Abstract
Objective
To evaluate if two different measures of synovial activation, baseline Hoffa-synovitis and effusion-synovitis, assessed by MRI, predict cartilage loss in the tibiofemoral joint at 30 months follow-up in subjects with neither cartilage damage nor tibiofemoral radiographic osteoarthritis (OA) of the knee.
Methods
Non-contrast enhanced MRI was performed using proton density-weighted fat-suppressed sequences in the axial and sagittal planes and a STIR sequence in the coronal plane. Hoffa-synovitis, effusion-synovitis and cartilage status were assessed semiquantitatively according to the WORMS scoring system. Included were knees that had neither radiographic OA nor MRI-detected tibio-femoral cartilage damage at the baseline visit. Presence of Hoffa-synovitis was defined as any grade ≥2 (range from 0–3) and effusion-synovitis as any grade ≥2 (range from 0–3). We performed logistic regression to examine the relation of presence of either measure to the risk of cartilage loss at 30 months adjusting for other potential confounders of cartilage loss.
Results
Of 514 knees included in the analysis, prevalence of Hoffa-synovitis and effusion-synovitis at the baseline visit was 8.4% and 10.3%, respectively. In the multivariable analysis, baseline effusion-synovitis was associated with an increased risk for cartilage loss (odds ratio (OR) = 2.7, 95% confidence intervals 1.4–5.1, p=0.002); however, no such an association was observed for baseline Hoffa-synovitis (OR =1.0, 95% confidence intervals 0.5–2.0).
Conclusions
Baseline effusion-synovitis, but not Hoffa-synovitis, predicted cartilage loss. Our findings suggest that effusion-synovitis, a reflection of inflammatory activity including joint effusion and synovitic thickening, may play a role in future development of cartilage lesions in knees without OA.
Keywords: Osteoarthritis, magnetic resonance imaging, effusion, synovitis, cartilage loss
INTRODUCTION
During recent years it has been increasingly accepted that osteoarthritis (OA) is a complex disease involving multiple joint tissues including the synovium, the subchondral and cortical bone, the menisci, the ligaments and cartilage leading eventually to joint failure.[1–4] Synovitis is an important feature in the disease process and is defined as inflammation of the synovium. It may manifest itself phenotypically as thickening of the synovial membrane or indirectly as joint effusion as the result of synovial activation.[5, 6] The exact pathophysiological mechanisms that lead to synovial inflammation remain largely unknown, but activated synovial macrophages seem to play a key role in the inflammatory processes leading to synovial activation.[7] It has been suggested that synovitis in OA most likely is a secondary phenomenon due to phagocytosis of intraarticular debris, the release of soluble cartilage matrix macromolecules, the presence of calcium pyrophosphate dehydrate, or calcium hydroxyapatite crystals.[8–10] Accepted is that synovitis and effusion are frequently present in OA and correlate with pain and other clinical outcomes.[11, 12]
It also has been suggested that synovitis in OA predisposes to further structural progression.[13, 14] One study using arthroscopy as a reference standard demonstrated a positive correlation between the severity of synovitis and the degree of progression of cartilage lesions over time, particularly in the medial patello-femoral compartment.[15]
Synovial inflammation already occurs in early stages of the OA process.[16–19] However, as multiple tissues are involved in the disease initiation process it remains unclear if synovitis and effusion have to be regarded as secondary collateral phenomena or if these inflammatory processes play an important independent role in disease initiation and later progression. To overcome this dilemma it seems appealing to investigate joints without macroscopic cartilage damage prior the occurrence of apparent radiographic features of OA.[17]
Both synovial thickening and joint effusion may be assessed non-invasively by MRI.[4, 6, 13, 20] Ideally, synovitis in OA is evaluated using contrast-enhanced imaging after i.v. administration of gadolinium-based contrast agents.[6, 12, 21] However, due to possible side effects and associated costs, contrast-enhanced MRI is usually not part of large epidemiological or clinical OA studies. Instead, current methods to assess synovial activation on non-enhanced MRI in large studies use a surrogate of signal changes in Hoffa’s fat pad (‘Hoffa-synovitis’) as it had been shown in a histologic correlation study that these signal alterations represent mild chronic synovitis, which seems to be a sensitive, albeit non-specific measure when using contrast-enhanced MRI as the reference.[13, 18, 22] Further, intraarticular fluid-equivalent signal is evaluated according to the extent of capsular distention.[4, 20] Recent evidence showed that this measure reflects a composite of true joint effusion (i.e. joint fluid) and synovial thickening (‘effusion-synovitis’).[21] These MRI-based semi-quantitative measures of synovitis are associated with pain severity and similarly change in synovitis and effusion is associated with change in pain severity in both directions.[12, 13, 23] High grade synovitis assessed on non-enhanced MRI seems to play a role in structural deterioration.[13]
In order to analyze the possible link between early synovial activation and later structural deterioration we wished to assess if knees without tibio-femoral radiographic OA and without cartilage damage but with evidence of MRI-detected Hoffa-synovitis or effusion-synovitis (composite score of joint fluid and synovial thickening) are at increased risk of cartilage loss at 30 months follow-up when compared to knees without these baseline features of synovial activation.
PATIENTS AND METHODS
Study Design and Subjects
Subjects were participants in The Multicenter Osteoarthritis (MOST) Study, a prospective epidemiological study of 3,026 persons aged 50 to 79 years with a goal of identifying risk factors for incident and progressive knee OA in a sample either with OA or at high risk of developing disease. Participants at high risk included those who were overweight or obese, had knee pain, aching or stiffness on most of the last 30 days, or had a history of knee injury that made it difficult to walk for at least one week, or had previous knee surgery.
