Abstract
Our knowledge of membrane estradiol signaling mechanisms and their interactions that regulate physiology and behavior has grown rapidly over the past three decades. The discovery of novel membrane estrogen receptors and their signaling mechanisms has started to reveal the complex timing and interactions of these various signaling mechanisms with classical genomic steroid actions within the nervous system to regulate physiology and behavior. The activation of the various estrogenic signaling mechanisms is site specific and differs across the estrous cycle acting through both classical genomic mechanisms and rapid membrane-initiated signaling to coordinate reproductive behavior and physiology. This review focuses on our current understanding of estrogenic signaling mechanisms to promote: 1) sexual receptivity within the arcuate nucleus of the hypothalamus, 2) estrogen positive feedback that stimulates de novo neuroprogesterone synthesis to trigger the luteinizing hormone surge important for ovulation and estrous cyclicity, and 3) alterations in energy balance.
Keywords: Estrogen Receptor, membrane estradiol receptors, lordosis, feeding, LH surge, neuroprogesterone
INTRODUCTION
In females, reproduction and energy balance are regulated by the fluctuation of the ovarian hormones estradiol and progesterone across the estrous/menstrual cycle [36, 134, 215, 387, 388]. The hormones of the hypothalamic pituitary gonadal (HPG) axis are important for coordinating ovulation, uterine endometrium development and reproductive behavior to maximize the possibility that copulation results in pregnancy. The HPG axis regulates the release of ovarian hormones through both negative and positive feedback mechanisms. Through diestrous day 1 and day 2 of the rat estrous cycle follicle stimulating hormone (FSH) and luteinizing hormone (LH) released from the pituitary stimulate ovarian follicle maturation, development and steroidogenesis. This produces slowly rising circulating estradiol levels regulated by steroid-induced negative feedback on the HPG axis. However, on proestrus estradiol levels rapidly rise as a part of the positive feedback mechanism and peak on the afternoon of proestrus. This rise in estradiol levels is essential for the induction of the luteinizing hormone surge that induces ovulation and luteinization of the follicular cells and production of progesterone. The sequential release of estradiol and progesterone acts simultaneously in reproductive neurocircuits and the organs of the reproductive tract coordinating reproductive behavior with the preparation of the uterine endometrium for copulation and subsequent implantation of the embryo. In addition to reproduction and reproductive behaviors, these hormones modulate other behaviors as well. For example, in conjunction with increased motivation to seek copulation, general activity is increased [105] and anxiety [84] and nociception is reduced. Further, behaviors associated with food intake and energy balance are modulated as well [36, 75, 134, 215, 317, 387, 388, 432].
Because the physiological and behavioral effects of these hormones seemed to require hours and days to be realized, as was the loss of function or behavior after castration, for decades the prevailing thought was that these hormones acted slowly over longer periods of time to alter the activity of neurons and neural circuits [24, 25]. To mediate the actions of steroid hormones and other molecules of communication, the receptor hypothesis was put forth. The receptor hypothesis stated that contained in target tissues were molecules that specifically interacted with hormones and in turn the biological activity of the receptor molecules was altered to change cellular activity. For estradiol, this hypothesis was supported by the findings of Glasscock and Hoekstra [131] and Jensen and Jacobson [164] demonstrating that the uterus and vagina of the sheep, goat and rat specifically concentrated 3H-estradiol that was given subcutaneously. The slower estradiol regulation of systems was bolstered by estrogen receptors (ER) and other steroid receptors being localized in the cytoplasm and translocated into the nucleus [32, 34, 129, 136, 184, 218, 300, 355, 398]. The discovery that two cytosolic/nuclear ER were present gave rise to the initial naming of ERα and ERβ [130]. Additionally, estradiol regulation of protein expression, such as progesterone receptor [33, 217, 218, 251], as well as numerous enzymes, neurotransmitters and cognate receptors [92, 213, 214, 312] provided support of this notion. Further, local infusions of either RNA transcription or protein synthesis inhibitors attenuated estradiol and estradiol and progesterone facilitation of sexual behavior [230, 231, 308, 311, 313]. Thus, these studies indicated that ER regulated genomic transcription. This was later confirmed when ER were demonstrated to be transcription factors that homodimerize to interact with estrogen response elements [26, 138, 139, 163, 190, 191, 419].
For decades the actions of estradiol were thought to be predominately through the slower estrogen response element mediated transcriptional mechanisms, even though during this same time period rapid actions of estradiol were being demonstrated in both peripheral and neural tissues [48, 182, 383, 430, 431]. For example, in uterine tissue estradiol increased cAMP production within seconds [383]. Furthermore, depending on the application, estradiol could increase or decrease neuronal activity within seconds to minutes indicating rapid regulation of neuronal activity [48, 182, 430, 431]. A small fraction of investigators continued to investigate the rapid actions of estradiol on neuronal function during this time [97, 180, 183, 257, 263, 393]. Additionally, studies in peripheral tissues continued to demonstrate rapid nongenomic actions of steroids [314] through Ca2+ mechanisms in granulosa cells [254, 352], endometrial cells [294-296] and oocytes [59, 391, 392]. Binding of estradiol and other steroids was demonstrated on synaptic plasma membranes [405] as well as on the membranes of peripheral tissues [295-297, 336, 337]. Many considered the findings that estradiol binding proteins were associated with the cell plasma membrane as artifact. Thus, in spite of the growing amount of relatively conclusive evidence in the 70’s and 80’s that estradiol had rapid actions in neural and reproductive tissues, most investigations and interpretations over the next two decades focused on slower transcriptional mechanisms thought to be associated with estrogen response elements on the DNA for steroid regulation of physiology and behavior. One of the larger technical hurdles to determining whether estradiol was signaling through the genome or membrane was the lack of estrogenic compounds that could act at membrane ER (mER). Since the mid 1990’s, greater attention was placed on the actions of mER [179, 181, 196, 197, 236] and their interactions with classical ER actions via the genome because of new molecular and pharmacological tools available to dissect the actions different types of ER’s. These advances included estradiol attached to large molecules (e.g. bovine serum albumin or biotin) to render estradiol membrane impermeable, selective estrogen receptor modulators (SERM’s) antisense/siRNA techniques to target specific known receptors, and ER knock-out mouse strains. Moreover, the advent of techniques like immunohistofluorescence, in situ hybridization, quantitative PCR, coupled with the development of a number of different transgenic mouse lines, has greatly facilitated our ability to identify the cellular phenotypes through which rapid estrogenic signaling is taking place. In addition to rapidly modulating ion flux and second messenger systems, estrogenic rapid signaling is conveyed through cascades that can act via the genome through phosphorylated cyclic AMP response element protein (pCREB) and intermediate early genes [3, 43, 141, 384]. Thus, the temporal complexity of the roles and interactions of the different types of ER and their signaling pathways regulating physiology and behaviors across the estrous/menstrual cycle are beginning to be revealed. Using new tools, novel ERs have been described and ERα and ERβ have been shown to be mER that complex with and signal through G protein-coupled receptors (GPCR) in both neurons and astrocytes. We review many of the mER signaling pathways and their timing and interactions with classical ER signaling in the regulation of female reproductive behavior, the estrogen positive feedback mechanism that triggers the luteinizing hormone (LH) surge and energy homeostasis.
Multiple ERs with multiple signaling mechanisms
Because of new molecular, anatomical and pharmacological techniques in the past thirty years, research has begun to further reveal the complexity of the steroid hormone timing, interactions and mechanisms involved in modulating physiology and behavior. The field has moved rapidly forward by the recent discoveries of multiple estrogen-binding proteins that are classified as ER, and splice variants of classical ERs, as well as multiple signaling pathways for various ER’s. Multiple types of mERs have been recently discovered. The classical nuclear transcription factors ERα and ERβ are trafficked to the membrane and functionally signal through GPCR’s [41, 42, 141]. ERαΔ4 is an ERα mRNA splice variant lacking exon 4 and is expressed in the hypothalamus to produce a 52-55 kDa immunoreactive membrane protein [94, 369]. ER-X is an estradiol binding membrane receptor that is preferentially activated by 17α-estradiol and not blocked by the ER antagonist ICI 182,780 [399, 400]. mER-Gαq is an undefined membrane protein that is activated by estradiol and the SERM, STX and inhibited by ICI 182,780 even in double ERα/ERβ knockout mice [198, 303, 304]. GPR30 is a G protein-coupled receptor that may be localized to the plasma and/or endoplasmic reticulum membranes that signals through adenylyl cyclase and intracellular Ca2+ [113, 114, 269, 321, 390, 396].
With the discovery of these other ER’s, the ER antagonist ICI 182,780 was also found to inhibit more than just ERα and ERβ. For example, ICI 182,780 inhibits the actions of estradiol or STX in proopiomelanocortin (POMC) and GnRH neurons indicating that ICI 182,780 also antagonizes the mER-Gαq [198, 303, 304, 433]. However, ICI 182,780 does not inhibit estradiol and G1 actions on the GPR30 [2, 111, 113, 269, 390]. While ICI 182,780 is a broad spectrum antagonist of ER-mediated response [246], much like naloxone for defining opioid activity, it may not be entirely useful in defining all ER-mediated signaling with the discovery of estradiol binding proteins like GPR30.
mERα and mERβ
The first two distinctly different cytosolic/nuclear ER’s were identified by Giambi et al., and the names ERα and ERβ were bestowed upon them [130]. A little over a decade later ERβ was officially identified and characterized [258]. These ER’s were shown to be ligand binding transcription factors that upon binding estradiol translocate into the nucleus to regulate DNA transcription [138, 139, 163, 190, 191, 419]. This was problematic for explaining how estradiol was rapidly acting through numerous membrane associated signaling pathways that include modulating electrophysiological properties of the membrane [48, 182, 430, 431], GPCR mechanisms that modulate the activity of MAPK [159], PKC [86], cAMP signaling [383], internal Ca2+ concentrations, and Ca2+ currents. However, Razandi et al., [320] demonstrated the potential for both ER and ER to act as mER by over-expressing ERα and ERβ in Chinese hamster ovary (CHO) cells. They demonstrated that these nuclear receptors were physically associated with the plasma membrane and could mediate intracellular signaling [320]. The membrane association of ERα and ERβ has been confirmed in vivo and in vitro in neurons, astrocytes and cancer cell lines using immunohistochemistry, western blotting, co-immunoprecipitation and surface biotinylation techniques [4, 40, 61, 93, 95, 151, 159, 193, 240, 281, 284, 285, 319, 338, 376, 414]. Although mERα and mERβ signal through GPCR mechanisms, as transcription factors, they lack the GPCR canonical structure [138, 139, 163, 190, 191, 419]. To signal as a GPCR, classical ERs are trafficked to the plasma membrane and then complex with and functionally signal through metabotropic glutamate receptors (mGluR’s) [43, 61, 85, 141, 245]. The trafficking of ERα and ERβ is associated with caveolin and palmitoylation of the ER [4, 286, 319]. ERα and ERβ that are trafficked to the plasma membrane, transactivate specific mGluR’s depending on the caveolin proteins within a given brain region [141]. For example, mERα within the arcuate nucleus (ARH) use calveolin-1 protein to functionally couple with mGluR1a (mERα-mGluR1a), whereas in the striatum, mERα and mERβ are guided by caveolin-3 to couple with mGluR3 [141]. Thus, mERα transactivates mGluRs to stimulate phospholipase C (PLC)/IP3 – MAPK pathways leading to the phosphorylation of CREB or release of internal Ca+2 stores [43, 61, 85, 245]. Although this review covers estradiol signaling through the ERα-mGluR1a complex within the hypothalamus to regulate reproduction, more detailed reviews of the trafficking cycle of classical ER-mGluR’s to the membrane and mER-mGluR signaling can be found in this volume by Micevych and Mermelstein, respectively.
ERαΔ4 – Splice variant of ERα
The brain tissue of rat and lizard express a Δ4 splice variant of ERα mRNA [369]. The splice variant has also been observed in the mammary carcinoma cells as well as brain, pituitary and breast tissue [39, 83, 121, 287, 291]. Recently ERαΔ4 mRNA was found through investigations of estradiol regulation of mERα trafficking in hypothalamic astrocytes. Using membrane protein biotinylation techniques, in addition to the full length 66kDA ERα protein, a 52-55 kDa ERα immunoreactive protein was also found that is the product of an alternatively spliced ERα mRNA missing exon 4 [94]. The 52-55 kDa protein lacks the nuclear localization signal which partially explains why it is trafficked to the membrane [40, 95, 283, 327]. Exon 4 is associated with the ligand binding domain, thus it is uncertain whether estradiol is required for ERαΔ4 signaling or its function. Unlike the full-length 66 kDa ERα, ERαΔ4 does not co-immunoprecipitate with mGluR1a suggesting that it may not complex with other GPCR to signal [192, 193].
mER-Gαq
mER-Gαq is a Gαq-coupled membrane-associated binding protein that is activated by estradiol and blocked by ICI 182,780 [303, 304]. However, mER-Gαq is distinguished by its responsiveness to the diphenylacrylamide SERM, STX [303]. Double ERα/ERβ knockout mice maintain responsiveness to STX that is inhibited by the ER antagonist ICI 182,780 [304]. STX has a low affinity for ERα or ERβ and does not activate signaling by these receptors [303]. Conversely, STX is extremely potent at the mER-Gαq, where it exhibits ~20X greater affinity for this receptor than does estradiol [198, 304, 433]. Activation of mER-Gαq induces signaling through PLC [303], as well as a PKC-PKA signaling cascade that can potentiate an ATP-sensitive potassium channel (KATP) channel in GnRH neurons [433]. In hypothalamic astrocytes, both estradiol and STX activate the same PLC pathway through activation of mGluR1a to increase [Ca2+] and progesterone synthesis [192]. Since STX continued to induce Ca2+ i release in a similar manner in astrocytes from ERαKO mice it indicated that STX and estradiol were signaling through separate receptors that converge on the mGluR1a PLC signaling pathway [192]. ERα is necessary for female reproductive behavior, and the induction of the LH surge [192, 271, 323, 425], yet some aspects of ERα and STX signaling appears to converge, leaving the exact role of the mER-Gαq unclear. Compounding this enigma is the receptor/binding protein mediating STX signaling has yet to be determined. Nonetheless, because STX lacks uterotropic actions it has the potential to be an important therapeutic agent [327].
ER-X
ER-X is another putative mER that is expressed mainly during development and following injury [399, 400]. It is an estradiol binding protein that is associated with caveolar-like proteins whose binding kinetics has been determined [400]. A distinguishing trait of ER-X is the lack of stereo-specificity of binding estradiol. ER-X signaling is more responsive to 17α-estradiol than 17β-estradiol stimulation [400]. Unlike ERα or ERβ progesterone can displace estradiol binding on ER-X [400]. Activation of ER-X induces MEK-dependent phosphorylation of ERK1 and ERK2 in a rapid and sustained manner [267, 365, 366]. This ER-X activity was not inhibited by ICI 182,780 [365]. In contrast, ERα activity inhibits these signaling pathways [365, 366]. As with the mER-Gαq, an issue slowing research into ER-X has been that the receptor has not been cloned.
GPR30
GPR30 (aka GPER) is a G protein-coupled receptor that has been associated with advanced development of cancers in female reproductive organs [112, 194, 370, 371]. In breast cancer cell lines where GPR30 is normally expressed or transfected to overexpress, estradiol increased adenylyl cyclase signaling and cAMP production compared to cells that lacked GPR30 but expressed ERβ [113, 114, 321, 396]. Interestingly, ER antagonists (tamoxifen and ICI 182,780) induced cAMP production in cells expressing GPR30 [114]. However, others did not observe estradiol induced cAMP or ERK phosphorylation in the SKBR3 cells [276, 277]. Further, they observed that COS-7 cells transfected with GPR30 did not bind estradiol compared to those transfected with ERα [277]. A number of studies observed GPR30 localized to either the plasma membrane or endoplasmic reticulum [111, 113, 120, 277, 321], but more a more recent study demonstrated that GPR30 is transported between the plasma membrane and perinuclear locations that include the endoplasmic reticulu m [63] which may account for the discrepancies among the studies. However, in neurons and astrocytes GPR30 has not been localized to the plasma membrane [40, 135, 192]. The GPR30 has also been shown to regulate rapid mobilization of intracellular Ca2+ stores making the localization of this receptor to the endoplasmic reticulum logical [269, 321, 390]. A current controversy is whether or not GPR30 should be classified as an ER [158, 204, 276, 277]. The ER antagonist ICI 182,780 does not block the actions of estradiol at GPR30 under several conditions [2, 111, 113, 269, 390] and actually appears to activate GPR30 at 1 μM levels [269]. It has been argued that GPR30 does not mediate estradiol signaling important for successful reproduction, since GPR30KO mice and mice with a GPR-30-lacz reporter that induces a deletion in the GPR30 coding sequence exhibit little to no effects on female fertility or reproductive organ phenotypes [276, 277]. However, recent studies indicate GPR30 may be involved in facilitating sexual receptivity. Rats primed with a behaviorally subthreshold dose of estradiol and then two days later infused into the third ventricle with either free estradiol or the GPR30 agonist, G1, exhibited increased sexual receptivity within 30 minutes that was inhibited by pretreatment with the GPR30 antagonist G-15 [208]. On the other hand, GPR30 activation desensitizes 5HT1A receptor-mediated increases in adrenocorticotropin hormone and oxytocin secretion, which indicates that it may very well play a role in regulating the stress axis [428].
Integration of mER and Classical ER signaling
Estradiol signaling through ERα is critical for successful reproduction in females. ERαKO mice do not display sexual receptivity, lack estrous cyclicity and are infertile [212, 271, 272]. Exogenous hormone treatments are incapable of rescuing sexual receptivity [271]. Both nongenomic and genomic signaling is disrupted in these animals. For example, estradiol-induced activation of medial preoptic nucleus (MPN) mu-opioid receptors (MOP) is regulated by the ERα-transactivation of mGluRa1 and is eliminated in the ERαKO but retained in ERβKO female mice [247]. Similarly, genomic actions of estradiol-induced progesterone receptor expression are attenuated or eliminated in a number of brain regions in mice indicating multiple site-specific ER mechanisms may be involved in regulating progesterone receptor expression [7, 354]. In intact female rodents, estradiol levels are low and begin to rise slowly on diestrus days 1 and 2 and then rise rapidly and peak on the afternoon of proestrus. On the surface, these distinct fluctuations in estradiol levels might seem a likely indicator of when slower classic ER genomic signaling may be favored over rapid mER mediated mechanisms of signaling. However, in our model systems for regulation of sexual receptivity and neuroprogesterone synthesis ERα-mGluR1a signaling occurs during different times of the cycle. For example, for sexual receptivity, ERα-mGluR1a signaling is activated when estradiol levels are low early in the cycle, whereas for the regulation of neuroprogesterone synthesis for triggering the LH surge the ERα-mGluR1a signaling occurs when estradiol levels the rise on the day of proestrus [85, 193]. Thus, the timing and integration of classic ER genomic and rapid mER initiated signaling are site-specific and complex. We will be reviewing the types of ER interactions and their timing in the regulation of sexual receptivity, the LH surge and energy homeostasis.
Estrogenic Signaling in Sexual Receptivity
Sexual receptivity (lordosis) can be facilitated in female rats by mimicking the estrous cycle with sequential treatment of a priming dose of estradiol followed by progesterone or by a longer exposure to higher levels of estradiol [35, 70, 289, 309, 373]. Progesterone treatment must be delayed about 24 hours after estradiol priming to facilitate lordosis [362] to allow for the expression of progesterone receptors through the “priming” actions of estradiol through direct genomic actions of ERα, ERβ [7, 354] and possibly through mER interactions that ultimately modulate transcription. For example, blocking classical opioid receptors with naloxone at the time of estradiol injection decreases the ability of progesterone 48 hours later to facilitate lordosis [401-404]. The activation of μ-opioid receptors in the medial preoptic nucleus are driven by estradiol binding mERα that complex and transactivate mGluR1a mERα-mGluR1a actions in the ARH [102] indicating the potential for an alternative indirect mechanism for estradiol to induce progesterone receptor expression that is initiated through mERα. In contrast, as the rat enters early menopause the brain is exposed to prolonged higher levels of estradiol [210]. At this point in her reproductive life, the rat appears to become a reflex ovulator where male sexual stimulation will induce a LH surge and ovulation [80]. In estradiol-only facilitation of lordosis, either a single large dose of estradiol or repeated injections of smaller doses of estradiol are required [35, 70, 289, 309, 362, 373]. Additionally, the onset of sexual receptivity in estradiol-only treated animals is about 48 hours after initial treatment, whereas with subsequent progesterone, lordosis can be induced about 24 hours after estradiol treatment [38, 362]. The ability of two different steroid regimens to induce sexual receptivity that appear to be associated with reproductive life history indicates that multiple neural pathways may mediate the same behavior. With new pharmacological, histological and molecular tools the temporal patterns of estradiol’s signaling actions important for lordosis and the LH surge have started to emerge.
An exceptional model neural circuit to study the temporal patterns of steroid signaling that regulate sexual receptivity is the β-endorphin (β-END) – MOP system circuit that originates in the ARH and projects to the MPN [65, 248]. β-END is one of several posttranslational by-products expressed in POMC neurons. There are different subpopulations of POMC neurons that can be distinguished both anatomically and by their sensitivity to MOP agonists and KATP channel modulators [28, 65, 155, 160, 234, 248]. Those POMC neurons involved in sexual behavior terminate in the MPN [65, 248], whereas those that regulate energy homeostasis (discussed at length below) project to the hypothalamic paraventricular nucleus (PVN; [28, 160, 234]). Estradiol upregulates the number of ARH neurons that project to the MPN that positively express POMC [339]. MOP activation in the MPN rapidly and robustly inhibits lordosis in maximally receptive females [290, 362, 367]. The MOP is a GPCR. When activated by endogenous and some exogenous ligands, MOP are rapidly internalized into early endosomes as part of the desensitization and downregulation processes [12, 174-176, 224, 239, 351]. MOP internalization can be easily visualized by immunohistochemistry and used as a measure of MOP activation, allowing us to determine whether the circuit has been turned on or off through endogenous mechanisms or exogenous manipulation [85, 102, 248, 362]. The activation/internalization of MPN MOP has been associated with the inhibition of lordosis in steroid primed rodents, whereas the deactivation of MPN MOP after estrogen priming is associated with facilitation of sexual receptivity [85, 102, 247, 248, 362, 364].
Interestingly, MPN MOP are initially deactivated in ovariectomized rats suggesting that this reproductive circuit is inactive when the ovarian hormones are absent [102]. Within 20 minutes of estradiol treatment MPN MOP are activated presumably through the release of β-END [102, 367], and MOP activation is maintained for at least 48 hours in rats that receive a priming dose of estradiol that does not induce sexual receptivity [340, 362]. Subsequent to estradiol priming, MPN MOP deactivation facilitates lordosis by either progesterone, exposure to a high dose of estradiol for 48 hours, or treatment with naloxone [5, 102, 181, 362]. In the intact female rat, MPN MOP activation fluctuates throughout the estrous cycle coincident with sexual receptivity – activated during diestrous days 1 and 2, deactivated on the evening of proestrus when sexually receptive and reactivated on the morning of estrus when she is no longer receptive [361] suggesting that the activity of this circuit is important for timing the onset and termination of sexual receptivity. Little is known about the MPN MOP neurons and we have begun studying their projections and phenotype. Preliminary tract tracing studies have revealed that a subpopulation of these neurons project to the ventromedial nucleus of the hypothalamus (VMH) region [359]. Since activation of MOP tends to be inhibitory to neurotransmission, neurotransmitters expressed by a subpopulation of MPN MOP neurons should be facilitative to lordosis motor output pathways of the VMH [55, 56, 73, 292, 293]. We have also observed that subpopulations of MPN MOP neurons have been colocalized with either ERα or opioid receptor-like receptor-1 (ORL-1; aka NOP) [299].
