(01)00143-1. [DOI] [PubMed] [Google Scholar]
- [9].Balthazart J, Baillien M, Ball GF. Rapid control of brain aromatase activity by glutamatergic inputs. Endocrinology. 2006;147:359–366. doi: 10.1210/en.2005-0845. [DOI] [PubMed] [Google Scholar]
- [10].Balthazart J, Baillien M, Charlier TD, Ball GF. Calcium-dependent phosphorylation processes control brain aromatase in quail. Eur. J. Neurosci. 2003;17:1591–1606. doi: 10.1046/j.1460-9568.2003.02598.x. [DOI] [PubMed] [Google Scholar]
- [11].Barry J, Dubois MP, Poulain P. LRF producing cells of the mammalian hypothalamus. A fluorescent antibody study. Z Zellforsch Mikrosk Anat. 1973;146:351–366. doi: 10.1007/BF02346227. [DOI] [PubMed] [Google Scholar]
- [12].Baulieu EE. Neurosteroids: of the nervous system, by the nervous system, for the nervous system. Recent Prog. Horm. Res. 1997;52:1–32. [PubMed] [Google Scholar]
- [13].Black MP, Balthazart J, Baillien M, Grober MS. Socially induced and rapid increases in aggression are inversely related to brain aromatase activity in a sex-changing fish, Lythrypnus dalli. Proc. Biol. Sci. 2005;272:2435–2440. doi: 10.1098/rspb.2005.3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Callard GV, Petro Z, Ryan KJ. Phylogenetic distribution of aromatase and other androgen-converting enzymes in the central nervous system. Endocrinology. 1978;103:2283–2290. doi: 10.1210/endo-103-6-2283. [DOI] [PubMed] [Google Scholar]
- [15].Campbell RE, Gaidamaka G, Han SK, Herbison AE. Dendro-dendritic bundling and shared synapses between gonadotropin-releasing hormone neurons. Proc. Natl. Acad. Sci. U. S. A. 2009;106:10835–10840. doi: 10.1073/pnas.0903463106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chaban VV, Lakhter AJ, Micevych P. A membrane estrogen receptor mediates intracellular calcium release in astrocytes. Endocrinology. 2004;145:3788–3795. doi: 10.1210/en.2004-0149. [DOI] [PubMed] [Google Scholar]
- [17].Charlier TD, Harada N, Balthazart J, Cornil CA. Human and quail aromatase activity is rapidly and reversibly inhibited by phosphorylating conditions. Endocrinology. 2011;152:4199–4210. doi: 10.1210/en.2011-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cheng YJ, Karavolas HJ. Conversion of progesterone to 5 alpha-pregnane-3,20-dione and 3 alpha-hydroxy-5 alpha-pregnan-20-one by rat medical basal hypothalami and the effects of estradiol and stage of estrous cycle on the conversion. Endocrinology. 1973;93:1157–1162. doi: 10.1210/endo-93-5-1157. [DOI] [PubMed] [Google Scholar]
- [19].Cheong RY, Kwakowsky A, Barad Z, Porteous R, Herbison AE, Abrahám IM. Estradiol acts directly and indirectly on multiple signaling pathways to phosphorylate cAMP-response element binding protein in GnRH neurons. Endocrinology. 2012;153:3792–3803. doi: 10.1210/en.2012-1232. [DOI] [PubMed] [Google Scholar]
- [20].Chongthammakun S, Terasawa E. Negative feedback effects of estrogen on luteinizing hormone-releasing hormone release occur in pubertal, but not prepubertal, ovariectomized female rhesus monkeys. Endocrinology. 1993;132:735–743. doi: 10.1210/endo.132.2.8425492. [DOI] [PubMed] [Google Scholar]
- [21].Christian CA, Glidewell-Kenney C, Jameson JL, Moenter SM. Classical estrogen receptor alpha signaling mediates negative and positive feedback on gonadotropin-releasing hormone neuron firing. Endocrinology. 2008;149:5328–5334. doi: 10.1210/en.2008-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chu Z, Andrade J, Shupnik MA, Moenter SM. Differential regulation of gonadotropin-releasing hormone neuron activity and membrane properties by acutely applied estradiol: dependence on dose and estrogen receptor subtype. J. Neurosci. 2009;29:5616–5627. doi: 10.1523/JNEUROSCI.0352-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Clasadonte J, Poulain P, Hanchate NK, Corfas G, Ojeda SR, Prevot V. Prostaglandin E2 release from astrocytes triggers gonadotropin-releasing hormone (GnRH) neuron firing via EP2 receptor activation. Proc. Natl. Acad. Sci. U. S. A. 2011;108:16104–16109. doi: 10.1073/pnas.1107533108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Clements JD, Lester RAJ, Tong G, Jahr CE, Westbrook GL. The time course of glutamate in the synaptic cleft. Science. 1992;258:1498–1501. doi: 10.1126/science.1359647. [DOI] [PubMed] [Google Scholar]
- [25].Cornil CA, Ball GF, Balthazart J. Functional significance of the rapid regulation of brain estrogen action: where do the estrogens come from? Brain Res. 2006;1126:2–26. doi: 10.1016/j.brainres.2006.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cornil CA, Charlier TD. Rapid behavioural effects of oestrogens and fast regulation of their local synthesis by brain aromatase. J. Neuroendocrinol. 2010;22:664–673. doi: 10.1111/j.1365-2826.2010.02023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Balthazart J. Estradiol rapidly activates male sexual behavior and affects brain monoamine levels in the quail brain. Behav. Brain Res. 2006;166:110–123. doi: 10.1016/j.bbr.2005.07.017. [DOI] [PubMed] [Google Scholar]