Subjects were recruited from two U.S. communities, Birmingham, Alabama and Iowa City, Iowa through mass mailing of letters and study brochures, supplemented by media and community outreach campaigns. The study protocol was approved by the Institutional Review Boards at the University of Iowa, University of Alabama, Birmingham, University of California, San Francisco and Boston University Medical Campus.
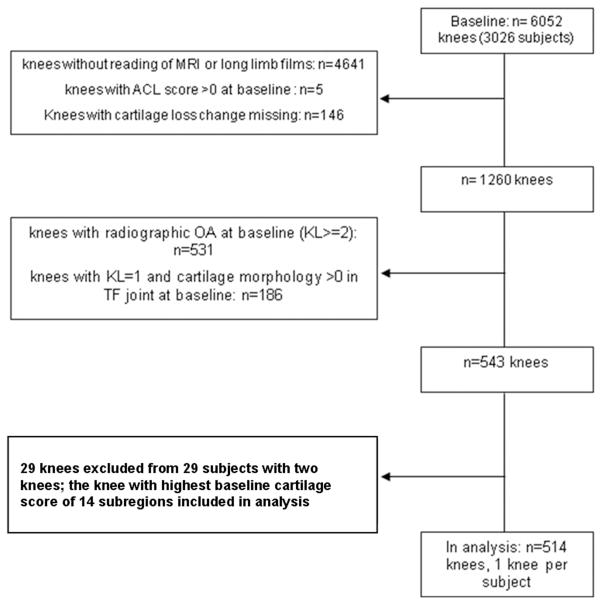
Subjects were excluded from MOST if they screened positive for rheumatoid arthritis [24], had ankylosing spondylitis, psoriatic arthritis, Reiter’s syndrome, had renal insufficiency that required hemo- or peritoneal dialysis, a history of cancer (except for non-melanoma skin cancer), had or planned to have bilateral knee replacement surgery, were unable to walk without assistance, or were planning to move out of the area in the next three years.[25] Subjects were further excluded if a diagnosis of rheumatoid arthritis or related diseases was established between the baseline and the follow-up visit. Figure 1 gives a detailed overview of the inclusion process for the present analysis.
Figure 1.
Flowchart of knee inclusion.
Radiographs
At baseline, all subjects underwent weight-bearing posteroanterior (PA) fixed flexion knee radiographs using the protocol by Peterfy et al. and a plexiglass positioning frame (SynaFlexer™).[26] A musculoskeletal radiologist and a rheumatologist experienced in reading study films, both blinded to case/control status and clinical data, graded all PA films according to the K/L scale. Radiographic tibiofemoral OA was considered present if K/L grade ≥2. If readers disagreed on the presence of radiographic OA, the film readings were adjudicated by a panel of 3 readers.
At the baseline clinic visit long-limb films were acquired with a 14-inch × 51-inch cassette. Mechanical alignment was measured as the angle formed by the intersection of the femoral and tibial mechanical axes. The femoral mechanical axis is the line from the center of the femoral head through the center of the knee, and the tibial mechanical axis is drawn as a line from the center of the ankle to the center of the knee. Neutral alignment was defined as 179–181 degrees, varus malalignment as ≤178 degrees and valgus malalignment as ≥182 degrees.
MRI Acquisition
MRIs were obtained in both knees with a 1.0T dedicated MR system (OrthOne™, ONI Medical Systems, Inc., Wilmington, MA) with a circumferential extremity coil using fat-suppressed fast spin echo PDw sequences in two planes, sagittal (TR 4800 ms, TE 35 ms, 3 mm slice thickness, 0 mm interslice gap, 32 slices, 288 × 192 matrix, 2 excitations (NEX), 140 mm2 field of view (FOV), echo train length (ETL) 8) and axial (TR 4680 ms, TE 13 ms, 3 mm slice thickness, 0 mm interslice gap, 20 slices, 288 × 192 matrix, 2 NEX, 140 mm2 FOV, ETL 8), and a STIR sequence in the coronal plane (TR 6650 ms, TE 15 ms, TI 100 ms, 3 mm slice thickness, 0 mm interslice gap, 28 slices, 256 × 192 matrix, 2 NEX, 140 mm2 FOV, ETL 8).
MRI Interpretation
Two musculoskeletal radiologists, blinded to clinical data, read BMLs and cartilage status according to the Whole-Organ Magnetic Resonance Imaging Score (WORMS) method.[4] Baseline and follow-up MRIs were read paired and with the chronological order known to the readers. MRI features were assessed simultaneously.
Signal alterations in the infrapatellar and intercondylar regions of Hoffa’s fat pad were scored from 0 to 3 as a surrogate for synovial thickening according to the literature as this feature is not part of the original WORMS system.[11, 13, 18] We will refer to these scores as ‘Hoffa-synovitis’ in the following sections, although acknowledging that these signal changes also include non-specific alterations not necessarily related to synovits.[22] (Figure 2A)
Figure 2.
