Abstract
Given the complexity of prostate cancer progression and metastasis, multimodalities that target different aspects of tumor biology, e.g., radiotherapy (RT) in conjunction with immunotherapy, may provide the best opportunities for promoting clinical benefits in patients with high risk localized prostate cancer. Here we show that intratumoral administration of unmodified dendritic cells (DCs) failed to synergize with fractionated RT. However, ionizing radiation combined with in situ vaccination with DCs, in which the immunosuppressive scavenger receptor A (SRA/CD204) has been downregulated by lentivirus-mediated gene silencing, profoundly suppressed the growth of two mouse prostate cancers (e.g., RM1 and TRAMP-C2), and prolonged the lifespan of tumor-bearing animals. Treatment of subcutaneous tumors with this novel combinatorial radio-immunotherapeutic regimen resulted in a significant reduction in distant experimental metastases. SRA/CD204-silenced DCs were highly efficient in generating antigen or tumor-specific T cells with increased effector functions (e.g., cytokine production and tumoricidal activity). SRA/CD204 silencing-enhanced tumor cell death was associated with elevated IFN-γ levels in tumor tissue and increased tumor-infiltrating CD8+ cells. IFN-γ neutralization or depletion of CD8+ cells abrogated the SRA/CD204 downregulation-promoted antitumor efficacy, indicating a critical role of IFN-γ-producing CD8+ T cells. Therefore, blocking SRA/CD204 activity significantly enhances the therapeutic potency of local RT combined with in situ DC vaccination by promoting a robust systemic antitumor immunity. Further studies are warranted to test this novel combinatorial approach for translating into improved clinical outcomes in prostate cancer patients.
Keywords: Prostate cancer, radiotherapy, dendritic cell, T cell, tumor immunity, IFN-γ, CD204, scavenger receptor A
Introduction
Prostate cancer is the most prevalent solid tumor and the second leading cause of cancer death among men in the United States (1). Although the majority of patients are currently diagnosed with clinically localized disease, many patients develop biochemical failure after local definite therapy, e.g., radiotherapy (RT), perhaps due to occult metastatic disease. Androgen deprivation therapy in addition to RT improves overall survival of patients at high risk for recurrence (2). This approach, however, is associated with significant side effects. Given that prostate cancer patients often present with low tumor burden, one potential treatment is the incorporation of immunotherapy into a standard RT protocol (3). With recent approval of an antigen-presenting cell vaccine sipuleucel-T (Provenge) by the US Food and Drug Administration, immunotherapy now represents an established treatment modality for metastatic prostate cancer (4, 5).
Several lines of evidence support an approach of combined RT and immunotherapy to achieve local tumor control while at the same time generating systemic immune responses (6). Local irradiation not only debulks tumor, but also generates an inflammatory microenvironment. Tumor antigens released by dying tumor cells are captured and processed by specialized antigen-presenting cells (e.g., DCs) in the context of the costimulatory or “danger” signals, resulting in effective T cell activation (7). RT also renders tumor cells more susceptible to recognition and attack by tumor-specific CTLs (8–10). Gulley et al. recently reported clinical trials in which RT combined with a PSA-targeted vaccine displayed a favorable toxicity profile and generated significant T cell responses in prostate cancer patients (11, 12). Indeed, several phase II/III clinical trials of RT in combination with therapeutic cancer vaccines, e.g., ProstAtakTM (NCT01436968), PSA/TRICOM (NCT00450619), L-BLP25 (Stimuvax, NCT01496131), for treatment of high risk prostate cancer are ongoing.
Induction of immunity against self-antigens is often restricted by multiple intrinsic inhibitory checkpoints or molecules (13, 14). Knowledge of these inhibitory regulators has led to great progress in development of immunotherapeutic strategies for cancer treatment (15). We have identified an immunosuppressive pathway in DCs involving the scavenger receptor SRA/CD204, which can dampen immune responses against several cancers, including prostate cancer (16, 17). SRA/CD204 attenuates the immunostimulatory capability of DCs and subsequent activation of CTLs in the context of vaccine therapy and tumor immunity (18, 19). Recently, we demonstrated that silencing SRA/CD204 in primary DCs markedly enhanced the potency of DC vaccines in mounting an effective antitumor T cell response (20).
Considering the fact that SRA/CD204 absence significantly increases the immunogenicity of ionizing radiation treated prostate cancer cells (17), we hypothesize that local RT-induced cancer cell damage in combination with in situ vaccination with SRA/CD204-downregulated DCs could achieve improved systemic antitumor efficacy. In the present study, we provide the first experimental evidence that intratumoral administration of SRA/CD204-silenced DCs profoundly enhances the control of RT-treated local prostate cancer as well as its metastases, which is mainly mediated by IFN-γ-producing CD8+ CTLs. Our data support the concept of inhibiting SRA/CD204 as a novel strategy to optimize DC-targeted immunotherapy that may be rationally combined with RT for treatment of human prostate cancer.
Materials and Methods
Mice and cell lines
Wild-type (WT) C57BL/6 mice were obtained from National Cancer Institute (Bethesda, MD). SRA/CD204 knockout mice (SRA−/−), OT-I mice bearing TCR specific for OVA257–264 (SIINFEKL) were purchased from The Jackson Laboratory (Bar Harbor, ME). All experiments and procedures involving mice were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University. Androgen-refractory mouse prostate cancer cell lines, RM1 (21) kindly provided by Dr. TC Thompson (Baylor College of Medicine, TX, USA) and TRAMP-C2 (22) were used. RM1 cells-expressing OVA (17) or luciferase were generated in our laboratory. All cell lines were maintained in DMEM supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. These cells have been screened for Mycoplasma and other pathogens before in vivo use. No authentication of the cell lines were conducted by the authors.
