Abstract
Noroviruses (NoVs) are a leading cause of epidemic acute gastroenteritis affecting millions of people worldwide. Understanding of NoV remains limited due to the lack of a cell culture system and small animal models. Currently, there are no available vaccines or antivirals against NoVs. In this study, an approach for large-scale production of anti-NoV antibodies for use as a potential treatment for NoV disease using passive immunization was evaluated. NoV-specific immunoglobulins (IgY) were produced by immunizing chickens with NoV P particles. The birds continuously produced high titers of antibodies in their eggs for at least 3 months, in which NoV-specific antibody levels reached 4.7-9.2 mg/egg yolk. The egg yolk antibodies strongly reacted with NoV P particles by both ELISA and Western blot and blocked NoV virus-like particle (VLP) and P particle binding to the histo-blood group antigen (HBGA) receptors with a BT50 of about 1:800. The blocking activity of the chicken IgY remained after an incubation at 70°C for 30 min or treatment at pH 4 to 9 for 3 h. These data suggested that chicken IgY could be a practical strategy for large-scale production of anti-NoV antibodies for potential use as passive immunization against NoV infection, as well as for diagnostic purposes.
Keywords: Norovirus, Immunoglobulin, IgY, Chicken, Norovirus P particle, Diarrhea
1. Introduction
Noroviruses (NoVs) are important pathogens, responsible for more than 90% of outbreaks of non-bacterial acute gastroenteritis. NoV outbreaks occur in a wide variety of settings including nursing homes, hospitals, day-care centers, cruise ships, restaurants, and catered events (Glass et al, 2009). Although NoV infection is usually mild and self-limited, severe cases have been observed in immunocompromised patients and the elderly (Schwartz et al., 2011). NoVs also result in over a million hospital admissions; with ~900,000 clinic visits and ~200,000 deaths of children under 5 years of age in developing countries (Patel et al., 2008). Unfortunately, there are no vaccines or antivirals available currently against NoVs (Tan and Jiang, 2010).
NoVs are non-enveloped RNA viruses that contain a single-stranded, positive sense RNA genome. The genome is encompassed by a protein capsid that is formed by a single major structural protein, the capsid protein (VP1) and a minor structural protein (VP2). The VP1 capsid protein can be divided into two major domains, the N-terminal shell (S) domain and the C-terminal protrusion (P) domain. The P domain can form dimers (Tan, Hegde, and Jiang, 2004), 12-mer small P particles (Tan et al., 2011a), and 24-mer P particles (Tan et al., 2008; Tan and Jiang, 2005) when it is expressed in E. coli. While all three of these P complexes are immunogenic and recognize HBGAs, the 24-mer P particle is particularly useful as a candidate vaccine because of its high immunogenicity, stability, and low cost of production (Tan et al., 2011b).
Passive immunization remains an effective strategy to prevent and treat infectious diseases. Oral administration of antibodies derived from mammalian serum has been described previously (Cooper and Paterson, 2009). However, the high cost of large-scale antibody production in mammals has limited its application. Passive immunization with monoclonal antibodies has also been shown to have lower levels of protection compared to polyclonal antibodies. The recently developed chicken IgY approach provides a useful alternative for large-scale production of polyclonal antibodies at a lower cost. Chicken IgYs are made in the blood and transferred to the egg yolk during embryo development (Xu et al., 2011). Since egg yolks are easily harvested, the IgY technology became a promising strategy to prevent and control infectious diseases, especially for gastrointestinal infections (Amaral et al., 2002; Liou et al., 2010; Vega et al., 2011).
This manuscript describes the production of NoV-specific IgY in the egg yolks of chickens immunized with NoV P particles. A large amount of high titer anti-NoV antibodies was obtained. These IgYs were stable at a wide range of temperatures and pHs, reacted strongly to NoV virus-like particles (VLPs) and P particles in both ELISA and Western blot techniques, and were capable of blocking NoV-HBGA receptor interactions. These data support the notion that NoV-specific IgY may be a useful option for large-scale production of NoV-specific antibodies for therapeutic use against NoVs.