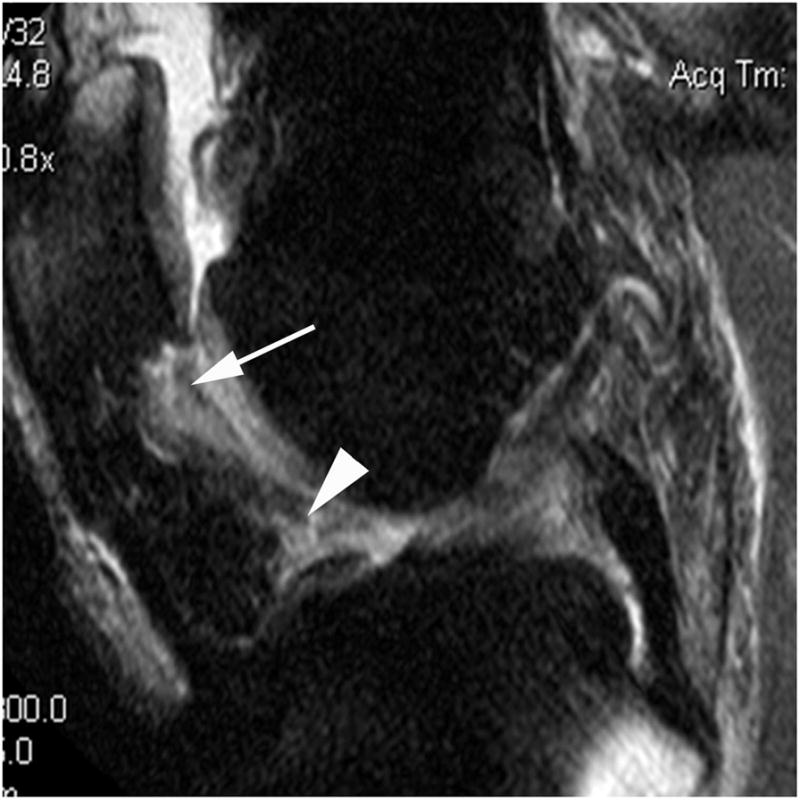
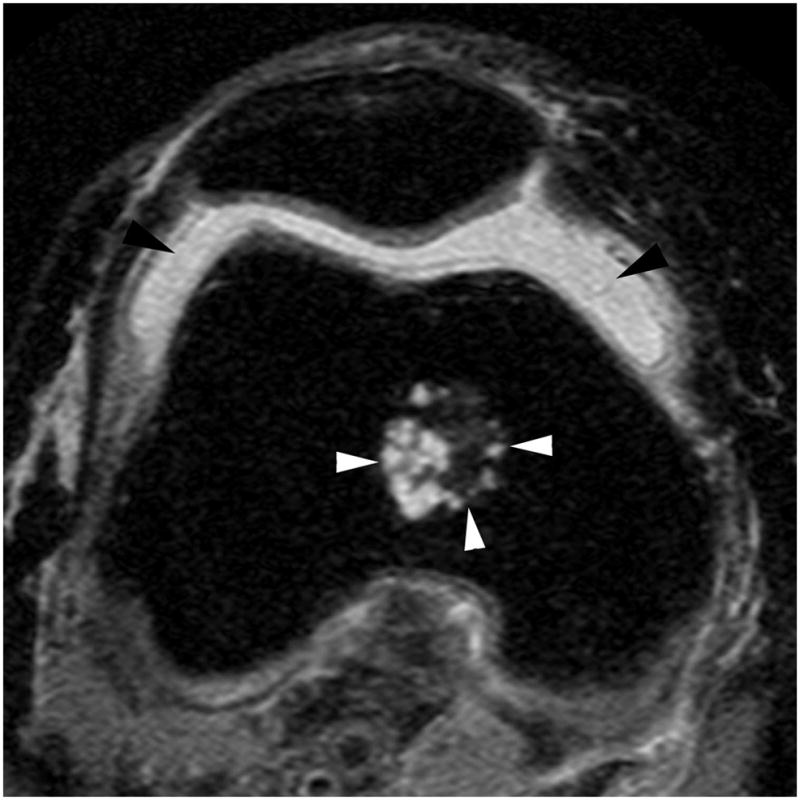
Figure 2A. Sagittal proton density-weighted MR image. Grade 2 Hoffa-synovitis is shown in the infrapatellar (arrow) and intercondylar (arrowhead) regions of Hoffa’s fat pad.
Figure 2B. Axial proton-density weighted MR image. Grade 2 joint effusion-synovitis is depicted as fluid-equivalent signal within the joint cavity (black arrowheads). Marked capsular distention is present. Note incidental finding of a metaphyseal enchondroma in the distal femur (white arrowheads).
WORMS uses a combined measure of joint effusion and synovitis based on the amount of intraarticular fluid-equivalent signal. This composite score is graded from 0 to 3 according to the estimated maximal distention of the synovial cavity. and was applied in addition to mentioned signal changes in Hoffa’s fat pad (Figure 2B).[4] We will refer to this scoring measure as ‘effusion-synovitis’ in the following sections to acknowledge both constituents of the composite score.
Cartilage signal and morphology were scored according to the WORMS system from 0 to 6 in 14 articular surface regions. In a modification of WORMS developed for longitudinal readings, a score of within-grade worsening for cartilage assessment was introduced to reflect any subtle within-grade progression that did not fulfill the criteria of a full-grade change. Any change including within-grade and full-grade or greater increases in cartilage damage in at least one of 10 tibio-femoral subregions was defined as cartilage loss.[2, 14] In addition, bone marrow lesions (BMLs) and meniscal damage were assessed according to the WORMS system at baseline. BML size was scored from 0–3 based on the extent of regional involvement. Meniscal status was graded from 0 to 4 in the anterior horn, the body segment, and the posterior horn of the medial and lateral meniscus. Furthermore meniscal extrusion of the medial and lateral meniscal body was scored on the coronal plane according to previous publications as this feature is not part of the WORMS score.[27, 28] The interreader reliability (weighted kappa) for the readings of the different features was 0.62 for BMLs, 0.65 for both, synovitis and joint effusion, 0.65 for meniscal extrusion, 0.78 for cartilage morphology and 0.80 for meniscal status.
Knees that showed typical radiologic signs of traumatic bone contusions, osteonecrosis, fracture or malignant bone infiltration were excluded from the analysis. However, of all analyzed MRIs only one knee showed a subacute tibial depression fracture at follow-up and was excluded.
