Abstract
Objective
We have previously demonstrated that pre- and post-treatment of animals with suberoylanilide hydroxamic acid (SAHA), a histone deacetylase inhibitor (HDACI), can improve survival in a mouse model of lipopolysaccharides (LPS)-induced severe shock. This study was to assess whether SAHA affects LPS/Toll like receptor4 (TLR4) signaling through acetylation of HSP90 and degradation of its client protein interleukin-1 receptor associated kinase 1 (IRAK1).
Methods and Results
RAW264.7 cells were exposed to LPS (1 μg/ml) for two hours followed by treatment with SAHA (10 μM) or one of HSP90 inhibitors, geldanamycin (GA) (3 μM). Sham (no SAHA, no LPS) macrophages served as a control. The cells were harvested at different time points, and time zero served as the reference point. LPS dramatically increased protein expression of myeloid differentiation factor 88 (MyD88) and IRAK1, and stimulated nuclear translocation of nuclear factor kB (NF-kB), leading to increases of gene expression and protein production of TNF-α and IL-6. Treatment with SAHA significantly attenuated these LPS- stimulated alterations. LPS or SAHA did not change the levels of HSP90 protein, but immunoprecipitation studies demonstrated that SAHA treatment enhanced acetylation of HSP90, and increased the dissociation of IRAK1, compared to the LPS control.
Conclusions
SAHA suppresses LPS/TLR4 signaling in LPS-stimulated macrophages through multiple possible mechanisms. It inhibits the function of HSP90 through hyperacetylation of the chaperone protein, which results in dissociation and degradation of the client protein IRAK1 and, at least in part, leads to a resultant decrease in nuclear translocation of NF-κB and attenuation of key pro-inflammatory cytokine expression.
Keywords: Suberoylanilide hydroxamic acid, Lipopolysaccharides, Toll-Like Receptor 4, HSP90, macrophages, inflammation, immune response, acetylation
INTRODUCTION
Despite the recent advances in antibiotics and critical care, the current mortality rate remains unacceptably high for patients who develop severe sepsis and septic shock (1). Recently, we have shown that pre (before insult) and post (after insult) treatment with suberoylanilide hydroxamic acid (SAHA), a histone deacetylase (HDAC) inhibitor (HDACI), can improve survival in a mouse model of lethal lipopolysaccharide (LPS) induced shock (2, 3). However, the mechanism remains largely unknown.
The detection of bacterial lipopolysaccharide (LPS) by macrophages is mediated by Toll-like receptor 4 (TLR4) (4, 5). Prominent components of the LPS/TLR4 pathway include MyD88, IL-1 receptor associated kinases (e.g. IRAK1 and IRAK4), TRAF6 (TNF receptor-associated factor 6), and transcription factors including NF-kB (nuclear factor kB). Activation of this signaling pathway can result in the release of critical pro-inflammatory cytokines that activate potent immune responses. However, an excessive LPS/TLR4 signal can result in overproduction of pro-inflammatory cytokines which can exaggerate systemic inflammation and worsen the sepsis physiology (6, 7).
Heat shock protein 90 (HSP90) is a molecular chaperone which can hold and stabilize client proteins such as IRAK1. Inhibition of HSP90 with its inhibitor geldanamycin (GA) in macrophages can result in rapid loss of IRAK1 protein, which impairs the ability of TLR4 to stimulate the release of inflammatory cytokines (8). More recently, Meng et al reported that carbamazepine, an HDACI, can promote Her-2 onco-protein degradation by modulating acetylation of HSP90 to inhibit proliferation of breast cancer cell (9). It remains unclear, however, whether SAHA exerts its protective effects by dampening the LPS/TLR4 signaling and the downstream cascade. Our goal was to test whether treatment with SAHA would attenuate inflammation in LPS-stimulated mouse macrophages through increased acetylation of HSP90 and degradation of IRAK1 in the TLR4 pathway.
MATERIALS AND METHODS
Cell Culture and Treatment
Murine macrophages RAW264.7 cells (American Type Culture Collection, Manassas, VA) were maintained in advanced DMEM (Invitrogen, Grand Island, NY) supplemented with 10% FBS (Invitrogen, Grand Island, NY). These cells were grown at 37 °C in a humidified incubator in 5% CO2 and 95% air. The cells were subcultured before 70-80% confluence and seeded at a density of 0.25 × 106 cells/mL, allowed to attach to the bottom of the 75 cm2 flasks for 24 hours and then serum starved (0.5% FBS) overnight before treatment with LPS (from S typhosa) (Sigma Chemical Co, St. Louis, MO) (final concentration:1 μg/mL). Two hours later, SAHA (Biomol International, Plymouth Meeting, PA) (final concentration: 10 μM) or GA (Cell Signaling Technology Inc, Danvers, MA) (final concentration: 3 μM) were added into the cell culture medium. Sham (no SAHA, no LPS) macrophages served as control. The cells were harvested in pellets and the cell culture supernatants were collected at different time points. The time of LPS treatment served as the reference point (time zero) on which all time points in this study were calculated. Whole cell lysate and cell fractionation were prepared from the cell pellets using Whole Cell Extraction Kit (Millipore Corporation, Temecula, CA) and ProteoExtractTM Subcellular Proteome Extraction Kit (EMD Biosciense, Inc., La Jolla, CA), respectively. The fractionated nucleus was used for analysis of nuclear translocation of NF-κB protein from cytosol.
