Abstract
The discovery of microRNA (miR) represents a novel paradigm in RNA-based regulation of gene expression and their dysregulation has become a hallmark of many a tumor. In virally associated cancers, the host–pathogen interaction could involve alteration in miR expression. Epstein–Barr virus (EBV)-encoded EBNA2 is indispensable for the capacity of the virus to transform B cells in vitro. Here, we studied how it affects cellular miRs. Extensive miR profiling of the virus-infected and EBNA2-transfected B lymphoma cells revealed that oncomiR miR-21 is positively regulated by this viral protein. Conversely, Burkitt's lymphoma (BL) cell lines infected with EBNA2 lacking P3HR1 strain did not show any increase in miR-21. EBNA2 increased phosphorylation of AKT and this was directly correlated with increased miR-21. In contrast, miR-146a was downregulated by EBNA2 in B lymphoma cells. Low miR-146a expression correlates with an elevated level of IRAK1 and type I interferon in EBNA2 transfectants. Taken together, the present data suggest that EBNA2 might contribute to EBV-induced B-cell transformation by altering miR expression and in particular by increasing oncomiR-like miR-21 and by affecting the antiviral responses of the innate immune system through downregulation of its key regulator miR-146a.
Keywords: EBV, EBNA2, DLBCL, microRNA, miR-21, miR-146a
Introduction
EBV is peerless in its capacity to transform human B lymphocytes. The resulting lymphoblastoid cell lines (LCLs) express six virally encoded nuclear proteins (EBNA1-6) and three membrane proteins (LMP1, 2A and 2B).1 EBNA2 is mandatory for the transforming capacity of the virus as P3HR1 strain which lacks EBNA2 coding region is devoid of this potential.2 Importantly, the reconstitution of EBNA2 into P3HR1 genome confers the virus its ability to provoke indefinite proliferation of the lymphoblasts.3 Using condition mutants in which EBNA2 has been fused to hormone-receptor-binding domain, it was shown that estradiol-mediated induction of EBNA2 was required for the infected B cells to start proliferation.4 The fact that EBNA2, together with EBNA-LP, is among the first proteins to be expressed when primary B cells are infected with the virus is consistent with its central role in causing B-cell transformation.5
Structurally, EBNA2 is a 497-amino-acid long nuclear protein and an activator of transcription. Interestingly, it does not bind directly to DNA but activates transcription through binding to adapter proteins. The case in point is RBP-Jk/CBF1.6 EBNA2 regulates CD23 and LMP2A through this transcription factor.7 In contrast, it regulates LMP1 promoter through PU1 and AP1 transcription factors.8 In addition, EBNA2 facilitates recruitment of TIFIIB, TIFIIH and p300CBP through its acidic domain.9 Besides viral genes, EBNA2 regulates cellular genes as well. The emergence of microarray technology has facilitated large-scale studies in which several human mRNAs were shown to be positively regulated by EBNA2.10 This virally encoded nuclear protein is also capable of negatively regulating human mRNAs.11, 12
The microRNAs (miRs) participate in gene regulation of a broad spectrum of biological pathways through fine-tuning of protein expression levels. Their aberrant expression is a hallmark of a variety of diseases. The initial evidence of their involvement in cancer came in light by studies in chronic lymphocytic leukemia.13 It is estimated that >50% human miRs are located to genomic regions, such as common breakpoints and fragile sites, involved in cancers.13 Some miRs are overexpressed in cancers in comparison with normal tissues, whereas others are downregulated. The former thus may be considered oncomiRs and the latter tumor supressor miRs.
EBV encodes its own miRs and can also influence cellular miRs.14 The initial evidence that this is so came from studies showing that EBV-negative and EBV-positive Burkitt's lymphomas (BLs) differ in their miR expression signature.15 Moreover, among EBV-positive BLs, it was shown that the miR profile of latency I and III expressors is diverse. In particular, miR-155 was highly expressed in latency III expressing cells.16 LMP1 was the driving force behind this increase17 and it is also responsible for increasing miR-146a expression.18, 19, 20 Recently, we have shown that downregulation of TCL1 provoked by LMP1 was due to an increase in miR-29b expression.20
Besides the noted requirement of EBNA2 for B-cell transformation, its expression in rat fibroblasts resulted in altered cellular morphology, increased colony formation in soft agar and tumorigenicity in nude mice.21 Mitotic check-point disruption and chromosomal instability caused by EBNA2 has been recently described.22 In EBNA2 transgenic mice, kidney tumors were frequently found.23 Given the importance of EBNA2 in EBV-driven transformation on the one hand, and the dysregulation of miRs in cancer on the other, we asked how this viral protein influences cellular miR expression. In the present study, we established microarray miR profile of EBNA2 expressing and nonexpressing EBV-infected cells and compared it with the same cells transfected with EBNA2 only and show that EBNA2 differentially regulates two major cellular miRs, namely miR-21 and miR-146a.
Materials and methods
Cells
U2932 is a previously characterized EBV-negative diffuse large B-cell lymphoma (DLBCL)-derived cell line.24 BL28 and BL41 are EBV-negative BLs. BL28 E95A and BL41 E95C are B95-8 virus convertants, and BL28 EHRA and BL41 EHRA are infected with the P3HR1 strain. Oma 4 is an EBV-negative variant of originally EBV-positive BL.25 Bjab is an EBV-negative B lymphoma cell line. Bjab K3 are estrogen-inducible EBNA2 transfectants.26 The Akata EBV-infected U2932, Bjab and Oma 4 convertants have been previously described.11 The B95-8 is a marmoset cell line which express all latent viral proteins. LCLAKE was established after infecting peripheral B lymphocytes with the recombinant EBV strain from Akata BL. The cells were grown in RPMI supplemented with 10% fetal calf serum and 100 IU of penicillin and 100 μg/ml streptomycin.
Infection with a recombinant EBV strain
Akata recombinant EBV producer cells were treated with anti IgG as previously described to obtain infectious virus particles.27 The details of infection protocol can be found in the Supplementary Materials and Methods.
Immunoblotting
The expression of EBNA2, LMP1, AKT, pAKT, IRAK1, β-actin and lamin B was verified by immunoblotting. The cells (107) were lysed in 1 ml of lysis buffer. Extracts equivalent to 106 cells were separated on discontinuous SDS–polyacrylamide gels and transferred to Protran (Amersham, Milan, Italy) filters. Antibody details are included in the Supplementary Materials and Methods.
Locked nucleic acid-based miRNA microarray
Total RNA was prepared using Trizol (Invitrogen, Monza, Italy) following the manufacturer's protocol. RNA quality was assessed by gel electrophoresis. Microarray experiments were performed using miRCURY LNA microRNA Array Power Labeling Kit (Exiqon, Vedbaek, Denmark). The experimental sample (U2932 EBVGFP cl-A, cl-B and U2932 EBNA2 cl-2) and a reference sample (U2932 MPA vector) were labeled in separate reactions with Hy5 and Hy3, respectively. Labeled experimental and the reference sample were combined, denatured, and then hybridized on custom-made slides (Mollenkopf), containing locked nucleic acid (LNA)-modified miR capture probes targeting all human, mouse and rat miRNAs listed in the miR BASE version 8.1. More Details of the method can be found in Supplementary Information.
Northern blotting
RNA was extracted by standard trizol method and suspended in DNAse- and RNAse-free water. Samples were run on 15% 7 M urea-acrylamide gels. Electrophoresis was performed at 20 mA for about 3 h at room temperature. The oligonucleotides to be used as probes were purchased from Sigma (Milan, Italy). After electrophoresis on urea-acrylamide gels, the RNA was transferred to hybond nylon filters at 10 V overnight at 4 °C. After the ultraviolet cross-linking, the filters were prehybridized in 6 × SSPE, 5 × DENHARDT and 0.5% SDS solution. Ten picomoles probes were radiolabelled using P32 γATP by standard reaction at 37 °C. Microspin G25 columns (Amersham) were used to separate radiolabelled probe from unincorporated γATP. The prehybridization and hybridization were performed in the same buffer, at 37 °C overnight. The filters were washed once in prewarmed washing solution containing 6 × SSPE and exposed in cassettes containing phosphor screen overnight. The signals were read by Amersham Typhoon 9200 phosphoimager and densitometry was performed with IMAGEQUANT software (Amersham). The filters were stripped in a solution containing 0.2 × SSPE and 0.2% SDS for 10 min at 95 °C and subsequently rehybridized with another probe.