This rapid estradiol MPN MOP activation was mimicked by membrane impermeable estradiol-biotin conjugate [85], and was eliminated in the ERαKO but not ERβKO mice indicating that estradiol initially signals through mERα [247]. Exactly where the initial effects of estradiol occur is not exactly clear. The evidence points to estradiol regulation of neurons or neural circuits that converge on the β-END neurons and not direct estradiol regulation of the β-END neuron. Anatomical evidence for ERα expression in β-END neurons is species dependent and lacking replication. In the rat, very few ARH β-END immunoreactive neurons (about 4%) were either observed to concentrate tritiated estradiol in their nuclei [255] or express ERα immunoreactivity [357]. In contrast, using immunocytochemical methods, about 74% of ARH β-END neurons in the female guinea pig express ERα [328]. Thus, the possibility exists that estradiol regulates β-END neurons directly. However, other physiological and anatomical evidence indicates that estradiol is acting through neurons or multisynaptic neural circuits that converge on the β-END neuron. In our model circuit an intermediary neuron mediating estradiol’s effects is an NPY neuron (Figure 1). Activation of NPY-Y1 receptors inhibits lordosis and reverses progesterone deactivation of MPN MOP [66, 248]. The NPY-Y1 receptor is expressed in β-END neurons that project to the MPN [66, 248], and like MOP, estradiol rapidly induces NPY-Y1 receptor internalization in β-END neurons that is reversed by progesterone [248]. Like β-END neurons, the evidence for ERα expression in NPY neurons is lacking replication and may be species dependent. In rats, approximately 10-20% of ARH NPY immunopositive neurons accumulate estradiol within the nucleus [346, 357]. However, in mice that express green fluorescent protein in agouti-related protein-NPY neurons, no colocalization of ERα and GFP was observed in the ARH [274]. In contrast, ERα mRNA was expressed in approximately 19% of individual ARH NPY neurons collected from ovariectomized mice [329]. Therefore the possibility exists that estradiol could act directly on NPY neurons or act on neurons upstream of the NPY neuron in our model system. Regardless of type of neuron in the ARH that expresses ERα, the estradiol signals rapidly through ERα transactivation of mGluR1a. Estradiol had been shown to signal through mGluR’s in the absence of glutamate [43] and this was shown to be the case for estradiol in the ARH [85, 86]. The mGluR1a receptor is expressed in ARH neurons and was shown to complex with ERα (mERα-mGluR1a) in membrane preparations from the ARH by coimmunoprecipitation [85, 86]. Pretreating the rats with the mGluR1a antagonist, LY 367385 eliminated estradiol induced MOP activation as well as the ability of a high dose of estradiol to induce sexual receptivity [85]. Conversely, activating mGluR1a concurrently with the priming dose of estradiol facilitated lordosis compared to the estradiol only treated females [85]. The mERα-mGluR1a activates the PKCΘ signaling pathway to activate MPN MOP and facilitate lordosis [86]. Blocking PKC activity inhibited both estradiol and mGluR1a agonist induced MPN MOP activation [86], and ARH infusions of a PKC antagonist 30 minutes prior to estradiol administration inhibited facilitation of lordosis [86]. Although the initial mERα-mGluR1a signaling through the PKCΘ pathway is necessary for estradiol facilitation, activating this pathway on its own is not sufficient to mimic estradiol facilitation of lordosis [86]. These findings indicate that estradiol dose is “measured” through the mERα-mGluR1a signaling that is important for determining whether estradiol will either prime the reproductive neurocircuits for progesterone facilitation of lordosis or induce sexual receptivity on its own. This circuit appears to mark important timing information for regulating the onset reproductive behavior and sexual receptivity, as well as mediate downstream priming effects of estradiol that are important for facilitation of lordosis by either high doses of estradiol or subsequent progesterone. The initial activation ARH-MPN lordosis inhibitory circuit prevents the rat from copulating prior to the other priming effects of ovarian hormones that are inducing uterine development and ovulation so that they are coordinated with sexual behavior.
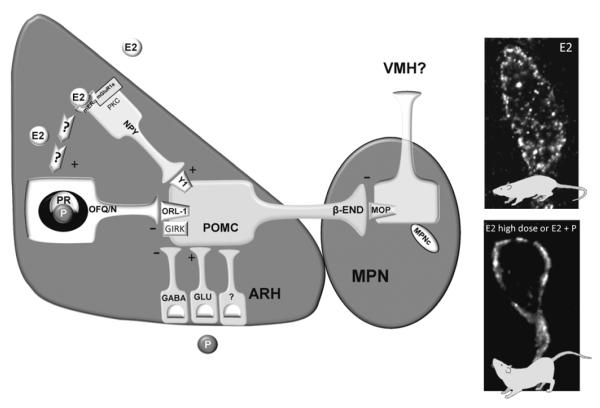
Figure 1.
Proposed model of estradiol regulation of lordosis circuitry within the ARH and medial preoptic nucleus (MPN). Estradiol binds to that signals through PKC to induce the release of neuropeptide Y (NPY; although the mERα-mGluR1a is depicted in the NPY neuron it may located in a neuron upstream of the NPY neuron). NPY then binds to the NPY-Y1 receptor (Y1) to excite (+) a subpopulation of POMC neurons involved in sexual behavior that project to the MPN. Estradiol rapidly activates this circuit and maintains activation of POMC neurons to induce the release of β-END from nerve terminals in the MPN. β-END binds to and activates and internalizes μ-opioid receptors (MOP) into early endosome to initially inhibit lordosis (E2 photomicrograph). A priming dose of estradiol maintains MOP activation for at least 30 hours inhibiting lordosis and increases ORL-1 expression in ARH POMC neurons that project to the MPN. This circuit is deactivated by longer (48 hours) exposure to high doses of estradiol (5-50 μg; E2 high dose photomicrograph) by 1) reducing NPY release through possibly the downregulation of mERα-mGluR1a in NPY neurons and 2) through an unknown mechanism increasing the release of OFQ/N to activate ORL-1 which decreases POMC excitation (-) through increasing GIRK currents. This reduces MPN MOP activation, recycling the MOP to the plasma membrane as part of the mechanism for facilitating lordosis. Following estradiol priming, progesterone also deactivates this circuit to facilitate lordosis. Although progesterone receptors are expressed in OFQ/N neuron, OFQ/N is not necessary for deactivating MPN MOP and progesterone may induce the release of other inhibitory neurotransmitters such as GABA to inhibit β-END release to facilitate lordosis [49, 85, 86, 102, 144, 248, 339, 340, 358, 362, 416].
An interesting question is how does estradiol signaling switch from activating the β-END-MPN MOP circuit and inhibiting sexual receptivity to reducing the output of the β-END neuron and facilitating lordosis? Both the high estradiol dose that facilitates lordosis and the priming dose maintain MPN MOP activation for least 24 hours [102, 222, 362]. However, somehow between 24 and 48 hours the high estradiol dose deactivates MPN MOP to facilitate lordosis [102, 222, 340]. This switch occurs while significant levels of estradiol remain in the circulation of the high dose animals [301]. Is it possible that mERα-mGluR1a is reduced? This was supported by blocking classical ERα signaling in estradiol primed animals. Treating estradiol primed rats with ER antagonists (tamoxifen or ICI 182,780) 44 hours after priming and testing 4 hours later facilitated lordosis and MPN MOP activation was reduced [124]. One of the potential mechanisms is that the high dose of estradiol down-regulates and removes ERα from the plasma membrane to reduce mERα-mGluR1a signaling that activates MPN MOP. Further, the priming dose and the high dose of estradiol both reduce the number of ARH neurons that express ERα [220]. However, western blot analysis indicates that the total amount of ARH ERα is not reduced by the priming dose of estradiol, whereas the high dose reduces total ERα [220]. Thus, it is possible that the priming dose of estradiol maintains or even increases the amount of ER α per cell which may signal to traffic ERα to the membrane [320] and maintains mERα-mGluR1a signaling to inhibit lordosis. On the other hand, the high dose of estradiol appears to down regulate ERα which would likely reduce the amount of ER trafficked to the membrane [94], and in turn reduce activation of ARH β-END neurons, and MPN MOP to facilitate lordosis. Thus, it appears a part of the mechanisms to facilitate lordosis is to remove excitatory mERα-mGluR1a signaling input to β-END neurons that project to the MPN.
Both high dose estradiol and estradiol + progesterone reduce MOP activation to facilitate lordosis. However, do these steroid priming paradigms act through similar neurociruits to inhibit or reduce the activity of the β-END neuron? Although both steroid paradigms decrease the activity of the β-END neuron to deactivate MPN MOP and facilitate lordosis, it appears that different neurotransmitters systems are used to inhibit the ARH β-END neuron as a mechanism to facilitate lordosis. The opioid, orphanin FQ (OFQ/N; aka nociceptin), facilitates lordosis in estradiol-primed nonreceptive female rats when infused into the ARH region, via its cognate receptor, ORL-1 [340, 358, 360]. Estradiol priming increases the expression ORL-1 expression in the VMH and ARH POMC (putative β-END) neurons that project to the MPN [339, 363]. Further, estradiol priming increases the number of immunopositive OFQ/N and progesterone receptor neurons in the ARH and their colocalization [144]. OFQ/N reduces MPN MOP activation through hyperpolarizing β-END neurons via G protein-coupled inward-rectifying potassium (GIRK) channels [107, 339, 415, 416]. Antagonizing OFQ-ORL-1 signaling in the ARH by either OFQ immunoneutralization or antagonism of ORL-1 with UFP-101 blocks high dose estradiol facilitation of lordosis by relieving OFQ inhibition on β-END as indicated by the increase in MPN MOP activation [340, 358]. These data indicate that the high dose of estradiol induces the release of OFQ/N in the ARH through an unknown mechanism. In contrast, these treatments had no effect on progesterone facilitation of lordosis and deactivation of MPN MOP [340, 358]. Thus, these results support the notion that estradiol only and estradiol + progesterone facilitation of lordosis activate different neurocircuits that converge on ARH β-END neurons to inhibit their output to the MPN. Although estradiol appears to ultimately induce OFQ/N release to activate ORL-1 in the ARH, OFQ/N induced GIRK currents are reduced 30 hours after estradiol priming [339, 416] which would reduce that inhibitory effects of OFQ/N on the activity of β-END neurons. This decrease in ORL-1 signaling fits with the priming and initial actions of estradiol to increase β-END activity. However, it raises the questions does the high dose of estradiol increase ORL-1 GIRK signaling to inhibit POMC neuronal activity to facilitate lordosis, and if so through what signaling pathway?
In summary, estradiol rapidly activates mERα-mGluR1a that signals through PKCΘ and either acts upstream of, or directly on ARH NPY neurons to release NPY (Figure 1). NPY then activates NPY-Y1 receptors to activate β-END neurons that project to the MPN, activating MOP for up to 48 hours inhibiting lordosis. Lordosis is facilitated by reducing excitatory input to β-END neuron through possibly downregulating mERα and increasing inhibitory input to the POMC neuron through OFQ/N release. Although estradiol increases the co-expression of progesterone receptor and OFQ/N, OFQ/N does not appear to be necessary for progesterone facilitation of lordosis and may act through the increase in GABA neurotransmission to inhibit β-END neurons to deactivate MPN MOP and facilitate lordosis.
Estradiol Signaling Regulating Neuroprogesterone Synthesis
The release of ovarian hormones across the cycle coordinates the facilitation of sexual behavior with ovulation to maximize the fertilization of the ovum. Multiple ER signaling pathways are used throughout the cycle. For example, as new follicles mature during diestrous days 1 and 2 they secrete the lower levels of estradiol into the circulation that produce negative feedback. During negative feedback, estradiol induces the expression of hypothalamic progesterone receptor-A that are required for induction of the GnRH and LH surges that induce ovulation [46, 62, 109, 195, 221, 315]. These progesterone receptor-A either directly or indirectly activate kisspeptin neurons in the region of the anteroventral periventricular nucleus (AVPV) to stimulate the surge release of GnRH [67, 68, 77, 149, 207, 237, 241]. However, circulating progesterone levels rise either in parallel or after the LH surge [108, 171, 372], and the LH surge can be induced with estradiol only in OVX/ADX rats [223, 243, 378] indicating that a peripheral source of progesterone is not necessary for LH surge generation.
Baulieu and colleagues demonstrated that the brain had the steroidogenic capacity to produce steroids de novo from cholesterol [22, 23, 71, 142, 168, 169, 186, 201, 232, 233, 342]. In particular astrocytes preferentially synthesize progesterone [434] and express ERα and ERβ indicating that estradiol could directly induce progesterone synthesis in hypothalamic astrocytes [61, 123, 284, 310]. Treating ADX/OVX rats with estradiol increased progesterone concentration site-specifically in the hypothalamus on the afternoon of the LH surge [243]. Further, inhibiting progesterone synthesis by blocking either P450 side chain cleavage (P450scc) or 3β-hydroxysteroid dehydrogenase (3β-HSD), the enzymes for progesterone synthesis, blocks the LH surge [243]. In addition, the estrous cycle can be arrested in proestrus by infusing an inhibitor of P450scc into the third ventricle on the morning of proestrus [242]. Vaginal cytology remained in a proestrous state, antral follicles were present but no corpora lutea were observed and uterine tissues were fluid filled, and estradiol levels were still raised in the circulation, but progesterone levels were low compared to intact control rats. The ability to block neurosteroidogenesis and arrest the cycle in proestrus indicates that peripheral steroidogenesis was maintained, but neurosteroidogenesis was needed for the LH surge to induce ovulation and luteinization in the intact rat [242].
Since the LH surge occurs about 48 to 56 hours after estradiol stimulation, it appeared that estradiol was acting through genomic mechanism to induce the surge. This appears true for the induction of progesterone receptor-A and possibly for the increase in 3β-HSD mRNA observed in the hypothalamus 24 hours after estradiol treatment [375]. However, the estradiol signaling that induces progesterone synthesis in astrocytes on the afternoon of proestrus appears to be by a similar mERα-mGluR1a complex observed in ARH neurons. In this case, mERα-mGluR1a is activated by the higher levels of estradiol produced during positive feedback on proestrus [85, 86, 192, 193].
To investigate estradiol’s mechanisms of action to induce neuroprogesterone synthesis, hypothalamic astrocyte cultures were generated from female mice. Hypothalamic astrocytes cultured from post-pubertal female mice treated with estradiol had increased progesterone synthesis compared to control treated cultures [244]. Although pre-pubertal astrocytes from female mice also synthesized progesterone, estradiol did not increase progesterone demonstrating that the astrocytic responsiveness to estradiol stimulation is developmentally regulated [244]. Further, estradiol did not induce progesterone synthesis from hypothalamic astrocyte cultures from males demonstrating the developmental and sexual differentiated responsiveness of the positive feedback system that is seen in the intact animal [243, 368]. This progesterone synthesis was blocked by ICI 182,780 indicating that ERα, ERβ or STX may be involved [244].
In the periphery, steroidogenesis is dependent on intracellular signaling pathways that are associated with increasing intracellular levels of Ca2+ [58, 117, 316, 333, 408, 412] suggesting a rapid nongenomic pathway that is mediated by estradiol. This indeed is the case. Estradiol rapidly increased intracellular calcium concentration ([Ca2+]i) within seconds to minutes after application [61, 192, 193]. This was mimicked by membrane impermeable E-6-BSA, and both estradiol and E-6-BSA actions were blockable by ICI 182,780 [245]. This [Ca2+]i increase is released from intracellular Ca2+ stores in the smooth endoplasmic reticulum and mediated through the phospholipase C/inositol trisphosphate (PLC/IP3) pathway to open IP3-gated Ca2+ channels [61, 245]. Eliciting the release of Ca2+ via IP3-gated channels with thapsigargin induced progesterone synthesis in post-pubertal hypothalamic astrocytes within 60 minutes [61, 192]. In the earlier studies, the astrocytes were incubated for 48 hours prior to assaying for progesterone synthesis [245]. However, these recent studies demonstrated that estradiol releases [Ca2+]i within seconds to minutes and progesterone synthesis is induced within one hour [193]. This estradiol signaling regulating [Ca2+]i was first demonstrated to be mediated through mERα-mGluR1a [193]. Estradiol produces a dose-dependent increase in [Ca2+]i that is blockable by either ICI 182,780 or antagonizing mGluR1a [193]. Activating mGluR1a with DHPG produced a similar increase in [Ca2+]i to estradiol alone, and treating these astrocytes with both estradiol and DHPG augmented the [Ca2+]I induced by each alone [193]. Thus, activation of progesterone synthesis can be regulated by both estradiol levels and whether glutamate neurotransmission is taking place to allow for tighter regulation of the LH surge by the integration of signaling produced during positive feedback [51, 147, 350].
Further studies indicate that other mER may be involved in this Ca2+ pathway through mGluR1a as well [192]. In these hypothalamic astrocytes rapid increases in [Ca2+]I similar to those produced by estradiol can be induced by treatment with any of the following SERMs: PPT (ERα agonist), DPN (ERβ agonist), STX (mER-Gαq agonist), or G-1 (GPR30 agonist) [192]. Estradiol and PPT did not induce [Ca2+]i in astrocytes cultured from ERαKO mice, but DPN, STX and G-1 did indicating that the latter use signaling pathway independent of ERα [192]. The increase in [Ca2+]i induced by all these SERMs are blocked by antagonizing mGluR1a, and all but G-1 are augmented by stimulating mGluR1a [192]. Although these mER appear to be signaling through a similar pathway to release [Ca2+]i, the pathways through which they induce progesterone synthesis are not the same. Estradiol, PPT, STX and G-1 induced progesterone synthesis, but DPN, the ERβ agonist, did not [192]. Interestingly, antagonizing mGluR1a blocked estradiol, PPT and STX progesterone synthesis, but had no effect on G-1 induction of progesterone [192]. Since STX acts in the absence of ERα, but estradiol requires its presence to increase [Ca2+]i and induce progesterone, it appears that both activate the PLC signaling pathway via different receptors that transactivate mGluR1a. In contrast, GPR30 uses a different signaling mechanism than either estradiol or STX. GPR30 and mGluR1a do not co-immunoprecipitate suggesting a lack of transactivation of mGluR1a [192]. The localization of GPR30 to the endoplasmic reticulum [63, 321] and lack of interaction with mGluR1a indicates that GPR30 may directly induce the release of intracellular stores of Ca2+ to induce progesterone synthesis. However, the role of GPR30 role in estradiol induction of neuroprogesterone synthesis is unclear, since estradiol requires ERα for its activity [192]. It is unlikely that ER-X plays a role in inducing progesterone synthesis in astrocytes since 17α-estradiol did not increase [Ca2+]i [193, 245]. It is possible that all of these estradiol mediated pathways with glutamate interactions integrate and “measure” the levels of estradiol across the estrus cycle through regulating levels of [Ca2+]i to indicate the appropriate time to induce progesterone synthesis to trigger the LH surge.
Estrogenic Signaling Involved in the Regulation of Energy Homeostasis
As described above, estrogens play a vital role in the control of reproduction. They work in conjunction with progesterone via a combination of classic genomic and mER initiated rapid signaling mechanisms to help regulate the development of ovarian follicles, endometrial thickness, gonadotropin output from the anterior pituitary, and female sexual receptivity; all for the expressed purpose of ensuring reproductive behavior is synchronized with ovulation so that it will ultimately result in fertilization of the secondary oocyte and implantation of the resultant blastocyst [76, 278, 362, 389, 403]. This next section will focus on estrogen-induced alterations in energy homeostasis that are coincident with the reproductive cycle, and describe what is currently known regarding the rapid signaling mechanisms through which these changes take place.
Estrogenic Regulation of Food Intake
It is well recognized that estrogens diminish appetite in rodent animal models as well as primates [52, 76, 98, 166, 279]. In rats the ERα agonist PPT decreased food intake and meal size whereas the ERβ agonist DPN was without effect [345]. In mice ERα silencing within the VMH leads to hyperphagia, obesity and glucose intolerance [261]. In guinea pigs both estradiol and the mER-Gαq agonist STX decrease food intake and meal frequency [327]. Thus the appetite-modulating properties of estradiol may be mediated through a combination of rapid mER initiated signaling mechanisms that involve ERα and mER-Gαq but not ERβ. In human females energy intake is at its lowest during the periovulatory phase, when estrogen levels are at their peak, and at its highest during the progesterone-dominated luteal phase [166]. These cyclical changes in energy intake are associated with decreased carbohydrate consumption around the time of ovulation, and increased fat consumption during the luteal phase [166].
Estrogenic Regulation of Energy Expenditure
Estrogens influence energy expenditure by modulating core body temperature and thermogenesis. In ob/ob and db/db mice estradiol increased energy expenditure, core body temperature and O2 and decreased the respiratory quotient [122]. It also prevented the fasting-induced decrease in energy expenditure, core body temperature and O2 consumption [122]. Conversely, ERα knockdown mice exhibited a decrease in total energy expenditure, an increase in the respiratory quotient, and negligible diet-induced thermogenesis [261]. In addition, ovariectomized rats display increased skin vasodilation and a left-shifting of the thermoneutral zone to lower ambient temperature threshold [78]. On the other hand, both estradiol and STX reduce core body temperature in ovariectomized guinea pigs and rats [78, 177, 327], which suggests the involvement of mER signaling in the control of energy expenditure. In addition, mitochondrial oxidative capacity in rodent brown adipocytes is at its lowest during proestrus, when estrogen levels are at their highest [302]. Estradiol also rapidly (within minutes) increases the firing rate of warm-sensitive neurons in the preoptic area (POA) [356], which would decrease heat production, promote heat loss and thereby lower core body temperature. These latter set of findings are consistent with those in humans, where core body temperature, measured either from the vagina or the esophagus, is lowest during the periovulatory phase and highest during the luteal phase of the ovarian cycle, and is positively correlated with the progesterone/estradiol ratio [53, 379, 381]. The idea that estrogens modulate core body temperature primarily through their effects on hypothalamic thermoregulatory centers is further substantiated by the fact that: 1) estradiol is without effect on the expression of uncoupling protein (UCP)1 or UCP2 in rodent brown adipocytes [326], and 2) mitochondrial GDP binding (an index of UCP1 activity) does not fluctuate over the course of the estrous cycle [302]. Moreover, estradiol failed to alter norepinephrine turnover in, and thus, sympathetic innervation of brown adipose tissue of either thermoneutral or cold-acclimated rats [265]. Furthermore, estradiol blunts the cold-induced activation of hypothalamic-pituitary-thyroid axis as measured via thyrotrophin-releasing hormone expression in the PVN as well as serum thyroid-stimulating hormone and triiodothyronine levels [410].
Estrogens elicit these effects in part by inducing synaptic plasticity at critical neuroanatomical substrates within the hypothalamic feeding circuitry; increasing the number of asymmetric excitatory contacts with anorexigenic POMC neurons and upregulating α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors in these cells [90, 122]. They also influence the release of, and the cellular responsiveness to, a number of different neurotransmitters/modulators (e.g. cannabinoids), adipokines like leptin and gut hormones such as cholecystokinin (CCK) and ghrelin, all of which are well-established as regulators of energy intake and expenditure. These interactions form the basis of the discussion that follows. For each of the sections below we lay the introductory groundwork and describe the current understanding of how these transmitters, modulators and hormones control energy homeostasis; followed by a discussion of what we know about how estrogenic signaling creates a negative energy balance by influencing their ability to function in this regard.
Estrogen-cannabinoid interactions
Cannabinoids exert pervasive effects in regulating energy homeostasis. They have long been known as an appetite stimulant in both humans [47, 228] and rodents [91, 161, 423]. As such, they have appreciable therapeutic efficacy in ameliorating the cachexia associated with HIV/AIDS and cancer [146, 157]. This hyperphagic effect is characterized by an inverted U-shaped dose-response relationship [177, 185], which would explain why high doses of cannabinoid CB1 receptor agonists actually decrease appetite and weight gain [31, 227, 335]. Cannabinoids affect energy expenditure through their actions in the POA/anterior hypothalamus, where they increase the activity of primary thermodetectors and heat-sensitive neurons, and decrease the activity of cold-sensitive neurons to ultimately bring about hypothermia [91, 318, 348]. Cannabinoids also act in the periphery where they reduce the expression of UCP1 in brown adipocytes, as well as respectively increase and decrease the expression of visfatin and adiponectin in white adipocytes, to ultimately inhibit thermogenesis [288].
Estrogens powerfully disrupt the cannabinoid regulation of energy balance. They rapidly and completely block the cannabinoid-induced hyperphagia, reduce the magnitude and duration of the cannabinoid-induced hypothermia, and occlude any further hypophagic effect of CB1 receptor antagonists [177]. This estrogenic antagonism can be attributed, in large part, to the downregulation of hypothalamic CB1 receptors [322], as well as the attenuation of the cannabinoid-induced inhibition of glutamate release at POMC synapses, and the cannabinoid-induced activation of Kv4.2 channels underlying an A-type K+ current (IA) in these cells (Figure 2) [162, 177, 268, 386]. Estradiol upregulates the expression of PKCΔ and the p85α subunit of phosphatidylinositol-3-kinase (PI3K) and in the ARH, and PKC as well as PI3K inhibition restores the ability of cannabinoid receptor agonists to presynaptically inhibit glutamatergic input onto POMC neurons [162, 417]. Estradiol also upregulated the expression of the α1 and α2 catalytic subunits of AMP-dependent protein kinase (AMPK), however it was the AMPK activator metformin that when co-perfused along with estradiol for 10-15 minutes actually abrogated the antagonism of the cannabinoid-induced hyperphagia and inhibition of glutamate release at POMC synapses [162]. This would suggest that estrogens acutely decrease AMPK expression in the mediobasal hypothalamus in a manner similar to that seen with leptin [249], and that the elevated level of AMPK expression observed by Jeffery and co-workers [162] is reflective of a compensatory response in the face of the negative energy balance created by week-long steroid treatment. The components of the signal transduction pathway that to this point we know contribute to the rapid estrogenic antagonism of cannabinoid signaling at POMC synapses are illustrated in Figure 2.