Analysis Approach
In order to minimize possible misclassification errors of low-grade Hoffa-synovitis and effusion-synovitis vs. normal imaging findings, Hoffa-synovitis was defined as any grade ≥2 and effusion-synovitis as any grade ≥2 (Figures 1 and 2). Knees with scores of either 0 or 1 were the reference. We compared frequencies of cartilage loss in knee plates according to presence or absence of Hoffa-synovitis and effusion-synovitis using chi square statistics. We examined the association between baseline Hoffa-synovitis and the risk of tibio-femoral cartilage loss using the logistic regression model. In the multivariable regression model, we adjusted for age, gender, body mass index, knee malalignment, and other structural lesions (i.e., patellofemoral cartilage damage, meniscus damage, meniscal extrusion, bone marrow lesions, and effusion-synovitis). We used the same approach to examine the relation of effusion-synovitis to the risk of tibio-femoral cartilage loss. In this analysis we also adjusted for Hoffa-synovitis. In an additional analysis we included only knees without any cartilage damage in all 14 articular subregions including the patello-femoral joint. Further, we explored if a lower threshold of “definite” Hoffa- and effusion-synovitis yielded comparable results (using grades 0 only as the reference). All statistical calculations were performed using SAS® software (Version 9.1 for Windows; SAS Institute; Cary, NC).
RESULTS
As shown in Table 1, of 514 subjects (514 knees) included in the analysis, the average age at baseline was 60 years and 56% were women. The mean body mass index was 29.1 kg/m2. The majority (92.8%) of knees had a K/L grade of 0, and the remaining knees (7.2%) had a K/L grade of 1. 17% of the knees had frequent pain symptoms, and more than 60% knees had either varus or valgus malalignment.
Table 1.
Characteristics of study sample (514 subjects, 1 knee per subject)
| Characteristic | |
|---|---|
| Age, years, mean (SD) | 60.1 (7.2) |
| Female, N (%) | 286 (55.6) |
| Ethnicity (white), N (%) | 454 (88.3) |
| BMI, kg/m2, mean (SD) | 29.1 (4.5) |
| Kellgren & Lawrence grade* (%) | |
| 0 | 477 (92.8) |
| 1 | 37 (7.2) |
| Frequent knee symptoms, N (%)*[1] | 85 (16.5) |
| Alignment*[2], N(%) | |
| Varus | 223 (43.4) |
| Neutral | 193 (37.5 |
| Valgus | 98 (19.1) |
knee-based characteristics
Frequent knee symptoms: reported knee pain, aching or stiffness on most days during the past 30 days both on telephone interview and at clinic visit.
Varus alignment: 2 degrees or higher varus; neutral alignment: 1 degree varus to 1 degree valgus; valgus alignment: 2 degrees or higher valgus
SD = standard deviation
85 (16.5%) subjects recalled a history of injury to the examined knee badly enough to limit walking for at least two days and 14 (2.7%) knees had surgery.
At the baseline visit the majority of knees had not or only exhibited low-grade (i.e. grade=1) bone marrow lesions, meniscal damage or meniscal extrusion. Slightly more than 9% knees showed Hoffa-synovitis (grade >1) and 10.3% presented with effusion-synovitis (grade >1) (Table 2).
Table 2.
Prevalence of specific features on baseline MRI
| Feature | Bone marrow lesions 1 | Meniscal damage 2 | Meniscal extrusion 4 | Hoffa-Synovitis | Effusion-Synovitis | Hoffa-Synovitis or Effusion-Synovitis |
|---|---|---|---|---|---|---|
| Maximum Grade | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) |
| 0 | 395 (76.8) | 321 (62.5) | 373 (72.6) | 282 (54.8) | 235 (45.7) | 163 (31.7) |
| 1 | 91 (17.7) | 122 (23.7) | 138 (26.8) | 185 (36.0) | 226 (44.0) | 265 (51.6) |
| 2 | 20 (3.9) | 59 (11.5) | 3 (0.6) | 40 (7.8) | 49 (9.5) | 75 (14.6) |
| 3 | 8 (1.6) | 12 (2.3) 3 | n/a | 7 (1.4) | 4 (0.8) | 11 (2.1) |
Maximum grade of 10 TF subregions.
Maximum grade of 3 meniscal subregions (anterior, body, posterior) in medial or lateral TF compartments.
including grades 3 and 4.
Maximum grade medial or lateral, measured in coronal plane.
Concerning anatomical distribution in regard to articular plates, cartilage loss occurred most frequently in the retropatellar subregions (17.5% of knees) and medial weight-bearing femur (15.0% of knees). Only 6.6% of knees exhibited cartilage loss in the lateral weight-bearing femur. Cartilage loss was more frequently observed in the lateral tibia in knees with Hoffa-synovitis when compared to knees without signs of Hoffa synovitis. Furthermore, cartilage loss was more frequently observed in the medial and lateral weight bearing femur in knees with effusion-synovitis. The anatomical distribution of cartilage loss is presented in Table 3. Of 137 knees with cartilage loss in any of the tibio-femoral subregions, 45 (32.9%) had also tibio-femoral joint space narrowing progression on the X-ray.
Table 3.