- [28].Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Dejace C, Ball GF, Balthazart J. Rapid decreases in preoptic aromatase activity and brain monoamine concentrations after engaging in male sexual behavior. Endocrinology. 2005;146:3809–3820. doi: 10.1210/en.2005-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cornil CA, Taziaux M, Baillien M, Ball GF, Balthazart J. Rapid effects of aromatase inhibition on male reproductive behaviors in Japanese quail. Horm. Behav. 2006;49:45–67. doi: 10.1016/j.yhbeh.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cross E, Roselli CE. 17beta-estradiol rapidly facilitates chemoinvestigation and mounting in castrated male rats. Am. J. Physiol. 1999;276:R1346–1350. doi: 10.1152/ajpregu.1999.276.5.R1346. [DOI] [PubMed] [Google Scholar]
- [31].de Seranno S, d’Anglemont de Tassigny X, Estrella C, Loyens A, Kasparov S, Leroy D, Ojeda SR, Beauvillain JC, Prevot V. Role of estradiol in the dynamic control of tanycyte plasticity mediated by vascular endothelial cells in the median eminence. Endocrinology. 2010;151:1760–1772. doi: 10.1210/en.2009-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dickens MJ, Cornil CA, Balthazart J. Acute stress differentially affects aromatase activity in specific brain nuclei of adult male and female quail. Endocrinology. 2011;152:4242–4251. doi: 10.1210/en.2011-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Drouva SV, Laplante E, Gautron JP, Kordon C. Effects of 17 beta-estradiol on LH-RH release from rat mediobasal hypothalamic slices. Neuroendocrinology. 1984;38:152–157. doi: 10.1159/000123883. [DOI] [PubMed] [Google Scholar]
- [34].Eghlidi DH, Haley GE, Noriega NC, Kohama SG, Urbanski HF. Influence of age and 17beta-estradiol on kisspeptin, neurokinin B, and prodynorphin gene expression in the arcuate-median eminence of female rhesus macaques. Endocrinology. 2010;151:3783–3794. doi: 10.1210/en.2010-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ellinwood WE, Hess DL, Roselli CE, Spies HG, Resko JA. Inhibition of aromatization stimulates luteinizing hormone and testosterone secretion in adult male rhesus monkeys. J. Clin. Endocrinol. Metab. 1984;59:1088–1096. doi: 10.1210/jcem-59-6-1088. [DOI] [PubMed] [Google Scholar]
- [36].Everett JW, Sawyer CH. The blocking effect of nembutal on the ovulatory discharge of gonadotrophin in the cyclic rat. Proc. Soc. Exp. Biol. Med. 1949;71:696–698. doi: 10.3181/00379727-71-17303. [DOI] [PubMed] [Google Scholar]
- [37].Everett JW, Sawyer CH. A 24-hour periodicity in the “LH-release apparatus” of female rats, disclosed by barbiturate sedation. Endocrinology. 1950;47:198–218. doi: 10.1210/endo-47-3-198. [DOI] [PubMed] [Google Scholar]
- [38].Eyigor O, Lin W, Jennes L. Identification of neurones in the female rat hypothalamus that express oestrogen receptor-alpha and vesicular glutamate transporter-2. J. Neuroendocrinol. 2004;16:26–31. doi: 10.1111/j.1365-2826.2004.01109.x. [DOI] [PubMed] [Google Scholar]
- [39].Fester L, Ribeiro-Gouveia V, Prange-Kiel J, Schassen C. von, Böttner M, Jarry H, Rune GM. Proliferation and apoptosis of hippocampal granule cells require local oestrogen synthesis. J. Neurochem. 2006;97:1136–1144. doi: 10.1111/j.1471-4159.2006.03809.x. [DOI] [PubMed] [Google Scholar]
- [40].Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol. Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- [41].Filardo EJ, Quinn JA, Frackelton AR, Jr., Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol. Endocrinol. 2002;16:70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- [42].Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. 17beta-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J. Neurophysiol. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- [43].Franceschini D. Lomet, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neurosci. Lett. 2006;401:225–230. doi: 10.1016/j.neulet.2006.03.039. [DOI] [PubMed] [Google Scholar]
- [44].Fuenzalida LC, Keen KL, Terasawa E. Colocalization of FM1-43, Bassoon, and GnRH-1: GnRH-1 release from cell bodies and their neuroprocesses. Endocrinology. 2011;152:4310–4321. doi: 10.1210/en.2011-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Geraudie P, Hinfray N, Gerbron M, Porcher JM, Brion F, Minier C. Brain cytochrome P450 aromatase activity in roach (Rutilus rutilus): seasonal variations and impact of environmental contaminants. Aquat. Toxicol. 2011;105:378–384. doi: 10.1016/j.aquatox.2011.07.009. [DOI] [PubMed] [Google Scholar]
- [46].Glidewell-Kenney C, Weiss J, Hurley LA, Levine JE, Jameson JL. Estrogen receptor alpha signaling pathways differentially regulate gonadotropin subunit gene expression and serum follicle-stimulating hormone in the female mouse. Endocrinology. 2008;149:4168–4176. doi: 10.1210/en.2007-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Grabauskas G. Time course of GABA in the synaptic clefts of inhibitory synapses in the rostral nucleus of the solitary tract. Neurosci. Lett. 2005;373:10–15. doi: 10.1016/j.neulet.2004.09.051. [DOI] [PubMed] [Google Scholar]