Short hairpin (sh) RNA–mediated gene silencing in DCs
Lentiviral (LV) vector encoding mouse SRA/CD204 shRNA were packaged using Phoenix cells co-transfected with pLKO.1 constructs and pMD.G and pCMVΔR8.91 (20). DCs were generated by culturing mouse bone marrow (BM) cells in the presence of granulocyte macrophage colony-stimulating factor (GM-CSF). Lentiviral infection of primary DCs was carried out as previously described (20, 23). Briefly, BM cells were cultured in media containing recombinant mouse GM-CSF (20 ng/ml, Preprotech, Rocky Hill, NJ). Day 3 BM-DCs were infected with lentiviruses in the presence of 8 μg/mL polybrene for 3 hours. Cells were then re-suspended and plated in 12-well plates at 1 × 106 cells/mL. Day 8 DCs were collected and used for studies.
Tumor treatment and immunization protocols
Mice were injected with 1×105 RM1 tumor cells or 2×106 TRAMP-C2 tumor cells subcutaneously (s.c.) in the right hind leg. When tumors reached 4–5 mm in diameter, mice were anesthetized using ketamine (80 mg/kg)/xylazine (10 mg/kg), and locally irradiated with a Picker 60Co source at 1 Gy/min. Mice received a single dose of 10 Gy on each of three consecutive days. For in situ DC immunization, mice were injected with DCs (2 × 106) into the tumors 24 and 72 hours after the last radiation treatment. In some experiments, CD4+, CD8+ T cells or NK cells were depleted by intraperitoneal (i.p.) injection of GK1.5, 2.43 or PK136 monoclonal antibodies (mAbs), respectively, as previously described (16).
Experimental lung metastases
C57BL/6 mice bearing s.c. RM1 tumors were established with lung metastases by intravenously (i.v.) injecting 5×104 RM1-luciferase cells in PBS (without Ca2+ or Mg2+). Only tumors in the flank were treated. Mice were injected i.p. with D-luciferin (150 mg/kg, Caliper Life Sciences, Hopkinton, MA) and examined using a Xenogen IVIS Imaging System. Lung metastatic nodules were also enumerated. In the case of TRAMP-C2 tumor, 1×106 cells were injected i.v. to generate pulmonary metastases.
In vitro T cell stimulation
DCs were pulsed with tumor cell lysates (1:1 ratio) for 5 hours. After washing, serially diluted DCs were incubated with 5×104 OT-I cells in a round-bottom 96-well microtiter plate. Cells were cultured for 60 hours and pulsed with 3H-thymidine (0.5 μCi/well) during the last 16 hours of culture period. T cell proliferation was assessed based on 3H-thymidine incorporation. Levels of IL-2 in the supernatants were determined using Enzyme-linked immunosorbent assay (ELISA).
Enzyme-linked immunosorbent spot (ELISPOT) assay and intracellular IFN-γ staining
Splenocytes or lymph node cells from treated mice were analyzed for the frequency of antigen-specific IFN-γ-secreting CD8+ T cells using ELISPOT assays as previously described (19). For intracellular cytokine staining, cells were stimulated with OVA257–264 peptides (AnaSpec Inc., San Jose, CA) for 3 days, followed by treatment with brefeldin A (BD GolgiPlug; BD Biosciences). Cells were then stained with Abs for CD8 and IFN-γ, and subjected to FACS analysis (19).
Cytotoxicity assays
An in vivo CTL assay to determine antigen-specific cytolytic activity of CD8+ T cells was performed as previously described (18). Briefly, naïve splenocytes that were labeled with 12.5 μM CFSE (i.e., CFSEhigh cells) using CellTraceTM 5-(and 6-) carboxyfluorescein diacetate succinimidyl ester cell proliferation kit (Molecular Probes, Eugene, OR), and pulsed with OVA257–264 served as targets. Control cells were labeled with 0.5 μM CFSE (i.e., CFSElow cells) and pulsed with irrelevant peptides. A mixture of these cell populations (total 5 × 106 cells) at 1:1 ratio was injected i.v. into treated mice, followed by FACS analysis of splenocytes 16 hours later.
Statistical analysis
Data are presented as mean ± SD. Differences between groups within experiments were analyzed using two-tailed unpaired Student t test. Animal survival data were analyzed using log-rank test. p values < .05 were considered statistically significant.
Results
SRA/CD204 dampens the therapeutic effectiveness of conventional RT
We previously showed that mouse prostate tumor RM1 cells treated with ionizing radiation displayed increased immunogenicity in SRA−/− mice (17). Therefore, we sought to determine whether absence of SRA/CD204 in tumor-bearing mice could amplify the antitumor efficacy of local RT. As previous reported, RM1 tumors grew aggressively at a similar rate in WT and SRA−/− mice before treatment (17). However, RM1 tumor growth was profoundly inhibited by RT in SRA−/− mice compared with WT mice (Fig. 1), suggesting that SRA/CD204 ablation sensitizes RM1 tumors to RT.
Figure 1. SRA/CD204 ablation improves the effectiveness of RT against established prostate tumors.
