Abstract
This review focuses on the effects of estrogens upon the cerebellum, a brain region long ignored as a site of estrogen action. Highlighted are the diverse effects of estradiol within the cerebellum, emphasizing the importance of estradiol signaling in cerebellar development, modulation of synaptic neurotransmission in the adult, and the potential influence of estrogens on various health and disease states. We also provide new data, consistent with previous studies, in which locally synthesized estradiol modulates cerebellar glutamatergic neurotransmission, providing one underlying mechanism by which the actions of estradiol can affect this brain region.
Keywords: estrogen receptor, glutamate, ataxia, Purkinje neuron, neuroprotection, estradiol, motor control, alcohol, hormone therapy
Introduction
Studies characterizing the effects of steroid hormones on neural function have often taken a logical and programmatic approach. By first utilizing methods to identify brain nuclei which contain steroid hormone receptors, researchers would then examine the cellular mechanisms of steroid action on those nerve cells. The work would culminate in an understanding of the physiological and behavioral functions nested within the neural circuitry encompassing the steroid responsive neurons [102]. For estradiol, the classic autoradiographic studies (e.g., [101]) highlighted the basal forebrain, including the hypothalamus, as the primary region in which cellular uptake of radioactive estradiol occurred. Many studies followed, demonstrating the importance of estradiol on a variety of behaviors related to reproduction [26, 39, 66, 101, 102].
As valuable as the initial autoradiography technique was to identify brain regions sensitive to estradiol, it became clear that this method is useful primarily in identifying areas in which estrogen receptor expression is abundant. The natural tendency by investigators, often stated explicitly, was to suggest that brain regions exhibiting lower radioactive signals were less, or not at all influenced by the hormone [3, 120, 155, 168]. In a simple system, in which estradiol had just one receptor and one mechanism of action, such an assumption could be easily justified. We now know, however, that there are multiple estrogen receptors, each of which can affect numerous cellular signaling pathways [79, 86, 87, 88, 109]. Many of these actions require only a few receptors to produce pronounced changes in cellular function. Thus, the conclusions drawn from these foundational autoradiography discoveries have led to many false negatives regarding neuronal tissue sensitive to hormonal manipulation.
One such brain area considered insensitive to estradiol has been the cerebellum. This structure, with unique cell types and cytoarchitecture [32, 60, 98], regulates a variety of behaviors including motor control, language, attention, and memory [16, 151]. Over many years, several key findings have emerged that redefine the cerebellum as a target of estradiol action. More sensitive detection methods have determined a much wider distribution of estrogen-responsive brain regions, including the cerebellum. Another critical observation has been that estrogens guide cerebellar development. Additionally, the cerebellum itself may be a source of estradiol, through local steroid hormone synthesis. Estradiol also appears to directly affect cerebellar glutamatergic neurotransmission, provides protection against toxic insults, and impacts a variety of health related cerebellar functions. Yet, remarkably, the canon that the cerebellum is unresponsive to estradiol remains. This review examines each of these topics in turn. In addition, we will offer new in vivo data demonstrating that estradiol modulates glutamate neurotransmission in the cerebellum through locally synthesized estradiol. We conclude that the coalescence of data in support of estradiol regulation of cerebellar functioning is persuasive. That said, our understanding of the roles of estrogen signaling in the cerebellum is still in its infancy.
Estrogen Receptors and Estradiol Synthesis in the Cerebellum
Before the first cloning of estrogen receptor α (ERα), estrogen responsive neurons were detected by examining for radiolabeled estradiol accumulation. As mentioned in the Introduction, this led to the identification and characterization of hypothalamic and other diencephalic structures involved in reproduction. Interestingly, estradiol uptake was also observed in the cerebellum, albeit to a far lesser extent [81, 82, 83]. This surprising finding led to the cerebellum being considered a target of estrogen action for the first time. However, the stated implications were extremely limited. The hypothesis put forward was that steroid hormones acting within the cerebellum could affect signaling to other brain regions more traditionally considered to be involved in reproduction [81, 83]. The idea that estradiol affects cerebellar function to influence a variety of non-reproductive behaviors was yet to be explicitly considered.
Detection of estrogen receptors is the current means for defining estrogen-responsive cells. ERα and estrogen receptor β (ERβ) are the two most studied receptors [47, 80, 86, 170]. GPR-30 (also termed GPER) is another estrogen-responsive receptor [51], and there are several more putative (not yet verified through genetic cloning) receptors (i.e., ER-X and the STX-sensitive ER-Gq) [106, 154]. Modern and more sensitive methodological techniques demonstrate that the adult cerebellum expresses both ERα and ERβ [58, 90, 105, 137, 138, 141]. ERα expression in the adult is localized primarily to granule cells, albeit at low concentrations [14, 58]. In contrast, the adult cerebellum expresses high levels of ERβ, primarily in Purkinje cells, but in cerebellar granule cells as well [14, 45, 47, 58, 75, 76, 90, 91, 101, 105, 138, 154, 175], but see [148]. GPR-30 is also expressed throughout the cerebellum, with expression most abundant within Purkinje neurons [51]. To date the cerebellum has not been examined for ER-X and the STX-sensitive ER-Gq.
Estrogen receptors can affect neuronal function through a variety of mechanisms. For a detailed review see [87]. Originally defined as ligand-gated transcription factors, both ERα and ERβ can also act at the surface membrane, triggering various intracellular signaling pathways. In comparison, GPR-30 was initially described as a membrane receptor, although recent evidence suggests it may primarily regulate signaling in the endoplasmic reticulum. Both ER-X and the STX-sensitive ER-Gq are believed to act solely as membrane receptors.
With the discovery that steroid hormones are not only produced by the gonads, but can also be produced de novo within the brain, a series of studies have investigated whether the cerebellum contains the appropriate machinery to produce neurosteroids. Purkinje cells express various hormone synthesizing enzymes, including the key enzyme in estradiol formation, cytochrome P450 aromatase [6, 124]. Importantly, experiments have not just been limited to rodent and avian models. A positron emission tomography (PET) study using a radiolabeled aromatase inhibitor tracer found elevated levels of aromatase within the human cerebellum [15]. Aromatase immunostaining of post-mortem cerebellar tissue further confirmed that aromatase is found within human Purkinje cells and interneurons [6].
Estrogens and Cerebellar Development
The development of the cerebellum has been detailed in several excellent reviews [2, 22, 89, 140]. In regard to the actions of estrogens, a different pattern of estradiol binding is observed during development compared to the adult, beginning in the late postnatal period [161] and continuing up until weaning [76]. Ikeda and Nagai [58] demonstrated that ERα mRNA is significantly higher in the cerebellum of neonatal rat pups compared to adults. Measurements of ERα protein were consistent with the PCR data. Elevated ERα protein and mRNA during development is mainly confined to Purkinje cells during dendritic growth and synapse formation that occurs around postnatal day 7, suggesting a role for ERα in Purkinje cell differentiation. ERβ expression in the cerebellum also varies with age. In the first postnatal week, ERβ immunoreactivity was localized to both granule and Purkinje cells. Similar to ERα, ERβ expression reaches peak levels during the initiation of Purkinje cell dendritic growth, slightly decreasing over time to the level maintained throughout adulthood [62].
The best described role for estrogens in the developing cerebellum involves Purkinje cell dendritic growth, and spine and synapse formation, based on a series of compelling studies by Tsutsui and colleagues. Initial discoveries found that estradiol promotes dendritic growth and spine formation, effects that were blocked by the estrogen receptor antagonist tamoxifen [124]. Interestingly, these phenomena were observed in the developing cerebella of both male and female rats. Additionally, this paper demonstrated mRNA expression of aromatase within the Purkinje cell, suggesting that locally synthesized estradiol may be responsible for these effects.
In a follow up study [129], work from this laboratory demonstrated the importance of endogenous estrogens in not only dendritic growth and spine density, but in synaptogenesis as well. Specifically, aromatase knockout mice exhibited a reduction in Purkinje cell dendritic length, spines and synapse number. Each of these effects in the knockout mice are reversed following administration of estradiol. Mechanistically, the actions of estradiol are less clear. The current model posits that locally derived estradiol activates nuclear ERβ, resulting in increased expression of the neurotrophin BDNF, which ultimately leads to changes in Purkinje cell structure and function [158]. Evidence in support of each step in the proposed signaling pathway varies. Local synthesis of estradiol is suggested not only because of the measured mRNA expression of aromatase within Purkinje cells [124], but also because there are no other obvious sources of estrogens in the male and female rodent during this age. Whether ERβ is the (sole) receptor responsible is less clear. A role for ERβ is based primarily on expression studies in the developing cerebellum that looked for this particular receptor. Additional support is derived from the ERβ knockout mouse that exhibits deficits in spatial learning, neuronal migration, and apoptosis [163]. However, while tamoxifen reduces the effects of both endogenous and experimentally administered estrogens, this anti-estrogen does not completely block the actions of these hormones on Purkinje cell development [48]. Whether incomplete inhibition by tamoxifen is due to a concentration/blockade issue, or because there are tamoxifen-insensitive estrogen signaling pathways present is unknown. Finally, BDNF expression is decreased in tamoxifen-treated and aromatase knockout animals; knockout animals exhibit increased BDNF expression following administration of estrogen benzoate [48]. The importance of BDNF on Purkinje cell development is well established [33, 118, 136]. Therefore, estradiol promotion of BDNF expression is likely to play an important role. That said, multiple mechanisms are involved in the complex process of Purkinje cell development. As an example, development and maintenance of excitatory synapses is an activity-dependent process. In other brain regions, estradiol-mediated changes in spine density require glutamate receptor activation [84, 172]. And as discussed below, locally synthesized estradiol promotes glutamatergic neurotransmission between parallel fibers and Purkinje cells in the adult cerebellum. Hence, BDNF signaling is most likely only one component of the many effects of estradiol on cerebellar development.