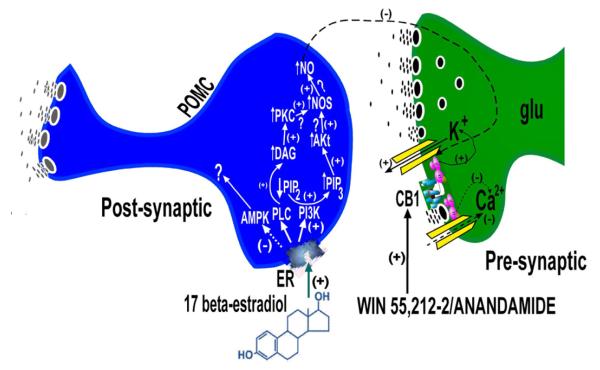
Figure 2.
Schemata depicting our current understanding for how estrogens disrupt presynaptic CB1 receptor-mediated signaling at glutamatergic inputs impinging upon a subpopulation of anorexigenic POMC neurons that project to the PVN and help regulate energy balance. A) Under hypoestrogenic conditions cannabinoid agonists such as anandamide and WIN 55,212-2 robustly inhibit glutamatergic input onto POMC neurons. The quiescence of the POMC neurons helps to bring about the cannabinoid-induced hyperphagia, hypothermia and alterations in energy expenditure. B) Estrogens rapidly uncouple c annabinoid CB1 receptors from their effector systems in the glutamatergic nerve terminal by activating PKC and PI3K, and by inhibiting AMPK. PKC and PI3K may, in turn, activate nNOS as has been shown in hypothalamic explants and in glucose-sensitive neurons in the VMH [57, 226]. The nitric oxide thus produced may then act transynaptically in a retrograde fashion to antagonize the cannabinoid-induced presynaptic inhibition.
Estrogen-NPY interactions
Activation of NPY neurons in the ARH promotes appetite and weight gain [103, 170, 422]. These neurons are glucose-sensitive. As such, NPY expression and neuronal activity are inversely proportional to circulating glucose concentrations, and increased by conditions of negative energy balance like that caused by fasting [30, 49, 116, 170, 203]. NPY levels are elevated in the ARH, PVN, MPN, suprachiasmatic nucleus and the anterior hypothalamus of genetic- and diet-induced obese rodents [27, 422], and circulating NPY concentrations are increased in the plasma of overweight and obese humans [18]. The NPY-induced orexigenesis is associated with decreased UCP expression in brown adipose tissue [189]. Additionally, agouti-related peptide (AgRP) colocalizes with NPY in the vast majority of these cells [45], and serves to increase appetite by antagonizing melanocortin (MC)4 receptor-mediated signaling [118, 145, 154].
Estrogens attenuate the orexigenic effect of AgRP [343]. Estradiol is sequestered in NPY-immunoreactive neurons [346], and as discussed above ERα signaling activates NPY neurons that are part of the limbic-hypothalamic circuit controlling female sexual behavior. This is evidenced by the internalization of Y1 receptors in β-endorphin neurons measured 30 hours following central administration of the steroid [248]. In contrast, estradiol activation of ERα that colocalizes with caveolin-1 in the plasma membrane of mHypoE-42 and mHypoA-2/12 neurons inhibits NPY secretion by stimulating activity of PI3K and AMPK [87]. Similar findings were encountered in N-38 cells, where estradiol stimulation of mERα inhibits NPY expression via activation of PI3K/Akt, ERK1/2, MAPK and CREB [397]. NPY neurons express an array of K+ channels (i.e., KCNQ5, Kir2.4, Kv4.1) that are upregulated by estradiol treatment [328]. Estradiol also augments the fasting- and XE991-sensitive M current, and blunts the fasting-induced c-Fos activation, in these cells [274, 329]. In addition, both estradiol and the mER-Gαq agonist, STX downregulate NPY expression in the ARH which indicates that this mER mechanism can modulate transcriptional regulation of this system [274, 330]. Moreover, NPY and AgRP levels positively correlate with changes in food intake over the course of the estrous cycle, and reach a nadir during the transition from proestrus to estrus [274].
Estrogen-melanin-concentrating hormone (MCH) interactions
MCH is expressed in neurons emanating from the lateral hypothalamus. These cells work in conjunction with orexin-containing somata in the same region to stimulate appetite and food intake [1, 103, 407]. MCH-containing nerve terminals appear to target the PVN, ARH and dorsomedial nucleus as MCH administered directly into these brain regions (but not the supraoptic nucleus, lateral hypothalamus, MPN, anterior hypothalamus or VMH) induces feeding in satiated rats [1]. This is associated with increased NPY and AgRP release, and decreased α-melanocyte-stimulating hormone (α-MSH) release, as observed in in vitro hypothalamic explants [1]. This latter finding is consistent with the fact that co-administration of α-MSH reverses the orexigenic effect of MCH [407]. The MCH-induced hyperphagia is connected to decreased energy expenditure and hypothermia [133]. Similar to NPY, fasting increases hypothalamic MCH expression [30], and MCH content is elevated in the lateral hypothalamus as well as other brain regions of ob/ob and db/db mice [252]. Conversely, ablation of MCH neurons results in an acquired leanness characterized by hypophagia, decreases in body weight, body length, body fat and circulating leptin levels, as well as elevated O2 consumption [6]. Unlike NPY neurons, however, MCH-containing cells are glucose-responsive, and as such their firing rate varies directly with ambient glucose levels [50]. This may be related to the role MCH plays in regulating arousal, as MCH knockout mice exhibit not only exaggerated weight loss in response to fasting but also marked hyperactivity and an appreciable reduction in rapid eye movement sleep [424].
Estrogen-induced anorexia has been reported in male MCH knockout mice [406]. However, the majority of the available evidence points to the fact that estrogens blunt the appetite-stimulating effect of MCH [238, 344]. The MCH-induced increase in food intake varies over the course of the estrus cycle, and is lower during estrous than it is during diestrous [344]. While estradiol produces biphasic variations in MCH expression in the diencephalon of ovariectomized cynomolgus monkeys [413], it also reduces MCH expression in ovariectomized rats [260], and attenuates the increased expression caused by energy restriction in AgRP over-expressing Ay mice [256]. Although ERα is not expressed in MCH neurons from male rat hypothalamus [262], very little else is known about the signaling mechanisms through which estrogens modulate MCH-induced hyperphagia.
Estrogen-serotonin interactions
Serotonergic neurons have cell bodies located primarily in the dorsal and median raphe, and are classified by an alpha-numeric system as the B1-B9 cell groups [411]. Their diffuse axonal projections ascend into the hypothalamus and forebrain (e.g., caudate-putamen, hippocampus, cortex), and descend into the spinal cord [115, 380, 411]. Serotonin is also released from enteroendocrine cells in the gut, where it activates 5HT3 receptors on vagal afferents in response to the presence of glucose in the lumen [29]. The 5HT3 receptor-mediated stimulation of these vagal afferents creates a negative feedback loop that conveys a satiety signal to the brainstem [29]. Collectively, these serotonergic cells play an important role in regulating neuroendocrine function, nociception, mood, stress and energy balance [127, 199, 380, 411]. With regard to the latter, disruption of the serotonergic system either through enhanced release with fenfluramine [235] or blockade of reuptake with fluoxetine [10] was at one time considered a promising strategy to curb appetite and, as a result, obesity. Targeted deletion of the 5HT2C receptor results in hyperphagia and late-onset obesity [270], whereas 5HT1B receptor knockout mice exhibit increased food intake and weight gain but not obesity [44].
Estrogens potentiate the appetite suppression caused by fenfluramine-induced disruption of serotonergic neurotransmission [324]. It is thought that fenfluramine increases serotonin levels at anorexigenic POMC synapses, which results in heightened activation of 5HT2C receptors on POMC neurons and an increase in their firing rate [74]. The 5HT2C receptors are Gq-coupled and therefore activate PLC to hydrolyze phosphatidylinositol bisphosphate , which uncouples GIRK channels from metabotropic Gi/o-coupled receptors like the GABAB receptor expressed in these cells to increase their excitability [307]. They also couple to transient receptor potential cation (TRPC) channels in a subset of POMC neurons [374]. However, PKC inhibitors were unable to reverse the attenuation in the GABAB response caused by 5HT2C receptor agonists [307], which indicates that PKC is not involved in the 5HT2C receptor-mediated excitation of POMC neurons. Estradiol uncouples GIRK channels from GABAB receptors in POMC neurons via a PLC/PKC/PKA pathway following activation of mER-Gαq, which was mimicked by STX [306]. This suggests that Gq-coupled mERs and 5HT2C receptors activate distinct yet overlapping signal transduction pathways, and helps explain the additive effects of estradiol and 5HT2C receptor agonists on POMC neuronal excitability and food intake when the two are combined [307]. Activation of Gi/o-coupled 5HT1B receptors directly inhibits NPY/AgRP neurons, and presynaptically inhibits inhibitory GABAergic input from NPY/AgRP neurons that impinges on POMC neurons [225, 429]. Estrogens desensitize 5HT1A receptor signaling in the PVN that is mediated, in part, by activation of GPR30 [334, 428]. Although 5HT1A and 5HT1B receptors are both metabotropic, Gi/o-coupled receptors [225], it is unknown whether estrogens similarly uncouple the latter from their effector system(s) in NPY/AgRP neurons or at POMC synapses.
Estrogen-ghrelin interactions
Ghrelin is a hormone released primarily from the oxyntic mucosa of the stomach [188]. It is also found in the perikarya of neurons in the ARH [211]. Its receptor is expressed in the ARH, VMH, PVN, area postrema, nucleus tractus solitarius (NTS), dorsal motor nucleus of the vagus (DMV) and in parasympathetic preganglionic neurons [188]. Both peripherally and centrally administered ghrelin stimulate food intake and do so, in large part, through a Ca2+-dependent increase in endogenous cannabinoid production in the hypothalamus that ultimately stimulates AMPK activity [187, 188]. AMPK then decreases malonyl-CoA and increases carnitine palmitoyltransferase to inhibit fatty acid synthesis in the VMH [209]. Ghrelin also upregulates UCP2 expression in the mitochondria of NPY/AgRP neurons [9]. This, in turn, elevates the NPY/AgRP expression and activity believed to be responsible for NPY/AgRP-induced inhibition of POMC neurons [9, 74]. These orexigenic effects appear to be due solely to the activation of central ghrelin receptors, as vagal deafferentation did not influence the ghrelin-induced hyperphagia [13]. In the periphery ghrelin modulates energy expenditure by decreasing AMPK expression in adipose tissue and liver [188], which would facilitate lipogenesis and attenuate gluconeogenesis. It also decreases the expression of UCP1 and UCP3 in brown adipose tissue [395], which would decrease thermogenesis and energy expenditure. Collectively, this is in keeping with the observations that ghrelin receptor knockout mice exhibit a reduced fat/lean ratio, increased energy expenditure and increased UCP1 expression in brown adipose tissue [216].
The orexigenic potency of ghrelin is sexually differentiated and negatively modulated by estrogens [69], which also decrease its expression in rat stomach [110]. While one study documented that estrogens were without effect on circulating ghrelin levels in normally cycling and postmenopausal women [79], another noted that estrogen replacement therapy increased them in postmenopausal women [178]. Still others contend that circulating ghrelin levels are elevated in exercising, estrogen-deficient women [82], and that they are sexually dimorphic, positively correlated with testosterone levels in men, and significantly reduced in men with hypogonadotropic hypogonadism [100, 140]. Recall that while estrogens rapidly decrease NPY secretion in part by increasing AMPK activity [87], they appear to attenuate cannabinoid signaling at POMC synapses in part by decreasing AMPK activity [162]. Given that the hyperphagic effect of ghrelin is due largely to the cannabinoid-induced stimulating of AMPK activity [187], it is compelling to speculate that estrogens abrogate ghrelin-induced orexigenesis by antagonizing the ability of ghrelin to promote hypothalamic endogenous cannabinoid production and the subsequent increase in AMPK activity– perhaps by activating mERα and/or mER-Gαq. Clearly, more work is necessary to provide a clearer picture of how estrogens influence ghrelin-induced changes in energy balance.
Estrogen-CCK interactions
CCK is a hormone secreted from endocrine cells in the proximal small intestine. It is released in response to the presence of fat and, to a lesser extent, protein in the duodenal lumen [29]. CCK conveys satiety primarily by activating CCK-A receptors on capsaicin-sensitive vagal afferents that, in turn, stimulate neurons in the NTS and PVN [125, 172, 253]. CCK-A receptors in the circumventricular area postrema also are activated by peripheral CCK [125, 253]. In the NTS, this CCK-A receptor-mediated satiety is due to the activation of a cAMP→ERK1/2→CREB signal transduction pathway in both POMC and tyrosine hydroxylase-containing neurons [17, 382]. CCK also augments excitatory glutamatergic synaptic input via the solitary tract that impinges on POMC neurons in the NTS [11]. In addition, the CCK-induced appetite suppression is blocked by prior administration of a MC4 antagonist into the fourth ventricle [382]. Interestingly, CCK appears to excite orexin/hypocretin neurons by activating a non-selective cation channel, which is thought to play a role in post-prandial somnolence [409].
Estrogens augment the CCK-induced suppression of food intake, enhance the CCK-induced satiation caused by the intraduodenal lipid infusion, and increase the potency of CCK to decrease meal size [14-16]. They do so by activating ERα-bearing cells in the NTS as assessed by immunocytochemical detection of ERα and c-Fos co-localization [16, 125, 394]. Estradiol also potentiates CCK-induced c-Fos expression in the PVN but not the area postrema [101]. The anorexigenic effect of CCK also varies over the course of the estrous cycle – with greater responsiveness observed during late diestrus as compared to metastrus [99]. This estrogenic modulation of CCK-induced hypophagia is not dependent on a change in the affinity or the number of CCK-A receptors as estradiol did not alter the K D or the Bmax in the NTS, area postrema or the VMH, but did upregulate pancreatic CCK-A receptors [126]. With regard to the latter, estrogens heighten the CCK-induced stimulation of amylase released from guinea pig pancreatic acini [143]. Opposite effects were observed in rat pancreas [37], but this may have to with differences in the dose, duration, and route of estradiol administration. In the hypothalamus, CCK primarily elicits increased firing in ARH units of unknown phenotype, the responsiveness of which is increased in slices from estradiol-treated animals [280]. This CCK-induced elevation in the firing rate may be due to disinhibition as CCK reportedly activates an IA as assessed from whole-cell patch recordings from ARH neurons [49].
Estrogen-leptin interactions
Leptin is the product of the ob gene, and is postprandially secreted by adipocytes [205]. It produces a marked anorexia, and also reduces adiposity and body weight [205]. This leptin-induced anorexia is sexually disparate – with females being more responsive than males [427]. Leptin suppresses appetite in a number of different ways. First of all, leptin depolarizes POMC neurons and increases their firing rate by activating TRPC channels [74, 305]. This leptin-induced excitation of POMC neurons is paralleled by increased c-Fos and POMC mRNA expression [19, 104], and is corroborated by the fact that leptin receptor knockdown in POMC neurons results in animals with altered hypothalamic neuropeptide expression, obesity and hyperleptinemia [19]. Secondly, leptin hyperpolarizes and thereby inhibits NPY/AgRP neurons [385], which is in accordance with the ability of leptin to inhibit basal or fasting-induced increases in NPY and AgRP mRNA expression [189, 250], to reduce c-fos expression [88], to decrease NPY release from hypothalamic explants [202], as well as to increase suppressor of cytokine signaling-3 expression in these cells [104]. These inhibitory effects on NPY/AgRP could very well be attributed to the ability of leptin to presynaptically inhibit glutamatergic input onto these cells [132], and activate KATP channels within these cells [377]. Thirdly, leptin reduces endogenous cannabinoid production in the hypothalamus [89], and prevents the cannabinoid-mediated retrograde inhibition of GABAergic input to MCH neurons in the lateral hypothalamus [165]. Finally, leptin also acts in the PVN, where it alters the excitability of second-order MC4 receptor-bearing neurons; decreasing firing in a population of descending neurons and increasing firing in a population projecting to the median eminence that includes corticotropin-releasing hormone immunopositive neurons [128]. Leptin elicits these effects by activating a receptor (OB-R) that ultimately stimulates janus kinase (JAK)2/signal transducer and activator of transcription (STAT)3 and Ras/ERK/MAPK pathways [165, 229, 264]. Further downstream leptin also decreases AMPK expression and activity in the ARH and PVN [249]. This decrease contributes to the ability of leptin to inhibit NPY release from mHypoA-59 cells [88]. In the VMH the leptin-induced decrease in AMPK activity leads to a reduction in neuronal nitric oxide synthase (nNOS) that, in turn, suppresses the firing rate of glucose-sensitive cells [57]. In addition, leptin increases the activity and expression of PI3K, the transcription factor forkhead box protein (FoxO1) and PLCγ in POMC neurons, which contributes to the enhanced neuronal activity and associated anorexia caused by the adipokine [119, 150, 305, 332]. This leptin-induced PI3K activation also participates in the decreased NPY secretion from mHypoA-59 cells [88], and in stimulating the activity of the mammalian Target of Rapamycin within the hypothalamus [72]. In the periphery leptin enhances energy expenditure by upregulating UCP expression in brown adipose tissue [189].
In many respects, the effects of estrogens on energy homeostasis are closely aligned with those of leptin. ERs and OB-R ultimately converge on nearly identical signal transduction pathways that include STAT3 and PI3K [122, 162, 328]. The ablation of ERα also abolishes the leptin-induced upregulation of POMC expression in diabetic, female Akita mice [152]. In addition, OB-R knockdown in POMC neurons of female mice decreases hypothalamic ERα expression, and ovariectomized POMC-deficient female mice accumulate more fat than do ovariectomized controls [353]. However, the estrogen-induced increase in asymmetric synapse formation onto POMC perikarya is independent of leptin signaling as this effect was observed in ob/ob and db/db mice [122]. Estrogens acutely increase circulating leptin concentrations in women [206], and stimulate leptin release in organ cultures of female (but not male) adipose tissue [60]. Leptin mRNA expression also is higher in premenopausal vs. postmenopausal women [106]. Conversely, in male mice and gonadally intact female rats the anorexia caused by chronic estradiol implantation is unassociated with serum, plasma or cerebrospinal fluid levels of leptin [325, 406]. On the other hand, estradiol and ERα selective agonists chronically administered to ovariectomized female rats reduce circulating leptin concentrations along with body weight, and visceral fat content [420]. This may represent genomic effects following ERα activation, or alternatively compensatory changes in the face of chronic steroid exposure.
Estrogen-insulin interactions
Insulin plays a vital role in regulating glucose homeostasis and energy balance. Insulin receptors and insulin receptor substrate are abundantly expressed in the ARH (including POMC and NPY/AgRP neurons), VMH, PVN, as well as brainstem nuclei such as the NTS, area postrema, DMV and the hypoglossal nucleus [282, 421]. Third ventricular administration of insulin reduces food intake and weight gain in lean but not obese Zucker rats [156]. This insulin insensitivity observed in these diabetic, obese animals is due to a downregulation of insulin receptors and a reduced tyrosine kinase activity of their β subunits [273, 426]. Conversely, the hypoglycemia caused by insulin overshoot is associated with increased orexin-B expression in the lateral hypothalamus, as well as c-Fos expression in the ARH, PVN, NTS and DMV, all of which likely account for the hypoglycemia-induced hyperphagia [54]. As with leptin, the insulin-induced hypophagia is sexually differentiated; however, in this case male rats are more sensitive than their female counterparts [427]. Once insulin has gained access to the hypothalamus, it can stimulate serotonin release in the PVN/VMH region [275]. In addition, insulin attenuates ghrelin-induced increases in intracellular Ca2+ within NPY neurons [219] . It can also inhibit glucocorticoid-induced increases in NPY expression in a GABA-dependent manner [347]. Like leptin, insulin reduces the expression and activity of AMPK in the ARH, PVN and lateral hypothalamus [249], and also signals through the JAK2/STAT3 pathway [332]. In addition, leptin enhances PI3K activity in POMC and NPY/AgRP neurons in the ARH [119, 298], and in glucose-sensitive neurons in the VMH [57]. It is currently held that the insulin-induced increase in PI3K activity stimulates FoxO1 in POMC and NPY/AgRP neurons [119, 332], hyperpolarizes POMC neurons via the activation of KATP channels [150, 298], and elicits increases in nNOS activity and depolarization of glucose-sensitive neurons in the VMH [57]. This same pathway also hyperpolarizes warm-sensitive neurons in the POA, which is responsible for the insulin-induced hyperthermia and thermogenesis [341]. In the periphery insulin respectively increases and decreases circulating concentrations of leptin and ghrelin [8].
Available evidence indicates that estrogens modulate insulin sensitivity. They increase the expression of glucose transporters (i.e., GLUT1, GLUT4) in brain, skeletal muscle and adipose tissue, and promote their translocation to the plasma membrane [21]. In addition, while there is some evidence to suggest that ERβ is diabetogenic, aromatase and ERα knockout mice exhibit increased adiposity, hyperinsulinemia, glucose intolerance and decreased glucose uptake in skeletal muscle [21, 167]. The ablation of ERα also blunts the insulin-induced upregulation of POMC expression [152]. Moreover, individuals with Turner’s syndrome have a higher body mass index, total fat mass and circulating leptin concentrations, a lower total lean body mass, and reduced oxygen consumption and physical activity than their age-matched controls [137]. On the other hand, while estradiol per se enhances the activity of Akt, AMPK and TBC1D1/4 in rat soleus muscle, it does not influence insulin-stimulated glucose uptake [331]. In addition, blood glucose levels fall more sharply during the luteal phase than they do during the follicular phase in normally cycling women engaged in prolonged exercise and fed a low-carbohydrate diet [200]. This latter observation may reflect a decreased capacity for hepatic gluconeogenesis rather than a true change in insulin sensitivity caused by the heightened levels of estrogens and progesterone. Moreover, estradiol attenuates the increase in NPY expression caused by recurrent insulin-induced hypoglycemia [266].
Concluding Remarks and Future Directions
In the past two decades, major advances have taken place in our understanding of ER signaling. Multiple estrogen binding proteins have been discovered that are located to the plasma and/or endoplasmic reticulum membranes. Further, ERα and ERβ are no longer considered only classical ligand binding transcriptions factors. They are trafficked to the plasma membrane and complex with and rapidly transactivate mGluR’s. These findings have transformed our views ER signaling and advanced our understanding of estradiol temporal regulation of cellular activity. In contrast to the 1970’s and 1980’s, most of the recent research has been investigating the mechanisms of the rapid actions of estrogen signaling. One of the major challenges ahead is determining the temporal and site specific integration of the different estrogen signaling mechanisms in the regulation behavioral, reproductive and homeostatic systems. Understanding the integration of these signaling mechanisms across the estrous/menstrual cycle as well as changes in signaling that occur during menopause will be important for directing drug discovery and therapies. Although we have demonstrated that a number of estrogen signaling pathways are associated with neuroprogesterone production that is associated with the LH surge, renewed investigation into the estrogen regulation of progesterone receptor synthesis and signaling will be interesting. Further, studying the sex differences in the expression of these recently discovered estrogen signaling pathways should be illuminating as well, since most of the studies have been performed in females. In the few studies that have involved males, rapid estrogen signaling mechanisms are not as prevalent [20, 43, 141, 148, 236]. Additionally, it will be interesting how these newly discovered signaling mechanisms are associated with localized aromatase activity in the central nervous system. In all likelihood, every estrogen binding protein/receptor has not been discovered and signaling mechanism defined for known ERs. Therefore, much of the near future research will be determining the integration of these signaling mechanisms to regulate complex behavior and physiological systems in reproduction and homeostatic systems.
It is also clear that estrogens exert powerful influences on energy intake and expenditure. Estrogens suppress appetite and food seeking behavior, and do so by altering synaptic plasticity and interacting with a number different neurotransmitters/modulators, adipokines, as well as gut and pancreatic hormones. In general, estrogens attenuate the actions of orexigenic agents, and potentiate the effects of anorexigenic substances. Although some data suggest that estrogens elevate energy expenditure and elicit thermogenesis, there is an impressive body of evidence demonstrating that they lower core body temperature through their actions within the hypothalamic thermoregulatory centers.
We have made great strides in furthering our understanding of how rapid estrogenic signaling impacts cannabinoid responsiveness, NPY synthesis and release, CCK sensitivity and serotonergic neurotransmission. We also know that POMC neurons are a critical neuroanatomical substrate upon which estrogens and these other systems interact. However, despite the ever-growing knowledge base concerning the signal transduction pathways utilized by MCH, ghrelin, leptin and insulin in the brainstem, hypothalamus, gut, liver, pancreas, adipose tissue and skeletal muscle, information on how rapid estrogenic signaling alters cellular responsiveness to these peptides to help bring about estrogen-induced changes in energy balance is comparatively lacking. In addition, while it is firmly established that the hypothalamus (in general) and POMC neurons (in particular) are integral to how rapid estrogenic signaling influences energy homeostasis, to date we do not have a clear picture of how such signaling in the NTS, DMV, vagal afferents, gut, pancreas, liver, adipose tissue and skeletal muscle contributes to the overall process. Moreover, there are the incretins such as glucagon-like peptide (GLP)-1 that are secreted postprandially from intestinal L cells in response to neural stimulation from the vagus and nutrient signals in the lumen; resulting in enhanced glucose-dependent insulin secretion [64, 153, 259]. The ability of GLP-1 to attenuate energy intake is due to effects of the incretin on both the peripheral and central nervous systems [81, 96, 173, 418]. In the hypothalamus, GLP-1 attenuates the fasting-induced increases in NPY/AgRP and AMPKα2 expression, as well as the decrease in POMC expression [349]. Unfortunately, very little is known about whether estrogens can rapidly modulate the effects of GLP-1 on energy balance. Therefore, we must continue our efforts on these various frontiers to gain a more thorough understanding of how rapid estrogenic signaling regulates energy homeostasis.