- [48].Heimovics SA, Prior NH, Maddison CJ, Soma KK. Rapid and widespread effects of 17b-estradiol on intracellular signaling in the male songbird brain: a seasonal comparison. Endocrinology. 2012;153:1364–1376. doi: 10.1210/en.2011-1525. [DOI] [PubMed] [Google Scholar]
- [49].Herbison AE. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr. Rev. 1998;19:302–330. doi: 10.1210/edrv.19.3.0332. [DOI] [PubMed] [Google Scholar]
- [50].Herbison AE. Rapid actions of oestrogen on gonadotropin-releasing hormone neurons; from fantasy to physiology? J. Physiol. 2009;587:5025–5030. doi: 10.1113/jphysiol.2009.179838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Herbison AE, Pape JR. New evidence for estrogen receptors in gonadotropin-releasing hormone neurons. Front. Neuroendocrinol. 2001;22:292–308. doi: 10.1006/frne.2001.0219. [DOI] [PubMed] [Google Scholar]
- [52].Herman MA, Jahr CE. Extracellular glutamate concentration in hippocampal slice. J. Neurosci. 2007;27:9736–9741. doi: 10.1523/JNEUROSCI.3009-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hess DL, Wilkins RH, Moossy J, Chang JL, Plant TM, McCormack JT, Nakai Y, Knobil E. Estrogen-induced gonadotropin surges in decerebrated female rhesus monkeys with medial basal hypothalamic peninsulae. Endocrinology. 1977;101:1264–1271. doi: 10.1210/endo-101-4-1264. [DOI] [PubMed] [Google Scholar]
- [54].Higo S, Hojo Y, Ishii H, Kominami T, Nakajima K, Poirier D, Kimoto T, Kawato S. Comparison of sex-steroid synthesis between neonatal and adult rat hippocampus. Biochem. Biophys. Res. Commun. 2009;385:62–66. doi: 10.1016/j.bbrc.2009.05.005. [DOI] [PubMed] [Google Scholar]
- [55].Hojo Y, Hattori T, Enami T, Furukawa A, Suzuki K, Ishii H, Mukai H, Morrison JH, Janssen WGM, Kominami S, Harada N, Kimoto T. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017α and P450 aromatase localized in neurons. Proc. Nat. Acad. Sci. U. S. A. 2004;101:865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hojo Y, Higo S, Ishii H, Ooishi Y, Mukai H, Murakami G, Kominami T, Kimoto T, Honma S, Poirier D, Kawato S. Comparison between hippocampus-synthesized and circulation derived sex steroids in the hippocampus. Endocrinology. 2009;150:5106–5112. doi: 10.1210/en.2009-0305. [DOI] [PubMed] [Google Scholar]
- [57].Hojo Y, Murakami G, Mukai H, Higo S, Hatanaka Y, Ogiue-Ikeda M, Ishii H, Kimoto T, Kawato S. Estrogen synthesis in the brain--role in synaptic plasticity and memory. Mol. Cell. Endocrinol. 2008;290:31–43. doi: 10.1016/j.mce.2008.04.017. [DOI] [PubMed] [Google Scholar]
- [58].Hrabovszky E, Kalló I, Szlávik N, Keller E, Merchenthaler I, Liposits Z. Gonadotropin-releasing hormone neurons express estrogen receptor-beta. J. Clin. Endocrinol. Metab. 2007;92:2827–2830. doi: 10.1210/jc.2006-2819. [DOI] [PubMed] [Google Scholar]
- [59].Hrabovszky E, Shughrue PJ, Merchenthaler I, Hajszán T, Carpenter CD, Liposits Z, Petersen SL. Detection of estrogen receptor-beta messenger ribonucleic acid and 125I-estrogen binding sites in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2000;141:3506–3509. doi: 10.1210/endo.141.9.7788. [DOI] [PubMed] [Google Scholar]
- [60].Hrabovszky E, Steinhauser A, Barabás K, Shughrue PJ, Petersen SL, Merchenthaler I, Liposits Z. Estrogen receptor-beta immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2001;142:3261–3264. doi: 10.1210/endo.142.7.8176. [DOI] [PubMed] [Google Scholar]
- [61].Huang RQ, Dillon GH. Functional analysis of GABA(A) receptors in nucleus tractus solitarius neurons from neonatal rats. Brain Res. 2001;921:183–194. doi: 10.1016/s0006-8993(01)03117-1. [DOI] [PubMed] [Google Scholar]
- [62].Jakab RL, Horvath TL, Leranth C, Harada N, Naftolin F. Aromatase immunoreactivity in the rat brain: gonadectomy-sensitive hypothalamic neurons and an unresponsive “limbic ring” of the lateral septum-bed nucleus-amygdala complex. J. Steroid Biochem. Mol. Biol. 1993;44:481–498. doi: 10.1016/0960-0760(93)90253-s. [DOI] [PubMed] [Google Scholar]
- [63].Karavolas HJ, Herf SM. Conversion of progesterone by rat medial basal hypothalamic tissue to 5 -pregnane-3,20-dione. Endocrinology. 1971;89:940–942. doi: 10.1210/endo-89-3-940. [DOI] [PubMed] [Google Scholar]
- [64].Kawato S, Hojo Y, Kimoto T. Histological and metabolism analysis of P450 expression in the brain. Methods Enzymol. 2002;357:241–249. doi: 10.1016/s0076-6879(02)57682-5. [DOI] [PubMed] [Google Scholar]
- [65].Kelly MJ, Moss RL, Dudley CA. Differential sensitivity of the preoptic-septal neurons to microelectrophoresed estrogen during the estrous cycle. Brain Res. 1976;114:152–157. doi: 10.1016/0006-8993(76)91017-9. [DOI] [PubMed] [Google Scholar]