2. Materials and methods
2.1 Preparation of Strain VA387 P Particles
Recombinant 24-mer P particles (P-CDCRGDCFC) of strain VA387 (GII.4) were expressed in E. coli (BL21, DE3) with an induction of 0.5 mM isopropyl-β-D-thiogalactopyranoside (IPTG) at room temperature (22 °C) overnight as described previously (Tan and Jiang, 2005; Tan et al., 2008). Purification of the glutathione S-transferase (GST)-P fusion protein was performed using resin of Glutathione Sepharose 4 Fast Flow (GE Healthcare life Sciences, NJ, USA) according to the manufacturer’s instructions. GST was removed from the target proteins by thrombin (GE Healthcare life Sciences, NJ, USA) cleavage either on beads or in solution (phosphate buffer saline, PBS, pH 7.4) at room temperature for 16 h.
2.2 Chickens and immunization
Ten, 20-week-old, healthy White Leghorn chickens were provided by the Guangdong bird breeding company (Guangzhou, China) and were randomly divided into two groups. Four chickens (immunization group) were immunized by injecting 50 μg of P particle antigen into different spots of the pectoral muscle three times in two week intervals. The first immunization included complete Freund’s adjuvant (Sigma, F5881, St Louis, USA), while the second and third boosters were administrated with incomplete Freund’s adjuvant (Sigma, F5506, St Louis, USA). The control group (n=6) was injected with PBS plus the corresponding adjuvant. Blood (1 ml) was collected from the wing vein before and after each immunization. Eggs were collected one week before immunization and every day after the first immunization for 16 weeks. The experimental protocol was reviewed and approved by the Ethics Commission for the Use of Animals of School of Public Health and Tropical Medicine, Southern Medical University.
2.3 Detection of NoV-specific IgY antibodies in serum by ELISA
Sera were collected from blood after an overnight incubation at 4 °C and centrifugation at 7000 × g for 10 min at 4°C. The serum was stored at −20°C until use. The NoV-specific IgY antibody titers of sera were measured by standard ELISAs. Briefly, ninety-six well microtiter plates (Dynex Immulon; Dynatech, Franklin, MA, USA) were coated with 100 μl of purified NoV P particle antigen (200 ng/well) and incubated overnight at 4°C. After blocking with 5% nonfat milk, serially diluted chicken sera were added to the antigen-coated wells and incubated at 37°C for 1 h. After washing, goat anti-chicken IgY-HRP (1:5000) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was added. The bound HRP was colorized by adding substrate reagent (BD OptEIA TMB Substrate Reagent Set, BD Biosciences, San Jose, CA, USA). The signal intensity was measured at 450 nm using a micro-plate reader (DTX 880 Multimode Reader, Beckman Coulter, Krefeld, Germany). Pre-immunized chicken sera and chicken sera after immunization with PBS were used as controls. Antigen-specific antibody titers were defined as the end-point dilutions with a cutoff signal intensity of 0.15.
2.4 Isolation and purification of yolk IgY
Eggs were stored at 4°C before IgY extraction. A water dilution method for IgY extraction from egg yolk (Akita and Nakai, 1992; Akita and Nakai, 1993) was used with some modifications. Briefly, egg yolks were separated from egg whites by egg separators and washed with deionized water. The egg yolk was diluted 10 times with PBS and then the suspension was adjusted to a final pH of 5 with 0.1 N HCl and kept overnight at 4°C. The supernatant containing the IgY was collected after centrifugation (10000 × g for 30 min at 4°C). Solid ammonium sulfate was added to the supernatant to reach 55% saturation and the mixture was kept at 4°C for 2 h. The precipitate was collected by centrifugation (10000 × g for 15 min at 4°C) and dissolved in 2-4 ml cold PBS, before addition of 50-100 ml (25 × volume of PBS) 33% saturated ammonium sulfate (SAS) solution to give a final 31.7% of SAS. The mixture was kept at 4°C for 2 h. Protein precipitate was collected again by centrifugation (10000 × g for 15 min at 4°C) and was then dissolved in 6-7 ml PBS (pH 7.4). After pasteurization at 60°C for 30 minutes, the IgY solution was stored at 4°C. The purity of the IgY was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by Coomassie blue staining.
