Abstract
Purpose
To evaluate whether a difference in central corneal thickness (CCT) between the paired eyes could be associated to worse glaucoma in the thinner cornea eye.
Methods
From 16 different glaucoma centres, at least 50 glaucomatous patients were saved on the Italian Glaucoma Register. Eight hundred and sixteen glaucomatous patients were found in the register. CCT, ophthalmoscopic cup/disc ratio, mean deviation (MD), pattern SD (PSD), and intraocular pressure (IOP). The difference (Δ) between the paired eyes was calculated for all the considered parameters and two subgroups were created on the basis of ΔCCT. Because the difference between the two eyes could be positive or negative, the absolute value of Δ was considered for all the measurements. Three different ΔCCT cutoffs were selected: 10, 15, and 20 μm. Student's t-test was used to compare the subgroups.
Results
When the entire group was divided in two subgroups using 20 μm as ΔCCT cutoff, no significant difference was found for ΔIOP (−0.38±2.53 (mean±SD) mm Hg and −0.07±2.35 mm Hg, respectively) between the two subgroups. Significant (P<0.001) difference was found for ΔMD (6.58±7.30 and 3.14±4.22 dB, respectively), ΔPSD (3.92±4.01 and 2.16±2.57, respectively), and ΔC/D (0.11±0.14 and 0.08±0.11, respectively) between the two subgroups. No significant correlation was found between ΔCCT and the other parameters.
Conclusion
The ΔCCT between the two eyes could be associated to a worse glaucoma in the thinner cornea eye.
Keywords: glaucoma, central corneal thickness, visual field damage, difference between eyes
Introduction
As glaucoma is a chronic asymmetric progressive degenerative disease of the optic nerve head, most patients with glaucoma can have a different stage of the disease between the two eyes.
Although central corneal thickness (CCT) measurements are usually highly symmetric between paired eyes, approximately 6–7% of individuals do manifest significant interocular CCT asymmetry.1, 2, 3 CCT has been identified as a substantial glaucoma risk factor,4, 5, 6, 7, 8 and glaucomatous damage often presents in an asymmetric fashion.9, 10 The percentage of normal-tension and high-tension glaucoma patients with unilateral VF loss has been estimated to be approximately 25–35%.9
Asymmetric CCT was already associated with asymmetric primary open-angle glaucoma (POAG) in a retrospective study.11 In a later study by the same group, however, asymmetric POAG was associated with asymmetric dynamic contour tonometry but not Goldmann applanation tonometry or CCT.12
The aim of the study was to evaluate whether a difference in CCT between paired eyes could be associated to worse glaucoma in the thinner cornea eye.
Patients and methods
This is a retrospective, cross-sectional study. Institutional review boards and ethics committees at the institutions gave their approval of this study. This study followed the principles of the Declaration of Helsinki.
From 16 different glaucoma centres, at least 50 glaucomatous patients for each centre were saved on the Italian Glaucoma Register (IGR). Glaucomatous eyes were diagnosed based upon having a reproducible and characteristic VF defect of three non-edge points, all of which were depressed on the pattern deviation plot at a P<5%, along with an asymmetrical cupping >0.2, the presence of a notch on the rim and/or an increased cupping >0.6 when measured as cup/disc ratio (CDR) and an open angle at gonioscopy.
All subjects underwent at least two Swedish interactive thresholding algorithm (SITA) standard 24-2 perimetry. Reliable tests had <30% fixation losses, false-negative and false-positive responses. Patients included in this study had a best-corrected visual acuity of 20/60 or better and a refractive error of +6.00 to −6.00 dioptres. Subjects were excluded for a history of diabetes or posterior pole pathology other than glaucoma. In addition, subjects were excluded for use of systemic steroids, any other systemic medication known to affect the retina, any neurological condition known to affect the VF, and any ocular surgery.
The diagnosis of glaucoma was based on visual field examination, optic nerve head analysis, intraocular pressure (IOP) measurements, and gonioscopy.13
CCT was measured with ultrasonic contact pachymeter. Pachymetry values were always obtained by the same observer in all the different centres. Patients were instructed to look straight ahead at a fixation target located at 3 m. After having pushed the button to initiate corneal thickness measurements, the probe tip was gently positioned to touch the patient's cornea at its centre. The pachymeter probe had to be perpendicular to the apex of the cornea. If the measurement was valid, a value appeared on the digital display. The mean value of three consecutive measurements was used for the statistical analysis.
