Summary
Cellular responsiveness to many neuromodulators is controlled by endocytosis of the transmembrane receptors that transduce their effects. Endocytic membrane trafficking of particular neuromodulator receptors exhibits remarkable diversity and specificity, determined largely by molecular sorting operations that guide receptors at trafficking branch-points after endocytosis. In this review, we discuss recent progress in elucidating mechanisms mediating the molecular sorting of neuromodulator receptors in the endocytic pathway. There is emerging evidence that endocytic trafficking of neuromodulator receptors, in addition to influencing longer-term cellular responsiveness under conditions of prolonged or repeated activation, may also affect the acute response. Physiological and pathological consequences of defined receptor trafficking events are only now being elucidated, but it is already apparent that endocytosis of neuromodulator receptors has a significant impact on the actions of therapeutic drugs. The present data also suggest, conversely, that mechanisms of receptor endocytosis and molecular sorting may themselves represent promising targets for therapeutic manipulation.
Introduction
The spatial and temporal actions of neuromodulators are determined by local sensitivity of target neurons, and this is fundamentally determined by mechanisms that control the number and functional activity of cognate receptors that are locally available for activation (Kenakin, 2004). These properties, in turn, are subject to dynamic regulation. Accordingly, achieving appropriate neuromodulation requires dynamic and local control of the number and activity of specific neuromodulator receptors expressed in target neurons.
Most neuromodulator receptors belong to the seven-transmembrane receptor (7TMR) family, also called G protein-coupled receptors because many of their downstream effects are transduced by activation of heterotrimeric GTP-binding proteins (G proteins). 7TMRs comprise the largest and most diverse family of signal-transducing receptors, as reviewed elsewhere (Rosenbaum et al., 2009; Gainetdinov et al., 2004). 7TMRs are typically subject to exquisite regulation by the coordinated actions of multiple mechanisms (Gainetdinov et al., 2004; Jean-Alphonse and Hanyaloglu, 2011). One general class of 7TMR regulatory mechanisms is through post-translational modification. 7TMR modification by phosphorylation, acylation and ubiquitylation can produce diverse effects on the ability of receptors to bind ligands and to interact with various cytoplasmic mediator and regulator proteins, as reviewed previously elsewhere (Gainetdinov et al., 2004; Qanbar and Bouvier, 2003; Shenoy, 2007a; Kirkin and Dikic, 2007). Another class of 7TMR regulatory mechanisms is through physical movement, or trafficking, from one membrane compartment or sub-domain to another. 7TMR membrane trafficking modifies cellular signaling responsiveness by dynamically altering the number of functional receptors available for activation by neuromodulators in target neurons, or in a particular subcellular location of the neuron.
Even closely related 7TMR family members can differ markedly in trafficking behaviors in both the biosynthetic and endocytic pathways, as reviewed elsewhere (Jean-Alphonse and Hanyaloglu, 2011; Sorkin and von Zastrow, 2009). For 7TMRs that transduce neuromodulator effects, diversity and specificity of membrane trafficking is perhaps most remarkable in the endocytic pathway. The present review focuses on what is presently known about how this regulation is achieved, and about functional consequences of 7TMR endocytic trafficking to the control of neuromodulator responsiveness. In doing so we shall focus on progress made through study of two subclasses of neuromodulatory 7TMR that have been characterized in considerable detail, catecholamine receptors and opioid neuropeptide receptors, and on functional consequences manifest at the level of ‘conventional’ 7TMR signaling mediated by allosteric coupling to heterotrimeric G proteins. The reader is directed to other reviews discussing additional diversity in membrane trafficking properties observed among various 7TMR family members (Tsao and von Zastrow, 2001; Wolfe and Trejo, 2007), and for information regarding ‘alternate’ 7TMR signaling by G protein-independent mechanisms such as arrestin-mediated scaffolding of downstream signaling components (Rajagopal et al., 2010).
7TMR endocytosis and differential effects of drugs
The first step in the endocytic trafficking of a 7TMR is its removal from the plasma membrane by packaging into an endocytic vesicle. Mammalian cells express multiple endocytic mechanisms (McMahon and Boucrot, 2011; Sandvig et al., 2011) that individual 7TMRs can potentially engage (Tsao and von Zastrow, 2001; Wolfe and Trejo, 2007). Many neuromodulatory 7TMRs are internalized by clathrin-coated pits (CCPs), which are complex and highly versatile endocytic machines capable of internalizing a wide variety of membrane cargoes in addition to 7TMRs (McMahon and Boucrot, 2011; Conner and Schmid, 2003). In studies that have carefully examined the endocytic process, 7TMRs undergo activation-induced accumulation in previously formed CCPs and do not appear to initiate CCP formation on their own; accordingly, a major determinant of 7TMR endocytic rate is the degree to which receptors concentrate in CCPs (Goodman et al., 1998; Puthenveedu and von Zastrow, 2006; Krupnick et al., 1997; Kang et al., 2009).
For many neuromodulatory 7TMRs that undergo regulated endocytosis via CCPs, receptor concentration in them is stimulated by activation-induced phosphorylation of receptors followed by phosphorylation-promoted association of receptors with beta-arrestins, as reviewed previously elsewhere (Goodman et al., 1998; Gainetdinov et al., 2004). Beta-arrestins bind both to activated 7TMRs and to components of the CCP (including clathrin heavy chain, the endocytic adaptor protein AP-2, and phosphatidylinositol 4,5-bisphosphate), thereby functioning as regulated endocytic adaptors (Goodman et al., 1996; Laporte et al., 1999; Gaidarov et al., 1999). Beta-arrestins can associate with CCPs after assembly of major structural components has already occurred (Santini et al., 2000; Puthenveedu and von Zastrow, 2006), explaining how 7TMRs concentrate in CCPs after their formation and in the presence of other endocytic cargoes. While there is presently no evidence for 7TMR packaging into specialized CCPs a priori, pre-existing clusters of activated 7TMR in the plasma membrane may initiate CCP formation in some cases (Puthenveedu and von Zastrow, 2006). Further, 7TMRs that associate with pre-existing CCPs appear to do so in a cooperative manner, producing a receptor-enriched CCP subset, and can influence the kinetics of subsequent CCP maturation events. This appears to be a means by which some 7TMRs, including beta-adrenergic catecholamine receptors (Puthenveedu and von Zastrow, 2006) and mu-opioid neuropeptide receptors (Henry et al., 2012) locally modify the properties of their enclosing CCP after the fact. 7TMR clustering in previously formed CCPs has been directly demonstrated in neurons (Yu et al., 2010) but subsequent ‘customization’ of CCP dynamics by locally accumulated 7TMRs has been shown only in non-neural cell models, and its functional significance remains largely unexplored in any system.
A number of 7TMRs undergo activation-induced internalization in vivo, but much remains to be learned about the conditions under which this occurs. Mu opioid neuropeptide receptors, for example, have long been known to internalize robustly throughout the brain and in spinal cord neurons following administration of certain opioid agonist drugs (Sternini et al., 1996; Keith et al., 1998; Trafton et al., 2000; Haberstock-Debic et al., 2003), but internalization elicited by endogenous neuropeptide release occurs to a smaller degree (Trafton et al., 2000) or is limited to particular brain regions (Mills et al., 2004). Regulated endocytosis of endogenous D1 dopaminergic catecholamine receptors has been clearly documented in the striatum following administration of direct or indirect agonist drugs (Berthet et al., 2009; Dumartin et al., 1998), occurs in primates in some pathological conditions (Guigoni et al., 2007), and in transporter-mutant mice exhibiting abnormally elevated extracellular dopamine concentration (Dumartin et al., 2000). However, it remains unclear to what degree D1 receptor endocytosis occurs in any brain region in response to truly physiological levels of neuromodulator. Based on present mechanistic understanding, efficient endocytosis of 7TMRs requires and is effectively driven by receptor occupancy by agonist, suggesting that differences observed in vivo correspond to quantitative differences in overall receptor occupancy produced by endogenously released neuromodulator relative to drugs. Supporting this idea, 7TMR internalization observed in vivo has been used successfully as a proxy for estimating local 7TMR activation both by endogenous ligands and drugs (Trafton et al., 2000; Mantyh et al., 1995).