A series of other studies examined the biochemical changes elicited by neonatal estrogens acting in the cerebellum by analyzing the levels of free amino acids in the developing rat brain [57]. Administration of estradiol from postnatal day 6 through 10 eliminates the normal developmental increase of glutamic acid and GABA in the cerebellum, as well as the typical decrease in glutamine [57]. Cavallotti et al. [23] focused on the effect of estradiol on the enzymes that degrade GABA, the principal neurotransmitter of Purkinje cells [32]. Immunohistochemical analysis revealed that estradiol treatment stimulates GABA transaminase and succinic semialdehyde dehydrogenase activity. Together, these studies suggest that estradiol may affect the balance between excitatory and inhibitory neurotransmission within the cerebellar cortex. This idea will be discussed again later in this review.
Estrogen Facilitation of Cerebellar Glutamatergic Signaling
While it has become clear that estradiol affects cerebellar function, the mechanisms of action are not well understood. Sheryl Smith and colleagues pioneered the concept that of estrogens augment glutamatergic neurotransmission in the adult. In their initial studies, estrogens applied either directly to the cerebellum or i.v. in anesthetized adult (ovariectomized) rats resulted in an increase in Purkinje cell responsiveness to microionotophoresis-applied glutamate. The local effects of estradiol enhancing cerebellar glutamate responsiveness were determined to be stereospecific [146]. Additionally, estradiol potentiates both AMPA and NMDA receptor-mediated excitation [144, 147].
While these studies were transformative, criticism regarding the use of anesthetized animals and a lack of behavioral relevance followed. To address these concerns, Smith and colleagues monitored the firing of individual Purkinje cells while female rats walked on a treadmill [147]. Subcutaneous injection of estradiol increases locomotor-induced Purkinje cell activity within 15 minutes of steroid administration [147], demonstrating effectiveness in an intact, awake animal and implicating estradiol in cerebellar locomotor function. These data parallel the finding that estradiol enhances striatal-mediated motor function [13].
In female rodents, ovarian estrogens enhance cerebellar Purkinje cell responsiveness to glutamate. More recent studies utilizing wire arrays have found augmented single unit activity with locomotor activity in multiple cells across consecutive estrous cycles coinciding with increased circulating estrogens [145]. Moreover, these effects of estradiol are not limited to locomotion. In female mice, estradiol enhanced long-term potentiation at the parallel fiber to Purkinje cell synapse, increased the density of these same glutamatergic synapses, and improved vestibulo-ocular reflex adaptation via ERβ signaling [4].
As we gain appreciation for the cellular actions of estradiol within the cerebellum, it is also important to consider the role the cerebellum plays in behavior. While originally believed to be involved solely in motor-related behaviors, recent findings have shown that the cerebellum influences language, attention, and memory [16, 151]. Hence, it would be unsurprising to find out that estrogen signaling in the cerebellum impacts such diverse functions as memory [103, 104], cognition [119, 130, 131, 132], locomotor activity [94] and mood [107].
While the number of laboratories examining the direct effects of estradiol on cerebellar function is limited, the work by Sheryl Smith and others demonstrate an enhancement of glutamatergic neurotransmission between the granule cell parallel fibers and Purkinje cells. Estradiol enhancement of glutamatergic neurotransmission has been described in additional brain regions, including the hippocampus and cerebral cortex [35, 67, 68, 123, 150, 162, 177].
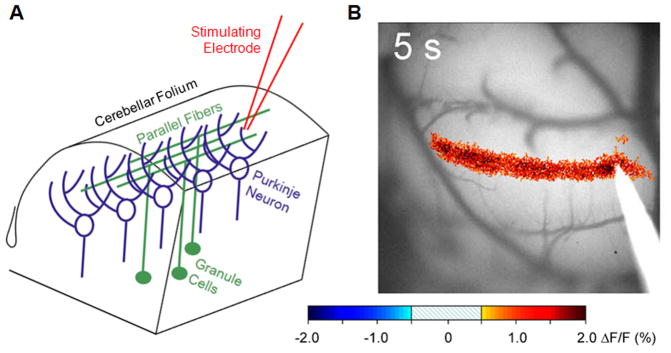
Utilizing novel in vivo imaging techniques, we find evidence in support of previous work, where locally- and gonadally-derived estrogens promote glutamatergic neurotransmission at the parallel fiber-Purkinje cell synapse. Here we will describe the first published data using this technique. Activity-dependent optical imaging experiments in the intact cerebellum have elucidated numerous mechanistic models to explain both normal cerebellar functioning as well as abnormal functioning in cerebellar ataxia [10, 31, 112, 164, 165]. This is an ideal approach to decipher the role of estradiol regarding its influence on glutamatergic neurotransmission at the parallel fiber-Purkinje cell synapse. Specifically, the imaging of mitochondrial flavoproteins allows the monitoring of parallel fiber-Purkinje cell synaptic transmission in vivo [110, 111]. Methodologically, upon exposing the cerebellum of an anesthetized mouse, a water-tight optical chamber is constructed and filled with artificial cerebrospinal fluid (aCSF). A microelectrode is placed just below the surface of the cerebellar cortex to stimulate parallel fibers (Figure 1A). The evoked action potentials along the parallel fibers promote the release of glutamate, which activate the post-synaptic Purkinje cells. The increase in flavoprotein fluorescence in response to parallel fiber stimulation is due to the postsynaptic activation of many hundreds of mediolaterally aligned Purkinje cells and is principally dependent upon AMPA receptors, with a smaller contribution from mGluR1 [110, 164, 165]. This activity response is imaged as a beam-like increase in intrinsic fluorescence (Figure 1B) and is stable over many hours of recording.
Figure 1.
Optical imaging of the parallel fiber-Purkinje neuron synapse. (A) Schematic diagram of cerebellar anatomy. The stimulating electrode is placed on the surface of the cerebellum after removal of the skull to activate multiple parallel fibers, leading to activation of Purkinje cells aligned in a mediolateral orientation. (B) Change in flavoprotein fluorescence within Cerebellar Purkinje neurons following parallel fiber activation. Changes in fluorescence are directly due to glutamatergic neurotransmission between the parallel fiber and the Purkinje neuron. Due to the circuitry of the cerebellum, electrode stimulation results in a “beam” of synaptic activity.
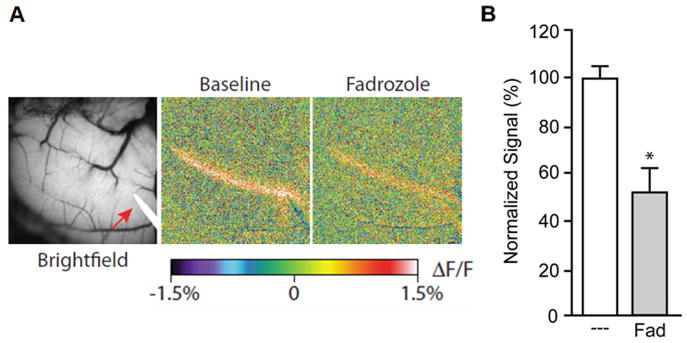
Using this technique, we find glutamatergic neurotransmission between the parallel fiber-Purkinje cell synapse to be reliant upon estrogen signaling. Application of estrogen receptor blockers to the aCSF resulted in a profound decrease in beam fluorescence (not shown). Electrophysiological recordings determined that estradiol directly impacts the efficacy of the glutamate synapse, and not the excitability of parallel fibers. Furthermore, locally synthesized estradiol appears to maintain robust neurotransmission. Applying the aromatase inhibitor fadrozole (1 μM) to the exposed folia diminished the beam amplitude within 30 minutes (Figure 2), supporting the hypothesis that local estrogen synthesis is essential for glutamatergic transmission within the parallel fiber-Purkinje cell network. This effect is not completely surprising, as the cerebellum is capable of synthesizing steroids. The rapid effects of fadrozole suggest estradiol is synthesized on demand for maintenance of parallel fiber-Purkinje cell neurotransmission.
Figure 2.
Local estrogen synthesis maintains glutamatergic neurotransmission between the parallel fiber-Purkinje cell synapse. (A) Bright field image and changes in fluorescence following parallel fiber activation. Fadrozole (1μM) added to the recording chamber surrounding the cerebellum decreased synaptic activity. (B) Fadrozole produced an approximate 50% reduction in synaptic beam fluorescence (n=4).
In a larger context, evidence is rapidly accumulating that estradiol supports neurotransmission, directly affecting the electrochemical state of the cell [8, 9]. Not only can estradiol immediately influence the membrane physiological properties of a neuron [65], but synthesis of estradiol from aromatase localized to presynaptic boutons appears to place estradiol in the category of a neurotransmitter [56, 96, 133, 157]. Interestingly, aromatase expression in glia may be an equally viable alternative [41, 125], as glial cells play an essential role in the support of neurotransmission [36]. While much of the work regarding local estradiol neurotransmission began in avian systems [126, 134], there is evidence of similar mechanisms in the mammalian brain, in which ERβ plays a principal role [67, 149, 150]. With ERβ expressed within the adult cerebellum, the study of estrogen support of cerebellar neurotransmission provides a tractable model in which to gain additional insights into this expanding field.
Health Implications
An important extension of the observations that estradiol affects cerebellar development and promotes neuronal excitatory neurotransmission in the adult, is the functional significance for human health. While these types of studies typically use systemic estrogen treatment, and thus the observed effects cannot be definitively attributed to the hormone acting directly and solely upon this brain region, the impact of estrogen treatment on cerebellar functioning is unequivocal.
Estradiol action has been implicated in neuroprotection from many diseases [28, 69, 93]. Recently, an emphasis has been placed on the role of estradiol in aging and neurodegenerative diseases such as Alzheimer’s disease (AD) [52]. Although not often associated with this disorder, the cerebellum influences cognitive function. Furthermore, estradiol has been consistently found to affect the cerebellum in afflicted/vulnerable humans as well as in animal models of AD.