CCT, ophthalmoscopic CDR, mean deviation (MD), pattern standard deviation (PSD), and IOP were considered in this study. Besides the difference (Δ) between the paired eyes was calculated for all the considered parameters. Because the difference was always calculated as ‘Right–Left', an absolute value was introduced to avoid negative values and to better analyse the difference in the group. Then two subgroups were created on the basis of the absolute ΔCCT and three different cutoffs were arbitrarily chosen to perform the analysis: 10, 15, and 20 μm.
The data were analysed by descriptive analysis and when the distribution of the data was normal, t-test and Pearson's r coefficient were used and when the distribution of the data was non-normal, Mann–Whitney test and Spearman's coefficient were used.
Results
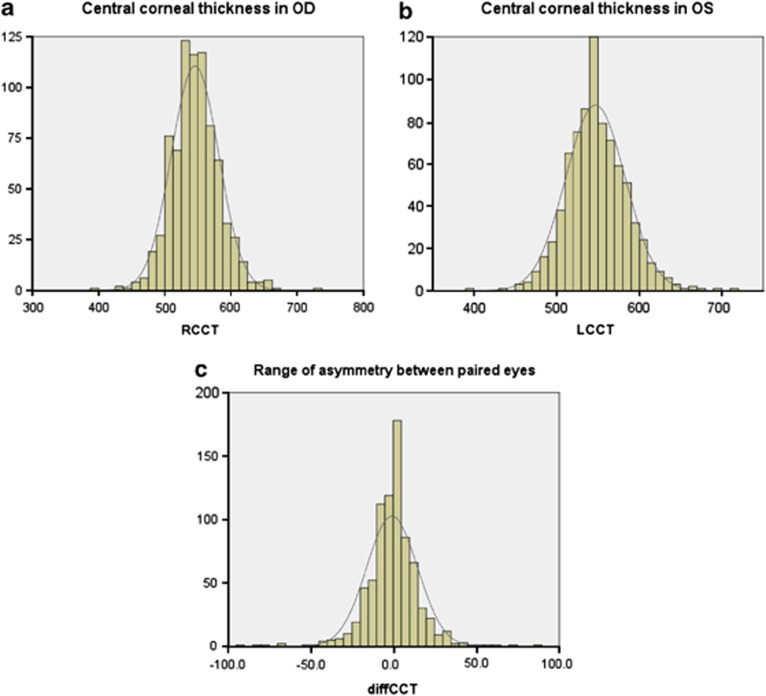
Eight hundred and sixteen patients were included in the IGR. Twenty-two patients were excluded because the CCT measurement was present for only one eye: five patients had only right measurements and ten only left measurements, and seven patients did not have the VF data for both eyes. All the patients were Caucasian. The descriptive analysis of the 794 included patients has been described in Table 1. No significant difference was found between paired eyes for IOP, CDR, MD, PSD, and CCT (Table 2). The distribution curves for CCT of both eyes and distribution of the CCT asymmetry between paired eyes have been shown in Figures 1a–c.
Table 1. Descriptive analysis of 794 patients.
| Mean | SD | |
|---|---|---|
| Age (year) | 65.52 | 12.78 |
| Right IOP (mm Hg) | 16.16 | 3.55 |
| Left IOP (mm Hg) | 16.28 | 3.31 |
| ΔIOP (mm Hg) | −0.12 | 2.38 |
| Absolute ΔIOP (mm Hg) | 1.45 | 1.90 |
| Right CDR | 0.55 | 0.21 |
| Left CDR | 0.56 | 0.21 |
| ΔCDR | −0.01 | 0.15 |
| Absolute ΔCDR | 0.87 | 0.11 |
| Right CCT (μm) | 545.68 | 35.82 |
| Left CCT (μm) | 546.89 | 36.09 |
| ΔCCT (μm) | −1.22 | 15.44 |
| Absolute ΔCCT (μm) | 10.41 | 11.46 |
| Right MD (dB) | −5.23 | 6.38 |
| Left MD (dB) | −5.37 | 6.58 |
| ΔMD (dB) | 0.14 | 6.22 |
| Absolute ΔMD (dB) | 3.70 | 5.00 |
| Right PSD | 4.53 | 3.61 |
| Left PSD | 4.49 | 3.49 |
| ΔPSD | 0.04 | 3.81 |
| Absolute ΔPSD | 2.45 | 2.92 |
Abbreviations: CCT, central corneal thickness; CDR, cup/disc area ratio; Δ, difference; IOP, intraocular pressure; MD, mean deviation; PSD, pattern SD.