- [66].Kenealy BP, Guerriero KA, Keen KL, Terasawa E. Direct administration of estradiol benzoate to the stalk median eminence of female ovariectomized rhesus macaques induces a rapid increase in GnRH release In vivo. Abstracts of the 42nd Annual Meeting of the Society for Neuroscience (No. 278.18), held; New Orleans, LA. October 13-17, 2012. [Google Scholar]
- [67].Kenealy BP, Keen KL, Terasawa E. Rapid action of estradiol in primate GnRH neurons: The role of estrogen receptor alpha and estrogen receptor beta. Steroids. 2011;76:861–866. doi: 10.1016/j.steroids.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kenealy BP, Keen KL, Terasawa E. Role of intracellular and extracellular sources of calcium in the rapid estrogen action in primate GnRH neurons. Abstracts of the 41st Annual Meeting of the Society for Neuroscience (No. 500.08), held; Washington DC. November 12-16, 2011. [Google Scholar]
- [69].Kenealy BP, Keen KL, Rønnekleiv OK, Terasawa E. STX a novel non-steroidal estrogenic compound, induces rapid action in primate GnRH neuronal calcium dynamics and peptide release. Endocrinology. 2011;152:3182–3191. doi: 10.1210/en.2011-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].King JC, Rubin BS. Dynamic changes in LHRH neurovascular terminals with various endocrine conditions in adults. Horm. Behav. 1994;28:349–356. doi: 10.1006/hbeh.1994.1031. [DOI] [PubMed] [Google Scholar]
- [71].Knobil E. The neuroendocrine control of the menstrual cycle. Recent Prog. Horm. Res. 1980;36:53–88. doi: 10.1016/b978-0-12-571136-4.50008-5. [DOI] [PubMed] [Google Scholar]
- [72].Kondo K, Okuno T, Eguchi T, Yasui T, Suzuki H, Nagahama S, Saruta T. Vascular action of high dose estrogen in rats. Endocrinol. Jpn. 1980;27:307–313. doi: 10.1507/endocrj1954.27.307. [DOI] [PubMed] [Google Scholar]
- [73].Konkle AT, McCarthy MM. Developmental time course of estradiol, testosterone, and dihydrotestosterone levels in discrete regions of male and female rat brain. Endocrinology. 2011;152:223–235. doi: 10.1210/en.2010-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kordon C, Kerdelhué B, Pattou E, Jutisz M, Sawyer CH. Immunocytochemical localization of LHRH in axons and nerve terminals of the rat median eminence. Proc. Soc. Exp. Biol. Med. 1974;174:122–127. doi: 10.3181/00379727-147-38294. [DOI] [PubMed] [Google Scholar]
- [75].Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc. Natl. Acad. Sci. U.S.A. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kretz O, Fester L, Wehrenberg U, Zhou L, Brauckmann S, Zhao S, Prange-Kiel J, Naumann T, Jarry H, Frotscher M, Rune GM. Hippocampal synapses depend on hippocampal estrogen synthesis. J. Neurosci. 2004;24:5913–5921. doi: 10.1523/JNEUROSCI.5186-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Kuiper GG, Enmark E, Pelto-Huikko M, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. U.S.A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Kuo J, Hamid N, Bondar G, Prossnitz ER, Micevych P. Membrane estrogen receptors stimulate intracellular calcium release and progesterone synthesis in hypothalamic astrocytes. J. Neurosci. 2010;30:12950–12957. doi: 10.1523/JNEUROSCI.1158-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Kurian JR, Keen KL, Terasawa E. Epigenetic changes coincide with in vitro primate GnRH neuronal maturation. Endocrinology. 2010;151:5359–5368. doi: 10.1210/en.2010-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Levine JE, Norman RL, Gliessman PM, Oyama TT, Bangsberg DR, Spies HG. In vivo gonadotropin-releasing hormone release and serum luteinizing hormone measurements in ovariectomized, estrogen-treated rhesus macaques. Endocrinology. 1985;117:711–721. doi: 10.1210/endo-117-2-711. [DOI] [PubMed] [Google Scholar]
- [81].Leranth C, MacLusky NJ, Shanabrough M, Naftolin F. Catecholaminergic innervation of luteinizing hormone-releasing hormone and glutamic acid decarboxylase immunopositive neurons in the rat medial preoptic area. An electron-microscopic double immunostaining and degeneration study. Neuroendocrinology. 1988;48:591–602. doi: 10.1159/000125068. [DOI] [PubMed] [Google Scholar]
- [82].Leranth C, Shanabrough M, Naftolin F. Estrogen induces ultrastructural changes in progesterone receptor-containing GABA neurons of the primate hypothalamus. Neuroendocrinology. 1991;54:571–579. doi: 10.1159/000125962. [DOI] [PubMed] [Google Scholar]
- [83].Lebesgue D, Traub M, Butte-Smith M. De, Chen C, Zukin RS, Kelly MJ, Etgen AM. Acute administration of non-classical estrogen receptor agonists attenuates ischemia-induced hippocampal neuron loss in middle aged female rats. PLoS One. 2010;5:e8642. doi: 10.1371/journal.pone.0008642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Le Goascogne C, Robel P, Gouézou M, Sananès N, Baulieu EE, Waterman M. Neurosteroids: cytochrome P-450scc in rat brain. Science. 1987;237:1212–1215. doi: 10.1126/science.3306919. [DOI] [PubMed] [Google Scholar]