2.5 Determination of NoV-specific IgY titers in egg yolk
NoV-specific IgY titer in egg yolk was determined by the aforementioned ELISA (2.3), in which IgY samples were serially diluted to determine the end-point dilution. To calculate the amount of total IgY and P particle-specific IgY, a standard curve was set up as follows: wells were coated with 100 μl serially diluted pure chicken IgY (Promega, G116A, Madison, WI, USA) at a concentration from 0.0075 μg/ml to 1 μg/ml. After washing with PBST (PBS containing 0.05% Tween), 100 μl of goat anti-chicken IgY-HRP (1:5000) (Santa Cruz Biotechnology, CA, USA) were added (37°C for 1 h). The bound HRP was colorized by substrate reagent (BD OptEIA TMB Substrate Reagent Set; BD Biosciences San Diego, CA, USA), followed by a reading of the signal intensity at 450 nm (DTX 880 Multimode Reader, Beckman Coulter, GmbH, Krefeld, Germany). The resulting standard curve of absorbance was used to quantify the relative concentration of total IgY and P particle specific IgY from the chickens by coating plates with either P particles or rabbit anti-chicken IgY antibodies (10 μg/ml, Sigma C2288, USA) to capture the total IgY or P particle-specific IgY. Alternatively, to compare the reactivity of IgY induced by NoV P particles to VLPs of strain VA387, plates were coated with either 100 μl NoV VLP (200 ng/well) or P particles in different dilutions, as described above.
2.6 Characterization of IgY by SDS-PAGE and Western blot analysis
NoV P particles were separated by conventional SDS-PAGE and visualized with Coomassie Blue stain. For Western analysis, proteins were transferred to nitrocellulose membranes. After blocking with 5% nonfat milk, the membrane was incubated with anti-NoV-specific IgY or non-specific IgY (1:2000) in 1% nonfat milk-PBS at 4°C overnight. After washing with PBST, the membrane was incubated with goat anti-IgY (Sigma, Steinhein, Germany, 1:5000) at 37°C for 1h. The bound HRP was detected by enhanced chemiluminescence (ECL) Western blotting detection reagents (GE Healthcare Life Sciences, Buckinghamshire, England). The ECL signals were captured by Hyper film ECL (GE Healthcare Life Sciences, Buckinghamshire, England).
2.7 HBGA binding and blocking assays
The saliva-based binding and blocking assays were carried out as described elsewhere (Huang et al., 2003, 2005; Feng and Jiang, 2007). Briefly, boiled human saliva samples with known HBGA phenotypes were diluted 1000-fold and coated on 96-well microtiter plates (Dynex Immulon; Dynatech, Franklin, MA, USA). After blocking with 5% nonfat milk in PBS, NoV P particles (250 ng/ml; VA387, GII.4) were added. The bound P particles were detected using serially diluted IgY (from 1:250~1:128,000), followed by the addition of HRP-conjugated goat anti-chicken IgY (1:5000). Guinea pig antibodies against NoV VLPs were also used in this HBGA binding assay, followed by the addition of HRP-conjugated goat anti-guinea pig IgG (1:5000).
The blocking effects of IgY on P particle binding to saliva samples were measured after pre-incubation of P particles with diluted IgY for 1 hour at 37°C before addition to the saliva-coated wells. Then a guinea pig anti-VA387 VLP antiserum (1:3333) was added, followed by the addition of HRP-conjugated goat anti-guinea pig IgG (1:5000). The blocking rates were calculated by comparing the optical densities (ODs) measured with and without blocking by the chicken IgYs. The IgYs from chickens immunized with PBS were used as negative controls (Huang et al., 2005).
2.8 Reactivity of IgY after treatments with various pHs and temperatures
Egg yolk IgY solution (1 ml, 1:4000, pH 7.4) in a test tube was heated to a given temperature (50 to 80°C) for up to 30 min. The heated mixtures were then cooled on ice. To determine pH stability, egg yolk IgY solution (1 ml, 1:40, pH 7.4) was diluted with 10 mM phosphate buffer containing 0.15 M NaCl. The pH of the solutions was adjusted with HCl or NaOH to a final pH of 2 to 11. The solution was incubated at 37°C for 3h, and then the pH of the solution was neutralized by adding 100 × PBS. The activity of the treated IgY was measured by HBGA blocking assay.