RM1 tumor-bearing WT and SRA−/− mice (n=5) were treated with or without RT. ** p < 0.01. The results shown are representative of at least three independent experiments.
In situ vaccination using SRA/CD204-deficient DCs enhances the effectiveness of RT by promoting CD8+ T cell activation
SRA/CD204 is expressed in antigen-presenting cells, and enhanced antitumor immunity in SRA−/− mice has been shown to result from increased functions of SRA−/− DCs exposed to tumor cell lysates (17) or stress proteins (19). We tested whether improved tumor control could be achieved by intratumoral delivery of SRA−/− DCs following RT (Fig. 2A). Our initial studies showed that fractionated RT (i.e., 3 consecutive 10 Gy) delayed tumor growth (Fig. 2B, left). Direct injections of WT or SRA−/− DCs without RT had no effect on tumor growth (data not shown). Surprisingly, administration of WT DCs to the irradiated RM1 tumors failed to provide any additional benefits (Fig. 2B, left). In contrast, in situ vaccination with SRA−/− DCs after RT resulted in increased tumor suppression compared with RT alone or RT plus WT DCs (Fig. 2B, middle). The enhanced tumor control in mice treated with RT plus SRA−/− DCs was also associated with significantly improved animal survival (Fig. 2B, right).
Figure 2. SRA/CD204 absence in DCs enhances the antitumor efficacy of RT combined with DC vaccination.
A. Scheme for the combined RT and in situ DC vaccination. B. RM1 tumor-bearing mice (n=5) were treated with RT alone, RT plus WT DCs or left untreated. WT DC vaccine failed to enhance tumor inhibition following RT (left). In contrast, administration of SRA−/− DC vaccine combined with RT led to improved tumor control (middle) and survival rate (right). ** p < 0.01, RT + WT DC vs RT + SRA−/− DC. C. Enhanced T cell activation by RT and SRA−/− DC vaccination. Splenocytes (top) and lymph node cells (bottom) from treated RM1-OVA tumor-bearing mice were stimulated with OVA257–264 peptides. The frequency of IFN-γ-producing CD8+ T cells was measured using ELISPOT (* p < 0.01). D. Resistance of SRA−/− DCs to tumor-mediated immunosuppression. OVA257–264-loaded DCs were co-cultured with OT-I cells in the presence of RM1 tumor conditioned media (TCM) at indicated concentrations. T cell proliferation was assessed using 3H-thymidine incorporation assays (top). Index ratios of T cell proliferation driven by DCs (SRA−/− DCs/WT DCs) are also presented (bottom). In addition, the levels of IL-2 and IFN-γ in the supernatants were determined using ELISA (* p < 0.01).
To determine whether the increased tumor control by combined RT and SRA−/− DC vaccine correlated with enhanced T cell activation, RM1 tumors expressing a non-“self” model antigen OVA (RM1-OVA) was used to facilitate monitoring of antigen-specific T cells. The frequency of IFN-γ-producing CD8+ cells reactive with OVA257–264 epitope increased in mice treated with RT plus WT DC. However, SRA−/− DCs were much more efficient than WT DCs in generating OVA-specific CD8+ T cells when combined with RT (Fig. 2C).
Tumor immune evasion often involves a complex array of immune suppressive mechanisms, including inhibition of T cell-stimulating activity of DCs in the tumor environment. We compared the capability of WT and SRA−/− DCs to stimulate OVA-specific CD8+ T cells in the presence of tumor-mediated immunosuppression. When co-cultured with naïve OT-I cells in RM1 tumor-conditioned media, OVA257–264-pulsed SRA−/− DCs were consistently more effective than WT DCs in stimulating OT-I cell proliferation and production of cytokine IL-2 and IFN-γ (Fig. 2D). Interestingly, exposure of WT DCs with tumor-conditioned media caused upregulation of SRA/CD204 protein levels (data not shown).
SRA/CD204 silencing in DCs enhances cross-presentation of antigen from dying tumor cells
BM-DCs were genetically modified by infection with lentiviral vectors (LV) encoding shRNA specific for SRA/CD204 or scrambled shRNA as previously described (20). SRA/CD204-silenced DCs (DC-SRA shRNA) produced more pro-inflammatory cytokine IL-6 (Fig. 3A) and chemokine IP-10 (Fig. 3B) than scrambled shRNA-treated DCs (i.e., DC-scram) upon exposure to dying RM1 tumor cells. The mRNA levels of the inflammatory genes (il6, ip10 and ifnβ) similarly increased in DC-SRA shRNA (Supplementary Fig. S1A–C). Elevation of IL-6 expression was further confirmed by intracellular cytokine staining (Supplementary Fig. S1D). These results are consistent with our previous observations that lack of SRA/CD204 sensitizes DCs to inflammatory signals associated with cell injury (17). Additionally, DC-SRA shRNA pulsed with RM1-OVA tumor lyastes were more efficient than mock cells in stimulating OVA-specific OT-I cell activation (Fig. 3C and 3D).
Figure 3. SRA/CD204 silencing in DCs promotes cross-presentation of RM1 tumor cell-associated antigens.