An interesting related question is whether drugs can produce different regulatory effects than endogenous neuromodulators after binding to the same 7TMRs. Naturally produced neuromodulators typically stimulate endocytosis of cognate 7TMRs after promoting receptor activation, at least when examined in cultured cell models. However, some drugs that activate the same receptors differ significantly in endocytic effects, even when applied at high concentration in such a controlled system. For example, opioid neuropeptides stimulate rapid endocytosis of mu opioid receptors whereas some non-peptide drugs, such as morphine, do so considerably less strongly (Keith et al., 1996; Sternini et al., 1996; Keith et al., 1998). A predictor of the endocytic activity of a particular drug is its relative agonist efficacy as estimated through traditional pharmacological analysis, with more efficacious agonists stimulating endocytosis generally more strongly than less efficacious drugs (McPherson et al., 2010). The precise nature of this relationship remains unclear, however, and this question goes to the larger issue of whether drugs can produce different effects on neuromodulatory processes than endogenous ligands. Early studies proposed the existence of drug-specific receptor conformational states (Von Zastrow et al., 1993; Keith et al., 1996), and subsequent studies support the hypothesis that opioid ligand effects are not adequately described by a single ‘dimension’ of agonist activity (Whistler et al., 1999; Borgland et al., 2003; Von Zastrow et al., 1993; Pradhan et al., 2010; Arttamangkul et al., 2006a). This concept remains controversial, however, particularly with regard to understanding the effects of morphine (McPherson et al., 2010; Molinari et al., 2010). Nevertheless, the general idea that some drugs promote regulated endocytosis of opioid receptors out of proportion to conventional estimates of relative agonist activity is increasingly recognized (Rivero et al., 2012).
Recent mechanistic data provide independent support for this concept because opioid receptor engagement with arrestins and subsequent clustering in CCPs, key initiating events affecting the rate of agonist-induced endocytosis, require multi-site phosphorylation of the receptor’s cytoplasmic tail. Detailed analysis of discrete phosphorylated receptor forms generated in intact cells, by quantitative mass spectrometry applied to isotope-labeled cells, indicates that this multi-site requirement renders endocytosis inherently non-linear with respect to receptor activation (Lau et al., 2011). This principle for generating non-linearity by multi-phosphorylation is reminiscent of how multi-phosphorylation can produce ‘ultrasensitive’ responses in other biological contexts (Nash et al., 2001; Ferrell, 1996), and is a particularly attractive strategy for integral membrane proteins such as 7TMRs because significant non-linearity can occur even in the presence of an excess local concentration of kinase (Dushek et al., 2011). Accordingly, non-linear control by multi-site phosphorylation may underlie how apparently complex differences in the regulatory effects of drugs - variously described in terms of ‘functional selectivity’, ‘multidimensional’ efficacy or ‘agonist bias’ - are manifest at the cellular level.
7TMR membrane trafficking after endocytosis
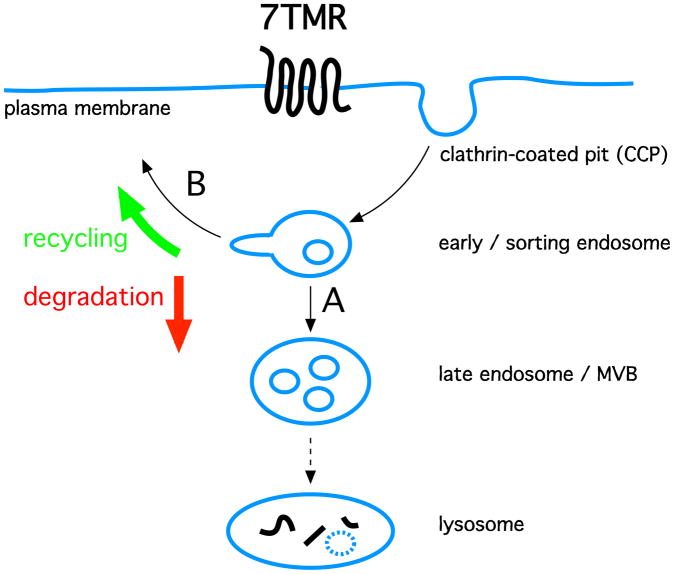
One function of 7TMR endocytosis is to initiate a multi-step trafficking pathway mediating receptor delivery to lysosomes, a proteolytic organelle in which many 7TMRs are destroyed (Fig. 1A). When a sufficient fraction of the overall cellular receptor pool is depleted through this pathway, as can occur under conditions of prolonged or repeated ligand-induced activation, cellular signaling responsiveness to neuromodulator is attenuated or ‘down-regulated’ (Tsao et al., 2001). Endocytic down-regulation of delta opioid neuropeptide receptors by delivery to lysosomes, first recognized in cultured neuroblastoma cells (Law et al., 1984), has been directly shown in vivo and correlated with development of physiological tolerance to opioid drugs (Pradhan et al., 2009; Scherrer et al., 2006).
Figure 1. Simplified outline of divergent 7TMR endocytic membrane traffic.
Key membrane compartments defining the endocytic pathway are indicated in blue, and black arrows indicate main routes of endocytic membrane traffic between compartments. Many 7TMRs (indicated as serpentine black line) enter the endocytic pathway after ligand-induced activation by clustering in CCPs. Molecular sorting operations occurring largely in early/sorting endosomes guide receptor trafficking itinerary between divergent routes mediating receptor delivery to late endosomes and then lysosomes (route A), or back to the plasma membrane (route B). This is a key decision because 7TMR trafficking to lysosomes results in proteolytic degradation (red arrow), while recycling returns receptors intact to the plasma membrane (green arrow).
Individual 7TMRs differ greatly in the efficiency with which they traffic to lysosomes after endocytosis, and this contributes to receptor-specific differences in endocytic regulation (Tsao and von Zastrow, 2000). Some 7TMRs appear to be remarkably stable after endocytosis. Radioligand binding assays of mu opioid receptors, for example, detect little down-regulation in most brain regions even after prolonged administration of agonist drugs (Sim-Selley et al., 2000; Yoburn et al., 1993). Instead, it is thought that the major trafficking itinerary of receptors after ligand-induced endocytosis is nondestructive recycling to the plasma membrane, which can occur repeatedly and efficiently under conditions of prolonged agonist exposure (Tanowitz and von Zastrow, 2003) (Fig. 1B). 7TMR recycling has long been recognized to be one means for supporting the ability of cells to sustain cellular responsiveness to a neuromodulator, or for achieving efficient recovery of responsiveness after a period of functional desensitization (Gainetdinov et al., 2004). An important caveat is that most studies investigating the functional consequences of 7TMR recycling are limited to cultured cell systems. However, the rapid recycling pathway traversed by adrenergic catecholamine receptors is essential for maintaining physiological catecholamine responsiveness of the heart (Odley et al., 2004). Conversely, disrupting the ability of mu opioid receptors to recycle efficiently in vivo produces enhanced physiological tolerance to the antinociceptive effects of opioids (Enquist et al., 2011).
Differences in the endocytic trafficking fate of otherwise similar 7TMRs can confer essentially opposite functional effects on longer-term cellular signaling responsiveness (Cao et al., 1999; Tanowitz and von Zastrow, 2003). In principle, discrete endocytic fates could be mediated by altogether different endocytic mechanisms or by molecular sorting of receptors after endocytosis. The former possibility has not been fully ruled out, and may apply to the regulation of some 7TMRs. However, there is compelling evidence that opioid and catecholamine receptors are subject to exquisitely selective molecular sorting after endocytosis by a shared, CCP-dependent early endocytic pathway (Tsao and von Zastrow, 2000; Puthenveedu et al., 2010). The following discussion will focus on such ‘post-endocytic’ sorting of 7TMRs, and the recycling-versus-degradation decision as a relatively extensively studied example.
7TMR sorting to lysosomes
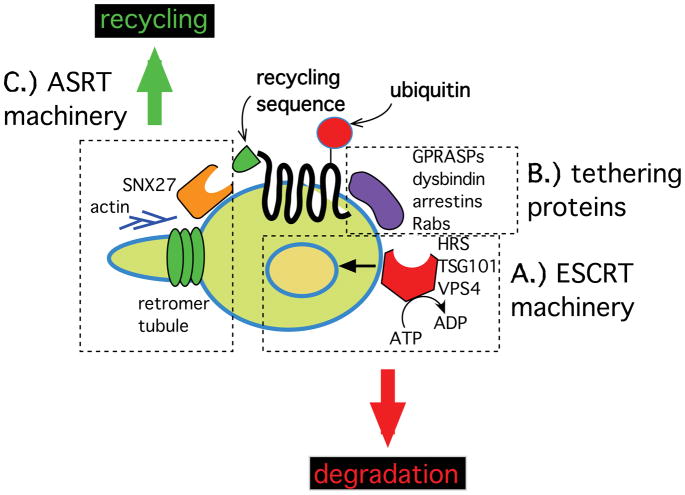
Many signaling receptors require ubiquitylation for endocytic delivery to lysosomes, and recycle efficiently to the plasma membrane when their ubiquitylation is prevented (Raiborg and Stenmark, 2009; Eden et al., 2012). Ubiquitin-directed sorting is mediated by a complex endosome-associated machinery, extensively conserved from yeast to humans, which is collectively called the endosomal sorting complex required for transport (ESCRT, Fig. 2A). A great deal is presently known about ESCRT structure and function, as discussed in excellent recent reviews (Hurley and Hanson, 2010; Henne et al., 2011; Raiborg and Stenmark, 2009). In brief, the ESCRT machinery associates with the endosome limiting membrane and functions both to 1) generate intralumenal membrane vesicles within late endosomes/multivesicular bodies, and 2) capture ubiquitylated membrane proteins after endocytosis and direct their selective packaging into intralumenal vesicles. Together these events prevent internalized receptors from recycling to the plasma membrane and promote the subsequent delivery of ubiquitin-marked receptors to lysosomes. Ubiquitin-directed sorting has been extensively demonstrated in mammalian cells for the EGF receptor tyrosine kinase (Raiborg and Stenmark, 2009; Eden et al., 2012) and there is increasing evidence for ubiquitin-directed sorting of various 7TMRs, as reviewed elsewhere (Marchese et al., 2008; Shenoy, 2007a; Kirkin and Dikic, 2007). In some cases specific ubiquitin ligases and hydrolases controlling 7TMR endocytic trafficking have been identified, as previously reviewed elsewhere (Hislop and von Zastrow, 2011; Marchese et al., 2008; Shenoy, 2007b), but the available information on this topic is presently limited to studies of 7TMR regulation in non-neural cell types.