EBNA2 and LMP1 transfection and selection
An EBNA2 expression vector J144-C1, the expression vector for LMP1 J132-G5 and the corresponding vector control pSV-MPA GPT (a kind gift of Professor Lars Rymo, Gothenburg University, Sweden) were individually transfected into U2932 DLBCL cells by electroporation (5 μg DNA, 230 V, 960 μF in 200 μl phosphate-buffered saline). Two days later, the cells were placed in a selection medium containing 1.5 μg/ml mycophenolic acid, 160 μg/ml xanthine and 10 μg/ml hypoxanthine. Drug-resistant clones were isolated after 4 weeks and used to verify EBNA2 and LMP1 expression. The latter was also transfected transiently together with pEGFP-N1 plasmid into U2932 and U2932 EBNA2 cl-2. The green fluorescent protein (GFP)-positive and -negative cells were sorted on a Becton-Dickinson (Milan, Italy) cell-sorter. Oma 4 and BL41 cells were electroporated with EBNA2 expressing vector J144-C1 and stable EBNA2 expressing clones were selected as described above. Bjab K3 cells transfected with estrogen-inducible EBNA2 were treated with 1 μM estradiol to induce EBNA2 expression.
Quantitative reverse transcription PCR
Total RNA from cell lines was isolated using Trizol (Invitrogen) according to the vending company's instructions. The integrity of RNA was routinely checked using 1% agarose gel. Further details of quantitative PCR (qPCR) for mature miRs, pri-miRs, IRAK1, IFNA2, IFNA4 and respective primers can be found as Supplementary Information.
LNA knockdown of miR-21
U2932 EBNA2 cl-2 and U2932 EBVGFP cl-B were transfected with 40 nM of LNA anti-miR-21 oligonucleotide (hsa-miR-21 miRCURY LNA, Exiqon) or scrambled oligonucleotide (Exiqon) by Lipofectamine 2000 (Invitrogen). After 48 h, the cells were harvested for total RNA and protein extraction.
Luciferase reporter activity assay
Five million cells each from U2932 MPA vector, U2932 EBNA2 and LMP1-transfected clones were electroporated with 10 μg of A547 construct containing 547-bp long miR-146a promoter cloned in frame with firefly luciferase gene (obtained from Addgene and previously described by Taganov et al.28) together with 5 ng pRL-TK plasmid containing renilla luciferase. The transfection efficiency was measured by calculating renilla expression ratio between the transfected and the untransfected cells. Firefly and renilla activities were measured using the Dual luciferase assay kit (Promega, Milan, Italy) by a luminometer (Perkin Elmer, Monza, Italy, 1420, Multilabel counter).
Results
Cellular miRNA expression profile of EBV-infected and EBNA2-transfected DLBCL cells
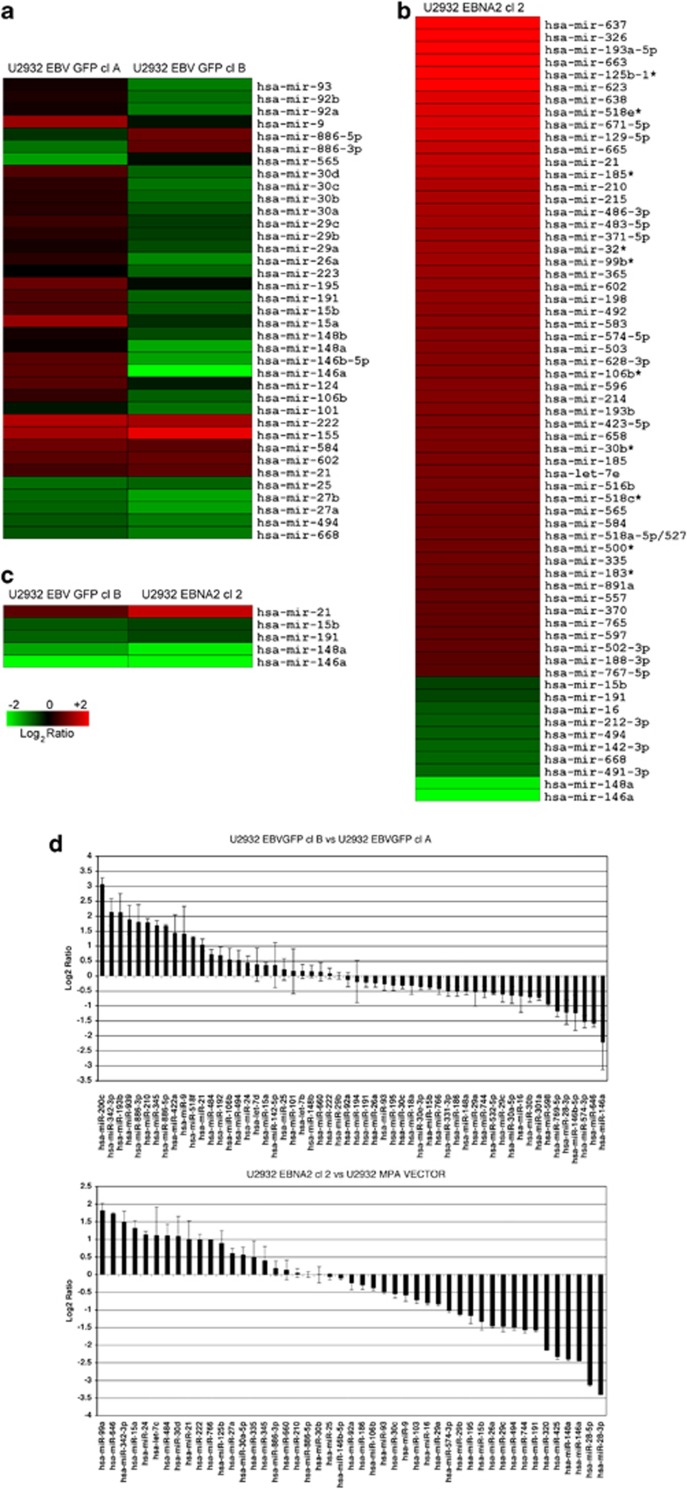
EBV infection of U2932 DLBCL cells and viral latent gene expression has been described in detail previously.11 The prime importance of EBNA2 in EBV-induced transformation prompted us to ask if its expression would alter miR profile of DLBCL cells. To this end, we compared miR expression profile of two clones of EBV-infected U2932 cells, which differ in EBNA2 expression, namely U2932 EBVGFP cl-A (EBNA2-) and U2932 EBVGFP cl-B (EBNA2+) (Figure 1a). Three different RNA preparations were generated from the control and EBV-infected and EBNA2-transfected cells. Fluorescence signals observed in triplicate array features at a level twofold that of background were considered to be indicative of miRNA expression. The expression of a total of 462 miRs was investigated. Among noteworthy alterations, miR-155 was expressed at a higher level in U2932 EBVGFP cl-B. miR-21 was also slightly more expressed in EBNA2-positive clone-B. Interestingly, among the differentially expressed miRs between these two EBV convertants, miR-146a expression was quite distinct. This miR was slightly upregulated in U2932 EBVGFP cl-A but notably downregulated in the EBNA2-positive U2932 EBVGFP cl-B. Other miRs with similar but less prominently different expression were miR-146b, -5p, -15b, -191, -26a and -30d. In order to find out how the differences in miR expression are related specifically to EBNA2, an expression vector for EBNA2 (J144 C-1) and its vector control plasmid were transfected into U2932 cells. Immunoblots showing EBNA2 expression are in Figure 2. miR expression microarray profile of the EBNA2-transfected clone, U2932 EBNA2 cl-2, is shown in Figure 1b. Among the most prominently altered miRs on the array, we find miR-637, miR-326, miR-193a-5p and miR-663. Interestingly, one of the most studied miR, namely miR-21, was also found upregulated in the EBNA2 transfectant. In contrast, among the downregulated miRs, we noted miR-146a and miR-148a. A comparison of miRs significantly altered in EBNA2-positive U2932 EBVGFP cl-B and the EBNA2-transfected U2932 EBNA2 cl-2 is shown in Figure 1c. The results obtained with microarray profiling were further validated by qPCR. These data are shown in Figure 1d and are essentially in concordance with those observed by microarray profiling. The corresponding heat map of Figure 1d is shown as Supplementary Figure 1.