Proportion of knees with cartilage loss according to articular plates
| Frequency of cartilage loss | Hoffa- Synovitis Absence (grades 0 and 1) | Hoffa- Synovitis Presence (maximum grade ≥ 2) | P-value * | Effusion- Synovitis Absence (grades 0 and 1) | Effusion- Synovitis Presence (grade ≥ 2) | P-value * | |
|---|---|---|---|---|---|---|---|
| N=514 | N=467 | N=47 | N=461 | N=53 | |||
| Retropatellar | 90 (17.5) | 78 (16.7) | 12 (25.5) | 0.131 | 79 (17.2) | 11 (20.8) | 0.516 |
| Trochlea | 49 (9.5) | 44 (9.4) | 5 (10.6) | 0.787 | 40 (8.7) | 9 (17.0) | 0.051 |
| Medial femur weight bearing | 77 (15.0) | 69 (14.8) | 8 (17.0) | 0.681 | 62 (13.5) | 15 (28.3) | 0.004* |
| Lateral femur weight bearing | 34 (6.6) | 26 (5.6) | 8 (17.0) | 0.003* | 23 (5.0) | 11 (20.8) | <.0001* |
| Medial Tibia | 53 (10.3) | 47 (10.1) | 6 (12.8) | 0.562 | 46 (10.0) | 7 (13.2) | 0.464 |
| Lateral tibia | 49 (9.5) | 40 (8.6) | 9 (19.2) | 0.019* | 40 (8.7) | 9 (17.0) | 0.051 |
| Patello-femoral (4 subregions) | 122 (23.7) | 108 (23.1) | 14 (29.8) | 0.306 | 106 (23.0) | 16 (30.2) | 0.244 |
| Tibio-femoral (10 subregions) ** | 137 (26.7) | 119 (25.5) | 18 (38.3) | 0.058 | 109 (23.6) | 28 (52.8) | <.0001* |
statistically significant at p<0.05
As shown in Table 4, baseline Hoffa-synovitis was not associated with an increased risk of cartilage loss at follow-up (adjusted odds ratio 1.0 [95% confidence intervals 0.5–2.1, p=0.89]). However, knees with baseline effusion-synovitis had an increased risk for cartilage loss (adjusted odds ratio 2.7 [95% confidence intervals 1.4–5.1, p=0.002]).
Table 4.
Longitudinal association between baseline effusion- synovitis and cartilage status at 30-months follow-up
| Synovitis and effusion at baseline (N = 514 knees) | Cartilage Status at follow-up | Crude OR (95% confidence interval) | Adjusted OR* (95% confidence interval) | |
|---|---|---|---|---|
| Total number of knees | Cartilage loss (%) | |||
| Hoffa-Synovitis Absence (grades 0 and 1) | 467 | 119 (25.5) | 1.0 (reference) | 1.0 (reference) |
| Hoffa-Synovitis Presence (maximum grade ≥ 2) | 47 | 18 (38.3) | 1.4 (0.7–2.7) | 1.0 (0.5–2.1) p =0.89 |
| Effusion-Synovitis Absence (grades 0 and 1) | 461 | 109 (23.6) | 1.0 (reference) | 1.0 (reference) |
| Effusion-Synovitis Presence (maximum grade ≥ 2) | 53 | 28 (52.8) | 3.4 (1.9–6.2) | 2.7 (1.4–5.1) p=0.002 |
Adjusted for age, sex, BMI, malalignment, and baseline effusion-synovitis, Hoffa-synovitis, patello-femoral cartilage damage, meniscus damage, meniscal extrusion,, and bone marrow lesions
We performed an additional analysis by limiting the knees that had no cartilage damage in all 14 articular subregions, including the patello-femoral joint (n = 85). Of them, only 2 knees showed any grade 2 Hoffa-synovitis or effusion-synovitis, 24 (28.2%) knees showed grade 1 Hoffa-synovitis and 16 (18.8%) exhibited grade 1 effusion-synovitis at baseline. During the follow-up period, only 13 (15.3%) knees developed any cartilage loss; thus prohibiting us from assessing association between either Hoffa-synovitis or effusion-synovitis and the risk of cartilage loss.
Using grades 0 only as the reference group neither baseline Hoffa-synovitis nor effusion-synovitis was associated with an increased risk of cartilage loss at follow-up (adjusted odds ratio 0.9 [95% confidence intervals 0.6–1.3, p=0.48] and adjusted odds ratio 1.5 [95% confidence intervals 0.9–2.3, p=0.11]).
DISCUSSION
In knees without radiographic OA we identified baseline joint effusion and synovitis assessed by a composite MRI measure, that we termed ‘effusion-synovitis’ as a predictor of future cartilage loss over a 30-month period. The imaging surrogate of signal alterations in Hoffa’s fat pad that we termed ‘Hoffa-synovitis’ was not predictive of later cartilage loss. We adjusted our results for possible confounders of future cartilage loss and thus could show that baseline effusion-synovitis as assessed on non-enhanced MRI is an independent predictor of cartilage detrioration and might play an important role in disease initiation.
OA is usually classified as a non-inflammatory disease as leukocyte count in OA synovial fluid is typically below the threshold defining an ‘inflammatory’ disorder.[29] However, synovial activation is commonly observed in OA. Clinically it manifests itself typically as ‘flares’ of pain.[30] On imaging and arthroscopy increased synovial thickness, hypervascularization and proliferation of hypertrophic and hyperemic synovial villi are observed.[6, 13, 15, 18, 21] Histology shows hypertrophy and hyperplasia with an increase in the number of synovial lining cells and infiltration of mononuclear cells with activated T cells, B cells and macrophages being the major components of the synovial infiltrate.[19, 31] The mediators affecting the activation of synovial macrophages have not been clearly identified, but are thought to to be caused by breakdown products of cartilage or meniscal degradation.[7, 8] Our results neither support nor contradict this hypothesis. Although we have analyzed knees without any MRI-detectable cartilage damage, early cartilage degradation prior to being visually apparent on MRI may have been present. Thus, the synovial activation we have observed in the form of synovitis and effusion might have been triggered by these breakdown products. On the other hand synovitis and effusion might have direct effects on cartilage matrix integrity that triggers further cartilage breakdown including surface damage.[7] Also stretching of the joint capsule as a result of effusion with consequent joint laxity leading eventually to microtrauma and possibly cartilage damage might play a role.