Table 2. Comparison between the paired eyes.
| Mean | SD | P-value between the two eyes | |
|---|---|---|---|
| Right CCT (μm) | 545.68 | 35.82 | |
| Left CCT (μm) | 546.89 | 36.09 | NS |
| Right CDR | 0.55 | 0.21 | |
| Left CDR | 0.56 | 0.21 | NS |
| Right MD (dB) | −5.23 | 6.38 | |
| Left MD (dB) | −5.37 | 6.58 | NS |
| Right PSD | 4.53 | 3.61 | |
| Left PSD | 4.49 | 3.49 | NS |
| Right IOP (mm Hg) | 16.16 | 3.55 | |
| Left IOP (mm Hg) | 16.28 | 3.31 | NS |
Abbreviations: CCT, central corneal thickness; CDR, cup/disc area ratio; IOP, intraocular pressure; MD, mean deviation; PSD, pattern SD.
Figure 1.
Distribution curve of central corneal thickness (CCT) for the right eye (R) (a) and left eye (L) (b) and of the central corneal thickness difference (diffCCT) between the paired eyes obtained by using the following formula: ‘Right–Left' (c).
Then the entire group was divided into two subgroups based on the magnitude of absolute ΔCCT between the two eyes. When the cutoff between the two subgroups was 10 μm, no significant difference was found for IOP, CDR, RPSD, LPSD, and ΔPSD, whereas a significant difference was found for RMD, LMD, absolute ΔMD, and absolute ΔPSD (Table 3).
Table 3. Comparison between subgroups based on different absolute ΔCCT cutoffs.
|
Cutoff 10 μm (336/458 patients) |
Cutoff 15 μm (189/605 patients) |
Cutoff 20 μm (128/666 patients) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | P-value | Mean | SD | P-value | Mean | SD | P-value | |
| Age (year) | |||||||||
| > | 66.33 | 12.16 | 66.50 | 12.00 | 67.40 | 11.81 | |||
| < | 64.85 | 13.25 | NS | 65.19 | 13.03 | NS | 65.15 | 12.94 | NS |
| Right IOP (mm Hg) | |||||||||
| > | 16.05 | 3.27 | 15.60 | 3.19 | 15.44 | 3.01 | |||
| < | 16.25 | 3.75 | NS | 16.34 | 3.64 | NS | 16.30 | 3.63 | NS |
| Left IOP (mm Hg) | |||||||||
| > | 16.25 | 3.25 | 15.81 | 3.43 | 15.81 | 3.56 | |||
| < | 16.31 | 3.35 | NS | 16.43 | 3.26 | NS | 16.37 | 3.25 | NS |
| ΔIOP (mm Hg) | |||||||||
| > | −0.19 | 2.60 | −0.21 | 2.57 | −0.38 | 2.53 | |||
| < | −0.06 | 2.21 | NS | −0.09 | 2.32 | NS | −0.07 | 2.35 | NS |
| Absolute ΔIOP (mm Hg) | |||||||||
| > | 1.64 | 2.02 | 1.66 | 1.97 | 1.62 | 1.97 | |||
| < | 1.29 | 1.80 | NS | 1.37 | 1.87 | NS | 1.40 | 1.89 | NS |
| Right CDR | |||||||||
| > | 0.56 | 0.21 | 0.57 | 0.21 | 0.58 | 0.22 | |||
| < | 0.55 | 0.20 | NS | 0.55 | 0.20 | NS | 0.55 | 0.20 | NS |
| Left CDR | |||||||||