Highlights.
A general background on membrane estrogen receptors (mER) is provided
mER activation of metabotropic glutamate receptor-1a (mER-mGluR1a) in reproduction
mER-mGluR1a signaling is important for estradiol facilitation of sexual behavior
mER-mGluR1a signaling is important for neuroprogesterone synthesis and LH surge
Estradiol signaling mechanisms that regulate energy homeostasis are reviewed
Acknowledgements
The research from the authors’ laboratories was supported by NIH grants: HD058638, HD04263 and DA024314.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERNCES
- [1].Abbott CR, Kennedy AR, Wren AM, Rossi M, Murphy KG, Seal LJ, Todd JF, Ghatei MA, Small CJ, Bloom SR. Identification of hypothalamic nuclei involved in the orexigenic effect of melanin-concentrating hormone. Endocrinology. 2003;144:3943–3949. doi: 10.1210/en.2003-0149. [DOI] [PubMed] [Google Scholar]
- [2].Abe H, Keen KL, Terasawa E. Rapid action of estrogens on intracellular calcium oscillations in primate luteinizing hormone-releasing hormone-1 neurons. Endocrinology. 2008;149:1155–1162. doi: 10.1210/en.2007-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Abraham IM, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signaling in mouse brain. Endocrinology. 2004;145:3055–3061. doi: 10.1210/en.2003-1676. [DOI] [PubMed] [Google Scholar]
- [4].Acconcia F, Ascenzi P, Bocedi A, Spisni E, Tomasi V, Trentalance A, Visca P, Marino M. Palmitoylation-dependent estrogen receptor alpha membrane localization: regulation by 17beta-estradiol. Molecular biology of the cell. 2005;16:231–237. doi: 10.1091/mbc.E04-07-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Acosta-Martinez M, Etgen AM. Activation of mu-opioid receptors inhibits lordosis behavior in estrogen and progesterone-primed female rats. Hormones and Behavior. 2002;41:88–100. doi: 10.1006/hbeh.2001.1741. [DOI] [PubMed] [Google Scholar]
- [6].Alon T, Friedman JM. Late-onset leanness in mice with targeted ablation of melanin concentrating hormone neurons. J Neurosci. 2006;26:389–397. doi: 10.1523/JNEUROSCI.1203-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Alves SE, McEwen BS, Hayashi S, Korach KS, Pfaff DW, Ogawa S. Estrogen-regulated progestin receptors are found in the midbrain raphe but not hippocampus of estrogen receptor alpha (ER alpha) gene-disrupted mice. Journal of Comparative Neurology. 2000;427:185–195. doi: 10.1002/1096-9861(20001113)427:2<185::aid-cne2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- [8].Anderwald C, Brabant G, Bernroider E, Horn R, Brehm A, Waldhausl W, Roden M. Insulin-dependent modulation of plasma ghrelin and leptin concentrations is less pronounced in type 2 diabetic patients. Diabetes. 2003;52:1792–1798. doi: 10.2337/diabetes.52.7.1792. [DOI] [PubMed] [Google Scholar]
- [9].Andrews ZB, Liu ZW, Walllingford N, Erion DM, Borok E, Friedman JM, Tschop MH, Shanabrough M, Cline G, Shulman GI, Coppola A, Gao XB, Horvath TL, Diano S. UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature. 2008;454:846–851. doi: 10.1038/nature07181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Anelli M, Bizzi A, Caccia S, Codegoni AM, Fracasso C, Garattini S. Anorectic activity of fluoxetine and norfluoxetine in mice, rats and guinea-pigs. The Journal of pharmacy and pharmacology. 1992;44:696–698. doi: 10.1111/j.2042-7158.1992.tb05500.x. [DOI] [PubMed] [Google Scholar]
- [11].Appleyard SM, Bailey TW, Doyle MW, Jin YH, Smart JL, Low MJ, Andresen MC. Proopiomelanocortin neurons in nucleus tractus solitarius are activated by visceral afferents: regulation by cholecystokinin and opioids. J Neurosci. 2005;25:3578–3585. doi: 10.1523/JNEUROSCI.4177-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Arden JR, Segredo V, Wang Z, Lameh J, Sadaee W. Phosphorylation and agonist-specific intracellular trafficking of an epitope-tagged mu-opioid receptor expressed in HEK 293 cells. Journal of Neurochemistry. 1995;65:1636–1645. doi: 10.1046/j.1471-4159.1995.65041636.x. [DOI] [PubMed] [Google Scholar]
- [13].Arnold M, Mura A, Langhans W, Geary N. Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. J Neurosci. 2006;26:11052–11060. doi: 10.1523/JNEUROSCI.2606-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity. Horm Behav. 2002;42:461–471. doi: 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- [15].Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones, Philosophical transactions of the Royal Society of London. Series B. Biological sciences. 2006;361:1251–1263. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Asarian L, Geary N. Estradiol enhances cholecystokinin-dependent lipid-induced satiation and activates estrogen receptor-alpha-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology. 2007;148:5656–5666. doi: 10.1210/en.2007-0341. [DOI] [PubMed] [Google Scholar]
- [17].Babic T, Townsend RL, Patterson LM, Sutton GM, Zheng H, Berthoud HR. Phenotype of neurons in the nucleus of the solitary tract that express CCK-induced activation of the ERK signaling pathway. American journal of physiology. Regulatory, integrative and comparative physiology. 2009;296:R845–854. doi: 10.1152/ajpregu.90531.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Baltazi M, Katsiki N, Savopoulos C, Iliadis F, Koliakos G, Hatzitolios AI. Plasma neuropeptide Y (NPY) and alpha-melanocyte stimulating hormone (a-MSH) levels in patients with or without hypertension and/or obesity: a pilot study. American journal of cardiovascular disease. 2011;1:48–59. [PMC free article] [PubMed] [Google Scholar]
- [19].Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr., Elmquist JK, Lowell BB. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- [20].Barabas K, Szego EM, Kaszas A, Nagy GM, Juhasz GD, Abraham IM. Sex differences in oestrogen-induced p44/42 MAPK phosphorylation in the mouse brain in vivo. Journal of neuroendocrinology. 2006;18:621–628. doi: 10.1111/j.1365-2826.2006.01447.x. [DOI] [PubMed] [Google Scholar]
- [21].Barros RP, Machado UF, Gustafsson JA. Estrogen receptors: new players in diabetes mellitus. Trends in molecular medicine. 2006;12:425–431. doi: 10.1016/j.molmed.2006.07.004. [DOI] [PubMed] [Google Scholar]
- [22].Baulieu EE. Steroid hormones in the brain: several mechanisms? In: Fuxe K, Gustafsson JA, editors. Steroid Hormone Regulation of the Brain. Pergamon; Oxford: 1981. pp. 3–14. [Google Scholar]
- [23].Baulieu EE. Neurosteroids: a new function in the brain. Biol Cell. 1991;71:3–10. doi: 10.1016/0248-4900(91)90045-o. [DOI] [PubMed] [Google Scholar]
- [24].Beach F. Hormones and Behavior. Hoeber; New York: 1948. [Google Scholar]
- [25].Beach FA. Sexual attractivity, proceptivity, and receptivity in female mammals. Horm Behav. 1976;7:105–138. doi: 10.1016/0018-506x(76)90008-8. [DOI] [PubMed] [Google Scholar]
- [26].Beato M, Herrlich P, Schutz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995;83:851–857. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- [27].Beck B, Burlet A, Nicolas JP, Burlet C. Hyperphagia in obesity is associated with a central peptidergic dysregulation in rats. The Journal of nutrition. 1990;120:806–811. doi: 10.1093/jn/120.7.806. [DOI] [PubMed] [Google Scholar]
- [28].Bell ME, Bhatnagar S, Akana SF, Choi S, Dallman MF. Disruption of arcuate/paraventricular nucleus connections changes body energy balance and response to acute stress. J Neurosci. 2000;20:6707–6713. doi: 10.1523/JNEUROSCI.20-17-06707.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Berthoud HR, Morrison C. The brain, appetite, and obesity. Annual review of psychology. 2008;59:55–92. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
- [30].Bertile F, Oudart H, Criscuolo F, Maho YL, Raclot T. Hypothalamic gene expression in long-term fasted rats: relationship with body fat. Biochemical and biophysical research communications. 2003;303:1106–1113. doi: 10.1016/s0006-291x(03)00481-9. [DOI] [PubMed] [Google Scholar]
- [31].Biscaia M, Marin S, Fernandez B, Marco EM, Rubio M, Guaza C, Ambrosio E, Viveros MP. Chronic treatment with CP 55,940 during the peri-adolescent period differentially affects the behavioural responses of male and female rats in adulthood. Psychopharmacology. 2003;170:301–308. doi: 10.1007/s00213-003-1550-7. [DOI] [PubMed] [Google Scholar]
- [32].Blaustein JD, Dudley SD, Gray JM, Roy EJ, Wade GN. Long-term retention of estradiol by brain cell nuclei and female rat sexual behavior. Brain Research. 1979;173:355–359. doi: 10.1016/0006-8993(79)90637-1. [DOI] [PubMed] [Google Scholar]
- [33].Blaustein JD, Feder HH. Cytoplasmic progestin receptors in female guinea pig brain and their relationship to refractoriness in expression of female sexual behavior. Brain Res. 1979;177:489–498. doi: 10.1016/0006-8993(79)90466-9. [DOI] [PubMed] [Google Scholar]
- [34].Blaustein JD, Feder HH. Nuclear progestin receptors in guinea pig brain measured by an in vitro exchange assay after hormonal treatments that affect lordosis. Endocrinol. 1980;106:1061–1069. doi: 10.1210/endo-106-4-1061. [DOI] [PubMed] [Google Scholar]
- [35].Blaustein JD, Finkbohner R, Delville Y. Estrogen-induced and estrogen-facilitated female rat sexual behavior is not mediated by progestin receptors. Neuroendocrinology. 1987;45:152–159. doi: 10.1159/000124717. [DOI] [PubMed] [Google Scholar]
- [36].Blaustein JD, Wade GN. Ovarian influences on the meal patterns of female rats. Physiology & Behavior. 1976;17:201–208. doi: 10.1016/0031-9384(76)90064-0. [DOI] [PubMed] [Google Scholar]
- [37].Blevins GT, Jr., Huang HS, Tangoku A, McKay DW, Rayford PL. Estrogens influence cholecystokinin stimulated pancreatic amylase release and acinar cell membrane cholecystokinin receptors in rat. Life sciences. 1991;48:1565–1574. doi: 10.1016/0024-3205(91)90281-f. [DOI] [PubMed] [Google Scholar]
- [38].Boling FL, Blandau RJ. The estrogen-progesterone induction of mating responses in the spayed female rat. Endocrinol. 1939;25:359–364. [Google Scholar]
- [39].Bollig A, Miksicek RJ. An estrogen receptor-alpha splicing variant mediates both positive and negative effects on gene transcription. Molecular endocrinology. 2000;14:634–649. doi: 10.1210/mend.14.5.0460. [DOI] [PubMed] [Google Scholar]
- [40].Bondar G, Kuo J, Hamid N, Micevych P. Estradiol-induced estrogen receptor-alpha trafficking. J Neurosci. 2009;29:15323–15330. doi: 10.1523/JNEUROSCI.2107-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Boulware MI, Kordasiewicz H, Mermelstein PG. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. Journal of Neuroscience. 2007;27:9941–9950. doi: 10.1523/JNEUROSCI.1647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Boulware MI, Mermelstein PG. Membrane estrogen receptors activate metabotropic glutamate receptors to influence nervous system physiology. Steroids. 2009;74:608–613. doi: 10.1016/j.steroids.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Bouwknecht JA, van der Gugten J, Hijzen TH, Maes RA, Hen R, Olivier B. Male and female 5-HT(1B) receptor knockout mice have higher body weights than wildtypes. Physiology & Behavior. 2001;74:507–516. doi: 10.1016/s0031-9384(01)00589-3. [DOI] [PubMed] [Google Scholar]
- [45].Broberger C, Johansen J, Johansson C, Schalling M, Hokfelt T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:15043–15048. doi: 10.1073/pnas.95.25.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Brom GM, Schwartz NB. Acute changes in the estrous cycle following ovariectomy in the golden hamster. Neuroendocrinology. 1968;3:366–377. doi: 10.1159/000121725. [DOI] [PubMed] [Google Scholar]
- [47].Budney AJ, Hughes JR, Moore BA, Novy PL. Marijuana abstinence effects in marijuana smokers maintained in their home environment. Archives of general psychiatry. 2001;58:917–924. doi: 10.1001/archpsyc.58.10.917. [DOI] [PubMed] [Google Scholar]
- [48].Bueno J, Pfaff DW. Single unit recording in hypothalamus and preoptic area of estrogen-treated and untreated ovariectomized female rats. Brain research. 1976;101:67–78. doi: 10.1016/0006-8993(76)90988-4. [DOI] [PubMed] [Google Scholar]
- [49].Burdakov D, Ashcroft FM. Cholecystokinin tunes firing of an electrically distinct subset of arcuate nucleus neurons by activating A-Type potassium channels. J Neurosci. 2002;22:6380–6387. doi: 10.1523/JNEUROSCI.22-15-06380.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Burdakov D, Luckman SM, Verkhratsky A. Glucose-sensing neurons of the hypothalamus, Philosophical transactions of the Royal Society of London. Series B. Biological sciences. 2005;360:2227–2235. doi: 10.1098/rstb.2005.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94:1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- [52].Butera PC, Czaja JA. Intracranial estradiol in ovariectomized guinea pigs: effects on ingestive behaviors and body weight. Brain research. 1984;322:41–48. doi: 10.1016/0006-8993(84)91178-8. [DOI] [PubMed] [Google Scholar]
- [53].Cagnacci A, Volpe A, Paoletti AM, Melis GB. Regulation of the 24-hour rhythm of body temperature in menstrual cycles with spontaneous and gonadotropin-induced ovulation. Fertility and sterility. 1997;68:421–425. doi: 10.1016/s0015-0282(97)00242-2. [DOI] [PubMed] [Google Scholar]
- [54].Cai XJ, Evans ML, Lister CA, Leslie RA, Arch JR, Wilson S, Williams G. Hypoglycemia activates orexin neurons and selectively increases hypothalamic orexin-B levels: responses inhibited by feeding and possibly mediated by the nucleus of the solitary tract. Diabetes. 2001;50:105–112. doi: 10.2337/diabetes.50.1.105. [DOI] [PubMed] [Google Scholar]
- [55].Calizo LH, Flanagan-Cato LM. Estrogen-induced dendritic spine elimination on female rat ventromedial hypothalamic neurons that project to the periaqueductal gray. J Comp Neurol. 2002;447:234–248. doi: 10.1002/cne.10223. [DOI] [PubMed] [Google Scholar]
- [56].Calizo LH, Flanagan-Cato LM. Hormonal-neural integration in the female rat ventromedial hypothalamus: triple labeling for estrogen receptor-alpha, retrograde tract tracing from the periaqueductal gray, and mating-induced Fos expression. Endocrinology. 2003;144:5430–5440. doi: 10.1210/en.2003-0331. [DOI] [PubMed] [Google Scholar]
- [57].Canabal DD, Song Z, Potian JG, Beuve A, McArdle JJ, Routh VH. Glucose, insulin, and leptin signaling pathways modulate nitric oxide synthesis in glucose-inhibited neurons in the ventromedial hypothalamus. American journal of physiology. Regulatory, integrative and comparative physiology. 2007;292:R1418–1428. doi: 10.1152/ajpregu.00216.2006. [DOI] [PubMed] [Google Scholar]
- [58].Carnegie JA, Tsang BK. Follicle-stimulating hormone--regulated granulosa cell steroidogenesis: involvement of the calcium-calmodulin system. American journal of obstetrics and gynecology. 1983;145:223–228. doi: 10.1016/0002-9378(83)90496-9. [DOI] [PubMed] [Google Scholar]
- [59].Carroll J, Swann K. Spontaneous cytosolic calcium oscillations driven by inositol trisphosphate occur during in vitro maturation of mouse oocytes. The Journal of biological chemistry. 1992;267:11196–11201. [PubMed] [Google Scholar]
- [60].Casabiell X, Pineiro V, Peino R, Lage M, Camina J, Gallego R, Vallejo LG, Dieguez C, Casanueva FF. Gender differences in both spontaneous and stimulated leptin secretion by human omental adipose tissue in vitro: dexamethasone and estradiol stimulate leptin release in women, but not in men. The Journal of clinical endocrinology and metabolism. 1998;83:2149–2155. doi: 10.1210/jcem.83.6.4849. [DOI] [PubMed] [Google Scholar]
- [61].Chaban VV, Lakhter AJ, Micevych P. A membrane estrogen receptor mediates intracellular calcium release in astrocytes. Endocrinology. 2004;145:3788–3795. doi: 10.1210/en.2004-0149. [DOI] [PubMed] [Google Scholar]
- [62].Chappell PE, Levine JE. Stimulation of gonadotropin-releasing hormone surges by estrogen. I. Role of hypothalamic progesterone receptors. Endocrinology. 2000;141:1477–1485. doi: 10.1210/endo.141.4.7428. [DOI] [PubMed] [Google Scholar]
- [63].Cheng SB, Graeber CT, Quinn JA, Filardo EJ. Retrograde transport of the transmembrane estrogen receptor, G-protein-coupled-receptor-30 (GPR30/GPER) from the plasma membrane towards the nucleus. Steroids. 2011;76:892–896. doi: 10.1016/j.steroids.2011.02.018. [DOI] [PubMed] [Google Scholar]
- [64].Cheong YH, Kim MK, Son MH, Kaang BK. Glucose exposure pattern determines glucagon-like peptide 1 receptor expression and signaling through endoplasmic reticulum stress in rat insulinoma cells. Biochemical and biophysical research communications. 2011;414:220–225. doi: 10.1016/j.bbrc.2011.09.061. [DOI] [PubMed] [Google Scholar]
- [65].Cheung S, Hammer R. Gonadal steroid hormone regulation of proopiomelanocortin gene expression in the arcuate neurons that innervate the medial preoptic are of the rat. Neuroendocrinology. 1995;62:283–292. doi: 10.1159/000127015. [DOI] [PubMed] [Google Scholar]
- [66].Clark JT, Kalra PS, Kalra SP. Neuropeptide Y stimulates feeding but inhibits sexual behavior in rats. Endocrinology. 1985;117:2435–2442. doi: 10.1210/endo-117-6-2435. [DOI] [PubMed] [Google Scholar]
- [67].Clarkson J, Han SK, Liu X, Lee K, Herbison AE. Neurobiological mechanisms underlying kisspeptin activation of gonadotropin-releasing hormone (GnRH) neurons at puberty. Molecular and cellular endocrinology. 2010;324:45–50. doi: 10.1016/j.mce.2010.01.026. [DOI] [PubMed] [Google Scholar]
- [68].Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Clegg DJ, Brown LM, Zigman JM, Kemp CJ, Strader AD, Benoit SC, Woods SC, Mangiaracina M, Geary N. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes. 2007;56:1051–1058. doi: 10.2337/db06-0015. [DOI] [PubMed] [Google Scholar]
- [70].Clemens LG, Weaver DR. The role of gonadal hormone in the activation of feminine sexual behavior. In: Adler N, Pfaff D, Goy RW, editors. Handbook of Behavioral Neurobiology. Plenum Press; New York: 1985. pp. 183–227. [Google Scholar]
- [71].Corpechot C, Robel P, Axelson M, Sjovall J, Baulieu EE. Characterization and measurement of dehydroepiandrosterone sulfate in rat brain. Proc. Natl. Acad. Sci. U.S.A. 1981;78:4704–4707. doi: 10.1073/pnas.78.8.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- [73].Cottingham SL, Femano PA, Pfaff DW. Electrical stimulation of the midbrain central gray facilitates reticulospinal activation of axial muscle EMG. Exp Neurol. 1987;97:704–724. doi: 10.1016/0014-4886(87)90127-0. [DOI] [PubMed] [Google Scholar]
- [74].Cowley MA, Cone RD, Enriori P, Louiselle I, Williams SM, Evans AE. Electrophysiological actions of peripheral hormones on melanocortin neurons. Annals of the New York Academy of Sciences. 2003;994:175–186. doi: 10.1111/j.1749-6632.2003.tb03178.x. [DOI] [PubMed] [Google Scholar]
- [75].Czaja JA, Butera PC. Body temperature and temperature gradients: changes during the estrous cycle and in response to ovarian steroids. Physiology & Behavior. 1986;36:591–596. doi: 10.1016/0031-9384(86)90339-2. [DOI] [PubMed] [Google Scholar]
- [76].Czaja JA, Goldfoot DA, Karavolas HJ. Comparative facilitation and inhibition of lordosis in the guinea pig with progesterone, 5-alpha-pregnane-3,2-dione, or 3-alpha-hydroxy-5-alpha-pregnan-20-one. Hormones and behavior. 1974;5:261–274. doi: 10.1016/0018-506x(74)90034-8. [DOI] [PubMed] [Google Scholar]
- [77].d’Anglemont de Tassigny X, Fagg LA, Carlton MB, Colledge WH. Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology. 2008;149:3926–3932. doi: 10.1210/en.2007-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Dacks PA, Rance NE. Effects of estradiol on the thermoneutral zone and core temperature in ovariectomized rats. Endocrinology. 2010;151:1187–1193. doi: 10.1210/en.2009-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Dafopoulos K, Chalvatzas N, Kosmas G, Kallitsaris A, Pournaras S, Messinis IE. The effect of estrogens on plasma ghrelin concentrations in women. Journal of endocrinological investigation. 2010;33:109–112. doi: 10.1007/BF03346563. [DOI] [PubMed] [Google Scholar]
- [80].Day JR, Morales TH, Lu JK. Male stimulation of luteinizing hormone surge, progesterone secretion and ovulation in spontaneously persistent-estrous, aging rats. Biol Reprod. 1988;38:1019–1026. doi: 10.1095/biolreprod38.5.1019. [DOI] [PubMed] [Google Scholar]
- [81].De Silva A, Salem V, Long CJ, Makwana A, Newbould RD, Rabiner EA, Ghatei MA, Bloom SR, Matthews PM, Beaver JD, Dhillo WS. The gut hormones PYY 3-36 and GLP-1 7-36 amide reduce food intake and modulate brain activity in appetite centers in humans. Cell metabolism. 2011;14:700–706. doi: 10.1016/j.cmet.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].De Souza MJ, West SL, Jamal SA, Hawker GA, Gundberg CM, Williams NI. The presence of both an energy deficiency and estrogen deficiency exacerbate alterations of bone metabolism in exercising women. Bone. 2008;43:140–148. doi: 10.1016/j.bone.2008.03.013. [DOI] [PubMed] [Google Scholar]
- [83].Deecher DC, Swiggard P, Frail DE, O’Connor LT. Characterization of a membrane-associated estrogen receptor in a rat hypothalamic cell line (D12) Endocrine. 2003;22:211–223. doi: 10.1385/ENDO:22:3:211. [DOI] [PubMed] [Google Scholar]
- [84].Devall AJ, Santos JM, Lovick TA. Estrous cycle stage influences on neuronal responsiveness to repeated anxiogenic stress in female rats. Behavioural brain research. 2011;225:334–340. doi: 10.1016/j.bbr.2011.07.038. [DOI] [PubMed] [Google Scholar]
- [85].Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P. Membrane estrogen receptor-alpha interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J Neurosci. 2007;27:9294–9300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Dewing P, Christensen A, Bondar G, Micevych P. Protein kinase C signaling in the hypothalamic arcuate nucleus regulates sexual receptivity in female rats.[see comment] Endocrinology. 2008;149:5934–5942. doi: 10.1210/en.2008-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Dhillon SS, Belsham DD. Estrogen inhibits NPY secretion through membrane-associated estrogen receptor (ER)-alpha in clonal, immortalized hypothalamic neurons. International journal of obesity. 2011;35:198–207. doi: 10.1038/ijo.2010.124. [DOI] [PubMed] [Google Scholar]
- [88].Dhillon SS, McFadden SA, Chalmers JA, Centeno ML, Kim GL, Belsham DD. Cellular leptin resistance impairs the leptin-mediated suppression of neuropeptide y secretion in hypothalamic neurons. Endocrinology. 2011;152:4138–4147. doi: 10.1210/en.2011-0178. [DOI] [PubMed] [Google Scholar]
