Abstract
The consumption of moderate amounts of cocoa products has been associated with reductions in the incidence of cardiovascular diseases. In animal studies, the flavanol (-)-epicatechin (Epi) yields cardioprotection. The effects may be partly due to its capacity to stimulate endothelial nitric oxide synthase (eNOS). The sustained activation of eNOS as observed with exercise, can serve as a trigger of muscle angiogenesis via the activation of VEGF related events. Experiments were pursued to examine the potential of Epi to stimulate myocardial angiogenesis and determine the effects that its combined use with exercise (Ex) may trigger. Hearts obtained from a previous study were used for this purpose. Animals received 1 mg/kg of Epi or water (vehicle) via oral gavage (twice daily). Epi and/or Ex (by treadmill) was provided for 15 days. Results indicate that Ex or Epi significantly stimulate myocardial angiogenesis by ~30% above control levels. The use of Epi-Ex lead to further significant increases (to ~50%). Effects were associated with increases in protein levels and/or activation of canonical angiogenesis pathway associated events (HIF1α, VEGF, VEGFR2, PI3K, PDK, AKT, eNOS, NO, cGMP, MMP-2/-9, Src-1 and CD31). Thus, the use of Epi may represent a safe and novel means to stimulate myocardial angiogenesis.
Keywords: angiogenesis, flavonoids, myocardium, ischemia
Introduction
Epidemiological evidence indicates a negative correlation between consumption of modest amounts of dark chocolate and incidence of cardiovascular diseases (CVD) including hypertension, atherosclerosis, stroke, myocardial infarction and heart failure [1-3]. Cacao seeds contain large amounts of flavonoids, in particular the subtype known as flavanols (up to 7% by weight) [4] . Main flavanols in cacao are catechins and epicatechins present in mono- or multimeric forms. Interest in the beneficial effects of flavanol consumption emerged from observations of Kuna Indians living off Panama. Kuna islanders have a very low incidence of CVD, in particular, hypertension. Studies attribute the low incidence of hypertension to the consumption of cacao and not other factors [5, 6]. The flavanol (-)-epicatechin (Epi) (and not other flavanols) appears to underlie the vasodilatory effects of cocoa as recently reported [7, 8].
We have examined the cardioprotective effects that Epi pre-treatment has in animal models of ischemia-reperfusion (I/R) [9] . The use of Epi at 1 mg/kg/day by gavage for 10 days prior to I/R led to notable reductions in infarct size (~50%) and the preservation of myocardial structure [9] . In a follow-up study, we reported on equivalent cardioprotective effects using a model of permanent coronary occlusion[10]. This first-in-kind result led us to evaluate possible mechanisms underlying Epi induced cardioprotection. It is well accepted that the activation of eNOS in the setting of myocardial ischemia can trigger cardioprotection [11] . In this regard, we have reported on the ability of Epi to acutely activate eNOS, stimulate the production of nitric oxide (NO) and trigger vasorelaxation related events in cultures of human coronary artery endothelial cells and in intact vessels [12, 13] . Thus, Epi induced activation of eNOS may partly explain the cardioprotective effects of the flavanol. However, the sustained administration of the flavanol as done in our studies likely triggers long-term eNOS/NO related events that may further contribute to reductions in infarct size.
It is well demonstrated that exercise training can condition tissues and/or organs such as the heart to become more resistant to ischemia [14] . Underlying mechanisms relate to the stimulation of angiogenesis via shear flow dependent effects on the vascular endothelial growth factor (VEGF)/eNOS/NO pathway [14]. The long-term consumption of the flavonoid resveratrol, has demonstrated cardioprotective effects and these actions have also been associated with VEGF/eNOS/NO stimulated angiogenesis [15]. However, no studies have examined the capacity of Epi to stimulate myocardial angiogenesis in spite of its reported capacity to activate eNOS/NO. It is thus, feasible that the long-term administration of Epi may favor the stimulation of cardiac angiogenesis via the activation of canonical or alternate pathways. Of interest, is the possibility that Epi effects on angiogenesis may be additive when combined with exercise.
Recently, we demonstrated in mice [16] that Epi increases fatigue resistance and oxidative capacity, as well as capillary density in skeletal muscle suggesting similar angiogenic effects in organs such as the heart. Thus, the major objectives of this study were to: 1) examine the capacity of Epi to stimulate cardiac angiogenesis and identify the signaling pathways associated with such responses, 2) contrast these effects to those triggered by exercise and, 3) examine the effects when both (Epi and exercise) are combined.
Materials and methods
Animals and Ethical Approval
We studied one year old, C57BL/6N male mice (N = 25; Harlan) that were randomized into four groups. Animals were placed four per cage and fed a standard diet without limitations. The room temperature was kept at 21°C with 12-hour light:dark cycles. All animal care and experimental procedures were approved by the University of California, San Diego Animal Care and Use Committee and conform to NIH and American Physiological Society standards.
Experimental Design and Approach
A between subjects design was used to determine the effects of Epi on cardiac angiogenesis of one year old mice. All animals performed an incremental treadmill test and then subsequently randomized into four groups: 1) water; 2) water-exercise (W-Ex); 3) (-)-epicatechin ((-)-Epi); and 4) (-)-epicatechin-exercise ((-)-Epi Ex). Groups 2 and 4 performed exercise on a rodent treadmill (model CL-4, Omnitech, Columbus, OH, USA) Monday through Friday during the study period. On the day after the final training session, all mice performed an incremental treadmill test. Approximately 48 h following the treadmill test, mice were sacrificed. Hearts were harvested and used for histological, biochemical and molecular analyses.
(-)-Epicatechin Administration
Mice in the Epi groups (3 and 4) were given 1 mg·kg of body mass−1 twice a day (morning and evening) for 15 consecutive days, whereas animals in the control groups (1 and 2) received the vehicle (water). Both Epi (Sigma-Aldrich, St. Louis, Missouri) and vehicle were administered via oral gavage.