Figure 2. Multiple sorting machineries associated with the endosome limiting membrane cooperate in determining the post-endocytic fate of 7TMRs.
Ubiquitin attached to the cytoplasmic surface of internalized 7TMRs (red circle) can engage the ‘endosomal sorting complex required for transport’ (ESCRT, box A), comprised of a set of multi-protein complexes including HRS and TSG101 that directly bind ubiquitinated receptors, and VPS4 that mediates ATP-dependent disassembly of the ESCRT complex and is required for efficient formation of membrane vesicles within the endosome lumen (blue circle within endosome). ESCRT-dependent transfer of ubiquitinated receptors from the endosome limiting membrane to intralumenal vesicles effectively prevents receptors from entering the recycling pathway, and enhances the accessibility of receptors to proteases following fusion of 7TMR-containing endocytic vesicles with lysosomes. A variety of proteins are proposed to interact with internalized 7TMRs in the endosome limiting membrane, effectively tethering them away from traversing the recycling pathway with bulk membrane flux (box B). Recycling sequences present in the 7TMR cytoplasmic surface (green cone) engage a discrete machinery associated with the endosome limiting membrane (box C), which prevents receptors from trafficking to lysosomes and actively promotes their recycling via membrane tubules that pinch off from endosomes and later fuse with the plasma membrane. Main components of the ‘actin-sorting nexin 27- retromer’ (ASRT) complex that mediates PDZ motif-directed recycling of 7TMRs such as beta-adrenergic receptors is diagrammed.
Some neuromodulatory 7TMRs do not require ubiquitylation to undergo efficient endocytic delivery to lysosomes, and there is evidence for additional machinery directing this process. For example, internalized delta opioid receptors can be effectively excluded from the recycling pathway and delivered to lysosomes even when their ubiquitylation is prevented by mutation of all cytoplasmic lysine residues (Tanowitz and Von Zastrow, 2002). Receptor ubiquitylation enhances but is not required for opioid receptor localization to intralumenal vesicles, and receptors can be delivered to lysosomes for inactivating proteolytic fragmentation even when transfer to intralumenal vesicles is blocked (Hislop et al., 2009; Henry et al., 2011). Nevertheless, irrespective of whether or not ubiquitylation of receptors is allowed to occur, the overall process of delta opioid receptor degradation requires the main components of the ESCRT machinery (Hislop et al., 2004). Accordingly, the present data suggest that discrete ubiquitin -independent and -dependent sorting mechanisms operate in series in the conserved ESCRT-dependent MVB pathway, with the ubiquitylation-independent mechanism operating effectively upstream and having the ability to effectively ‘force’ internalized receptors to traffic to lysosomes even when their ubiquitylation is prevented (Henry et al., 2011). Evidence for ubiquitylation-independent sorting of internalized 7TMRs to lysosomes, as for the ubiquitin-directed sorting mechanism discussed above, is presently limited primarily to studies of non-neural cell types.
The biochemical basis for ubiquitylation-independent lysosomal delivery of 7TMRs remains poorly understood in any system. One possibility is that internalized receptors are guided to lysosomes simply through ‘piggybacking’ on a ubiquitin-directed cargo, as proposed for ubiquitin-independent trafficking in yeast (MacDonald et al., 2012), but this seems unlikely for delta opioid receptors because of the above-noted differences in basic features of receptor down-regulation. Another possibility is that there exists additional machinery directing some 7TMRs to lysosomes (Fig. 2B). Early studies identified a cytoplasmic protein that binds the cytoplasmic tail of delta opioid receptors irrespective of ubiquitylation, and is highly expressed in brain (Whistler et al., 2002). Over-producing a C-terminal fragment in transfected fibroblastic cells inhibited ligand-induced down-regulation of co-expressed delta opioid receptors, leading to the suggestion that this protein represents a putative ‘G protein-coupled receptor-associated sorting protein’ (GASP). A family of related GASP proteins (now called GPRASPs) was subsequently identified, which are widely expressed in mammals but not in yeast (Abu-Helo and Simonin, 2010). Supporting the potential in vivo significance of this mechanism in neurons, genetic knockout of the originally identified GASP protein (GPRASP1) in mice blocked cocaine-induced down-regulation of D2 dopamine receptors in brain (Thompson et al., 2010). Independent biochemical studies suggested that GPRASPs provide alternate connectivity of internalized receptors to the ESCRT machinery (Marley and von Zastrow, 2010), potentially explaining enhanced recycling of D2 dopamine receptors observed in the cortex of dysbindin-knockout mice (Ji et al., 2009). The precise functional role(s) of GPRASPs remain unclear, however, and other studies have suggested distinct or additional roles in 7TMR sorting or signaling (Abu-Helo and Simonin, 2010). There is also evidence that additional protein interactions engaged by 7TMRs, including with conventional beta-arrestins as well as so-called alpha-arrestins that are thought to share structural features, may prevent internalized 7TMRs from exiting endosomes or provide alternate connectivity of receptors to the ubiquitylation/ESCRT machinery (Shenoy et al., 2009; Nabhan et al., 2010). Moreover Rab-family small GTP binding proteins, long known to be master regulators of both the biosynthetic and endocytic pathways, have been observed to affect the endocytic sorting of particular 7TMRs through direct interaction (Seachrist and Ferguson, 2003; Esseltine et al., 2011). Endocytic trafficking effects have been reported for direct 7TMR interaction with several Rab family members (Rabs 4, 8 and 11) but, to our knowledge, all of the evidence regarding a discrete tethering function of Rabs is presently limited to 7TMR trafficking in non-neural cells.
7TMR recycling to the plasma membrane
Another clue to the existence of additional, ubiquitylation-independent endocytic sorting machinery relevant to neuromodulatory 7TMR regulation is that efficient recycling of some 7TMRs requires a discrete cytoplasmic sorting determinant that can clearly operate irrespective of receptor ubiquitylation. Beta-adrenergic receptors, for example, require a PDZ domain-interacting sequence for efficient plasma membrane recycling in both fibroblasts (Cao et al., 1999) and neurons (Yudowski et al., 2006; Yu et al., 2010), and the sorting activity of this sequence does not require cytoplasmic lysine residues that represent potential sites of receptor ubiquitylation (Hanyaloglu and von Zastrow, 2007). A variety of such ‘recycling sequences’ have been identified in other 7TMRs, but not all are PDZ motifs. An interesting example is the mu opioid receptor, whose recycling is promoted by a discrete, PDZ-independent C-terminal sequence that is devoid of lysine residues and critically depends on two leucine residues separated by two other amino acids (L-x-x-L) (Yu et al., 2010; Tanowitz and von Zastrow, 2003). This system of endocytic fate determination confers additional regulation and diversity of 7TMR regulation. For example, phosphorylation of the PDZ motif present in the beta-adrenergic receptor tail blocks its recycling activity, and results in flexible re-routing of internalized beta-adrenergic receptors to the lysosomal down-regulation pathway (Cao et al., 1999). Alternative splicing of mu opioid receptor transcripts creates variant receptors that lack the ‘L-x-x-L’ recycling sequence, and thus preferentially down-regulate rather than recycle after endocytosis (Tanowitz et al., 2008). Both PDZ-dependent sequences derived from beta-adrenergic receptors and the discrete PDZ-independent sequence derived from mu opioid receptors have been explicitly shown to promote efficient sorting of internalized 7TMRs into the recycling pathway in neurons (Yu et al., 2010).