Immunoprecipitation (IP)
Each sample of whole cell lysate was incubated with 15 μg antibody against HSP90 (Millipore Corporation, Billerica, MA) with rotation over night at 4°C and then 50 μl TrueBlot Anti-Mouse Ig IP Beads (eBioscience, Inc., San Diego, CA) were added. The mixtures were incubated for two hours with rotation at room temperature to allow Ag-Ab complex to bind to the beads. The supernatants were separated by spin and removed. The (pelleted) beads were washed three times with Lysis Buffer. 30 μl of double-distilled water and 6 μl of SDS- sample buffer (6X, redc.) (Boston Bioproducts, Ashland, MA) were added into each pelleted bead. Heated for 5 min at 99°C, samples were prepared for following western blot analysis.
Western Blot Analysis
Equal amounts of whole cell lysate, nuclear fraction and immunoprecipitate SDS samples were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA). The membranes were blocked in 0.05% PBS-tween (PBST) containing 5% milk (Bio-Rad Laboratories) and then incubated with primary antibodies against following proteins: MyD88 and IRAK1, NF-κB p65 (Cell Signaling Technology Inc, Danvers, MA), HSP90 (mouse Hsp90 monoclonal antibody, AC88; Millipore Corporation, Billerica, MA), acetyl-lysine (Millipore Corporation) and actin (Sigma-Aldrich, Inc., St. Louis, MO), at 4 °C overnight. The primary antibodies were detected by incubation with horseradish peroxidase-coupled second antibodies against rabbit or mouse IgG (Amersham Biosciences, Piscataway, NJ) (1:3,000 in PBST with 5% milk) at room temperature for 2 hours. The chemiluminescence detections were performed by using Western Lighting Chemiluminescence Reagent Plus (Perkin-Elmer LAS, Inc., Boston, MA). Films were developed using a standard photographic procedure and quantitative analysis of detected bands was carried out by densitometer scanning using the VersaDoc Imaging System (Bio-Rad Laboratories).
RNA Isolation and Real-Time Polymerase Chain Reaction (Real-time PCR)
Total RNA was prepared from macrophage cell pellets in RNAlater solution using the RNeasy Mini Kit (Qiagen, Valencia, CA), and mRNA was reverse transcribed into cDNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) according to the manufacture’s instructions. Equal amounts of cDNA were submitted for PCR in the presence of SYBR Green Master Mix (Roche Diagnosis, Indianapolis, IN) and forward and reverse primers using the ABI PRISM 7300 Real-Time PCR detection machine (Applied Biosystems). The primers for TNF-α (forward: 5′-CCC ACT CTG ACC CCT TTA CT-3′; reverse: 5′- TTT GAG TCC TTG ATG GTC GT-3′) and IL-6 (forward: 5′-CTA CCC CAA TTT CCA ATG CT-3′; reverse: 5′-ACC ACA GTG AGG AAT GTC CA-3′) were synthesized by Real Time Primers (Elkins Park, PA). GAPDH (forward: 5′-CCTGGAGAAACCTGCCAAGTAT-3′; reverse: 5′-CTCGGCCGCCTGCTT-3′) was used as an internal control and was synthesized by Invitrogen (Carlsbad, CA). PCR was performed with 40 cycles of 15 seconds at 95 °C, and 1 minute at 60 °C. Each sample was run in triplicate. Relative quantification of mRNA expression was performed using the ΔΔCt method where ΔCt is the calculated difference in the Ct (threshold cycle) values between TNF-α, IL-6 and GAPDH, and ΔΔCt is the calculated difference between the ΔCt for a given sample and the ΔCt for sham, such that the relative quantity of mRNA, normalized to GAPDH and sham, is calculated as 2−ΔΔCt.
Evaluation of LPS-induced TNF-α and IL-6 Secretion by RAW264.7 Cells
Quantitative determination in the cell culture medium was made using the Quantikine Enzyme-Linked Immunosorbent Assay (ELISA) Kit (R&D Systems, Minneapolis, MN) for TNF-α and IL-6 according to manufacturer’s instruction. The concentration of cytokine was measured by optical densitometry at 450 nm in a SpectramaxPlus 384 microplate reader (Molecular Devices, Sunnyvale, CA). All of the analyses were performed in triplicate.
Statistical Analysis
All quantitative data are described as the means ± SD (standard deviation). Data were analyzed using Microsoft Office Excel 2003. Statistical differences were determined by Student t tests. A p<0.05 was considered as statistically significant.