Determination of (-)-Epicatechin in Plasma
At the time of sacrifice blood samples were obtained from all groups. Plasma samples were extracted as follows (1); 40 μl of vitamin C-EDTA solution (200 mg vitamin C and 1 mg EDTA in 1 ml dd-water) and 40 μl β-glucuronidase and sulphatase crude solution was added to plasma samples (400 μl). The mixture was incubated at 37°C for 45 min with intermittent mixing. Plasma was extracted with acetonitrile (1 ml) and centrifuged at 13,000 rpm for 5 min at 4 °C. The supernatant was transferred to a tube containing 100 mg alumina. Two washing steps were performed with 2 ml of Tris-HCl pH 7.0 buffer followed by a further wash with 2 ml methanol. Between each wash, the heterogeneous solution was centrifuged at 13,000 rpm for 5 min at 4°C and the supernatant was discarded. An aliquot of 250 μl of perchloric acid (0.25 mol/l) was added to the alumina, vortexed for 1 min and centrifuged at 13,000 rpm for 1 min at 4°C. The supernatant was transferred to another tube and the resulting solution was evaporated to dryness under high vacuum. Thereafter the dry solid was dissolved in 80 μl 5 % mobile phase B (95 % mobile phase A), centrifuged for 1 min, then 40 μl of the sample was loaded for analysis.
A Shiseido CAPCELL MG III C-18 column (Catalog number 92744, ID 2.0 mm x length 50mm) coupled to ThermoFinnigan LCQ deca mass spectrometer was employed (LC-MS) for analysis using positive ion mode atmospheric pressure chemical ionization (APCI) source. For LC separation, 2.5% acetonitrile in water with 0.1% trifluoroacetic acid (TFA) was used as mobile phase A, and 90% ACN with 0.1% TFA was used as mobile phase B. A gradient of 5% mobile phase B to 95% mobile phase B in 14 min was applied with a flow of 200 μl/min. The 200 μl/min LC eluent flow was then mixed with a 300 μl/min 50% methanol/50% water make-up flow before being delivered to the APCI source for MS analysis. Selected ion monitoring (SIM) mode was used to monitor the molecular ion peak of EPICA (m/z 291, [M+H]+) and internal standard (Chlortetracycline) (m/z 479, [M+H]+). Each unknown sample was spiked with 50 ng of internal standard, and 50% of the sample was loaded for LC-MS analysis to quantify the amount of Epi in each sample.
Incremental Treadmill Tests
On at least two occasions prior to the test all mice were familiarized with the treadmill (model CL-4, Omnitech, Columbus, OH) at a slow speed (≈5 m.min−1) at 10° incline for approximately 5-10 minutes. The incremental test consisted of warm up at 4 m.min−1 for two minutes followed by an increase of 2 m.min−1 every minute thereafter. A shock grid (0.2 milliamps) and air jets at the back of the treadmill were used to discourage the mice from stopping while the treadmill belt was moving. Exhaustion was determined when the mouse was no longer able to maintain its normal running position on the treadmill and/or was unwilling to run as indicated by the frequent contact (i.e., touching the shock grid with each stride) or sitting on the shock grid consistent with previous studies from our laboratory. For 15 days, mice in groups 2 and 4 underwent treadmill training at approximately 14 m.min−1 (50% of maximal treadmill speed) at 10° incline for 30-minutes five times per week. Results of this test were previously reported and will not be discussed (15).
Nitric oxide products and cGMP measurements
NO products (i.e., nitrite and nitrate anions; NOx) were measured as per instructions (Cayman chemicals) with some modifications. Briefly ~50 mg of myocardium was homogenized on dry ice in 0.5 ml of cold PBS (pH 7.4) and centrifuged (10,000 g) for 20 min at 4°C. Pellet was recovered to measure total protein content (Bradford Assay Bio-Rad) and supernatant was centrifuged (100,000 g) for 30 min at 4°C. Supernatant was recovered and filtered through 0.20 μm sterile syringe filter (Corning). Samples were ultrafiltered through 10 kDa molecular weight cut-off (Amicon ultra-0.5 10K Millipore), and centrifuged (10,000 g) for 15 min at 4°C. Total NOx were measured in eluted fraction in 96 well plate as per kit specifications.
To measure intracellular cGMP, 50 mg of myocardium was homogenized on dry ice in 0.25 ml of cold 5% trichloroacetic acid (TCA) in water plus 10 μM sildenafil (Sigma). The homogenate was centrifuged (1500 g) for 10 min at 4°C. The supernatant was transferred to a new test tube and TCA was removed using 1 ml of water-saturated ether via mixing. The top ether phase was discarded, and the TCA extraction was repeated 2 times. The remaining aqueous phase was heated at 70°C, 5 min, and cGMP was measured per instructions (Cayman chemicals).
Western Blotting
Approximately 50 mg of the heart were homogenized with a polytron in 500 μl lysis buffer (1% triton X-100, 20 mM Tris, 140 mM NaCl, 2 mM EDTA, and 0.1% SDS) with protease and phosphatase inhibitor cocktails (P2714 and P2850, Sigma-Aldrich, St. Louis, Missouri) supplemented with 0.15 mM PMSF, 5 mM Na3VO4 and 3 mM NaF. Homogenates were passed through an insulin syringe five times, sonicated for 30 min at 4°C and centrifuged (12,000 g) for 10 min at 4°C. The total protein content was measured in the supernatant using the Bradford method. A total of 40 μg of protein was loaded onto a 4% - 15% precast TGX polyacrylamide gel (BioRad), electrotransferred to a nitrocellulose membrane using a semidry system (12 V, 50 minutes) for low molecular weight proteins or using a miniprotean (70 V, 2 h) for high molecular weight proteins. The membranes were incubated for 1 h in blocking solution (5% nonfat dry milk in TBS plus 0.1% Tween 20 [TBS-T]), followed by a 3-h incubation at room temperature with primary mouse monoclonal antibodies. Antibodies used included HIF-1α, (Novus Biologicals, Inc.), VEGF, VEGFR1/2, (Abcam, Inc.), p-eNOS (Ser 617) (Upstate, Inc.), p-VGFR2 (Tyr 1054), PI3K, p-PI3K (Tyr458/Tyr199), pyruvate dehydrogenase kinase (PDK)1, p-PDK1 (Ser241), protein kinase B (AKT), p-AKT (Ser473), p-eNOS (Ser1177), heat shock protein 90 (HSP90), calmodulin1 (CaM1), caveolin-1 (Cav-1), p-Src (Tyr416), GAPDH (Cell Signaling, Inc.), eNOS, matrix metalloproteinase (MMP)-2, MMP-9 (AnaSpec, Inc.), steroid receptor coactivator-1 (Src-1) (Santa Cruz Biotechnology Inc.), CD31 (Cell Applications, Inc.). An antibody against total eNOS was used for the immunoprecipitation, for this assay we followed standard methodologies previously published (12). Membranes were washed (3X for 5 min) in TBS-T and incubated 1 h at room temperature in the presence of HRP-conjugated secondary antibodies (Cell Signaling, Inc.) diluted 1:10,000 in blocking solution. Membranes were again washed 3 times in TBS-T, and the immunoblots were developed using an ECL Plus detection kit (Amersham-GE). The band intensities were digitally quantified using ImageJ software (http://www.nih.gov).
