Abstract
The nitric oxide (NO) prodrug JS-K is shown to have anticancer activity. To profile the molecular events associated with anticancer effects of JS-K, HL-60 leukemia cells were treated with JS-K and subjected to microarray and real-time RT-PCR analysis. JS-K induced concentration- and time-dependent gene expression changes in HL-60 cells corresponding to the cytolethality effects. The apoptotic genes (caspases, Bax, and TNF-α) were induced, and differentiation-related genes (CD14, CD11b, and vimentin) were increased. For acute phase protein genes, some were increased (p53, c-jun) while others were suppressed (c-myc, cyclin E). The expression of anti-angiogenesis genes thrombospondin-1 and CD36 and genes involved in tumor cell migration such as tissue inhibitors of metalloproteinases, were also increased by JS-K. Confocal analysis confirmed key gene changes at the protein levels. Thus, multiple molecular events are associated with JS-K effects in killing HL-60, which could be molecular targets for this novel anticancer NO prodrug.
Keywords: JS-K, Nitric oxide donor, HL-60 cells, gene expression, confocal analysis
Introduction
Nitric oxide (NO) inhibits the growth and induces differentiation of acute myeloid leukemia cells [1,2]. Expression of human monocyte differentiation markers CD11b and CD14, and tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) transcripts in leukemia cells can be increased by NO [1.2]. Spontaneous NO generating diazeniumdiolate compounds induce apoptosis in acute myeloid leukemia HL-60 and U937 cells [3], with the long half-life (20 hr) NO donor DETA-NO being the most active. The major obstacle for the use of NO-generating compounds in vivo to treat malignant disease is NO-induced vasodilation [4]. An alternative approach is the use of NO-generating prodrugs that would be activated in the targeted cells, thus avoiding the vascular effects of the drug. For instance, esterase-activated diazeniumdiolates that do not release NO spontaneously but rather are enzymatically activated to release NO are cytotoxic to leukemia cells [5]. However, such a drug design is unlikely to provide enough selectivity because esterases are ubiquitous. Many malignant cells, including leukemia cells, overexpress glutathione S-transferases (GST) which result in resistance to cytotoxic compounds through glutathione (GSH) conjugation and cellular efflux via multi-drug resistance (MRP) pumps, or by inhibition of mitogen activated protein kinases (MAPK) [6]. Consequently, GST constitutes an attractive enzyme system for the targeting of cytotoxic agents to cancer cells.
O2-arylated diazeniumdiolates are designed to release NO upon conjugation with GSH in a reaction catalyzed by GST. O2-(2,4-dinitrophenyl) 1-[(4-ethoxyxarbonyl)piperazin-1-yl]diazen-1-ium-1,2-diolate (JS-K), the lead compound from this family is potently cytotoxic to human myeloid leukemia HL-60 cells in vitro and in vivo [7]. JS-K also inhibits hepatoma Hep 3B cell proliferation [8], enhances arsenic- and cisplatin-induced cytolethality in arsenic-transformed rat liver cells [9], and induces apoptosis in human multiple myeloma cell lines [10]. In the National Cancer Institute 51-cell panel screen, JS-K was effective against leukemia, renal cancer cells, prostate cancer cells, and brain cancer cells [11,12]. In in vivo murine models, JS-K was effective in inhibiting the growth of HL-60 (leukemia) [7], PPC-1 (prostate cancer) [7], JM-1 (hepatoma) [11], and OPM1 cells (myeloma) [10]. Consequently, JS-K is a lead compound establishing a new class of cancer chemotherapeutic agents [12].
Mechanisms for the antineoplastic effects of JS-K are not completely elucidated. In HL-60 cells, JS-K induces differentiation and apoptosis. JS-K-induced apoptosis in HL-60 cells occurs through activation of both the intrinsic and extrinsic pathways [7,13]. Apoptosis induction by JS-K is mediated, at least in part, by cytochrome c release and caspase activation [13]. The goal of the present study was to use microarray and real-time polymerase chain reaction (RT-PCR) analysis to identify genes that are modulated by JS-K in killing HL-60 cells. Cofocal image analysis was also performed on selected proteins. Results demonstrate that multiple molecular events are likely involved in the antitumor effects of JS-K, including activation of caspases, modulation of cell growth and differentiation genes, increased expression of anti-angiogenesis genes and genes inhibiting tumor cell migration, all of which could be potential molecular targets for anticancer effects of JS-K.
Materials and Methods
Materials
JS-K was synthesized as previously described [14]. The Clontech Human Cancer Arrays (600 genes) were purchased from Clontech (Palo Alto, CA). All the primers for RT-PCR analysis were synthesized by Sigma-Genosys (The Woodlands, TX). All other chemicals were of reagent grade.
Cell culture and JS-K treatment
Human myeloid leukemia HL-60 cells (ATCC, Manassas, VA) were cultured in RPMI-1640 supplemented with 10% fetal bovine serum (FBS) and penicillin/streptomycin. Cells were cultured at 37°C in a 5% CO2 humidified atmosphere. JS-K stock (5 mM) was prepared with dimethyl sulfoxide (DMSO) and diluted in phosphate buffered saline (PBS) before addition to cultures. The final concentration of DMSO added to the cultures was less than 0.1% and the media containing 0.1% DMSO was used as controls. JS-K was added to HL-60 cells at concentrations of 0, 0.25, 0.5, and 1.0 μM, and cells were harvested 2, 4, 6, and 24 hr later.
RNA isolation
At the end of JS-K treatments, cells were harvested by centrifugation and total RNA was isolated with TRIzol agent (Invitrogen, Carlsbad, CA), followed by purification and DNase-I digestion with RNeasy columns (Qiagen, Valencia, CA). The quality of RNA was determined by the 260/280 ratios, and by gel electrophoresis to visualize the integrity of 18S and 28S bands.