| > | 0.58 | 0.21 | 0.60 | 0.21 | 0.59 | 0.22 | |||
| < | 0.55 | 0.20 | NS | 0.55 | 0.20 | NS | 0.56 | 0.20 | NS |
| ΔCDR | |||||||||
| > | −0.02 | 0.16 | −0.02 | 0.18 | −0.02 | 0.18 | |||
| < | 0.00 | 0.13 | NS | 0.00 | 0.13 | NS | −0.01 | 0.14 | NS |
| Absolute ΔCDR | |||||||||
| > | 0.10 | 0.13 | 0.11 | 0.14 | 0.11 | 0.14 | |||
| < | 0.08 | 0.11 | NS | 0.08 | 0.11 | P<0.001 | 0.08 | 0.11 | <0.001 |
| Right CCT (μm) | |||||||||
| > | 546.16 | 39.18 | 546.45 | 40.94 | 546.36 | 43.89 | |||
| < | 545.32 | 33.18 | NS | 545.43 | 34.10 | NS | 545.54 | 34.09 | NS |
| Left CCT (μm) | |||||||||
| > | 548.55 | 39.56 | 549.69 | 40.90 | 550.95 | 43.20 | |||
| < | 545.67 | 33.30 | NS | 546.01 | 34.44 | NS | 546.11 | 34.54 | NS |
| Right MD (dB) | |||||||||
| > | −5.95 | 7.17 | −6.91 | 7.72 | −7.47 | 8.35 | |||
| < | −4.70 | 5.68 | <0.001 | −4.70 | 5.81 | <0.001 | −4.80 | 5.84 | <0.001 |
| Left MD (dB) | |||||||||
| > | −6.37 | 7.67 | −7.11 | 8.17 | −7.02 | 8.73 | |||
| < | −4.64 | 5.54 | <0.001 | −4.83 | 5.90 | <0.001 | −5.06 | 6.04 | <0.001 |
| ΔMD (dB) | |||||||||
| > | 0.42 | 8.27 | 0.19 | 9.47 | −0.45 | 9.83 | |||
| < | −0.06 | 4.12 | NS | 0.13 | 4.78 | NS | 0.26 | 5.25 | NS |
| Absolute ΔMD (dB) | |||||||||
| > | 5.22 | 6.42 | 6.32 | 7.05 | 6.58 | 7.30 | |||
| < | 2.58 | 3.21 | <0.001 | 2.88 | 3.82 | <0.001 | 3.14 | 4.22 | <0.001 |
| Right PSD | |||||||||
| > | 4.90 | 3.93 | 5.39 | 4.22 | 5.55 | 4.44 | |||
| < | 4.26 | 3.34 | NS | 4.26 | 3.36 | <0.001 | 4.33 | 3.40 | <0.001 |
| Left PSD | |||||||||
| > | 4.81 | 3.81 | 5.25 | 4.10 | 5.04 | 4.20 | |||
| < | 4.25 | 3.22 | NS | 4.25 | 3.24 | <0.001 | 4.38 | 3.33 | <0.001 |
| ΔPSD | |||||||||
| > | 0.09 | 4.83 | 0.14 | 5.42 | 0.51 | 5.60 | |||
| < | 0.01 | 2.85 | NS | 0.01 | 3.15 | NS | −0.05 | 3.36 | NS |
| Absolute ΔPSD | |||||||||
| > | 3.28 | 3.54 | 3.78 | 3.88 | 3.92 | 4.01 | |||
| < | 1.83 | 2.18 | <0.001 | 2.03 | 2.41 | <0.001 | 2.16 | 2.57 | <0.001 |
Abbreviations: CCT, central corneal thickness; CDR, cup/disc area ratio; Δ, difference; IOP, intraocular pressure; MD, mean deviation; PSD, pattern SD.
When the cutoff between the two subgroups was 15 μm, a significant difference was found for absolute Δ of CDR, RMD, LMD, absolute ΔMD, RPSD, LPSD, and absolute PSDΔ (Table 3).
When the cutoff between the two subgroups was 20 μm, a significant difference was found for absolute Δ of CDR, RMD, LMD, absolute ΔMD, RPSD, LPSD, and absolute ΔPSD (Table 3).