Figure 1.
Global miR expression profile by microarray and qPCR of EBV-infected and EBNA2-transfected U2932: The experimental samples and the vector-transfected U2932 were labeled with Hy5 and Hy3, respectively. Three different RNA preparations were generated from the controls, EBV-infected and EBNA2-transfected cells. Red signals represent increase and the green color represents miRs whose expression is downregulated. Custom-made slides containing LNA-based capture probes (Exiqon library from the miR BASE version 8.1) representing >460 miRs were spotted. (a) Two EBV-infected clones of U2932 cells, U2932 EBVGFP cl-A (EBNA2 negative) and U2932 EBVGFP cl-B (EBNA2 positive), were compared for their miR profile. (b) Overview of miR profiling of EBNA2-transfected U2932 cells. (c) A comparison of principally altered miRs in U2932 EBVGFP cl-B and U2932 EBNA2 cl-2. The differences for both miR-21 and miR-146a expression in comparison to the parental U2932 cells were statistically significant (P<0.05), as measured by Student's t-test. The number of independent samples is n=3. (d) List of miRs statistically differentially expressed in U2932 EBVGFP cl-B (EBNA2 expressor) vs U2932 EBVGFP cl-A (EBNA2 non expressor) upper panel and in EBNA2 overexpressed U2932 EBNA2 cl-2 vs parental vector U2932 MPA vector (lower panel). The P-value is ⩽0.05 for the miRNA listed (Mann–Whitney test).
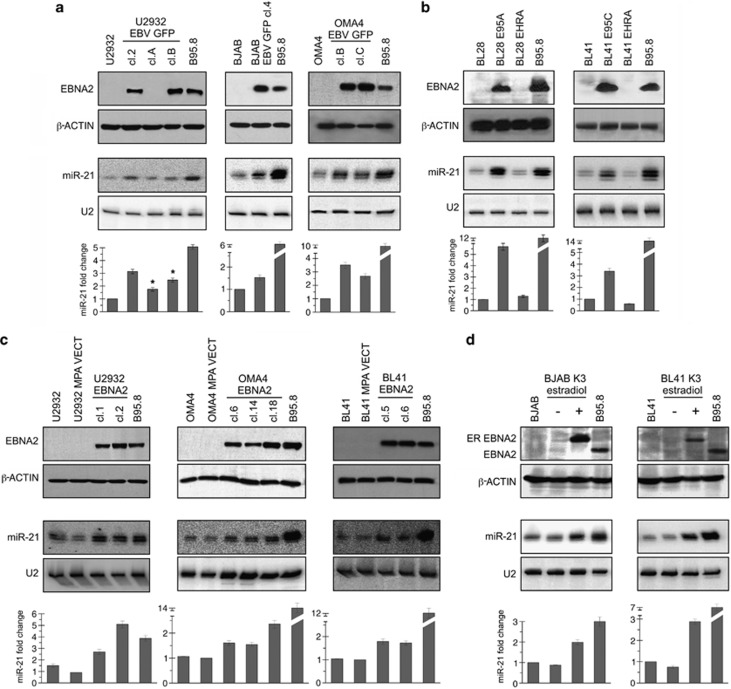
Figure 2.
EBNA2 and miR-21 expression in EBV-infected and EBNA2-transfected B tumor cells. (a) U2932 (DLBCL), Bjab (B-cell lymphoma) and Oma 4 (BL) were infected with the recombinant Akata strain of EBV. EBNA2 expression in the resulting convertants is shown by immunoblotting, using PE2 monoclonal antibodies. β-Actin expression was verified as loading control. The lower panels show miR-21 expression by northern blotting. End-labeled LNA antisense oligonucleotide was used as a probe. As a control for comparable loading and RNA quality, U2 expression was analyzed. ‘*' indicates a P-value of <0.05 by one sided t-test. (b) miR-21 expression was verified in isogenic pairs of BL28 and BL41, infected with the wild-type B95-8 and EBNA2 lacking P3HR1 viral strains. The upper panels show EBNA2 expression as detected by immunoblotting. The lower panels show miR-21 expression by northern blotting. The histograms are mean (±s.d.; n=3) densitometric analysis of northern blot data on miR-21 expression. The fold changes are normalized values against the house keeping U2 RNA. (c) An expression vector for EBNA2 was electroporated into U2932, Oma 4 and BL41 cells. EBNA2 expressing clones were established from all three cell lines. The expression of EBNA2 was analyzed by immunoblotting with PE2 monoclonal antibodies. β-Actin expression is used as loading control. miR-21 expression as evaluated by northern blotting is shown in the lower panels. (d) Estradiol-inducible EBNA2 was transfected into Bjab (Bjab K3) and BL41 (BL41 K3) cells. Estradiol-treated and -untreated cells were tested for EBNA2 and miR-21 expression. The ER–EBNA2 fusion protein was of higher molecular weight, EBNA2 induction was accompanied by an increase in miR-21 expression. β-Actin expression is the protein loading control and U2 expression is RNA loading and quality control. B95-8 was used as positive control both for EBNA2 and miR-21. The mean (±s.d.; n=3) densitometric analysis of miR-21 northern blots is shown below. The fold changes are normalized values against the house keeping U2 RNA.
Regulation of miR-21 in EBV-infected (in vitro) B tumor cells
As miR-21 is highly expressed in several human tumors, we chose to further study the regulation of this miR by EBNA2. To this end, we first asked how in vitro EBV infection of EBV-negative BLs influence its expression. Three EBV-negative cell lines were infected with a recombinant EBV derived from Akata.27 The expression of EBNA2 as determined by immunoblotting is shown in the top panels of Figure 2. β-actin expression was analyzed as protein loading control. Figure 2a shows that latency III expressing clone U2932 EBVGFP cl-2 and -B has higher miR-21 expression as analyzed by northern blotting. The EBNA2-negative U2932 EBVGFP cl-A expressed slightly higher miR-21 in comparison with the uninfected parental cells, however, this increase was lower than twofold. This minor miR-21 increase in U2932 EBVGFP cl-A is most likely due to LMP1 expression, as we have previously shown.20 Similarly, Bjab and Oma4 EBV conventants expressing latency III (thus EBNA2 positive) expressed higher miR-21 (Figure 2a).
To further clarify the role of EBNA2 in miR-21 regulation, we chose two BLs, BL28 and BL41, which were infected with the two prototype EBV strains B95-8 and P3HR1. As seen in Figure 2b, the EBNA2-positive B95-8 convertants of both these BLs show a significant increase in miR-21, whereas their P3HR1-infected counterparts and thus lacking EBNA2 show no change in miR-21, underlining the important role of EBNA2 in miR-21 increase.
The lower panels show mean densitometric analysis of northern blots (n=3) showing miR-21 expression.
EBNA2 induces miR-21 and pAKT expression
To confirm that EBNA2 alone is sufficient to induce miR-21 expression, an expression vector for EBNA2, J144-C1 and its empty vector control were electroporated into U2932, Oma 4 and BL41 cell lines. Figure 2c (top two rows) shows EBNA2 and β-actin expression by immunoblotting in the transfected cells. Northern blot analysis of miR-21 in lower panels suggests that EBNA2 alone is sufficient to increase miR-21 expression.
The effects of EBNA2 on miR-21 expression were further investigated in Bjab K3 and BL41 K3 EBNA2 transfectants in which EBNA2 expression was induced by treatment with estrogen. Bjab K3 and BL41 K3 cells were treated with 1 μM β-estradiol for 2 days. In Figure 2d, top row shows induction of estrogen receptor (ER)-EBNA2 after the hormone treatment. The EBNA2 induction was accompanied by an increase in miR-21 expression in these cells. The lower panels are mean densitometric analysis of miR-21 northern blots.