The biochemical machinery that mediates sequence-directed recycling has only recently begun to come into focus, based largely on study of PDZ motif-directed recycling of beta-adrenergic receptors (Fig. 2C). The critical trans-acting protein recognizing the 2C recycling sequence present in the adrenergic receptor cytoplasmic tail is sorting nexin 27 (SNX27) (Lauffer et al., 2010). Sorting nexins comprise a diverse family of cytoplasmic proteins that share a phosphoinositide-binding ‘SNX-PX’ domain linking them to endosome and/or plasma membranes, and members of the sorting nexin family are found in diverse organisms (Worby and Dixon, 2002; Carlton et al., 2005). SNX27, an early endosome-associating sorting nexin that is the only known family member to possess a PDZ domain, is restricted to metazoans. Depleting SNX27 inhibits recycling of both the beta-1 and beta-2 adrenergic receptors and increases receptor delivery to lysosomes, effectively phenocopying mutation of the respective C-terminal recycling sequences. SNX27 is highly expressed in neurons and its expression is subject to robust regulation by psychostimulant drugs (Kajii et al., 2003). Accordingly, mechanistic elucidation of the sequence-directed recycling machinery suggests the existence of still more flexibility in the control of neuromodulatory 7TMR trafficking in vivo.
SNX27 acts as an adaptor to deliver internalized adrenergic receptors to a multi-protein sorting machinery assembled at the base of tubular extensions of the endosome limiting membrane. These tubules were initially recognized in live cell imaging as sites associated with a dynamic Arp2/3 -dependent actin network, and from which internalized beta-adrenergic receptors exit endosomes for return to the plasma membrane (Puthenveedu et al., 2010). These tubules were then found to associate also with the retromer complex, a multi-protein complex previously known to function in endosome-to-Golgi delivery of selected membrane cargoes (Bonifacino and Hurley, 2008), and studies of adrenergic receptor recycling revealed an additional role of the retromer complex in supporting ‘direct’ endosome-to-plasma membrane delivery (Temkin et al., 2011). SNX27 appears to associate both with the actin polymerization machinery and with the retromer complex through an additional multi-protein complex, the WASH complex (Temkin et al., 2011), which regulates Arp2/3 -mediated actin nucleation and associates with the retromer complex at the base of endosome tubules (Gomez and Billadeau, 2009). Together, these findings led to the identification of an ‘actin-SNX27-retromer tubule’ (ASRT) interaction network, which represents a discrete sorting machinery directing specific 7TMRs from the endosome limiting membrane into the rapid recycling pathway (Fig. 2C). The range of endocytic cargoes that are sorted by the ASRT machinery remains to be determined, and ASRT function in neurons is only beginning to be explored. However PDZ motif-directed recycling clearly occurs in neurons, as noted above, and all known components of the ASRT machinery are highly expressed in brain.
Trans-acting sorting effects
The discussion up to now would suggest that 7TMRs are sorted completely independently of one another. While there is indeed remarkable specificity in the endocytic itinerary of even closely related 7TMRs, and this is apparent even when homologous receptors are co-expressed at supra-physiological levels, accumulating evidence points to the ability of some neuromodulatory 7TMRs to influence the trafficking properties of others in trans.
The most obvious source of trans-effects on 7TMR trafficking is through physical oligomerization of receptors. There is now abundant evidence that 7TMRs can form homotypic and heterotypic interactions, although the functional significance of oligomer formation remains unclear for many 7TMRs (Milligan and Bouvier, 2005). Briefly summarized, some 7TMRs (such as GABA-B and metabotropic glutamate receptors) assemble during or shortly after biosynthesis into a stable heterodimer that is essential for biological activity, and these core heterodimers may subsequently assemble into higher-order oligomers (Kniazeff et al., 2011). For other 7TMRs, and probably for the majority, oligomer formation is more variable and can occur transiently, with receptors maintaining functional competence as monomers (Whorton et al., 2007) and rapidly converting between monomeric and oligomeric forms (Hern et al., 2010; Kasai et al., 2011).
Much remains to be learned about biophysical and physiological aspects of 7TMR oligomer formation, but there has been evidence for many years supporting a role in receptor membrane traffic. Studies of the Ste2p mating pheromone 7TMR in yeast showed that an endocytic defect of a mutant Ste2p construct was rescued in trans by expression of wild type Ste2p, suggesting that one 7TMR can physically ‘drag’ another into the endocytic pathway by oligomer formation (Overton and Blumer, 2000). Similar trans-effects have been widely observed in the regulated endocytosis of mammalian 7TMRs, including for opioid neuropeptide receptors in native neurons (He et al., 2002), and there is evidence from study of non-neural cell models that oligomer formation can affect the regulatory trafficking of 7TMRs after endocytosis (Cao et al., 2005). Given extensive and growing evidence that 7TMRs can form oligomers, and that such interactions can affect endocytic trafficking, the ability of co-expressed receptors to sort in a receptor-specific manner is even more remarkable. An interesting question that remains unexplored is how 7TMR oligomerization is controlled to produce trans-effects on some trafficking decisions while allowing other trafficking decisions to occur independently.
A distinct type of 7TMR trans-regulation was discovered serendipitously in non-neural cells, based on the observation that simultaneous activation of the V2 vasopressin receptor can inhibit agonist-induced endocytosis of other co-expressed 7TMRs including adrenergic and opioid receptors (Klein et al., 2001). The mechanism turned out to involve V2 receptor-mediated sequestration of the available cellular pool of beta-arrestins to endosomes, based on persistent phosphorylation of receptors that renders their affinity for arrestins unusually high (Oakley et al., 2000). Verifying this, over-expressing beta-arrestins or mutating phosphorylation sites in the V2 receptor cytoplasmic tail to reduce arrestin binding blocked the trans-inhibition effect and effectively rescued agonist-induced endocytosis of the co-expressed 7TMRs (Klein et al., 2001). Subsequent studies established a similar mechanisms of trans-inhibition in native neurons expressing relevant neuromodulatory 7TMR combinations at endogenous levels: First, NK1 and NK3 neurokinin receptors in myenteric neurons (Schmidlin et al., 2002) and, second, NK1 and mu opioid receptors both in medium spiny neurons cultured from amygdala and in locus coeruleus neurons examined in an acute brain slice preparation (Yu et al., 2009). For both 7TMR pairs, endocytic inhibition was associated with impaired desensitization of a corresponding receptor-linked downstream signaling response. It remains to be determined how widespread this mechanism of trans-regulation is among neuromodulator receptors, and what functional consequences such trans-regulation produces in vivo. Based on first principles, one might expect such trans-regulation to be quite widespread, particularly in neuronal sub-compartments such as dendrites and axons where the locally available pool of arrestins is likely to be restricted.
Signaling consequences of 7TMR endocytosis
It is traditionally thought that 7TMR endocytosis regulates cellular responsiveness to prolonged or repeated exposure to neuromodulator (Fig. 1), and there is increasingly strong support for this hypothesis in vivo. Recent studies of delta opioid receptor regulation provide a clear example. A GFP-tagged delta opioid receptor, expressed at near-endogenous levels in mutant mice, exhibited agonist-induced endocytosis and was subsequently delivered to lysosomes in CNS-derived neurons (Scherrer et al., 2006). Interestingly, the occurrence of this trafficking process correlated temporally with the development of physiological tolerance to subsequent antinociceptive effects of the drug (Pradhan et al., 2009). A different agonist drug, which does not strongly promote receptor endocytosis, failed to elicit this component of physiological tolerance but both drugs elicited a slower form of tolerance, apparently through endocytosis-independent downstream adaptation(s) (Pradhan et al., 2010). These results, in addition to demonstrating a role of endocytic trafficking in attenuating physiological opioid responsiveness, elegantly illustrate the existence of discrete ‘layers’ of homeostatic control impacting tissue responsiveness to a neuromodulator over different time scales.
Other studies of opioid receptor regulation suggest still more complexity across receptors and systems. Agonist-induced endocytosis of an epitope-tagged mu opioid receptor, expressed at near-endogenous levels in the locus coeruleus of mutant mice, was visualized in acute brain slices by two-photon fluorescence microscopy. Rapid endocytosis of receptors occurred following application of several opioid agonists, but not after application of even high concentrations of morphine (Arttamangkul et al., 2008). However, morphine was able to produce desensitization of the acute signaling response. Further, previous studies from the same group showed that blocking endocytosis of endogenous mu opioid receptors did not impair enkephalin-induced desensitization of signaling, nor did it detectably affect recovery from desensitization after washout of the opioid peptide (Arttamangkul et al., 2006b). Thus it appears that receptor endocytosis is not essential for rapid functional desensitization or recovery from desensitization, even after receptor activation by an agonist that robustly promotes endocytosis over a similar time scale.
Interestingly, when animals were rendered opioid tolerant by repeated administration of morphine prior to preparation of the brain slice, rapid desensitization of the enkephalin-induced electrophysiological response still occurred but its recovery after agonist washout was inhibited (Quillinan et al., 2011). Chemical inhibition of GRK2, a kinase that can promote mu opioid receptor endocytosis by phosphorylating relevant residues in the receptor’s cytoplasmic tail, produced a rapid recovery from desensitization similar to that observed in brain slices prepared from drug-naive animals. These results suggest that regulated endocytosis of mu opioid receptors functions as a discrete, second mechanism of functional desensitization that is engaged under conditions of excessively prolonged neuromodulator receptor activation, such as that produced by chronic drug administration.