Microarray analysis
Approximately 5 μg of total RNA was converted to [α-32P]-dATP-labeled cDNA probe using MuLV reverse transcriptase and the Atlas human cancer cDNA synthesis primer mix, and then purified with a NucleoSpin column (Clontech, Palo Alto, CA). The human cancer membrane array (588 genes, Clontech, Palo Alto, CA) were used for analysis. The membranes were prehybridized with Expresshyb from Clontech for 2 hr at 68°C, followed by hybridization with the cDNA probe overnight at 68°C. The membranes were then washed four times in 2X SSC/1% SDS, 30 min each, and two times in 0.1X SSC/0.5% SDS for 30 min. The membranes were then sealed with plastic wrap and exposed to a Molecular Dynamics Phosphoimage Screen. Images were analyzed densitometrically using the AtlasImage software (Clontech, version 2.01). Gene expression intensities were first corrected with the external background and then globally normalized as described previously [25].
Real-time RT-PCR analysis
Total RNA was reverse transcribed with MMLV reverse transcriptase and oligodT primers. The PCR primers were designed with Primer Express software (Applied Biosystems, Foster City, CA, USA) and listed in Table 1. The Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) was used for real-time RT-PCR analysis. Differences in gene expression between groups were calculated using cycle time (Ct) values, which were normalized against β-actin as relative and expressed to control.
Table 1.
Primer sequences for real-time RT-PCR analysis
| Gene | GenBank Number | Forward | Reverse |
|---|---|---|---|
| β-actin | X00351 | GTCCACCTTCCAGCAGATGTG | GCATTTGCGGTGGACGAT |
| Bax | NM_004324 | CCGCCGTGGACACAGACT | TTGAAGTTGCCGTCAGAAAACA |
| Caspase3 | NM_004346 | TGGTTCATCCAGTCGCTTTG | CCCGGGTAAGAATGTGCATAA |
| Caspase8 | NM_001228 | ACCAGGCAGGGCTCAAATTT | GCACTGGCTGTTTGCTTCAG |
| c-jun | J04111 | CCAAAGGCTAGTGCGATGTTTC | GGTCACAGCACATGCCACTT |
| c-myc | V00568 | GAGGCGAACACACAACGTCTT | CGCAACAAGTCCTCTTCAGAAA |
| CD11b | M82856 | CGCCCTCTTCCTTTGAATCTC | AACCACAAGGAAGCCACCAA |
| CD14 | M86511 | TAACCTGACACTGGACGGGAAT | CACGCCGGAGTTCATTGAG |
| CD36 | NM_031561 | GGCTAAATGAGACTGGGACCAT | CCAGGCCCAGGAGCTTTATT |
| Cyclin E | X75888 | GCCCTTAAGTGGCGTCTAAGC | CGTTGACATAGGCCACTTGGA |
| EGR1 | X52541 | TGAACGCAAGAGGCATACCA | CCGAAGAGGCCACAACACTT |
| IL1-1β- | NM_000576 | CTTAAAGCCCGCCTGACAGA | TCAGAATGTGGGAGCGAATG |
| MMP9 | NM_004994 | GGACGATGCCTGCAACGT | GTACTTCCCATCCTTGAACAAATACAG |
| MMP11 | X57766 | CCGCCTCTACTGGAAGTTTGA | TCGGCACAGCCAAAGAAGT |
| p53 | M14694 | CCCAGCCAAAGAAGAAACCA | CAGCTCTCGGAACATCTCGAA |
| TIMP1 | NM003254 | TCCCTGCGGTCCCAGATA | GTGGGAACAGGGTGGACACT |
| TIMP2 | NM003255 | TCACCCTCTGTGACTTCATCGT | CCATCTGGTACCTGTGGTTCAG |
| TIMP3 | NM000362 | AACTTGGGTGAAGGCTGAGTGT | CCTCACCAAGGCCTAACAGATG |
| TNF-α | NM_000594 | CCTCAACCTCTTCTGGCTCAAA | CTGAATCCCAGGTTTCGAAGTG |
| TSP1, thrombospondin-1 | X14787 | CATGCCACGGCCAACAA | GGCCCAGGTAGTTGCACTTG |
| VIM, vimentin | X56134 | CGCCAACTACATCGACAAGGT | ACTTGCCTTGGCCCTTGAG |
Immuncytochemistry
HL-60 cells were collected by centrifugation and fixed with 3% paraformaldehyde/0.25glutaldehyde in PBS for 10 min, followed by permeabilization with 0.1% Triton X-100 for 10 min at room temperature. After washing with PBS, cells were blocked for 60 min with.1% bovine serum albumin (BSA) in PBS for 30 min, and were incubated at 37°C for 1 h with primary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) diluted with 1%BSA in PBS (1:100). After rinsing 3 times in PBS, secondary antibodies [Alexa Fluor 488 conjugated goat anti-mouse IgG (H+L) (Invitrogen) for TIMP1, c-myc and CD14 (1:1000) and Alexa Fluor 543 goat anti-rabbit IgG (H+L) (Invitrogen) for caspase-3 (1:500) diluted in PBS containing 1% BSA] were added to the cells and incubated at 37°C for 1 h. Propidium iodide (1 mg/mL) in 1% BSA was used to stain cell nuclei (1:1000) at room temperature for 10 min. After further washing with PBS, cells were transferred to Teflon imaging chamber for confocal microscopy. Negative controls were treated similarly except they were not exposed to primary antibody. Cells were imaged using a Zeiss LSM 510 laser scanning confocal microscope (Carl Zeiss Inc., Thornwood, NY) with a Zeiss C-Apochromat 40x/1.2 water objective lens. Data reported are the results of single experiments that are representative of three replicate experiments.
Statistical analysis
For microarray analysis, pooled cell samples were performed in triplicate and the mean and SEM were calculated for statistical analysis [25]; for RT-PCR analysis, individual samples (n = 3) were used. For comparisons of gene expression between two groups, the Students’ t test was used. For comparisons among 3 or more groups, data were analyzed using a one-way analysis of variance, followed by Duncan’s multiple range tests. The level of significance was set at P < 0.05 in all cases.