Nonsignificant correlation was found between absolute ΔCCT and ΔIOP, ΔCDR, absolute ΔCDR, ΔMD, and ΔPSD. Significant but weak correlation was found for the absolute ΔMD, for which a weak correlation was found when all the patients were considered and in two subgroups; less and greater than 10 μm, but no significant correlation was found in the other subgroups: greater than 15 and 20 μm. Similar results were found for absolute ΔPSD, but weaker (Table 4).
Table 4. Correlation between absolute ΔCCT and the other parameters in different subgroups.
| All the patients | Abs ΔCCT<10 μm | Abs ΔCCT>10 μm | Abs ΔCCT>15 μm | Abs ΔCCT>20 μm | |
|---|---|---|---|---|---|
| ΔIOP (mm Hg) | |||||
| r | −0.02 | 0.02 | 0.00 | 0.01 | 0.07 |
| P-value | NS | NS | NS | NS | NS |
| Absolute ΔIOP (mm Hg) | |||||
| r | 0.09 | −0.02 | 0.06 | 0.07 | 0.12 |
| P-value | NS | NS | NS | NS | NS |
| ΔCDR | |||||
| r | −0.02 | 0.03 | 0.01 | 0.06 | 0.05 |
| P-value | NS | NS | NS | NS | NS |
| Absolute ΔCDR | |||||
| r | 0.09 | 0.09 | 0.03 | −0.08 | −0.10 |
| P-value | 0.02 | NS | NS | NS | NS |
| ΔMD (dB) | |||||
| r | −0.04 | −0.12 | −0.10 | −0.11 | −0.09 |
| P-value | NS | 0.01 | NS | NS | NS |
| Absolute ΔMD (dB) | |||||
| r | 0.28 | 0.16 | 0.16 | 0.05 | 0.03 |
| P-value | 0.00 | 0.00 | 0.00 | NS | NS |
| ΔPSD | |||||
| r | 0.07 | 0.07 | 0.09 | 0.12 | 0.09 |
| P-value | NS | NS | NS | NS | NS |
| Absolute ΔPSD | |||||
| r | 0.26 | 0.11 | 0.14 | 0.06 | 0.04 |
| P-value | 0.000 | 0.02 | 0.011 | NS | NS |
Abbreviations: Abs, absolute value; CCT, central corneal thickness; CDR, cup/disc area ratio; Δ, difference; IOP, intraocular pressure; MD, mean deviation; PSD, pattern SD.
Discussion
Glaucoma is an asymmetric disease and in many patients visual field shows significant difference between the two eyes. Also the asymmetry between the right and left optic nerve head of the same subject is considered to be an important predictor of the disease.14 Progressive optic disc cupping has been shown to precede visual field loss in patients with OH,15, 16 and in unilateral glaucoma.17 The asymmetry of the CDR between the two eyes has been shown by some but not all studies to predict the development of glaucomatous visual field loss in patients with elevated IOP.18, 19, 20 Clinically, asymmetry in certain optic disc parameters between the two eyes might assist in differentiating patients with glaucoma from those without glaucoma. In particular, an asymmetry of CDR >0.2 between the two eyes can be suspicious of glaucoma.13 Even with the new imaging device the asymmetry is one of the parameter that the computerized systems are able to calculate and are useful to interpret the results of the printout.
The importance of CCT and anterior corneal curvature is known because the flatter the cornea, the easier the applanation to the accuracy of IOP measurement by Goldmann applanation. In clinical practice, many ophthalmologists have begun to routinely measure CCT, and large variations in corneal thickness have been documented, with the expected result. The aim of this study was to evaluate whether a difference in CCT between paired eyes could be correlated to a worse glaucoma. CCT has been shown to be an independent risk factor for glaucoma,4 and the relationship between pachymetry values and the risk of glaucoma damage is still controversial. No correlation between the thickness of the central cornea, of the peripapillary retinal nerve fibres21 and of the lamina cribrosa22 was found in nonglaucomatous human eyes; it is not known whether hystomorphometry of the lamina cribrosa or peripapillary nerve fibre layer thickness in glaucomatous eyes would show a relationship with corneal thickness. There is no consensus on the influence of pachymetry values on the likelihood of progression of glaucomatous damage in established glaucoma. Kim and Chen23 and Herndon et al6 proved the association of thinner central cornea values with VF progression in glaucoma patients. Jonas et al7 and Chauhan et al24 found an association between lower CCT and worse baseline VF, but the lower CCT was not associated with the progression of glaucomatous optic nerve neuropathy.