- [85].Lerma J, Herranz AS, Herreras O, Abraira V, Martin Del Rio R. In vivo determination of extracellular concentration of amino acids in the rat hippocampus. A method based on brain dialysis and computerized analysis. Brain Res. 1986;384:145–155. doi: 10.1016/0006-8993(86)91230-8. [DOI] [PubMed] [Google Scholar]
- [86].Li GL, Liu XC, Lin HR. Seasonal changes of serum sex steroids concentration and aromatase activity of gonad and brain in red-spotted grouper (Epinephelus akaara) Anim. Reprod. Sci. 2007 May;99(1-2):156–66. doi: 10.1016/j.anireprosci.2006.05.015. [DOI] [PubMed] [Google Scholar]
- [87].London SE, Boulter J, Schlinger BA. Cloning of the zebra finch androgen synthetic enzyme CYP17: a study of its neural expression throughout posthatch development. J. Comp. Neurol. 2003;467:496–508. doi: 10.1002/cne.10936. [DOI] [PubMed] [Google Scholar]
- [88].London SE, Clayton DF. Genomic and neural analysis of the estradiol-synthetic pathway in the zebra finch. BMC. Neurosci. 2010;11:46. doi: 10.1186/1471-2202-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].London SE, Monks DA, Wade J, Schlinger BA. Widespread capacity for steroid synthesis in the avian brain and song system. Endocrinology. 2006;147:5975–5987. doi: 10.1210/en.2006-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].London SE, Remage-Healey L, Schlinger BA. Neurosteroid production in the songbird brain: a re-evaluation of core principles. Front. Neuroendocrinol. 2009;30:302–314. doi: 10.1016/j.yfrne.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].MacLusky NJ, Luine VN, Hajszan T, Leranth C. The 17alpha and 17beta isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology. 2005;146:287–293. doi: 10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- [92].MacLusky NJ, Naftolin F, Goldman-Rakic PS. Estrogen formation and binding in the cerebral cortex of the developing rhesus monkey. Proc. Natl. Acad. Sci. U.S.A. 1986;83:513–516. doi: 10.1073/pnas.83.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Maeda K, Ohkura S, Uenoyama Y, Wakabayashi Y, Oka Y, Tsukamura H. H. Okamura H.Neurobiological mechanisms underlying GnRH pulse generation by the hypothalamus. Brain Res. 2010 Dec 10;1364:103–15. doi: 10.1016/j.brainres.2010.10.026. [DOI] [PubMed] [Google Scholar]
- [94].Mayer C, Acosta-Martinez M, Dubois SL, Wolfe A, Radovick S, Boehm U, Levine JE. Timing and completion of puberty in female mice depend on estrogen receptor alpha-signaling in kisspeptin neurons. Proc. Natl. Acad. Sci. U.S.A. 2010;107:22693–22698. doi: 10.1073/pnas.1012406108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].McCullough LD, Blizzard K, Simpson ER, Oz OK, Hurn PD. Aromatase cytochrome P450 and extragonadal estrogen play a role in ischemic neuroprotection. J. Neurosci. 2003;23:8701–8705. doi: 10.1523/JNEUROSCI.23-25-08701.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Mellon SH. Neurosteroids: biochemistry, modes of action, and clinical relevance. J. Clin. Endocrinol. Metab. 1994;78:1003–1008. doi: 10.1210/jcem.78.5.8175951. [DOI] [PubMed] [Google Scholar]
- [97].Mizuno M, Terasawa E. Search for neural substrates mediating inhibitory effects of oestrogen on pulsatile luteinising hormone-releasing hormone release in vivo in ovariectomized female rhesus monkeys (Macaca mulatta) J. Neuroendocrinol. 2005;17:238–245. doi: 10.1111/j.1365-2826.2005.01295.x. [DOI] [PubMed] [Google Scholar]
- [98].Mukai H, Kimoto T, Hojo Y, Kawato S, Murakami G, Higo S, Hatanaka Y, Ogiue-Ikeda M. Modulation of synaptic plasticity by brain estrogen in the hippocampus. Biochim. Biophys. Acta. 2010;1800:1030–1044. doi: 10.1016/j.bbagen.2009.11.002. [DOI] [PubMed] [Google Scholar]
- [99].Munetsuna E, Hattori M, Komatsu S, Sakimoto Y, Ishida A, Sakata S, Hojo Y, Kawato S, Yamazaki T. Social isolation stimulates hippocampal estradiol synthesis. Biochem. Biophys. Res. Commun. 2009;379:480–484. doi: 10.1016/j.bbrc.2008.12.076. [DOI] [PubMed] [Google Scholar]
- [100].Naftolin F, Horvath TL, Jakab RL, Leranth C, Harada N, Balthazart J. Aromatase immunoreactivity in axon terminals of the vertebrate brain. An immunocytochemical study on quail, rat, monkey, and human tissues. Neuroendocrinology. 1996;63:149–155. doi: 10.1159/000126951. [DOI] [PubMed] [Google Scholar]
- [101].Naftolin F, Ryan KJ, Petro Z. Aromatization of androstenedione by the diencephalons. J. Clin. Endocrinol. Metab. 1971;33:368–370. doi: 10.1210/jcem-33-2-368. [DOI] [PubMed] [Google Scholar]
- [102].Navarro CE, Saeed SA, Murdock C, Martinez-Fuentes AJ, Arora KK, Krsmanovic LZ, Catt KJ. Regulation of cyclic adenosine 3′,5′-monophosphate signaling and pulsatile neurosecretion by Gi-coupled plasma membrane estrogen receptors in immortalized gonadotrophin-releasing hormone neurons. Mol. Endocrinol. 2003;17:1792–1804. doi: 10.1210/me.2003-0040. [DOI] [PubMed] [Google Scholar]