A–B. SRA/CD204 downregulation renders DCs more responsive to stimulation with dying tumor cells. DCs were infected with lentiviruses encoding scrambled shRNA or SRA shRNA, and co-cultured with RM1 cell lysates prepared after ionizing radiation. Protein levels of IL-6 (A) and IP-10 (B) in the media were determined using ELISA. The efficiency of SRA/CD204 silencing in DCs was assessed using immunoblotting (A, insert). C–D. Increased CD8+ T cell activation by SRA/CD204-silenced DCs. DCs were pulsed with RM1-OVA cell lysates, and co-cultured with naïve OT-I cells. T cell proliferation was assessed using 3H-thymidine incorporation assays (C). The IL-2 concentration in the supernatant was determined using ELISA (D). * p< 0.01. The results shown represent three experiments.
RT combined with SRA/CD204-silenced DC vaccine results in enhanced therapeutic efficacy against local tumors and distant metastases
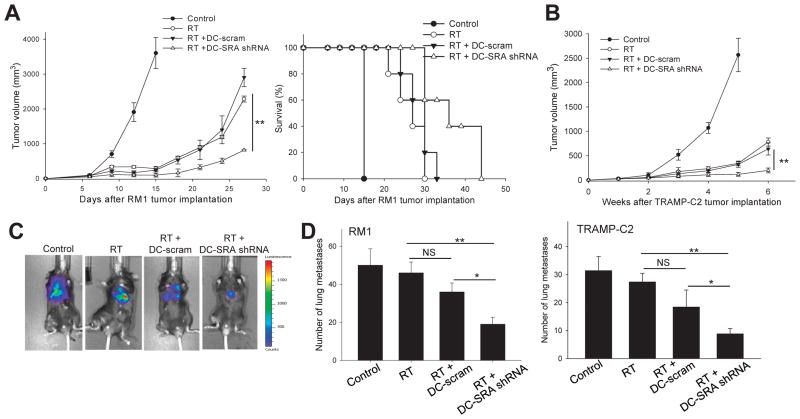
Using a mouse prostate cancer RM1 model, we evaluated the antitumor response augmented by RT and in situ vaccination with SRA/CD204-silenced DCs. DC-SRA shRNA, but not DC-scram, promoted growth inhibition of RM1 tumors and prolonged the survival of RT-treated mice (Fig. 4A). The similar results were also obtained in a different mouse model of prostate cancer (Fig. 4B), TRAMP-C2, which was derived from spontaneous prostate cancers arising in TRAMP mice (22).
Figure 4. In situ vaccination with SRA/CD204-silenced DCs enhances the antitumor efficacy of RT.
A. RM1 tumor-bearing mice (n=5) received RT alone, RT plus DC-scram or DC-SRA shRNA, or left untreated. Tumor growth (left) and survival of RM1 tumor-bearing mice (right) were monitored. ** p<0.01, RT+DC-SRA shRNA vs RT+DC-scram. B. Enhanced suppression of TRAMP-C2 prostate tumor by SRA/CD204-targeted combinatorial therapy. * p < 0.01. C–D. Treatment of local prostate tumors with RT plus DC-SRA shRNA reduces distant metastases. Mice (n=5) were simultaneously established with s.c. ‘primary’ RM1 tumors in the flank and lung metastases through i.v. injection of RM1-luciferase cells. Tumors in the flank were subjected to treatment only. The metastases in the lungs were assessed using bioluminescence imaging (C). Lung tissues were collected from RM1-tumor (left) or TRAMP-C2 tumor (right)-bearing mice following treatment of s.c. tumors, and metastatic tumor nodules in the lungs were counted (D). * p < 0.05, ** p < 0.01. Data are representative of two experiments with similar results.
Although local RT of the primary tumor can prevent development of subsequent systemic metastases, tumor radiation fails to control pre-existing systemic disease. We used an experimental lung metastasis model to assess the ability of the combination therapy to control distant metastases. RM1-luciferase tumor cells were injected i.v. into WT mice one day before the implantation of s.c. RM1 tumors. Treatment of s.c. tumors with RT alone or RT plus DC-scram vaccine partially inhibited lung metastases. However, RT plus DC-SRA shRNA was far more effective than other treatment modalities in controlling metastases (Fig. 4C and 4D, left). The result was also validated using the metastasis model of TRAMP-C2 tumor (Fig. 4D, right). Mice receiving RT plus DC-SRA shRNA did not display any signs of pathology or autoimmune toxicity in the prostate (Supplementary Fig. S2A) and other organs (data not shown).
RT combined with DC-SRA shRNA vaccination enhances activation of antigen-specific CTLs
The enhanced antitumor effect observed after the combinatorial treatment using DC-SRA shRNA prompted us to examine systemic immune responses. Upon stimulation with OVA257–264 peptide, splenocytes from RM1-OVA tumor-bearing mice treated with RT plus DC-SRA shRNA exhibited increased proliferation (Fig. 5A, top) and IL-2 production (Fig. 5A, bottom) compared to those from mice treated with RT plus DC-scram. Mice receiving RT plus DC-SRA shRNA also showed increased frequency of OVA-specific, IFN-γ-expressing CD8+ T cells, as shown by intracellular IFN-γ staining assays (Fig. 5B) and ELISPOT assays (data not shown). Considering that OVA is a non-“self” antigen and there is no pre-existing tolerance in mice, we assessed T cell recognition of six-transmembrane epithelial antigen of the prostate (STEAP), an antigen highly expressed in advanced human prostate cancers and mouse prostate tumors (24, 25). Integration of DC-SRA shRNA vaccine into the RT protocol effectively primed CD8+ T cells recognizing mSTEAP326–335 (Fig. 5C, left) or RM1 tumor cells (Fig. 5C, right). T cell functions assays showed that RT-treated RM1-OVA tumor-bearing mice receiving DC-SRA shRNA vaccine developed a stronger cytolytic response against OVA257–264-pulsed targets in vivo (Fig. 5D) and RM1-OVA tumor cells in vitro (Supplementary Fig. S2B) compared to other treatment modalities.