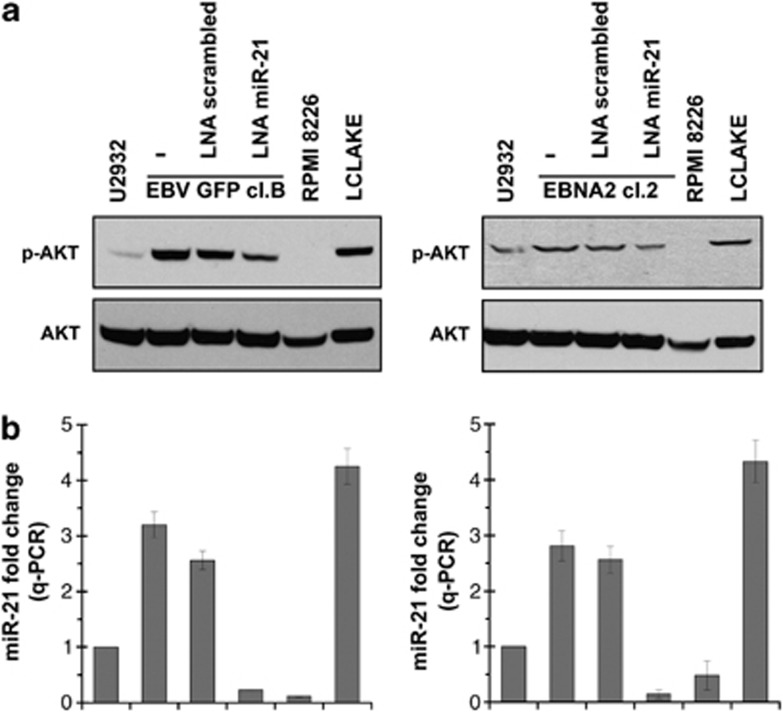
One of the known miR-21 targets, PDCD4, was unchanged in U2932 EBNA2 cells (data not shown). In order to investigate the biological significance of miR-21 induction by EBNA2, we tested pAKT expression. miR-21 has been previously shown to induce pAKT which is important for cellular proliferation.29, 30 As shown in Figure 3a, U2932 EBNA2 transfectants expressed higher pAKT levels. That higher pAKT in EBNA2 transfectants is due to increased miR-21 was verified by transfecting antisense LNA miR-21 oligonucleotides. Indeed the diminished miR-21 expression (Figure 3b) was accompanied by reduced pAKT expression (Figure 3a). A confirmation of these data was obtained in four additional cell lines, namely, BL41 E95C, BL41 EBNA2 cl-5, Bjab EBVGFP cl-4 and Bjab K3 (inducible EBNA2 expressors) and they are shown in Supplementary Figure 2.
Figure 3.
pAKT expression and the effect of miR-21 knockdown in EBNA2 expressing U2932 cells. (a) pAKT and total AKT expression was analyzed by immunoblotting in U2932 EBV-convertant and EBNA2-transfected clone. The role of miR-21 in the regulation of pAKT was further studied by knocking down miR-21 by transfecting antisense LNA miR-21 oligonucleotide. RPMI 8226 and LCLAKE were used as negative and positive controls, respectively. (b) Expression of miR-21 as analyzed by taqman qPCR in the corresponding cells as in (a). The figure represents mean of three experiments, each performed in triplicates. The values are normalized to an internal U6 RNA control.
Regulation of miR-21 expression by EBNA2
miR expression can be regulated by at least two principal mechanisms. The first being an increase at the transcriptional level. The second being post-transcriptional regulation that can involve increased processing and/or maturation. To understand whether EBNA2 increases the primary transcript (pri-miR) encoding miR-21, we designed primers shown in Supplementary Table 1 in which at least one of the two primers correspond to sequences that flank the Drosha cleavage site. A quantitative reverse transcription (qRT)-PCR analysis of pri-miR-21 transcript in EBV-converted U2932 clones and EBNA2 transfectants of the same line was performed. The pri-miR-21 transcript levels were slightly lower in the EBV-infected U2932 cells but did not significantly differ between EBNA2-negative U2932 EBVGFP cl-A and EBNA2-positive U2932 EBVGFP cl-B (Supplementary Figure 3a). The expression of the pri-miR-21 was still lower in EBNA2-transfected U2932 cells (Supplementary Figure 3b). These data suggest that EBNA2 might affect miR-21 expression at post-transcriptional processing level. This was further confirmed by the lack of miR-21 promoter activity in EBNA2 transfectants (Supplementary Figure 4).
Differential regulation of mature miR-146a and its primary transcript by LMP1 and EBNA2
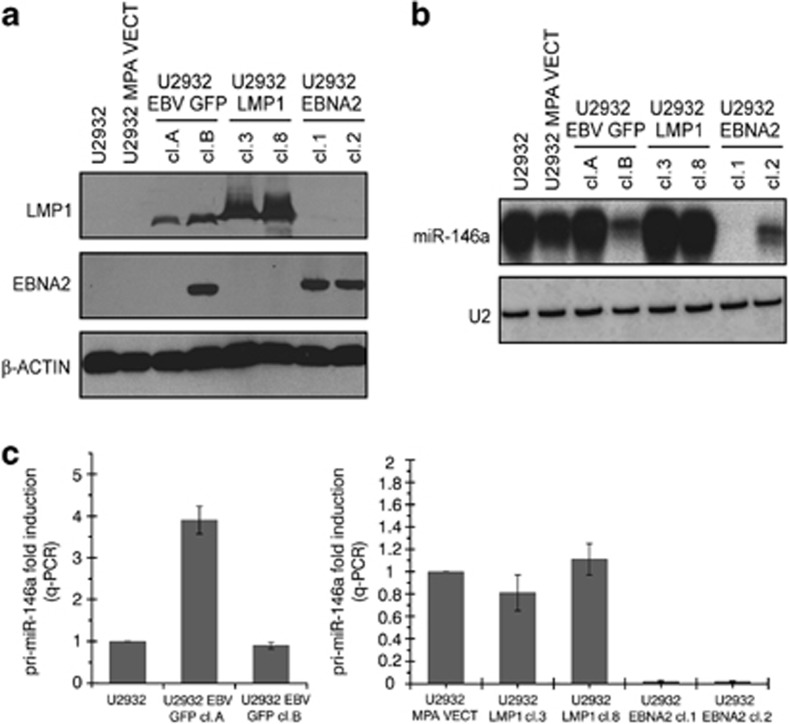
Previously, we and others have shown that one of the miRs consistently upregulated by LMP1 is miR-146a.18, 19, 20 Interestingly, our initial microarray profiling of EBNA2-transfected U2932 cells showed that this virally encoded nuclear antigen has the opposite effect on miR-146a expression (Figures 1b and c). These data were further validated by northern blotting. Figure 4a shows LMP1 and EBNA2 expression in EBV-converted, LMP1- and EBNA2-transfected U2932 cells. The northern blots shown in Figure 4b for the detection of miR-146a clearly demonstrate that while LMP1 moderately increased this miR, its expression was severely reduced in EBNA2 transfectants and also in EBV-infected EBNA2-positive cl-B. Next we studied the levels of pri-miR-146a transcripts in these cells by qRT-PCR. The primers for pri-miR-146a detection have been previously described.28 In EBNA2-negative but LMP1-positive U2932 EBVGFP cl-A, a significant increase in pri-miR-146a was observed. However, no alteration in pri-miR-146a was seen in EBNA2- and LMP1-positive U2932 EBVGFP cl-B when compared with the uninfected parental cells (Figure 4c). The right panel of Figure 6c shows that LMP1 did not affect pri-miR-146a, whereas in EBNA2 transfectants there was almost a complete block in miR-146a transcription.
Figure 4.
Mature and primary miR-146a expression in EBV-infected, LMP1- and EBNA2-transfected U2932 cells. (a) U2932 cells were transfected with expression vector for LMP1 and EBNA2 individually. Stable LMP1 and EBNA2 expressing clones were analyzed for expression of these proteins by S12 and PE2 monoclonal antibodies, using immunoblotting. EBV-infected U2932 cells were also included in this experiment. β-Actin expression was verified to check protein loading. (b) miR-146a expression in U2932 LMP1 and EBNA2 transfectants with corresponding vector control cell lines and in EBV-infected U2932 clones was verified by northern blotting. LNA oligonucleotide corresponding to sequences complementary to miR-146a was used as a probe. U2 expression was used to verify RNA loading and quality. (c) Two separate RNA preparations of U2932 parentals and EBV-infected clones EBVGFP cl-A (EBNA2 negative) and cl-B (EBNA2 positive) were tested for pri-miR-146a levels (left panel). Each experiment was performed in triplicates. In the right panel, U2932 LMP1- and EBNA2-transfected clones were tested for pri-miR-146a.