Results
JS-K alters gene expression in HL-60 cells in a concentration- and time-dependent manner
In order to determine gene expression changes, cDNA microarrays were performed following treatment of HL-60 cells with 0.5 μM JS-K for 2, 4, 6, and 24 hr (time-course) and at 6 hr after treatment with 0, 0.25, 0.5, and 1.0 μM JS-K (dose-response) in triplicates. The expression of approximately 10% or the 600 genes on the human cancer array was significantly altered. Among these significantly altered genes, 20 were selected from the time-course (Table 2) and dose-response (Table 3) studies. Altered genes included genes related to apoptosis, acute phase protein genes, cell growth regulators, matrix metalloproteases (MMPs), tissue inhibitors of metalloproteases (TIMPs) and others. In general, JS-K-induced gene expression changes occurred in a time- and concentration-dependent manner. The highest changes in gene expression occurred after 24 hr treatments; while at the 6 hr time-point, the highest concentration(1.0 μM) produced the most significant changes. Thus, HL-60 cells treated with various concentrations of JS-K (0, 0.25, 0.5, and 1.0 μM) for 24 hr were further analyzed with RT-PCR for the expression of selected genes based on the potential biological significance.
Table 2.
Microarray analysis of JS-K time-response in human HL-60 myeloid leukemia cells at 0.5 μM concentration
| Protein/gene | Control at 6 h | JS-K2 h | JS-K4 h | JS-K6 h | JS-K24 h | |
|---|---|---|---|---|---|---|
| Apoptosis-related genes | ||||||
| Survivin, apoptosis inhibitor | U75285 | 2562 (1.0) | 0.97 | 1.05 | 1.40 | 1.58* |
| TFAR15, apoptosis-related protein | AF022385 | 560 (1.0) | 1.61 | 1.01 | 1.99* | 2.42* |
| Bcl2A1, BCL-2-related protein A1 | U29680 | 4087 (1.0) | 0.73 | 0.95 | 0.88 | 2.43* |
| Casp4, caspase-4 precursor | U28014 | 15634 (1.0) | 1.12 | 1.15 | 1.23 | 1.42* |
| Casper, a FADD- and caspase-related apoptosis inducer | Y14040 | 1579 (1.0) | 1.64 | 1.35 | 1.42 | 2.45* |
| DAPK1, death-associated protein kinase 1 | X76104 | 1315 (1.0) | 0.91 | 0.85 | 0.80 | 1.73* |
| Acute phase protein genes | ||||||
| EGR1, early growth response protein 1 | X52541 | 2902 (1.0) | 0.86 | 1.41 | 0.72 | 1.78* |
| C-fgr proto-oncogene | M19722 | 12190 (1.0) | 0.77 | 0.94 | 1.00 | 2.06* |
| SOD1, cytosolic superoxide dismutase 1 | K00065 | 24504 (1.0) | 0.91 | 0.93 | 0.80 | 0.48* |
| PIG7, p53-induced gene 7 | AF010312 | 14070 (1.0) | 0.97 | 0.99 | 1.03 | 2.14* |
| TRAF-interacting protein and NF-kappaB activator | U59863 | 2126 (1.0) | 0.85 | 1.25 | 1.18 | 1.86* |
| Cell growth related genes | ||||||
| CDK6, cell division protein kinase 6 | X66365 | 42997 (1.0) | 0.95 | 1.10 | 1.22 | 1.30* |
| CDK9, cell division protein kinase 9 | L25676 | 6319 (1.0) | 0.83 | 1.07 | 1.13 | 1.33* |
| p19, cyclin-dependent kinase 4 inhibitor; p19-INK4D | U40343 | 5795 (1.0) | 0.97 | 1.44 | 1.57 | 3.06* |
| HBEGF, heparin-binding EGF-like growth factor | M60278 | 3400 (1.0) | 1.27 | 1.31 | 0.93 | 2.28* |
| IGFR, insulin-like growth factor I receptor | X04434 | 3241 (1.0) | 0.78 | 0.78 | 0.86 | 0.46* |
| IGF1A, insulin-like growth factor IA precursor | M27544 | 2561 (1.0) | 0.97 | 1.07 | 0.35 | 0.36* |
| MMPs, TIMPs, and others | ||||||
| MMP11, matrix metalloproteinase 11; stromelysin 3 | X57766 | 3880 (1.0) | 0.98 | 1.10 | 1.53* | 2.66* |
| TIMP1, metalloproteinase inhibitor 1 precursor | X03124 | 5093 (1.0) | 0.99 | 1.13 | 1.40* | 2.46* |
| TIMP2, tissue inhibitor of metalloproteinases 2 | J05593 | 856 (1.0) | 0.54 | 0.32 | 1.33 | 2.32* |
| CDH5, cadherin 5; VE-cadherin | X79981 | 46856 (1.0) | 1.00 | 1.04 | 1.14 | 1.24* |
| VIM, vimentin | X56134 | 6951 (1.0) | 1.77 | 1.66 | 1.81* | 2.16* |
The base expression levels (control at 6 h) were given in numbers, and were used as (1.0) to compare with time course changes. Data are fold changes over controls from average of three hybridizations.
Significantly different from controls p < 0.05.
Table 3.