Estrogen therapy initiated at the onset of menopause reduces the risk or delays the onset of AD in women [175, 176]. Likewise, administration of the androgen/estrogen precursor dehydroepiandrosterone (DHEA) improved memory performance in both normally aged mice [37, 38] and in a mouse model of AD [78]. Interestingly, the conversion of DHEA into the high affinity estrogen receptor agonist,Δ5-androstene-3β, 17β-diol occurs in abundance within the cerebellum compared to the frontal cortex and hippocampus of animals modeling AD [167].
Hormone therapy remains a controversial treatment for cognitive impairment associated with age-related cognitive decline or pathological aging, such as AD in post-menopausal women. Meta-analysis of clinical trials have not generally supported the use of hormone therapy to treat cognitive decline in post-menopausal women [74]. However, these findings cannot be extended to specific subgroups of women, such as young menopausal women, who could potentially still benefit from such therapies. A longitudinal study was conducted comparing cognitive performance of healthy post-menopausal women with risk factors for AD who had been receiving either estradiol hormone therapy or conjugated equine estrogen therapy for least one year [173]. Women who received estradiol displayed better verbal memory performance when compared to women given conjugated equine estrogens, independent of age, IQ, years of education, risk factors for AD, duration of estrogen exposure, concurrent progesterone use, or menopause status (natural or surgically produced) [173]. Additionally, fMRI studies demonstrate that gray matter volumes increase in the cerebellum of post-menopausal women given estrogens, and are greater in women who underwent treatment in the past versus women who were currently undergoing treatment [17, 42]. Further, gray matter volume is decreased in the cerebellum of females who had never been given hormone therapy compared to both young females and older females on hormone therapy, suggesting that estradiol is protective for cerebellar neurons [117].
Estradiol also impacts vasodilatation, affecting both cerebral and cerebellar circulation. In adult rats, estradiol increases blood flow within 10 minutes of administration to many areas of the brain, including the cerebellum [43]. It has been suggested that estradiol exerts these effects through a nitric oxide and cGMP-dependent mechanism [99, 169]. Interestingly, post-menopausal women exhibit approximately a 5% increase in cerebellar blood flow following estrogen replacement therapy compared to their pre-therapy blood flow measurements [143]. Further, this cerebellar blood flow increase correlates with an increase in serum levels of estradiol [143]. Intranasal administration of estrogens also results in an increase in cerebellar blood flow in post-menopausal women [64].
Endogenous estrogens may also provide a neuroprotective role in the cerebellar ataxias [139]. Chemically-induced cerebellar ataxia in castrated male rats was mitigated by estrogen implants. Specifically, estradiol significantly decreased neuronal death. This study also established that the inferior olivary nucleus of adult male rats express aromatase, the enzyme responsible for the conversion of testosterone to estradiol. Correspondingly, inhibition of aromatase using this model led to an increase in cell death within the inferior olive [139], demonstrating that the estradiol produced within the inferior olive provides neuroprotection. As a side note, the targeting of aromatase has been a treatment for various reproductive cancers. As an example, administration of the aromatase inhibitor aminoglutethimide is beneficial in the treatment of breast carcinomas in post-menopausal women [127]. However, one of the major side effects is a significant increase in the observance of ataxia [127].
Not surprisingly, balance and coordination, two known functions of the cerebellum, have also been studied in post-menopausal women with regard to the potential therapeutic effects of estrogen therapy [21]. Postural stability and balance are known to decrease with age. Impaired balance and mobility are risk factors in post-menopausal women and contribute to at least one fall per year in 33% of women aged 60 or older [5, 11]. Trans-dermal estrogen treatment in post-menopausal women increased balance performance [46], potentially implicating a neuroprotective role of estradiol in cerebellar function.
Friedreich’s ataxia is the most common form of inherited ataxia in the world, affecting primarily spinocerebellar and other spinal neurons. The disease shows no sex bias in incidence [18, 49, 73, 100, 135]. However, epidemiological studies indicate a better prognosis in female patients [73], suggesting a possible role of estradiol in the prevention and treatment of Friedreich’s ataxia [30]. Consistent with this hypothesis, estradiol protects against the effects of oxidative damage in fibroblasts of patients with Friedreich’s ataxia [115]. It is not known whether estradiol acting within the cerebellum impacts the progression of Friedreich’s ataxia or whether this is due to estradiol action within the spinal cord and/or another site.
Additional evidence points to cerebellar involvement in a psychoneuroendocrine disorder, premenstrual dysphoric disorder (PMDD). Affective symptoms of PMDD include irritability, anger, depression, mood swings, anxiety, fatigue, and difficulty concentrating (DSM-IV). Although triggered by ovulation [7], these symptoms cannot be attributed to differences in circulating hormone levels of women with PMDD compared to non-affected women [121, 122]. A PET study mapping the functional brain abnormalities associated with PMDD across the menstrual cycle found an increase in cerebellar activity, particularly in the right cerebellar vermis. This increase in cerebellar activity correlated with worsening of mood in the same PMDD subjects, suggesting a role of the cerebellum in the affective state of patients with PMDD [107]. The mechanisms for estradiol action on cerebellar function in these and other health related findings, remains unknown.
An ongoing dilemma regarding the effects of estrogens on human health is the apparent duality regarding hormonal benefit versus detriment. None is probably more intensively studied or debated than the use of estrogens regarding stroke [95, 97, 116]. Various animal models found that estrogens ameliorate the effects of stroke [24, 25, 29, 55, 59, 71, 85, 108, 142, 156]. Yet, hormonal therapy in post menopausal women increases the likelihood of stroke and worsens symptoms [12, 53, 128, 166]. Some of these discrepancies across animal models/human subjects may be related to age [72, 95] and duration of hormone deprivation [6], but these findings also highlight potential differences between the actions of endogenous estrogens and those exogenously administered. Concentration and duration-dependent factors in estradiol treatment may also play a role. Hence, it becomes increasingly difficult to integrate various findings into a simplified model of estradiol action. Within the cerebellum, estrogen-mediated neuroprotection from ethanol toxicity falls within this category. Ironically, this is probably the most studied area regarding the cerebellum and estrogens.
Ethanol use and abuse profoundly affects many areas of the brain, though some regions are more susceptible to ethanol’s toxic actions. The cerebellum is particularly sensitive to ethanol [61]. The effects of chronic ethanol use and withdrawal on the cerebellum can be so profound that ataxias are common signs in alcoholics (e.g. [152]). In long-term alcoholics, a characteristic pattern of cerebellar degeneration is seen, particularly within the vermis [61]. Many neuronal cell types are affected, though most of the focus has been on the atrophy of Purkinje cell dendrites, the loss of Purkinje neurons, and shrinkage of cerebellar white matter [61, 174]. While in humans it is difficult to disentangle whether these neuropathology’s result from alcohol use or dietary (especially thiamine) deficiency [34, 77], the observation that similar cerebellar pathology occurs in controlled animal studies [34] suggest that these degenerative processes are due to ethanol use itself.
Ethanol use in adults would be expected to have potential toxic actions, just as it does during fetal and early neonatal development [61]. However, much of the adverse neurological effects in adult animals result from the consequences of ethanol withdrawal. Withdrawal following ethanol use produces a characteristic pattern of symptoms in both animals and humans [44]. Studies in rats use a paradigm of chronic ethanol administration, typically as part of the animal’s diet, followed by a transfer to an ethanol-free diet (e.g. [63]). In this paradigm, estradiol is administered chronically during ethanol administration and withdrawal. Animals withdrawing from ethanol model human disease by exhibiting a widespread reduction in Purkinje cells, particularly in the vermis, i.e. the midline subdivision of the cerebellum separating the left and right hemispheres [1, 63, 159, 160]. Concurrent estradiol treatment to rats counteracts the effects of ethanol and ethanol withdrawal, maintaining control numbers of Purkinje neurons [63].
The neurodegeneration produced by ethanol withdrawal is accompanied by ataxia. Ethanol withdrawal produces a decrease in accelerated rotarod performance consistent with cerebellar ataxia [63, 113, 114]. Degraded rotarod performance is typically associated with Purkinje cell loss [19]. As estradiol protects against Purkinje cell loss during withdrawal, the corresponding maintenance of rotarod performance by estradiol treatment is predictable [63, 113, 114].
Underlying ethanol-induced toxicity and estradiol-mediated neuroprotection is the balance between GABAergic and glutamatergic neurotransmission. Glutamate-induced cell death is a proposed mechanism for cerebellar neuronal toxicity during ethanol withdrawal, as the magnitude of elevated extracellular glutamate levels measured during withdrawal correlates with the severity of behavior [34]. To the degree that GABA neurotransmission can modulate glutamate-induced neuronal excitability, ethanol withdrawal can be modulated through pharmacological manipulation of GABA-A receptor activity [70]. Specifically, GABA-A agonists reduce the severity of ethanol withdrawal, whereas antagonists potentiate withdrawal. Notably, increased GABAergic signaling, in addition to acting as a counterbalance to excitatory stimuli, can also directly inhibit glutamatergic neurotransmission, [20, 32, 40, 50].
How does estradiol signaling within the cerebellum fit within this model? This question may be hard to answer unequivocally. Chronic estradiol treatment mimics the action of GABA-A agonists by reducing the severity of withdrawal symptoms [113, 114]. Additionally, the GABA-A antagonist bicuculline counteracts the effects of estradiol on preventing Purkinje cell loss following ethanol withdrawal. Bicuculline also eliminates the ability of estradiol to maintain rotarod performance following withdrawal. Based on these data, the assumption is that GABA is the common mechanism of action mediating estradiol protection against neurotoxicity and motor loss. In numerous brain regions, estradiol promotes GABA synthesis [54, 92, 171], suggesting a potential mechanism. However, the effects of estradiol and the GABA-A agonist muscimol are additive [114], suggesting estradiol may recruit additional signaling mechanisms independent of GABA-A receptors.