The reliability of central corneal thickness measurement has been much less controversial than that of IOP measurements.25 Techniques to measure CCT include optical (mean CCT 530 mm) and ultrasound (mean CCT 544 mm), with the latter being easier to perform. Also corneal hysteresis might also interfere with IOP measurements; it appears as though this corneal variable describes the response of the cornea to rapid deformation. Congdon et al26 suggested that the relationship between glaucoma and corneal features was more complex than simple anatomic thickness.
Circardian fluctuations in CCT have been found; they were small and, although statistically significant, did not seem to interfere with the circardian IOP measurements. However, no evidence that the 24-h change in IOP was due to the change in corneal biomechanical properties, was shown.27, 28
In this study, the CCT difference between paired eyes was analysed and the results suggested that a CCT difference >10 μm between the two eyes, could be associated to a worse glaucoma in the thinner cornea eye. However, the weak correlation we found, outlining that there was not a linear distribution between CCT and damage, because of the distribution of the data. In particular when the subgroups were analysed, the subgroup with a greater ΔCCT (>20 μm) showed a more significant difference between the eyes for VF indices and optic disc parameter (Table 3), but in this group no significant correlation between ΔCCT and the other parameters was found, suggesting that the difference in CCT were not correlated to the amount of the functional and structural damage. However, only the 16% of the considered patients had a ΔCCT >20 μm between paired eyes, whereas 84% of the patients had a ΔCCT <20 μm, 76% <15 μm and 58% of the patients had a ΔCCT <10 μm. In the latter group, a very weak but significant correlation was found between absolute ΔCCT and absolute ΔMD and PSD. However, to have a ΔCCT>20 μm could be a risk factor for the thinner cornea eye for receiving a late diagnosis of glaucoma in that eye.
Part of these data agreed with Anand et al's29 study that showed that POAG patients with asymmetric VF damage had symmetric CCT values. Furthermore, they found that CCT measurements were highly symmetric in paired eyes in >93% of the patients.
A possible bias of this study could be the presence of many centres involved, because of some subjective measurements (CDR or CCT measurement) that could change from one centre to another.
In conclusion, from these data most of the glaucomatous patients has a symmetric CCT and there is no correlation with the structural/functional damage; however, a difference in CCT measurements >15 μm between the two eyes had to be taken in consideration clinically: the thinner eye could be associated to a worse glaucoma.
Acknowledgments
The IGR is grateful to AISG and SIGLA for their support.
Appendix
The Italian Glaucoma Register:
M Iester1, P Brusini2, P Frezzotti3, GL Manni4, D Paoli5, M Rolando1, T Rolle6, M Uva7, R Altafini8, M Figus9, E Martini10, G Milano11, A Perdicchi12, L Rossetti13, P Fogagnolo13, A Rossi14, G Rossi11, F Scrimieri15, M Vetrugno16
1Laboratorio clinico anatomo-funzionale per la diagnosi e il trattamento del glaucoma e delle malattie neurooftalmologiche, Clinica Oculistica, Department of Neurological Sciences, Ophthalmology, Genetic, University of Genoa, Italy, 2Divisione di Oculistica, Ospedale di Udine, 3Clinica Oculistica, University of Siena, 4Clinica Oculistica, University Tor Vergata, Rome, 5Divisione di Oculistica, Ospedale di Monfalcone, 6Clinica Oculistica, University of Torino, 7Clinica Oculistica, University of Catania, 8Divisione di Oculistica, Ospedale di Bassano, 9Divisione di Oculistica, Ospedale di Sassuolo, 10Clinica Oculistica, University of Pisa, 11Clinica Oculistica, University of Pavia, 12Clinica Oculistica, University of La Sapienza II, Rome, 13Clinica Oculistica, San Paolo, University of Milan, 14Clinica Oculistica, University of Milan, 15Divisione di Oculistica, Ospedale di Tricase, 16Clinica Oculistica, University of Bari
The authors declare no conflict of interest.