An intriguing additional observation made in the same study (Quillinan et al., 2011) was that the GRK2-dependent component of persistent desensitization occurred only in brain slices prepared from animals rendered opioid tolerant with morphine, but not from animals rendered tolerant with methadone. One possible explanation for this difference could be that methadone simply produced less tolerance in these experiments, but this was not evident by behavioral assessment. Another possibility is that the secondary form of opioid desensitization is drug-specific, engaged by morphine but not by drugs such as methadone that promote endocytosis of mu opioid receptors relatively robustly. If this is the case, further investigation could identify a rational basis for improving the therapeutic efficacy of opioid drugs. For example, one might expect pharmacological manipulations that increase receptor endocytosis to lessen the development of physiological tolerance after prolonged or repeated drug administration. Further, based on what is presently known about delta opioid receptor regulation in vivo, one might expect manipulations that increase lysosomal delivery of internalized receptors to enhance or accelerate the development of physiological tolerance.
Another interesting horizon in the relationship between neuromodulator receptor trafficking and function regards the effect of receptor endocytosis on the acute signaling response. As discussed above, it is generally believed that 7TMRs are functionally uncoupled from G proteins before endocytosis, and remain unable to elicit ‘classical’ G protein-linked signaling until after re-insertion to the plasma membrane by recycling. This view is being re-evaluated based on live cell imaging data indicating that agonist-induced endocytosis of some 7TMRs, such as the D1 dopaminergic catecholamine receptor, occurs remarkably rapidly and on a similar time scale as the acute biochemical signaling response. Further, experimental manipulations that impair D1 receptor endocytosis reduce the rate of initial cAMP accumulation detected in cells after acute agonist application, and also inhibit the ability of a D1-specific agonist to produce a cAMP-dependent enhancement of neuronal excitability in a brain slice preparation (Kotowski et al., 2011). The mechanistic basis for this endocytosis-dependent enhancement of acute D1 receptor-mediated signaling remains incompletely understood, but a simple hypothesis is that D1 receptors activate adenylyl cyclase both from the plasma membrane and endosomes (Fig. 3). This is plausible because both heterotrimeric G proteins and adenylyl cyclases have been detected on endosomes and there is evidence that endosomes may contribute to a non-canonical mechanism of prolonged 7TMR signaling (Calebiro et al., 2009; Vilardaga et al., 2012). However, it has not been directly determined whether or not internalized 7TMRs can indeed elicit a ‘conventional’ mode of acute G protein-linked signaling from the endosome membrane.
Figure 3. Proposed subcellular locations of D1 dopamine receptor-mediated signaling.
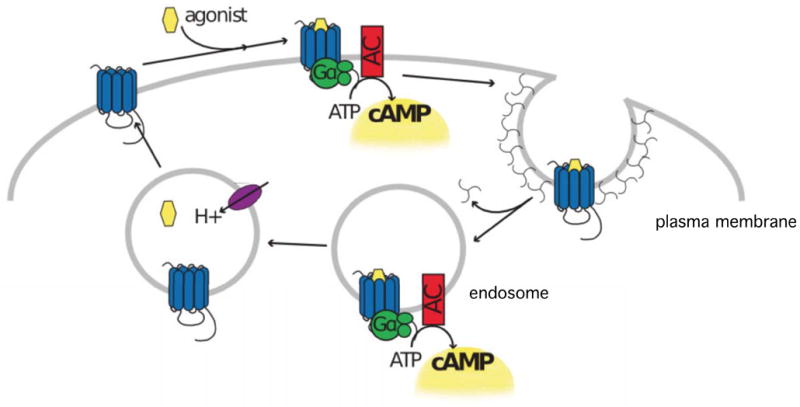
It has long been known that D1-type dopamine receptors (blue) mediate acute cAMP signaling through activating Gs/Golf heterotrimeric G proteins (green) in the plasma membrane (gray line, as indicated). Recent evidence, as discussed in the text, suggests that D1 receptors may also mediate acute G protein activation from endosomes, resulting in receptor-elicited cAMP production from both the plasma membrane and endosome membrane (yellow).
Conclusion and future perspectives
This review attempts to summarize the present understanding of mechanisms and functional consequences of endocytic membrane trafficking of neuromodulatory 7TMRs, focusing on catecholamine and opioid neuropeptide receptors as important and relatively well characterized examples. There has been significant recent progress in understanding molecular sorting operations that determine the membrane trafficking itinerary of these 7TMRs after entry to the endocytic pathway. Much remains unknown about the mechanistic basis of 7TMR sorting, particularly ubiquitylation-independent trafficking to lysosomes and the role of cytoskeletal dynamics in sequence-directed recycling, and little is known about the operation of any specific 7TMR sorting mechanism in neurons.
One particularly interesting area for future study concerns the organization of specific 7TMR trafficking mechanisms with respect to the highly differentiated and polarized architecture of neurons. There is already evidence for enhanced endocytosis of opioid receptors in dendrites following systemic administration of opioid drugs (Haberstock-Debic et al., 2003), and for reduced functional desensitization of various 7TMRs including opioid receptors in presynaptic relative to post-synaptic compartments (Wetherington and Lambert, 2002; Pennock et al., 2012). However, much remains to be learned about how 7TMR regulatory machineries are compartmentalized in neurons, and if there are differences in the regulated endocytic trafficking of receptors produced by local compared to global receptor activation. Related to this is the question of which membrane domain(s) are the source of physiologically salient 7TMR signaling. The traditional view is that G protein-linked signaling is restricted to the plasma membrane and based on rapid diffusion of downstream mediators. However, it is increasingly clear that even classical ‘diffusible’ mediators such as cAMP are spatially restricted through local synthesis and destruction (Willoughby et al., 2006), and neuromodulators such as opioid neuropeptides exhibit a limited range of action in neural tissue (Banghart and Sabatini, 2012). Accordingly, the precise subcellular location of 7TMR activation is likely to be an important parameter in neuromodulation, particularly for projection neurons and neurons with extensive dendritic arbors. Better understanding the compartmentation of 7TMR trafficking and signaling will surely add to our understanding of the basic physiology of endogenous neuromodulation, and may provide useful insight to how systemically administered drugs differentially impact the function of neural circuits.
Additional questions arise from the observation that some 7TMRs affect one another’s endocytic regulation in trans, either by direct physical interaction or through alternative mechanisms such as depletion of the local pool of functional arrestin. We are only at the beginning of investigating the functional consequences of such trans-regulatory effects in vivo. Whereas mechanistic studies of 7TMR biology generally investigate the effects of activating a single 7TMR or receptor class in isolation, it is increasingly recognized that CNS neurons typically co-express multiple distinct types of neuromodulatory 7TMR (Bartfai et al., 2012). Thus trans-regulatory effects on 7TMR trafficking might be a widespread but previously overlooked phenomenon in vivo, with potentially major implications both to physiology and for understanding drug effects on neuromodulation.
It is clear that endocytic membrane trafficking of endogenous neuromodulatory 7TMRs occurs after exogenous administration of agonist drugs and in some pathological states, but it remains largely unresolved whether endocytic trafficking of 7TMRs also represents a significant regulatory process under conditions of normal physiological activation by endogenous neuromodulators. Future investigation of this question could provide important insight to the pathological basis of neuropsychiatric disease and guide the search for improved therapeutics to manage complex disorders, such as mood and anxiety syndromes, in which disturbed neuromodulatory tone is a prominent feature.