3. Results
3.1 Characterization of specific anti-NoV IgY in chicken sera and egg yolk
The serum and egg yolk NoV-specific IgYs of individual chickens were measured by ELISA. Steady increases in serum NoV-specific IgY titers were observed after the first immunization and reached a peak at the 6th week. The serum IgY titers remained high for at least 10 weeks after the peak. Sera of chickens immunized with the PBS-adjuvant control did not have any reactivity to NoV P particles (Fig. 1a). The anti-NoV antibody titers in the eggs were not detected until the third week after immunization and then continued to increase, reaching a peak during the 7th week after the first immunization. All four birds continued making high titers of NoV-specific antibodies in their eggs (200,000~500,000), with little variation for at least 13 weeks (4-16 weeks after immunization) (Fig. 1b). These antibody titers were similar to the IgG (400,000) from guinea pigs immunized with VLPs of strain VA387. The chicken IgY reacted strongly with the VA387 VLPs (Fig. 1c).
Fig. 1.
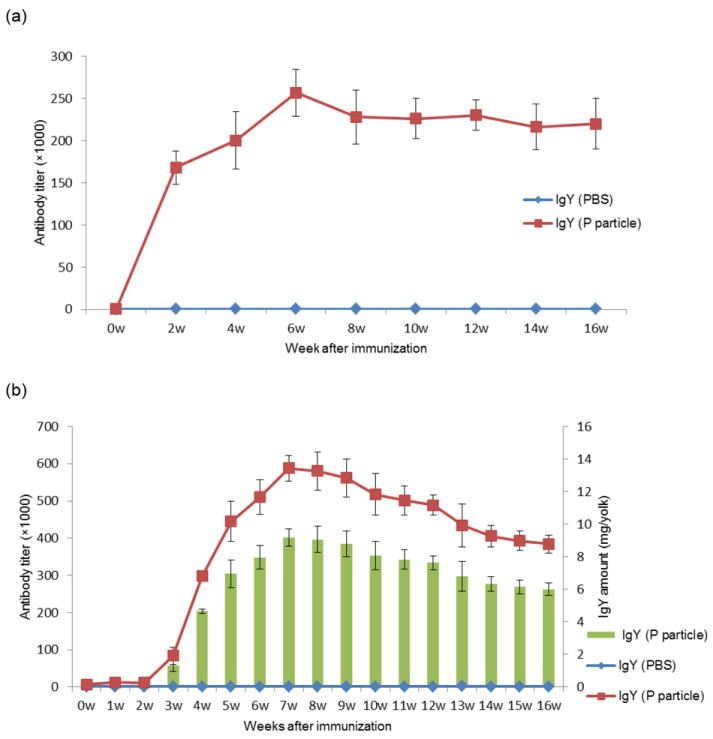
Kinetics of serum (a) and egg yolk (b) anti-NoV IgY response of chickens after immunization with NoV P particles. NoV-specific IgY was determined by ELISA against GII.4 (VA387) P particle antigens. The amount of NoV-specific IgY per egg yolk (green columns) was also determined (b) (right-hand Y-axis). Comparable reactivities of egg yolk IgY with NoV GII.4 (VA387) P particles (PP) and virus-like particles (VLP) were also observed (c).
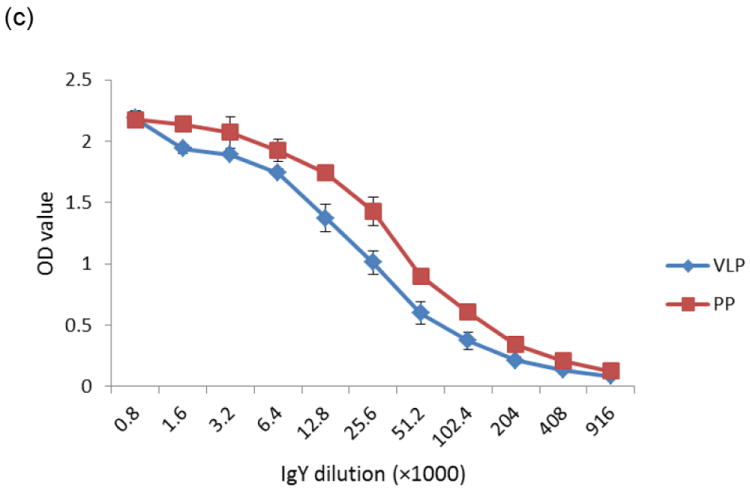
The concentration of total IgY was determined by SDS-PAGE with standard BSA (bovine serum albumin standard II, Bio-rad) and verified by ELISA using an IgY standard. The concentration of specific anti-NoV IgY was also determined by ELISA. After comparison with the IgY standard curve, the total IgY level in each yolk was estimated to be 186 ± 21 mg. The specific anti-NoV IgY levels increased gradually from the third week after the first immunization with an average of 4.7 - 9.2 mg/yolk from the fourth to sixteenth week, accounting for about 2.5-5.0% of the total IgY (Fig. 1b). Analysis of the concentrated egg IgY by SDS-PAGE revealed a major band of ~68 kDa heavy chain and a minor band of ~28 kDa light chain and an unknown protein band of ~40 kDa (Fig. 2a). NoV-specific IgY also reacted with NoV P particles in a Western blot analysis (Fig. 2c).