Figure 5. SRA/CD204 silencing enhances tumor-specific T cell responses after RT combined with in situ DC vaccination.
A. Increased activation of antigen-specific CD8+ T cells. RM1-OVA tumor-bearing mice were treated with RT alone or RT plus DC vaccines. Splenocytes were stimulated with OVA257–264 and analyzed for T cell proliferation (top) and IL-2 production (bottom). B. The percentage of IFN-γ-producing CD8+ T cells was assessed using intracellular cytokine staining assays. C. Enhanced immune cell recognition of prostate tumor antigen STEAP and RM1 tumor cells. Splenocytes were stimulated with mSTEAP326–335 (left), or irradiated RM1 cells (right). IL-2 or IFN-γ production was assessed using ELISA. * p < 0.01. Data are representative of two experiments in which at least of 3 mice of each group were analyzed. D. Increased cytolytic activity of effector T cells. One week after DC vaccination, RM1-OVA tumor-bearing mice (n=3) were injected i.v. with OVA257–264-pulsed, CFSEhigh splenocytes mixed with CFSElow splenocytes pulsed with irrelevant peptides. Spleens were analyzed 16 hours later using flow cytometry. The percentage of killing of antigen-positive target is shown in parentheses. Representative histograms from three experiments with similar results are shown.
SRA/CD204 silencing enhanced antitumor efficacy is dependent on IFN-γ-producing CD8+ T cells
TUNEL assays showed that RT plus DC-SRA shRNA induced more RM1 tumor cell death than RT alone or RT plus DC-scram. In parallel, RT plus DC-SRA shRNA also resulted in a significant increase in tumor-infiltrating CD8+ cells (Fig. 6A). Levels of CD4+ cells in the tumor tissues were comparable among treatment groups (Supplementary Fig. S3). Interestingly, increased tumor infiltration by CD11c+ cells, presumably DCs, was also seen in mice receiving SRA/CD204-silenced DCs (Supplementary Fig. S3). These tumor-resident CD11c+ cells were unlikely to be ex vivo genetically engineered DCs, because the majority of DCs injected post-RT migrated out of the tumor site within 24 hours (data not shown).
Figure 6. SRA/CD204 silencing-enhanced RM1 tumor control depends on IFN-γ production by CD8+ T cells.
A. RM1 tumor cell death is associated with increased tumor-infiltrating CD8+ cells. One week after DC vaccination, tumor cell death was examined by TUNNEL assays. Nuclei were counterstained with DAPI. Cryosections of tumor tissues were stained with Abs for CD8 (green). Scale bar indicates 50 μm. Quantitative analysis of infiltrating cells was conducted by counting positive cells in at least five randomly selected fields of view (right). * p < 0.05. B. CD8+ cells primarily contribute to IFN-γ production. IFN-γ levels increased in RM1 tumor tissues after treatment with RT plus DC-SRA shRNA, as determined using ELISA (left). Depletion of CD8 cells prior to combined therapy reduced IFN-γ levels in tumor tissues (middle). In addition, tumor-infiltrating CD8+ cells were analyzed for IFN-γ expression with intracellular cytokine staining assays (right). C. CD8+ cells are required for SRA/CD204 silencing-enhanced tumor inhibition. RM1 tumor-bearing mice (n=5) were depleted of effector cell subsets before the combinatorial therapy. D. IFN-γ is critical for SRA/CD204 silencing-facilitated antitumor activity. RM1 tumor-bearing mice (n=5) receiving RT plus DC-SRA shRNA were administrated with IFN-γ neutralizing Abs or control IgG. NS, not significant; * p < 0.05; ** p < 0.01. Data shown are representative of two experiments.
ELISA analysis of tumor tissues showed that the levels of IFN-γ, a cytokine critical for effective antitumor immunity, were significantly higher in mice receiving RT plus DC-SRA shRNA vaccination than other treatment groups (Fig. 6B, left). Removal of CD8+ cells by antibody depletion substantially decreased the intratumoral levels of IFN-γ, whereas depletion of CD4+ or NK1.1 cells only showed a slight effect (Fig. 6B, middle). Intracellular staining showed that tumor-infiltrating CD8+ cells from mice treated with RT and DC-SRA shRNA displayed markedly increased expression of IFN-γ (Fig. 6B, right) as well as granzyme B (Supplementary Fig. S4). Following radiation treatment, mice receiving DC-SRA shRNA showed a dramatic increase in the percentage of IFN-γ+CD8+ cells compared to IFN-γ+CD4+ cells in the spleen (Supplementary Fig. S5). Furthermore, depletion of CD8+, not CD4+ or NK1.1 cells, abolished SRA/CD204 silencing-enhanced antitumor efficacy (Fig. 6C). Lastly, blocking IFN-γ using neutralizing antibodies also abrogated the therapeutic efficacy of the combinatorial therapy (Fig. 6D), suggesting that IFN-γ-producing CD8+ cells represents a key factor mediating SRA/CD204 silencing-promoted antitumor immunity.