Microarray analysis of JS-K concentration-response in human HL-60 myeloid leukemia cells at 6 h time point
| Protein/gene | GenBank Access# | Control at 6 h | JS-K 0.25 μM | JS-K 0.5 μM | JS-K 1.0 μM |
|---|---|---|---|---|---|
| Apoptosis related genes | |||||
| MGMT, 6-O-methylguanine-DNA methyltransferase | M29971 | 2978 (1.0) | 0.69 | 0.75 | 1.27* |
| Bcl2, apoptosis regulator | M14745 | 4895(1.0) | 1.16 | 1.12 | 1.44* |
| Bad, bcl-2 binding component | U66879 | 1862 (1.0) | 2.03* | 1.33 | 1.65* |
| Bak, bcl2 homologous killer | U23765 | 2934 (1.0) | 1.03 | 1.23 | 1.52* |
| Casp8, caspase-8 precursor | U60520 | 3896 (1.0) | 1.73* | 1.55* | 1.57* |
| Casper, a FADD- and caspase-related apoptosis inducer | Y14040 | 1579 (1.0) | 1.48 | 1.42 | 1.61* |
| DR5, death receptor 5; cytotoxic TRAIL receptor 2 | AF016268 | 2856 (1.0) | 1.72* | 1.25 | 1.46* |
| TRAIL, TNF-related apoptosis inducing ligand | U57059 | 282 (1.0) | 1.61 | 1.06 | 2.66* |
| TRADD, TNFR1-associated death domain protein | L41690 | 793 (1.0) | 1.27 | 1.19 | 2.05* |
| Acute phase protein genes | |||||
| JNK1, c-jun N-terminal kinase 1 | L26318 | 2520 (1.0) | 0.95 | 1.18 | 1.51* |
| Ku (p70/p80) subunit; nuclear factor IV | M30938 | 3380 (1.0) | 1.53 | 1.33 | 1.38* |
| TRAF-interacting protein and NF-kappaB activator | U59863 | 2126 (1.0) | 0.82 | 1.18 | 1.64* |
| Cell growth related genes | |||||
| p19, cyclin-dependent kinase 4 inhibitor, p19-INK4D | U40343 | 5795 (1.0) | 1.61 | 1.57 | 1.41* |
| CCND1, G1/S-specific cyclin D1 | X59798 | 1255 (1.0) | 0.63 | 0.38* | 0.33* |
| CCNE, G1/S-specific cyclin E | M73812 | 2318 (1.0) | 0.52 | 0.16* | 0.15* |
| GADD45, growth arrest & DNA-damage-inducible protein | M60974 | 4696 (1.0) | 1.06 | 0.97 | 1.26* |
| VEGFR1, vascular endothelial growth factor receptor 1 | X51602 | 17352 (1.0) | 1.47 | 1.36* | 1.44* |
| MMPs, TIMPs, and others | |||||
| MMP11, matrix metalloproteinase 11; stromelysin 3 | X57766 | 3880 (1.0) | 1.26 | 1.53 | 1.44* |
| TIMP1, metalloproteinase inhibitor 1 precursor | X03124 | 5093 (1.0) | 1.11 | 1.40* | 1.42* |
| VIM, vimentin | X56134 | 6951 (1.0) | 1.52 | 1.81* | 1.67* |
The base expression levels (control at 6 h) were given in numbers, and were used as (1.0) to compare with dose-dependent changes. Data are fold changes over controls from average of three hybridizations.
Significantly different from controls p < 0.05.
JS-K induced apoptotic gene expression correspondence to apoptosis in HL-60 cells
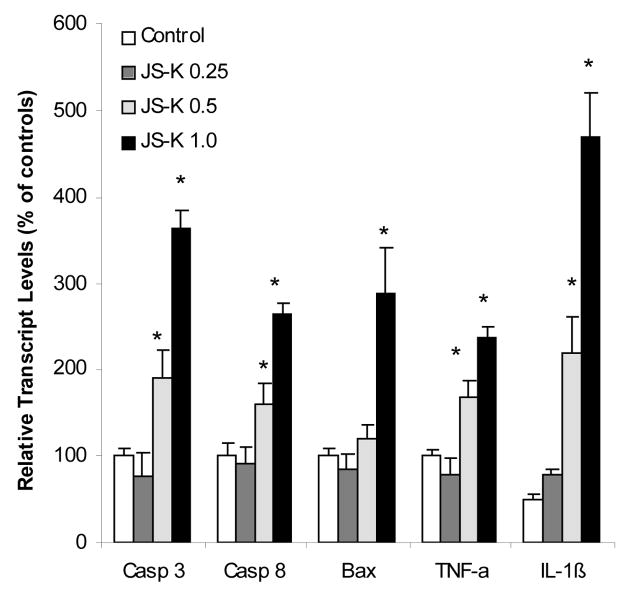
JS-K treatment of HL-60 cells induces apoptosis [7, 13]. Consistent with JS-K-induced apoptosis in HL-60 cells (data not shown), the expression of caspase (Casp)-3 (apoptosis executor) and Casp-8 (apoptosis initiator) were increased about 3 fold in JS-K-treated cells (Fig. 1). In addition to Casp activation, the apoptosis protein gene Casper (data not shown) was also increased 3 fold. Expressions of TNF-α and IL-1β were increased 2.5 and 9 folds, respectively (Fig. 1), in agreement with previous observations of the effects of NO on HL-60 cells [1]. RT-PCR confirmed and extended microarray results. One can therefore conclude that gene expression analysis clearly indicates that apoptotic pathways were activated by JS-K in HL-60 cells.
Figure 1.
Effect of JS-K on the expression of apoptosis-related genes. HL-60 cells were treated with 0, 0.25, 0.5, and 1.0 μM JS-K for 24 hr, and total purified RNA was subjected to real-time RT-PCR analysis. Data are mean and SEM (n = 3). *Significantly different from controls, p < 0.05.