Acknowledgments
The authors thank members of their laboratories and numerous other colleagues for valuable contributions, suggestions and critical discussion. Work in the authors’ laboratories is supported by the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abu-Helo A, Simonin F. Identification and biological significance of G protein-coupled receptor associated sorting proteins (GASPs) Pharmacology & therapeutics. 2010;126:244–250. doi: 10.1016/j.pharmthera.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Arttamangkul S, Quillinan N, Low MJ, von Zastrow M, Pintar J, Williams JT. Differential activation and trafficking of micro-opioid receptors in brain slices. Molecular pharmacology. 2008;74:972–979. doi: 10.1124/mol.108.048512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arttamangkul S, Torrecilla M, Kobayashi K, Okano H, Williams JT. Separation of mu-opioid receptor desensitization and internalization: endogenous receptors in primary neuronal cultures. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006a;26:4118–4125. doi: 10.1523/JNEUROSCI.0303-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arttamangkul S, Torrecilla M, Kobayashi K, Okano H, Williams JT. Separation of mu-opioid receptor desensitization and internalization: endogenous receptors in primary neuronal cultures. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006b;26:4118–4125. doi: 10.1523/JNEUROSCI.0303-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banghart MR, Sabatini BL. Photoactivatable neuropeptides for spatiotemporally precise delivery of opioids in neural tissue. Neuron. 2012;73:249–259. doi: 10.1016/j.neuron.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartfai T, Buckley PT, Eberwine J. Drug targets: single-cell transcriptomics hastens unbiased discovery. Trends in pharmacological sciences. 2012;33:9–16. doi: 10.1016/j.tips.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthet A, Porras G, Doudnikoff E, Stark H, Cador M, Bezard E, Bloch B. Pharmacological analysis demonstrates dramatic alteration of D1 dopamine receptor neuronal distribution in the rat analog of L-DOPA-induced dyskinesia. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:4829–4835. doi: 10.1523/JNEUROSCI.5884-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Hurley JH. Retromer. Current opinion in cell biology. 2008;20:427–436. doi: 10.1016/j.ceb.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Connor M, Osborne PB, Furness JB, Christie MJ. Opioid agonists have different efficacy profiles for G protein activation, rapid desensitization, and endocytosis of mu-opioid receptors. The Journal of biological chemistry. 2003;278:18776–18784. doi: 10.1074/jbc.M300525200. [DOI] [PubMed] [Google Scholar]
- Calebiro D, Nikolaev VO, Gagliani MC, de Filippis T, Dees C, Tacchetti C, Persani L, Lohse MJ. Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS biology. 2009;7:e1000172. doi: 10.1371/journal.pbio.1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao TT, Brelot A, von Zastrow M. The composition of the beta-2 adrenergic receptor oligomer affects its membrane trafficking after ligand-induced endocytosis. Molecular pharmacology. 2005;67:288–297. doi: 10.1124/mol.104.003608. [DOI] [PubMed] [Google Scholar]
- Cao TT, Deacon HW, Reczek D, Bretscher A, von Zastrow M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the beta2-adrenergic receptor. Nature. 1999;401:286–290. doi: 10.1038/45816. [DOI] [PubMed] [Google Scholar]
- Carlton J, Bujny M, Rutherford A, Cullen P. Sorting nexins--unifying trends and new perspectives. Traffic (Copenhagen, Denmark) 2005;6:75–82. doi: 10.1111/j.1600-0854.2005.00260.x. [DOI] [PubMed] [Google Scholar]
- Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- Dumartin B, Caillé I, Gonon F, Bloch B. Internalization of D1 dopamine receptor in striatal neurons in vivo as evidence of activation by dopamine agonists. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1998;18:1650–1661. doi: 10.1523/JNEUROSCI.18-05-01650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumartin B, Jaber M, Gonon F, Caron MG, Giros B, Bloch B. Dopamine tone regulates D1 receptor trafficking and delivery in striatal neurons in dopamine transporter-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:1879–1884. doi: 10.1073/pnas.97.4.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dushek O, van der Merwe PA, Shahrezaei V. Ultrasensitivity in multisite phosphorylation of membrane-anchored proteins. Biophysical journal. 2011;100:1189–1197. doi: 10.1016/j.bpj.2011.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden ER, Huang F, Sorkin A, Futter CE. The role of EGF receptor ubiquitination in regulating its intracellular traffic. Traffic (Copenhagen, Denmark) 2012;13:329–337. doi: 10.1111/j.1600-0854.2011.01305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enquist J, Kim JA, Bartlett S, Ferwerda M, Whistler JL. A novel knock-in mouse reveals mechanistically distinct forms of morphine tolerance. The Journal of pharmacology and experimental therapeutics. 2011;338:633–640. doi: 10.1124/jpet.111.179754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esseltine JL, Dale LB, Ferguson SSG. Rab GTPases bind at a common site within the angiotensin II type I receptor carboxyl-terminal tail: evidence that Rab4 regulates receptor phosphorylation, desensitization, and resensitization. Molecular pharmacology. 2011;79:175–184. doi: 10.1124/mol.110.068379. [DOI] [PubMed] [Google Scholar]
- Ferrell JE. Tripping the switch fantastic: how a protein kinase cascade can convert graded inputs into switch-like outputs. Trends in biochemical sciences. 1996;21:460–466. doi: 10.1016/s0968-0004(96)20026-x. [DOI] [PubMed] [Google Scholar]
- Gaidarov I, Krupnick JG, Falck JR, Benovic JL, Keen JH. Arrestin function in G protein-coupled receptor endocytosis requires phosphoinositide binding. The EMBO journal. 1999;18:871–881. doi: 10.1093/emboj/18.4.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Desensitization of G protein-coupled receptors and neuronal functions. Annual review of neuroscience. 2004;27:107–144. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- Gomez TS, Billadeau DD. A FAM21-containing WASH complex regulates retromer-dependent sorting. Developmental cell. 2009;17:699–711. doi: 10.1016/j.devcel.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman OB, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- Goodman OB, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. Role of arrestins in G-protein-coupled receptor endocytosis. Advances in pharmacology (San Diego, Calif) 1998;42:429–433. doi: 10.1016/s1054-3589(08)60780-2. [DOI] [PubMed] [Google Scholar]
- Guigoni C, Doudnikoff E, Li Q, Bloch B, Bezard E. Altered D(1) dopamine receptor trafficking in parkinsonian and dyskinetic non-human primates. Neurobiology of disease. 2007;26:452–463. doi: 10.1016/j.nbd.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Haberstock-Debic H, Wein M, Barrot M, Colago EEO, Rahman Z, Neve RL, Pickel VM, Nestler EJ, von Zastrow M, Svingos AL. Morphine acutely regulates opioid receptor trafficking selectively in dendrites of nucleus accumbens neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:4324–4332. doi: 10.1523/JNEUROSCI.23-10-04324.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanyaloglu AC, von Zastrow M. A novel sorting sequence in the beta2-adrenergic receptor switches recycling from default to the Hrs-dependent mechanism. The Journal of biological chemistry. 2007;282:3095–3104. doi: 10.1074/jbc.M605398200. [DOI] [PubMed] [Google Scholar]
- He L, Fong J, von Zastrow M, Whistler JL. Regulation of opioid receptor trafficking and morphine tolerance by receptor oligomerization. Cell. 2002;108:271–282. doi: 10.1016/s0092-8674(02)00613-x. [DOI] [PubMed] [Google Scholar]
- Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Developmental cell. 2011;21:77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Henry AG, Hislop JN, Grove J, Thorn K, Marsh M, von Zastrow M. Regulation of endocytic clathrin dynamics by cargo ubiquitination. Developmental cell. 2012;23:519–532. doi: 10.1016/j.devcel.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry AG, White IJ, Marsh M, von Zastrow M, Hislop JN. The role of ubiquitination in lysosomal trafficking of δ-opioid receptors. Traffic (Copenhagen, Denmark) 2011;12:170–184. doi: 10.1111/j.1600-0854.2010.01145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hern JA, Baig AH, Mashanov GI, Birdsall B, Corrie JET, Lazareno S, Molloy JE, Birdsall NJM. Formation and dissociation of M1 muscarinic receptor dimers seen by total internal reflection fluorescence imaging of single molecules. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:2693–2698. doi: 10.1073/pnas.0907915107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hislop JN, Henry AG, Marchese A, von Zastrow M. Ubiquitination regulates proteolytic processing of G protein-coupled receptors after their sorting to lysosomes. The Journal of biological chemistry. 2009;284:19361–19370. doi: 10.1074/jbc.M109.001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hislop JN, Marley A, Von Zastrow M. Role of mammalian vacuolar protein-sorting proteins in endocytic trafficking of a non-ubiquitinated G protein-coupled receptor to lysosomes. The Journal of biological chemistry. 2004;279:22522–22531. doi: 10.1074/jbc.M311062200. [DOI] [PubMed] [Google Scholar]