Immunoprecipitation assays
Immunoprecipitation assays were performed as described previously [Ramirez-Sanchez et al 2012] with some modifications. Briefly, 100 mg of heart tissue were lysed with 250 μl of nondenaturing extraction buffer (0.5%, Triton X-100, 50 mmol/L Tris-HCl, pH 7.4, 0.15 mol/L NaCl, 0.5 mmol/L EDTA) and supplemented with protease and phosphatase inhibitor cocktail plus 0.1 mmol/L PMSF, 2 mmol/L Na3VO4 and 1 mmol/L NaF (Sigma). Homogenates were passed through an insulin syringe three times and incubated on ice with shaking for 25 min and centrifuged (15 min) at 12,000 x g at 4°C. A total of 0.5 mg protein was pre-cleared by adding 1 μg of normal rabbit IgG control and 20 μL protein A/G-agarose (Santa Cruz) and mixed for 30 min (4°C) with subsequent centrifugation at 12,000 x g for 10 min at 4°C. The supernatant was recovered and incubated at 4°C under mild agitation for 3 h with 10 μl of immunoprecipitating anti-eNOS (49G3) antibody (Cell Signaling). Twenty microliters of protein A/G-Sepharose was added, and the mixture was incubated overnight at 4°C with shaking. The immunoprecipitated mixture was centrifuged at 12,000 x g for 15 min at 4°C, and the supernatant was recovered and stored at 4°C for later analysis. The pellet was washed three times with extraction buffer under shaking 15 min and centrifuged at 12,000 x g for 15 min at 4°C. The immunoprecipitated proteins in the pellet and those remaining in the supernatant were applied to a precast 4% - 15% SDS-PAGE (Bio-rad) for immunoblotting.
Capillary staining
Sections from hearts from all four groups were stained using an antibody directed against CD-31 [17] and counterstained with hematoxylin. Heart crosssections (5 μm thick) obtained from similar regions of the free wall of the left ventricle, were viewed under a light microscope (40x magnification, Jenalumar, Zeiss, Jena, Germany), and digital images of the sections were recorded (Sony Model DXC-960MD, Sony Corp., Tokyo, Japan). Capillaries were quantified by a blinded individual using 10 randomly chosen digital images of the same size derived from 3 nonconsecutive sections of each heart.
Statistical Analyses
All data are presented as mean ± SEM from n=3-5 per group. Separate one-way ANOVA were performed to compare the relevant group means for each dependent variable. When appropriate post hoc Tukey’s HSD was used to determine which means were significantly different from each other. An α level at p ≤ 0.05 was selected for all statistical comparisons.
Results
Detection of plasma Epi concentration
Animals were treated for 2 weeks with 1 mg.kg body mass−1 of EPI or vehicle. Epi plasma levels were 0.67±0.38 μM in the Epi treated groups (Epi and Epi-Ex), but undetectable in both control group and exercise group.
Effects on heart capillarity
Figure 1A illustrates differences in heart capillary density observed amongst the 4 groups determined by histochemistry. As shown, exercise triggered an angiogenic effect (~30% above control) that was also induced to similar levels by Epi (~30%). When both were combined, the effects increased further (to ~50%) suggesting an additive outcome. In order to corroborate the increase in capillaries number, the presence of CD31 was analyzed in heart homogenates. CD31 protein levels were significantly increased (30-50%, Fig. 1B) on Ex, Epi and Epi-Ex groups.
Figure 1.
Effects of exercise and/or Epi on myocardial capillary density. A) Capillary density was assessed using microphotographs of sections obtained from hearts from water (W), exercise (Ex), epicatechin (Epi) and epicatechin plus exercise (Epi-Ex) groups, immunostained using an antibody against CD31 (as an endothelial marker) and counterstained with haematoxylin. Mean ± SE of at least 10 images (randomly chosen, 40X) from 3 nonconsecutive sections of 3-5 hearts per group. (B) CD31 levels were assessed by Western blots. *p < 0.05 vs. water; **significantly different from Ex or Epi.
