Abstract
Latent inhibition (LI) manifests as poorer conditioning to a stimulus that has previously been experienced without consequence. There is good evidence of dopaminergic modulation of LI, as the effect is reliably disrupted by the indirect dopamine (DA) agonist amphetamine. The disruptive effects of amphetamine on LI are reversed by both typical and atypical antipsychotics, which on their own are able to facilitate LI. However, the contribution of different DA receptors to these effects is poorly understood. Amphetamine effects on another stimulus selection procedure, overshadowing, have been suggested to be D1-mediated. Thus, in the current experiments, we systematically investigated the role of D1 receptors in LI. First, we tested the ability of the full D1 agonist SKF 81297 to abolish LI and compared the effects of this drug on LI and overshadowing. Subsequently, we examined whether the D1 antagonist SCH 23390 can lead to the emergence of LI under conditions that do not produce the effect in normal animals (weak pre-exposure). Finally, we tested the ability of SCH 23390 to block amphetamine-induced disruption of LI. We found little evidence that direct stimulation of D1 receptors abolishes LI (although there was some attenuation of LI at 0.4 mg/kg SKF 81297). Similarly, SCH 23390 failed to enhance LI. However, SCH 23390 did block amphetamine-induced disruption of LI. These data indicate that, while LI may be unaffected by selective manipulation of activity at D1 receptors, the effects of amphetamine on LI are to some extent dependent on actions at D1 receptors.
Keywords: D1 receptor, latent inhibition, overshadowing, SCH 23390, SKF 81297
Introduction
Latent inhibition (LI) manifests as poorer conditioning to a stimulus that has previously been experienced (‘pre-exposed’) without consequence (Lubow & Moore, 1959). There is compelling evidence that the normal effect of pre-exposure at conditioning is modulated by dopaminergic mechanisms (Weiner, 1990, 2003). First, LI is abolished by the indirect dopamine (DA) agonist amphetamine and this effect is antagonized by both typical and atypical antipsychotics (e.g. Crider et al. 1982; Solomon et al. 1981; Weiner et al. 1984). Moreover, under experimental conditions that do not produce LI in controls, antipsychotics given on their own are able to facilitate LI (Feldon & Weiner, 1991; Shadach et al. 2000; Weiner & Feldon, 1987). Further studies have shown that both the disruption and enhancement of LI by dopaminergic drugs is modulated by the mesolimbic DA system. For example, infusions of amphetamine into the nucleus accumbens disrupt LI whereas haloperidol microinjected into the shell enhances LI (Joseph et al. 2000; Nelson et al. 2011a, b). Disruption to LI manifests as increased conditioning to a stimulus that would normally be treated as irrelevant. As aberrant processing of stimulus salience has been posited to contribute to the cognitive inflexibility seen in schizophrenia (Bleuler, 1911; Cassaday & Moran, 2010; Gray et al. 1991, 1999; Kapur, 2003, 2004), LI has gained widespread acceptance as a preclinical model for schizophrenic attention disorder.
LI is just one of a series of procedures employed to examine the neural substrates of stimulus selection. For example, overshadowing procedures use the relative intensity of competing cues to manipulate associability. Normally a more intense stimulus acquires associative strength at the expense of a relatively less intense stimulus (Pavlov, 1927). Overshadowing has similarly been shown to be disrupted by acute treatment with amphetamine (O'Tuathaigh & Moran, 2002, 2004; O'Tuathaigh et al. 2003).