Fig.2.
Western blot analysis of anti-NoV chicken IgY against NoV P particle. Partially purified chicken egg yolk IgY was analyzed by SDS-PAGE (a). Lane 1: protein standards (Bio-RAD), lane 2: purified egg yolk IgY with indications of heavy and light chains. The partially purified IgY (1:4000 dilution) interacts with GII.4 (VA387) P particles (c) following SDS-PAGE (b). Lane1: protein standards, lane 2: NoV P particles.
3.2 IgY antibodies block NoV binding to HBGA receptors
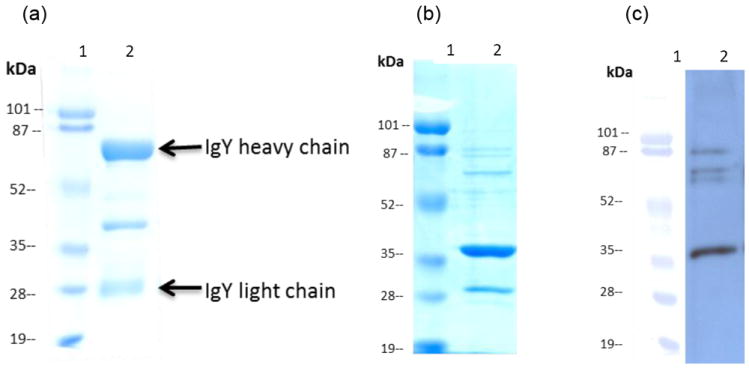
The egg yolk IgY antibodies induced by P particles can be used in an ELISA as a detection antibody to measure NoV/receptor interaction. Partially purified IgY reacted strongly with both NoV VLPs or P particles in saliva-based HBGA binding assays, similar to guinea pig IgG after immunization with VLPs of strain VA387 (Fig. 3a). In addition, the egg yolk IgY antibodies were able to block NoV VLP and P particle binding to HBGA receptors (Fig. 3b) with a BT50 (a serum dilution with 50% blocking activity) of about 1:800.
Fig.3.
Chicken IgY induced by NoV P particles blocked the binding of NoV norovirus P particles to HBGA receptors. (a) Partially purified IgY induced by NoV P particle (red line) in a wide range of dilutions can be used as a detection antibody to measure binding of NoV P particles to various saliva samples in saliva-based HBGA binding assays. The results were similar to those using guinea pig IgG induced by VLPs of strain VA387 (green line). Each data point represents an average value of six binding assays using six different type A salivas. (b) The IgY induced by NoV P particles blocked binding of norovirus VLPs to HBGA receptors (blue line). On the other hand, IgY after immunization with PBS neither reacted with P particles (blue line, a) nor showed detectable blocking (red line, b).