LMP1 cannot derepress EBNA2-induced block in miR-146a expression
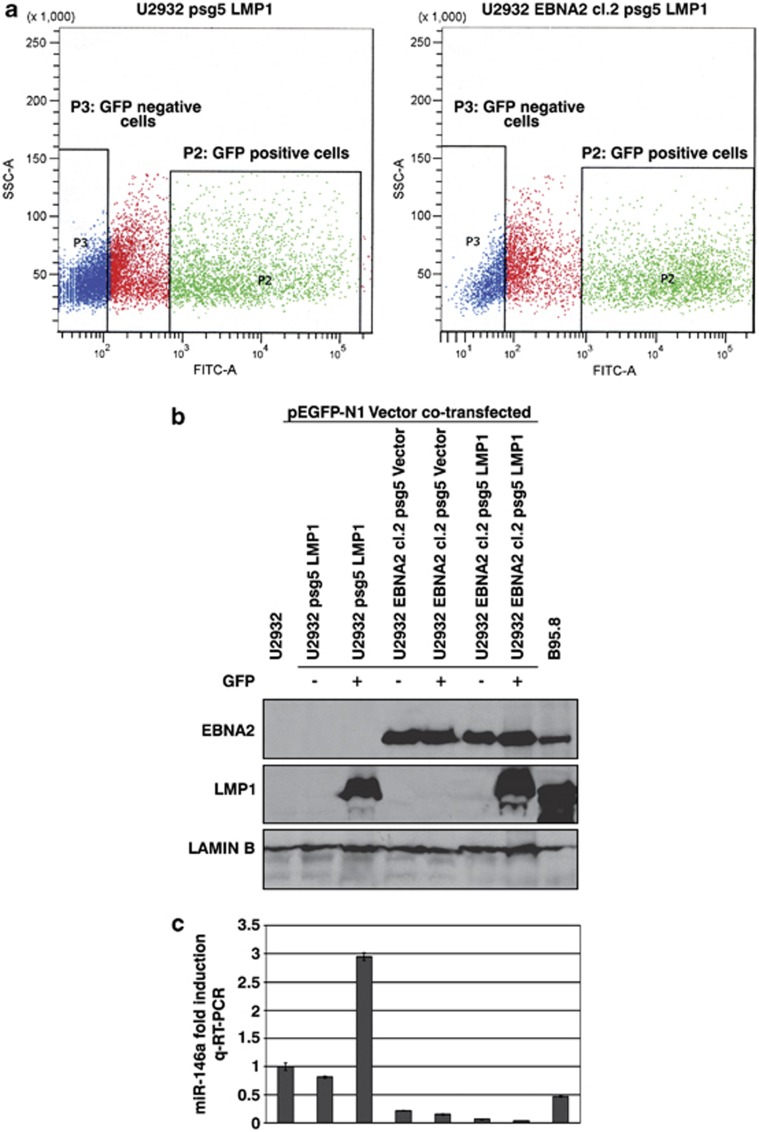
As in EBNA2-LMP1-positive EBV-infected U2932 EBVGFP cl-B the expression of miR-146a was downregulated, it appeared that the block imposed by EBNA2 on miR-146a expression is dominant over the induction of miR-146a by LMP1 in this cell line. In order to clarify this, LMP1 and pEGFP-N1 plasmids were cotransfected into U2932 and U2932 EBNA2 cl-2 cells. The GFP-positive (P2 quadrant, green) and -negative (P3 quadrant, blue) cells were sorted on a cell sorter (Figure 5a). The sorted cells were analyzed for EBNA2 and LMP1 expression as seen in Figure 5b. The parental U2932 cells which were GFP positive, expressed LMP1 whereas GFP-negative cells did not express LMP1. Similarly, U2932 EBNA2 cl-2 GFP-positive cells expressed LMP1 but the GFP-negative cells did not. Subsequently, miR-146a expression was analyzed in the GFP-sorted U2932 and U2932 EBNA2 cl-2 by qRT-PCR. As expected, the parental U2932 cells positive for GFP and LMP1 had higher miR-146a expression (Figure 5c, third column). In contrast, the negative effect of EBNA2 on miR-146a expression in GFP+/LMP1+ U2932 EBNA2 Cl-2 persisted in spite of high LMP1 expression (Figure 5c, the penultimate column). The data suggest that transient expression of LMP1 does not alleviate EBNA2-mediated block on miR-146a expression. This is consistent with low miR-146a expression in U2932 EBVGFP cl-B in which both EBNA2 and LMP1 are expressed. The higher expression of miR-146a in LCL control as against the latency III convertant U2932 EBVGFP cl-B is likely due to different cellular background.
Figure 5.
The effect of LMP1 on miR-146a expression in U2932- and EBNA2-transfected cells. (a) U2932 and U2932 EBNA2 cl-2 cells were transiently cotransfected with pSG5 LMP1 and pEGFP-N1 expression vectors. The GFP-positive and -negative cells were sorted. The P2 quadrant (green) and P3 quadrant (blue) show GFP-positive and -negative cells, respectively. (b) EBNA2, LMP1 and lamin B expression as seen by immunoblotting in GFP-sorted parental U2932 and its EBNA2-transfected derivative U2932 EBNA2 cl-2. (c) Expression of miR-146a by Taqman qPCR in corresponding cell lines. The figure represents a mean of three experiments.
miR-146a promoter activity in EBNA2 and LMP1 expressing cells
To further clarify the role of the inhibition of transcription of miR-146a by EBNA2, we tested the miR-146a promoter activity in U2932 EBNA2-transfected cells. A reporter construct in which miR-146a promoter is linked to a luciferase gene was transfected into U2932 parental line and its EBNA2 and LMP1 expressing derivatives. Supplementary Figure 5 shows that while EBNA2 severely blocked luciferase activity, LMP1 had no such effect. The data suggest that the increase in mature miR-146a expression by LMP1 is likely due to enhanced post-transcriptional processing of the primary transcript and might involve positive regulation of Drosha, Dicer or argonaute proteins. In contrast, these data suggest that the inhibition of miR-146a expression is due to repression of its transcription by EBNA2.