- Hislop JN, von Zastrow M. Role of ubiquitination in endocytic trafficking of G-protein-coupled receptors. Traffic (Copenhagen, Denmark) 2011;12:137–148. doi: 10.1111/j.1600-0854.2010.01121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley JH, Hanson PI. Membrane budding and scission by the ESCRT machinery: it’s all in the neck. Nature reviews Molecular cell biology. 2010;11:556–566. doi: 10.1038/nrm2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean-Alphonse F, Hanyaloglu AC. Regulation of GPCR signal networks via membrane trafficking. Molecular and cellular endocrinology. 2011;331:205–214. doi: 10.1016/j.mce.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Ji Y, Yang F, Papaleo F, Wang H-X, Gao W-J, Weinberger DR, Lu B. Role of dysbindin in dopamine receptor trafficking and cortical GABA function. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:19593–19598. doi: 10.1073/pnas.0904289106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajii Y, Muraoka S, Hiraoka S, Fujiyama K, Umino A, Nishikawa T. A developmentally regulated and psychostimulant-inducible novel rat gene mrt1 encoding PDZ-PX proteins isolated in the neocortex. Molecular psychiatry. 2003;8:434–444. doi: 10.1038/sj.mp.4001258. [DOI] [PubMed] [Google Scholar]
- Kang DS, Kern RC, Puthenveedu MA, von Zastrow M, Williams JC, Benovic JL. Structure of an arrestin2-clathrin complex reveals a novel clathrin binding domain that modulates receptor trafficking. The Journal of biological chemistry. 2009;284:29860–29872. doi: 10.1074/jbc.M109.023366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai RS, Suzuki KGN, Prossnitz ER, Koyama-Honda I, Nakada C, Fujiwara TK, Kusumi A. Full characterization of GPCR monomer-dimer dynamic equilibrium by single molecule imaging. The Journal of cell biology. 2011;192:463–480. doi: 10.1083/jcb.201009128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith DE, Anton B, Murray SR, Zaki PA, Chu PC, Lissin DV, Monteillet-Agius G, Stewart PL, Evans CJ, von Zastrow M. mu-Opioid receptor internalization: opiate drugs have differential effects on a conserved endocytic mechanism in vitro and in the mammalian brain. Molecular pharmacology. 1998;53:377–384. [PubMed] [Google Scholar]
- Keith DE, Murray SR, Zaki PA, Chu PC, Lissin DV, Kang L, Evans CJ, von Zastrow M. Morphine activates opioid receptors without causing their rapid internalization. The Journal of biological chemistry. 1996;271:19021–19024. doi: 10.1074/jbc.271.32.19021. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Principles: receptor theory in pharmacology. Trends in pharmacological sciences. 2004;25:186–192. doi: 10.1016/j.tips.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Kirkin V, Dikic I. Role of ubiquitin- and Ubl-binding proteins in cell signaling. Current opinion in cell biology. 2007;19:199–205. doi: 10.1016/j.ceb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Klein U, Müller C, Chu P, Birnbaumer M, von Zastrow M. Heterologous inhibition of G protein-coupled receptor endocytosis mediated by receptor-specific trafficking of beta-arrestins. The Journal of biological chemistry. 2001;276:17442–17447. doi: 10.1074/jbc.M009214200. [DOI] [PubMed] [Google Scholar]
- Kniazeff J, Prézeau L, Rondard P, Pin J-P, Goudet C. Dimers and beyond: The functional puzzles of class C GPCRs. Pharmacology & therapeutics. 2011;130:9–25. doi: 10.1016/j.pharmthera.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Kotowski SJ, Hopf FW, Seif T, Bonci A, von Zastrow M. Endocytosis promotes rapid dopaminergic signaling. Neuron. 2011;71:278–290. doi: 10.1016/j.neuron.2011.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupnick JG, Santini F, Gagnon AW, Keen JH, Benovic JL. Modulation of the arrestin-clathrin interaction in cells. Characterization of beta-arrestin dominant-negative mutants. The Journal of biological chemistry. 1997;272:32507–32512. doi: 10.1074/jbc.272.51.32507. [DOI] [PubMed] [Google Scholar]
- Laporte SA, Oakley RH, Zhang J, Holt JA, Ferguson SS, Caron MG, Barak LS. The beta2-adrenergic receptor/betaarrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:3712–3717. doi: 10.1073/pnas.96.7.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau EK, Trester-Zedlitz M, Trinidad JC, Kotowski SJ, Krutchinsky AN, Burlingame AL, von Zastrow M. Quantitative encoding of the effect of a partial agonist on individual opioid receptors by multisite phosphorylation and threshold detection. Science signaling. 2011;4:ra52. doi: 10.1126/scisignal.2001748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffer BE, Melero C, Temkin P, Lei C, Hong W, Kortemme T, von Zastrow M. SNX27 mediates PDZ-directed sorting from endosomes to the plasma membrane. The Journal of cell biology. 2010;190:565–574. doi: 10.1083/jcb.201004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law PY, Hom DS, Loh HH. Down-regulation of opiate receptor in neuroblastoma x glioma NG108-15 hybrid cells. Chloroquine promotes accumulation of tritiated enkephalin in the lysosomes. The Journal of biological chemistry. 1984;259:4096–4104. [PubMed] [Google Scholar]
- MacDonald C, Stringer DK, Piper RC. Sna3 Is an Rsp5 Adaptor Protein that Relies on Ubiquitination for Its MVB Sorting. Traffic. 2012;13:586–598. doi: 10.1111/j.1600-0854.2011.01326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyh PW, DeMaster E, Malhotra A, Ghilardi JR, Rogers SD, Mantyh CR, Liu H, Basbaum AI, Vigna SR, Maggio JE. Receptor endocytosis and dendrite reshaping in spinal neurons after somatosensory stimulation. Science (New York, NY) 1995;268:1629–1632. doi: 10.1126/science.7539937. [DOI] [PubMed] [Google Scholar]
- Marchese A, Paing MM, Temple BRS, Trejo J. G protein-coupled receptor sorting to endosomes and lysosomes. Annual review of pharmacology and toxicology. 2008;48:601–629. doi: 10.1146/annurev.pharmtox.48.113006.094646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marley A, von Zastrow M. Dysbindin promotes the post-endocytic sorting of G protein-coupled receptors to lysosomes. PloS one. 2010;5:e9325. doi: 10.1371/journal.pone.0009325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nature reviews Molecular cell biology. 2011;12:517–533. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- McPherson J, Rivero G, Baptist M, Llorente J, Al-Sabah S, Krasel C, Dewey WL, Bailey CP, Rosethorne EM, Charlton SJ, et al. μ-opioid receptors: correlation of agonist efficacy for signalling with ability to activate internalization. Molecular pharmacology. 2010;78:756–766. doi: 10.1124/mol.110.066613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G, Bouvier M. Methods to monitor the quaternary structure of G protein-coupled receptors. The FEBS journal. 2005;272:2914–2925. doi: 10.1111/j.1742-4658.2005.04731.x. [DOI] [PubMed] [Google Scholar]
- Mills RH, Sohn RK, Micevych PE. Estrogen-induced mu-opioid receptor internalization in the medial preoptic nucleus is mediated via neuropeptide Y-Y1 receptor activation in the arcuate nucleus of female rats. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:947–955. doi: 10.1523/JNEUROSCI.1366-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari P, Vezzi V, Sbraccia M, Grò C, Riitano D, Ambrosio C, Casella I, Costa T. Morphine-like opiates selectively antagonize receptor-arrestin interactions. The Journal of biological chemistry. 2010;285:12522–12535. doi: 10.1074/jbc.M109.059410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabhan JF, Pan H, Lu Q. Arrestin domain-containing protein 3 recruits the NEDD4 E3 ligase to mediate ubiquitination of the beta2-adrenergic receptor. EMBO reports. 2010;11:605–611. doi: 10.1038/embor.2010.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash P, Tang X, Orlicky S, Chen Q, Gertler FB, Mendenhall MD, Sicheri F, Pawson T, Tyers M. Multisite phosphorylation of a CDK inhibitor sets a threshold for the onset of DNA replication. Nature. 2001;414:514–521. doi: 10.1038/35107009. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS. Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein-coupled receptors delineate two major classes of receptors. The Journal of biological chemistry. 2000;275:17201–17210. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- Odley A, Hahn HS, Lynch RA, Marreez Y, Osinska H, Robbins J, Dorn GW. Regulation of cardiac contractility by Rab4-modulated beta2-adrenergic receptor recycling. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:7082–7087. doi: 10.1073/pnas.0308335101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton MC, Blumer KJ. G-protein-coupled receptors function as oligomers in vivo. Current biology: CB. 2000;10:341–344. doi: 10.1016/s0960-9822(00)00386-9. [DOI] [PubMed] [Google Scholar]
- Pennock RL, Dicken MS, Hentges ST. Multiple Inhibitory G-Protein-Coupled Receptors Resist Acute Desensitization in the Presynaptic But Not Postsynaptic Compartments of Neurons. 2012;32:10192–10200. doi: 10.1523/JNEUROSCI.1227-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Becker JA, Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, Massotte D, Gavériaux-Ruff C, Kieffer BL. In vivo delta opioid receptor internalization controls behavioral effects of agonists. PLoS ONE. 2009;4:e5425. doi: 10.1371/journal.pone.0005425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Walwyn W, Nozaki C, Filliol D, Erbs E, Matifas A, Evans C, Kieffer BL. Ligand-directed trafficking of the δ-opioid receptor in vivo: two paths toward analgesic tolerance. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:16459–16468. doi: 10.1523/JNEUROSCI.3748-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthenveedu MA, Lauffer B, Temkin P, Vistein R, Carlton P, Thorn K, Taunton J, Weiner OD, Parton RG, von Zastrow M. Sequence-dependent sorting of recycling proteins by actin-stabilized endosomal microdomains. Cell. 2010;143:761–773. doi: 10.1016/j.cell.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthenveedu MA, von Zastrow M. Cargo regulates clathrin-coated pit dynamics. Cell. 2006;127:113–124. doi: 10.1016/j.cell.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Qanbar R, Bouvier M. Role of palmitoylation/depalmitoylation reactions in G-protein-coupled receptor function. Pharmacology & therapeutics. 2003;97:1–33. doi: 10.1016/s0163-7258(02)00300-5. [DOI] [PubMed] [Google Scholar]