Discussion
Given that RT is the primary treatment option for high-risk localized prostate cancer and the biochemical relapse rate of many of these patients is high, combining RT with immunotherapy represents an attractive strategy to improve clinical outcomes. In the present study, we provide the first experimental evidence that silencing SRA/CD204 in DCs markedly enhances antitumor effectiveness of RT combined with in situ DC vaccination. The amplified systemic antitumor immunity by SRA/CD204 downregulation in DCs not only confers an additional inhibitory effect on RT-treated tumors, but also limits experimental metastatic outgrowth. Our studies establish the feasibility and efficacy of this novel combinatorial radio-immunotherapy in the two preclinical models of prostate cancer.
DC vaccine represents a promising immunotherapeutic approach for cancer eradication (26). While DCs are often loaded ex vivo with tumor antigens or tumor lysates to induce antitumor immune responses in conventional DC vaccination, in situ DC vaccination in a setting of local RT has also been reported to provide additional antitumor benefits (27). The failure of WT DCs or DC-scram to synergize with local RT for controlling established RM1 tumor in our initial study was unexpected, which may be attributed to its poorly immunogenic nature or the immune suppression mediated by this tumor line. However, absence or silencing of SRA/CD204 in DCs was able to effectively restore T cell-mediated antitumor immune responses when these modified DCs were introduced to the tumors treated with ionizing radiation. This finding was also confirmed using a second mouse model of prostate cancer (i.e., TRAMP-C2), which lends further support to the previously proposed role of SRA/CD204 as an immune suppressor capable of attenuating DC functions (16–20, 28).
Local RT is accompanied by the release of tumor antigen and endogenous ‘danger’ signals (29), such as stress proteins (30), oxidized LDL (31) and high-mobility-group box 1 protein (HMGB1) (32). It is likely that SRA/CD204 limits the functional activation of DCs exposed to these damage-associated molecules or ‘danger’ signals (33, 34). Out data support this hypothesis by showing that SRA/CD204 silencing renders DCs more responsive to stimulation with dying RM1 tumor cells, as evidenced by increased expression of inflammatory mediators and T cell-stimulating capability. These results also coincide with our recent observation of significantly heightened immunogenicity of dying prostate tumor cells in SRA−/− mice (17). In addition, lack of SRA/CD204 in DCs also enhances antigen cross-presentation, CTL activation and antitumor responses induced by stress proteins as immunostimulators (16, 19).
It was reported that dying tumor cells produced by cancer therapies (e.g., RT or chemotherapy) augment a TLR4-dependent immune response (35, 36), which has been attributed to an essential role of TLR4 signaling in processing and cross-presentation of tumor cell-associated antigens by DCs. Recently, our molecular studies revealed that SRA/CD204 attenuated TLR4-engaged NF-κB signaling in DCs through blockade of TRAF6 ubiquitination and oligomerization (37). The similar molecular mechanism may underlie SRA/CD204-mediated immune suppression in DCs upon stimulation with ‘danger’ molecules released by ionizing radiation-treated RM1 tumor cells. SRA/CD204 downregulation may also enable DCs more resistant to tumor-mediated immune suppression, as shown in the current study and a recent report that SRA/CD204 contribute to DC dysfunction in tumor-bearing mice and cancer patients (28). In situ DC vaccination described here can target the highly individualized tumor antigens as well as inflammatory signals released from dying cancer cells following RT. Considering the immunomodulating effects of RT, e.g., sensitizing cancer cells to T cell-mediated attack (8–10), and promoting T cell trafficking (38), we expect that RT and conventional DC vaccination with antigen-loaded, SRA/CD204-silenced DCs should also display enhanced synergistic antitumor efficacy.
Combining RT and in situ vaccination with SRA/CD204-silenced DCs is highly effective in stimulating a systemic, antigen-specific CTL-mediated antitumor immune response. As a result, a significant elevation in the number of tumor-infiltrating CD8+ cells, the intratumoral IFN-γ levels, and the frequency of IFN-γ-expressing CD8+ cells is achieved following the combinatorial therapy, which also positively correlates with increased tumor cell death. The supporting evidence also derives from the result showing that antitumor efficacy of RT and DC-SRA shRNA vaccination is abrogated after IFN-γ neutralization or in the absence of CD8+ cells. Together with the observation of diminished IFN-γ level in the tumor bed upon CD8+ cell depletion, we conclude that CD8+ T cells predominantly contribute to the improved tumor control by SRA/CD204-silenced DC vaccine following RT.
Our findings contrast to the previous study by Teitz-Tennenbaum et al (39), in which enhanced therapeutic efficacy of adoptively transferred T cells following RT and DC administration was associated with a selective increase in proliferation and function of CD4+ T cells. Several differences between these two studies may contribute to this discrepancy. While our research is centered on SRA silencing-enhanced endogenous tumor-reactive T cell response, their study primarily focused on the activity of adoptively transferred T cells. Treatment regimens used in our study (i.e., RT plus genetically engineered DCs) is also different from the one employed by Teitz-Tennenbaum et al. (DC vaccination following RT and lymphodepletion plus adoptive T cell transfer). The different observations could also derive from the use of different tumor models. It should be noted that the contribution of CD4+ T cells to tumor suppression was not directly examined in their study.