Although both LI and overshadowing can be disrupted by amphetamine, there is evidence to suggest that the pharmacological profile of these effects may differ. For example, the disruptive effects of amphetamine on LI are blocked by typical antipsychotics, such as haloperidol, as well as by atypical antipsychotics (e.g. Gosselin et al. 1996; Warburton et al. 1994; Weiner et al. 1996). It has therefore been suggested that the effects of these drugs on LI are meditated by their actions at D2 receptors (e.g. Weiner, 2003). On the other hand, the disruptive effects of amphetamine on overshadowing are not blocked by the D2 antagonists haloperidol, raclopride or sulpriride but are reversed by the D1 antagonist SCH 23390 (O'Tuathaigh & Moran, 2002, 2004). These data indicate that amphetamine effects on LI and overshadowing are differentially sensitive to antagonism of D1 and D2 receptors. Consistent with this dissociable pharmacological profile, the partial D1 agonist SKF 38393 is able to disrupt overshadowing but not LI (Feldon et al. 1991; Loskutova et al. 2010; O'Tuathaigh & Moran, 2002; Zelikowsky & Fanselow, 2010).
However, recent evidence has suggested that D1 receptors may, under certain circumstances, be involved in the mediation of LI (Bay-Richter et al. 2009). Moreover, to date, the effects of a full D1 agonist have not been tested on LI and although there is evidence that D1 antagonists do not potentiate LI on their own (Trimble et al. 2002), the ability of a D1 antagonist to reverse amphetamine-induced disruption of LI has not been examined. Thus, in the current experiments we compared the effects of the full D1 agonist SK 81297 (at two doses) on both LI and overshadowing. Where disruptive effects of the agonist were demonstrated, expt 2 followed up on these results, examining the effects the effects of a D1 antagonist (SCH 23390), now using experimental parameters suitable to test for behavioural enhancement (reduced number of stimulus pre-exposures to produce a weaker LI effect). Finally, using the same parameters as expt 1 [30 conditioned stimulus (CS) pre-exposures, which yield robust LI in normal animals and are suitable to show amphetamine-induced abolition], we examined whether SCH 23390 would block the disruptive effects of amphetamine on LI.
Experimental procedures
Subjects
Subjects were adult male Wistar rats (Charles River, UK), caged in pairs on a 12-h light/dark cycle (lights on 07:00 hours) with food and water ad libitum. Rats were handled for approximately 10 min/d for 1 wk. In expts 1a, 1b and 2, there were 72 animals run in a single replication. Expt 3 was run in two replications with 48 rats per replication.
All procedures were carried out in accordance with the UK Animals Scientific Procedures Act 1986, Project Licence number: PPL 40/3163. The UK Act ensures full compliance with the ‘Principles of laboratory animal care’ (NIH publication No. 86-23, revised 1985).
Apparatus
Six identical fully automated conditioning chambers, housed within sound-attenuating cases containing ventilation fans (Cambridge Cognition, UK), were used. Each of the inner conditioning chambers consisted of a plain steel box (25 cm×25 cm×22 cm high) with a Plexiglas door (27 cm×21 cm high) at the front. The floor was a shock grid with steel bars 1 cm apart and 1 cm above the lip of a 7 cm deep sawdust tray. A waterspout was mounted on one wall. The spout was 5 cm above the floor and connected to a lickometer supplied by a pump. Licks were registered by a break in the photo beam within the spout, which also triggered water delivery of 0.05 ml per lick. The waterspout was illuminated when water was available. A loudspeaker for the presentation of auditory stimuli was set in the roof. In all but expt 2b, a 5 s flashing light, provided by the three wall-mounted stimulus lights and the house light flashing both on (0.5 s) and off (0.5 s) served as the CS. In the overshadowing condition (expt 1 only), the 5 s light CS was presented in compound with a 5 s mixed frequency noise set at 85 dB (including background noise from the fans). In expt 2b, the 5 s mixed frequency noise (again set at 85 dB including background noise from the fans) was used as the CS. Footshock of 1 s duration and 1 mA intensity provided the unconditioned stimulus (UCS). This was delivered through the grid floor by a constant current shock generator (pulsed voltage: output square wave 10 ms on, 80 ms off, 370 V peak under no load conditions; MISAC Systems, UK). Stimulus control and data collection was by an Acorn Archimedes RISC computer programmed in Basic with additional interfacing using an Arachnid extension (Cambridge Cognition).