- [103].Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E. Involvement of G-protein coupled receptor 30 (GPR30) in rapid action of estrogen in primate LHRH neurons. Mol. Endocrinol. 2008;23:349–359. doi: 10.1210/me.2008-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Norman RL, Spies HG. Cyclic ovarian function in a male macaque: additional evidence for a lack of sexual differentiation in the physiological mechanisms that regulate the cyclic release of gonadotropins in primates. Endocrinology. 1986;118:2608–2610. doi: 10.1210/endo-118-6-2608. [DOI] [PubMed] [Google Scholar]
- [105].Nyitrai G, Kékesi KA, Juhász G. Extracellular level of GABA and Glu: in vivo microdialysis-HPLC measurements. Curr. Top. Med. Chem. 2006;6:935–940. doi: 10.2174/156802606777323674. [DOI] [PubMed] [Google Scholar]
- [106].O’Byrne KT, Thalabard JC, Grosser PM, Wilson RC, Williams CL, Chen MD, Ladendorf D, Hotchkiss J, Knobil E. Radiotelemetric monitoring of hypothalamic gonadotropin-releasing hormone pulse generator activity throughout the menstrual cycle of the rhesus monkey. Endocrinology. 1991;129:1207–1214. doi: 10.1210/endo-129-3-1207. [DOI] [PubMed] [Google Scholar]
- [107].Ojeda SR, Ma YJ, Lee BJ, Prevot V. Glia-to-neuron signaling and the neuroendocrine control of female puberty. Recent Prog. Horm. Res. 2000;55:197–223. [PubMed] [Google Scholar]
- [108].Pasmanik M, Callard GV. Changes in brain aromatase and 5 alpha-reductase activities correlate significantly with seasonal reproductive cycles in goldfish (Carassius auratus) Endocrinology. 1988;122:1349–1356. doi: 10.1210/endo-122-4-1349. [DOI] [PubMed] [Google Scholar]
- [109].Peterson RS, Yarram L, Schlinger BA, Saldanha CJ. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proc. Bio. Sci. 2005;272:2089–2096. doi: 10.1098/rspb.2005.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Plant TM. A comparison of the neuroendocrine mechanisms underlying the initiation of the preovulatory LH surge in the human, Old World monkey and rodent. Front. Neuroendocrinol. 2012 Mar 3; doi: 10.1016/j.yfrne.2012.02.002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [111].Pintér O, Péczely P, Zsebok S, Zelena D. Seasonal changes in courtship behavior, plasma androgen levels and in hypothalamic aromatase immunoreactivity in male free-living European starlings (Sturnus vulgaris) Gen. Comp. Endocrinol. 2011;172:151–157. doi: 10.1016/j.ygcen.2011.02.002. [DOI] [PubMed] [Google Scholar]
- [112].Prange-Kiel J, Jarry H, Schoen M, Kohlmann P, Lohse C, Zhou L, Rune GM. Gonadotropin-releasing hormone regulates spine density via its regulatory role in hippocampal estrogen synthesis. J. Cell Biol. 2008;180:417–426. doi: 10.1083/jcb.200707043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Prevot V, Bellefontaine N, Baroncini M, Sharif A, Hanchate NK, Parkash J, Campagne C, de Seranno S. Gonadotrophin-releasing hormone nerve terminals, tanycytes and neurohaemal junction remodelling in the adult median eminence: functional consequences for reproduction and dynamic role of vascular endothelial cells. J. Neuroendocrinol. 2010;22:639–649. doi: 10.1111/j.1365-2826.2010.02033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Prevot V, Croix D, Rialas CM, Poulain P, Fricchione GL, Stefano GB, Beauvillain JC. Estradiol coupling to endothelial nitric oxide stimulates gonadotropin-releasing hormone release from rat median eminence via a membrane receptor. Endocrinology. 1999;140:652–659. doi: 10.1210/endo.140.2.6484. [DOI] [PubMed] [Google Scholar]
- [115].Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Rønnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J. Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Qiu J, Bosch MA, Tobias SC, Krust A, Graham SM, Murphy SJ, Korach KS, Chambon P, Scanlan TS, Rønnekleiv OK, Kelly MJ. A G-protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J. Neurosci. 2006;26:5649–5655. doi: 10.1523/JNEUROSCI.0327-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Qiu J, Rønnekleiv OK, Kelly MJ. Modulation of hypothalamic neuronal activity through a novel G-protein-coupled estrogen membrane receptor. Steroids. 2008;73:985–991. doi: 10.1016/j.steroids.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Radovick S, Ticknor CM, Nakayama Y, Notides AC, Rahman A, Weintraub BD, Cutler GB, Jr, Wondisford FE. Evidence for direct estrogen regulation of the human gonadotropin-releasing hormone gene. J. Clin. Invest. 1991;88:1649–1655. doi: 10.1172/JCI115479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Rance NE, Krajewski SJ, Smith MA, Cholanian M, Dacks PA. Neurokinin B and the hypothalamic regulation of reproduction. Brain Res. 2010;1364:116–128. doi: 10.1016/j.brainres.2010.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidy during social interactions. Nat. Neurosci. 2008;11:1327–1334. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Richter TA, Keen KL, Terasawa E. Synchronization of Ca(2+) oscillations among primate LHRH neurons and nonneuronal cells in vitro. J. Neurophysiol. 2002;88:1559–1567. doi: 10.1152/jn.2002.88.3.1559. [DOI] [PubMed] [Google Scholar]