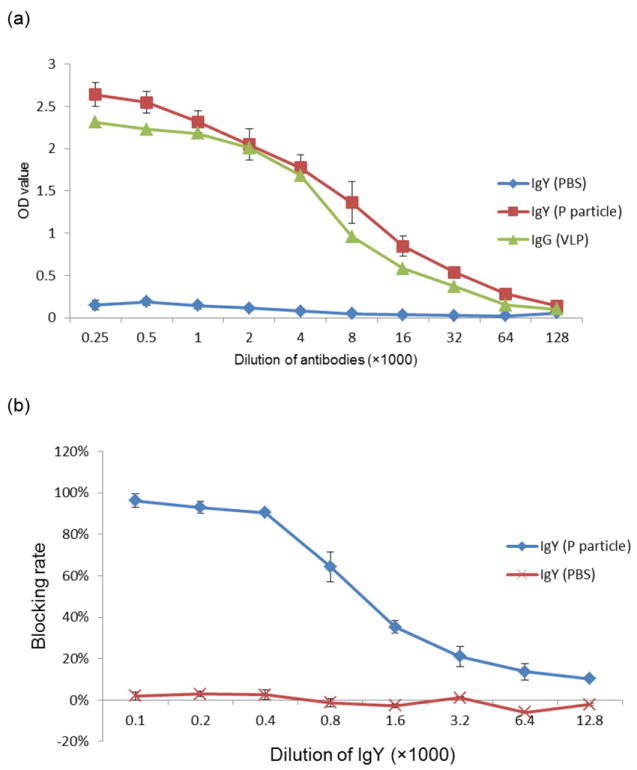
3.3 Reactivity of specific anti-NoV IgY after treatment with various pHs and temperatures
IgY remained reactive to NoV P particles after heat treatment at 70°C or below for 30 min, as shown by its blocking capability (Fig. 4a). IgY also retained blocking ability after an incubation at pH 4-9 at 37°C for 3 h (Fig. 4b).
Fig.4.
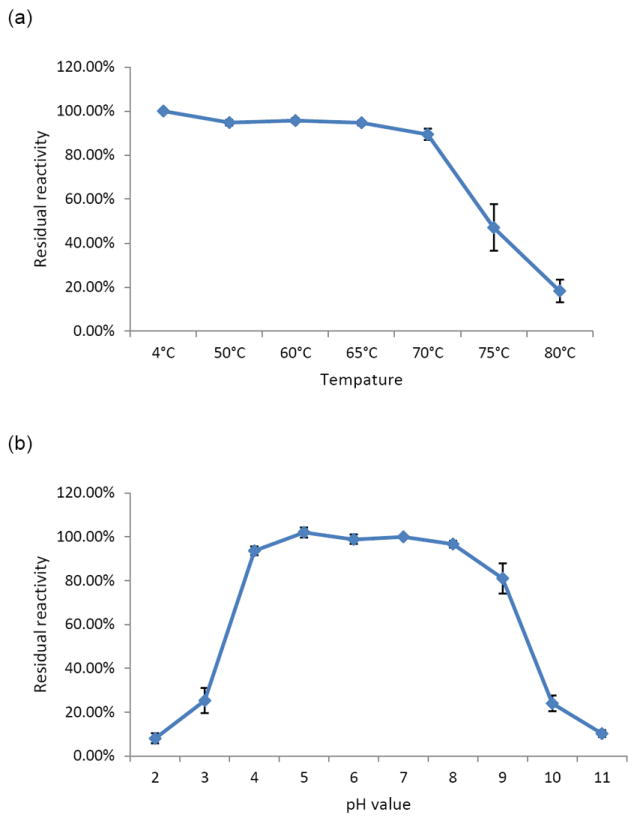
Effect of temperature (a) and pH (b) on the ability of IgY to block NoV P particle binding to HBGAs. The GII.4 (VA387) P particles and a type A saliva sample were used in the blocking assay. The chicken IgY was treated with the indicated range of temperatures for 30 min (a) or with the indicated range of pHs for 3 hours (b) before being tested in the blocking assay. A percentage (%) of residual blocking activity in comparison with an untreated sample is shown. Each assay was performed in triplicate and each data point represents the average of these assays.
4. Discussion
IgY has been successfully used to treat and prevent infectious diseases in humans and domestic animals (Dias da Silva and Tambourgi, 2010; Vega et al., 2011). White leghorn chickens are easily raised and have high egg productivity. After immunization with a small dose of antigen, a chicken can continuously produce eggs containing antigen-specific antibodies in their yolks (Xu et al., 2011). A chicken usually lays 280 eggs/year and an egg yolk (12-15 ml) usually contains 150-200 mg IgY, of which 2 to 10% are specific antibodies (Nguyen et al., 2010). Thus, a chicken is referred as a small “factory” for antibody production.
In this study, a continual increase of NoV-specific IgY in egg yolks was observed starting in the third week after the initial immunization. NoV specific antibody reached 4.7-9.2 mg/egg yolk between the fourth and the sixteenth weeks, which accounted for 2.5-5% of the total IgY. During this period, 340 eggs were collected from 4 immunized chickens and thus, a total of ~2,400 mg specific anti-NoV IgY was obtained. Producing an equal amount of antibody would require 100-125 guinea pigs assuming that 15-20 ml sera can be collected from each animal. Strikingly, high levels of antigen-specific egg yolk IgY can be produced up to 2 years when the bird is continuously boosted every three months. Furthermore, collection of yolk antibody is non-invasive without affecting the immunized chickens. Finally, the NoV-specific IgY showed excellent reactivity at a wide range of temperature and pHs (this report), an indication of highly stable IgY, important for clinical use.