Effects on known mediators/inducers of angiogenesis
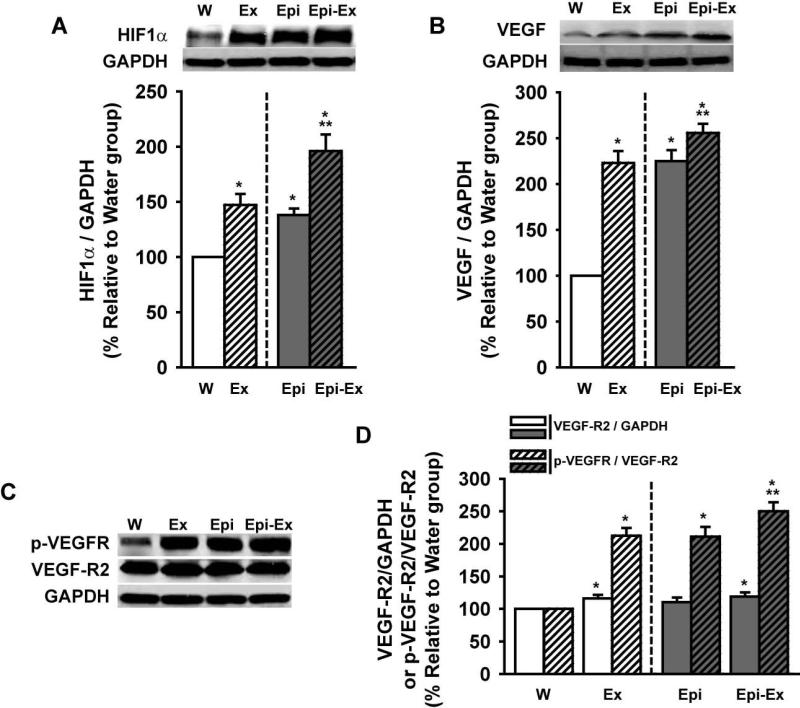
All Western blots assays were in the linear range. In Figure 2, the effects of exercise and/or Epi on HIF1α (Fig. 2A), VEGF (Fig. 2B) and VEGF-R2/p-VEGFR (Fig. 2C and 2D) levels are illustrated. Exercise and Epi alone induced significant increases in HIF1α protein levels (40-45%) and the effects increased further (i.e., additive effect) when combined (~90%). Exercise and Epi alone significantly increased VEGF protein levels (~125%) and these effects were enhanced (albeit to a modest degree) to ~160% when combined. Exercise alone and the combination of exercise + Epi induced a modest (~10%), but significant increase in VEGF-R2 protein levels. However, VEGF receptor phosphorylation (p-VEGFR) increased ~110% on either exercise or Epi groups and to ~150%, on Epi-Ex group.
Figure 2.
Effects of exercise and/or Epi on myocardial (A) Hypoxia inducible factor 1α HIF1α), (B) vascular endothelial growth factor (VEGF), (C, D) VEGF-R2 and p-VEGFR levels from water (W), exercise (Ex), epicatechin (Epi) and epicatechin plus exercise (Epi-Ex) groups. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control. Mean ± SE relative to W group (100%) values. *p < 0.05 vs. water; **significantly different from Ex or Epi.
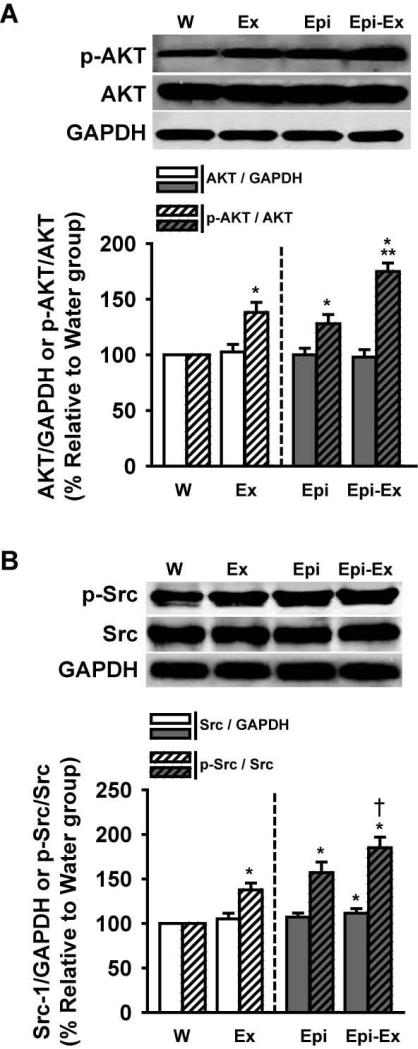
Exercise and/or Epi did not alter protein levels of PI3K (Fig. 3A) and PDK (Fig. 3B), but triggered significant increases in their phosphorylated states (50-55% for p-PI3K, Fig. 3A and 35-55% for p-PDK, Fig. 3B). Interestingly, p-PI3K was increased further on Epi-Ex group (~80%, Fig. 3A), but not for p-PDK (Fig. 3B). Figure 4A shows that protein levels of AKT were not changed in any of the groups vs. control, but p-AKT was increased on both Ex and Epi groups (30-40%) where the combination of both, yielded an apparent additive effect (~75%). Similarly, protein levels of Src were not changed with exercise or Epi (Fig. 4B). Interestingly, Src levels were significantly increased on Epi-ex group (~10%, Fig. 4B). The phosphorylation of Src (p-Src, Fig. 4B) increased with either exercise or Epi (40-60%) and was further enhanced with their combination (~80%).
Figure 3.
Effects of exercise and/or Epi on myocardial (A) Phosphoinositol kinase 3 (PI3K), p-PI3K and (B) PDK1, p-PDK1 levels from water (W), exercise (Ex), epicatechin (Epi) and epicatechin plus exercise (Epi-Ex) groups. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control. Mean ± SE relative to W group (100%) values. *p < 0.05 vs. water; **significantly different from Ex or Epi.
Figure 4.
Effects of exercise and/or Epi on myocardial (A) AKT, p-AKT and (B) Src, p-SRC levels from water (W), exercise (Ex), epicatechin (Epi) and epicatechin plus exercise (Epi-Ex) groups. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control. Mean ± SE relative to W group (100%) values. *p < 0.05 vs. water; **significantly different from Ex or Epi.