Drugs
SKF 81297 (Tocris Bioscience, UK) at a dose of 0.8 mg/kg (expt 1a) and 0.4 mg/kg (expt 1b) was administered (s.c.) 15 min prior to the pre-exposure and conditioning stages of the experiment.
In expt 2, SCH 23390 (Tocris Bioscience) was administered at doses of 0.025 mg/kg or 0.05 mg/kg s.c. 15 min before both the pre-exposure and the conditioning stages.
In expt 3, d-amphetamine (Amph; Sigma, UK) was administered at a dose of 1 mg/kg s.c. 15 min before both pre-exposure and conditioning. SCH 23390 (Tocris Bioscience) at a dose of 0.02 mg/kg was given 15 min before pre-exposure and conditioning. Animals in the Amph+SCH 23390 group received an injection of Amph (1 mg/kg s.c.) immediately followed by an injection of SCH 23390 (0.02 mg/kg s.c.) 15 min before each stage of the LI procedure. The SCH 23390, Amph and saline controls animals each received an additional saline injection so that all animals in expt 3 received two injections.
In each experiment, drugs were dissolved in physiological saline to an injection volume of 1 ml/kg. All doses were calculated as the salt. Controls received an equivalent volume of saline. Reshape and test sessions were all conducted drug-free.
The 1 mg/kg dose of amphetamine is well-established as a dose that disrupts LI (e.g. Nelson et al. 2011a). SCH 23390 doses in the range of 0.01–0.05 mg/kg have previously been shown to antagonize amphetamine abolition of overshadowing as well as block the disruptive effects of nicotine on LI (O'Tuathaigh & Moran, 2002; Young et al. 2005). The dose of SCH 23390 was reduced to 0.02 mg/kg in expt 3, based on the results of expt 2, and to match that demonstrated to reverse the effects of nicotine on LI measured in the same fear conditioning procedure (Young et al. 2005). The SKF 81297 doses were chosen on the basis of their effects in an operant conditioning paradigm (Cheung et al. 2007).
Procedure
Water deprivation was introduced 1 d prior to shaping. Thereafter, the animals received 1 h and 15 min of ad libitum access to water in their home cage in addition to water in the experimental chambers. The stages of the conditioned emotional response procedure used in expts 1–3 were as follows.
Pre-training
Rats were shaped for 1 d until all drank from the waterspout and were individually assigned to a conditioning box for the duration of the experiment.
There then followed 5 d pre-training, in which rats drank in the experimental chamber for 15 min each day (timed from first lick). The drinking spout was illuminated throughout, but no other stimuli were presented in this phase. Latency to first lick was measured as an indicator of habituation to the experimental context. Total number of licks was also recorded each day to assess any pre-existing differences in drinking (prior to conditioning).
Pre-exposure
Animals were placed in the chambers where the pre-exposed (PE group) animals received 30×5 s light CS presentations (expts 1 and 3) and 10×5 s light CS presentations (expt 2a), with an average inter-stimulus interval of 60 s. In expt 2b, the PE group were given 10×5 s noise CS presentations. The non-pre-exposed (NPE) control animals and overshadowed groups (expt 1 only) were confined to the chambers for an identical period of time (30 or 10 min) without receiving any CS presentations. Water was not available within the chamber and the waterspout was not illuminated during the pre-exposure session.
Conditioning
Conditioning was conducted on the day following pre-exposure. No water was available within the chamber and the waterspout was not illuminated. There were two conditioning trials, in which the UCS footshock was delivered following termination of the CS. The first pairing of CS and UCS was presented after 5 min had elapsed and the second pairing was 5 min after the first, followed by a further 5 min left in the apparatus. In the absence of drinking, there were no behavioural measures to record.
Reshaping
On the day following conditioning, animals were reshaped following the same procedure as in pre-training sessions. This was in order to re-establish drinking after conditioning. Reshaping also provided measures of conditioning to the box context (latency to first lick).