RESULTS
Impact of SAHA on levels of MyD88, IRAK1 and HSP90 proteins
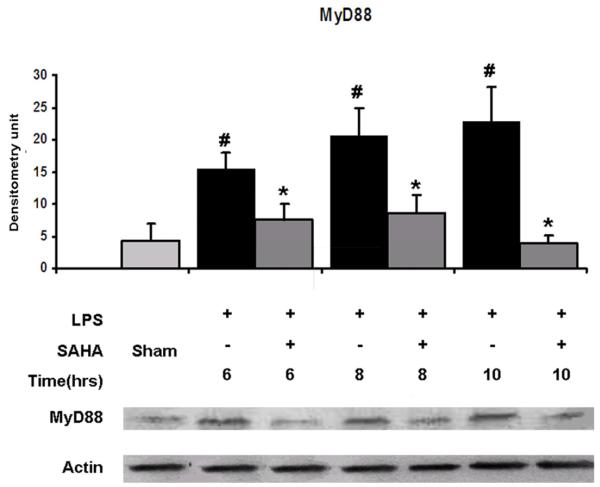
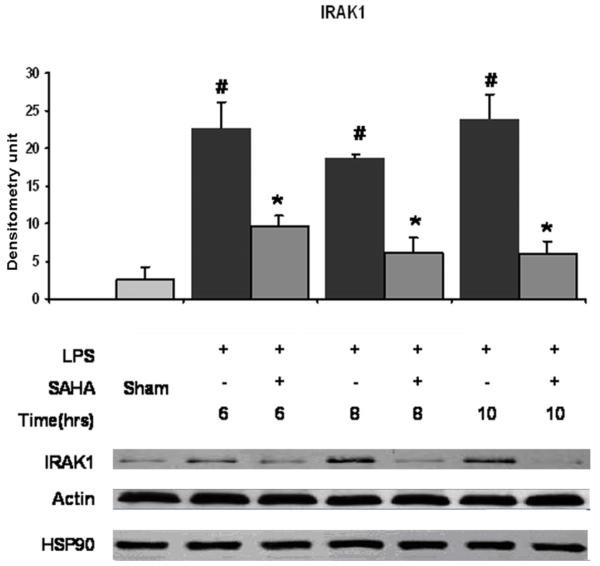
Cellular levels of MyD88 and IRAK1 proteins in RAW264.7 cells at 6, 8 and 10 hours were examined by immunoblotting with anti- MyD88 and anti-IRAK1 antibodies. Normally, RAW264.7 cells express low levels of MyD88 and IRAK1 proteins. LPS increased MyD88 and IRAK1 protein expression in a time-dependent manner. Post-treatment of the cells with SAHA significantly inhibited increase of MyD88 (Fig. 1, p <0.05) and IRAK1 (Fig. 2, p <0.05) protein levels in cells. There was no significant difference in the HSP90 protein levels among groups of sham, LPS and LPS with SAHA treatment at all the time points (Fig. 2). These results indicate that LPS induces expression of MyD88 and IRAK1 proteins in the LPS/TLR4 signal transduction pathway, and SAHA post-treatment attenuates these changes without alteration in HSP90 protein levels.
FIG. 1. Effects of SAHA on protein expression of MyD88 in LPS -stimulated macrophages.
Cellular levels of MyD88 protein in RAW264.7 cells at 6, 8 and 10 hours were examined by immunoblots with anti- MyD88 and anti-actin antibodies. Specific bands were quantified by densitometry and expressed as mean values ± SD (n = 3). LPS significantly increased and SAHA decreased the MyD88 protein expression, respectively. # denotes a significant difference compared with the sham group (p < 0.05). * denotes a significant difference compared with the LPS group (p < 0.05). Values at time zero used as the reference point.
FIG. 2. Effects of SAHA on protein expression of IRAK1 and HSP90 in LPS-stimulated macrophages.
Cellular levels of IRAK1 and HSP90 proteins in RAW264.7 cells at 6, 8, and 10 hours were examined by immunoblots. Specific bands were quantified by densitometry and expressed as mean values ± SD (n = 3). LPS significantly increased and SAHA decreased IRAK1 protein levels, respectively. Neither LPS nor SAHA affected expression of HSP90. # denotes a significant difference compared with the sham group (p < 0.05). * denotes a significant difference compared with the LPS group (p < 0.05). Values at time zero used as the reference point.
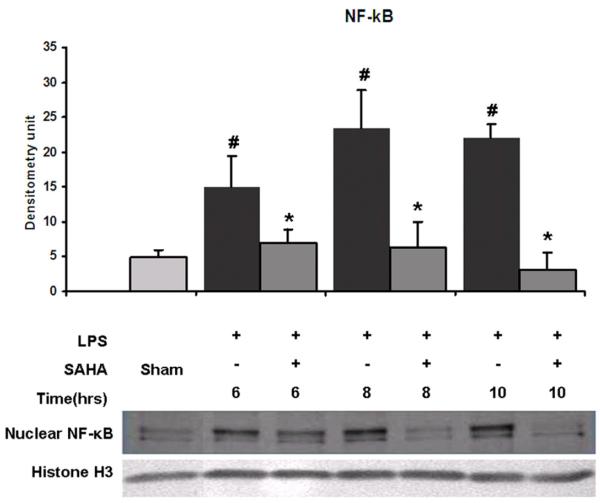
Effect of SAHA on nuclear translocation of NF-κB
We examined the levels of NF-κB protein in the nucleus fraction isolated from all groups at various time points. LPS exposure increased levels of NF-κB at all time points compared to sham. SAHA treatment significantly decreased NF-κB protein levels in the nuclear fraction compared to the sham and LPS groups (Fig. 3, p <0.05). These results suggest that SAHA treatment attenuates nuclear translocation of NF-κB.
FIG. 3. Effects of SAHA on nuclear translocation of NF-κB in LPS-stimulated macrophages.