Effects on eNOS and nitric oxide products
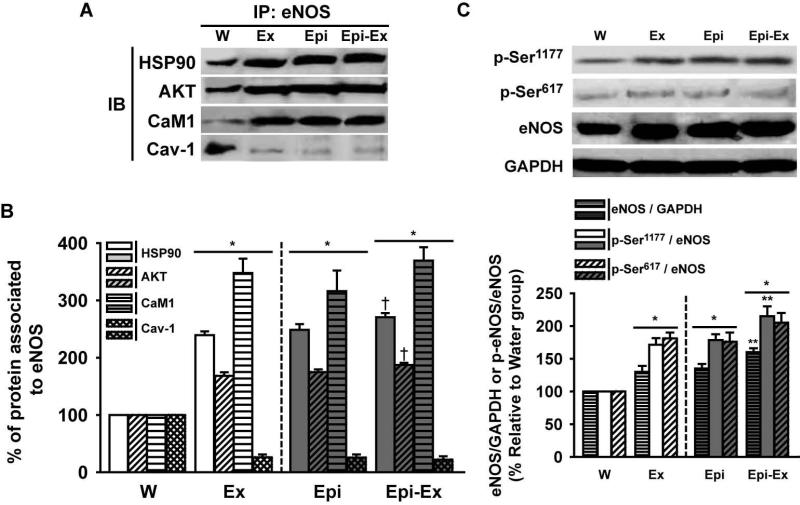
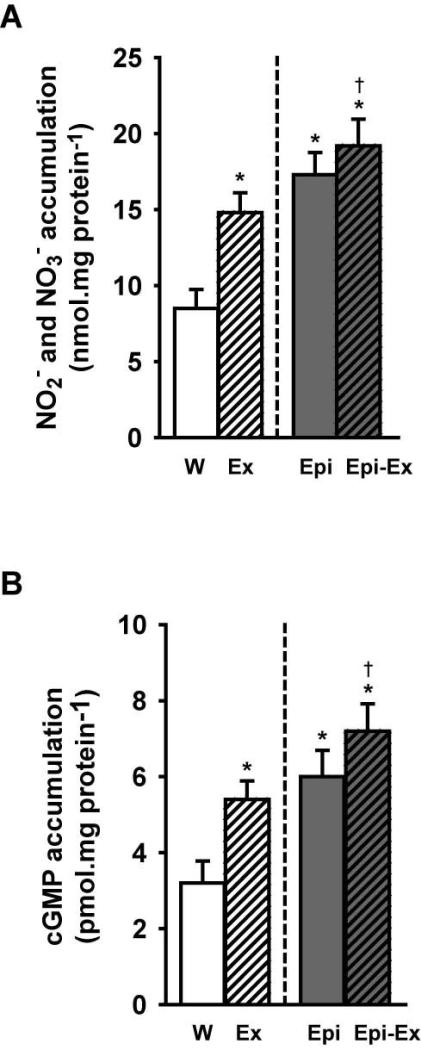
One of the downstream signaling pathways of angiogenesis is the activation of eNOS. Figures 5A and 5B illustrate the uncoupling that the signaling molecules HSP90, AKT and CaM1 have from Cav-1 (i.e., towards eNOS) when myocardium was stimulated by exercise and/or Epi. As observed, a significant activation and displacement of these molecules occurred (from the cell membrane to the cytoplasm) upon treatment. Furthermore, the protein levels of eNOS were significantly elevated in all groups compared to the control group (~30-40%; Fig. 5C), and the combination of Epi and exercise yielded an apparent additive effect (~65%). As well as for total eNOS, the treatments increased the phosphorylation status of eNOS on the residues Ser617 and Ser1177, which are correlated with eNOS enzymatic activation. Once both eNOS levels and activity were increased on either exercise or Epi or Epi-Ex groups, we decided to measure whether the production of nitric oxide was increased in these groups by measuring the accumulation of both NOx levels (NO −2 and NO −3, Fig. 6A) and cGMP (Fig. 6B). Exercise and Epi increased NOx (to 15-17 nmol/mg protein) and cGMP (5-6 pmol/mg protein) and the effects were modestly enhanced when combined (to ~19 nmol/mg protein and to ~7 pmol/mg protein, for NOx and cGMP, respectively).
Figure 5.
(A) (B) Effects of exercise and/or Epi on myocardial HSP-90, AKT, CAM1 and Cav-1 protein levels from water (W), exercise (Ex), epicatechin (Epi) and epicatechin plus exercise (Epi-Ex) groups. GAPDH was used as a loading. An antibody against total eNOS was used for the immunoprecipitation. (C) eNOS, p-Ser617, p-Ser1177 levels. Mean±SEM relative to W group (100%) values. *p < 0.05 vs. water; **significantly different from Ex or Epi, †significantly different vs. exercise only.
Figure 6.
Effects of exercise and/or Epi on myocardial (A) NOx and, (B) cGMP levels as assessed by bioassays from water (W), exercise (Ex), epicatechin (Epi) and epicatechin plus exercise (Epi-Ex) groups. Mean ± SE. *p < 0.05 vs. water; **significantly different from Ex or Epi, †significantly different vs. exercise only.
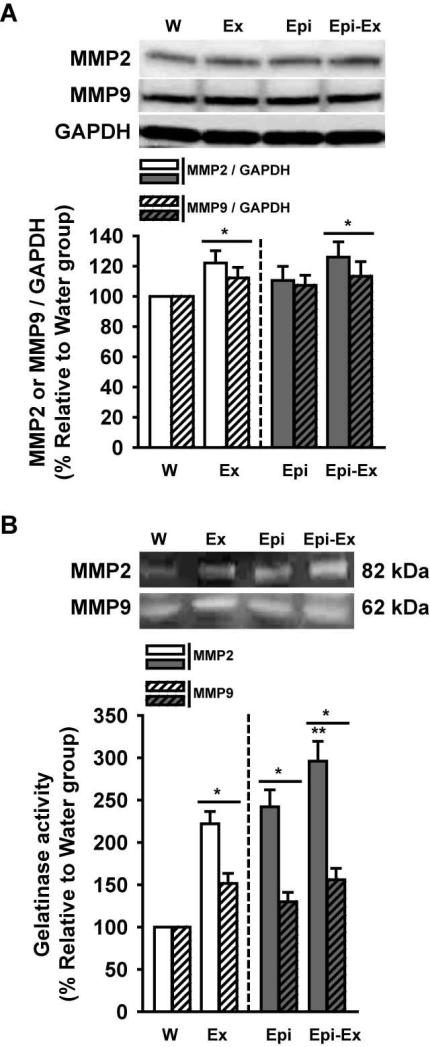
Effects on matrix metalloproteinases
MMPs play crucial roles in angiogenesis by allowing for tissue remodeling to occur. Thus, we assayed for changes in MMP protein abudance and activity for those deemed relevant. Figure 7 reports on the effects of exercise and/or Epi on MMP-2/-9 protein levels (Fig. 7A) and their activity (Fig. 7B). MMP-2 and MMP-9 protein levels (92 and 72 KDa respectively) increased in both exercise groups (Ex and Epi-Ex; 10-20%) 92 KDa forms. However, there was no change in the Epi group vs. control. Increases in MMP-2 activity levels (82 KDa form) were observed with exercise or Epi (125-150%) and demonstrated a greater effect when combined (~200%). MMP-9 activity levels (62 KDa form) increased with exercise and/or Epi to similar extents (40-50%). Zymography results suggest that most of the forms detected represented the non-zymogen (i.e. truncated and active) varieties of the enzymes.