Another potential mechanism is estradiol regulation of glutamatergic neurotransmission. At first this appears counterintuitive, since as previously described, endogenous estradiol potentiates glutamatergic neurotransmission. Thus, the straightforward assumption would be that estradiol would exacerbate ethanol withdrawal. However, down-regulation of excitatory synapses may be a case of compensatory action following chronic estradiol treatment. Within multiple brain regions, chronic activation of ERβ leads to down-regulation of glutamatergic neurotransmission (in parallel to increased GABAergic signaling) [153]. Hence, if similar mechanisms occur within the cerebellum, we predict that while chronic estradiol administration is neuroprotective, acute estradiol would worsen the effects of ethanol withdrawal. Future research could test this hypothesis.
Conclusions and Future Directions
Once thought to be extraneous regarding estradiol action, the cerebellum is now known to be a brain structure affected by this hormone, leading to alterations in a variety of behaviors. Although not described in detail here, there is also growing evidence that the cerebellum contributes to sex differences in the pathology of autism, attention-deficit hyperactivity disorder, schizophrenia, and depression (for a review see [27]). Whether this is due to sex differences in cerebellar physiology and/or differences in hormone production between males and females remain to be fully determined. Additional studies determining the mechanism by which estradiol acts upon the cerebellum and the ultimate functional impact estrogens have on cerebellar-mediated behaviors are clearly warranted.
Highlights.
Estrogen action in the cerebellum has been observed for years, but often ignored
Estradiol affects cerebellar development
Estradiol promotes glutamate neurotransmission within the cerebellum
Cerebellar estradiol is impacts a variety of health issues
Acknowledgments
This work was supported by DA013680, Core funding NS062158, NS048944, NS077661 and NSF IOS-1146016.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allsop J, Turner B. Cerebellar degeneration associated with chronic alcoholism. J Neurol Sci. 1966;3:238–58. doi: 10.1016/0022-510x(66)90024-4. [DOI] [PubMed] [Google Scholar]
- 2.Altman J, Bayer SA. Development of the cerebellar system in relation to its evolution, structures and functions. CRC Press; New York, NY: 1997. [Google Scholar]
- 3.Amateau SK, Alt JJ, Stamps CL, McCarthy MM. Brain estradiol content in newborn rats: sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology. 2004;145:2906–17. doi: 10.1210/en.2003-1363. [DOI] [PubMed] [Google Scholar]
- 4.Andreescu CE, Milojkovic BA, Haasdijk ED, Kramer P, De Jong FH, Krust A, De Zeeuw CI, De Jeu MT. Estradiol improves cerebellar memory formation by activating estrogen receptor beta. J Neurosci. 2007;27:10832–9. doi: 10.1523/JNEUROSCI.2588-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold CM, Busch AJ, Schachter CL, Harrison L, Olszynski W. The relationship of intrinsic fall risk factors to a recent history of falling in older women with osteoporosis. J Orthop Sports Phys Ther. 2005;35:452–60. doi: 10.2519/jospt.2005.35.7.452. [DOI] [PubMed] [Google Scholar]
- 6.Azcoitia I, Yague JG, Garcia-Segura LM. Estradiol synthesis within the human brain. Neuroscience. 2011;191:139–47. doi: 10.1016/j.neuroscience.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Backstrom T, Andersson A, Andree L, Birzniece V, Bixo M, Bjorn I, Haage D, Isaksson M, Johansson IM, Lindblad C, Lundgren P, Nyberg S, Odmark IS, Stromberg J, Sundstrom-Poromaa I, Turkmen S, Wahlstrom G, Wang M, Wihlback AC, Zhu D, Zingmark E. Pathogenesis in menstrual cycle-linked CNS disorders. Ann N Y Acad Sci. 2003;1007:42–53. doi: 10.1196/annals.1286.005. [DOI] [PubMed] [Google Scholar]
- 8.Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29:241–9. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Balthazart J, Cornil CA, Taziaux M, Charlier TD, Baillien M, Ball GF. Rapid changes in production and behavioral action of estrogens. Neuroscience. 2006;138:783–91. doi: 10.1016/j.neuroscience.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 10.Barnes JA, Ebner BA, Duvick LA, Gao W, Chen G, Orr HT, Ebner TJ. Abnormalities in the climbing fiber-Purkinje cell circuitry contribute to neuronal dysfunction in ATXN1 [82Q] mice. J Neurosci. 2011;31:12778–89. doi: 10.1523/JNEUROSCI.2579-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrett-Connor E, Weiss TW, McHorney CA, Miller PD, Siris ES. Predictors of falls among postmenopausal women: results from the National Osteoporosis Risk Assessment (NORA) Osteoporos Int. 2009;20:715–22. doi: 10.1007/s00198-008-0748-2. [DOI] [PubMed] [Google Scholar]
- 12.Bath PM, Gray LJ. Association between hormone replacement therapy and subsequent stroke: a meta-analysis. BMJ. 2005;330:342. doi: 10.1136/bmj.38331.655347.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becker JB, Snyder PJ, Miller MM, Westgate SA, Jenuwine MJ. The influence of estrous cycle and intrastriatal estradiol on sensorimotor performance in the female rat. Pharmacol Biochem Behav. 1987;27:53–9. doi: 10.1016/0091-3057(87)90476-x. [DOI] [PubMed] [Google Scholar]
- 14.Belcher SM. Regulated expression of estrogen receptor alpha and beta mRNA in granule cells during development of the rat cerebellum. Brain Res Dev Brain Res. 1999;115:57–69. doi: 10.1016/s0165-3806(99)00050-4. [DOI] [PubMed] [Google Scholar]
- 15.Biegon A, Kim SW, Alexoff DL, Jayne M, Carter P, Hubbard B, King P, Logan J, Muench L, Pareto D, Schlyer D, Shea C, Telang F, Wang GJ, Xu Y, Fowler JS. Unique distribution of aromatase in the human brain: in vivo studies with PET and [N-methyl-11C]vorozole. Synapse. 2010;64:801–7. doi: 10.1002/syn.20791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bloedel JR, Bracha V. Duality of cerebellar motor and cognitive functions. Int Rev Neurobiol. 1997;41:613–34. doi: 10.1016/s0074-7742(08)60373-6. [DOI] [PubMed] [Google Scholar]
- 17.Boccardi M, Ghidoni R, Govoni S, Testa C, Benussi L, Bonetti M, Binetti G, Frisoni GB. Effects of hormone therapy on brain morphology of healthy postmenopausal women: a Voxel-based morphometry study. Menopause. 2006;13:584–91. doi: 10.1097/01.gme.0000196811.88505.10. [DOI] [PubMed] [Google Scholar]
- 18.Bradley JL, Blake JC, Chamberlain S, Thomas PK, Cooper JM, Schapira AH. Clinical, biochemical and molecular genetic correlations in Friedreich’s ataxia. Hum Mol Genet. 2000;9:275–82. doi: 10.1093/hmg/9.2.275. [DOI] [PubMed] [Google Scholar]
- 19.Breton P, Bizot JC, Buee J, De La Manche I. Brain neurotoxicity of Penitrem A: electrophysiological, behavioral and histopathological study. Toxicon. 1998;36:645–55. doi: 10.1016/s0041-0101(97)00084-6. [DOI] [PubMed] [Google Scholar]
- 20.Callaway JC, Lasser-Ross N, Ross WN. IPSPs strongly inhibit climbing fiber-activated [Ca2+]i increases in the dendrites of cerebellar Purkinje neurons. J Neurosci. 1995;15:2777–87. doi: 10.1523/JNEUROSCI.15-04-02777.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cangussu LM, Nahas-Neto J, Petri Nahas EA, Rodrigues Barral AB, de Buttros AD, Uemura G. Evaluation of postural balance in postmenopausal women and its relationship with bone mineral density- a cross sectional study. BMC Musculoskelet Disord. 2012;13:2. doi: 10.1186/1471-2474-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carletti B, Rossi F. Neurogenesis in the cerebellum. Neuroscientist. 2008;14:91–100. doi: 10.1177/1073858407304629. [DOI] [PubMed] [Google Scholar]
- 23.Cavallotti C, Iacopino L, Amenta F. Stimulatory effect of beta-estradiol treatment on GABA-degradative enzymes within rat cerebellar cortex. Neurosci Lett. 1983;39:205–9. doi: 10.1016/0304-3940(83)90078-2. [DOI] [PubMed] [Google Scholar]
- 24.Connell BJ, Crosby KM, Richard MJ, Mayne MB, Saleh TM. Estrogen-mediated neuroprotection in the cortex may require NMDA receptor activation. Neuroscience. 2007;146:160–9. doi: 10.1016/j.neuroscience.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 25.Culmsee C, Vedder H, Ravati A, Junker V, Otto D, Ahlemeyer B, Krieg JC, Krieglstein J. Neuroprotection by estrogens in a mouse model of focal cerebral ischemia and in cultured neurons: evidence for a receptor-independent antioxidative mechanism. J Cereb Blood Flow Metab. 1999;19:1263–9. doi: 10.1097/00004647-199911000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Davis PG, McEwen BS, Pfaff DW. Localized behavioral effects of tritiated estradiol implants in the ventromedial hypothalamus of female rats. Endocrinology. 1979;104:898–903. doi: 10.1210/endo-104-4-898. [DOI] [PubMed] [Google Scholar]