RT is commonly combined with androgen deprivation therapy (ADT) for treatment of patients with high-risk and locally advanced prostate cancer today (40). The addition of neoadjuvant and adjuvant ADT can significantly delay tumor progression, and improve disease-free and overall survival. Considering that immunotherapy (e.g., SRA-targeted DC vaccine) engages a complementary antitumor mechanism by targeting the host immune system, it therefore has the potential to further promote the additive or synergistic antitumor effects of multimodality therapy (e.g., RT plus ADT), leading to effective eradication of recurrence and metastases.
In summary, we have demonstrated that selective downregulation of SRA/CD204 in DCs can overcome immune tolerance and enhance antitumor potency of RT combined with in situ DC-based vaccination. Strategically combining RT with SRA/CD204-silenced DCs could alter or modify the tumor environment, shifting the balance in favor of immune activation. Further studies are warranted to determine whether multimodality therapy combining standard RT, SRA/CD204-targeted vaccines and/or other novel approaches that effectively break tumor-mediated immune regulatory mechanisms (41) can be successfully translated into improved clinical outcomes.
Supplementary Material
Acknowledgments
We gratefully thank Dr. Tim Thompson (Baylor College of Medicine, TX, USA) for kindly providing us with the RM1 cell line.
Grant support: This work was supported in part by American Cancer Society Scholarship RSG-08-187-01-LIB(X-Y.Wang), NIH CA129111(X-Y.Wang), CA154708 (X-Y.Wang), CA099326 (J.R.Subjeck), CA90881 (R.B.Mikkelsen), CA097318 (P.B.Fisher), DOD Prostate Cancer Research Program W81XWH-10-PCRP-SIDA (P.B.Fisher and X-Y. Wang), Harrison Endowed Scholarship (X-Y. Wang) and NCI Cancer Center Support Grants to Massey Cancer Center.
Abbreviations
- RT
Radiotherapy
- SRA
scavenger receptor A
- DC
dendritic cell
- CTL
cytotoxic T lymphocytes
Footnotes
Disclosure: All authors disclosed no conflict of interest
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Hanks GE, Pajak TF, Porter A, Grignon D, Brereton H, Venkatesan V, et al. Phase III Trial of Long-Term Adjuvant Androgen Deprivation After Neoadjuvant Hormonal Cytoreduction and Radiotherapy in Locally Advanced Carcinoma of the Prostate: The Radiation Therapy Oncology Group Protocol 92-02. J Clin Oncol. 2003;21:3972–8. doi: 10.1200/JCO.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 3.Cha E, Fong L. Immunotherapy for prostate cancer: biology and therapeutic approaches. J Clin Oncol. 2011;29:3677–85. doi: 10.1200/JCO.2010.34.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 5.Rotow J, Gameiro SR, Madan RA, Gulley JL, Schlom J, Hodge JW. Vaccines as monotherapy and in combination therapy for prostate cancer. Clin Transl Sci. 2010;3:116–22. doi: 10.1111/j.1752-8062.2010.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharp HJ, Wansley EK, Garnett CT, Chakraborty M, Camphausen K, Schlom J, et al. Synergistic antitumor activity of immune strategies combined with radiation. Front Biosci. 2007;12:4900–10. doi: 10.2741/2436. [DOI] [PubMed] [Google Scholar]
- 7.Hodge JW, Guha C, Neefjes J, Gulley JL. Synergizing radiation therapy and immunotherapy for curing incurable cancers. Opportunities and challenges Oncology (Williston Park) 2008;22:1064–70. discussion 75, 80–1, 84. [PMC free article] [PubMed] [Google Scholar]
- 8.Chakraborty M, Abrams SI, Camphausen K, Liu K, Scott T, Coleman CN, et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol. 2003;170:6338–47. doi: 10.4049/jimmunol.170.12.6338. [DOI] [PubMed] [Google Scholar]
- 9.Garnett CT, Palena C, Chakraborty M, Tsang KY, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004;64:7985–94. doi: 10.1158/0008-5472.CAN-04-1525. [DOI] [PubMed] [Google Scholar]
- 10.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–71. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gulley JL, Arlen PM, Bastian A, Morin S, Marte J, Beetham P, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res. 2005;11:3353–62. doi: 10.1158/1078-0432.CCR-04-2062. [DOI] [PubMed] [Google Scholar]
- 12.Lechleider RJ, Arlen PM, Tsang KY, Steinberg SM, Yokokawa J, Cereda V, et al. Safety and immunologic response of a viral vaccine to prostate-specific antigen in combination with radiation therapy when metronomic-dose interleukin 2 is used as an adjuvant. Clin Cancer Res. 2008;14:5284–91. doi: 10.1158/1078-0432.CCR-07-5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fong L, Small EJ. Anti-cytotoxic T-lymphocyte antigen-4 antibody: the first in an emerging class of immunomodulatory antibodies for cancer treatment. J Clin Oncol. 2008;26:5275–83. doi: 10.1200/JCO.2008.17.8954. [DOI] [PubMed] [Google Scholar]
- 14.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nature Reviews Immunology. 2004;4:336–47. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 15.Palucka K, Ueno H, Banchereau J. Recent Developments in Cancer Vaccines. The Journal of Immunology. 2011;186:1325–31. doi: 10.4049/jimmunol.0902539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang XY, Facciponte J, Chen X, Subjeck JR, Repasky EA. Scavenger receptor-A negatively regulates antitumor immunity. Cancer Res. 2007;67:4996–5002. doi: 10.1158/0008-5472.CAN-06-3138. [DOI] [PubMed] [Google Scholar]