The aim of our study was not to analyze the cause of synovial activation itself, but rather to assess if synovial activation, once present, is an independent risk factor of later progression, which clearly seems to be the case. This supports findings of an earlier analysis from the MOST study where synovitis and effusion predicted rapid cartilage loss in knees with only minor cartilage damage (albeit statistically only borderline significant).[14]
We observed that baseline Hoffa-synovitis did not predict progression, but effusion-synovitis did. These results have to be interpreted carefully in light of the manifestations of synovitis and effusion on non-enhanced MRI that are commonly employed in large OA studies such as the MOST study. Signal changes assessed in Hoffa’s fat pad have been used for a long time to evaluate synovitis on non-enhanced MRI and have shown clinically relevant associations.[11, 13, 18, 23] We have previously shown that these signal changes are a sensitive, but non-specific measure using contrast enhanced MRI as a reference, which might result in over-interpretation of these MRI findings towards inflammatory activity although a non-inflammatory cause might be the reason for these MRI findings.[22] A recent comparative study assessing joint effusion on non-enhanced and contrast-enhanced MRI showed that the commonly applied measure of water-isointense signal within the joint cavity and the amount of capsular distention is a weak measure of true joint effusion. In fact, hyperintensity within the joint cavity has to be interpreted as a composite of synovial thickening and joint effusion and thus, the measurement is truly a reflection of both.[21] Consequently, there might be reason to assume that the composite measurement of ‘effusion-synovitis’ is potentially a better marker of synovial activation on non-enhanced MRI than the surrogate and non-specific measure of signal changes in Hoffa’s fat pad. Ideally, our findings need to be verified on a large study employing contrast-enhanced MRI in order to resolve the question if synovitis or joint effusion or both, predict structural progression. In a histologic correlation study, the degree of synovial thickening on contrast-enhanced MRI correlated with qualitative macroscopic analysis and with microscopic features such as surface fibrin deposition, fibrosis, edema, congestion, and infiltration.[32] Using contrast enhanced imaging for assessment, MRI-detected synovitis seems to be present to a relevant extent in OA knees despite the absence of joint effusion.[21] The fact that we did not find positive associations for the alternative definition of the reference group using only grades 0 as the reference confirms the difficulty of defining a threshold between “normal” or “physiologic” and “abnormal” or “pathologic” using semiquantitative (and also quantitative) approaches. For this reason it seems appropriate to use a more conservative definition for disease or pathology as we have in the present study, which is also in analogy to radiography-based studies that define Kellgren-Lawrence grades 0 and 1 as knees without radiographic OA.[25, 33, 34]
One possible shortcoming of our study is the fact that we employed 1.0T extremity MRI, which has been questioned to yield inferior image quality when compared to 1.5T or 3T large bore systems. These issues to the extent they exist seem not to affect semi-quantitative scoring of knee OA. In a comparative exercise scoring knees of subjects, which had received a 1.0T extremity MRI scan and a 1.5T large bore examination of the same knee on the same day, we could show good agreement, sensitivity and specificityfor all assessed features.[35] We believe that the slice thickness of 3 mm employed in MOST MRIs was adequate in order to detect discrete focal defects but also more wide-spread cartilage damage. In fact, standard intermediate-weighted sequences seem to be superiorly suited to pick up small focal cartilage defects when compared to high-resolution 3D sequences such as FLASH, SPGR, DESS or similar, as TSE sequences offer superior contrast between joint fluid and cartilage surface.[36, 37, 38]
As one of the main aims of our study was to assess change in cartilage over time, the MRIs were read not blinded to time point. This might result in a slight tendency to read more change in comparison to a blinded reading. However, it has been shown that scoring without knowing the chronological sequence substantially decreases sensitivity in the detection of clinically relevant changes in comparison with scoring in chronological order and that it does not introduce false positive changes.[39, 40] These studies showed that blinding to time point can lead to misclassification of the longitudinal change in a feature and that it may compromise the assessment of the relation of that feature and its outcome.[41] However, we have to acknowledge that to date longitudinal OA studies comparing semiquantitative MRI assessment blinded and non-blinded to chronological order are missing.
As there were only few knees without any cartilage damage in both, the tibio-femoral and patello-femoral joints, we included knees knees without tibiofemoral cartilage damage and without or with patellofemoral cartilage lesions. Adjustment was performed for presence of patello-femoral cartilage damage as a possible confounder of subsequent cartilage loss.
We did not analyze clinical symptoms or functional parameters at baseline or follow-up as this was not the focus of our study and would have gone beyond the scope of this work. However, several studies have shown that synovitis seems to be strongly associated with pain and is clinically relevant.[12, 13, 23] Subjects that developed an inflammatory disorder such as rheumatoid arthritis or a related disease between baseline and the follow-up visit were excluded from the analysis.
Despite having analyzed knees without cartilage damage, the concomitant presence of subchondral BMLs and meniscal damage in about 20–30% of the analyzed knees suggests that early features of OA are apparent prior to the manifestation of cartilage loss.[42]
In summary, we found that baseline joint effusion and synovitis assessed on non-enhanced MRI in knees without tibio-femoral radiographic OA but at risk for developing OA are predictors of later cartilage loss. The two MRI measures applied to non-contrast enhanced images showed different results and the composite measure of effusion and synovitis seemed to be predictive while Hoffa’s signal changes were not. This leads to the assumption that joint effusion might play an additional role in predicting cartilage loss. Contrast-enhanced MRI needs to verify these findings in the future. Our results support the importance of further research in the field of targeted inhibition of synovial activation in early OA in order to eventually avoid progressive cartilage degradation and functional impairment.
Acknowledgments
Funding Source:
Supported by NIH grants from the National Institute of Aging to Drs. Lewis (U01-AG-18947), Torner (U01-AG-18832), Nevitt (U01-AG-19069), and Felson (U01-AG-18820) and NIH AR47785
We would like to thank the participants and staff of the MOST study at the clinical sites in Birmingham, AL and Iowa City, IA and at the Coordinating Center at UCSF, San Francisco, CA.