- 27.Dean SL, McCarthy MM. Steroids, sex and the cerebellar cortex: implications for human disease. Cerebellum. 2008;7:38–47. doi: 10.1007/s12311-008-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dhandapani KM, Brann DW. Role of astrocytes in estrogen-mediated neuroprotection. Exp Gerontol. 2007;42:70–5. doi: 10.1016/j.exger.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 29.Dubal DB, Kashon ML, Pettigrew LC, Ren JM, Finklestein SP, Rau SW, Wise PM. Estradiol protects against ischemic injury. J Cereb Blood Flow Metab. 1998;18:1253–8. doi: 10.1097/00004647-199811000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Dykens JA, Moos WH, Howell N. Development of 17alpha-estradiol as a neuroprotective therapeutic agent: rationale and results from a phase I clinical study. Ann N Y Acad Sci. 2005;1052:116–35. doi: 10.1196/annals.1347.008. [DOI] [PubMed] [Google Scholar]
- 31.Ebner TJ, Wang X, Gao W, Cramer SW, Chen G. Parasagittal Zones in the Cerebellar Cortex Differ in Excitability, Information Processing, and Synaptic Plasticity. Cerebellum. 2011;11:418–9. doi: 10.1007/s12311-011-0347-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eccles JC, Ito M, Szentagothai J. The Cerebellum as a Neuronal Machine. Springer-Verlag; Berlin, Germany: 1967. [Google Scholar]
- 33.Ernfors P, Lee KF, Jaenisch R. Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature. 1994;368:147–50. doi: 10.1038/368147a0. [DOI] [PubMed] [Google Scholar]
- 34.Fadda F, Rossetti ZL. Chronic ethanol consumption: from neuroadaptation to neurodegeneration. Prog Neurobiol. 1998;56:385–431. doi: 10.1016/s0301-0082(98)00032-x. [DOI] [PubMed] [Google Scholar]
- 35.Fester L, Zhou L, Butow A, Huber C, von Lossow R, Prange-Kiel J, Jarry H, Rune GM. Cholesterol-promoted synaptogenesis requires the conversion of cholesterol to estradiol in the hippocampus. Hippocampus. 2009;19:692–705. doi: 10.1002/hipo.20548. [DOI] [PubMed] [Google Scholar]
- 36.Fields RD, Stevens-Graham B. New insights into neuron-glia communication. Science. 2002;298:556–62. doi: 10.1126/science.298.5593.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flood JF, Roberts E. Dehydroepiandrosterone sulfate improves memory in aging mice. Brain Res. 1988;448:178–81. doi: 10.1016/0006-8993(88)91116-x. [DOI] [PubMed] [Google Scholar]
- 38.Flood JF, Smith GE, Roberts E. Dehydroepiandrosterone and its sulfate enhance memory retention in mice. Brain Res. 1988;447:269–78. doi: 10.1016/0006-8993(88)91129-8. [DOI] [PubMed] [Google Scholar]
- 39.Floody OR, Pfaff DW. Aggressive behavior in female hamsters: the hormonal basis for fluctuations in female aggressiveness correlated with estrous state. J Comp Physiol Psychol. 1977;91:443–64. doi: 10.1037/h0077341. [DOI] [PubMed] [Google Scholar]
- 40.Gao W, Chen G, Reinert KC, Ebner TJ. Cerebellar cortical molecular layer inhibition is organized in parasagittal zones. J Neurosci. 2006;26:8377–87. doi: 10.1523/JNEUROSCI.2434-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garcia-Ovejero D, Azcoitia I, Doncarlos LL, Melcangi RC, Garcia-Segura LM. Glia-neuron crosstalk in the neuroprotective mechanisms of sex steroid hormones. Brain Res Brain Res Rev. 2005;48:273–86. doi: 10.1016/j.brainresrev.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 42.Ghidoni R, Boccardi M, Benussi L, Testa C, Villa A, Pievani M, Gigola L, Sabattoli F, Barbiero L, Frisoni GB, Binetti G. Effects of estrogens on cognition and brain morphology: involvement of the cerebellum. Maturitas. 2006;54:222–8. doi: 10.1016/j.maturitas.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 43.Goldman H, Skelley EB, Sandman CA, Kastin AJ, Murphy S. Hormones and regional brain blood flow. Pharmacol Biochem Behav. 1976;5:165–9. doi: 10.1016/0091-3057(76)90347-6. [DOI] [PubMed] [Google Scholar]
- 44.Goldstein DB, Pal N. Alcohol dependence produced in mice by inhalation of ethanol: grading the withdrawal reaction. Science. 1971;172:288–90. doi: 10.1126/science.172.3980.288. [DOI] [PubMed] [Google Scholar]
- 45.Gottfried-Blackmore A, Croft G, McEwen BS, Bulloch K. Transcriptional activity of estrogen receptors ERalpha and ERbeta in the EtC.1 cerebellar granule cell line. Brain Res. 2007;1186:41–7. doi: 10.1016/j.brainres.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 46.Hammar ML, Lindgren R, Berg GE, Moller CG, Niklasson MK. Effects of hormonal replacement therapy on the postural balance among postmenopausal women. Obstet Gynecol. 1996;88:955–60. doi: 10.1016/s0029-7844(96)00356-0. [DOI] [PubMed] [Google Scholar]
- 47.Handa RJ, Ogawa S, Wang JM, Herbison AE. Roles for oestrogen receptor beta in adult brain function. J Neuroendocrinol. 2012;24:160–73. doi: 10.1111/j.1365-2826.2011.02206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haraguchi S, Sasahara K, Shikimi H, Honda S, Harada N, Tsutsui K. Estradiol promotes purkinje dendritic growth, spinogenesis, and synaptogenesis during neonatal life by inducing the expression of BDNF. Cerebellum. 2012;11:416–7. doi: 10.1007/s12311-011-0342-6. [DOI] [PubMed] [Google Scholar]
- 49.Harding AE. Classification of the hereditary ataxias and paraplegias. Lancet. 1983;1:1151–5. doi: 10.1016/s0140-6736(83)92879-9. [DOI] [PubMed] [Google Scholar]
- 50.Hausser M, Clark BA. Tonic synaptic inhibition modulates neuronal output pattern and spatiotemporal synaptic integration. Neuron. 1997;19:665–78. doi: 10.1016/s0896-6273(00)80379-7. [DOI] [PubMed] [Google Scholar]
- 51.Hazell GG, Yao ST, Roper JA, Prossnitz ER, O’Carroll AM, Lolait SJ. Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. J Endocrinol. 2009;202:223–36. doi: 10.1677/JOE-09-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Henderson VW, Brinton RD. Menopause and mitochondria: windows into estrogen effects on Alzheimer’s disease risk and therapy. Prog Brain Res. 2010;182:77–96. doi: 10.1016/S0079-6123(10)82003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Henderson VW, Lobo RA. Hormone therapy and the risk of stroke: perspectives 10 years after the Women’s Health Initiative trials. Climacteric. 2012;15:229–34. doi: 10.3109/13697137.2012.656254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herbison AE. Estrogen regulation of GABA transmission in rat preoptic area. Brain Res Bull. 1997;44:321–6. doi: 10.1016/s0361-9230(97)00210-4. [DOI] [PubMed] [Google Scholar]
- 55.Hoffman GE, Merchenthaler I, Zup SL. Neuroprotection by ovarian hormones in animal models of neurological disease. Endocrine. 2006;29:217–31. doi: 10.1385/ENDO:29:2:217. [DOI] [PubMed] [Google Scholar]
- 56.Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, Harada N, Kimoto T, Kawato S. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc Natl Acad Sci U S A. 2004;101:865–70. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hudson DB, Vernadakis A, Timiras PS. Regional changes in amino acid concentration in the developing brain and the effects of neonatal administration of estradiol. Brain Res. 1970;23:213–22. doi: 10.1016/0006-8993(70)90040-5. [DOI] [PubMed] [Google Scholar]
- 58.Ikeda Y, Nagai A. Differential expression of the estrogen receptors alpha and beta during postnatal development of the rat cerebellum. Brain Res. 2006;1083:39–49. doi: 10.1016/j.brainres.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 59.Inagaki T, Kaneko N, Zukin RS, Castillo PE, Etgen AM. Estradiol attenuates ischemia-induced death of hippocampal neurons and enhances synaptic transmission in aged, long-term hormone-deprived female rats. PLoS One. 2012;7:e38018. doi: 10.1371/journal.pone.0038018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ito M. Cerebellar circuitry as a neuronal machine. Prog Neurobiol. 2006;78:272–303. doi: 10.1016/j.pneurobio.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 61.Jaatinen P, Rintala J. Mechanisms of ethanol-induced degeneration in the developing, mature, and aging cerebellum. Cerebellum. 2008;7:332–47. doi: 10.1007/s12311-008-0034-z. [DOI] [PubMed] [Google Scholar]
- 62.Jakab RL, Wong JK, Belcher SM. Estrogen receptor beta immunoreactivity in differentiating cells of the developing rat cerebellum. J Comp Neurol. 2001;430:396–409. doi: 10.1002/1096-9861(20010212)430:3<396::aid-cne1039>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 63.Jung ME, Yang SH, Brun-Zinkernagel AM, Simpkins JW. Estradiol protects against cerebellar damage and motor deficit in ethanol-withdrawn rats. Alcohol. 2002;26:83–93. doi: 10.1016/s0741-8329(01)00199-9. [DOI] [PubMed] [Google Scholar]
- 64.Kaya E, Sahin FK, Koken G, Kose M, Cevrioglu AS. Acute effect of intranasal estrogen on cerebral and cerebellar perfusion in postmenopausal women. Maturitas. 2008;59:72–82. doi: 10.1016/j.maturitas.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 65.Kelly MJ, Levin ER. Rapid actions of plasma membrane estrogen receptors. Trends Endocrinol Metab. 2001;12:152–6. doi: 10.1016/s1043-2760(01)00377-0. [DOI] [PubMed] [Google Scholar]
- 66.Kow LM, Pfaff DW. Induction of lordosis in female rats: two modes of estrogen action and the effect of adrenalectomy. Horm Behav. 1975;6:259–76. doi: 10.1016/0018-506x(75)90013-6. [DOI] [PubMed] [Google Scholar]