IRAK1 and type I interferon expression in EBV-infected and EBNA2/LMP1-transfected U2932 cells
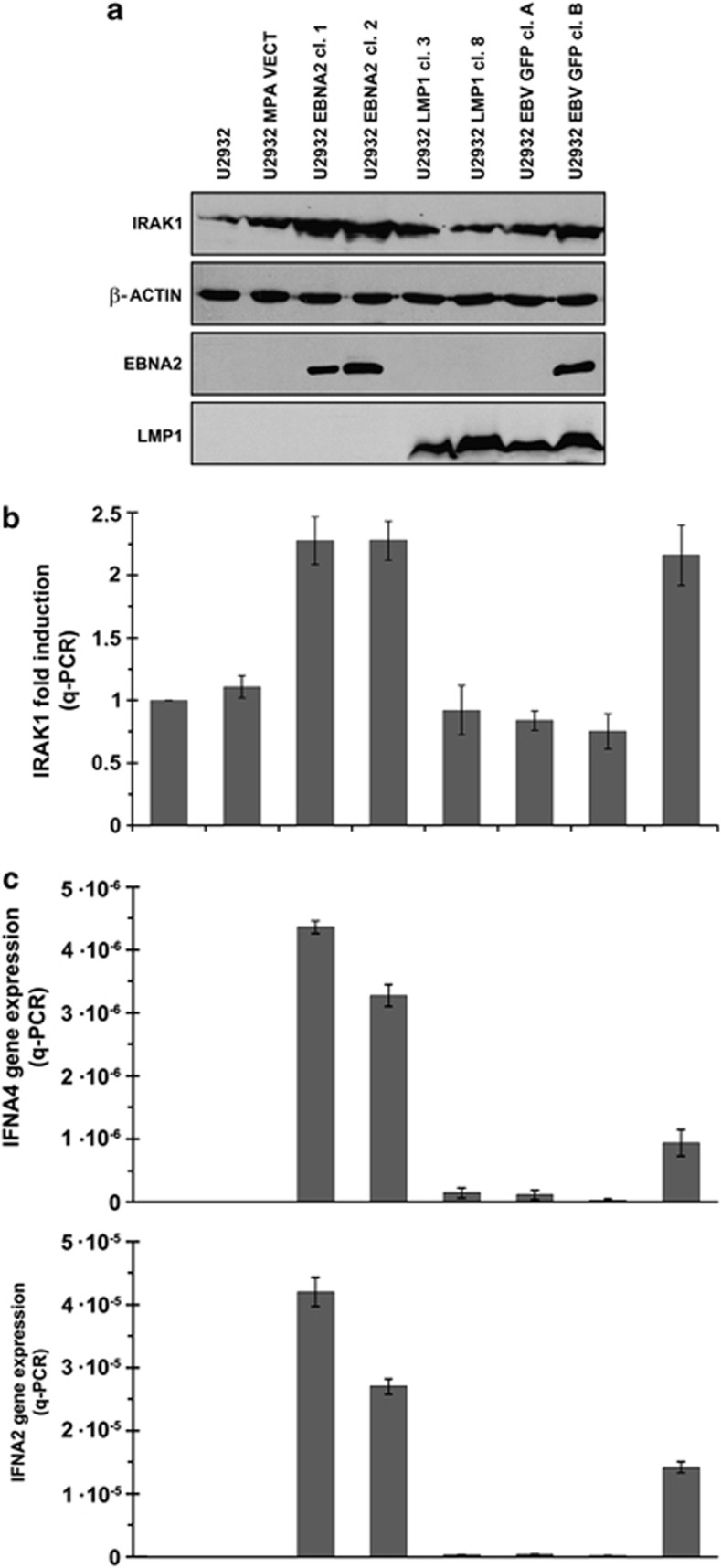
IRAK1 is one of the validated targets of miR-146a.28 As a consequence of differential regulation of this miR by EBNA2 and LMP1, we asked if IRAK1 levels would change accordingly. Figure 6 shows IRAK1 expression by immunoblotting (a) and taqman qPCR (b) in U2932 EBV convertants and LMP1/EBNA2 transfectants. While LMP1 had no influence on IRAK1 expression, EBNA2 increased its levels. Similarly, EBNA2-positive U2932 EBVGFP cl-B has higher IRAK1 levels compared with EBNA2-negative U2932 EBVGFP cl-A (Figure 6). EBNA2 and LMP1 expression in the transfected clones is shown in lower panels (Figure 6a).
Figure 6.
IRAK1 and type I interferon expression in EBV-infected and EBNA2/LMP1-transfected cells: (a) Immunoblotting was performed to evaluate IRAK1 protein expression. A monoclonal anti-IRAK1 antibody is used. EBNA2 and LMP1 expression was verified by using respective monoclonal antibody. (b) qPCR was employed to assess IRAK1 transcript levels in EBV-converted and EBNA2/LMP1-transfected U2932 cell, using IRAK1-specific taqman primers. The experiment represents mean of three experiments, each performed in triplicates, from two different RNA extraction. The values are normalized against an internal GAPDH mRNA control. (c) IFNA4 (top panel) and IFNA2 (lower panel) were quantified by qRT-PCR in total RNA-derived from U2932 EBV-infected and -transfected with the empty vector (MPA), with EBNA2 or with LMP1. Levels of IFNA2 and IFNA4 mRNAs were normalized to the GAPDH level using the formula 2−ΔΔCt; the values shown are means±s.d. of triplicate determinations.
As miR-146a is a key regulator of innate immune system and type I IFN pathway, we reasoned that as a consequence of differential regulation of this miR by EBNA2 and LMP1, the levels of type I IFN might be different in cells expressing these two viral proteins. Thus, we tested two type I IFN, IFNA4 and IFNA2 expression by qRT-PCR in EBNA2/LMP1-transfected and EBV-infected U2932 cells. Figure 6c shows that indeed EBNA2 expressors had higher levels of IFNA4 (Figure 6c, upper panel) and IFNA2 (Figure 6c, lower panel). However, transcripts of both these IFNs were reduced in the LMP1 transfectants.
Proliferation, apoptosis and cell cycle related changes in EBNA2 expressing U2932 cells
The biological role of EBNA2-induced changes in miR-21 and miR-146 was investigated by testing the cell proliferation, changes in cell cycle and apoptosis in U2932 cells. The expression of apoptosis-related genes BCL2 and BCLxS was analyzed. No significant alteration in antiapoptotic BCL2 expression was observed but we did find that proapoptotic BCLxS was reduced in EBNA2 expressing U2932 cells (Supplementary Figure 6a). The number of apoptotic cells were reduced in EBNA2 U2932 cells (Supplementary Figure 6b). Additionally, p21 and p27 expression was reduced but CDK4 was slightly increased (Supplementary Figure 6c). When LNA miR-21 was transfected, p21 was the only protein reconstituted (Supplementary Figure 6d). LNA miR-21 transfection did not affect bcl2, bclxS or p27 expression (data not shown). There was an increase in number of cells in S phase in U2932 EBNA2 expressors (Supplementary Figure 6e). We also noted higher in vitro proliferation and clonability in semisolid medium of EBNA2 expressing U2932 in comparison with controls (Supplementary Figures 6f and g). LNA miR-21 transfection reduced the growth of EBNA2 expressing U2932 cells in vitro as well as in semisolid medium (Supplementary Figures 6g and f).
Discussion
EBNA2 has a pivotal role in EBV-induced B-cell transformation as indicated by the fact that viral strains lacking the gene cannot immortalize primary B cells. In a successful B-cell transformation, however, the virus brings profound alterations in cellular gene expression and phenotype. The recent emergence of miRs as fine regulators of gene expression and their dysregulated expression in cancers suggest that transforming viruses might achieve this end by regulating these regulators. That EBV can influence cellular miR expression has been shown by us and others.20, 31 However, the individual contribution of EBV genes in miR regulation has been mainly studied for LMP1.18, 19, 20 To the best of our knowledge, this is the first study which investigates how EBNA2 affects miR expression. Using EBV-infected B tumor cells and EBNA2 transfectants, we demonstrated that EBNA2 can positively regulate miR-21 and negatively affects miR-146a expression.
Deregulated miR-21 expression is a hallmark of many tumors including glioblastoma, lung cancer, squamous cell carcinoma and breast cancer and chronic lymphocytic leukemia.32, 33, 34, 35, 36 A landmark study recently demonstrated that overexpression of miR-21 in an in vivo system leads to proliferation of pre-malignant lymphoid phenotype.37 The critical role of this miR in tumorigenesis was demonstrated by showing that by knocking down its expression, tumor cells were more prone to apoptosis.38 In addition, breast cancer cell growth and metastasis was suppressed when miR-21 was knocked down.39 The altered expression in miR-21 expression has been documented in BL with type III EBV latency and in LCLs.31, 40 However, which EBV protein is responsible for the observed miR-21 increase was not investigated in these previous studies. We now identify that among EBV-encoded proteins, EBNA2 alone is sufficient to increase its expression. We further show that EBNA2 does not upregulate miR-21 primary transcripts. This is consistent with a report by Cameron et al.,40 which showed that the increase in mature miR-21 in latency III cells is not correlated with a corresponding increase in the primary transcripts. Taken together, we surmise that the mature miR-21 increase by EBNA2 is most probably due to post-transcriptional mechanisms such as increased processing. Among the targets of miR-21, tumor suppressor PDCD4 and TIMP have been identified.41, 42 However, in our DLBCL model system, we did not detect any significant change in PDCD4 expression (data not shown).
The miR expression profiling of EBV-infected latency III expressing DLBCL cells highlighted that miR-146a was strongly downregulated. Interestingly, this miR was seen upregulated in LCLs and we confirm this.40 This difference could be related to diverse cellular background. Previously, it was reported that EBV infection of the virus-negative BLs results in an increase in miR-146a and this was attributed to LMP1.18, 19, 40 However, LMP1-EBNA2 expression in these EBV convertants was not demonstrated. It is erroneous to assume that in vitro EBV infection would regularly lead to a type III latency profile. Indeed there are several examples in the literature that show that LMP1 and/or EBNA2 might not be expressed in the in vitro-infected BLs and DLBCL.11, 43 In addition, it has been reported by Godshalk et al.31 that de novo infection of primary B cells initially decreases miR-146a. Perhaps more significantly, we found that the observed decrease in miR-146a expression in latency III expressor U2932 EBVGFP cl-B was due to EBNA2 expression, as the same cells expressing only EBNA2 showed a strong downregulation of this miR. This datum is consistent with miR-146a decrease observed by Godshalk et al.31 after de novo EBV infection, as EBNA2, together with EBNA5, is among the first EBV latent proteins to be expressed upon fresh infection.