- [89].Di Marzo V, Goparaju SK, Wang L, Liu J, Batkai S, Jarai Z, Fezza F, Miura GI, Palmiter RD, Sugiura T, Kunos G. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- [90].Diano S, Naftolin F, Horvath TL. Gonadal steroids target AMPA glutamate receptor-containing neurons in the rat hypothalamus, septum and amygdala: a morphological and biochemical study. Endocrinology. 1997;138:778–789. doi: 10.1210/endo.138.2.4937. [DOI] [PubMed] [Google Scholar]
- [91].Diaz S, Farhang B, Hoien J, Stahlman M, Adatia N, Cox JM, Wagner EJ. Sex differences in the cannabinoid modulation of appetite, body temperature and neurotransmission at POMC synapses. Neuroendocrinology. 2009;89:424–440. doi: 10.1159/000191646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Dohanich GP, Witcher JA, Weaver DR, Clemens LG. Alteration of muscarinic binding in specific brain areas following estrogen treatment. Brain Res. 1982;241:347–350. doi: 10.1016/0006-8993(82)91075-7. [DOI] [PubMed] [Google Scholar]
- [93].Dominguez R, Hu E, Zhou M, Baudry M. 17beta-estradiol-mediated neuroprotection and ERK activation require a pertussis toxin-sensitive mechanism involving GRK2 and beta-arrestin-1. J Neurosci. 2009;29:4228–4238. doi: 10.1523/JNEUROSCI.0550-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Dominguez R, Kuo J, Dewing P, Micevych P. Trafficking of membrane ER involves membrane-initiated estrogen signaling in immortalized hypothalamic N-38 neurons. Endocrinology. 2011 doi: 10.1016/j.steroids.2012.12.008. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Dominguez R, Micevych P. Estradiol rapidly regulates membrane estrogen receptor alpha levels in hypothalamic neurons. The Journal of Neuroscience. 2010;30:12589–12596. doi: 10.1523/JNEUROSCI.1038-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Dossat AM, Lilly N, Kay K, Williams DL. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J Neurosci. 2011;31:14453–14457. doi: 10.1523/JNEUROSCI.3262-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Drouva SV, Laplante E, Kordon C. Effects of ovarian steroids on in vitro release of LHRH from mediobasal hypothalamus. Neuroendocrinology. 1983;37:336–341. doi: 10.1159/000123572. [DOI] [PubMed] [Google Scholar]
- [98].Dubuc PU. Effects of estrogen on food intake, body weight, and temperature of male and female obese mice. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine. 1985;180:468–473. doi: 10.3181/00379727-180-42204. [DOI] [PubMed] [Google Scholar]
- [99].Dulawa SC, Vanderweele DA. Cholecystokinin and estradiol synergistically potentiate satiety in rats. Peptides. 1994;15:913–918. doi: 10.1016/0196-9781(94)90050-7. [DOI] [PubMed] [Google Scholar]
- [100].Duran C, Yonem A, Ustun I, Ozcan O, Ipcioglu OM, Basekim CC. Plasma ghrelin levels in males with idiopathic hypogonadotropic hypogonadism. Endocrine. 2008;34:81–86. doi: 10.1007/s12020-008-9102-x. [DOI] [PubMed] [Google Scholar]
- [101].Eckel LA, Houpt TA, Geary N. Estradiol treatment increases CCK-induced c-Fos expression in the brains of ovariectomized rats. American journal of physiology. Regulatory, integrative and comparative physiology. 2002;283:R1378–1385. doi: 10.1152/ajpregu.00300.2002. [DOI] [PubMed] [Google Scholar]
- [102].Eckersell CB, Popper P, Micevych PE. Estrogen-induced alteration of mu-opioid receptor immunoreactivity in the medial preoptic nucleus and medial amygdala. J Neurosci. 1998;18:3967–3976. doi: 10.1523/JNEUROSCI.18-10-03967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Edwards CM, Abusnana S, Sunter D, Murphy KG, Ghatei MA, Bloom SR. The effect of the orexins on food intake: comparison with neuropeptide Y, melanin-concentrating hormone and galanin. The Journal of endocrinology. 1999;160:R7–12. doi: 10.1677/joe.0.160r007. [DOI] [PubMed] [Google Scholar]
- [104].Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, Elmquist JK. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–786. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- [105].Fahrbach SE, Meisel RL, Pfaff DW. Preoptic implants of estradiol increase wheel running but not the open field activity of female rats. Physiology & Behavior. 1985;35:985–992. doi: 10.1016/0031-9384(85)90270-7. [DOI] [PubMed] [Google Scholar]
- [106].Fajardo ME, Malacara JM, Martinez-Rodriguez HG, Barrera-Saldana HA. Hormone and metabolic factors associated with leptin mRNA expression in pre- and postmenopausal women. Steroids. 2004;69:425–430. doi: 10.1016/j.steroids.2004.03.013. [DOI] [PubMed] [Google Scholar]
- [107].Farhang B, Pietruszewski L, Lutfy K, Wagner EJ. The role of the NOP receptor in regulating food intake, meal pattern, and the excitability of proopiomelanocortin neurons. Neuropharmacology. 2010;59:190–200. doi: 10.1016/j.neuropharm.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Feder HH, Brown-Grant K, Corker CS. Pre-ovulatory progesterone, the adrenal cortex and the ‘critical period’ for luteinizing hormone release in rats. The Journal of endocrinology. 1971;50:29–39. doi: 10.1677/joe.0.0500029. [DOI] [PubMed] [Google Scholar]
- [109].Ferin M, Tempone A, Zimmering PE, Van de Wiele RL. Effect of antibodies to 17beta-estradiol and progesterone on the estrous cycle of the rat. Endocrinology. 1969;85:1070–1078. doi: 10.1210/endo-85-6-1070. [DOI] [PubMed] [Google Scholar]
- [110].Ferrer-Lorente R, Fernandez-Lopez JA, Alemany M, Cabot C. Short-term oral oleoyl-estrone decreases the expression of ghrelin in the rat stomach. Regulatory peptides. 2009;152:79–81. doi: 10.1016/j.regpep.2008.09.004. [DOI] [PubMed] [Google Scholar]
- [111].Filardo E, Quinn J, Pang Y, Graeber C, Shaw S, Dong J, Thomas P. Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology. 2007;148:3236–3245. doi: 10.1210/en.2006-1605. [DOI] [PubMed] [Google Scholar]
- [112].Filardo EJ, Graeber CT, Quinn JA, Resnick MB, Giri D, DeLellis RA, Steinhoff MM, Sabo E. Distribution of GPR30, a seven membrane-spanning estrogen receptor, in primary breast cancer and its association with clinicopathologic determinants of tumor progression. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12:6359–6366. doi: 10.1158/1078-0432.CCR-06-0860. [DOI] [PubMed] [Google Scholar]
- [113].Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- [114].Filardo EJ, Quinn JA, Frackelton AR, Jr., Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol. 2002;16:70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- [115].Fink K, Boing C, Gothert M. Presynaptic 5-HT autoreceptors modulate N-methyl-D-aspartate-evoked 5-hydroxytryptamine release in the guinea-pig brain cortex. European journal of pharmacology. 1996;300:79–82. doi: 10.1016/0014-2999(96)00042-8. [DOI] [PubMed] [Google Scholar]
- [116].Fioramonti X, Contie S, Song Z, Routh VH, Lorsignol A, Penicaud L. Characterization of glucosensing neuron subpopulations in the arcuate nucleus: integration in neuropeptide Y and pro-opio melanocortin networks? Diabetes. 2007;56:1219–1227. doi: 10.2337/db06-0567. [DOI] [PubMed] [Google Scholar]
- [117].Flores JA, Aguirre C, Sharma OP, Veldhuis JD. Luteinizing hormone (LH) stimulates both intracellular calcium ion ([Ca2+]i) mobilization and transmembrane cation influx in single ovarian (granulosa) cells: recruitment as a cellular mechanism of LH-[Ca2+]i dose response. Endocrinology. 1998;139:3606–3612. doi: 10.1210/endo.139.8.6162. [DOI] [PubMed] [Google Scholar]
- [118].Fong TM, Mao C, MacNeil T, Kalyani R, Smith T, Weinberg D, Tota MR, Van der Ploeg LH. ART (protein product of agouti-related transcript) as an antagonist of MC-3 and MC-4 receptors. Biochemical and biophysical research communications. 1997;237:629–631. doi: 10.1006/bbrc.1997.7200. [DOI] [PubMed] [Google Scholar]
- [119].Fukuda M, Jones JE, Olson D, Hill J, Lee CE, Gautron L, Choi M, Zigman JM, Lowell BB, Elmquist JK. Monitoring FoxO1 localization in chemically identified neurons. J Neurosci. 2008;28:13640–13648. doi: 10.1523/JNEUROSCI.4023-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Funakoshi T, Yanai A, Shinoda K, Kawano MM, Mizukami Y. G protein-coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochemical and biophysical research communications. 2006;346:904–910. doi: 10.1016/j.bbrc.2006.05.191. [DOI] [PubMed] [Google Scholar]
- [121].Fuqua SA, Fitzgerald SD, Allred DC, Elledge RM, Nawaz Z, McDonnell DP, O’Malley BW, Greene GL, McGuire WL. Inhibition of estrogen receptor action by a naturally occurring variant in human breast tumors. Cancer research. 1992;52:483–486. [PubMed] [Google Scholar]
- [122].Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, Bechmann I, Leranth C, Allerand D. Toran-, Priest CA, Roberts JL, Gao XB, Mobbs C, Shulman GI, Diano S, Horvath TL. Anorectic estrogen mimics leptin’s effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nature medicine. 2007;13:89–94. doi: 10.1038/nm1525. [DOI] [PubMed] [Google Scholar]
- [123].Garcia-Segura LM, Naftolin F, Hutchison JB, Azcoitia I, Chowen JA. Role of astroglia in estrogen regulation of synaptic plasticity and brain repair. Journal of neurobiology. 1999;40:574–584. [PubMed] [Google Scholar]
- [124].Garcia BL, Mana A, Kim A, Sinchak K. Society for Neuroscience, Abstract Viewer/Itinerary Planner. Society for Neuroscience; Washington, DC: San Diego: 2010. Antagonism of estrogen receptors facilitates sexual receptivity through opioid circuits in the arcuate nucleus of the hypothalamus and the medial preoptic nucleus in estradiol primed non-receptive female rats. 2010. [Google Scholar]
- [125].Geary N. Estradiol, CCK and satiation. Peptides. 2001;2:1251–1263. doi: 10.1016/s0196-9781(01)00449-1. [DOI] [PubMed] [Google Scholar]
- [126].Geary N, Smith GP, Corp ES. The increased satiating potency of CCK-8 by estradiol is not mediated by upregulation of NTS CCK receptors. Brain research. 1996;719:179–186. doi: 10.1016/0006-8993(96)00099-6. [DOI] [PubMed] [Google Scholar]
- [127].Gerra G, Fertonani G, Tagliavini P, Zaimovic A, Delsignore R, Maestri D, Avanzini P, Caccavari R, Brambilla F. Serotonin function in detoxified heroin abusers: prolactin and cortisol responses to fenfluramine challenge. Psychiatry research. 1995;58:153–160. doi: 10.1016/0165-1781(95)02665-j. [DOI] [PubMed] [Google Scholar]
- [128].Ghamari-Langroudi M, Srisai D, Cone RD. Multinodal regulation of the arcuate/paraventricular nucleus circuit by leptin. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:355–360. doi: 10.1073/pnas.1016785108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Giambiagi N, Pasqualini JR. Immunorecognition of cytosol and nuclear estradiol receptor of fetal guinea pig uterus using monoclonal antibody. Endocrinology. 1982;110:1067–1069. doi: 10.1210/endo-110-3-1067. [DOI] [PubMed] [Google Scholar]
- [130].Giambiagi N, Pasqualini JR, Greene G, Jensen EV. Recognition of two forms of the estrogen receptor in the guinea-pig uterus at different stages of development by a monoclonal antibody to the human estrogen receptor. Dynamics of the translocation of these two forms to the nucleus. Journal of steroid biochemistry. 1984;20:397–400. doi: 10.1016/0022-4731(84)90241-3. [DOI] [PubMed] [Google Scholar]
- [131].Glascock RF, Hoekstra WG. Selective accumulation of tritium-labelled hexoestrol by the reproductive organs of immature female goats and sheep. The Biochemical journal. 1959;72:673–682. doi: 10.1042/bj0720673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Glaum SR, Hara M, Bindokas VP, Lee CC, Polonsky KS, Bell GI, Miller RJ. Leptin, the obese gene product, rapidly modulates synaptic transmission in the hypothalamus. Molecular pharmacology. 1996;50:230–235. [PubMed] [Google Scholar]
- [133].Glick M, Segal-Lieberman G, Cohen R, Kronfeld-Schor N. Chronic MCH infusion causes a decrease in energy expenditure and body temperature, and an increase in serum IGF-1 levels in mice. Endocrine. 2009;36:479–485. doi: 10.1007/s12020-009-9252-5. [DOI] [PubMed] [Google Scholar]
- [134].Gong EJ, Garrel D, Calloway DH. Menstrual cycle and voluntary food intake. The American journal of clinical nutrition. 1989;49:252–258. doi: 10.1093/ajcn/49.2.252. [DOI] [PubMed] [Google Scholar]
- [135].Gorosito SV, Lorenzo AG, Cambiasso MJ. Estrogen receptor alpha is expressed on the cell-surface of embryonic hypothalamic neurons. Neuroscience. 2008;154:1173–1177. doi: 10.1016/j.neuroscience.2008.05.001. [DOI] [PubMed] [Google Scholar]
- [136].Gorski J, Shyamala G, Toft D. Interrelationships of nuclear and cytoplasmic estrogen receptors. Current topics in developmental biology. 1969;4:149–167. doi: 10.1016/s0070-2153(08)60483-4. [DOI] [PubMed] [Google Scholar]
- [137].Gravholt CH, Hjerrild BE, Mosekilde L, Hansen TK, Rasmussen LM, Frystyk J, Flyvbjerg A, Christiansen JS. Body composition is distinctly altered in Turner syndrome: relations to glucose metabolism, circulating adipokines, and endothelial adhesion molecules. European journal of endocrinology / European Federation of Endocrine Societies. 2006;155:583–592. doi: 10.1530/eje.1.02267. [DOI] [PubMed] [Google Scholar]
- [138].Green S, Chambon P. A superfamily of potentially oncogenic hormone receptors. Nature. 1986;324:615–617. doi: 10.1038/324615a0. [DOI] [PubMed] [Google Scholar]
- [139].Green S, Kumar V, Theulaz I, Wahli W, Chambon P. The N-terminal DNA-binding ‘zinc finger’ of the oestrogen and glucocorticoid receptors determines target gene specificity. The EMBO journal. 1988;7:3037–3044. doi: 10.1002/j.1460-2075.1988.tb03168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Greenman Y, Rouach V, Limor R, Gilad S, Stern N. Testosterone is a strong correlate of ghrelin levels in men and postmenopausal women. Neuroendocrinology. 2009;89:79–85. doi: 10.1159/000151768. [DOI] [PubMed] [Google Scholar]
- [141].Grove-Strawser D, Boulware MI, Mermelstein PG. Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience. 2010;170:1045–1055. doi: 10.1016/j.neuroscience.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Guennoun R, Fiddes RJ, Gouezou M, Lombes M, Baulieu EE. A key enzyme in the biosynthesis of neurosteroids, 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4-isomerase (3 beta-HSD), is expressed in rat brain. Brain Res Mol Brain Res. 1995;30:287–300. doi: 10.1016/0169-328x(95)00016-l. [DOI] [PubMed] [Google Scholar]
- [143].Guo YS, Singh P. Effect of estradiol on pancreatic amylase and cholecystokinin binding in ovariectomized guinea pigs. Journal of steroid biochemistry. 1989;33:459–464. doi: 10.1016/0022-4731(89)90337-3. [DOI] [PubMed] [Google Scholar]
- [144].Haase J, Mana A, Chinn E, Sinchak K. Society for Neuroscience, Abstract Viewer/Itinerary Planner. Society for Neuroscience; Washington, DC: 2008. Estradiol upregulates expression and colocalization progesterone receptor and orphanin FQ/nociceptin immunopositive neurons in the arcuate nucleus of the hypothalamus; pp. 781–789. Washington, DC, 2008. [Google Scholar]
- [145].Hanada R, Nakazato M, Matsukura S, Murakami N, Yoshimatsu H, Sakata T. Differential regulation of melanin-concentrating hormone and orexin genes in the agouti-related protein/melanocortin-4 receptor system. Biochemical and biophysical research communications. 2000;268:88–91. doi: 10.1006/bbrc.1999.2081. [DOI] [PubMed] [Google Scholar]
- [146].Haney M, Rabkin J, Gunderson E, Foltin RW. Dronabinol and marijuana in HIV(+) marijuana smokers: acute effects on caloric intake and mood. Psychopharmacology. 2005;181:170–178. doi: 10.1007/s00213-005-2242-2. [DOI] [PubMed] [Google Scholar]
- [147].Hawkins RA, Freedman B, Marshall A, Killen E. Oestradiol-17 beta and prolactin levels in rat peripheral plasma. British journal of cancer. 1975;32:179–185. doi: 10.1038/bjc.1975.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Heimovics SA, Prior NH, Maddison CJ, Soma KK. Rapid and widespread effects of 17beta-estradiol on intracellular signaling in the male songbird brain: a seasonal comparison. Endocrinology. 2012;153:1364–1376. doi: 10.1210/en.2011-1525. [DOI] [PubMed] [Google Scholar]
- [149].Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V) Brain research reviews. 2008;57:277–287. doi: 10.1016/j.brainresrev.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. The Journal of clinical investigation. 2008;118:1796–1805. doi: 10.1172/JCI32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Hirahara Y, Matsuda K, Gao W, Arvanitis DN, Kawata M, Boggs JM. The localization and non-genomic function of the membrane-associated estrogen receptor in oligodendrocytes. Glia. 2009;57:153–165. doi: 10.1002/glia.20742. [DOI] [PubMed] [Google Scholar]
- [152].Hirosawa M, Minata M, Harada KH, Hitomi T, Krust A, Koizumi A. Ablation of estrogen receptor alpha (ERalpha) prevents upregulation of POMC by leptin and insulin. Biochemical and biophysical research communications. 2008;371:320–323. doi: 10.1016/j.bbrc.2008.04.073. [DOI] [PubMed] [Google Scholar]
- [153].Holst JJ, McGill MA. Potential new approaches to modifying intestinal GLP-1 secretion in patients with type 2 diabetes mellitus: focus on bile acid sequestrants. Clinical drug investigation. 2012;32:1–14. doi: 10.2165/11595370-000000000-00000. [DOI] [PubMed] [Google Scholar]
- [154].Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- [155].Ibrahim N, Bosch MA, Smart JL, Qiu J, Rubinstein M, Ronnekleiv OK, Low MJ, Kelly MJ. Hypothalamic proopiomelanocortin neurons are glucose responsive and express K(ATP) channels. Endocrinology. 2003;144:1331–1340. doi: 10.1210/en.2002-221033. [DOI] [PubMed] [Google Scholar]
- [156].Ikeda H, West DB, Pustek JJ, Figlewicz DP, Greenwood MR, Porte D, Jr., Woods SC. Intraventricular insulin reduces food intake and body weight of lean but not obese Zucker rats. Appetite. 1986;7:381–386. doi: 10.1016/s0195-6663(86)80006-x. [DOI] [PubMed] [Google Scholar]
- [157].Inui A. Cancer anorexia-cachexia syndrome: current issues in research and management. CA: a cancer journal for clinicians. 2002;52:72–91. doi: 10.3322/canjclin.52.2.72. [DOI] [PubMed] [Google Scholar]
- [158].Isensee J, Meoli L, Zazzu V, Nabzdyk C, Witt H, Soewarto D, Effertz K, Fuchs H, Gailus-Durner V, Busch D, Adler T, de Angelis MH, Irgang M, Otto C, Noppinger PR. Expression pattern of G protein-coupled receptor 30 in LacZ reporter mice. Endocrinology. 2009;150:1722–1730. doi: 10.1210/en.2008-1488. [DOI] [PubMed] [Google Scholar]
- [159].Ivanova T, Karolczak M, Beyer C. Estrogen stimulates the mitogen-activated protein kinase pathway in midbrain astroglia. Brain research. 2001;889:264–269. doi: 10.1016/s0006-8993(00)03149-8. [DOI] [PubMed] [Google Scholar]
- [160].Jacobowitz DM, O’Donohue TL. alpha-Melanocyte stimulating hormone: immunohistochemical identification and mapping in neurons of rat brain. Proceedings of the National Academy of Sciences of the United States of America. 1978;75:6300–6304. doi: 10.1073/pnas.75.12.6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Jamshidi N, Taylor DA. Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats. British journal of pharmacology. 2001;134:1151–1154. doi: 10.1038/sj.bjp.0704379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Jeffery GS, Peng KC, Wagner EJ. The role of phosphatidylinositol-3-kinase and AMP-activated kinase in the rapid estrogenic attentuation of cannabinoid-induced changes in energy homeostasis. Pharmaceuticals. 2011;4:630–651. [Google Scholar]
- [163].Jensen EV, Greene GL, Closs LE, DeSombre ER, Nadji M. In: Recent Progress in Hormone Research. Greep RO, editor. Academic; New York: 1982. pp. 1–34. [DOI] [PubMed] [Google Scholar]
- [164].Jensen EV, Jacobson HI. Fate of steroid estrogens in target tissues. In: Pincus G, Vollmer EP, editors. Biological activities of steroids in relation to cancer. Academic Press; New York: 1960. pp. 161–174. [Google Scholar]
- [165].Jo YH, Chen YJ, Chua SC, Jr., Talmage DA, Role LW. Integration of endocannabinoid and leptin signaling in an appetite-related neural circuit. Neuron. 2005;48:1055–1066. doi: 10.1016/j.neuron.2005.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Johnson WG, Corrigan SA, Lemmon CR, Bergeron KB, Crusco AH. Energy regulation over the menstrual cycle. Physiology & Behavior. 1994;56:523–527. doi: 10.1016/0031-9384(94)90296-8. [DOI] [PubMed] [Google Scholar]
- [167].Jones ME, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, Oz OK, Leury BJ, Robertson KM, Yao S, Simpson ER. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:12735–12740. doi: 10.1073/pnas.97.23.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Jung-Testas I, Alliot F, Pessac B, Robel P, Baulieu EE. Immunocytochemical localization of cytochrome P-450scc in cultured rat oligodendrocytes. C R Acad Sci III. 1989;308:165–170. [PubMed] [Google Scholar]
- [169].Jung-Testas I, Hu ZY, Baulieu EE, Robel P. Neurosteroids: biosynthesis of pregnenolone and progesterone in primary cultures of rat glial cells. Endocrinol. 1989;125:2083–2091. doi: 10.1210/endo-125-4-2083. [DOI] [PubMed] [Google Scholar]
- [170].Kalra SP, Dube MG, Sahu A, Phelps CP, Kalra PS. Neuropeptide Y secretion increases in the paraventricular nucleus in association with increased appetite for food. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:10931–10935. doi: 10.1073/pnas.88.23.10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Kalra SP, Kalra PS. Temporal interrelationships among circulating levels of estradiol, progesterone and LH during the rat estrous cycle: effects of exogenous progesterone. Endocrinology. 1974;95:1711–1718. doi: 10.1210/endo-95-6-1711. [DOI] [PubMed] [Google Scholar]
- [172].Kang KS, Yahashi S, Azuma M, Matsuda K. The anorexigenic effect of cholecystokinin octapeptide in a goldfish model is mediated by the vagal afferent and subsequently through the melanocortin- and corticotropin-releasing hormone-signaling pathways. Peptides. 2010;31:2130–2134. doi: 10.1016/j.peptides.2010.07.019. [DOI] [PubMed] [Google Scholar]
- [173].Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology. 2011;152:3103–3112. doi: 10.1210/en.2011-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Keith D, Murray S, Zaki P, Chu P, Lisson D, Kang L, Aimi J, Evans C, von Zastrow M. Rapid endocytosis of opioid receptors: Differential regulation by opioid peptide and morphine. Soc. Neurosci. Abstr. 1995;21:1353. [Google Scholar]
- [175].Keith DE, Anton B, Murray SR, Zaki PA, Chu PC, Lissin DV, Monteillet-Agius G, Stewart PL, Evans CJ, von Zastrow M. mu-Opioid receptor internalization: opiate drugs have differential effects on a conserved endocytic mechanism in vitro and in the mammalian brain. Molecular Pharmacology. 1998;53:377–384. [PubMed] [Google Scholar]
- [176].Keith DE, Murray SR, Zaki PA, Chu PC, Lissin DV, Kang L, Evans CJ, von Zastrow M. Morphine activates opioid receptors without causing their rapid internalization. J Bio Chem. 1996;271:19021–19024. doi: 10.1074/jbc.271.32.19021. [DOI] [PubMed] [Google Scholar]