- 67.Kramar EA, Chen LY, Brandon NJ, Rex CS, Liu F, Gall CM, Lynch G. Cytoskeletal changes underlie estrogen’s acute effects on synaptic transmission and plasticity. J Neurosci. 2009;29:12982–93. doi: 10.1523/JNEUROSCI.3059-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kretz O, Fester L, Wehrenberg U, Zhou L, Brauckmann S, Zhao S, Prange-Kiel J, Naumann T, Jarry H, Frotscher M, Rune GM. Hippocampal synapses depend on hippocampal estrogen synthesis. J Neurosci. 2004;24:5913–21. doi: 10.1523/JNEUROSCI.5186-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kumar P, Kale RK, McLean P, Baquer NZ. Protective effects of 17beta estradiol on altered age related neuronal parameters in female rat brain. Neurosci Lett. 2011;502:56–60. doi: 10.1016/j.neulet.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 70.Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology (Berl) 2009;205:529–64. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lebesgue D, Chevaleyre V, Zukin RS, Etgen AM. Estradiol rescues neurons from global ischemia-induced cell death: multiple cellular pathways of neuroprotection. Steroids. 2009;74:555–61. doi: 10.1016/j.steroids.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leon RL, Li X, Huber JD, Rosen CL. Worsened outcome from middle cerebral artery occlusion in aged rats receiving 17beta-estradiol. Endocrinology. 2012;153:3386–93. doi: 10.1210/en.2011-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leone M, Brignolio F, Rosso MG, Curtoni ES, Moroni A, Tribolo A, Schiffer D. Friedreich’s ataxia: a descriptive epidemiological study in an Italian population. Clin Genet. 1990;38:161–9. doi: 10.1111/j.1399-0004.1990.tb03566.x. [DOI] [PubMed] [Google Scholar]
- 74.Lethaby A, Hogervorst E, Richards M, Yesufu A, Yaffe K. Hormone replacement therapy for cognitive function in postmenopausal women. Cochrane Database Syst Rev. 2008:CD003122. doi: 10.1002/14651858.CD003122.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.MacLusky NJ, Chaptal C, McEwen BS. The development of estrogen receptor systems in the rat brain and pituitary: postnatal development. Brain Res. 1979;178:143–60. doi: 10.1016/0006-8993(79)90094-5. [DOI] [PubMed] [Google Scholar]
- 76.MacLusky NJ, Lieberburg I, McEwen BS. The development of estrogen receptor systems in the rat brain: perinatal development. Brain Res. 1979;178:129–42. doi: 10.1016/0006-8993(79)90093-3. [DOI] [PubMed] [Google Scholar]
- 77.Martin FH, Siddle DA. The interactive effects of alcohol and temazepam on P300 and reaction time. Brain Cogn. 2003;53:58–65. doi: 10.1016/s0278-2626(03)00203-3. [DOI] [PubMed] [Google Scholar]
- 78.Maurice T, Su TP, Privat A. Sigma1 (sigma 1) receptor agonists and neurosteroids attenuate B25–35-amyloid peptide-induced amnesia in mice through a common mechanism. Neuroscience. 1998;83:413–28. doi: 10.1016/s0306-4522(97)00405-3. [DOI] [PubMed] [Google Scholar]
- 79.McDevitt MA, Glidewell-Kenney C, Jimenez MA, Ahearn PC, Weiss J, Jameson JL, Levine JE. New insights into the classical and non-classical actions of estrogen: evidence from estrogen receptor knock-out and knock-in mice. Mol Cell Endocrinol. 2008;290:24–30. doi: 10.1016/j.mce.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McEwen BS. Invited review: Estrogens effects on the brain: multiple sites and molecular mechanisms. J Appl Physiol. 2001;91:2785–801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- 81.McEwen BS, Pfaff DW. Factors influencing sex hormone uptake by rat brain regions. I. Effects of neonatal treatment, hypophysectomy, and competing steroid on estradiol uptake. Brain Res. 1970;21:1–16. doi: 10.1016/0006-8993(70)90016-8. [DOI] [PubMed] [Google Scholar]
- 82.McEwen BS, Pfaff DW, Zigmond RE. Factors influencing sex hormone uptake by rat brain regions. 3. Effects of competing steroids on testosterone uptake. Brain Res. 1970;21:29–38. doi: 10.1016/0006-8993(70)90018-1. [DOI] [PubMed] [Google Scholar]
- 83.McEwen BS, Pfaff DW, Zigmond RE. Factors influencing sex hormone uptake by rat brain regions. II. Effects of neonatal treatment and hypophysectomy on testosterone uptake. Brain Res. 1970;21:17–28. doi: 10.1016/0006-8993(70)90017-x. [DOI] [PubMed] [Google Scholar]
- 84.McEwen BS, Woolley CS. Estradiol and progesterone regulate neuronal structure and synaptic connectivity in adult as well as developing brain. Exp Gerontol. 1994;29:431–6. doi: 10.1016/0531-5565(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 85.Merchenthaler I, Dellovade TL, Shughrue PJ. Neuroprotection by estrogen in animal models of global and focal ischemia. Ann N Y Acad Sci. 2003;1007:89–100. doi: 10.1196/annals.1286.009. [DOI] [PubMed] [Google Scholar]
- 86.Mermelstein PG. Membrane-localised oestrogen receptor alpha and beta influence neuronal activity through activation of metabotropic glutamate receptors. J Neuroendocrinol. 2009;21:257–62. doi: 10.1111/j.1365-2826.2009.01838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mermelstein PG, Micevych PE. Nervous system physiology regulated by membrane estrogen receptors. Rev Neurosci. 2008;19:413–24. doi: 10.1515/revneuro.2008.19.6.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Micevych P, Kuo J, Christensen A. Physiology of membrane oestrogen receptor signalling in reproduction. J Neuroendocrinol. 2009;21:249–56. doi: 10.1111/j.1365-2826.2009.01833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Millen KJ, Gleeson JG. Cerebellar development and disease. Curr Opin Neurobiol. 2008;18:12–9. doi: 10.1016/j.conb.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144:2055–67. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- 91.Mohamed MK, Abdel-Rahman AA. Effect of long-term ovariectomy and estrogen replacement on the expression of estrogen receptor gene in female rats. Eur J Endocrinol. 2000;142:307–14. doi: 10.1530/eje.0.1420307. [DOI] [PubMed] [Google Scholar]
- 92.Mong JA, Nunez JL, McCarthy MM. GABA mediates steroid-induced astrocyte differentiation in the neonatal rat hypothalamus. J Neuroendocrinol. 2002;14:45–55. doi: 10.1046/j.1365-2826.2002.00737.x. [DOI] [PubMed] [Google Scholar]
- 93.Moorthy K, Yadav UC, Mantha AK, Cowsik SM, Sharma D, Basir SF, Baquer NZ. Estradiol and progesterone treatments change the lipid profile in naturally menopausal rats from different age groups. Biogerontology. 2004;5:411–9. doi: 10.1007/s10522-004-3190-7. [DOI] [PubMed] [Google Scholar]
- 94.Morris NM, Udry JR. Variations in pedometer activity during the menstrual cycle. Obstet Gynecol. 1970;35:199–201. [PubMed] [Google Scholar]
- 95.Moura PJ, Petersen SL. Estradiol acts through nuclear- and membrane-initiated mechanisms to maintain a balance between GABAergic and glutamatergic signaling in the brain: implications for hormone replacement therapy. Rev Neurosci. 2010;21:363–80. doi: 10.1515/revneuro.2010.21.5.363. [DOI] [PubMed] [Google Scholar]
- 96.Naftolin F, Horvath TL, Jakab RL, Leranth C, Harada N, Balthazart J. Aromatase immunoreactivity in axon terminals of the vertebrate brain. An immunocytochemical study on quail, rat, monkey and human tissues. Neuroendocrinology. 1996;63:149–55. doi: 10.1159/000126951. [DOI] [PubMed] [Google Scholar]
- 97.Nelson HD, Walker M, Zakher B, Mitchell J. Menopausal Hormone Therapy for the Primary Prevention of Chronic Conditions: A Systematic Review to Update the U.S. Preventive Services Task Force Recommendations. Ann Intern Med. 2012;157:104–13. doi: 10.7326/0003-4819-157-2-201207170-00466. [DOI] [PubMed] [Google Scholar]
- 98.Palay SL, Chan-Palay V. Cerebellar cortex: cytology and organization. Springer-Verlag; New York, NY: 1974. [Google Scholar]
- 99.Palmon SC, Williams MJ, Littleton-Kearney MT, Traystman RJ, Kosk-Kosicka D, Hurn PD. Estrogen increases cGMP in selected brain regions and in cerebral microvessels. J Cereb Blood Flow Metab. 1998;18:1248–52. doi: 10.1097/00004647-199811000-00011. [DOI] [PubMed] [Google Scholar]
- 100.Pandolfo M. Molecular genetics and pathogenesis of Friedreich ataxia. Neuromuscul Disord. 1998;8:409–15. doi: 10.1016/s0960-8966(98)00039-x. [DOI] [PubMed] [Google Scholar]
- 101.Pfaff D, Keiner M. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J Comp Neurol. 1973;151:121–58. doi: 10.1002/cne.901510204. [DOI] [PubMed] [Google Scholar]
- 102.Pfaff DW. Estrogens and Brain Function: Neural Analysis of a Hormone-Controlled Mammalian Reproductive Behavior. Springer-Verlag; New York: 1980. [Google Scholar]
- 103.Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology. 1992;17:485–95. doi: 10.1016/0306-4530(92)90007-t. [DOI] [PubMed] [Google Scholar]
- 104.Phillips SM, Sherwin BB. Variations in memory function and sex steroid hormones across the menstrual cycle. Psychoneuroendocrinology. 1992;17:497–506. doi: 10.1016/0306-4530(92)90008-u. [DOI] [PubMed] [Google Scholar]
- 105.Price RH, Jr, Handa RJ. Expression of estrogen receptor-beta protein and mRNA in the cerebellum of the rat. Neurosci Lett. 2000;288:115–8. doi: 10.1016/s0304-3940(00)01221-0. [DOI] [PubMed] [Google Scholar]