Nuclear fractions were subjected to western blotting with anti- NF-κB p65 and anti-Histone H3 antibodies. Specific bands were quantified by densitometry and expressed as mean values ± SD (n = 3). LPS significantly increased and SAHA decreased the nuclear translocation of NF-κB, respectively.# denotes a significant difference compared with the sham group (p < 0.05). * denotes a significant difference compared with the LPS group (p < 0.05). Values at time zero used as the reference point. Histone H3 serves as an internal control for equal sample loading.
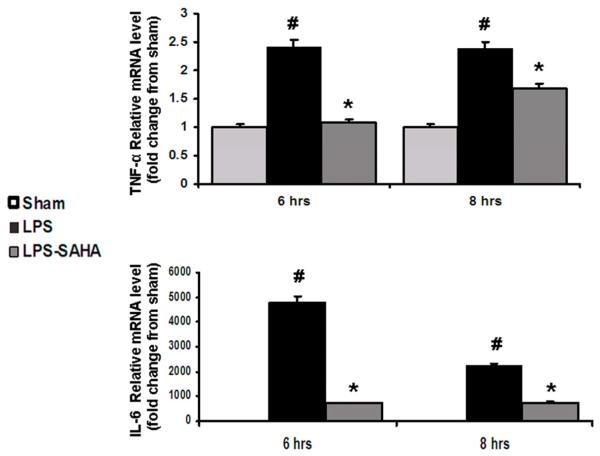
Effect of SAHA on gene expression and protein levels of TNF-α and IL-6
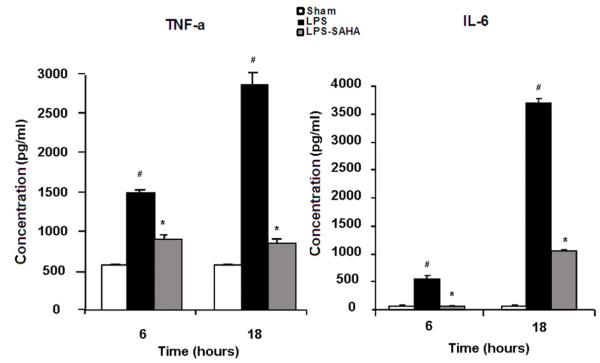
Real-time PCR was employed to measure expression of TNF-α and IL-6 genes. LPS stimulated expression of TNF-α and IL-6 genes, whereas SAHA treatment significantly decreased their transcription (Fig. 4, p < 0.05). Further analyses of cell culture supernatant by ELISA confirmed that extracellular levels of TNF-α and IL-6 proteins were consistent with their gene expression patterns (Fig. 5, p <0.05). These results suggest that inhibition of nuclear translocation of NF-κB by SAHA leads to a reduction in TNF-α and IL-6 gene expression and protein levels.
FIG. 4. Effects of SAHA on gene expression of TNF-α and IL-6 in LPS-stimulated macrophages.
TNF-α and IL-6 mRNA levels were determined by real-time PCR and expressed as mean values ± SD (n = 3). LPS significantly increased and SAHA decreased the expression of TNF-α and IL-6 genes, respectively. # denotes a significant difference compared with the sham group (p < 0.05). *denotes a significant difference compared with the LPS group (p < 0.05). Values at time zero used as the reference point.
FIG. 5. Effects of SAHA on protein secretion of TNF-α and IL-6 in LPS-stimulated macrophages.
Concentrations of TNF-α and IL-6 in the cell culture mediums were determined by ELISA. The cytokine concentration was expressed as mean values ± SD (n = 3). LPS significantly increased and SAHA decreased the TNF-α and IL-6 secretion from the macrophages, respectively. # denotes a significant difference compared with the sham group (p < 0.05). * denotes a significant difference compared with the LPS group (p < 0.05). Values at time zero used as the reference point.
Impact of SAHA induced HSP90 acetylation on intracellular IRAK1 protein level
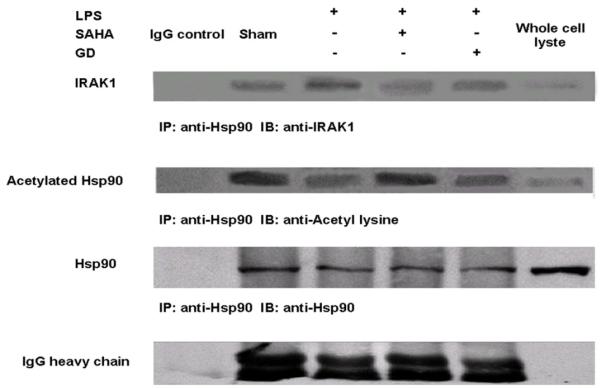
We immunoprecipitated HSP90 protein complex with anti-HSP90 antibody. Further immunoblottings were employed to detect: (1) IRAK1 protein with anti-IRAK1 antibody, and (2) acetylated HSP90 protein with anti-acetyl lysine antibody. As shown in Figure 6, there were no significant changes among all treatment groups in term of HSP90 protein concentration (the second panel from the bottom). However, LPS stimulation increased association of IRAK1 protein with HSP90, suggesting that more IRAK1 interacted with HSP90 (the top panel). By contrast, SAHA treatment dramatically induced hyperacetylation of HSP90 protein and decreased association of IRAK1 protein with HSP90. Geldanamycin (GA), an inhibitor of HSP90, decreased association of IRAK1 with HSP90 protein compared to LPS insult without increasing acetylation of HSP90. These results indicate that SAHA can induce hyperacetylation of HSP90 protein, which disrupts its chaperon function and results in disassociation of IRAK1 protein.