- [122].Rohmann KN, Schlinger BA, Saldanha CJ. Subcellular compartmentalization of aromatase is sexually dimorphic in the adult zebra finch brain. Dev. Neurobiol. 2007;67:1–9. doi: 10.1002/dneu.20303. [DOI] [PubMed] [Google Scholar]
- [123].Romanò N, Lee K, Abrahám IM, Jasoni CL, Herbison AE. Nonclassical estrogen modulation of presynaptic GABA terminals modulates calcium dynamics in gonadotropin-releasing hormone neurons. Endocrinology. 2008;149:5335–5344. doi: 10.1210/en.2008-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Roselli CE, Horton LE, Resko JA. Time-course and steroid specificity of aromatase induction in rat hypothalamus-preoptic area. Biol. Reprod. 1987;37:628–633. doi: 10.1095/biolreprod37.3.628. [DOI] [PubMed] [Google Scholar]
- [125].Saitoh Y, Luchansky LL, Claude P, Terasawa E. Transplantation of the fetal olfactory placode restores reproductive cycles in female rhesus monkeys (Mucaca mulatta) bearing lesions in the medial basal hypothalamus. Endocrinology. 1995;136:2760–2769. doi: 10.1210/endo.136.6.7750501. [DOI] [PubMed] [Google Scholar]
- [126].Saldanha CJ, Remage-Healey L, Schlinger BA. Synaptocrine signaling: steroid synthesis and action at the synapse. Endocr. Rev. 2011;32:532–549. doi: 10.1210/er.2011-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Sar M, Sahu A, Crowley WR, Kalra SP. Localization of neuropeptide-Y immunoreactivity in estradiol-concentrating cells in the hypothalamus. Endocrinology. 1990;127:2752–2756. doi: 10.1210/endo-127-6-2752. [DOI] [PubMed] [Google Scholar]
- [128].Schally AV, Arimura A, Kastin AJ, Matsuo H, Baba Y, Redding TW, Nair RM, Debeljuk L, White WF. Gonadotropin-releasing hormone: one polypeptide regulates secretion of luteinizing and follicle-stimulating hormones. Science. 1971;173:1036–1038. doi: 10.1126/science.173.4001.1036. [DOI] [PubMed] [Google Scholar]
- [129].Schlinger BA, Callard GV. Localization of aromatase in synaptosomal and microsomal subfractions of quail (Coturnix coturnix japonica) brain. Neuroendocrinology. 1989;49:434–441. doi: 10.1159/000125149. [DOI] [PubMed] [Google Scholar]
- [130].Selmanoff MK, Brodkin LD, Weiner RI, Siiteri PK. Aromatization and 5alpha-reduction of androgens in discrete hypothalamic and limbic regions of the male and female rat. Endocrinology. 1977;101:841–848. doi: 10.1210/endo-101-3-841. [DOI] [PubMed] [Google Scholar]
- [131].Sétáló G, Vigh S, Schally AV, Arimura A, Flerkó B. LH-RH-containing neural elements in the rat hypothalamus. Endocrinology. 1975;96:135–142. doi: 10.1210/endo-96-1-135. [DOI] [PubMed] [Google Scholar]
- [132].Sharifi N, Reuss AE, Wray S. Prenatal LHRH neurons in nasal explant cultures express estrogen receptor beta transcript. Endocrinology. 2002;143:2503–2507. doi: 10.1210/endo.143.7.8897. [DOI] [PubMed] [Google Scholar]
- [133].Shivers BD, Harlan RE, Morrell JI, Pfaff DW. Absence of oestradiol concentration in cell nuclei of LHRH-immunoreactive neurons. Nature. 1983;304:345–347. doi: 10.1038/304345a0. [DOI] [PubMed] [Google Scholar]
- [134].Skinner DC, Dufourny L. Oestrogen receptor beta-immunoreactive neurons in the ovine hypothalamus: distribution and colocalisation with gonadotropin-releasing hormone. J. Neuroendocrinol. 2005;17:29–39. doi: 10.1111/j.1365-2826.2005.01271.x. [DOI] [PubMed] [Google Scholar]
- [135].Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146:2976–2984. doi: 10.1210/en.2005-0323. [DOI] [PubMed] [Google Scholar]
- [136].Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology. 1975;96:219–226. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
- [137].Soma KK, Schlinger BA, Wingfield JC, Saldanha CJ. Brain aromatase, 5 alpha-reductase, and 5 beta-reductase change seasonally in wild male song sparrows: relationship to aggressive and sexual behavior. J. Neurobiol. 2003;56:209–221. doi: 10.1002/neu.10225. [DOI] [PubMed] [Google Scholar]
- [138].Steimer T, Hutchison JB. Aromatization of testosterone within a discrete hypothalamic area associated with the behavioral action of androgen in the male dove. Brain Res. 1980;192:586–591. doi: 10.1016/0006-8993(80)90912-9. [DOI] [PubMed] [Google Scholar]
- [139].Sun J, Chu Z, Moenter SM. Diurnal in vivo and rapid in vitro effects of estradiol on voltage-gated calcium channels in gonadotropin-releasing hormone neurons. J. Neurosci. 2010;30:3912–3923. doi: 10.1523/JNEUROSCI.6256-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Szego CM, Davis JS. Adenosine 3′,5′-monophosphate in rat uterus: acute elevation by estrogen. Proc. Natl. Acad. Sci. U.S.A. 1967;58:1711–1718. doi: 10.1073/pnas.58.4.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Tata JR. One hundred years of hormones. EMBO Rep. 2005;6:490–496. doi: 10.1038/sj.embor.7400444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Temple JL, Laing E, Sunder A, Wray S. Direct action of estradiol on gonadotropin-releasing hormone-1 neuronal activity via a transcription-dependent mechanism. J. Neurosci. 2004;24:6326–6333. doi: 10.1523/JNEUROSCI.1006-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Temple JL, Wray S. Bovine serum albumin-estrogen compounds differentially alter gonadotropin-releasing hormone-1 neuronal activity. Endocrinology. 2005;146:558–563. doi: 10.1210/en.2004-1117. [DOI] [PubMed] [Google Scholar]