Footnotes
Competing interest statement: Dr. Guermazi has received consultancies, speaking fees, and/or honoraria (less than $10,000 each) from Facet Solutions, Genzyme, and Stryker, and (more than $10,000) from Merck Serono, and is the President of Boston Imaging Core Lab (BICL). He receives research grant from General Electric Healthcare. Dr. Roemer is Vice President and shareholder of BICL. Dr. Crema is shareholder of BICL.
None of the other authors have declared any possible conflict of interest.
Copyright: The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd to permit this article (if accepted) to be published in ARD and any other BMJPGL products and sublicences such use and exploit all subsidiary rights, as set out in our licence (http://ARD.bmjjournals.com/ifora/licence.pdf).
References
- 1.Englund M, Guermazi A, Roemer FW, et al. Meniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons: The Multicenter Osteoarthritis Study. Arthritis Rheum. 2009;60:831–39. doi: 10.1002/art.24383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roemer FW, Guermazi A, Javaid MK, et al. Change in MRI-detected subchondral bone marrow lesions is associated with cartilage loss: the MOST Study. A longitudinal multicentre study of knee osteoarthritis. Ann Rheum Dis. 2009;68:1461–65. doi: 10.1136/ard.2008.096834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Felson DT, McLaughlin S, Goggins J, et al. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med. 2003;139:330–6. doi: 10.7326/0003-4819-139-5_part_1-200309020-00008. [DOI] [PubMed] [Google Scholar]
- 4.Peterfy CG, Guermazi A, Zaim S, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–90. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Rhodes LA, Grainger AJ, Keenan AM, et al. The validation of simple scoring methods for evaluating compartment-specific synovitis detected by MRI in knee osteoarthritis. Rheumatology (Oxford) 2005;44:1569–73. doi: 10.1093/rheumatology/kei094. [DOI] [PubMed] [Google Scholar]
- 6.Loeuille D, Rat AC, Goebel JC, et al. Magnetic resonance imaging in osteoarthritis: which method best reflects synovial membrane inflammation? Correlations with clinical, macroscopic and microscopic features. Osteoarthritis Cartilage. 2009;17:1186–92. doi: 10.1016/j.joca.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Aigner N, Van der Kraan P, Van den Berg W. Osteoarthritis and Inflammation -inflammatory changes in osteoarthritis synoviopathy. In: Buckwalter J, Lotz M, Stoltz J-F, editors. Osteoarthritis, Inflammation and Degradation: A Continuum. Amsterdam, NL: IOS Press; 2007. pp. 219–235. [Google Scholar]
- 8.Myers SL, Flusser D, Brandt KD, et al. Prevalence of cartilage shards in synovium and their association with synovitis in patients with early and end stage osteoarthritis. J Rheumatol. 1992;19:1247–51. [PubMed] [Google Scholar]
- 9.Boniface RJ, Cain PR, Evans CH. Articular responses to purified cartilage proteoglycans. Arthritis Rheum. 1998:258–66. doi: 10.1002/art.1780310214. [DOI] [PubMed] [Google Scholar]
- 10.Fuerst M, Lammers L, Schafer F, et al. Investigation of calcium crystals in OA knees. Rheumatol Int. 2010;30:623–31. doi: 10.1007/s00296-009-1032-2. [DOI] [PubMed] [Google Scholar]
- 11.Hill CL, Gale DG, Chaisson CE, et al. Knee effusions, popliteal cysts, and synovial thickening: association with knee pain in osteoarthritis. J Rheumatol. 2001;28:1330–7. [PubMed] [Google Scholar]
- 12.Baker K, Grainger A, Niu J, et al. Relation of synovitis to knee pain using contrast-enhanced MRIs. Ann Rheum Dis. 2010;69:1779–83. doi: 10.1136/ard.2009.121426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill CL, Hunter DJ, Niu J, et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2007;66:1599–603. doi: 10.1136/ard.2006.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roemer FW, Zhang Y, Niu J, et al. Tibiofemoral joint osteoarthritis: risk factors for MR-depicted fast cartilage loss over a 30-month period in the multicenter osteoarthritis study. Radiology. 2009;252:772–80. doi: 10.1148/radiol.2523082197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ayral X, Pickering EH, Woodworth TG, et al. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis - results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage. 2005;13:361–7. doi: 10.1016/j.joca.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Benito MJ, Veale DJ, FitzGerald O, et al. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64:1263–7. doi: 10.1136/ard.2004.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roemer FW, Guermazi A, Hunter DJ, et al. The association of meniscal damage with joint effusion in persons without radiographic osteoarthritis: the Framingham and MOST osteoarthritis studies. Osteoarthritis Cartilage. 2009;17:748–53. doi: 10.1016/j.joca.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandez-Madrid F, Karvonen RL, Teitge RA, Miller PR, et al. Synovial thickening detected by MR imaging in osteoarthritis of the knee confirmed by biopsy as synovitis. Magn Reson Imaging. 1995;13:177–83. doi: 10.1016/0730-725x(94)00119-n. [DOI] [PubMed] [Google Scholar]
- 19.Myers SL, Brandt KD, Ehlich JW, et al. Synovial inflammation in patients with early osteoarthritis of the knee. J Rheumatol. 1990;17:1662–9. [PubMed] [Google Scholar]