- 17.Guo C, Yi H, Yu X, Hu F, Zuo D, Subjeck JR, et al. Absence of scavenger receptor A promotes dendritic cell-mediated cross-presentation of cell-associated antigen and antitumor immune response. Immunol Cell Biol. 2012;90:101–8. doi: 10.1038/icb.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yi H, Yu X, Gao P, Wang Y, Baek SH, Chen X, et al. Pattern recognition scavenger receptor SRA/CD204 down-regulates Toll-like receptor 4 signaling-dependent CD8 T-cell activation. Blood. 2009;113:5819–28. doi: 10.1182/blood-2008-11-190033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qian J, Yi H, Guo C, Yu X, Zuo D, Chen X, et al. CD204 suppresses large heat shock protein-facilitated priming of tumor antigen gp100-specific T cells and chaperone vaccine activity against mouse melanoma. J Immunol. 2011;187:2905–14. doi: 10.4049/jimmunol.1100703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yi H, Guo C, Yu X, Gao P, Qian J, Zuo D, et al. Targeting the immunoregulator SRA/CD204 potentiates specific dendritic cell vaccine-induced T cell response and antitumor immunity. Cancer Res. 2011;71:6611–20. doi: 10.1158/0008-5472.CAN-11-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson TC, Southgate J, Kitchener G, Land H. Multistage carcinogenesis induced by ras and myc oncogenes in a reconstituted organ. Cell. 1989;56:917–30. doi: 10.1016/0092-8674(89)90625-9. [DOI] [PubMed] [Google Scholar]
- 22.Foster BA, Gingrich JR, Kwon ED, Madias C, Greenberg NM. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res. 1997;57:3325–30. [PubMed] [Google Scholar]
- 23.He Y, Zhang J, Mi Z, Robbins P, Falo LD., Jr Immunization with lentiviral vector-transduced dendritic cells induces strong and long-lasting T cell responses and therapeutic immunity. J Immunol. 2005;174:3808–17. doi: 10.4049/jimmunol.174.6.3808. [DOI] [PubMed] [Google Scholar]
- 24.Hubert RS, Vivanco I, Chen E, Rastegar S, Leong K, Mitchell SC, et al. STEAP: A prostate-specific cell-surface antigen highly expressed in human prostate tumors. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:14523–8. doi: 10.1073/pnas.96.25.14523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Hernandez MdlL, Gray A, Hubby B, Kast WM. In vivo effects of vaccination with six-transmembrane epithelial antigen of the prostate: a candidate antigen for treating prostate cancer. Cancer Res. 2007;67:1344–51. doi: 10.1158/0008-5472.CAN-06-2996. [DOI] [PubMed] [Google Scholar]
- 26.Schuler G. Dendritic cells in cancer immunotherapy. European Journal of Immunology. 2010;40:2123–30. doi: 10.1002/eji.201040630. [DOI] [PubMed] [Google Scholar]
- 27.Teitz-Tennenbaum S, Li Q, Rynkiewicz S, Ito F, Davis MA, McGinn CJ, et al. Radiotherapy potentiates the therapeutic efficacy of intratumoral dendritic cell administration. Cancer Res. 2003;63:8466–75. [PubMed] [Google Scholar]
- 28.Herber DL, Cao W, Nefedova Y, Novitskiy SV, Nagaraj S, Tyurin VA, et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med. 2010;16:880–6. doi: 10.1038/nm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McBride WH, Chiang CS, Olson JL, Wang CC, Hong JH, Pajonk F, et al. A sense of danger from radiation. Radiat Res. 2004;162:1–19. doi: 10.1667/rr3196. [DOI] [PubMed] [Google Scholar]
- 30.Todryk SM, Melcher AA, Dalgleish AG, Vile RG. Heat shock proteins refine the danger theory. Immunology. 2000;99:334–7. doi: 10.1046/j.1365-2567.2000.00002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–61. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–8. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 33.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 34.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–89. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 36.Apetoh L, Ghiringhelli F, Tesniere A, Criollo A, Ortiz C, Lidereau R, et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev. 2007;220:47–59. doi: 10.1111/j.1600-065X.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 37.Yu X, Yi H, Guo C, Zuo D, Wang Y, Kim HL, et al. Pattern pecognition scavenger receptor CD204 attenuates toll-like receptor 4-induced NF-{kappa}B activation by directly inhibiting ubiquitination of tumor necrosis factor (TNF) receptor-associated factor 6. J Biol Chem. 2011;286:18795–806. doi: 10.1074/jbc.M111.224345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–23. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 39.Teitz-Tennenbaum S, Li Q, Davis MA, Wilder-Romans K, Hoff J, Li M, et al. Radiotherapy combined with intratumoral dendritic cell vaccination enhances the therapeutic efficacy of adoptive T-cell transfer. J Immunother. 2009;32:602–12. doi: 10.1097/CJI.0b013e3181a95165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Payne H, Mason M. Androgen deprivation therapy as adjuvant/neoadjuvant to radiotherapy for high-risk localised and locally advanced prostate cancer: recent developments. Br J Cancer. 2011;105:1628–34. doi: 10.1038/bjc.2011.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kudo-Saito C, Schlom J, Camphausen K, Coleman CN, Hodge JW. The requirement of multimodal therapy (vaccine, local tumor radiation, and reduction of suppressor cells) to eliminate established tumors. Clin Cancer Res. 2005;11:4533–44. doi: 10.1158/1078-0432.CCR-04-2237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.