Figure 7.
Effects of exercise and/or Epi on myocardial MMP-2 and MMP-9 protein (A) or activity (B) levels as assessed by Western blots and gelatin zymography, respectively. From water (W), exercise (Ex), epicatechin (Epi) and epicatechin plus exercise (Epi-Ex) groups. GAPDH was used as a loading control in the Western blot analysis. Mean ± SE relative to W group (100%) values. *p < 0.05 vs. water; **significantly different from Ex or Epi.
Discussion
As evidence accumulates as to the potential health benefits of moderate chocolate consumption on cardiovascular morbidity and mortality a need arises as to identify the bioactive component(s) responsible for such effects and their mechanisms of action.
In this study we evaluated the effects of the most abundant cacao flavanol Epi on myocardial angiogenesis and related signaling pathways. Unique results demonstrate that Epi has the capacity to stimulate myocardial angiogenesis in a magnitude similar to that generated by moderate exercise training. The combined use of exercise and Epi triggered an enhanced angiogenesis response which suggests that the effects at the doses and/or duration of Epi or levels of training tested can act in a non-overlapping, complementary fashion. Signaling responses noted with Epi treatment paralleled those triggered by exercise and were further reinforced when combined. The nature of downstream responses observed suggest that Epi effects on angiogenesis are mediated at least in part, through canonical “physiological” signaling pathways.
There are multiple disease conditions where a vigorous and sustained angiogenesis response stimulated using safe, economical and non-invasive approaches would be desirable as a means to alleviate ischemic conditions. In spite of the simplicity of the idea, the translation to patients with heart disease has proven problematic. Studies have been pursued using VEGF and other cytokines as a means to stimulate myocardial angiogenesis (for review see[18]). So far, no large clinical trial has shown substantial and sustained benefit for ischemic conditions, in spite of supportive preclinical data. Thus, the area of therapeutic angiogenesis remains identified as an unmet medical need [19].
There are multiple reports demonstrating that endurance exercise training enhances the distribution of blood flow through the myocardium (for review, see[20]). The effects of endurance training can be observed in both normal [21, 22] and in failing post-infarcted hearts [23]. In 4 week post-infarcted failing hearts, 10 weeks of treadmill exercise training led to increases in capillary and arteriole formation which coupled to increases in myocardial blood flow, coronary reserve and conductance and decreases in resistance [23]. A hallmark study on exercise training effects on heart vascularization [22] demonstrated that 3 weeks of intense endurance training induces a significant increase in myocardial capillaries which was followed by increases in arteriole density and total vascular bed cross-sectional area (increased by up to 37% after 16 weeks). Interestingly, the period of time that the animals from the present study were submitted to exercise training and/or Epi (i.e., 2 weeks) is within the period of time that demonstrated to increase capillary density in myocardium in response to training [22]. However, it remains to be determined if continued exposure to Epi treatment also lead to increases in the development of arterioles as seen with training [22]. Recently, our group reported that 15 days of Epi treatment enhances treadmill exercise performance in mice, which led to increased skeletal muscle capillarity [16]. In this study, we observed that exercise training increased plantaris muscle capillary density by ~17%, but increased ~56% with Epi and ~94% when combined. Thus, there are striking differences in the responses generated by each of the treatments and suggest greater degrees of angiogenic plasticity in skeletal muscle when compared to myocardium. Although skeletal muscle aerobic capacity and peripheral oxygen diffusion are limiting factors for aerobic performance, cardiac output also plays an important role during exercise [24]. It is thus also possible, that enhanced exercise performance after Epi treatment was also a result of increased myocardial capillarity allowing for greater cardiac blood flow. As pointed above, the results from the present work suggest that Epi and exercise yield similar effects of cardiac capillarization. Thus, the use of Epi could potentially be seen as a new way to promote myocardial vascularization in situations in which cardiac perfusion is diminished and the practice of exercise is limited or restricted.
It has been suggested that endothelial cell generated NO plays a critical role in capillary development [25, 26]. The binding of VEGF to its receptor VEGFR-2 results in an autophosphorylation of a tyrosine residue which leads to NO production [27]. Thus, there is an intricate relationship between eNOS, VEGF and angiogenesis. Studies examining the effects of Epi on VEGF and downstream molecules on skeletal or cardiac muscle are so far, nonexistent. However, a large body of data has been accumulating on the effects of high flavanol chocolate on vascular function evidencing vasodilating and antihypertensive effects (for review see [4]). High flavanol chocolate and Epi can stimulate vasodilation in an NO dependent manner [8]. Recent studies have also reported on the effects of Epi on human coronary artery endothelial cell eNOS phosphorylation and NO production evidencing the possible existence of a G-protein associated cell membrane Epi receptor [12]. In the present investigation, we observed increases in VEGF and VEGF-R2 protein expression in animals treated with Epi. We also observed that p-VEGF-R2 was ~2-fold greater in the Epi group. It should be noted, that for both VEGF and p-VEGF-R2 increases in the Epi only group were similar to those seen in the exercise only group. The combination of exercise and Epi treatment increased VEGF protein expression above and beyond the Epi only group which was not seen for the VEGF receptor. A downstream consequence of these events was the noted increases in the phosphorylation levels of eNOS activation residues with Epi as well as with exercise. These findings again, suggest that Epi may activate similar VEGF signaling pathways as those from exercise.