- 106.Qiu J, Ronnekleiv OK, Kelly MJ. Modulation of hypothalamic neuronal activity through a novel G-protein-coupled estrogen membrane receptor. Steroids. 2008;73:985–91. doi: 10.1016/j.steroids.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rapkin AJ, Berman SM, Mandelkern MA, Silverman DH, Morgan M, London ED. Neuroimaging evidence of cerebellar involvement in premenstrual dysphoric disorder. Biol Psychiatry. 2011;69:374–80. doi: 10.1016/j.biopsych.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rau SW, Dubal DB, Bottner M, Gerhold LM, Wise PM. Estradiol attenuates programmed cell death after stroke-like injury. J Neurosci. 2003;23:11420–6. doi: 10.1523/JNEUROSCI.23-36-11420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Raz L, Khan MM, Mahesh VB, Vadlamudi RK, Brann DW. Rapid estrogen signaling in the brain. Neurosignals. 2008;16:140–53. doi: 10.1159/000111559. [DOI] [PubMed] [Google Scholar]
- 110.Reinert KC, Dunbar RL, Gao W, Chen G, Ebner TJ. Flavoprotein autofluorescence imaging of neuronal activation in the cerebellar cortex in vivo. J Neurophysiol. 2004;92:199–211. doi: 10.1152/jn.01275.2003. [DOI] [PubMed] [Google Scholar]
- 111.Reinert KC, Gao W, Chen G, Ebner TJ. Flavoprotein autofluorescence imaging in the cerebellar cortex in vivo. J Neurosci Res. 2007;85:3221–32. doi: 10.1002/jnr.21348. [DOI] [PubMed] [Google Scholar]
- 112.Reinert KC, Gao W, Chen G, Wang X, Peng YP, Ebner TJ. Cellular and metabolic origins of flavoprotein autofluorescence in the cerebellar cortex in vivo. Cerebellum. 2011;10:585–99. doi: 10.1007/s12311-011-0278-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rewal M, Jung ME, Simpkins JW. Role of the GABA-A system in estrogen-induced protection against brain lipid peroxidation in ethanol-withdrawn rats. Alcohol Clin Exp Res. 2004;28:1907–15. doi: 10.1097/01.alc.0000148100.78628.e7. [DOI] [PubMed] [Google Scholar]
- 114.Rewal M, Jung ME, Wen Y, Brun-Zinkernagel AM, Simpkins JW. Role of the GABAA system in behavioral, motoric, and cerebellar protection by estrogen during ethanol withdrawal. Alcohol. 2003;31:49–61. doi: 10.1016/j.alcohol.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 115.Richardson TE, Yang SH, Wen Y, Simpkins JW. Estrogen protection in Friedreich’s ataxia skin fibroblasts. Endocrinology. 2011;152:2742–9. doi: 10.1210/en.2011-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ritzel RM, Capozzi LA, McCullough LD. Sex, stroke, and inflammation: The potential for estrogen-mediated immunoprotection in stroke. Horm Behav. 2012 doi: 10.1016/j.yhbeh.2012.04.007. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Robertson D, Craig M, van Amelsvoort T, Daly E, Moore C, Simmons A, Whitehead M, Morris R, Murphy D. Effects of estrogen therapy on age-related differences in gray matter concentration. Climacteric. 2009;12:301–9. doi: 10.1080/13697130902730742. [DOI] [PubMed] [Google Scholar]
- 118.Rocamora N, Garcia-Ladona FJ, Palacios JM, Mengod G. Differential expression of brain-derived neurotrophic factor, neurotrophin-3, and low-affinity nerve growth factor receptor during the postnatal development of the rat cerebellar system. Brain Res Mol Brain Res. 1993;17:1–8. doi: 10.1016/0169-328x(93)90065-w. [DOI] [PubMed] [Google Scholar]
- 119.Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, de Andrade M, Melton LJ., 3rd Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074–83. doi: 10.1212/01.wnl.0000276984.19542.e6. [DOI] [PubMed] [Google Scholar]
- 120.Rogers LC, de Boer I, Junier MP, Ojeda SR. Estradiol Increases Neural-Specific Class II-beta-Tubulin mRNA Levels in the Developing Female Hypothalamus by Regulating mRNA Stability. Mol Cell Neurosci. 1993;4:424–31. doi: 10.1006/mcne.1993.1053. [DOI] [PubMed] [Google Scholar]
- 121.Rubinow DR, Hoban MC, Grover GN, Galloway DS, Roy-Byrne P, Andersen R, Merriam GR. Changes in plasma hormones across the menstrual cycle in patients with menstrually related mood disorder and in control subjects. Am J Obstet Gynecol. 1988;158:5–11. doi: 10.1016/0002-9378(88)90765-x. [DOI] [PubMed] [Google Scholar]
- 122.Rubinow DR, Schmidt PJ. Gonadal steroid regulation of mood: the lessons of premenstrual syndrome. Front Neuroendocrinol. 2006;27:210–6. doi: 10.1016/j.yfrne.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 123.Rune GM, Frotscher M. Neurosteroid synthesis in the hippocampus: role in synaptic plasticity. Neuroscience. 2005;136:833–42. doi: 10.1016/j.neuroscience.2005.03.056. [DOI] [PubMed] [Google Scholar]
- 124.Sakamoto H, Mezaki Y, Shikimi H, Ukena K, Tsutsui K. Dendritic growth and spine formation in response to estrogen in the developing Purkinje cell. Endocrinology. 2003;144:4466–77. doi: 10.1210/en.2003-0307. [DOI] [PubMed] [Google Scholar]
- 125.Saldanha CJ, Duncan KA, Walters BJ. Neuroprotective actions of brain aromatase. Front Neuroendocrinol. 2009;30:106–18. doi: 10.1016/j.yfrne.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Saldanha CJ, Remage-Healey L, Schlinger BA. Synaptocrine signaling: steroid synthesis and action at the synapse. Endocr Rev. 2011;32:532–49. doi: 10.1210/er.2011-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Santen RJ, Boucher AE, Santner SJ, Henderson IC, Harvey H, Lipton A. Inhibition of aromatase as treatment of breast carcinoma in postmenopausal women. J Lab Clin Med. 1987;109:278–89. [PubMed] [Google Scholar]
- 128.Sare GM, Gray LJ, Bath PM. Association between hormone replacement therapy and subsequent arterial and venous vascular events: a meta-analysis. Eur Heart J. 2008;29:2031–41. doi: 10.1093/eurheartj/ehn299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sasahara K, Shikimi H, Haraguchi S, Sakamoto H, Honda S, Harada N, Tsutsui K. Mode of action and functional significance of estrogen-inducing dendritic growth, spinogenesis, and synaptogenesis in the developing Purkinje cell. J Neurosci. 2007;27:7408–17. doi: 10.1523/JNEUROSCI.0710-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schilder CM, Seynaeve C, Beex LV, Boogerd W, Linn SC, Gundy CM, Huizenga HM, Nortier JW, van de Velde CJ, van Dam FS, Schagen SB. Effects of tamoxifen and exemestane on cognitive functioning of postmenopausal patients with breast cancer: results from the neuropsychological side study of the tamoxifen and exemestane adjuvant multinational trial. J Clin Oncol. 2010;28:1294–300. doi: 10.1200/JCO.2008.21.3553. [DOI] [PubMed] [Google Scholar]
- 131.Schilder CM, Seynaeve C, Linn SC, Boogerd W, Beex LV, Gundy CM, Nortier JW, van de Velde CJ, van Dam FS, Schagen SB. Cognitive functioning of postmenopausal breast cancer patients before adjuvant systemic therapy, and its association with medical and psychological factors. Crit Rev Oncol Hematol. 2010;76:133–41. doi: 10.1016/j.critrevonc.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 132.Schilder CM, Seynaeve C, Linn SC, Boogerd W, Gundy CM, Beex LV, van Dam FS, Schagen SB. The impact of different definitions and reference groups on the prevalence of cognitive impairment: a study in postmenopausal breast cancer patients before the start of adjuvant systemic therapy. Psychooncology. 2010;19:415–22. doi: 10.1002/pon.1595. [DOI] [PubMed] [Google Scholar]
- 133.Schlinger BA, Callard GV. Estrogen receptors in quail brain: a functional relationship to aromatase and aggressiveness. Biol Reprod. 1989;40:268–75. doi: 10.1095/biolreprod40.2.268. [DOI] [PubMed] [Google Scholar]
- 134.Schlinger BA, Remage-Healey L. Neurosteroidogenesis: insights from studies of songbirds. J Neuroendocrinol. 2012;24:16–21. doi: 10.1111/j.1365-2826.2011.02150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schulz JB, Boesch S, Burk K, Durr A, Giunti P, Mariotti C, Pousset F, Schols L, Vankan P, Pandolfo M. Diagnosis and treatment of Friedreich ataxia: a European perspective. Nat Rev Neurol. 2009;5:222–34. doi: 10.1038/nrneurol.2009.26. [DOI] [PubMed] [Google Scholar]
- 136.Schwartz PM, Borghesani PR, Levy RL, Pomeroy SL, Segal RA. Abnormal cerebellar development and foliation in BDNF-/- mice reveals a role for neurotrophins in CNS patterning. Neuron. 1997;19:269–81. doi: 10.1016/s0896-6273(00)80938-1. [DOI] [PubMed] [Google Scholar]
- 137.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–25. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 138.Shughrue PJ, Merchenthaler I. Distribution of estrogen receptor beta immunoreactivity in the rat central nervous system. J Comp Neurol. 2001;436:64–81. [PubMed] [Google Scholar]
- 139.Sierra A, Azcoitia I, Garcia-Segura L. Endogenous estrogen formation is neuroprotective in model of cerebellar ataxia. Endocrine. 2003;21:43–51. doi: 10.1385/endo:21:1:43. [DOI] [PubMed] [Google Scholar]
- 140.Sillitoe RV, Joyner AL. Morphology, molecular codes, and circuitry produce the three-dimensional complexity of the cerebellum. Annu Rev Cell Dev Biol. 2007;23:549–77. doi: 10.1146/annurev.cellbio.23.090506.123237. [DOI] [PubMed] [Google Scholar]
- 141.Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- 142.Simpkins JW, Rajakumar G, Zhang YQ, Simpkins CE, Greenwald D, Yu CJ, Bodor N, Day AL. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J Neurosurg. 1997;87:724–30. doi: 10.3171/jns.1997.87.5.0724. [DOI] [PubMed] [Google Scholar]