As a leading cause of viral gastroenteritis, NoVs remain hard to prevent and control due to their wide spread nature and lack of effective therapeutic and prophylactic methods. Thus, passive immunization could be useful for high-risk populations, such as young children, the elderly, and immunocompromised patients, whose immunity may be immature or weakened. Administration of NoV-specific IgY antibodies could help to prevent persistent infections with NoVs.
In this study, the NoV specific IgYs were also able to block the binding of NoV VLPs/P particles to HBGAs. This is an important indication that the IgY is likely to neutralize, and therefore prevent, NoV infection and illness. A direct correlation between the ability of an antibody to block VLP-HBGA binding and protection against NoV infection and illness was observed in a recent NoV challenge study (Reeck et al., 2010). An additional study also showed that pre-existing HBGA blocking antibodies protected children from a GII.4 infection (Nurminen et al., 2011). Thus, the IgYs developed in this study are likely to be effective for prevention and treatment of NoV infection and illness using a passive immunization approach.
Highlights.
▶NoV-specific immunoglobulins (IgY) were produced by immunizing chickens with NoV P particles. ▶The egg yolk antibodies strongly reacted with NoV P particles and blocked NoV virus-like particles (VLPs) and P particles binding to the histo-blood group antigen (HBGA) receptors. ▶The chicken IgY could be an efficient approach for large production of high titers of anti-NoV antibodies potentially for passive immunization against NoV infection and other purposes.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grants 30700716 and 30901992), Medical Scientific Research Foundation of Guangdong Province (B2010174), Doctoral Fund of Ministry of Education (20104433120015), Grant from School of Public Health and Tropical Medicine of Southern Medical University (GW201209) of China, and the National Institute of Health of the United States (R01 AI 055649, R01 AI 37093, R01 AI089634 and P01 HD 13021) and the Agriculture and Food Research Initiative Competitive Grants Program of the USDA National Institute of Food and Agriculture, NIFA Award No: 2011-68003-30005. We thank Dr. Christina Quigley for scientific discussion and editing of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akita EM, Nakai S. Immunoglobulins from Egg-Yolk - Isolation and Purification. Journal of Food Science. 1992;57:629–634. [Google Scholar]
- Akita EM, Nakai S. Production and purification of Fab’ fragments from chicken egg yolk immunoglobulin Y (IgY) J Immunol Methods. 1993;162:155–64. doi: 10.1016/0022-1759(93)90380-p. [DOI] [PubMed] [Google Scholar]
- Amaral JA, Tino De Franco M, Carneiro-Sampaio MM, Carbonare SB. Anti-enteropathogenic Escherichia coli immunoglobulin Y isolated from eggs laid by immunised Leghorn chickens. Res Vet Sci. 2002;72:229–34. doi: 10.1053/rvsc.2002.0551. [DOI] [PubMed] [Google Scholar]
- Cooper HM, Paterson Y. Production of polyclonal antisera. Curr Protoc Neurosci. 2009;Chapter 5(Unit 5):5. doi: 10.1002/0471142301.ns0505s48. [DOI] [PubMed] [Google Scholar]
- Dias da Silva W, Tambourgi DV. IgY: a promising antibody for use in immunodiagnostic and in immunotherapy. Vet Immunol Immunopathol. 2010;135:173–80. doi: 10.1016/j.vetimm.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Jiang X. Library screen for inhibitors targeting norovirus binding to histo-blood group antigen receptors. Antimicrob Agents Chemother. 2007;51:324–31. doi: 10.1128/AAC.00627-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass RI, Parashar UD, Estes MK. Norovirus gastroenteritis. N Engl J Med. 2009;361:1776–85. doi: 10.1056/NEJMra0804575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Farkas T, Marionneau S, Zhong W, Ruvoen-Clouet N, Morrow AL, Altaye M, Pickering LK, Newburg DS, LePendu J, Jiang X. Noroviruses bind to human ABO, Lewis, and secretor histo-blood group antigens: identification of 4 distinct strain-specific patterns. J Infect Dis. 2003;188:19–31. doi: 10.1086/375742. [DOI] [PubMed] [Google Scholar]