JS-K induced the differentiation genes in HL-60 cells
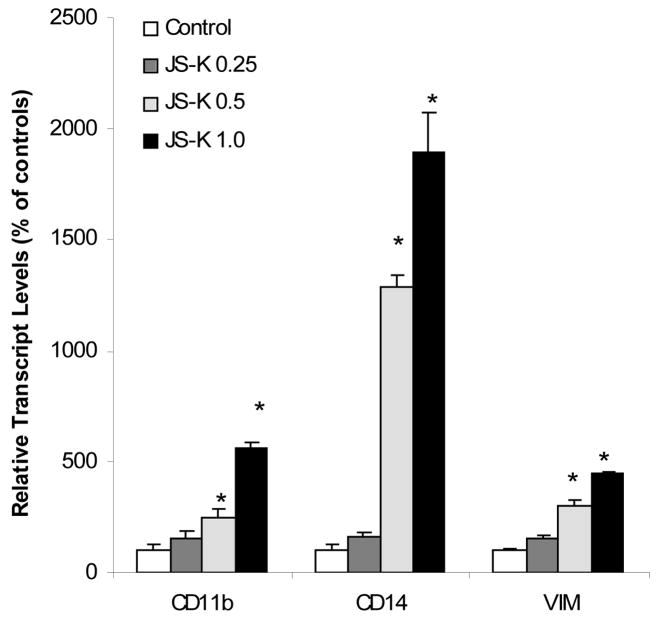
An important effect of NO on leukemia cells (including NO generated by JS-K) is the induction of differentiation along the monocytic pathway [1,2,7]. Thus, the effects of JS-K on monocytic differentiation markers were examined. As shown in Fig. 2, JS-K treatment increased the expression of CD11b and CD14. The increase in CD14 was as high as 19-fold at the highest dose of JS-K. Expression of vimentin (VIM), a gene associated with the late differentiation of HL-60 cells [15], was also increased by 4.5 folds (Fig. 2).
Figure 2.
Effect of JS-K on the expression of cell differentiation-related genes. HL-60 cells were treated with 0, 0.25, 0.5, and 1.0 μM JS-K for 24 hr, and total purified RNA was subjected to real-time RT-PCR analysis. Data are mean and SEM (n = 3). *Significantly different from controls, p < 0.05.
JS-K altered the expression of acute phase response genes in HL-60 cells
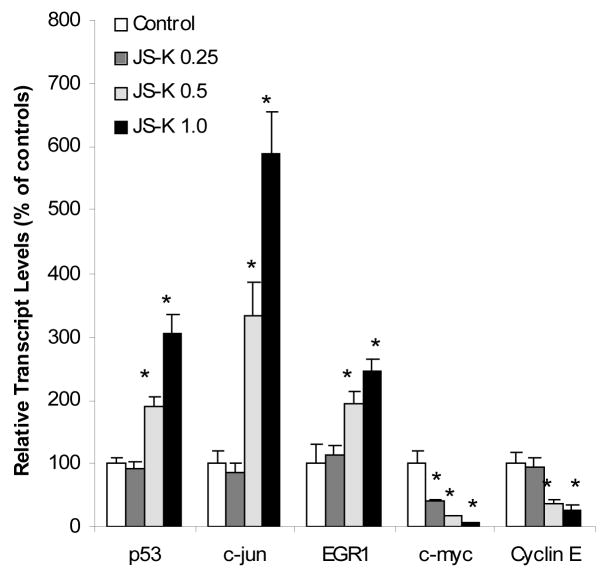
JS-K is known to activate acute phase proteins such as c-jun and c-fos and MAP kinases in Hep3B hepatoma cells, CAsE cells, and multiple myeloma cells [8–10]. In microarray analysis, acute phase protein genes EGR1, c-fgr, MAP kinases (JNK), and p53-induced protein PIG7 were all increased; while cell growth related genes such as cyclins and insulin-like growth factors (IGFs) were decreased (Table 2 and 3). Real-time RT-PCR (Fig. 3) shows that JS-K increased the expression of p53, c-jun, and EGR1 in a dose-dependent fashion, but decreased the expression of c-myc and cyclin E. In HL-60 cells, the expression of c-myc had been shown to be depressed by the NO donor sodium nitroprusside and NO gas [1], and the downregulation of c-myc by valproic acid was associated with HL-60 cell growth arrest [16].
Figure 3.
Effect of JS-K on the expression of acute phase protein genes. HL-60 cells were treated with 0, 0.25, 0.5, and 1.0 μM JS-K for 24 hr, and total purified RNA was subjected to real-time RT-PCR analysis. Data are mean and SEM (n = 3). *Significantly different from controls, p < 0.05.
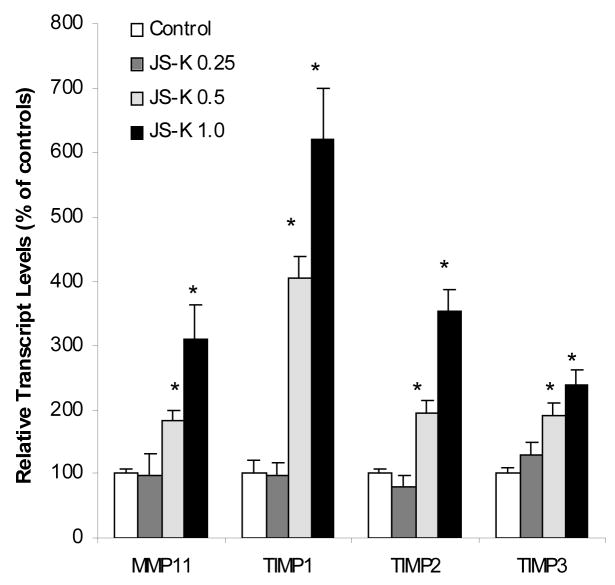
JS-K increased the expression of genes of anti-angiogenesis and anti-tumor cell migration
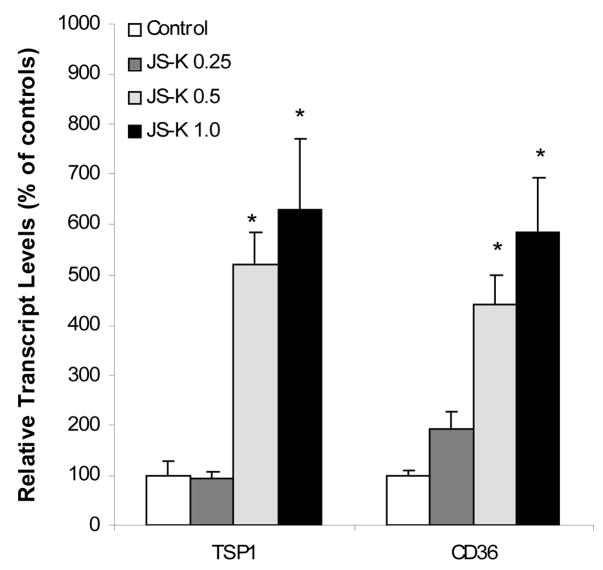
JS-K has been shown to inhibit angiogenesis in vitro [17]. Thus, we examined the effect of JS-K on anti-angiogenesis gene expression in HL-60 cells. Expression of the anti-angiogenesis gene thrombospodin-1 (TSP-1) and its receptor CD36 were significantly increased by JS-K in a concentration-dependent manner (Fig. 4). One of the interesting findings from microarray analysis was alterations in expression of matrix metalloproteinases (MMPs) and tissue inhibitor of matrix metalloproteinases (TIMPs) (Table 2 and 3). To confirm microarray findings, the expression of MMP11, TIMP1, TIMP2, and TIMP 3 were examined (Fig. 5). Consistent with microarray findings, these genes were all upregulated over 3 folds, and up to 6-fold increase in TIMP1 expression was evident. Of note is that TIMP1 plays an antiangiogenesis role in mammalian cells and the inhibition of TIMP1 enhances angiogenesis both in vivo and in vitro [18].