- [177].Kellert BA, Nguyen MC, Nguyen C, Nguyen QH, Wagner EJ. Estrogen rapidly attenuates cannabinoid-induced changes in energy homeostasis. European journal of pharmacology. 2009;622:15–24. doi: 10.1016/j.ejphar.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Kellokoski E, Poykko SM, Karjalainen AH, Ukkola O, Heikkinen J, Kesaniemi YA, Horkko S. Estrogen replacement therapy increases plasma ghrelin levels. The Journal of clinical endocrinology and metabolism. 2005;90:2954–2963. doi: 10.1210/jc.2004-2016. [DOI] [PubMed] [Google Scholar]
- [179].Kelly M, Wagner E. Estrogen modulation of G-protein-coupled receptors. Trends in Endocrinology and Metabolism. 1999;10:369–374. doi: 10.1016/s1043-2760(99)00190-3. [DOI] [PubMed] [Google Scholar]
- [180].Kelly MJ, Kuhnt U, Wuttke W. Hyperpolarization of hypothalamic parvocellular neurons by 17 beta-estradiol and their identification through intracellular staining with procion yellow. Exp Brain Res. 1980;40:440–447. doi: 10.1007/BF00236152. [DOI] [PubMed] [Google Scholar]
- [181].Kelly MJ, Lagrange AH, Wagner EJ, Ronnekleiv OK. Rapid effects of estrogen to modulate G protein-coupled receptors via activation of protein kinase A and protein kinase C pathways. Steroids. 1999;64:64–75. doi: 10.1016/s0039-128x(98)00095-6. [DOI] [PubMed] [Google Scholar]
- [182].Kelly MJ, Moss RL, Dudley CA. Differential sensitivity of preoptic-septal neurons to microelectrophoresed estrogen during the estrous cycle. Brain Res. 1976;114:152–157. doi: 10.1016/0006-8993(76)91017-9. [DOI] [PubMed] [Google Scholar]
- [183].Kelly MJ, Ronnekleiv OK, Eskay RL. Identification of estrogen-responsive LHRH neurons in the guinea pig hypothalamus. Brain Res Bull. 1984;12:399–407. doi: 10.1016/0361-9230(84)90112-6. [DOI] [PubMed] [Google Scholar]
- [184].King WJ, Greene GL. Monoclonal antibodies localize oestrogen receptor in the nuclei of target cells. Nature. 1984;307:745–747. doi: 10.1038/307745a0. [DOI] [PubMed] [Google Scholar]
- [185].Koch JE. Delta(9)-THC stimulates food intake in Lewis rats: effects on chow, high-fat and sweet high-fat diets. Pharmacology, biochemistry, and behavior. 2001;68:539–543. doi: 10.1016/s0091-3057(01)00467-1. [DOI] [PubMed] [Google Scholar]
- [186].Koenig HL, Schumacher M, Ferzaz B, Thi AN, Ressouches A, Guennoun R, Jung-Testas I, Robel P, Akwa Y, Baulieu EE. Progesterone synthesis and myelin formation by Schwann cells. Science. 1995;268:1500–1503. doi: 10.1126/science.7770777. [DOI] [PubMed] [Google Scholar]
- [187].Kola B, Farkas I, Christ-Crain M, Wittmann G, Lolli F, Amin F, Harvey-White J, Liposits Z, Kunos G, Grossman AB, Fekete C, Korbonits M. The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. PloS one. 2008;3:e1797. doi: 10.1371/journal.pone.0001797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Kola B, Korbonits M. Shedding light on the intricate puzzle of ghrelin’s effects on appetite regulation. The Journal of endocrinology. 2009;202:191–198. doi: 10.1677/JOE-09-0056. [DOI] [PubMed] [Google Scholar]
- [189].Kotz CM, Briggs JE, Pomonis JD, Grace MK, Levine AS, Billington CJ. Neural site of leptin influence on neuropeptide Y signaling pathways altering feeding and uncoupling protein. The American journal of physiology. 1998;275:R478–484. doi: 10.1152/ajpregu.1998.275.2.R478. [DOI] [PubMed] [Google Scholar]
- [190].Krust A, Green S, Argos P, Kumar V, Walter P, Bornert JM, Chambon P. The chicken oestrogen receptor sequence: homology with v-erbA and the human oestrogen and glucocorticoid receptors. The EMBO journal. 1986;5:891–897. doi: 10.1002/j.1460-2075.1986.tb04300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Kumar V, Green S, Stack G, Berry M, Jin JR, Chambon P. Functional domains of the human estrogen receptor. Cell. 1987;51:941–951. doi: 10.1016/0092-8674(87)90581-2. [DOI] [PubMed] [Google Scholar]
- [192].Kuo J, Hamid N, Bondar G, Prossnitz ER, Micevych P. Membrane estrogen receptors stimulate intracellular calcium release and progesterone synthesis in hypothalamic astrocytes. Journal of Neuroscience. 2010;30:12950–12957. doi: 10.1523/JNEUROSCI.1158-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Kuo J, Hariri OR, Bondar G, Ogi J, Micevych P. Membrane estrogen receptor-alpha interacts with metabotropic glutamate receptor type 1a to mobilize intracellular calcium in hypothalamic astrocytes. Endocrinology. 2009;150:1369–1376. doi: 10.1210/en.2008-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Kuo WH, Chang LY, Liu DL, Hwa HL, Lin JJ, Lee PH, Chen CN, Lien HC, Yuan RH, Shun CT, Chang KJ, Hsieh FJ. The interactions between GPR30 and the major biomarkers in infiltrating ductal carcinoma of the breast in an Asian population. Taiwanese journal of obstetrics & gynecology. 2007;46:135–145. doi: 10.1016/S1028-4559(07)60007-2. [DOI] [PubMed] [Google Scholar]
- [195].Labhsetwar AP. Role of estrogens in ovulation: A study using the estrogen-antagonist, I.C.I. 46,474. Endocrinology. 1970;87:542–551. doi: 10.1210/endo-87-3-542. [DOI] [PubMed] [Google Scholar]
- [196].Lagrange A, Wagner E, Ronnekleiv O, Kelly M. Estrogen rapidly attenuates a GABAB response in hypothalamic neurons. Neuroendocrinology. 1996;64:114–123. doi: 10.1159/000127106. [DOI] [PubMed] [Google Scholar]
- [197].Lagrange AH, Ronnekleiv K, Kelly MJ. Estradiol-17 β and μ-opioid peptides rapidly hyperpolarize GnRH Neurons: A cellular mechanism of negative feedback. Endocrinol. 1995;136:2341–2344. doi: 10.1210/endo.136.5.7720682. [DOI] [PubMed] [Google Scholar]
- [198].Lagrange AH, Ronnekleiv OK, Kelly MJ. Modulation of G protein-coupled receptors by an estrogen receptor that activates protein kinase A. Mol Pharmacol. 1997;51:605–612. doi: 10.1124/mol.51.4.605. [DOI] [PubMed] [Google Scholar]
- [199].Lanfumey L, La Cour C. Mannoury, Froger N, Hamon M. 5-HT-HPA interactions in two models of transgenic mice relevant to major depression. Neurochemical research. 2000;25:1199–1206. doi: 10.1023/a:1007683810230. [DOI] [PubMed] [Google Scholar]
- [200].Lavoie JM, Dionne N, Helie R, Brisson GR. Menstrual cycle phase dissociation of blood glucose homeostasis during exercise. Journal of applied physiology. 1987;62:1084–1089. doi: 10.1152/jappl.1987.62.3.1084. [DOI] [PubMed] [Google Scholar]
- [201].Le Goascogne C, Robel P, Gouezou M, Sananes N, Baulieu EE, Waterman M. Neurosteroids: cytochrome P-450scc in rat brain. Science. 1987;237:1212–1215. doi: 10.1126/science.3306919. [DOI] [PubMed] [Google Scholar]
- [202].Lee J, Morris MJ. Modulation of neuropeptide Y overflow by leptin in the rat hypothalamus, cerebral cortex and medulla. Neuroreport. 1998;9:1575–1580. doi: 10.1097/00001756-199805110-00059. [DOI] [PubMed] [Google Scholar]
- [203].Lee K, Li B, Xi X, Suh Y, Martin RJ. Role of neuronal energy status in the regulation of adenosine 5′-monophosphate-activated protein kinase, orexigenic neuropeptides expression, and feeding behavior. Endocrinology. 2005;146:3–10. doi: 10.1210/en.2004-0968. [DOI] [PubMed] [Google Scholar]
- [204].Levin ER. G protein-coupled receptor 30: estrogen receptor or collaborator? Endocrinology. 2009;150:1563–1565. doi: 10.1210/en.2008-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Levin N, Nelson C, Gurney A, Vandlen R, de Sauvage F. Decreased food intake does not completely account for adiposity reduction after ob protein infusion. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:1726–1730. doi: 10.1073/pnas.93.4.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Lin KC, Sagawa N, Yura S, Itoh H, Fujii S. Simultaneous increases of leptin and gonadotropin-releasing hormone following exogenous estrogen administration in women with normally menstrual cycle. Endocrine journal. 2005;52:449–454. doi: 10.1507/endocrj.52.449. [DOI] [PubMed] [Google Scholar]
- [207].Liu X, Lee K, Herbison AE. Kisspeptin excites gonadotropin-releasing hormone neurons through a phospholipase C/calcium-dependent pathway regulating multiple ion channels. Endocrinology. 2008;149:4605–4614. doi: 10.1210/en.2008-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Long NP, Chhorvann S, Sinchak K. In estradiol primed rats subsequent free estradiol rapidly facilitates lordosis through G-protein coupled receptor 30 (GPR30). Society for Neuroscience, 2012 Neuroscience Meeting Planner; Washington, DC: New Orleans: Society for Neuroscience; 2012. 2012. [Google Scholar]
- [209].Lopez M, Lage R, Saha AK, Perez-Tilve D, Vazquez MJ, Varela L, Sangiao-Alvarellos S, Tovar S, Raghay K, Rodriguez-Cuenca S, Deoliveira RM, Castaneda T, Datta R, Dong JZ, Culler M, Sleeman MW, Alvarez CV, Gallego R, Lelliott CJ, Carling D, Tschop MH, Dieguez C, Vidal-Puig A. Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin. Cell metabolism. 2008;7:389–399. doi: 10.1016/j.cmet.2008.03.006. [DOI] [PubMed] [Google Scholar]
- [210].Lu KH, Hopper BR, Vargo TM, Yen SS. Chronological changes in sex steroid, gonadotropin and prolactin secretions in aging female rats displaying different reproductive states. Biol.Reprod. 1979;21:193–203. doi: 10.1095/biolreprod21.1.193. [DOI] [PubMed] [Google Scholar]
- [211].Lu S, Guan JL, Wang QP, Uehara K, Yamada S, Goto N, Date Y, Nakazato M, Kojima M, Kangawa K, Shioda S. Immunocytochemical observation of ghrelin-containing neurons in the rat arcuate nucleus. Neuroscience letters. 2002;321:157–160. doi: 10.1016/s0304-3940(01)02544-7. [DOI] [PubMed] [Google Scholar]
- [212].Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci U S A. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Luine V, Park D, Joh T, Reis D, McEwen B. Immunochemical demonstration of increased choline acetyltransferase concentration in rat preoptic area after estradiol administration. Brain Res. 1980;191:273–277. doi: 10.1016/0006-8993(80)90332-7. [DOI] [PubMed] [Google Scholar]
- [214].Luine VN, Khylchevskaya RI, McEwen BS. Effect of gonadal steroids on activities of monoamine oxidase and choline acetylase in rat brain. Brain Res. 1975;86:293–306. doi: 10.1016/0006-8993(75)90704-0. [DOI] [PubMed] [Google Scholar]
- [215].Lyons PM, Truswell AS, Mira M, Vizzard J, Abraham SF. Reduction of food intake in the ovulatory phase of the menstrual cycle. The American journal of clinical nutrition. 1989;49:1164–1168. doi: 10.1093/ajcn/49.6.1164. [DOI] [PubMed] [Google Scholar]
- [216].Ma X, Lin L, Qin G, Lu X, Fiorotto M, Dixit VD, Sun Y. Ablations of ghrelin and ghrelin receptor exhibit differential metabolic phenotypes and thermogenic capacity during aging. PloS one. 2011;6:e16391. doi: 10.1371/journal.pone.0016391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].MacLusky NJ, McEwen BS. Oestrogen modulates progestin receptor concentrations in some rat brain regions but not in others. Nature. 1978;274:276–278. doi: 10.1038/274276a0. [DOI] [PubMed] [Google Scholar]
- [218].MacLusky NJ, McEwen BS. Progestin receptors in the rat brain: Distribution and properties of cytoplasmic progestin binding sites. Endocrin. 1980;106:192–202. doi: 10.1210/endo-106-1-192. [DOI] [PubMed] [Google Scholar]
- [219].Maejima Y, Kohno D, Iwasaki Y, Yada T. Insulin suppresses ghrelin-induced calcium signaling in neuropeptide Y neurons of the hypothalamic arcuate nucleus. Aging. 2011;3:1092–1097. doi: 10.18632/aging.100400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Mahavongtrakul M, Kanjiya SM, Garcia MP, Charukulvanich P, Sinchak K. Society for Neuroscience, Abstract Viewer/Itinerary Planner. Society for Neuroscience; Washington, DC: 2011. Estradiol down regulates estrogen receptor-α in a behaviorally relevant and dose dependent manner in the arcuate nucleus. Washington, D.C., 2011. [Google Scholar]
- [221].Mahesh VB, Brann DW. Regulation of the preovulatory gonadotropin surge by endogenous steroids. Steroids. 1998;63:616–629. doi: 10.1016/s0039-128x(98)00075-0. [DOI] [PubMed] [Google Scholar]
- [222].Mana A, Garcia BL, Fuentes KN, Sinchak K. Society for Neuroscience, Abstract Viewer/Itinerary Planner. Society for Neuroscience; Washington, DC: Chicago: 2009. Differential activation/deactivation of lordosis circuit is dependent on estradiol dosage. 2009. [Google Scholar]
- [223].Mann DR, Korowitz CD, Macfarland LA, Cost MG. Interactions of the light-dark cycle, adrenal glands and time of steroid administration in determining the temporal sequence of LH and prolactin release in female rats. Endocrinol. 1976;99:1252–1262. doi: 10.1210/endo-99-5-1252. [DOI] [PubMed] [Google Scholar]
- [224].Mantyh PW, Allen CJ, Ghilardi JR, Rogers SD, Mantyh CR, Liu H, Basbaum AI, Vigna SR, Maggio JE. Rapid endocytosis of a G protein-coupled receptor: Substance P evoked internalization of its receptor in the rat striatum in vivo. Proc Natl Acad Sci U S A. 1995;92:2622–2626. doi: 10.1073/pnas.92.7.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Marston OJ, Garfield AS, Heisler LK. Role of central serotonin and melanocortin systems in the control of energy balance. European journal of pharmacology. 2011;660:70–79. doi: 10.1016/j.ejphar.2010.12.024. [DOI] [PubMed] [Google Scholar]
- [226].Matagne V, Lebrethon MC, Gerard A, Bourguignon JP. Kainate/estrogen receptor involvement in rapid estradiol effects in vitro and intracellular signaling pathways. Endocrinology. 2005;146:2313–2323. doi: 10.1210/en.2004-1265. [DOI] [PubMed] [Google Scholar]
- [227].Mateos B, Borcel E, Loriga R, Luesu W, Bini V, Llorente R, Castelli MP, Viveros MP. Adolescent exposure to nicotine and/or the cannabinoid agonist CP 55,940 induces gender-dependent long-lasting memory impairments and changes in brain nicotinic and CB(1) cannabinoid receptors. Journal of psychopharmacology. 2011;25:1676–1690. doi: 10.1177/0269881110370503. [DOI] [PubMed] [Google Scholar]
- [228].Mattes RD, Engelman K, Shaw LM, Elsohly MA. Cannabinoids and appetite stimulation. Pharmacology, biochemistry, and behavior. 1994;49:187–195. doi: 10.1016/0091-3057(94)90475-8. [DOI] [PubMed] [Google Scholar]
- [229].McCowen KC, Chow JC, Smith RJ. Leptin signaling in the hypothalamus of normal rats in vivo. Endocrinology. 1998;139:4442–4447. doi: 10.1210/endo.139.11.6301. [DOI] [PubMed] [Google Scholar]
- [230].Meisel RL, Pfaff DW. RNA and protein synthesis inhibitors: effects on sexual behavior in female rats. Brain Res Bull. 1984;12:187–193. doi: 10.1016/0361-9230(84)90188-6. [DOI] [PubMed] [Google Scholar]
- [231].Meisel RL, Pfaff DW. Specificity and neural sites of action of anisomycin in the reduction or facilitation of female sexual behavior. Hormones and Behavior. 1985;19:237–251. doi: 10.1016/0018-506x(85)90024-8. [DOI] [PubMed] [Google Scholar]
- [232].Mellon SH. Neurosteroids: biochemistry, modes of action, and clinical relevance. J Clin Endocrinol Metab. 1994;78:1003–1008. doi: 10.1210/jcem.78.5.8175951. [DOI] [PubMed] [Google Scholar]
- [233].Mellon SH, Deschepper CF. Neurosteroid biosynthesis: genes for adrenal steroidogenic enzymes are expressed in the brain. Brain Res. 1993;629:283–292. doi: 10.1016/0006-8993(93)91332-m. [DOI] [PubMed] [Google Scholar]
- [234].Melnick I, Pronchuk N, Cowley MA, Grove KL, Colmers WF. Developmental switch in neuropeptide Y and melanocortin effects in the paraventricular nucleus of the hypothalamus. Neuron. 2007;56:1103–1115. doi: 10.1016/j.neuron.2007.10.034. [DOI] [PubMed] [Google Scholar]
- [235].Mennini T, Bizzi A, Caccia S, Codegoni A, Fracasso C, Frittoli E, Guiso G, Padura IM, Taddei C, Uslenghi A, et al. Comparative studies on the anorectic activity of d-fenfluramine in mice, rats, and guinea pigs. Naunyn-Schmiedeberg’s archives of pharmacology. 1991;343:483–490. doi: 10.1007/BF00169550. [DOI] [PubMed] [Google Scholar]
- [236].Mermelstein P, Becker J, Surmeister D. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. Journal of Neuroscience. 1996;16:595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Messina MM, Boersma G, Overton JM, Eckel LA. Estradiol decreases the orexigenic effect of melanin-concentrating hormone in ovariectomized rats. Physiology & Behavior. 2006;88:523–528. doi: 10.1016/j.physbeh.2006.05.002. [DOI] [PubMed] [Google Scholar]
- [239].Mestek A, Hurley JH, Bye LS, Campbell AD, Chen Y, Tian M, Liu J, Schulman H, Yu L. The human mu opioid receptor. Journal of Neuroscience. 1995;15:2396–2406. doi: 10.1523/JNEUROSCI.15-03-02396.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Mhyre AJ, Dorsa DM. Estrogen activates rapid signaling in the brain: role of estrogen receptor alpha and estrogen receptor beta in neurons and glia. Neuroscience. 2006;138:851–858. doi: 10.1016/j.neuroscience.2005.10.019. [DOI] [PubMed] [Google Scholar]
- [241].Micevych P, Chaban V, Quesada A, Sinchak K. Oestrogen modulates cholecystokinin: opioid interactions in the nervous system. Pharmacol Toxicol. 2002;91:387–397. doi: 10.1034/j.1600-0773.2002.910618.x. [DOI] [PubMed] [Google Scholar]
- [242].Micevych P, Sinchak K. Estradiol regulation of progesterone synthesis in the brain. Mol Cell Endocrinol. 2008;290:44–50. doi: 10.1016/j.mce.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Micevych P, Sinchak K, Mills RH, Tao L, LaPolt P, Lu JK. The luteinizing hormone surge is preceded by an estrogen-induced increase of hypothalamic progesterone in ovariectomized and adrenalectomized rats. Neuroendocrinology. 2003;78:29–35. doi: 10.1159/000071703. [DOI] [PubMed] [Google Scholar]
- [244].Micevych PE, Chaban V, Ogi J, Dewing P, Lu JK, Sinchak K. Estradiol stimulates progesterone synthesis in hypothalamic astrocyte cultures. Endocrinology. 2007;148:782–789. doi: 10.1210/en.2006-0774. [DOI] [PubMed] [Google Scholar]
- [245].Micevych PE, Chaban V, Ogi J, Lakhter A, Lu JKH, Sinchak K. Estradiol stimulates progesterone synthesis in hypothalamic astrocyte cultures. Endocrinology. 2007;148:782–789. doi: 10.1210/en.2006-0774. [DOI] [PubMed] [Google Scholar]
- [246].Micevych PE, Kelly MJ. Membrane Estrogen Receptor Regulation of Hypothalamic Function. Neuroendocrinology. 2012 doi: 10.1159/000338400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Micevych PE, Rissman EF, Gustafsson JA, Sinchak K. Estrogen receptor-alpha is required for estrogen-induced mu-opioid receptor internalization. J Neurosci Res. 2003;71:802–810. doi: 10.1002/jnr.10526. [DOI] [PubMed] [Google Scholar]
- [248].Mills RH, Sohn RK, Micevych PE. Estrogen-induced mu-opioid receptor internalization in the medial preoptic nucleus is mediated via neuropeptide Y-Y1 receptor activation in the arcuate nucleus of female rats. J Neurosci. 2004;24:947–955. doi: 10.1523/JNEUROSCI.1366-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- [250].Mizuno TM, Mobbs CV. Hypothalamic agouti-related protein messenger ribonucleic acid is inhibited by leptin and stimulated by fasting. Endocrinology. 1999;140:814–817. doi: 10.1210/endo.140.2.6491. [DOI] [PubMed] [Google Scholar]
- [251].Moguilewsky M, Raynaud JP. The relevance of hypothalamic and hypophyseal progestin receptor regulation in the induction and inhibtion of sexual behavior in the female rat. Endocrinol. 1979;105:516–522. doi: 10.1210/endo-105-2-516. [DOI] [PubMed] [Google Scholar]
- [252].Mondal MS, Nakazato M, Matsukura S. Characterization of orexins (hypocretins) and melanin-concentrating hormone in genetically obese mice. Regulatory peptides. 2002;104:21–25. doi: 10.1016/s0167-0115(01)00345-7. [DOI] [PubMed] [Google Scholar]
- [253].Monnikes H, Lauer G, Arnold R. Peripheral administration of cholecystokinin activates c-fos expression in the locus coeruleus/subcoeruleus nucleus, dorsal vagal complex and paraventricular nucleus via capsaicin-sensitive vagal afferents and CCK-A receptors in the rat. Brain research. 1997;770:277–288. doi: 10.1016/s0006-8993(97)00865-2. [DOI] [PubMed] [Google Scholar]
- [254].Morley P, Whitfield JF, Vanderhyden BC, Tsang BK, Schwartz JL. A new, nongenomic estrogen action: the rapid release of intracellular calcium. Endocrinology. 1992;131:1305–1312. doi: 10.1210/endo.131.3.1505465. [DOI] [PubMed] [Google Scholar]
- [255].Morrell JI, McGinty JF, Pfaff DW. A subset of beta-endorphin- or dynorphin-containing neurons in the medial basal hypothalamus accumulates estradiol. Neuroendocrinology. 1985;41:417–426. doi: 10.1159/000124212. [DOI] [PubMed] [Google Scholar]
- [256].Morton GJ, Mystkowski P, Matsumoto AM, Schwartz MW. Increased hypothalamic melanin concentrating hormone gene expression during energy restriction involves a melanocortin-independent, estrogen-sensitive mechanism. Peptides. 2004;25:667–674. doi: 10.1016/j.peptides.2004.02.007. [DOI] [PubMed] [Google Scholar]
- [257].Moss RL, Dudley CA. Molecular aspects of the interaction between estrogen and the membrane excitability of hypothalamic nerve cells. Progress in brain research. 1984;61:3–22. doi: 10.1016/S0079-6123(08)64426-X. [DOI] [PubMed] [Google Scholar]
- [258].Mosselman S, Polman J, Dijkema R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392:49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- [259].Mulherin AJ, Oh AH, Kim H, Grieco A, Lauffer LM, Brubaker PL. Mechanisms underlying metformin-induced secretion of glucagon-like peptide-1 from the intestinal L cell. Endocrinology. 2011;152:4610–4619. doi: 10.1210/en.2011-1485. [DOI] [PubMed] [Google Scholar]
- [260].Murray JF, Baker BI, Levy A, Wilson CA. The influence of gonadal steroids on pre- pro melanin-concentrating hormone mRNA in female rats. Journal of neuroendocrinology. 2000;12:53–59. doi: 10.1046/j.1365-2826.2000.00425.x. [DOI] [PubMed] [Google Scholar]
- [261].Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang XJ, Clegg DJ, Kaplitt MG, Ogawa S. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Muschamp JW, Hull EM. Melanin concentrating hormone and estrogen receptor-alpha are coexstensive but not coexpressed in cells of male rat hypothalamus. Neuroscience letters. 2007;427:123–126. doi: 10.1016/j.neulet.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Nabekura J, Oomura Y, Minami T, Mizuno Y, Fukuda A. Mechanism of the rapid effect of 17 beta-estradiol on medial amygdala neurons. Science. 1986;233:226–228. doi: 10.1126/science.3726531. [DOI] [PubMed] [Google Scholar]
- [264].Nakashima K, Narazaki M, Taga T. Overlapping and distinct signals through leptin receptor (OB-R) and a closely related cytokine signal transducer, gp130. FEBS letters. 1997;401:49–52. doi: 10.1016/s0014-5793(96)01430-5. [DOI] [PubMed] [Google Scholar]
- [265].Nava MP, Fernandez A, Abelenda M, Puerta M. Dissociation between brown adipose tissue thermogenesis and sympathetic activity in rats with high plasma levels of oestradiol. Pflugers Archiv : European journal of physiology. 1994;426:40–43. doi: 10.1007/BF00374668. [DOI] [PubMed] [Google Scholar]