FIG. 6. Effects SAHA on acetylation of HSP90 and association of IRAK1 with HSP90 in LPS stimulated macrophages.
The IRAK1 and acetylated HSP90 proteins in HSP90 protein complexes were examined by immunoprecipitation and immunoblots. LPS significantly increased and SAHA decreased association of IRAK1 protein with HSP90, respectively. Moreover, SAHA increased HSP90 acetylation compared to the LPS group. Ig G heavy chain, derived from external antibodies, serves as an internal control for equal amount of antibody used in the experiment.
DISCUSSION
We have previously reported that in murine model of LPS-induced severe shock, SAHA significantly attenuates organ injury and improves survival. This protective effect is equally pronounced regardless of whether the treatment is administered pre- or post- LPS insult (2, 3). In the present study, we explored the underlying mechanisms by focusing on the impact of SAHA treatment on TLR4 signaling in a model of LPS-insulted mouse macrophages. Using this potent HDACI, our experiments revealed some important findings: 1) SAHA decreases expression of MyD88 and IRAK1 proteins, prevents nuclear translocation of NF-kB, suppresses transcription of TNF-α and IL-6 genes, and attenuates secretion of these pro-inflammatory cytokines. This protective effect is present even when the cells are treated two hours after the LPS insult; 2) Treatment with SAHA hyperacetylates HSP90, which impairs its chaperone function and results in degradation of its client protein IRAK1. These data suggest that SAHA attenuates the LPS/TLR4 cascade mediated inflammatory reaction, at least in part, by interrupting the functions of IRAK1. Our study is the first to show the HDACI-mediated HSP90 hyperacetylation and degradation of IRAK1 in LPS-stimulated macrophages. These results are consistent with studies of HDACI-induced HSP90 acetylation and client protein degradation in cancer cells (9).
SAHA is a pan-inhibitor of HDAC, including HDAC6, and it has been shown to reduce serum levels of TNF-α, IL-1β, IL-6, and IFN-γ in an in vivo LPS-induced endotoxemia animal model, and in LPS stimulated peripheral blood mononuclear cell (PBMC) (2, 11). In the current in vitro study, we have identified the mechanisms behind these observations by demonstrating that SAHA decreases translocation of NF-κB into the nucleus, and suppresses transcription of TNF-α and IL-6 genes, and subsequent secretion of these cytokines. Historically, SAHA’s anti-inflammatory effects have been attributed to the inhibition of NF-κB, and kinases such as mitogen-activated protein kinases (MAPK, p38, ERK), MAPK phosphatase-1(MKP-1), etc, which are down-stream targets of LPS/TLR4 signaling pathway (12, 13). Studies had not explored proteins that are up-stream of NF-κB until recently, when we reported that the anti-inflammatory effects of SAHA may be due to inhibition of MyD88 protein in the TLR4-MyD88 pathway (3). Following up on these results, we now have shown that SAHA also decreases IRAK1 in addition to MyD88 protein, which are all keys components of the LPS/TLR4-MyD88 dependent pathway.
Once activated by LPS, TLR4 recruits its downstream adaptors including MyD88. In mice, MyD88 is a universal adapter cytosolic protein that is used by all TLRs (except TLR3) to activate the transcription factor NF-κB. It is an essential mediator for the LPS-TLR 4 signaling (14, 15). MyD88- deficient mice are resistant to the LPS-induced septic shock and MyD88-deficient macrophages fail to produce pro-inflammatory cytokines after LPS stimulation (16). Data from our laboratory has shown that SAHA suppresses gene and protein expression of MyD88 in lung tissue following LPS insult in mice (3). Here the finding that SAHA suppresses expression of MyD88 in murine macrophages supports our previous in vivo observations. SAHA inhibited MyD88 expression in lung tissue and attenuated LPS-induced acute lung injury (3).
As a component of the LPS/TLR4 pathway that is downstream of MyD88, IRAK1 advances the signaling cascade. IRAK-1-deficient mice are less susceptible to the lethal effects of LPS than their wild-type counterparts (17), and murine macrophages lacking IRAK-1 exhibit an impaired ability to activate NF-κB pathway and multiple MAPK and to secrete TNF-α when stimulated with LPS (17-19). These findings demonstrate the importance of this kinase in the pathway. The present study reports for the first time that SAHA suppresses expression of IRAK1 in macrophages following LPS activation.