Figure 4.
Effect of JS-K on the expression of anti-angiogenesis-related genes. HL-60 cells were treated with 0, 0.25, 0.5, and 1.0 μM JS-K for 24 hr, and total purified RNA was subjected to real-time RT-PCR analysis. Data are mean and SEM (n = 3). *Significantly different from controls, p < 0.05.
Figure 5.
Effect of JS-K on the expression of MMPs and TIMPs. HL-60 cells were treated with 0, 0.25, 0.5, and 1.0 μM JS-K for 24 hr, and total purified RNA was subjected to real-time RT-PCR analysis. Data are mean and SEM (n = 3). *Significantly different from controls, p < 0.05.
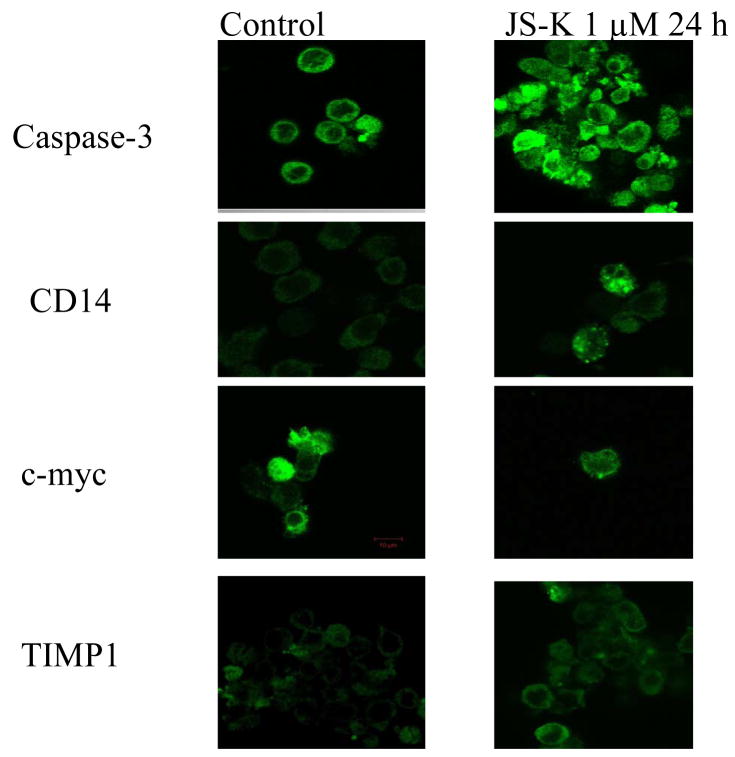
Confocal imaing analysis of JS-K altered protein expression in HL-60 cells
To verify the above gene expression changes, confocal imaging analysis was conducted to examine the expression and localization of relevant proteins at the cellular levels (Fig. 6). The intensity of apoptosis executor caspase-3 was markedly enhanced, especially in apoptotic cells; the expression of CD14 was also markedly enhanced, mainly in the cytoplasma; while the expression of c-myc was attenuated, consistent with mRNA data; and the expression of TIMP1 was universally increased, mainly located in the cytosol of HL-60 cells.
Figure 6.
Representative confocal image analysis of the expression of caspase-3, CD14, c-myc, and TIMP1. HL-60 cells were treated with 1.0 μM JS-K or leave untreated for 24 hr. Cells were then harvested, fixed, permeabilized, and incubated with specific primary antibodies and fluorescence labelled secondary antibodies for confocal imaging as detailed in Methods.
Discussion
This study initially used cDNA microarray to profile gene expression changes produced by JS-K in human leukemia HL-60 cells. Both time-course and dose-response studies showed that JS-K modulated the expression of genes related to apoptosis, cell differentiation, acute phase response, anti-angiogenesis, and tumor cell migration. Real-time RT-PCR and confocal image analysis at the 24 hr time point verified the selected key gene expression changes at both transcript levels and the protein levels. Thus, multiple molecular targets are clearly involved in anticancer effects of JS-K.
NO and NO donors have long been recognized to induce human leukemia cell apoptosis and differentiation [1,2,7]. The apoptotic effects of NO prodrugs are also associated with acute phase protein/gene induction (e.g., c-jun and c-fos) and MAP kinase pathway activation (e.g., JNK and ERK) [8–10,19]. The TNF-α p53, and Fas/CD95 pathways, as well as activation of caspase cascades and apoptotic proteins (such as Bad, Bax, and Mcl-1), are involved in JS-K-induced apoptosis in various tumor cells [7,10,13]. The current gene expression analysis clearly demonstrated that JS-K induced apoptosis and apoptosis-related gene expression are a major mechanism for tumor cell killing.
Induction of human myeloid cell differentiation is an important anti-leukemic mechanism for NO donors, including JS-K [1,2,7]. In the present study, the expression of CD14, a marker of monocytic differentiation, was increased up to 19 fold by JS-K, an effect too dramatic to ignore. Expression of CD11b and vimentin was also increased about 4 fold. Both molecules are known to be involved in NO-induced differentiation in HL-60 cells [1,2,15]. The induction of leukemia cell differentiation is an important mechanism for JS-K’s anti-leukemic properties.