Test
On the day following reshape, the animals were placed in the conditioning chambers and underwent an extinction test to the light (or noise in expt 2b) CS. Water was available throughout the test and the waterspout was illuminated. Once the animals had made 50 licks, the CS was presented for 15 min. The latency to make 50 licks in the absence of the CS (‘A’ period) provided a measure of any individual variation in baseline lick responding. This was compared with the time taken to complete 50 licks following CS onset (‘B’ period) in a suppression ratio (A/(A+B)) to assess the level of conditioning to the CS, adjusted for any individual variation in drink rate.
Assessment of locomotor activity (expt 4)
In order to confirm whether SCH 23390 is able to block the development of amphetamine sensitization, rats that had previously been treated with either Amph or Amph+SCH 23390 were given an amphetamine challenge and underwent an assessment of locomotor activity. On the day following the CS test, rats received a 0.5 mg/kg amphetamine challenge (s.c.) 10 min before being placed in a novel arena, where locomotor activity was monitored for 30 min. The arena consisted of a Perspex box (39×23.5×24.5 cm) with an exchangeable floor and metal grid lid. This was fitted with two layers of parallel infrared photocell beams, which, when broken, registered activity, recorded by a computer.
Design and analysis
Expts 1a and 1b were run in a 3×2 factorial design with between subject factors of conditioning group (control, PE and overshadowed) and drug (saline and SKF 81297). Expts 2a and 2b were run in a 2×3 factorial design with between subject factors of conditioning group (NPE and PE) and drug (saline and two doses of SCH 23390). Finally, expt 3 was run in a 2×4 factorial design with between subject factors of conditioning group (NPE and PE) and drug (saline, SCH 23390, Amph and Amph+SCH 23390). The pre-training data were subject to an additional repeated measures factor of day. Statistical analysis was performed using analysis of variance (ANOVA) with α set at p<0.05 for the rejection of the null hypothesis. Significant interactions were analysed with simple main effects based on the pooled error term. Where appropriate, LSD post-hoc tests were used to explore differences between groups. Raw latency data (time to first lick at reshape) were log transformed so that their distribution was suitable for parametric analysis.
Results
Expt 1a: effect of 0.8 mg/kg SKF 81297 on overshadowing and LI produced by 30 CS pre-exposures
Pre-exposure
Analysis of the latencies to first lick over the 5 d pre-training revealed an effect of day (F4,264=38.05, p<0.001) as latencies to first lick declined. This trend was unaffected by to-be-conditioned or subsequent drug group (max F8,264=1.15, p=0.33).
Reshape
Overall, the SKF 81297-treated animals took longer to make the first lick in the reshape session following conditioning (F1,66=7.43, p<0.01) [mean log s (±s.e.m.) saline=0.91 (±0.13); SKF 81297=1.36 (±0.11)]. There was also an effect of group (F1,66=5.37, p<0.05) as the overshadowed group had shorter latencies than both the control (p<0.05) and PE group animals (p<0.05). There was no interaction (F<1).
Test
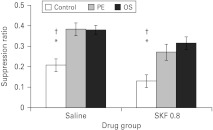
There were no differences in the A period between any of the experimental groups (max F2,66=1.18, p=0.31). The mean suppression ratios to the light CS are displayed in Fig. 1. Inspection of this figure suggests that, although the animals treated with SKF 81297 had overall lower suppression ratios (i.e. greater conditioned suppression), they nonetheless showed robust LI and overshadowing. This observation was confirmed by ANOVA, which yielded an effect of group (F2,66=21.92, p<0.001) and drug (F1,66=12.39, p<0.001) but no interaction. Thus, at a dose of 0.8 mg/kg SKF 81297 produced no selective effects on either LI or overshadowing.
Fig. 1.
The effect of 0.8 mg/kg SKF 81297 (SKF) on latent inhibition and overshadowing. Mean suppression ratios to the light conditioned stimulus (±s.e.m.) for control (white bars), pre-exposed (PE; light grey bars) and overshadowed (OS, dark grey bars) rats that had previously been treated with either saline or 0.8 mg/kg SKF. * Indicates significant difference from similarly treated PE group (p<0.05). † Indicates significant difference from similarly treated OS group (p<0.05).