In this study we also evaluated “upstream” signaling pathways associated with angiogenesis including the kinases PI3K, PDK, AKT, Src and the signaling molecule CaM [28]. For PI3K, PDK, AKT and Src, no changes in proteins levels were observed. However, the phosphorylation of these proteins (which typically reflects their activation status) increased to similar magnitudes in the exercise and Epi groups. With the exception of PDK, essentially additive effects on phosphorylation status were observed when combined treatment was used. As expected these events led to increases in NOX (an indirect measure of NO levels) and cGMP. Also critical to the angiogenesis process, is the degradation of surrounding extracellular matrix by MMPs so as to allow for sprouting to occur [28]. Whereas exercise and combination treatment increased MMP-2 and -9 protein and activity levels, Epi only increased the activity of the enzymes. Thus, there appears to be a distinct effect of Epi on MMP activity that may be related with a limited angiogenic effect.
Integrating published evidence with our results illustrates a possible scheme of signaling events associated with the pro-angiogenic actions of Epi and exercise (Figure 8). In the case of exercise, the upstream triggering event is likely associated with mechanotransduction whereas that of Epi may involve the binding to receptors [13]. We and others have generated indirect evidence for the existence of Epi receptors. The data suggests that GPCR like molecules are involved. This, field can be considered as emerging and is likely to provide additional direct and indirect evidence for the involvement of such molecules in the near future.
Figure 8.
Possible pathways associated with exercise and/or Epicatechin angiogenic induced effects in tissues/organs
There is precedent for the stimulatory effects of other flavonoids or drugs on angiogenesis. Most of the results generated so far on flavonoids, are derived from the use of resveratrol. In 2005, Kaga et al reported on resveratrol enhanced myocardial angiogenesis both in vivo and in vitro via VEGF dependent pathways [29]. Human coronary artery endothelial cells exposed to resveratrol demonstrated significantly accelerated tubular morphogenesis. Rats treated with resveratrol (1 mg/kg/day) for 14 days and subsequently undergoing a permanent coronary artery occlusion demonstrated reduced infarct size, improved function and increased capillary density of ~40% in peri-infarct myocardium. In a subsequent study, the stimulatory effects of resveratrol on infarcted myocardium were associated with the upregulation of eNOS [15]. Thus, the magnitude of changes in capillarity reported for resveratrol effects are comparable to those we report in this study with exercise and/or Epi. Other examples include statins which are a class of drugs that have demonstrated to exert pleiotropic actions and can yield cardioprotective outcomes unrelated to their cholesterol lowering effects [30]. Outcomes from clinical and experimental studies suggest that statins (as Epi) have the ability to stimulate eNOS as well as angiogenesis [10] . However, no clear consensus has emerged as to their possible use as a therapeutic modality to stimulate angiogenesis. Erythropoietin, is a growth factor known for its pleiotropic properties that has also been evaluated for its angiogenic potential [31]. However, the latest data generated from human trials using erythropoietin has cast a wide shadow over its potential to be used in indications beyond those to promote red blood cell production [32]. Thus, beyond exercise per se, the use of the above mentioned molecules to alleviate ischemic conditions via angiogenesis remains unproven. It should be noted that flavonoids have also been reported to inhibit angiogenesis, particular in the setting of tumor growth. Such actions while apparently contrasting with those reported by us and others may be explain by the unusual angiogenic control that tumors typically express and the fact that flavonoids used for those purposes typically are provided at concentrations far higher than the used in this study. Thus, there is the possibility that dual effects can be exerted on the basis of the doses used.
The functional implications of the effects of Epi on myocardial angiogenesis are difficult to quantify in particular, as this molecule is pleiotropic. However, one can reasonably surmise that the cardioprotective effects demonstrated by the use of Epi pre-treatment in rodent models of I/R and permanent coronary occlusion are partly related to myocardial angiogenesis [9, 10]. It is also difficult to determine if in the long run, Epi induced angiogenesis may lead to adverse secondary effects. Arguing against this point of view is the accumulating body of evidence indicating that the consumption of flavanol-rich chocolate is associated with a significant reduction for cardiovascular risks [1]. A limitation of this study is the lack of an extensive dose-response design using Epi and/or its combination with several levels or types of exercise such as swimming. Thus, additional work is necessary in order to provide more insight into possible physiological, structural and molecular interactions between Epi and exercise.
Conclusion
We report for the first time that myocardial angiogenic potential of Epi and contrast its capacity to stimulate capillary formation with exercise. Responses triggered by Epi appear to largely parallel those of exercise but given their additive character it suggests that Epi may also act through distinct upstream receptors and mediators. Well-structured clinical trials should be considered where the angiogenic potential of Epi-rich chocolate and/or pure Epi is examined for its ability to promote new blood vessel formation.
Acknowledgements
We would like to thank Brianna Potter and Taylor Coe for their technical assistance.
Funding sources This study was supported by a contract to Dr. M. Malek with Cardero Therapeutics Inc., NIH AT4277, HL43617, P60-MD000220 grants to Dr. Villarreal. Dr. Taub was supported by an American College of Cardiology/Merck Fellowship. Part of the work was performed at the National Center for Microscopy and Imaging Research supported by NIH RR004050.