Footnotes
This study has been presented in part at Association for Research and Vision in Ophthalmology (ARVO) meeting 1–5 May 2011, in Ft Lauderdale, FL, USA.
References
- Brandt JD, Beiser JA, Kass MA, Gordon MO. Central corneal thickness in the Ocular Hypertension Treatment Study (OHTS) Ophthalmology. 2001;108:1779–1788. doi: 10.1016/s0161-6420(01)00760-6. [DOI] [PubMed] [Google Scholar]
- Myrowitz EH, Kouzis AC, O'Brien TP. High interocular corneal symmetry in average simulated keratometry, central corneal thickness, and posterior elevation. Optom Vis Sci. 2005;82:428–431. doi: 10.1097/01.opx.0000162666.83092.e4. [DOI] [PubMed] [Google Scholar]
- Hahn S, Azen S, Ying-Lai M, Varma R. Central corneal thickness in Latinos. Invest Ophthalmol Vis Sci. 2003;44:1508–1512. doi: 10.1167/iovs.02-0641. [DOI] [PubMed] [Google Scholar]
- Gordon MO, Beiser JA, Brandt JD, Heuer DK, Higginbotham EJ, Jonnson CA, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–720. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- Medeiros FA, Sample PA, Zangwill LM, Bowd C, Aihara M, Weinreb RN. Corneal thickness as a risk factor for visual field loss in patients with preperimetric glaucomatous optic neuropathy. Am J Ophthalmol. 2003;136:805–813. doi: 10.1016/s0002-9394(03)00484-7. [DOI] [PubMed] [Google Scholar]
- Herndon LW, Weizer JS, Stinnett SS. Central corneal thickness as a risk factor for advanced glaucoma damage. Arch Ophthalmol. 2004;122:17–21. doi: 10.1001/archopht.122.1.17. [DOI] [PubMed] [Google Scholar]
- Jonas JB, Stroux A, Velten I, Juenemann A, Martus P, Budde WM. Central corneal thickness correlated with glaucoma damage and rate of progression. Invest Ophthalmol Vis Sci. 2005;46:1269–1274. doi: 10.1167/iovs.04-0265. [DOI] [PubMed] [Google Scholar]
- Sullivan-Mee M, Halverson KD, Saxon GB, Saxon MC, Shafer KM, Sterling JA, et al. The relationship between central corneal thickness-adjusted intraocular pressure and glaucomatous visual-field loss. Optometry. 2005;76:228–238. doi: 10.1016/s1529-1839(05)70298-0. [DOI] [PubMed] [Google Scholar]
- Poinoosawmy D, Fontana L, Wu JX, Bruce CV, Hitchings RA. Frequency of asymmetric visual field defects in normal-tension and high-tension glaucoma. Ophthalmology. 1998;105:988–991. doi: 10.1016/S0161-6420(98)96049-3. [DOI] [PubMed] [Google Scholar]
- Chen PP. Correlation of visual field progression between eyes in patients with open-angle glaucoma. Ophthalmology. 2002;109:2093–2099. doi: 10.1016/s0161-6420(02)01241-1. [DOI] [PubMed] [Google Scholar]
- Sullivan-Mee M, Gentry JM, Qualls C. Relationship between symmetric central corneal thickness and glaucomatous visual field loss within the same patient. Optom Vis Sci. 2006;83:516–519. doi: 10.1097/01.opx.0000218433.49803.e7. [DOI] [PubMed] [Google Scholar]
- Sullivan-Mee M, Halverson KD, Qualls C. Clinical comparison of Pascal dynamic contour tonometry and Goldmann applanation tonometry in asymmetric open-angle glaucoma. J Glaucoma. 2007;16:694–699. doi: 10.1097/IJG.0b013e3180408dc6. [DOI] [PubMed] [Google Scholar]
- European Glaucoma Society Terminology and Guidelines for Glaucoma, European Glaucoma SocietyII ed.Vol 1.1,Dogma: Savona; 20031–3. [Google Scholar]
- Varma R, Tielsch JM, Quigley HA, Hilton SC, Katz J, Spaeth GL, et al. Race-, age-, gender-, and refractive error-related differences in the normal optic disc. Arch Ophthalmol. 1994;112:1068–1076. doi: 10.1001/archopht.1994.01090200074026. [DOI] [PubMed] [Google Scholar]