- 143.Slopien R, Junik R, Meczekalski B, Halerz-Nowakowska B, Maciejewska M, Warenik-Szymankiewicz A, Sowinski J. Influence of hormonal replacement therapy on the regional cerebral blood flow in postmenopausal women. Maturitas. 2003;46:255–62. doi: 10.1016/s0378-5122(03)00144-0. [DOI] [PubMed] [Google Scholar]
- 144.Smith SS. Estrogen administration increases neuronal responses to excitatory amino acids as a long-term effect. Brain Res. 1989;503:354–7. doi: 10.1016/0006-8993(89)91691-0. [DOI] [PubMed] [Google Scholar]
- 145.Smith SS. Sensorimotor-correlated discharge recorded from ensembles of cerebellar Purkinje cells varies across the estrous cycle of the rat. J Neurophysiol. 1995;74:1095–108. doi: 10.1152/jn.1995.74.3.1095. [DOI] [PubMed] [Google Scholar]
- 146.Smith SS, Waterhouse BD, Woodward DJ. Locally applied estrogens potentiate glutamate-evoked excitation of cerebellar Purkinje cells. Brain Res. 1988;475:272–82. doi: 10.1016/0006-8993(88)90615-4. [DOI] [PubMed] [Google Scholar]
- 147.Smith SS, Woodward DJ, Chapin JK. Sex steroids modulate motor-correlated increases in cerebellar discharge. Brain Res. 1989;476:307–16. doi: 10.1016/0006-8993(89)91251-1. [DOI] [PubMed] [Google Scholar]
- 148.Snyder MA, Smejkalova T, Forlano PM, Woolley CS. Multiple ERbeta antisera label in ERbeta knockout and null mouse tissues. J Neurosci Methods. 2010;188:226–34. doi: 10.1016/j.jneumeth.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Srivastava DP, Waters EM, Mermelstein PG, Kramar EA, Shors TJ, Liu F. Rapid estrogen signaling in the brain: implications for the fine-tuning of neuronal circuitry. J Neurosci. 2011;31:16056–63. doi: 10.1523/JNEUROSCI.4097-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Srivastava R, Chaturvedi CM. Effect of estrogen and tamoxifen on the shell gland AVT and VT3R of scotosensitive and scotorefractory Japanese quail. Gen Comp Endocrinol. 2010;167:104–12. doi: 10.1016/j.ygcen.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 151.Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–34. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- 152.Sullivan EV, Deshmukh A, Desmond JE, Mathalon DH, Rosenbloom MJ, Lim KO, Pfefferbaum A. Contribution of alcohol abuse to cerebellar volume deficits in men with schizophrenia. Arch Gen Psychiatry. 2000;57:894–902. doi: 10.1001/archpsyc.57.9.894. [DOI] [PubMed] [Google Scholar]
- 153.Tan XJ, Dai YB, Wu WF, Kim HJ, Barros RP, Richardson TI, Yaden BC, Warner M, McKinzie DL, Krishnan V, Gustafsson JA. Reduction of dendritic spines and elevation of GABAergic signaling in the brains of mice treated with an estrogen receptor beta ligand. Proc Natl Acad Sci U S A. 2012;109:1708–12. doi: 10.1073/pnas.1121162109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Toran-Allerand CD. Estrogen and the brain: beyond ER-alpha, ER-beta, and 17beta-estradiol. Ann N Y Acad Sci. 2005;1052:136–44. doi: 10.1196/annals.1347.009. [DOI] [PubMed] [Google Scholar]
- 155.Torres-Aleman I, Rejas MT, Pons S, Garcia-Segura LM. Estradiol promotes cell shape changes and glial fibrillary acidic protein redistribution in hypothalamic astrocytes in vitro: a neuronal-mediated effect. Glia. 1992;6:180–7. doi: 10.1002/glia.440060305. [DOI] [PubMed] [Google Scholar]
- 156.Toung TJ, Traystman RJ, Hurn PD. Estrogen-mediated neuroprotection after experimental stroke in male rats. Stroke. 1998;29:1666–70. doi: 10.1161/01.str.29.8.1666. [DOI] [PubMed] [Google Scholar]
- 157.Tremere LA, Pinaud R. Brain-generated estradiol drives long-term optimization of auditory coding to enhance the discrimination of communication signals. J Neurosci. 2011;31:3271–89. doi: 10.1523/JNEUROSCI.4355-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tsutsui K. Neurosteroids in the Purkinje cell: biosynthesis, mode of action and functional significance. Mol Neurobiol. 2008;37:116–25. doi: 10.1007/s12035-008-8024-1. [DOI] [PubMed] [Google Scholar]
- 159.Victor M. Deficiency diseases of the nervous system secondary to alcoholism. Postgrad Med. 1971;50:75–9. doi: 10.1080/00325481.1971.11697591. [DOI] [PubMed] [Google Scholar]
- 160.Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff syndrome. A clinical and pathological study of 245 patients, 82 with post-mortem examinations. Contemp Neurol Ser. 1971;7:1–206. [PubMed] [Google Scholar]
- 161.Vito CC, Fox TO. Embryonic rodent brain contains estrogen receptors. Science. 1979;204:517–9. doi: 10.1126/science.432656. [DOI] [PubMed] [Google Scholar]
- 162.von Schassen C, Fester L, Prange-Kiel J, Lohse C, Huber C, Bottner M, Rune GM. Oestrogen synthesis in the hippocampus: role in axon outgrowth. J Neuroendocrinol. 2006;18:847–56. doi: 10.1111/j.1365-2826.2006.01484.x. [DOI] [PubMed] [Google Scholar]
- 163.Wang L, Andersson S, Warner M, Gustafsson JA. Estrogen receptor (ER)beta knockout mice reveal a role for ERbeta in migration of cortical neurons in the developing brain. Proc Natl Acad Sci U S A. 2003;100:703–8. doi: 10.1073/pnas.242735799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang X, Chen G, Gao W, Ebner T. Long-term potentiation of the responses to parallel fiber stimulation in mouse cerebellar cortex in vivo. Neuroscience. 2009;162:713–22. doi: 10.1016/j.neuroscience.2009.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang X, Chen G, Gao W, Ebner TJ. Parasagittally aligned, mGluR1-dependent patches are evoked at long latencies by parallel fiber stimulation in the mouse cerebellar cortex in vivo. J Neurophysiol. 2011;105:1732–46. doi: 10.1152/jn.00717.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, Kotchen T, Curb JD, Black H, Rossouw JE, Aragaki A, Safford M, Stein E, Laowattana S, Mysiw WJ. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. JAMA. 2003;289:2673–84. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 167.Weill-Engerer S, David JP, Sazdovitch V, Liere P, Schumacher M, Delacourte A, Baulieu EE, Akwa Y. In vitro metabolism of dehydroepiandrosterone (DHEA) to 7alpha-hydroxy-DHEA and Delta5-androstene-3beta,17beta-diol in specific regions of the aging brain from Alzheimer’s and non-demented patients. Brain Res. 2003;969:117–25. doi: 10.1016/s0006-8993(03)02288-1. [DOI] [PubMed] [Google Scholar]
- 168.Westley BR, Salaman DF. Nuclear binding of the oestrogen receptor of neonatal rat brain after injection of oestrogens and androgens; localization and sex differences. Brain Res. 1977;119:375–88. doi: 10.1016/0006-8993(77)90317-1. [DOI] [PubMed] [Google Scholar]
- 169.White RE, Darkow DJ, Lang JL. Estrogen relaxes coronary arteries by opening BKCa channels through a cGMP-dependent mechanism. Circ Res. 1995;77:936–42. doi: 10.1161/01.res.77.5.936. [DOI] [PubMed] [Google Scholar]
- 170.Wilson ME, Westberry JM. Regulation of oestrogen receptor gene expression: new insights and novel mechanisms. J Neuroendocrinol. 2009;21:238–42. doi: 10.1111/j.1365-2826.2009.01830.x. [DOI] [PubMed] [Google Scholar]
- 171.Wojtowicz T, Lebida K, Mozrzymas JW. 17beta-estradiol affects GABAergic transmission in developing hippocampus. Brain Res. 2008;1241:7–17. doi: 10.1016/j.brainres.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 172.Woolley CS, McEwen BS. Estradiol regulates hippocampal dendritic spine density via an N-methyl-D-aspartate receptor-dependent mechanism. J Neurosci. 1994;14:7680–7. doi: 10.1523/JNEUROSCI.14-12-07680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wroolie TE, Kenna HA, Williams KE, Powers BN, Holcomb M, Khaylis A, Rasgon NL. Differences in verbal memory performance in postmenopausal women receiving hormone therapy: 17beta-estradiol versus conjugated equine estrogens. Am J Geriatr Psychiatry. 2011;19:792–802. doi: 10.1097/JGP.0b013e3181ff678a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zahr NM, Pitel AL, Chanraud S, Sullivan EV. Contributions of studies on alcohol use disorders to understanding cerebellar function. Neuropsychol Rev. 2010;20:280–9. doi: 10.1007/s11065-010-9141-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhao L, Brinton RD. Estrogen receptor alpha and beta differentially regulate intracellular Ca(2+) dynamics leading to ERK phosphorylation and estrogen neuroprotection in hippocampal neurons. Brain Res. 2007;1172:48–59. doi: 10.1016/j.brainres.2007.06.092. [DOI] [PubMed] [Google Scholar]
- 176.Zhao L, Yao J, Mao Z, Chen S, Wang Y, Brinton RD. 17beta-Estradiol regulates insulin-degrading enzyme expression via an ERbeta/PI3-K pathway in hippocampus: relevance to Alzheimer’s prevention. Neurobiol Aging. 2010;32:1949–63. doi: 10.1016/j.neurobiolaging.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhou L, Fester L, von Blittersdorff B, Hassu B, Nogens H, Prange-Kiel J, Jarry H, Wegscheider K, Rune GM. Aromatase inhibitors induce spine synapse loss in the hippocampus of ovariectomized mice. Endocrinology. 2010;151:1153–60. doi: 10.1210/en.2009-0254. [DOI] [PubMed] [Google Scholar]