- 20.Hunter DJ, Lo GH, Gale D, Grainger AJ, Guermazi A, Conaghan PG. The reliability of a new scoring system for knee osteoarthritis MRI and the validity of bone marrow lesion assessment: BLOKS (Boston Leeds Osteoarthritis Knee Score) Ann Rheum Dis. 2008;67:206–11. doi: 10.1136/ard.2006.066183. [DOI] [PubMed] [Google Scholar]
- 21.Roemer FW, Javaid MK, Guermazi A, et al. Anatomical distribution of synovitis in knee osteoarthritis and its association with joint effusion assessed on non-enhanced and contrast-enhanced MRI. Osteoarthritis Cartilage. 2010;18:1269–74. doi: 10.1016/j.joca.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Roemer FW, Guermazi A, Zhang Y, et al. Hoffa’s Fat Pad: Evaluation on Unenhanced MR Images as a Measure of Patellofemoral Synovitis in Osteoarthritis. AJR Am J Roentgenol. 2009;192:1696–1700. doi: 10.2214/AJR.08.2038. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Nevitt MC, Niu J, et al. Reversible MRI features and knee pain fluctuation: the MOST study. Arthritis Rheum. 2010 in print. [Google Scholar]
- 24.Karlson EW, Sanchez-Guerrero J, Wright EA, et al. A connective tissue disease screening questionnaire for population studies. Ann Epidemiol. 1995;5:297–302. doi: 10.1016/1047-2797(94)00096-c. [DOI] [PubMed] [Google Scholar]
- 25.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterfy C, Li J, Zaim S, et al. Comparison of fixed-flexion positioning with fluoroscopic semi-flexed positioning for quantifying radiographic joint-space width in the knee: test-retest reproducibility. Skeletal Radiol. 2003;32:128–32. doi: 10.1007/s00256-002-0603-z. [DOI] [PubMed] [Google Scholar]
- 27.Hunter DJ, Zhang YQ, Niu JB, et al. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum. 2006;54:795–801. doi: 10.1002/art.21724. [DOI] [PubMed] [Google Scholar]
- 28.Hunter DJ, Zhang YQ, Tu X, et al. Change in joint space width: hyaline articular cartilage loss or alteration in meniscus? Arthritis Rheum. 2006;54:2488–95. doi: 10.1002/art.22016. [DOI] [PubMed] [Google Scholar]
- 29.Dougados M. Synovial fluid cell analysis. Bailleres Clin Rheumatol. 1996;10:519–34. doi: 10.1016/s0950-3579(96)80047-1. [DOI] [PubMed] [Google Scholar]
- 30.Dougados M. Clinical assessment of osteoarthritis in clinical trials. Curr Opin Rheumatol. 1995;7:87–91. doi: 10.1097/00002281-199503000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura H, Yoshino S, Kato T, Tsuruha J, Nishioka K. T-cell mediated inflammatory pathway in osteoarthritis. Osteoarthritis Cartilage. 1999;7:401–2. doi: 10.1053/joca.1998.0224. [DOI] [PubMed] [Google Scholar]
- 32.Loeuille D, Chary-Valckenaere I, Champigneulle J, et al. Macroscopic and microscopic features of synovial membrane inflammation in the osteoarthritic knee: correlating magnetic resonance imaging findings with disease severity. Arthritis Rheum. 2005 Nov;52(11):3492–501. doi: 10.1002/art.21373. [DOI] [PubMed] [Google Scholar]
- 33.Felson DT, Zhang Y, Hannan MT, Naimark A, Weissman BN, Aliabadi P, Levy D. The incidence and natural history of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1995;38:1500–5. doi: 10.1002/art.1780381017. [DOI] [PubMed] [Google Scholar]
- 34.Niu J, Zhang YQ, Torner J, Nevitt M, Lewis CE, Aliabadi P, Sack B, Clancy M, Sharma L, Felson DT. Is obesity a risk factor for progressive radiographic knee osteoarthritis? Arthritis Rheum. 2009;61:329–35. doi: 10.1002/art.24337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roemer FW, Lynch JA, Niu J, et al. A comparison of dedicated 1.0 T extremity MRI vs large-bore 1. 5 T MRI for semiquantitative whole organ assessment of osteoarthritis: the MOST study. Osteoarthritis Cartilage. 2010;18:168–174. doi: 10.1016/j.joca.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohr A. The value of water-excitation 3D FLASH and fat-saturated PDw TSE MR imaging for detecting and grading articular cartilage lesions of the knee. Skeletal Radiol. 2003;32:396–402. doi: 10.1007/s00256-003-0635-z. [DOI] [PubMed] [Google Scholar]
- 37.Stahl R, Luke A, Ma CB, et al. Prevalence of pathologic findings in asymptomatic knees of marathon runners before and after a competition in comparison with physically active subjects-a 3. 0 T magnetic resonance imaging study. Skeletal Radiol. 2008;37:627–638. doi: 10.1007/s00256-008-0491-y. [DOI] [PubMed] [Google Scholar]
- 38.Roemer FW, Kwoh CK, Hannon MJ, et al. Semiquantitative assessment of focal cartilage damage at 3T MRI: A comparative study of dual echo at steady state (DESS) and intermediate-weighted (IW) fat suppressed fast spin echo sequences. Eur J Radiol. 2010 Sep 10; doi: 10.1016/j.ejrad.2010.07.025. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 39.Bruynesteyn K, Van Der Heijde D, Boers M, et al. Detecting radiological changes in rheumatoid arthritis that are considered important by clinical experts: influence of reading with or without known sequence. J Rheumatol. 2002;29:2306–12. [PubMed] [Google Scholar]
- 40.Ross PD, Huang C, Karpf D, et al. Blinded reading of radiographs increases the frequency of errors in vertebral fracture detection. J Bone Miner Res. 1996;11:1793–800. doi: 10.1002/jbmr.5650111124. [DOI] [PubMed] [Google Scholar]
- 41.Felson DT, Nevitt MC. Blinding images to sequence in osteoarthritis: evidence from other diseases. Osteoarthritis Cartilage. 2009;17:281–3. doi: 10.1016/j.joca.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guermazi A, Hunter DJ, Roemer FW, et al. MRI prevalence of different features of knee osteoarthritis in persons with normal knee X-rays. Arhritis Rheum. 2007;56 (suppl):S128. [Google Scholar]