- Pederson JE, Anderson DR. The mode of progressive disc cupping in ocular hypertension and glaucoma. Arch Ophthalmol. 1980;98:490–495. doi: 10.1001/archopht.1980.01020030486010. [DOI] [PubMed] [Google Scholar]
- Tuulonen A, Airaksinen PJ. Initial glaucomatous optic disk and retinal nerve fiber layer abnormalities and their progression. Am J Ophthalmol. 1991;111:485–490. doi: 10.1016/s0002-9394(14)72385-2. [DOI] [PubMed] [Google Scholar]
- Zeyen TG, Caprioli J. Progression of disc and field damage in early glaucoma. Arch Ophthalmol. 1993;111:62–65. doi: 10.1001/archopht.1993.01090010066028. [DOI] [PubMed] [Google Scholar]
- Yablonski ME, Zimmerman TJ, Kass MA, Becker B. Prognostic significance of optic disk cupping in ocular hypertensive patients. Am J Ophthalmol. 1980;89:585–592. doi: 10.1016/0002-9394(80)90071-9. [DOI] [PubMed] [Google Scholar]
- Tholen A, Tremmel L, Maurer W, Robert Y, Hendrickson P. Lateral differences indicate future glaucoma. Graefes Arch Clin Exp Ophthalmol. 1992;230:29–35. doi: 10.1007/BF00166759. [DOI] [PubMed] [Google Scholar]
- Kitazawa Y, Horie T, Aoki S, Suzuki M, Nishioka K. Untreated ocular hypertension. A long-term prospective study. Arch Ophthalmol. 1977;95:1180–1184. doi: 10.1001/archopht.1977.04450070078004. [DOI] [PubMed] [Google Scholar]
- Iester M, Mermoud A. Retinal nerve fiber layer and physiological central corneal thickness. J Glaucoma. 2001;10:158–162. doi: 10.1097/00061198-200106000-00003. [DOI] [PubMed] [Google Scholar]
- Jonas JB, Holbach L. Central corneal thickness and thickness of the lamina cribrosa in human eyes. Invest Ophthalmol Vis Sci. 2005;46:1275–1279. doi: 10.1167/iovs.04-0851. [DOI] [PubMed] [Google Scholar]
- Kim WJ, Chen PP. Central corneal pachymetry and visual field progression in patients with open-angle glaucoma. Ophthalmology. 2004;111:2126–2132. doi: 10.1016/j.ophtha.2004.04.029. [DOI] [PubMed] [Google Scholar]
- Chauhan BC, Hutchison DM, LeBlanc RP, Artes PH, Nicolela MT. Central corneal thickness and progression of the visual field and optic disc in glaucoma. Br J Ophthalmol. 2005;89:1008–1012. doi: 10.1136/bjo.2004.062927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iester M, Mete M, Figus M, Frezzotti P. Incorporating corneal pachymetry into the management of glaucoma. J Cataract Refract Surg. 2009;35:1623–1628. doi: 10.1016/j.jcrs.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Congdon NG, Broman AT, Bandeen-Roche K, Grover D, Quigley HA. Central corneal thickness and corneal hysteresis associated with glaucoma damage. Am J Ophthalmol. 2006;141:868–875. doi: 10.1016/j.ajo.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Kida T, Liu JHK, Weinreb RN. Effect of 24-hour corneal biomechanical changes on intraocular pressure measurement. Invest Ophthalmol Vis Sci. 2006;47:4422–4426. doi: 10.1167/iovs.06-0507. [DOI] [PubMed] [Google Scholar]
- Fogagnolo P, Rossetti L, Mazzolani F, Orzalesi N. Circardian variations in central thickness and intraocular pressure in patients with glaucoma. Br J Ophthalmol. 2006;90:24–28. doi: 10.1136/bjo.2005.079285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A, De Moraes CG, Teng CC, Tello C, Liebmann JM, Ritch R. Corneal hysteresis and visual field asymmetry in open angle glaucoma. Invest Ophthalmol Vis Sci. 2010;51:6514–6518. doi: 10.1167/iovs.10-5580. [DOI] [PubMed] [Google Scholar]