JS-K is known to activate acute phase proteins such as c-jun and c-fos and MAP kinases in Hep3B hepatoma cells, CAsE cells, and multiple myeloma cells [8–10]. In microarray analysis, a number of acute phase protein genes were increased, including p53 and early growth response protein-1 (EGR1). A recent work demonstrates that JS-K inhibition of ubiquitin-activating enzyme E1 activity may preferentially induce apoptosis in p53-expressing tumor cells [20]. In contrast to upregulation of other acute proteins, the expression of c-myc, another major acute phase protein, was decreased by JS-K at both transcript and protein levels, together with decreased expression of cyclin E. These effects may be specific for myeloid leukemia cells, as it has been reported that the expression of c-myc was decreased by the NO donor sodium nitroprusside and NO gas [1], and the downregulation of c-myc is associated with HL-60 cell growth arrest [16].
JS-K has been shown to block angiogenesis by inhibiting proliferation, migration, and cord formation in human umbilical vein endothelial cell cultures [17]. This promoted us to examine the expression of anti-angiogenesis genes, and indeed, two of anti-angiogenesis protein genes, TSP-1 and its receptor CD36, were significantly increased by JS-K, supporting a role for JS-K as an anti-angiogenesis modulator. NO has been shown to have a dual, biphasic response in tumor biology, including the modulation of the gatekeeper TSP-1 in tumor angiogenesis [21,22]. TSP-1 is known to act through its receptor CD36 to inhibit cell proliferation and tumor cell angiogenesis [22]. Activation of TSP-1 and CD36 is also an important pathway to initiate tumor cell apoptosis via MAP kinases and to induce p53-mediated cascade with resultant cell growth arrest [23]. It should also be noted that increased expression of TIMP1 in the present study can also exert anti-angiogenic effect [18,24].
Microarray analysis revealed that JS-K increased the expression of MMPs and TIMPs, which was further verified by real-time RT-PCR analysis. In breast cancer cells, induction of TIMP2 and TIMP1 by JS-K are thought to play an important role is JS-K’s anti-invasive effects against breast cancer MDA-MB-231, F10, and MCF-7/COX2 cells [25]. MMPs and TIMPS regulate extracellular matrix turnover and remodeling during normal development and pathogenesis. They also play important roles in tumor cell signaling, tumor cell progression, angiogenesis, and tumor metastasis [24–26]. The dramatic induction of TIMPs by JS-K in HL-60 myeloid leukemia cells is a novel finding, and its contribution to inhibit tumor cell migration and metastasis is worthy of further investigation.
The exact mechanism by which JS-K effects these gene expression changes in acute myeloid leukemia cells are unknown. As a NO generator, JS-K is bound to cause significant oxidative stress with resultant changes in stress response genes. Kiziltepe et al have also observed that JS-K induces DNA strand breaks in multiple myeloma cells [10]. A similar mechanism is likely at play in myeloid leukemia cells with resultant induction of stress response genes. However, NO delivery to malignant cells is not the only cytotoxic mechanism for JS-K. Arylation of free protein thiols with resultant modification of protein function is also likely to play a role in JS-K’s anti-neoplastic effects [11]. Furthermore, Townsend et al have shown that the JS-K derivative PABA/NO is cytotoxic to ovarian cancer cells through a mechanism involving protein thiol glutathionylation [27]. It is therefore likely that JS-K modulates gene expression through modification of key transcription factors or signaling molecules via arylation and glutathionylation reactions.
In summary, this gene expression analysis revealed several potential mechanisms by which JS-K kills HL-60 cells in a concentration- and time-dependent manner. These mechanisms include activation of the p53- and TNF-α-mediated apoptotic pathways via acute phase responses, activation of the caspase cascade and induction of differentiation in myelogenous leukemia cells. In addition, JS-K induction of anti-angiogenesis gene expression and increased expression of TIMPs suggest a potential effect against tumor cell invasion and metastasis. These results could shed light on molecular targets for anticancer effects of JS-K, a lead O2-arylated diazeniumdiolate NO prodrug.