Translocation of transcription factor NF-κB into the nucleus (a downstream result of LPS/TLR4 signaling cascade) controls the expression of pro-inflammatory cytokines. It has previously been reported that SAHA suppresses the LPS-induced NF-kB p65 nuclear accumulation in E11 human synovial fibroblasts cells and THP-1 monocytic cells (20). While confirming these findings in murine macrophages, we also discovered that SAHA decreases pro-inflammatory cytokines (TNF-α and IL-6) at gene and protein levels. Furthermore, we found that SAHA decreases IRAK1 via hyperacetylation of HSP90 protein. HSP90 protein is a highly abundant molecular chaperone with hundreds of client proteins. Many of these client proteins are central players in key signal transduction pathways (21) and the majority of them are protein kinases, including IRAK1 (8, 22). HSP90 consists of an amino-terminal intrinsic ATPase domain, a central client-binding region, and a carboxy-terminal dimerization domain (23, 24). A common feature of HSP90 is that the chaperone binds its clients – in nearly mature conformations, retaining and releasing them in an activity cycle driven by ATP hydrolysis and regulated by binding of co-chaperones. Thus, HSP90 controls the biogenesis, stability, and activity of its client proteins. GA, an ansamycin antibiotic, can compete with ATP for binding at the N-terminal site of HSP90, thereby disrupting its chaperon functions. Upon the pharmacological inhibition of HSP90 by GA, the client protein undergoes degradation. For example, GA can cause rapid loss of IRAK1 protein in the macrophages (8). Inhibition of HSP90 and degradation of its client proteins have been targeted to treat a wide range of diseases, in which cancer is the most widely studied (25).
In addition to its inhibitors, the chaperone function of HSP90 is regulated by a number of posttranslational modifications. Reversible acetylation has been implicated as a regulatory posttranslational modification of HSP90 (26). Histone deacetylase inhibitors can cause acetylation of HSP90, and result in a reduced interaction with several of its client proteins, such as p53, Raf1, Bcl- Abl, glucorticoid receptor and Herb2 (27). Furthermore, besides the ability to interact with its client proteins, binding of ATP by HSP90 is also compromised by acetylation (27). HDAC6 has been shown to regulate the acetylation and chaperon activity of HSP90. Inactivation of HDAC6 leads to HSP90 hyperacetylation, its dissociation from p23 and loss of chaperone activity (28). Loss of HSP90 activity prevents the maturation of the glucocorticoid receptor, affecting ligand binding, nuclear translocation, and transcriptional activation (29, 30). By focusing on protein kinases IRAK1 (fairly upstream in the TLR4 signaling pathway) we have found SAHA can decrease IRAK1 by disassociating it from its chaperone protein through hyperacetylation of HSP90.
This study has several limitations that should be kept in mind. It was by design an in vitro mechanistic study, which could not address survival, organ function and other outcome measures. However, we have performed numerous survival (and organ function) studies in small and large animal models that have been summarized elsewhere (31). Moreover, the in vitro pattern of inflammatory cytokine production (TNF-α) found in this study is the same as that reported previously in vivo (2). LPS injection increases cytokines and SAHA treatment attenuates them both in vitro and in vivo.
Similarly, as our goal was to use a well-controlled and simple model, we decided to use LPS insult which is only one variable in the etiology of septic shock. Clearly, cellular response to bacterial infection may be much more complex. Similarly, we focused our attention on critical components of a well described pathway, and it is highly likely that many more pathways are involved that are equally important. Thus, we may be looking at one piece of a very complex jigsaw puzzle.
In our present study, the dose of SAHA was relatively higher, compared to what is used in clinical practice (32). We selected this large dose based on our previous study published in Shock (2) and the data generated by others that have looked at macrophages’ response to TLR4 agonist LPS (33). In a previous study (2), we didn’t find cell toxicity when macrophages RAW264.7 were treated with 10 M of SAHA. Therefore, we wanted to use the same dose of SAHA not only to ensure an effect, but also to be able to compare our current results with prior findings.
Experimentally, a broad range of LPS (1 ng/ml to 1 g/ml or even more) have been used to stimulate RAW 264.7 macrophages in cell culture. In fact, 1 μg/ml of LPS was used specifically for TLR-4 elicitation in RAW 264.7 macrophages (34). The LPS concentration used here (1 μg/ml) compares well with our previous studies (2) as well as other researchers (35, 36). In prior (2) and current studies, macrophages were cultured in DMEM medium containing 0.5% FBS before stimulation with LPS (1 g/ml). Starvation of the cells with low concentration of FBS (0.5 – 1%) has been used by many investigators to study LPS stimulated RAW 264.7 cells (37, 38). In conclusion, in the in vitro study using LPS-insulted macrophages, we have shown that treatment with SAHA suppresses inflammatory cytokines by preventing nuclear translocation of NF-κB, and by inhibiting its upstream proteins (MyD88 and IRAK1) in the LPS/TLR4 signaling cascade. In addition, hyperacetylation of chaperon HSP90 leads to degradation of its client protein IRAK1, which may also be, at least in part, responsible for a decrease in IRAK1 levels in the cells.