- Quillinan N, Lau EK, Virk M, von Zastrow M, Williams JT. Recovery from mu-opioid receptor desensitization after chronic treatment with morphine and methadone. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:4434–4443. doi: 10.1523/JNEUROSCI.4874-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458:445–452. doi: 10.1038/nature07961. [DOI] [PubMed] [Google Scholar]
- Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nature reviews Drug discovery. 2010;9:373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero G, Llorente J, McPherson J, Cooke A, Mundell SJ, McArdle CA, Rosethorne EM, Charlton SJ, Krasel C, Bailey CP, et al. Endomorphin-2: A Biased Agonist at the μ-Opioid Receptor. Molecular pharmacology. 2012;82:178–188. doi: 10.1124/mol.112.078659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K, Pust S, Skotland T, van Deurs B. Clathrin-independent endocytosis: mechanisms and function. Current opinion in cell biology. 2011;23:413–420. doi: 10.1016/j.ceb.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Santini F, Penn RB, Gagnon AW, Benovic JL, Keen JH. Selective recruitment of arrestin-3 to clathrin coated pits upon stimulation of G protein-coupled receptors. Journal of cell science. 2000;113(Pt 1):2463–2470. doi: 10.1242/jcs.113.13.2463. [DOI] [PubMed] [Google Scholar]
- Scherrer G, Tryoen-Tóth P, Filliol D, Matifas A, Laustriat D, Cao YQ, Basbaum AI, Dierich A, Vonesh JL, Gavériaux-Ruff C, et al. Knockin mice expressing fluorescent delta-opioid receptors uncover G protein-coupled receptor dynamics in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9691–9696. doi: 10.1073/pnas.0603359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidlin F, Déry O, Bunnett NW, Grady EF. Heterologous regulation of trafficking and signaling of G protein-coupled receptors: beta-arrestin-dependent interactions between neurokinin receptors. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:3324–3329. doi: 10.1073/pnas.052161299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seachrist JL, Ferguson SSG. Regulation of G protein-coupled receptor endocytosis and trafficking by Rab GTPases. Life sciences. 2003;74:225–235. doi: 10.1016/j.lfs.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Shenoy SK. Seven-transmembrane receptors and ubiquitination. Circulation research. 2007a;100:1142–1154. doi: 10.1161/01.RES.0000261939.88744.5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SK. Seven-transmembrane receptors and ubiquitination. Circulation research. 2007b;100:1142–1154. doi: 10.1161/01.RES.0000261939.88744.5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SK, Modi AS, Shukla AK, Xiao K, Berthouze M, Ahn S, Wilkinson KD, Miller WE, Lefkowitz RJ. Beta-arrestin-dependent signaling and trafficking of 7-transmembrane receptors is reciprocally regulated by the deubiquitinase USP33 and the E3 ligase Mdm2. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:6650–6655. doi: 10.1073/pnas.0901083106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim-Selley LJ, Selley DE, Vogt LJ, Childers SR, Martin TJ. Chronic heroin self-administration desensitizes mu opioid receptor-activated G-proteins in specific regions of rat brain. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20:4555–4562. doi: 10.1523/JNEUROSCI.20-12-04555.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin A, von Zastrow M. Endocytosis and signalling: intertwining molecular networks. Nature reviews Molecular cell biology. 2009;10:609–622. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternini C, Spann M, Anton B, Keith DE, Bunnett NW, von Zastrow M, Evans C, Brecha NC. Agonist-selective endocytosis of mu opioid receptor by neurons in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:9241–9246. doi: 10.1073/pnas.93.17.9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanowitz M, Hislop JN, von Zastrow M. Alternative splicing determines the post-endocytic sorting fate of G-protein-coupled receptors. The Journal of biological chemistry. 2008;283:35614–35621. doi: 10.1074/jbc.M806588200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanowitz M, Von Zastrow M. Ubiquitination-independent trafficking of G protein-coupled receptors to lysosomes. The Journal of biological chemistry. 2002;277:50219–50222. doi: 10.1074/jbc.C200536200. [DOI] [PubMed] [Google Scholar]
- Tanowitz M, von Zastrow M. A novel endocytic recycling signal that distinguishes the membrane trafficking of naturally occurring opioid receptors. The Journal of biological chemistry. 2003;278:45978–45986. doi: 10.1074/jbc.M304504200. [DOI] [PubMed] [Google Scholar]
- Temkin P, Lauffer B, Jäger S, Cimermancic P, Krogan NJ, von Zastrow M. SNX27 mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signalling receptors. Nature cell biology. 2011;13:715–721. doi: 10.1038/ncb2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D, Martini L, Whistler JL. Altered ratio of D1 and D2 dopamine receptors in mouse striatum is associated with behavioral sensitization to cocaine. PloS one. 2010;5:e11038. doi: 10.1371/journal.pone.0011038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trafton JA, Abbadie C, Marek K, Basbaum AI. Postsynaptic signaling via the [mu]-opioid receptor: responses of dorsal horn neurons to exogenous opioids and noxious stimulation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20:8578–8584. doi: 10.1523/JNEUROSCI.20-23-08578.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao P, Cao T, von Zastrow M. Role of endocytosis in mediating downregulation of G-protein-coupled receptors. Trends in pharmacological sciences. 2001;22:91–96. doi: 10.1016/s0165-6147(00)01620-5. [DOI] [PubMed] [Google Scholar]
- Tsao PI, von Zastrow M. Diversity and specificity in the regulated endocytic membrane trafficking of G-protein-coupled receptors. Pharmacology & therapeutics. 2001;89:139–147. doi: 10.1016/s0163-7258(00)00107-8. [DOI] [PubMed] [Google Scholar]
- Tsao PI, von Zastrow M. Type-specific sorting of G protein-coupled receptors after endocytosis. The Journal of biological chemistry. 2000;275:11130–11140. doi: 10.1074/jbc.275.15.11130. [DOI] [PubMed] [Google Scholar]
- Vilardaga J-P, Gardella TJ, Wehbi VL, Feinstein TN. Non-canonical signaling of the PTH receptor. Trends in pharmacological sciences. 2012;33:423–431. doi: 10.1016/j.tips.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherington JP, Lambert NA. Differential desensitization of responses mediated by presynaptic and postsynaptic A1 adenosine receptors. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:1248–1255. doi: 10.1523/JNEUROSCI.22-04-01248.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistler JL, Chuang HH, Chu P, Jan LY, von Zastrow M. Functional dissociation of mu opioid receptor signaling and endocytosis: implications for the biology of opiate tolerance and addiction. Neuron. 1999;23:737–746. doi: 10.1016/s0896-6273(01)80032-5. [DOI] [PubMed] [Google Scholar]
- Whistler JL, Enquist J, Marley A, Fong J, Gladher F, Tsuruda P, Murray SR, von Zastrow M. Modulation of postendocytic sorting of G protein-coupled receptors. Science (New York, NY) 2002;297:615–620. doi: 10.1126/science.1073308. [DOI] [PubMed] [Google Scholar]
- Whorton MR, Bokoch MP, Rasmussen SGF, Huang B, Zare RN, Kobilka B, Sunahara RK. A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:7682–7687. doi: 10.1073/pnas.0611448104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby D, Wong W, Schaack J, Scott JD, Cooper DM. An anchored PKA and PDE4 complex regulates subplasmalemmal cAMP dynamics. The EMBO journal. 2006;25:2051–2061. doi: 10.1038/sj.emboj.7601113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe BL, Trejo J. Clathrin-dependent mechanisms of G protein-coupled receptor endocytosis. Traffic (Copenhagen, Denmark) 2007;8:462–470. doi: 10.1111/j.1600-0854.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- Worby CA, Dixon JE. Sorting out the cellular functions of sorting nexins. Nature reviews Molecular cell biology. 2002;3:919–931. doi: 10.1038/nrm974. [DOI] [PubMed] [Google Scholar]
- Yoburn B, Billings B, Duttaroy A. Opioid receptor regulation in mice. J Pharmacol Exp Ther. 1993;265:314–320. [PubMed] [Google Scholar]
- Yu YJ, Arttamangkul S, Evans CJ, Williams JT, von Zastrow M. Neurokinin 1 receptors regulate morphine-induced endocytosis and desensitization of mu-opioid receptors in CNS neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:222–233. doi: 10.1523/JNEUROSCI.4315-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YJ, Dhavan R, Chevalier MW, Yudowski GA, von Zastrow M. Rapid delivery of internalized signaling receptors to the somatodendritic surface by sequence-specific local insertion. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:11703–11714. doi: 10.1523/JNEUROSCI.6282-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudowski GA, Puthenveedu MA, von Zastrow M. Distinct modes of regulated receptor insertion to the somatodendritic plasma membrane. Nature neuroscience. 2006;9:622–627. doi: 10.1038/nn1679. [DOI] [PubMed] [Google Scholar]
- Von Zastrow M, Keith DE, Evans CJ. Agonist-induced state of the delta-opioid receptor that discriminates between opioid peptides and opiate alkaloids. Molecular pharmacology. 1993;44:166–172. [PubMed] [Google Scholar]