Footnotes
Disclosures Drs. Francisco Villarreal and Pam Taub are co-founders of Cardero Therapeutics, Inc.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Buitrago-Lopez A, et al. Chocolate consumption and cardiometabolic disorders: systematic review and meta-analysis. BMJ. 2011;343:d4488. doi: 10.1136/bmj.d4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mostofsky E, et al. Chocolate intake and incidence of heart failure: a population-based prospective study of middle-aged and elderly women. Circ Heart Fail. 2010;3(5):612–6. doi: 10.1161/CIRCHEARTFAILURE.110.944025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher ND, Hollenberg NK. Flavanols for cardiovascular health: the science behind the sweetness. J Hypertens. 2005;23(8):1453–9. doi: 10.1097/01.hjh.0000174605.34027.9d. [DOI] [PubMed] [Google Scholar]
- 4.Corti R, et al. Cocoa and cardiovascular health. Circulation. 2009;119(10):1433–41. doi: 10.1161/CIRCULATIONAHA.108.827022. [DOI] [PubMed] [Google Scholar]
- 5.McCullough ML, et al. Hypertension, the Kuna, and the epidemiology of flavanols. J Cardiovasc Pharmacol. 2006;47(Suppl 2):S103–9. doi: 10.1097/00005344-200606001-00003. discussion 119-21. [DOI] [PubMed] [Google Scholar]
- 6.Heiss C, et al. Improvement of endothelial function with dietary flavanols is associated with mobilization of circulating angiogenic cells in patients with coronary artery disease. J Am Coll Cardiol. 2010;56(3):218–24. doi: 10.1016/j.jacc.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 7.Ottaviani JI, et al. The stereochemical configuration of flavanols influences the level and metabolism of flavanols in humans and their biological activity in vivo. Free Radic Biol Med. 2011;50(2):237–44. doi: 10.1016/j.freeradbiomed.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Schroeter H, et al. (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci U S A. 2006;103(4):1024–9. doi: 10.1073/pnas.0510168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamazaki KG, et al. Short- and long-term effects of (-)-epicatechin on myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2008;295(2):H761–7. doi: 10.1152/ajpheart.00413.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamazaki KG, et al. Effects of (-)-epicatechin on myocardial infarct size and left ventricular remodeling after permanent coronary occlusion. J Am Coll Cardiol. 2010;55(25):2869–76. doi: 10.1016/j.jacc.2010.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones SP, Bolli R. The ubiquitous role of nitric oxide in cardioprotection. J Mol Cell Cardiol. 2006;40(1):16–23. doi: 10.1016/j.yjmcc.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Ramirez-Sanchez I, et al. (-)-epicatechin activation of endothelial cell endothelial nitric oxide synthase, nitric oxide, and related signaling pathways. Hypertension. 2010;55(6):1398–405. doi: 10.1161/HYPERTENSIONAHA.109.147892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramirez-Sanchez I, et al. (-)-Epicatechin induces calcium and translocation independent eNOS activation in arterial endothelial cells. Am J Physiol Cell Physiol. 2011;300(4):C880–7. doi: 10.1152/ajpcell.00406.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bloor CM. Angiogenesis during exercise and training. Angiogenesis. 2005;8(3):263–71. doi: 10.1007/s10456-005-9013-x. [DOI] [PubMed] [Google Scholar]
- 15.Fukuda S, et al. Resveratrol ameliorates myocardial damage by inducing vascular endothelial growth factor-angiogenesis and tyrosine kinase receptor Flk-1. Cell Biochem Biophys. 2006;44(1):43–9. doi: 10.1385/CBB:44:1:043. [DOI] [PubMed] [Google Scholar]
- 16.Nogueira L, et al. (-)-Epicatechin Enhances Fatigue Resistance and Oxidative Capacity in Mouse Muscle. J Physiol. 2011 doi: 10.1113/jphysiol.2011.209924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ismail JA, et al. Immunohistologic labeling of murine endothelium. Cardiovasc Pathol. 2003;12(2):82–90. doi: 10.1016/s1054-8807(02)00166-7. [DOI] [PubMed] [Google Scholar]
- 18.Zachary I, Morgan RD. Therapeutic angiogenesis for cardiovascular disease: biological context, challenges, prospects. Heart. 2011;97(3):181–9. doi: 10.1136/hrt.2009.180414. [DOI] [PubMed] [Google Scholar]
- 19.Banquet S, et al. Arteriogenic therapy by intramyocardial sustained delivery of a novel growth factor combination prevents chronic heart failure. Circulation. 2011;124(9):1059–69. doi: 10.1161/CIRCULATIONAHA.110.010264. [DOI] [PubMed] [Google Scholar]
- 20.Hudlicka O, Brown M, Egginton S. Angiogenesis in skeletal and cardiac muscle. Physiol Rev. 1992;72(2):369–417. doi: 10.1152/physrev.1992.72.2.369. [DOI] [PubMed] [Google Scholar]
- 21.Gigante B, et al. Placenta growth factor is not required for exercise-induced angiogenesis. Angiogenesis. 2004;7(3):277–84. doi: 10.1007/s10456-004-4179-1. [DOI] [PubMed] [Google Scholar]
- 22.White FC, et al. Exercise training in swine promotes growth of arteriolar bed and capillary angiogenesis in heart. J Appl Physiol. 1998;85(3):1160–8. doi: 10.1152/jappl.1998.85.3.1160. [DOI] [PubMed] [Google Scholar]
- 23.Leosco D, et al. Exercise promotes angiogenesis and improves beta-adrenergic receptor signalling in the post-ischaemic failing rat heart. Cardiovasc Res. 2008;78(2):385–94. doi: 10.1093/cvr/cvm109. [DOI] [PubMed] [Google Scholar]
- 24.Wagner PD. Determinants of maximal oxygen transport and utilization. Annu Rev Physiol. 1996;58:21–50. doi: 10.1146/annurev.ph.58.030196.000321. [DOI] [PubMed] [Google Scholar]
- 25.Morbidelli L, et al. Nitric oxide mediates mitogenic effect of VEGF on coronary venular endothelium. Am J Physiol. 1996;270(1 Pt 2):H411–5. doi: 10.1152/ajpheart.1996.270.1.H411. [DOI] [PubMed] [Google Scholar]
- 26.Ziche M, et al. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest. 1994;94(5):2036–44. doi: 10.1172/JCI117557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 28.Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100(6):782–94. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- 29.Kaga S, et al. Resveratrol enhances neovascularization in the infarcted rat myocardium through the induction of thioredoxin-1, heme oxygenase-1 and vascular endothelial growth factor. J Mol Cell Cardiol. 2005;39(5):813–22. doi: 10.1016/j.yjmcc.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Elewa HF, et al. Diverse effects of statins on angiogenesis: new therapeutic avenues. Pharmacotherapy. 2010;30(2):169–76. doi: 10.1592/phco.30.2.169. [DOI] [PubMed] [Google Scholar]
- 31.Jaquet K, et al. Erythropoietin and VEGF exhibit equal angiogenic potential. Microvasc Res. 2002;64(2):326–33. doi: 10.1006/mvre.2002.2426. [DOI] [PubMed] [Google Scholar]
- 32.Najjar SS, et al. Intravenous erythropoietin in patients with ST-segment elevation myocardial infarction: REVEAL: a randomized controlled trial. JAMA. 2011;305(18):1863–72. doi: 10.1001/jama.2011.592. [DOI] [PMC free article] [PubMed] [Google Scholar]