Why EBNA2 negatively regulates miR-146a and what biological significance this bears is a highly interesting question and open to speculation at the moment. This miR has an important role in regulating genes involved in innate immune response. Among its targets, IRAK1 and TRAF6 were verified by Dr David Baltimore's group.28 In this context, it is relevant to note that in a hormone conditional EBNA2 LCL, when EBNA2 was expressed after tamoxifen treatment, one of the mRNA upregulated was that of IRAK1. In view of the present data, we suggest that the IRAK1 upregulation by EBNA2 observed by us and Zhao et al.12 could be a direct result of negative regulation of miR-146a by this viral protein. More recent studies indicate that miR-146a regulates type I interferon production through Rig-1 pathway.44, 45 It was thus reasonable to expect that one result of miR-146a downregulation provoked by EBNA2 would be higher expression of these cytokines. Indeed, EBNA2 transfectants did express higher interferon levels. This datum is also consistent with a previous study where EBNA2 expression in B cells led to increased type I interferon expression.46 The consequential question thus is, why a viral protein should activate antiviral pathway through upregulating type I interferon? Other DNA virus-encoded proteins such as SV40 large T antigen and adenovirus E1A have been previously implicated in the induction of interferon production.47, 48 A constant low level of interferon production induced by viral genes might eventually make the virus-infected cells refractory to this cytokine. This is consistent with previously reported resistance to interferon provoked by EBNA2 in BL cell lines.49
Further studies will be required to investigate precise molecular mechanisms on how EBNA2 affects miR expression and in particular miR-21 and miR-146a. However, the data suggest that EBNA2 might contribute to EBV-induced B-cell transformation by a positive regulation of oncomiR miR-21 and by interfering with the innate immunity through regulating a key regulator of it, namely miR-146a. Additionally, this miR has been attributed with oncosuppressive functions.50 Our data thus suggest that EBNA2 may promote B-cell transformation by simultaneously increasing an oncomiR and reducing a miR with noted tumor suppressing functions.
Acknowledgments
This work was partially supported by grants from MIUR, Associazione Italiana per la ricerca sul Cancro (AIRC), progetto strategico ISS 9ACF/1 of Ministero della salute, Fondazione Italiana per la sclerosi multipla (2007/R/17 to AF and 2009/R/7 to EC), Pasteur Cenci-Bolognetti foundation and Sixth Research Framework Programme of the European Union, Project RIGHT (LSHB-CT-2004 005276) and SIROCCO (037900).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
Supplementary Material
References
- Rowe M, Kelly GL, Bell AI, Rickinson AB. Burkitt's lymphoma: the Rosetta Stone deciphering Epstein-Barr virus biology. Semin Cancer Biol. 2009;19:377–388. doi: 10.1016/j.semcancer.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabson M, Gradoville L, Heston L, Miller G. Non-immortalizing P3J-HR-1 Epstein-Barr virus: a deletion mutant of its transforming parent, Jijoye. J Virol. 1982;44:834–844. doi: 10.1128/jvi.44.3.834-844.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchini A, Kieff E, Longnecker R. Marker rescue of a transformation-negative Epstein-Barr virus recombinant from an infected Burkitt lymphoma cell line: a method useful for analysis of genes essential for transformation. J Virol. 1993;67:606–609. doi: 10.1128/jvi.67.1.606-609.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochner N, Eick D, Zimber-Strobl U, Pawlita M, Bornkamm GW, Kempkes B. Epstein-Barr virus nuclear antigen 2 is a transcriptional suppressor of the immunoglobulin mu gene: implications for the expression of the translocated c-myc gene in Burkitt's lymphoma cells. EMBO J. 1996;15:375–382. [PMC free article] [PubMed] [Google Scholar]
- Brady G, MacArthur GJ, Farrell PJ. Epstein-Barr virus and Burkitt lymphoma. J Clin Pathol. 2007;60:1397–1402. doi: 10.1136/jcp.2007.047977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman SR, Johannsen E, Tong X, Yalamanchili R, Kieff E. The Epstein-Barr virus nuclear antigen 2 transactivator is directed to response elements by the J kappa recombination signal binding protein. Proc Natl Acad Sci USA. 1994;91:7568–7572. doi: 10.1073/pnas.91.16.7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laux G, Dugrillon F, Eckert C, Adam B, Zimber-Strobl U, Bornkamm GW. Identification and characterization of an Epstein-Barr virus nuclear antigen 2-responsive cis element in the bidirectional promoter region of latent membrane protein and terminal protein 2 genes. J Virol. 1994;68:6947–6958. doi: 10.1128/jvi.68.11.6947-6958.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannsen E, Koh E, Mosialos G, Tong X, Kieff E, Grossman SR. Epstein-Barr virus nuclear protein 2 transactivation of the latent membrane protein 1 promoter is mediated by J kappa and PU.1. J Virol. 1995;69:253–262. doi: 10.1128/jvi.69.1.253-262.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Grossman SR, Kieff E. Epstein-Barr virus nuclear protein 2 interacts with p300, CBP, and PCAF histone acetyltransferases in activation of the LMP1 promoter. Proc Natl Acad Sci USA. 2000;97:430–435. doi: 10.1073/pnas.97.1.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchesi W, Brady G, Dittrich-Breiholz O, Kracht M, Russ R, Farrell PJ. Differential gene regulation by Epstein-Barr virus type 1 and type 2 EBNA2. J Virol. 2008;82:7456–7466. doi: 10.1128/JVI.00223-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccellato F, Anastasiadou E, Rosato P, Kempkes B, Frati L, Faggioni A, et al. EBNA2 interferes with the germinal center phenotype by downregulating BCL6 and TCL1 in non-Hodgkin's lymphoma cells. J Virol. 2007;81:2274–2283. doi: 10.1128/JVI.01822-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Maruo S, Cooper AR, Chase M, Johannsen E, Kieff E, et al. RNAs induced by Epstein-Barr virus nuclear antigen 2 in lymphoblastoid cell lines. Proc Natl Acad Sci USA. 2006;103:1900–1905. doi: 10.1073/pnas.0510612103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calin GA, Croce CM. Chromosomal rearrangements and microRNAs: a new cancer link with clinical implications. J Clin Invest. 2007;117:2059–2066. doi: 10.1172/JCI32577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S, Zavolan M, Grasser FA, Chien M, Russo JJ, Ju J, et al. Identification of virus-encoded microRNAs. Science. 2004;304:734–736. doi: 10.1126/science.1096781. [DOI] [PubMed] [Google Scholar]
- Kluiver J, van den Berg A, de Jong D, Blokzijl T, Harms G, Bouwman E, et al. Regulation of pri-microRNA BIC transcription and processing in Burkitt lymphoma. Oncogene. 2007;26:3769–3776. doi: 10.1038/sj.onc.1210147. [DOI] [PubMed] [Google Scholar]
- Kluiver J, Haralambieva E, de Jong D, Blokzijl T, Jacobs S, Kroesen BJ, et al. Lack of BIC and microRNA miR-155 expression in primary cases of Burkitt lymphoma. Genes Chromosomes Cancer. 2006;45:147–153. doi: 10.1002/gcc.20273. [DOI] [PubMed] [Google Scholar]