- [144].Terasawa E. Developmental changes in the positive feedback effect of estrogen on luteinizing hormone release in ovariectomized female rhesus monkeys. Endocrinology. 1985;117:2490–2497. doi: 10.1210/endo-117-6-2490. [DOI] [PubMed] [Google Scholar]
- [145].Terasawa E. Luteinizing hormone-releasing hormone (LHRH) neurons: mechanism of pulsatile LHRH release. Vitam. Horm. 2001;63:91–129. doi: 10.1016/s0083-6729(01)63004-8. [DOI] [PubMed] [Google Scholar]
- [146].Terasawa E, Quanbeck CD, Schulz CA, Burich AJ, Luchansky LL, Claude P. A primary cell culture system of luteinizing hormone releasing hormone neurons derived from embryonic olfactory placode in the rhesus monkey. Endocrinology. 1993;133:2379–2390. doi: 10.1210/endo.133.5.8404690. [DOI] [PubMed] [Google Scholar]
- [147].Terasawa E, Rodriguez-Sierra JF, Dierschke DJ, Bridson WE, Goy RW. Positive feedback effect of progesterone on luteinizing hormone (LH) release in cyclic female rhesus monkeys: LH response occurs in two phases. J. Clin. Endocrinol. Metab. 1980;51:1245–1250. doi: 10.1210/jcem-51-6-1245. [DOI] [PubMed] [Google Scholar]
- [148].Thomas P, Alyea R, Pang Y, Peyton C, Dong J, Berg AH. Conserved estrogen binding and signaling functions of the G protein-coupled estrogen receptor 1 (GPER) in mammals and fish. Steroids. 2010;75:595–602. doi: 10.1016/j.steroids.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES, Jr., Nethrapalli IS, Tinnikov AA. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J. Neurosci. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Wacker DW, Wingfield JC, Davis JE, Meddle SL. Seasonal changes in aromatase and androgen receptor, but not estrogen receptor mRNA expression in the brain of the free-living male song sparrow, Melospiza melodia morphna. J. Comp. Neurol. 2010;518:3819–3835. doi: 10.1002/cne.22426. [DOI] [PubMed] [Google Scholar]
- [151].Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J. Neurosci. 2010;30:3124–3132. doi: 10.1523/JNEUROSCI.5848-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Wehrenberg U, Prange-Kiel J, Rune GM. Steroidogenic factor-1 expression in marmoset and rat hippocampus: co-localization with StAR and aromatase. J. Neurochem. 2001;76:1879–1886. doi: 10.1046/j.1471-4159.2001.00207.x. [DOI] [PubMed] [Google Scholar]
- [153].Weick RF, Dierschke DJ, Karsch FJ, Butler WR, Hotchkiss J, Knobil E. Periovulatory time courses of circulating gonadotropic and ovarian hormones in the rhesus monkey. Endocrinology. 1973;93:1140–1147. doi: 10.1210/endo-93-5-1140. [DOI] [PubMed] [Google Scholar]
- [154].Wintermantel TM, Campbell RE, Porteous R, Bock D, Gröne HJ, Todman MG, Korach KS, Greiner E, Pérez CA, Schütz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Yagi K. Changes in firing rates of single preoptic and hypothalamic units following an intravenous administration of estrogen in the castrated female rat. Brain Res. 1973;53:343–352. doi: 10.1016/0006-8993(73)90219-9. [DOI] [PubMed] [Google Scholar]
- [156].Yamaji T, Dierschke DJ, Hotchkiss J, Bhattacharya AN, Surve AH, Knobil E. Estrogen induction of LH release in the rhesus monkey. Endocrinology. 1971;89:1034–1041. doi: 10.1210/endo-89-4-1034. [DOI] [PubMed] [Google Scholar]
- [157].Yang LC, Zhang QG, Zhou CF, Yang F, Zhang YD, Wang RM, Brann DW. Extranuclear estrogen receptors mediate the neuroprotective effects of estrogen in the rat hippocampus. PLoS One. 2010;5:e9851. doi: 10.1371/journal.pone.0009851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Yilmaz MB, Wolfe A, Cheng YH, Glidewell-Kenney C, Jameson JL, Bulun SE. Aromatase promoter I.f is regulated by estrogen receptor alpha (ESR1) in mouse hypothalamic neuronal cell lines. Biol. Reprod. 2009;81:956–965. doi: 10.1095/biolreprod.109.077206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Zhang C, Kelly MJ, Rønnekleiv OK. 17Beta-estradiol rapidly increases K(ATP) activity in GnRH via a protein kinase signaling pathway. Endocrinology. 2010;151:4477–4484. doi: 10.1210/en.2010-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Zhao C, Fujinaga R, M M. Tanaka, A Yanai, Nakahama K, Shinoda K. Region-specific expression and sex-steroidal regulation on aromatase and its mRNA in the male rat brain: immunohistochemical and in situ hybridization analyses. J. Comp. Neurol. 2007:557–573. doi: 10.1002/cne.21193. [DOI] [PubMed] [Google Scholar]