- Huang P, Farkas T, Zhong W, Tan M, Thornton S, Morrow AL, Jiang X. Norovirus and histo-blood group antigens: demonstration of a wide spectrum of strain specificities and classification of two major binding groups among multiple binding patterns. J Virol. 2005;79:6714–22. doi: 10.1128/JVI.79.11.6714-6722.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou JF, Chang CW, Tailiu JJ, Yu CK, Lei HY, Chen LR, Tai C. Passive protection effect of chicken egg yolk immunoglobulins on enterovirus 71 infected mice. Vaccine. 2010;28:8189–96. doi: 10.1016/j.vaccine.2010.09.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HH, Tumpey TM, Park HJ, Byun YH, Tran LD, Nguyen VD, Kilgore PE, Czerkinsky C, Katz JM, Seong BL, Song JM, Kim YB, Do HT, Nguyen T, Nguyen CV. Prophylactic and therapeutic efficacy of avian antibodies against influenza virus H5N1 and H1N1 in mice. PLoS One. 2010;5:e10152. doi: 10.1371/journal.pone.0010152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurminen K, Blazevic V, Huhti L, Rasanen S, Koho T, Hytonen VP, Vesikari T. Prevalence of norovirus GII-4 antibodies in Finnish children. J Med Virol. 2011;83:525–31. doi: 10.1002/jmv.21990. [DOI] [PubMed] [Google Scholar]
- Patel MM, Widdowson MA, Glass RI, Akazawa K, Vinje J, Parashar UD. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg Infect Dis. 2008;14:1224–31. doi: 10.3201/eid1408.071114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeck A, Kavanagh O, Estes MK, Opekun AR, Gilger MA, Graham DY, Atmar RL. Serological correlate of protection against norovirus-induced gastroenteritis. J Infect Dis. 2010;202:1212–8. doi: 10.1086/656364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S, Vergoulidou M, Schreier E, Loddenkemper C, Reinwald M, Schmidt-Hieber M, Flegel WA, Thiel E, Schneider T. Norovirus gastroenteritis causes severe and lethal complications after chemotherapy and hematopoietic stem cell transplantation. Blood. 2011;117:5850–6. doi: 10.1182/blood-2010-12-325886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M, Fang P, Chachiyo T, Xia M, Huang P, Fang Z, Jiang W, Jiang X. Noroviral P particle: structure, function and applications in virus-host interaction. Virology. 2008;382:115–23. doi: 10.1016/j.virol.2008.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M, Fang PA, Xia M, Chachiyo T, Jiang W, Jiang X. Terminal modifications of norovirus P domain resulted in a new type of subviral particles, the small P particles. Virology. 2011a;410:345–52. doi: 10.1016/j.virol.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M, Hegde RS, Jiang X. The P domain of norovirus capsid protein forms dimer and binds to histo-blood group antigen receptors. J Virol. 2004;78:6233–42. doi: 10.1128/JVI.78.12.6233-6242.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M, Huang P, Xia M, Fang PA, Zhong W, McNeal M, Wei C, Jiang W, Jiang X. Norovirus P particle, a novel platform for vaccine development and antibody production. J Virol. 2011b;85:753–64. doi: 10.1128/JVI.01835-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M, Jiang X. The p domain of norovirus capsid protein forms a subviral particle that binds to histo-blood group antigen receptors. J Virol. 2005;79:14017–30. doi: 10.1128/JVI.79.22.14017-14030.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M, Jiang X. Norovirus gastroenteritis, carbohydrate receptors, and animal models. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega C, Bok M, Chacana P, Saif L, Fernandez F, Parreno V. Egg yolk IgY: protection against rotavirus induced diarrhea and modulatory effect on the systemic and mucosal antibody responses in newborn calves. Vet Immunol Immunopathol. 2011;142:156–69. doi: 10.1016/j.vetimm.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Li X, Jin L, Zhen Y, Lu Y, Li S, You J, Wang L. Application of chicken egg yolk immunoglobulins in the control of terrestrial and aquatic animal diseases: A review. Biotechnol Adv. 2011 doi: 10.1016/j.biotechadv.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