- [266].Nedungadi TP, Briski KP. Effects of estradiol on acute and recurrent insulin-induced hypoglycemia-associated patterns of arcuate neuropeptide Y, proopiomelanocortin, and cocaine- and amphetamine-related transcript gene expression in the ovariectomized rat. Neuroendocrinology. 2007;86:270–276. doi: 10.1159/000109678. [DOI] [PubMed] [Google Scholar]
- [267].Nethrapalli IS, Tinnikov AA, Krishnan V, Lei CD, Toran-Allerand CD. Estrogen activates mitogen-activated protein kinase in native, nontransfected CHO-K1, COS-7, and RAT2 fibroblast cell lines. Endocrinology. 2005;146:56–63. doi: 10.1210/en.2004-1106. [DOI] [PubMed] [Google Scholar]
- [268].Nguyen QH, Wagner EJ. Estrogen differentially modulates the cannabinoid-induced presynaptic inhibition of amino acid neurotransmission in proopiomelanocortin neurons of the arcuate nucleus. Neuroendocrinology. 2006;84:123–137. doi: 10.1159/000096996. [DOI] [PubMed] [Google Scholar]
- [269].Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E. Involvement of G protein-coupled receptor 30 (GPR30) in rapid action of estrogen in primate LHRH neurons. Molecular endocrinology. 2009;23:349–359. doi: 10.1210/me.2008-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Nonogaki K, Strack AM, Dallman MF, Tecott LH. Leptin-independent hyperphagia and type 2 diabetes in mice with a mutated serotonin 5-HT2C receptor gene. Nature medicine. 1998;4:1152–1156. doi: 10.1038/2647. [DOI] [PubMed] [Google Scholar]
- [271].Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor alpha gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139:5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- [272].Ogawa S, Taylor JA, Lubahn DB, Korach KS, Pfaff DW. Reversal of sex roles in genetic female mice by disruption of estrogen receptor gene. Neuroendocrinology. 1996;64:467–470. doi: 10.1159/000127154. [DOI] [PubMed] [Google Scholar]
- [273].Olefsky JM, Garvey WT, Henry RR, Brillon D, Matthaei S, Freidenberg GR. Cellular mechanisms of insulin resistance in non-insulin-dependent (type II) diabetes. The American journal of medicine. 1988;85:86–105. doi: 10.1016/0002-9343(88)90401-9. [DOI] [PubMed] [Google Scholar]
- [274].Olofsson LE, Pierce AA, Xu AW. Functional requirement of AgRP and NPY neurons in ovarian cycle-dependent regulation of food intake. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15932–15937. doi: 10.1073/pnas.0904747106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Orosco M, Rouch C, Gerozissis K. Activation of hypothalamic insulin by serotonin is the primary event of the insulin-serotonin interaction involved in the control of feeding. Brain research. 2000;872:64–70. doi: 10.1016/s0006-8993(00)02449-5. [DOI] [PubMed] [Google Scholar]
- [276].Otto C, Fuchs I, Kauselmann G, Kern H, Zevnik B, Andreasen P, Schwarz G, Altmann H, Klewer M, Schoor M, Vonk R, Fritzemeier KH. GPR30 does not mediate estrogenic responses in reproductive organs in mice. Biology of reproduction. 2009;80:34–41. doi: 10.1095/biolreprod.108.071175. [DOI] [PubMed] [Google Scholar]
- [277].Otto C, Rohde-Schulz B, Schwarz G, Fuchs I, Klewer M, Brittain D, Langer G, Bader B, Prelle K, Nubbemeyer R, Fritzemeier KH. G protein-coupled receptor 30 localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology. 2008;149:4846–4856. doi: 10.1210/en.2008-0269. [DOI] [PubMed] [Google Scholar]
- [278].Owen JA., Jr. Physiology of the menstrual cycle. The American journal of clinical nutrition. 1975;28:333–338. doi: 10.1093/ajcn/28.4.333. [DOI] [PubMed] [Google Scholar]
- [279].Palmer K, Gray JM. Central vs. peripheral effects of estrogen on food intake and lipoprotein lipase activity in ovariectomized rats. Physiology & Behavior. 1986;37:187–189. doi: 10.1016/0031-9384(86)90404-x. [DOI] [PubMed] [Google Scholar]
- [280].Pan JT, Kow LM, Pfaff DW. Single-unit activity of hypothalamic arcuate neurons in brain tissue slices. Effects of anterior pituitary hormones, cholecystokinin-octapeptide, and neurotransmitters. Neuroendocrinology. 1986;43:189–196. doi: 10.1159/000124527. [DOI] [PubMed] [Google Scholar]
- [281].Pappas TC, Gametchu B, Watson CS. Membrane estrogen receptors identified by multiple antibody labeling and impeded-ligand binding. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1995;9:404–410. doi: 10.1096/fasebj.9.5.7896011. [DOI] [PubMed] [Google Scholar]
- [282].Pardini AW, Nguyen HT, Figlewicz DP, Baskin DG, Williams DL, Kim F, Schwartz MW. Distribution of insulin receptor substrate-2 in brain areas involved in energy homeostasis. Brain research. 2006;1112:169–178. doi: 10.1016/j.brainres.2006.06.109. [DOI] [PubMed] [Google Scholar]
- [283].Pasqualini C, Guivarc’h D, Barnier JV, Guibert B, Vincent JD, Vernier P. Differential subcellular distribution and transcriptional activity of sigmaE3, sigmaE4, and sigmaE3-4 isoforms of the rat estrogen receptor-alpha. Molecular endocrinology. 2001;15:894–908. doi: 10.1210/mend.15.6.0642. [DOI] [PubMed] [Google Scholar]
- [284].Pawlak J, Karolczak M, Krust A, Chambon P, Beyer C. Estrogen receptor-alpha is associated with the plasma membrane of astrocytes and coupled to the MAP/Src-kinase pathway. Glia. 2005;50:270–275. doi: 10.1002/glia.20162. [DOI] [PubMed] [Google Scholar]
- [285].Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Molecular endocrinology. 2006;20:1996–2009. doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- [286].Pedram A, Razandi M, Sainson RC, Kim JK, Hughes CC, Levin ER. A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem. 2007;282:22278–22288. doi: 10.1074/jbc.M611877200. [DOI] [PubMed] [Google Scholar]
- [287].Perlman WR, Matsumoto M, Beltaifa S, Hyde TM, Saunders RC, Webster MJ, Rubinow DR, Kleinman JE, Weickert CS. Expression of estrogen receptor alpha exon-deleted mRNA variants in the human and non-human primate frontal cortex. Neuroscience. 2005;134:81–95. doi: 10.1016/j.neuroscience.2005.03.055. [DOI] [PubMed] [Google Scholar]
- [288].Perwitz N, Fasshauer M, Klein J. Cannabinoid receptor signaling directly inhibits thermogenesis and alters expression of adiponectin and visfatin. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2006;38:356–358. doi: 10.1055/s-2006-925401. [DOI] [PubMed] [Google Scholar]
- [289].Pfaff DW. Nature of sex hormone effects on rat sex behavior: Specificity of effects and individual patterns of response. J. Comp. Physiol. Psychol. 1970;73:349–358. doi: 10.1037/h0030242. [DOI] [PubMed] [Google Scholar]
- [290].Pfaus JG, Pfaff DW. Mu-, delta-, and kappa-opioid receptor agonists selectively modulate sexual behaviors in the female rat: differential dependence on progesterone. Horm. Behav. 1992;26:457–473. doi: 10.1016/0018-506x(92)90014-m. [DOI] [PubMed] [Google Scholar]
- [291].Pfeffer U, Fecarotta E, Castagnetta L, Vidali G. Estrogen receptor variant messenger RNA lacking exon 4 in estrogen-responsive human breast cancer cell lines. Cancer research. 1993;53:741–743. [PubMed] [Google Scholar]
- [292].Pfeifle JK, Edwards DA. Midbrain lesions eliminate sexual receptivity but spare sexual motivation in female rats. Physiol Behav. 1983;31:385–389. doi: 10.1016/0031-9384(83)90206-8. [DOI] [PubMed] [Google Scholar]
- [293].Pfeifle JK, Shivers M, Edwards DA. Parasagittal hypothalamic knife cuts and sexual receptivity in the female rat. Physiol Behav. 1980;24:145–150. doi: 10.1016/0031-9384(80)90026-8. [DOI] [PubMed] [Google Scholar]
- [294].Pietras RJ, Szego CM. Specific binding sites for oestrogen at the outer surfaces of isolated endometrial cells. Nature. 1977;265:69–72. doi: 10.1038/265069a0. [DOI] [PubMed] [Google Scholar]
- [295].Pietras RJ, Szego CM. Estrogen receptors in uterine plasma membrane. Journal of steroid biochemistry. 1979;11:1471–1483. doi: 10.1016/0022-4731(79)90124-9. [DOI] [PubMed] [Google Scholar]
- [296].Pietras RJ, Szego CM. Metabolic and proliferative responses to estrogen by hepatocytes selected for plasma membrane binding-sites specific for estradiol-17beta. Journal of cellular physiology. 1979;98:145–159. doi: 10.1002/jcp.1040980116. [DOI] [PubMed] [Google Scholar]
- [297].Pietras RJ, Szego CM. Partial purification and characterization of oestrogen receptors in subfractions of hepatocyte plasma membranes. The Biochemical journal. 1980;191:743–760. doi: 10.1042/bj1910743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [298].Plum L, Ma X, Hampel B, Balthasar N, Coppari R, Munzberg H, Shanabrough M, Burdakov D, Rother E, Janoschek R, Alber J, Belgardt BF, Koch L, Seibler J, Schwenk F, Fekete C, Suzuki A, Mak TW, Krone W, Horvath TL, Ashcroft FM, Bruning JC. Enhanced PIP3 signaling in POMC neurons causes KATP channel activation and leads to diet-sensitive obesity. The Journal of clinical investigation. 2006;116:1886–1901. doi: 10.1172/JCI27123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [299].Polovin G, Bowlby R, Garcia BL, Thach V, Tea P, Seng H, Sinchak K. Society for Neuroscience, Abstract Viewer/Itinerary Planner. Society for Neuroscience; Washington, DC: New Orleans, LA, USA: 2012. Subpopulation of m-opioid receptor neurons in the medial preoptic nucleus express estrogen receptor-a and opioid receptor-like receptor-1. 2012. [Google Scholar]
- [300].Press MF, Nousek-Goebl N, King WJ, Herbst AL, Greene GL. Immunohistochemical assessment of estrogen receptor distribution in the human endometrium throughout the menstrual cycle. Laboratory investigation; a journal of technical methods and pathology. 1984;51:495–503. [PubMed] [Google Scholar]
- [301].Priest CA, Vink KL, Micevych PE. Temporal regulation by estrogen of beta-preprotachykinin mRNA expression in the rat ventromedial nucleus of the hypothalamus. Brain Res. Mol. Brain Res. 1995;28:61–71. doi: 10.1016/0169-328x(94)00184-g. [DOI] [PubMed] [Google Scholar]
- [302].Puerta M, Rocha M, Gonzalez-Covaleda S, McBennett SM, Andrews JF. Changes in cytochrome oxidase activity in brown adipose tissue during oestrous cycle in the rat. European journal of endocrinology / European Federation of Endocrine Societies. 1998;139:433–437. doi: 10.1530/eje.0.1390433. [DOI] [PubMed] [Google Scholar]
- [303].Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [304].Qiu J, Bosch MA, Tobias SC, Krust A, Graham SM, Murphy SJ, Korach KS, Chambon P, Scanlan TS, Ronnekleiv OK, Kelly MJ. A G-protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci. 2006;26:5649–5655. doi: 10.1523/JNEUROSCI.0327-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [305].Qiu J, Fang Y, Ronnekleiv OK, Kelly MJ. Leptin excites proopiomelanocortin neurons via activation of TRPC channels. J Neurosci. 2010;30:1560–1565. doi: 10.1523/JNEUROSCI.4816-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [306].Qiu J, Ronnekleiv OK, Kelly MJ. Modulation of hypothalamic neuronal activity through a novel G-protein-coupled estrogen membrane receptor. Steroids. 2008;73:985–991. doi: 10.1016/j.steroids.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [307].Qiu J, Xue C, Bosch MA, Murphy JG, Fan W, Ronnekleiv OK, Kelly MJ. Serotonin 5-hydroxytryptamine2C receptor signaling in hypothalamic proopiomelanocortin neurons: role in energy homeostasis in females. Molecular pharmacology. 2007;72:885–896. doi: 10.1124/mol.107.038083. [DOI] [PubMed] [Google Scholar]
- [308].Quadagno D, Shryne J, Gorski R. The inhibition of steroid-induced sexual behavior by intrahypothalamic actinomycin-D. Hormones and Behavior. 1971;2:1–10. [Google Scholar]
- [309].Quadagno DM, McCullough J, Langan R. The effect of varying amounts of exogenous estradiol benzoate on estrous behavior in the rat. Horm Behav. 1972;3:175–179. doi: 10.1016/0018-506x(72)90029-3. [DOI] [PubMed] [Google Scholar]
- [310].Quesada A, Romeo HE, Micevych P. Distribution and localization patterns of estrogen receptor-beta and insulin-like growth factor-1 receptors in neurons and glial cells of the female rat substantia nigra: localization of ERbeta and IGF-1R in substantia nigra. J Comp Neurol. 2007;503:198–208. doi: 10.1002/cne.21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [311].Rainbow T, Davis P, McEwen B. Anisomycin inhibits the activation of sexual behavior by estradiol and progesterone. Brain Res. 1980;194:548–555. doi: 10.1016/0006-8993(80)91240-8. [DOI] [PubMed] [Google Scholar]
- [312].Rainbow TC, Degroff V, Luine VN, McEwen BS. Estradiol 17 beta increases the number of muscarinic receptors in hypothalamic nuclei. Brain Res. 1980;198:239–243. doi: 10.1016/0006-8993(80)90362-5. [DOI] [PubMed] [Google Scholar]
- [313].Rainbow TC, McGinnis MY, Davis PG, McEwen BS. Application of anisomycin to the lateral ventromedial nucleus of the hypothalamus inhibits the activation of sexual behavior by estradiol and progesterone. Brain Research. 1982;233:417–423. doi: 10.1016/0006-8993(82)91217-3. [DOI] [PubMed] [Google Scholar]
- [314].Rambo CO, Szego CM. Estrogen action at endometrial membranes: alterations in luminal surface detectable within seconds. The Journal of cell biology. 1983;97:679–685. doi: 10.1083/jcb.97.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [315].Rao IM, Mahesh VB. Role of progesterone in the modulation of the preovulatory surge of gonadotropins and ovulation in the pregnant mare’s serum gonadotropin-primed immature rat and the adult rat. Biol Reprod. 1986;35:1154–1161. doi: 10.1095/biolreprod35.5.1154. [DOI] [PubMed] [Google Scholar]
- [316].Rao MC, Palfrey HC, Nash NT, Greisman A, Jayatilak PG, Gibori G. Effects of estradiol on calcium-specific protein phosphorylation in the rat corpus luteum. Endocrinology. 1987;120:1010–1018. doi: 10.1210/endo-120-3-1010. [DOI] [PubMed] [Google Scholar]
- [317].Rashotte ME, Ackert AM, Overton JM. Ingestive behavior and body temperature during the ovarian cycle in normotensive and hypertensive rats. American journal of physiology. Regulatory, integrative and comparative physiology. 2002;282:R216–225. doi: 10.1152/ajpregu.00676.2000. [DOI] [PubMed] [Google Scholar]
- [318].Rawls SM, Cabassa J, Geller EB, Adler MW. CB1 receptors in the preoptic anterior hypothalamus regulate WIN 55212-2 [(4,5-dihydro-2-methyl-4(4-morpholinylmethyl)-1-(1-naphthalenyl-carbonyl)-6H-pyrr olo[3,2,1ij]quinolin-6-one]-induced hypothermia. The Journal of pharmacology and experimental therapeutics. 2002;301:963–968. doi: 10.1124/jpet.301.3.963. [DOI] [PubMed] [Google Scholar]
- [319].Razandi M, Alton G, Pedram A, Ghonshani S, Webb P, Levin ER. Identification of a structural determinant necessary for the localization and function of estrogen receptor alpha at the plasma membrane. Molecular and cellular biology. 2003;23:1633–1646. doi: 10.1128/MCB.23.5.1633-1646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [320].Razandi M, Pedram A, Greene G, Levin E. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- [321].Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- [322].Riebe CJ, Hill MN, Lee TT, Hillard CJ, Gorzalka BB. Estrogenic regulation of limbic cannabinoid receptor binding. Psychoneuroendocrinology. 2010;35:1265–1269. doi: 10.1016/j.psyneuen.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [323].Rissman EF, Early AH, Taylor JA, Korach KS, Lubahn DB. Estrogen receptors are essential for female sexual receptivity. Endocrinology. 1997;138:507–510. doi: 10.1210/endo.138.1.4985. [DOI] [PubMed] [Google Scholar]
- [324].Rivera HM, Eckel LA. The anorectic effect of fenfluramine is increased by estradiol treatment in ovariectomized rats. Physiology & Behavior. 2005;86:331–337. doi: 10.1016/j.physbeh.2005.08.004. [DOI] [PubMed] [Google Scholar]
- [325].Rocha M, Grueso E, Puerta M. The anorectic effect of oestradiol does not involve changes in plasma and cerebrospinal fluid leptin concentrations in the rat. The Journal of endocrinology. 2001;171:349–354. doi: 10.1677/joe.0.1710349. [DOI] [PubMed] [Google Scholar]
- [326].Rodriguez AM, Monjo M, Roca P, Palou A. Opposite actions of testosterone and progesterone on UCP1 mRNA expression in cultured brown adipocytes. Cellular and molecular life sciences : CMLS. 2002;59:1714–1723. doi: 10.1007/PL00012499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [327].Roepke TA, Bosch MA, Rick EA, Lee B, Wagner EJ, Seidlova-Wuttke D, Wuttke W, Scanlan TS, Ronnekleiv OK, Kelly MJ. Contribution of a membrane estrogen receptor to the estrogenic regulation of body temperature and energy homeostasis. Endocrinology. 2010;151:4926–4937. doi: 10.1210/en.2010-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [328].Roepke TA, Malyala A, Bosch MA, Kelly MJ, Ronnekleiv OK. Estrogen regulation of genes important for K+ channel signaling in the arcuate nucleus. Endocrinology. 2007;148:4937–4951. doi: 10.1210/en.2007-0605. [DOI] [PubMed] [Google Scholar]
- [329].Roepke TA, Qiu J, Smith AW, Ronnekleiv OK, Kelly MJ. Fasting and 17beta-estradiol differentially modulate the M-current in neuropeptide Y neurons. J Neurosci. 2011;31:11825–11835. doi: 10.1523/JNEUROSCI.1395-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [330].Roepke TA, Xue C, Bosch MA, Scanlan TS, Kelly MJ, Ronnekleiv OK. Genes associated with membrane-initiated signaling of estrogen and energy homeostasis. Endocrinology. 2008;149:6113–6124. doi: 10.1210/en.2008-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [331].Rogers NH, Witczak CA, Hirshman MF, Goodyear LJ, Greenberg AS. Estradiol stimulates Akt, AMP-activated protein kinase (AMPK) and TBC1D1/4, but not glucose uptake in rat soleus. Biochemical and biophysical research communications. 2009;382:646–650. doi: 10.1016/j.bbrc.2009.02.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [332].Romanatto T, Cesquini M, Amaral ME, Roman EA, Moraes JC, Torsoni MA, Cruz-Neto AP, Velloso LA. TNF-alpha acts in the hypothalamus inhibiting food intake and increasing the respiratory quotient--effects on leptin and insulin signaling pathways. Peptides. 2007;28:1050–1058. doi: 10.1016/j.peptides.2007.03.006. [DOI] [PubMed] [Google Scholar]
- [333].Rossato M, Nogara A, Merico M, Ferlin A, Garolla A, Foresta C. Store-operated calcium influx and stimulation of steroidogenesis in rat Leydig cells: role of Ca(2+)-activated K(+) channels. Endocrinology. 2001;142:3865–3872. doi: 10.1210/endo.142.9.8373. [DOI] [PubMed] [Google Scholar]
- [334].Rossi DV, Dai Y, Thomas P, Carrasco GA, DonCarlos LL, Muma NA, Li Q. Estradiol-induced desensitization of 5-HT1A receptor signaling in the paraventricular nucleus of the hypothalamus is independent of estrogen receptor-beta. Psychoneuroendocrinology. 2010;35:1023–1033. doi: 10.1016/j.psyneuen.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [335].Rubino T, Vigano D, Realini N, Guidali C, Braida D, Capurro V, Castiglioni C, Cherubino F, Romualdi P, Candeletti S, Sala M, Parolaro D. Chronic delta 9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:2760–2771. doi: 10.1038/sj.npp.1301664. [DOI] [PubMed] [Google Scholar]
- [336].Sadler SE, Bower MA, Maller JL. Studies of a plasma membrane steroid receptor in Xenopus oocytes using the synthetic progestin RU 486. Journal of steroid biochemistry. 1985;22:419–426. doi: 10.1016/0022-4731(85)90448-0. [DOI] [PubMed] [Google Scholar]
- [337].Sadler SE, Maller JL. Identification of a steroid receptor on the surface of Xenopus oocytes by photoaffinity labeling. The Journal of biological chemistry. 1982;257:355–361. [PubMed] [Google Scholar]
- [338].Sakuma S, Tokuhara D, Hattori H, Matsuoka O, Yamano T. Expression of estrogen receptor alpha and beta in reactive astrocytes at the male rat hippocampus after status epilepticus. Neuropathology : official journal of the Japanese Society of Neuropathology. 2009;29:55–62. doi: 10.1111/j.1440-1789.2008.00946.x. [DOI] [PubMed] [Google Scholar]
- [339].Sanathara NM, Moraes J, Borgquist A, Tavitian N, Wagner EJ, Sinchak K. Estradiol modulation of nociceptin system expression and signalling in proopiomelanocortin arcuate nucleus neurons that project to the medial preoptic nucleus. J Neurosci. (submitted) [Google Scholar]
- [340].Sanathara NM, Moraes J, Kanjiya S, Sinchak K. Orphanin FQ in the mediobasal hypothalamus facilitates sexual receptivity through the deactivation of medial preoptic nucleus mu-opioid receptors. Hormones and Behavior. 2011;60:540–548. doi: 10.1016/j.yhbeh.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [341].Sanchez-Alavez M, Tabarean IV, Osborn O, Mitsukawa K, Schaefer J, Dubins J, Holmberg KH, Klein I, Klaus J, Gomez LF, Kolb H, Secrest J, Jochems J, Myashiro K, Buckley P, Hadcock JR, Eberwine J, Conti B, Bartfai T. Insulin causes hyperthermia by direct inhibition of warm-sensitive neurons. Diabetes. 2010;59:43–50. doi: 10.2337/db09-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [342].Sanne JL, Krueger KE. Expression of cytochrome P450 side-chain cleavage enzyme and 3 beta-hydroxysteroid dehydrogenase in the rat central nervous system: a study by polymerase chain reaction and in situ hybridization. J. Neurochem. 1995;65:528–536. doi: 10.1046/j.1471-4159.1995.65020528.x. [DOI] [PubMed] [Google Scholar]
- [343].Santollo J, Eckel LA. Estradiol decreases the orexigenic effect of neuropeptide Y, but not agouti-related protein, in ovariectomized rats. Behavioural brain research. 2008;191:173–177. doi: 10.1016/j.bbr.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [344].Santollo J, Eckel LA. The orexigenic effect of melanin-concentrating hormone (MCH) is influenced by sex and stage of the estrous cycle. Physiology & Behavior. 2008;93:842–850. doi: 10.1016/j.physbeh.2007.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [345].Santollo J, Wiley MD, Eckel LA. Acute activation of ER alpha decreases food intake, meal size, and body weight in ovariectomized rats. American journal of physiology. Regulatory, integrative and comparative physiology. 2007;293:R2194–2201. doi: 10.1152/ajpregu.00385.2007. [DOI] [PubMed] [Google Scholar]
- [346].Sar M, Sahu A, Crowley WR, Kalra SP. Localization of neuropeptide-Y immunoreactivity in estradiol-concentrating cells in the hypothalamus. Endocrinology. 1990;127:2752–2756. doi: 10.1210/endo-127-6-2752. [DOI] [PubMed] [Google Scholar]
- [347].Sato I, Arima H, Ozaki N, Watanabe M, Goto M, Hayashi M, Banno R, Nagasaki H, Oiso Y. Insulin inhibits neuropeptide Y gene expression in the arcuate nucleus through GABAergic systems. J Neurosci. 2005;25:8657–8664. doi: 10.1523/JNEUROSCI.2739-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [348].Schmeling WT, Hosko MJ. Effect of delta 9-tetrahydrocannabinol on hypothalamic thermosensitive units. Brain research. 1980;187:431–442. doi: 10.1016/0006-8993(80)90213-9. [DOI] [PubMed] [Google Scholar]