Acknowledgments
The authors thank Drs. Chikara Kojima and Jean-Francois Coppin and for their review of this manuscript. Research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Centre for Cancer Research, the Federal funds from the National Cancer Institute, National Institutes of Health, under contract No. NO1-CO-12400. Research was also supported by a Translational Research Award from the Leukemia and Lymphoma Society and NCI grant R01CA84496 (PJS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Magrinat G, Mason SN, Shami PJ, Weinberg JB. Nitric oxide modulation of human leukemia cell differentiation and gene expression. Blood. 1992;80:1880–1884. [PubMed] [Google Scholar]
- 2.Shami PJ, et al. Nitric oxide modulation of the growth and differentiation of freshly isolated acute non-lymphocytic leukemia cells. Leuk Res. 1995;19:527–533. doi: 10.1016/0145-2126(95)00013-e. [DOI] [PubMed] [Google Scholar]
- 3.Shami PJ, Sauls DL, Weinberg JB. Schedule and concentration-dependent induction of apoptosis in leukemia cells by nitric oxide. Leukemia. 1998;12:1461–1466. doi: 10.1038/sj.leu.2401131. [DOI] [PubMed] [Google Scholar]
- 4.Keefer LK. Progress toward clinical application of the nitric oxide-releasing diazeniumdiolates. Annu Rev Pharmacol Toxicol. 2003;43:585–607. doi: 10.1146/annurev.pharmtox.43.100901.135831. [DOI] [PubMed] [Google Scholar]
- 5.Saavedra JE, et al. Esterase-sensitive nitric oxide donors of the diazeniumdiolate family: in vitro antileukemic activity. J Med Chem. 2000;43:261–269. doi: 10.1021/jm9903850. [DOI] [PubMed] [Google Scholar]
- 6.Townsend DM, Tew KD. The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene. 2003;22:7369–7375. doi: 10.1038/sj.onc.1206940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shami PJ, et al. JS-K, a glutathione/glutathione S-transferase-activated nitric oxide donor of the diazeniumdiolate class with potent antineoplastic activity. Mol Cancer Ther. 2003;2:409–417. [PubMed] [Google Scholar]
- 8.Ren Z, et al. JS-K, a novel non-ionic diazeniumdiolate derivative, inhibits Hep 3B hepatoma cell growth and induces c-Jun phosphorylation via multiple MAP kinase pathways. J Cell Physiol. 2003;197:426–434. doi: 10.1002/jcp.10380. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, et al. Nitric oxide prodrugs and metallochemotherapeutics: JS-K and CB-3-100 enhance arsenic and cisplatin cytolethality by increasing cellular accumulation. Mol Cancer Ther. 2004;3:709–714. [PubMed] [Google Scholar]
- 10.Kiziltepe T, et al. JS-K, a GST-activated nitric oxide generator, induces DNA double-strand breaks, activates DNA damage response pathways, and induces apoptosis in vitro and in vivo in human multiple myeloma cells. Blood. 2007;110:709–718. doi: 10.1182/blood-2006-10-052845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shami PJ, et al. Antitumor activity of JS-K [O2-(2,4-dinitrophenyl) 1-[(4-ethoxycarbonyl)piperazin-1-yl]diazen-1-ium-1,2-diolate] and related O2-aryl diazeniumdiolates in vitro and in vivo. J Med Chem. 2006;49:4356–4366. doi: 10.1021/jm060022h. [DOI] [PubMed] [Google Scholar]
- 12.Chakrapani H, et al. Synthesis, mechanistic studies, and anti-proliferative activity of glutathione/glutathione S-transferase-activated nitric oxide prodrugs. Bioorg Med Chem. 2008;16:9764–9771. doi: 10.1016/j.bmc.2008.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Udupi V, Yu M, Malaviya S, Saavedra JE, Shami PJ. JS-K, a nitric oxide prodrug, induces cytochrome c release and caspase activation in HL-60 myeloid leukemia cells. Leuk Res. 2006;30:1279–1283. doi: 10.1016/j.leukres.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Saavedra JE, et al. The secondary amine/nitric oxide complex ion R(2)N[N(O)NO] (−) as nucleophile and leaving group in S9N)Ar reactions. J Org Chem. 2001;66:3090–3098. doi: 10.1021/jo0016529. [DOI] [PubMed] [Google Scholar]
- 15.Rius C, Aller P. Vimentin expression as a late event in the in vitro differentiation of human promonocytic cells. J Cell Sci. 1992;101:395–401. doi: 10.1242/jcs.101.2.395. [DOI] [PubMed] [Google Scholar]
- 16.Cheng YC, et al. Downregulation of c-Myc is critical for valproic acid-induced growth arrest and myeloid differentiation of acute myeloid leukemia. Leuk Res. 2007;31:1403–1411. doi: 10.1016/j.leukres.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Shami PJ, Kaur G, Thillainatham L, Jia L, Saavedra JE, Keefer LK. JS-K, a novel nitric oxide (NO) generator, shows potent anti-angiogenic activity. Blood. 2004;104(Suppl 1):931a. [Google Scholar]
- 18.Reed MJ, Koike T, Sadoun E, Sage EH, Puolakkainen P. Inhibition of TIMP1 enhances angiogenesis in vivo and cell migration in vitro. Microvasc Res. 2003;65:9–17. doi: 10.1016/s0026-2862(02)00026-2. [DOI] [PubMed] [Google Scholar]
- 17.Townsend DM, Findlay VL, Tew KD. Glutathione S-transferases as regulators of kinase pathways and anticancer drug targets. Methods Enzymol. 2005;401:287–307. doi: 10.1016/S0076-6879(05)01019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitagaki J, et al. Nitric oxide prodrug JS-K inhibits ubiquitin E1 and kills tumor cells retaining wild-type p53. Oncogene. 2008 Nov 3; doi: 10.1038/onc.2008.401. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ridnour LA, et al. The biphasic nature of nitric oxide responses in tumor biology. Antioxid Redox Signal. 2006;8:1329–1337. doi: 10.1089/ars.2006.8.1329. [DOI] [PubMed] [Google Scholar]
- 19.Roberts DD, Isenberg JS, Ridnour LA, Wink DA. Nitric oxide and its gatekeeper thrombospondin-1 in tumor angiogenesis. Clin Cancer Res. 2007;13:795–798. doi: 10.1158/1078-0432.CCR-06-1758. [DOI] [PubMed] [Google Scholar]
- 20.Yamauchi M, Imajoh-Ohmi S, Shibuya M. Novel antiangiogenic pathway of thrombospondin-1 mediated by suppression of the cell cycle. Cancer Sci. 2007;98:1491–1497. doi: 10.1111/j.1349-7006.2007.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chirco R, Liu XW, Jung KK, Kim HR. Novel functions of TIMPs in cell signaling. Cancer Metastasis Rev. 2006;25:99–113. doi: 10.1007/s10555-006-7893-x. [DOI] [PubMed] [Google Scholar]
- 22.Simeone AM, et al. TIMP-2 mediates the anti-invasive effects of the nitric oxide-releasing prodrug JS-K in breast cancer cells. Breast Cancer Res. 2008;10:R44. doi: 10.1186/bcr2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 24.Townsend DM, et al. A glutathione S-transferase pi-activated prodrug causes kinase activation concurrent with S-glutathionylation of proteins. Mol Pharmacol. 2006;69:501–508. doi: 10.1124/mol.105.018523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J, Xie Y, Ward JM, Diwan BA, Waalkes MP. Toxicogenomic analysis of aberrant gene expression in liver tumors and nontumorous livers of adult mice exposed in utero to inorganic arsenic. Toxicol Sci. 2004;77:249–257. doi: 10.1093/toxsci/kfh055. [DOI] [PubMed] [Google Scholar]