Acknowledgements
This work was funded by a grant from NIH RO1 GM084127 to HBA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Opal SM. The host response to endotoxin, antilipopolysaccharide strategies, and the management of severe sepsis. Int J Med Microbiol. 2007;297:365–377. doi: 10.1016/j.ijmm.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Li Y, Liu B, Zhao H, Sailhamer EA, Fukudome EY, Zhang X, Kheirbek T, Finkelstein RA, Velmahos GC, deMoya M, Hales CA, Alam HB. Protective effect of suberoylanilide hydroxamic acid against LPS-induced septic shock in rodents. Shock. 2009;32:517–523. doi: 10.1097/SHK.0b013e3181a44c79. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Liu B, Fukudome EY, Kochanek AR, Finkelstein RA, Chong W, Jin G, Lu J, deMoya MA, Velmahos GC, Alam HB. Surviving lethal septic shock without fluid resuscitation in a rodent model. Surgery. 2010;148:246–254. doi: 10.1016/j.surg.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 5.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 6.Sands KE, Bates DW, Lanken PN, Graman PS, Hibberd PL, Kahn KL, Parsonnet J, Panzer R, Orav EJ, Snydman DR, Black E, Schwartz JS, Moore R, Johnson BL, Jr., Platt R. Epidemiology of sepsis syndrome in 8 academic medical centers. JAMA. 1997;278:234–240. [PubMed] [Google Scholar]
- 7.Friedman G, Silva E, Vincent JL. Has the mortality of septic shock changed with time. Crit Care Med. 1998;26:2078–2086. doi: 10.1097/00003246-199812000-00045. [DOI] [PubMed] [Google Scholar]
- 8.De Nardo D, Masendycz P, Ho S, Cross M, Fleetwood AJ, Reynolds EC, Hamilton JA, Scholz GM. A central role for the Hsp90.Cdc37 molecular chaperone module in interleukin-1 receptor-associated-kinase-dependent signaling by toll-like receptors. J Biol Chem. 2005;280:9813–9822. doi: 10.1074/jbc.M409745200. [DOI] [PubMed] [Google Scholar]
- 9.Meng Q, Chen X, Sun L, Zhao C, Sui G, Cai L. Carbamazepine promotes Her-2 protein degradation in breast cancer cells by modulating HDAC6 activity and acetylation of Hsp90. Mol Cell Biochem. 2011;348:165–171. doi: 10.1007/s11010-010-0651-y. [DOI] [PubMed] [Google Scholar]
- 10.Kawada J, Zou P, Mazitschek R, Bradner JE, Cohen J. Tubacin kills Epstein-Barr virus (EBV)- Burkitt lymphoma cells by inducing reactive oxygen species and EBV lymphoblastoid cells by indicing apoptosis. J Biol Chem. 2009;284:17102–17109. doi: 10.1074/jbc.M809090200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leoni F, Zaliani A, Bertolini G, Porro G, Pagani P, Pozzi P, Dona G, Fossati G, Sozzani S, Azam T, Bufler P, Fantuzzi G, Goncharov I, Kim SH, Pomerantz BJ, Reznikov LL, Siegmund B, Dinarello CA, Mascagni P. The antitumor histone deacetylase inhibitor suberoylanilide hydroxamic acid exhibits antiinflammatory properties via suppression of cytokines. Proc Natl Acad Sci U S A. 2002;99:2995–3000. doi: 10.1073/pnas.052702999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao W, Bao C, Padalko E, Lowenstein CJ. Acetylation of mitogen-activated protein kinase phosphatase-1 inhibits Toll-like receptor signaling. J Exp Med. 2008;205:1491–1503. doi: 10.1084/jem.20071728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finkelstein RA, Li Y, Liu B, Shuja F, Fukudome E, Velmahos GC, deMoya M, Alam HB. Treatment with histone deacetylase inhibitor attenuates MAP kinase mediated liver injury in a lethal model of septic shock. J Surg Res. 2010;163:146–154. doi: 10.1016/j.jss.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janssens S, Beyaert R. A universal role for MyD88 in TLR/IL-1R-mediated signaling. Trends Biochem Sci. 2002;27:474–482. doi: 10.1016/s0968-0004(02)02145-x. [DOI] [PubMed] [Google Scholar]
- 15.Beutler B. Tlr4: central component of the sole mammalian LPS sensor. Curr Opin Immunol. 2000;12:20–26. doi: 10.1016/s0952-7915(99)00046-1. [DOI] [PubMed] [Google Scholar]
- 16.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 17.Swantek JL, Tsen MF, Cobb MH, Thomas JA. IL-1 receptor-associated kinase modulates host responsiveness to endotoxin. J Immunol. 2000;164:4301–4306. doi: 10.4049/jimmunol.164.8.4301. [DOI] [PubMed] [Google Scholar]
- 18.Kanakaraj P, Schafer PH, Cavender DE, Wu Y, Ngo K, Grealish PF, Wadsworth SA, Peterson PA, Siekierka JJ, Harris CA, Fung-Leung WP. Interleukin (IL)-1 receptor-associated kinase (IRAK) requirement for optimal induction of multiple IL-1 signaling pathways and IL-6 production. J Exp Med. 1998;187:2073–2079. doi: 10.1084/jem.187.12.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas JA, Allen JL, Tsen M, Dubnicoff T, Danao J, Liao XC, Cao Z, Wasserman SA. Impaired cytokine signaling in mice lacking the IL-1 receptor-associated kinase. J Immunol. 1999;163:978–984. [PubMed] [Google Scholar]