- [349].Seo S, Ju S, Chung H, Lee D, Park S. Acute effects of glucagon-like peptide-1 on hypothalamic neuropeptide and AMP activated kinase expression in fasted rats. Endocrine journal. 2008;55:867–874. doi: 10.1507/endocrj.k08e-091. [DOI] [PubMed] [Google Scholar]
- [350].Shaikh AA, Shaikh SA. Adrenal and ovarian steroid secretion. Endocrinology. 1975;96:37–44. doi: 10.1210/endo-96-1-37. [DOI] [PubMed] [Google Scholar]
- [351].Sharma SK, Klee WA, Nirenberg M. Opiate-dependent modulation of adenylate cyclase. Proceedings of the National Academy of Sciences of the United States of America. 1977;74:3365–3369. doi: 10.1073/pnas.74.8.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [352].Shears SB. Regulation of the metabolism of 1,2-diacylglycerols and inositol phosphates that respond to receptor activation. Pharmacology & therapeutics. 1991;49:79–104. doi: 10.1016/0163-7258(91)90023-f. [DOI] [PubMed] [Google Scholar]
- [353].Shi H, Sorrell JE, Clegg DJ, Woods SC, Seeley RJ. The roles of leptin receptors on POMC neurons in the regulation of sex-specific energy homeostasis. Physiology & Behavior. 2010;100:165–172. doi: 10.1016/j.physbeh.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [354].Shughrue PJ, Lubahn DB, NegroVilar A, Korach KS, Merchenthaler I. Responses in the brain of estrogen receptor alpha-disrupted mice. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:11008–11012. doi: 10.1073/pnas.94.20.11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [355].Shyamala G, Gorski J. Estrogen receptors in the rat uterus. Studies on the interaction of cytosol and nuclear binding sites. The Journal of biological chemistry. 1969;244:1097–1103. [PubMed] [Google Scholar]
- [356].Silva NL, Boulant JA. Effects of testosterone, estradiol, and temperature on neurons in preoptic tissue slices. The American journal of physiology. 1986;250:R625–632. doi: 10.1152/ajpregu.1986.250.4.R625. [DOI] [PubMed] [Google Scholar]
- [357].Simonian SX, Spratt DP, Herbison AE. Identification and characterization of estrogen receptor alpha-containing neurons projecting to the vicinity of the gonadotropin-releasing hormone perikarya in the rostral preoptic area of the rat. Journal of Comparative Neurology. 1999;411:346–358. doi: 10.1002/(sici)1096-9861(19990823)411:2<346::aid-cne13>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- [358].Sinchak K, Dewing P, Cook M, Micevych P. Release of orphanin FQ/nociceptin in the medial preoptic nucleus and ventromedial nucleus of the hypothalamus facilitates lordosis. Horm Behav. 2007;51:406–412. doi: 10.1016/j.yhbeh.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [359].Sinchak K, Garcia BL, Bowlby R, Charukulvanich P, Garcia MP, Sanathara NM. Society for Neuroscience, Abstract Viewer/Itinerary Planner. Society for Neuroscience; Washington, DC: San Diego: 2010. Mu-opioid receptor neurons and opioid receptor-like receptor neurons in the medial preoptic nucleus project to the region of the ventromedial nucleus of the hypothalamus; p. 88.83. 2010. [Google Scholar]
- [360].Sinchak K, Hendricks DG, Baroudi R, Micevych PE. Orphanin FQ/nociceptin in the ventromedial nucleus facilitates lordosis in female rats. Neuroreport. 1997;8:3857–3860. doi: 10.1097/00001756-199712220-00004. [DOI] [PubMed] [Google Scholar]
- [361].Sinchak K, Micevych P. Visualizing activation of opioid circuits by internalization of G protein-coupled receptors. Molecular Neurobiology. 2003;27:197–222. doi: 10.1385/MN:27:2:197. [DOI] [PubMed] [Google Scholar]
- [362].Sinchak K, Micevych PE. Progesterone blockade of estrogen activation of μ-opioid receptors regulates reproductive behavior. J Neurosci. 2001;21:5723–5729. doi: 10.1523/JNEUROSCI.21-15-05723.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [363].Sinchak K, Romeo HE, Micevych PE. Site-specific estrogen and progestin regulation of orphanin FQ/nociceptin and nociceptin opioid receptor mRNA expression in the female rat limbic hypothalamic system. J Comp Neurol. 2006;496:252–268. doi: 10.1002/cne.20949. [DOI] [PubMed] [Google Scholar]
- [364].Sinchak K, Shahedi K, Dewing P, Micevych P. Sexual receptivity is reduced in the female mu-opioid receptor knockout mouse. Neuroreport. 2005;16:1697–1700. doi: 10.1097/01.wnr.0000181585.49130.93. [DOI] [PubMed] [Google Scholar]
- [365].Singh M, Setalo G, Jr., Guan X, Frail DE, Toran-Allerand CD. Estrogen-induced activation of the mitogen-activated protein kinase cascade in the cerebral cortex of estrogen receptor-alpha knock-out mice. J Neurosci. 2000;20:1694–1700. doi: 10.1523/JNEUROSCI.20-05-01694.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [366].Singh M, Setalo G, Jr., Guan X, Warren M, Toran-Allerand CD. Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants: convergence of estrogen and neurotrophin signaling pathways. J Neurosci. 1999;19:1179–1188. doi: 10.1523/JNEUROSCI.19-04-01179.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [367].Sirinathsinghji DJS. Regulation of lor dosis behavior in the female rat by corticotropin-releasing factor, beta-endorphin/corticotropin and luteinizing hormone-releasing hormone neuronal systems in the medial preoptic area. Brain Res. 1986;375:149–156. doi: 10.1016/0006-8993(86)90957-1. [DOI] [PubMed] [Google Scholar]
- [368].Sisk CL, Richardson HN, Chappell PE, Levine JE. In vivo gonadotropin-releasing hormone secretion in female rats during peripubertal development and on proestrus. Endocrinology. 2001;142:2929–2936. doi: 10.1210/endo.142.7.8239. [DOI] [PubMed] [Google Scholar]
- [369].Skipper JK, Young LJ, Bergeron JM, Tetzlaff MT, Osborn CT, Crews D. Identification of an isoform of the estrogen receptor messenger RNA lacking exon four and present in the brain. Proceedings of the National Academy of Sciences, USA. 1993;90:7172–7175. doi: 10.1073/pnas.90.15.7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [370].Smith HO, Arias-Pulido H, Kuo DY, Howard T, Qualls CR, Lee SJ, Verschraegen CF, Hathaway HJ, Joste NE, Prossnitz ER. GPR30 predicts poor survival for ovarian cancer. Gynecologic oncology. 2009;114:465–471. doi: 10.1016/j.ygyno.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [371].Smith HO, Leslie KK, Singh M, Qualls CR, Revankar CM, Joste NE, Prossnitz ER. GPR30: a novel indicator of poor survival for endometrial carcinoma. American journal of obstetrics and gynecology. 2007;196:386, e381–389. doi: 10.1016/j.ajog.2007.01.004. discussion 386 e389-311. [DOI] [PubMed] [Google Scholar]
- [372].Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology. 1975;96:219–226. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
- [373].Sodersten P, Eneroth P. Serum levels of oestradiol-17 beta and progesterone in relation to receptivity in intact and ovariectomized rats. J Endocrinol. 1981;89:45–54. doi: 10.1677/joe.0.0890045. [DOI] [PubMed] [Google Scholar]
- [374].Sohn JW, Xu Y, Jones JE, Wickman K, Williams KW, Elmquist JK. Serotonin 2C receptor activates a distinct population of arcuate pro-opiomelanocortin neurons via TRPC channels. Neuron. 2011;71:488–497. doi: 10.1016/j.neuron.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [375].Soma KK, Sinchak K, Lakhter A, Schlinger BA, Micevych PE. Neurosteroids and female reproduction: estrogen increases 3beta-HSD mRNA and activity in rat hypothalamus. Endocrinology. 2005;146:4386–4390. doi: 10.1210/en.2005-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [376].Song RX, Barnes CJ, Zhang Z, Bao Y, Kumar R, Santen RJ. The role of Shc and insulin-like growth factor 1 receptor in mediating the translocation of estrogen receptor alpha to the plasma membrane. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:2076–2081. doi: 10.1073/pnas.0308334100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [377].Spanswick D, Smith MA, Groppi VE, Logan SD, Ashford ML. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature. 1997;390:521–525. doi: 10.1038/37379. [DOI] [PubMed] [Google Scholar]
- [378].Sridaran R, Blake CA. Effects of long-term adrenalectomy on periovulatory increases in serum gonadotrophins and ovulation in rats. J Endocrinol. 1980;84:75–82. doi: 10.1677/joe.0.0840075. [DOI] [PubMed] [Google Scholar]
- [379].Stachenfeld NS, Silva C, Keefe DL. Estrogen modifies the temperature effects of progesterone. Journal of applied physiology. 2000;88:1643–1649. doi: 10.1152/jappl.2000.88.5.1643. [DOI] [PubMed] [Google Scholar]
- [380].Stamford JA. Descending control of pain. British journal of anaesthesia. 1995;75:217–227. doi: 10.1093/bja/75.2.217. [DOI] [PubMed] [Google Scholar]
- [381].Stephenson LA, Kolka MA. Esophageal temperature threshold for sweating decreases before ovulation in premenopausal women. Journal of applied physiology. 1999;86:22–28. doi: 10.1152/jappl.1999.86.1.22. [DOI] [PubMed] [Google Scholar]
- [382].Sutton GM, Duos B, Patterson LM, Berthoud HR. Melanocortinergic modulation of cholecystokinin-induced suppression of feeding through extracellular signal-regulated kinase signaling in rat solitary nucleus. Endocrinology. 2005;146:3739–3747. doi: 10.1210/en.2005-0562. [DOI] [PubMed] [Google Scholar]
- [383].Szego CM, Davis JS. Adenosine 3′,5′-monophosphate in rat uterus: acute elevation by estrogen. Proc Natl Acad Sci U S A. 1967;58:1711–1718. doi: 10.1073/pnas.58.4.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [384].Szego EM, Barabas K, Balog J, Szilagyi N, Korach KS, Juhasz G, Abraham IM. Estrogen induces estrogen receptor alpha-dependent cAMP response element-binding protein phosphorylation via mitogen activated protein kinase pathway in basal forebrain cholinergic neurons in vivo. J Neurosci. 2006;26:4104–4110. doi: 10.1523/JNEUROSCI.0222-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [385].Takahashi KA, Cone RD. Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons. Endocrinology. 2005;146:1043–1047. doi: 10.1210/en.2004-1397. [DOI] [PubMed] [Google Scholar]
- [386].Tang SL, Tran V, Wagner EJ. Sex differences in the cannabinoid modulation of an A-type K+ current in neurons of the mammalian hypothalamus. Journal of neurophysiology. 2005;94:2983–2986. doi: 10.1152/jn.01187.2004. [DOI] [PubMed] [Google Scholar]
- [387].Tarttelin MF, Gorski RA. Variations in food and water intake in the normal and acyclic female rat. Physiology & Behavior. 1971;7:847–852. doi: 10.1016/0031-9384(71)90050-3. [DOI] [PubMed] [Google Scholar]
- [388].ter Haar MB. Circadian and estrual rhythms in food intake in the rat. Hormones and behavior. 1972;3:213–219. doi: 10.1016/0018-506x(72)90034-7. [DOI] [PubMed] [Google Scholar]
- [389].Terasawa E, Goldfoot DA, Davis GA. Pentobarbital inhibition of progesterone-induced behavioral estrus in ovariectomized guinea pigs. Brain research. 1976;107:375–383. doi: 10.1016/0006-8993(76)90234-1. [DOI] [PubMed] [Google Scholar]
- [390].Terasawa E, Noel SD, Keen KL. Rapid action of oestrogen in luteinising hormone-releasing hormone neurones: the role of GPR30. Journal of neuroendocrinology. 2009;21:316–321. doi: 10.1111/j.1365-2826.2009.01839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [391].Tesarik J, Mendoza C. Nongenomic effects of 17 beta-estradiol on maturing human oocytes: relationship to oocyte developmental potential. The Journal of clinical endocrinology and metabolism. 1995;80:1438–1443. doi: 10.1210/jcem.80.4.7714121. [DOI] [PubMed] [Google Scholar]
- [392].Tesarik J, Mendoza C. Direct non-genomic effects of follicular steroids on maturing human oocytes: oestrogen versus androgen antagonism. Human reproduction update. 1997;3:95–100. doi: 10.1093/humupd/3.2.95. [DOI] [PubMed] [Google Scholar]
- [393].Teyler TJ, Vardaris RM, Lewis D, Rawitch AB. Gonadal steroids: effects on excitability of hippocampal pyramidal cells. Science. 1980;209:1017–1018. doi: 10.1126/science.7190730. [DOI] [PubMed] [Google Scholar]
- [394].Thammacharoen S, Lutz TA, Geary N, Asarian L. Hindbrain administration of estradiol inhibits feeding and activates estrogen receptor-alpha-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology. 2008;149:1609–1617. doi: 10.1210/en.2007-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [395].Theander-Carrillo C, Wiedmer P, Cettour-Rose P, Nogueiras R, Perez-Tilve D, Pfluger P, Castaneda TR, Muzzin P, Schurmann A, Szanto I, Tschop MH, Rohner-Jeanrenaud F. Ghrelin action in the brain controls adipocyte metabolism. The Journal of clinical investigation. 2006;116:1983–1993. doi: 10.1172/JCI25811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [396].Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- [397].Titolo D, Mayer CM, Dhillon SS, Cai F, Belsham DD. Estrogen facilitates both phosphatidylinositol 3-kinase/Akt and ERK1/2 mitogen-activated protein kinase membrane signaling required for long-term neuropeptide Y transcriptional regulation in clonal, immortalized neurons. J Neurosci. 2008;28:6473–6482. doi: 10.1523/JNEUROSCI.0514-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [398].Toft D, Shyamala G, Gorski J. A receptor molecule for estrogens: studies using a cell-free system. Proceedings of the National Academy of Sciences of the United States of America. 1967;57:1740–1743. doi: 10.1073/pnas.57.6.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [399].Toran-Allerand CD. Novel sites and mechanisms of oestrogen action in the brain. Novartis Foundation symposium. 2000;230:56–69. doi: 10.1002/0470870818.ch6. discussion 69-73. [DOI] [PubMed] [Google Scholar]
- [400].Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES, Jr., Nethrapalli IS, Tinnikov AA. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [401].Torii M, Kubo K. The effects of intraventricular injection of beta-endorphin on initial estrogen action to induce lordosis behavior. Physiol Behav. 1994;55:157–162. doi: 10.1016/0031-9384(94)90024-8. [DOI] [PubMed] [Google Scholar]
- [402].Torii M, Kubo K, Sasaki T. Naloxone and initial estrogen action to induce lordosis in ovariectomized rats: the effect of a cut between the septum and preoptic area. Neurosci Lett. 1995;195:167–170. doi: 10.1016/0304-3940(95)11809-b. [DOI] [PubMed] [Google Scholar]
- [403].Torii M, Kubo K, Sasaki T. Influence of opioid peptides on the priming action of estrogen on lordosis in ovariectomized rats. Neurosci Lett. 1996;212:68–70. doi: 10.1016/0304-3940(96)12763-4. [DOI] [PubMed] [Google Scholar]
- [404].Torii M, Kubo K, Sasaki T. Facilitatory and inhibitory effects of beta-endorphin on lordosis in female rats: relation to time of administration. Horm Behav. 1999;35:271–278. doi: 10.1006/hbeh.1999.1526. [DOI] [PubMed] [Google Scholar]
- [405].Towle AC, Sze PY. Steroid binding to synaptic plasma membrane: differential binding of glucocorticoids and gonadal steroids. Journal of steroid biochemistry. 1983;18:135–143. doi: 10.1016/0022-4731(83)90079-1. [DOI] [PubMed] [Google Scholar]
- [406].Tritos NA, Segal-Lieberman G, Vezeridis PS, Maratos-Flier E. Estradiol-induced anorexia is independent of leptin and melanin-concentrating hormone. Obesity research. 2004;12:716–724. doi: 10.1038/oby.2004.84. [DOI] [PubMed] [Google Scholar]
- [407].Tritos NA, Vicent D, Gillette J, Ludwig DS, Flier ES, Maratos-Flier E. Functional interactions between melanin-concentrating hormone, neuropeptide Y, and anorectic neuropeptides in the rat hypothalamus. Diabetes. 1998;47:1687–1692. doi: 10.2337/diabetes.47.11.1687. [DOI] [PubMed] [Google Scholar]
- [408].Tsang BK, Carnegie JA. Calcium requirement in the gonadotropic regulation of rat granulosa cell progesterone production. Endocrinology. 1983;113:763–769. doi: 10.1210/endo-113-2-763. [DOI] [PubMed] [Google Scholar]
- [409].Tsujino N, Yamanaka A, Ichiki K, Muraki Y, Kilduff TS, Yagami K, Takahashi S, Goto K, Sakurai T. Cholecystokinin activates orexin/hypocretin neurons through the cholecystokinin A receptor. J Neurosci. 2005;25:7459–7469. doi: 10.1523/JNEUROSCI.1193-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [410].Uribe RM, Zacarias M, Corkidi G, Cisneros M, Charli JL, Joseph-Bravo P. 17beta-Oestradiol indirectly inhibits thyrotrophin-releasing hormone expression in the hypothalamic paraventricular nucleus of female rats and blunts thyroid axis response to cold exposure. Journal of neuroendocrinology. 2009;21:439–448. doi: 10.1111/j.1365-2826.2009.01861.x. [DOI] [PubMed] [Google Scholar]
- [411].Van de Kar LD. Neuroendocrine pharmacology of serotonergic (5-HT) neurons. Annual review of pharmacology and toxicology. 1991;31:289–320. doi: 10.1146/annurev.pa.31.040191.001445. [DOI] [PubMed] [Google Scholar]
- [412].Van der Kraak G. Role of calcium in the control of steroidogenesis in preovulatory ovarian follicles of the goldfish. General and comparative endocrinology. 1991;81:268–275. doi: 10.1016/0016-6480(91)90011-t. [DOI] [PubMed] [Google Scholar]
- [413].Viale A, Kerdelhue B, Nahon JL. 17beta-estradiol regulation of melanin-concentrating hormone and neuropeptide-E-I contents in cynomolgus monkeys: a preliminary study. Peptides. 1999;20:553–559. doi: 10.1016/s0196-9781(99)00007-8. [DOI] [PubMed] [Google Scholar]
- [414].Wade CB, Robinson S, Shapiro RA, Dorsa DM. Estrogen receptor (ER)alpha and ERbeta exhibit unique pharmacologic properties when coupled to activation of the mitogen-activated protein kinase pathway. Endocrinology. 2001;142:2336–2342. doi: 10.1210/endo.142.6.8071. [DOI] [PubMed] [Google Scholar]
- [415].Wagner EJ, Ronnekleiv OK, Grandy DK, Kelly MJ. The peptide orphanin FQ inhibits beta-endorphin neurons and neurosecretory cells in the hypothalamic arcuate nucleus by activating an inwardly-rectifying K+ conductance. Neuroendocrinology. 1998;67:73–82. doi: 10.1159/000054301. [DOI] [PubMed] [Google Scholar]
- [416].Wagner EJ, Tavitian N, Borgquist A, Sinchak K. Society for Neuroscience, 2011 Abstract Viewer/Itinerary Planner. Society for Neuroscience; Washington, DC: 2011. Estrogenic modulation of the pleiotropic actions of orphanin FQ/nociceptin at proopiomelanocortin synapses. Washington, DC. [Google Scholar]
- [417].Washburn N, Borgquist A, Wang K, Jeffery GS, Kelly MJ, Wagner EJ. Receptor Subtypes and Signal Transduction Mechanisms Contributing to the Estrogenic Attenuation of Cannabinoid-Induced Changes in Energy Homeostasis. Neuroendocrinology. 2012 doi: 10.1159/000338669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [418].Washington MC, Raboin SJ, Thompson W, Larsen CJ, Sayegh AI. Exenatide reduces food intake and activates the enteric nervous system of the gastrointestinal tract and the dorsal vagal complex of the hindbrain in the rat by a GLP-1 receptor. Brain research. 2010;1344:124–133. doi: 10.1016/j.brainres.2010.05.002. [DOI] [PubMed] [Google Scholar]
- [419].Webster NJ, Green S, Jin JR, Chambon P. The hormone-binding domains of the estrogen and glucocorticoid receptors contain an inducible transcription activation function. Cell. 1988;54:199–207. doi: 10.1016/0092-8674(88)90552-1. [DOI] [PubMed] [Google Scholar]
- [420].Weigt C, Hertrampf T, Zoth N, Fritzemeier KH, Diel P. Impact of estradiol. ER subtype specific agonists and genistein on energy homeostasis in a rat model of nutrition induced obesity, Molecular and cellular endocrinology. 2012;351:227–238. doi: 10.1016/j.mce.2011.12.013. [DOI] [PubMed] [Google Scholar]
- [421].Werther GA, Hogg A, Oldfield BJ, McKinley MJ, Figdor R, Allen AM, Mendelsohn FA. Localization and characterization of insulin receptors in rat brain and pituitary gland using in vitro autoradiography and computerized densitometry. Endocrinology. 1987;121:1562–1570. doi: 10.1210/endo-121-4-1562. [DOI] [PubMed] [Google Scholar]
- [422].Wilding JP, Gilbey SG, Mannan M, Aslam N, Ghatei MA, Bloom SR. Increased neuropeptide Y content in individual hypothalamic nuclei, but not neuropeptide Y mRNA, in diet-induced obesity in rats. The Journal of endocrinology. 1992;132:299–304. doi: 10.1677/joe.0.1320299. [DOI] [PubMed] [Google Scholar]
- [423].Williams CM, Rogers PJ, Kirkham TC. Hyperphagia in pre-fed rats following oral delta9-THC. Physiology & Behavior. 1998;65:343–346. doi: 10.1016/s0031-9384(98)00170-x. [DOI] [PubMed] [Google Scholar]
- [424].Willie JT, Sinton CM, Maratos-Flier E, Yanagisawa M. Abnormal response of melanin-concentrating hormone deficient mice to fasting: hyperactivity and rapid eye movement sleep suppression. Neuroscience. 2008;156:819–829. doi: 10.1016/j.neuroscience.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [425].Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [426].Woods SC, Gibbs J. The regulation of food intake by peptides. Annals of the New York Academy of Sciences. 1989;575:236–243. doi: 10.1111/j.1749-6632.1989.tb53246.x. [DOI] [PubMed] [Google Scholar]
- [427].Woods SC, Gotoh K, Clegg DJ. Gender differences in the control of energy homeostasis. Experimental biology and medicine. 2003;228:1175–1180. doi: 10.1177/153537020322801012. [DOI] [PubMed] [Google Scholar]
- [428].Xu H, Qin S, Carrasco GA, Dai Y, Filardo EJ, Prossnitz ER, Battaglia G, Doncarlos LL, Muma NA. Extra-nuclear estrogen receptor GPR30 regulates serotonin function in rat hypothalamus. Neuroscience. 2009;158:1599–1607. doi: 10.1016/j.neuroscience.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [429].Xu Y, Elmquist JK, Fukuda M. Central nervous control of energy and glucose balance: focus on the central melanocortin system. Annals of the New York Academy of Sciences. 2011;1243:1–14. doi: 10.1111/j.1749-6632.2011.06248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [430].Yagi K. Effects of estrogen on the unit activity of the rat hypothalamus. Nihon seirigaku zasshi. Journal of the Physiological Society of Japan. 1970;32:692–693. [PubMed] [Google Scholar]
- [431].Yagi K. Changes in firing rates of single preoptic and hypothalamic units following an intravenous administration of estrogen in the castrated female rat. Brain research. 1973;53:343–352. doi: 10.1016/0006-8993(73)90219-9. [DOI] [PubMed] [Google Scholar]
- [432].Yochim JM, Spencer F. Core temperature in the female rat: effect of ovariectomy and induction of pseudopregnancy. The American journal of physiology. 1976;231:361–365. doi: 10.1152/ajplegacy.1976.231.2.361. [DOI] [PubMed] [Google Scholar]
- [433].Zhang C, Kelly MJ, Ronnekleiv OK. 17Beta-estradiol rapidly increases K(ATP) activity in GnRH via a protein kinase signaling pathway. Endocrinology. 2010;151:4477–4484. doi: 10.1210/en.2010-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [434].Zwain IH, Yen SS. Neurosteroidogenesis in astrocytes, oligodendrocytes, and neurons of cerebral cortex of rat brain. Endocrinol. 1999;140:3843–3852. doi: 10.1210/endo.140.8.6907. [DOI] [PubMed] [Google Scholar]