- Lu F, Weidmer A, Liu CG, Volinia S, Croce CM, Lieberman PM. Epstein-Barr virus-induced miR-155 attenuates NF-kappaB signaling and stabilizes latent virus persistence. J Virol. 2008;82:10436–10443. doi: 10.1128/JVI.00752-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron JE, Yin Q, Fewell C, Lacey M, McBride J, Wang X, et al. The Epstein-Barr Virus latent membrane protein 1 (LMP1) induces cellular microRNA-146a, a modulator of lymphocyte signaling pathways. J Virol. 2008;82:1946–1958. doi: 10.1128/JVI.02136-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motsch N, Pfuhl T, Mrazek J, Barth S, Grasser FA. Epstein-Barr virus-encoded latent membrane protein 1 (LMP1) induces the expression of the cellular microRNA miR-146a. RNA Biol. 2007;4:131–137. doi: 10.4161/rna.4.3.5206. [DOI] [PubMed] [Google Scholar]
- Anastasiadou E, Boccellato F, Vincenti S, Rosato P, Bozzoni I, Frati L, et al. Epstein-Barr virus encoded LMP1 downregulates TCL1 oncogene through miR-29b. Oncogene. 2010;29:1316–1328. doi: 10.1038/onc.2009.439. [DOI] [PubMed] [Google Scholar]
- Shimakage M, Kurata A, Inoue H, Okamoto Y, Yutsudo M, Hakura A. Tumorigenicity of EBNA2-transfected cells. FEBS Lett. 1995;371:245–248. doi: 10.1016/0014-5793(95)00889-h. [DOI] [PubMed] [Google Scholar]
- Pan SH, Tai CC, Lin CS, Hsu WB, Chou SF, Lai CC, et al. Epstein-Barr virus nuclear antigen 2 disrupts mitotic checkpoint and causes chromosomal instability. Carcinogenesis. 2009;30:366–375. doi: 10.1093/carcin/bgn291. [DOI] [PubMed] [Google Scholar]
- Tornell J, Farzad S, Espander-Jansson A, Matejka G, Isaksson O, Rymo L. Expression of Epstein-Barr nuclear antigen 2 in kidney tubule cells induce tumors in transgenic mice. Oncogene. 1996;12:1521–1528. [PubMed] [Google Scholar]
- Amini RM, Berglund M, Rosenquist R, Von Heideman A, Lagercrantz S, Thunberg U, et al. A novel B-cell line (U-2932) established from a patient with diffuse large B-cell lymphoma following Hodgkin lymphoma. Leuk Lymphoma. 2002;43:2179–2189. doi: 10.1080/1042819021000032917. [DOI] [PubMed] [Google Scholar]
- Trivedi P, Zhang QJ, Chen F, Minarovits J, Ekman M, Biberfeld P, et al. Parallel existence of Epstein-Barr virus (EBV) positive and negative cells in a sporadic case of Burkitt lymphoma. Oncogene. 1995;113:505–510. [PubMed] [Google Scholar]
- Kempkes B, Spitkovsky D, Jansen-Durr P, Ellwart JW, Kremmer E, Delecluse HJ, et al. B-cell proliferation and induction of early G1-regulating proteins by Epstein-Barr virus mutants conditional for EBNA2. EMBO J. 1995;14:88–96. doi: 10.1002/j.1460-2075.1995.tb06978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruo S, Yang L, Takada K. Roles of Epstein-Barr virus glycoproteins gp350 and gp25 in the infection of human epithelial cells. J Gen Virol. 2001;82:2373–2383. doi: 10.1099/0022-1317-82-10-2373. [DOI] [PubMed] [Google Scholar]
- Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka Y, Tagawa H, Takahashi N, Watanabe A, Guo YM, Iwamoto K, et al. Aberrant overexpression of microRNAs activate AKT signaling via down-regulation of tumor suppressors in natural killer-cell lymphoma/leukemia. Blood. 2009;114:3265–3275. doi: 10.1182/blood-2009-06-222794. [DOI] [PubMed] [Google Scholar]
- Testa JR, Bellacosa A. AKT plays a central role in tumorigenesis. Proc Natl Acad Sci USA. 2001;98:10983–10985. doi: 10.1073/pnas.211430998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godshalk S, Bhaduri-McIntosh ES, Slack FJ. Epstein-Barr virus-mediated dysregulation of human microRNA expression. Cell Cycle. 2008;7:3595–3600. doi: 10.4161/cc.7.22.7120. [DOI] [PubMed] [Google Scholar]
- Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TS, Liu XB, Wong BY, Ng RW, Yuen AP, Wei WI. Mature miR-184 as Potential Oncogenic microRNA of squamous cell carcinoma of tongue. Clin Cancer Res. 2008;14:2588–2592. doi: 10.1158/1078-0432.CCR-07-0666. [DOI] [PubMed] [Google Scholar]
- Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- Fulci V, Chiaretti S, Goldoni M, Azzalin G, Carucci N, Tavolaro S, et al. Quantitative technologies establish a novel microRNA profile of chronic lymphocytic leukemia. Blood. 2007;109:4944–4951. doi: 10.1182/blood-2006-12-062398. [DOI] [PubMed] [Google Scholar]
- Medina PP, Nolde M, Slack FJ. Oncomir addiction in an in vivo model of microRNa-21 induced pre-B cell lymphoma. Nature. 2010;467:86–90. doi: 10.1038/nature09284. [DOI] [PubMed] [Google Scholar]
- Si ML, Zhu S, Wu H, Lu Z, Wu F, Mo YY. miR-21-mediated tumor growth. Oncogene. 2007;26:2799–2803. doi: 10.1038/sj.onc.1210083. [DOI] [PubMed] [Google Scholar]
- Zhu S, Wu H, Wu F, Nie D, Sheng S, Mo YY. MicroRNA-21 targets tumorsuppressor genes in invasion and metastasis. Cell Res. 2008;18:350–359. doi: 10.1038/cr.2008.24. [DOI] [PubMed] [Google Scholar]
- Cameron JE, Fewell C, Yin Q, McBride J, Wang X, Lin Z, et al. Epstein-Barr virus growth/latency III program alters cellular microRNA expression. Virology. 2008b;382:257–266. doi: 10.1016/j.virol.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, Post S, et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–2136. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- Baek D, Villon J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masucci MG, Torsteindottir S, Colombani J, Brautbar C, Klein E, Klein G. Down-regulation of class I HLA antigens and of the Epstein-Barr virus-encoded latent membrane protein in Burkitt lymphoma lines. Proc Natl Acad Sci USA. 1987;84:4567–4571. doi: 10.1073/pnas.84.13.4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J, Wang P, Lin L, Liu X, Ma F, An H, et al. MicroRNA-146a feedback inhibits RIG-I-dependent Type I IFN production in macrophages by targeting TRAF6, IRAK1, and IRAK2. J Immunol. 2009;183:2150–2158. doi: 10.4049/jimmunol.0900707. [DOI] [PubMed] [Google Scholar]
- Tang Y, Luo X, Cui H, Ni X, Yuan M, Guo Y, et al. MicroRNA-146A contributes to a normal activation of the type I interferon pathway in human lupus by targeting the key signaling proteins. Arthritis Rheum. 2009;60:1065–1075. doi: 10.1002/art.24436. [DOI] [PubMed] [Google Scholar]
- Kanda K, Kempkes B, Bornkamm GW, von Gabain A, Decker T. The Epstein-Barr virus nuclear antigen 2 (EBNA2), a protein required for B lymphocyte immortalization, induces the synthesis of type I interferon in Burkitt's lymphomacell lines. Biol Chem. 1999;380:213–221. doi: 10.1515/BC.1999.029. [DOI] [PubMed] [Google Scholar]
- Hiscott J, Wong A, Alper D, Xanthoudakis S. Transactivation of type 1 interferon promoters by simian virus 40 T antigen. Mol Cell Biol. 1988;8:3397–3405. doi: 10.1128/mcb.8.8.3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsch U, Pine R, Zimmer SG, Babiss LE. Induced expression of the endogenous beta interferon gene in adenovirus type 5-transformed rat fibroblasts. J Virol. 1992;66:1884–1890. doi: 10.1128/jvi.66.4.1884-1890.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda K, Decker T, Aman P, Wahlstrom M, von Gabain A, Kallin B. The EBNA2-related resistance towards alpha interferon (IFN-alpha) in Burkitt's lymphoma cells effects induction of IFN-induced genes but not the activation of transcription factor ISGF-3. Mol Cell Biol. 1992;12:4930–4936. doi: 10.1128/mcb.12.11.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaumik D, Scott GK, Schokrpur S, Patil CK, Campisi J, Benz CC. Expression of microRNA-146 suppresses NF-kB actitivity with redution of metastatic potential in breast cancer cells. Oncogene. 2008;27:5643–5647. doi: 10.1038/onc.2008.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.