Expt 1b: effect of 0.4 mg/kg SKF 81297 on overshadowing and LI produced by 30 CS pre-exposures
Pre-training
Latencies to first lick declined over the 5 d pre-training (F4,264=19.57, p<0.001). The rate of decline did differ by the to-be-conditioned group (F4,264=3.47, p<0.05) but by day 5 there were no differences between the groups (all Fs<1).
Reshape
None of the experimental groups differed in the latency to first lick in the reshape session following conditioning (F2,66=1.66, p=0.19).
Test
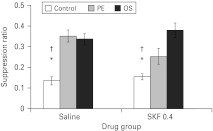
Analysis of the A periods revealed an effect of group (F2,66=3.17, p<0.05) as the control group had higher A periods than the PE group (p<0.05). This effect interacted with drug (F2,66=3.29, p<0.05) as the SKF 81297 controls took longer to make 50 licks compared to the PE (p<0.05) and overshadowed (p<0.05) groups. As the suppression ratio used to measure conditioning to the light explicitly takes account of baseline differences in responding, these effects on the A period are unlikely to account for the test results.
ANOVA revealed an effect of conditioning group (F2,66=31.4, p<0.001) but – as is clear from Fig. 2 – the effects of the conditioning treatment were not equivalent across the two drug groups and there was a conditioning group by drug interaction (F2,66=3.70, p<0.05). The 0.4 mg/kg dose of SKF 81297 was without effect on conditioning in the control and overshadowing groups (max F1,66=1.2, p=0.28) but clearly reduced LI as PE group animals treated with SKF 81297 showed greater conditioning to the light compared to their saline-injected counterparts (F1,66=6.19, p<0.05). It should be noted, however, that this effect reflects an attenuation of LI rather than an abolition as the pre-exposed SKF 81297-treated animals nonetheless showed reduced suppression relative to their non-pre-exposed controls (p<0.05).
Fig. 2.
The effect of 0.4 mg/kg SKF 81297 (SKF) on latent inhibition and overshadowing. Mean suppression ratios to the light conditioned stimulus (±s.e.m.) for control (white bars), pre-exposed (PE, light grey bars) and overshadowed (OS, dark grey bars) rats that had previously been treated with either saline or 0.4 mg/kg SKF. * Indicates significant difference from similarly treated PE group (p<0.05). † Indicates significant difference from similarly treated OS group (p<0.05).
Expt 2a: effect of SCH 23390 on LI produced by 10 light CS pre-exposures
Pre-training
The latency to first lick declined over the 5 d pre-training in all the experimental groups (F4,260=23.73, p<0.001). This trend was unaffected by either drug or conditioning group-to-be (max F8,260=1.61, p=0.123).
Reshape
Analysis of the latency to first lick in the reshape session revealed no effect of conditioning group (F<1) but an effect of drug (F2,65=9.27, p<0.001) as both doses of SCH 23390 reduced the latency to first lick relative to saline-injected animals (min p<0.01). There was no drug×conditioning group interaction (F2,65=2.73, p=0.073).
Light test
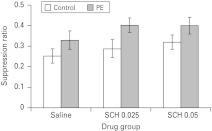
There was no effect of either drug nor of conditioning group on the A period latencies (max F2,65=1.62, p=0.21). The mean suppression ratios to the light CS are presented in Fig. 3. ANOVA revealed a main effect of conditioning group (F2,65=8.15, p<0.01), but no effect of drug (F2,65=1.74, p=0.184) nor an interaction (F<1), indicating that neither dose of SCH 23390 had any effect on the level of LI produced with 10 pre-exposures.
Fig. 3.
The effect of 0.025 or 0.05 mg/kg SCH 23990 (SCH) on latent inhibition [10 light conditioned stimulus (CS) pre-exposures]. Mean suppression ratios to the light CS (±s.e.m.) for non-pre-exposed (control, white bars) and pre-exposed (PE, light grey bars) rats that had previously been treated with either saline or SCH.