- 20.Choo QY, Ho PC, Tanaka Y, Lin HS. Histone deacetylase inhibitors MS-275 and SAHA induced growth arrest and suppressed lipopolysaccharide-stimulated NF-kappaB p65 nuclear accumulation in human rheumatoid arthritis synovial fibroblastic E11 cells. Rheumatology (Oxford) 2010;49:1447–1460. doi: 10.1093/rheumatology/keq108. [DOI] [PubMed] [Google Scholar]
- 21.Salminen A, Paimela T, Suuronen T, Kaarniranta K. Innate immunity meets with cellular stress at the IKK complex: regulation of the IKK complex by HSP70 and HSP90. Immunol Lett. 2008;117:9–15. doi: 10.1016/j.imlet.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 22.Citri A, Harari D, Shohat G, Ramakrishnan P, Gan J, Lavi S, Eisenstein M, Kimchi A, Wallach D, Pietrokovski S, Yarden Y. Hsp90 recognizes a common surface on client kinases. J Biol Chem. 2006;281:14361–14369. doi: 10.1074/jbc.M512613200. [DOI] [PubMed] [Google Scholar]
- 23.Schulze-Lefert P. Plant immunity: the origami of receptor activation. Curr Biol. 2004;14:R22–24. [PubMed] [Google Scholar]
- 24.Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70- based chaperone machinery. Exp Biol Med (Maywood) 2003;228:111–133. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- 25.Pacey S, Banerji U, Judson I, Workman P. Hsp90 inhibitors in the clinic. Handb Exp Pharmacol. 2006;172:331–358. doi: 10.1007/3-540-29717-0_14. [DOI] [PubMed] [Google Scholar]
- 26.Yu X, Guo ZS, Marcu MG, Neckers L, Nguyen DM, Chen GA, Schrump DS. Modulation of p53, ErbB1, ErbB2, and Raf-1 expression in lung cancer cells by depsipeptide FR901228. J Natl Cancer Inst. 2002;94:504–513. doi: 10.1093/jnci/94.7.504. [DOI] [PubMed] [Google Scholar]
- 27.Wandinger SK, Richter K, Buchner J. The Hsp90 chaperone machinery. J Biol Chem. 2008;283:18473–18477. doi: 10.1074/jbc.R800007200. [DOI] [PubMed] [Google Scholar]
- 28.Kekatpure VD, Dannenberg AJ, Subbaramaiah K. HDAC6 modulates Hsp90 chaperone activity and regulates activation of aryl hydrocarbon receptor signaling. J Biol Chem. 2009;284:436–445. doi: 10.1074/jbc.M808999200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Kovacs JJ, Murphy PJ, Gaillard S, Zhao X, Wu JT, Nicchitta CV, Yoshida M, Toft DO, Pratt WB, Yao TP. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol Cell. 2005;18:601–607. doi: 10.1016/j.molcel.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 30.Murphy PJ, Morishima Y, Kovacs JJ, Yao TP, Pratt WB. Regulation of the dynamics of hsp90 action on the glucocorticoid receptor by acetylation/deacetylation of the chaperone. J Biol Chem. 2005;280:33792–33799. doi: 10.1074/jbc.M506997200. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Alam HB. Modulation of acetylation: creating a pro-survival and anti-inflammatory phenotype in lethal hemorrhagic and septic shock. J Biomed Biotechnol. 2011;2011:523481. doi: 10.1155/2011/523481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelly WK, Richon VM, O’Connor O, Curley T, MacGregor-Curtelli B, Tong W, Klang M, Schwartz L, Richardson S, Rosa E, Drobnjak M, Cordon-Cordo C, Chiao JH, Rifkind R, Marks PA, Scher H. Phase I clinical trial of histone deacetylase inhibitor: suberoylanilide hydroxamic acid administered intravenously. Clin Cancer Res. 2003;9:3578–3588. [PubMed] [Google Scholar]
- 33.Halili MA, Andrews MR, Labzin LI, Schroder K, Matthias G, Cao C, Lovelace E, Reid RC, Le GT, Hume DA, Irvine KM, Matthias P, Fairlie DP, Sweet MJ. Differential effects of selective HDAC inhibitors on macrophage inflammatory responses to the Toll-like receptor 4 agonist LPS. J Leukoc Biol. 2010;87:1103–1114. doi: 10.1189/jlb.0509363. [DOI] [PubMed] [Google Scholar]
- 34.Premkumar V, Dey M, Dorn R, Raskin I. MyD88-dependent and independent pathways of Toll-Like Receptors are engaged in biological activity of triptolide in ligand-stimulated macrophages. BMC Chem Biol. 2010;10:3. doi: 10.1186/1472-6769-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang D, Lou J, Ouyang C, Chen W, Liu Y, Liu X, Cao X, Wang J, Lu L. Ras-related protein Rab 10 facilitates TLR4 signaling by promoting replenishment of TLR4 onto the plasma membrane. Proc Natl Acad Sci USA. 2010;107:13806–13811. doi: 10.1073/pnas.1009428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vijayan V, Baumgart-Vogt E, Naidu S, Qian G, Immenschuh S. Bruton’s tyrosine kinase is required for TLR-dependent heme oxygenase-1 gene activation via Nrf2 in macrophages. J Immunol. 2011;187:817–827. doi: 10.4049/jimmunol.1003631. [DOI] [PubMed] [Google Scholar]
- 37.Wu W, Mosteller RD, Broek D. Sphingosine kinase protects lipopolysaccharide-activated macrophages from apoptosis. Mol Cell Biol. 2004;24:7359–7369. doi: 10.1128/MCB.24.17.7359-7369.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tajima T, Murata T, Aritake K, Urade Y, Hirai H, Nakamura M, Ozaki H, Hori M. Lipopolysaccharide induces macrophage migration via prostaglandin D(2) and prostaglandin E(2) J Pharmacol Exp Ther. 2008;326:493–501. doi: 10.1124/jpet.108.137992. [DOI] [PubMed] [Google Scholar]