Expt 2b: effect of SCH 23390 on LI produced by 10 noise CS pre-exposures
Pre-training
There were no differences between any of the groups in terms of the latency to first lick or total amount drunk in the pre-training session (all Fs<1).
Reshape
There was a main effect of drug (F2,66=5.66, p<0.05) as both doses of SCH 23390 reduced latencies to first lick in the reshape session (both p<0.05). There was, however, no effect of conditioning group and no interaction (both Fs<1).
Noise test
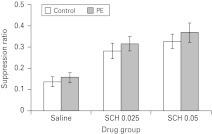
None of the groups differed in the time to make 50 licks in the absence of the noise CS (max F2,66=2.86, p=0.64). Figure 4 displays the mean suppression ratios to the noise CS. As is clear from this figure, both doses of SCH 23390 led to an overall reduction in the conditioned suppression to the noise CS relative to saline-injected animals. However, there was no evidence of differential conditioning to the noise CS by conditioning group. ANOVA revealed an effect of drug (F2,66=19.41, p<0.05) but no effect of conditioning group or interaction (max F1,66=1.45, p=0.23).
Fig. 4.
The effect of 0.025 or 0.05 mg/kg SCH 23990 (SCH) on latent inhibition [10 noise conditioned stimulus (CS) pre-exposures]. Mean suppression ratios to the noise CS (±s.e.m.) for non-pre-exposed (control, white bars) and pre-exposed (PE, light grey bars) rats that had previously been treated with either saline or SCH.
Expt 3: effect of SCH 23390 on amphetamine-induced abolition of LI produced by 30 CS pre-exposures
Pre-training
Latencies to first lick declined over the 5 d pre-training (F4,352=33.89, p<0.001). This trend interacted with the to-be-conditioned group but not drug (F4,352=3.68, p<0.05). The PE group's latencies overall declined more slowly but by day 5 there were no differences between the groups (all Fs<1).
Reshape
Analysis of the time to first to lick revealed no effect of group (F1,88=1.93, p=0.17) but a significant effect of drug (F3,88=16.38, p<0.001). This effect arose because the saline-treated animals took longer to make the first lick compared to the SCH 23390 (p<0.001) and Amph+SCH 23390 (p<0.05) groups but less time relative to animals treated with Amph alone (p<0.05). These effects of drug were not influenced by conditioning group as there was no group×drug interaction (F<1).
Test
There was no effect of either drug or conditioning group on the time to make licks 2–50 in the absence of the CS (F3,88=1.79, p=0.16).
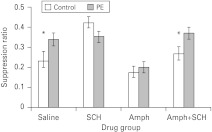
As is clear from Fig. 5, the level of conditioning seen to the CS differed by drug and group. ANOVA revealed no effect of group (F1,88=3.19, p=0.078), an effect of drug (F3,88=12.82, p<0.001) and also a group×drug interaction (F3,88=3.21, p<0.05). As expected, there was an effect of pre-exposure in saline-treated animals (F1,88=5.41, p<0.05) but this effect was abolished in animals-treated with Amph alone (F<1). However, co-administration of SCH 23390 in Amph-treated animals blocked the Amph-induced abolition of LI, in that statistically there was a significant pre-exposure effect in these animals (F1,88=4.88, p<0.05). Unexpectedly, in animals treated with SCH 23390, there was no evidence of differential conditioning by group and (if anything) there was less conditioned suppression in the NPE relative to the PE group, but this trend failed to reach statistical significance (F1,88=2.24, p=0.138). This disruption of LI in animals treated with SCH 23390 appeared to be mediated by drug action in the NPE condition as the level of conditioned suppression in the SCH 23390 NPE group was lower than all other NPE drug groups (p<0.05) but the SCH 23390 PE differed only from the Amph PE group (p<0.05).
Fig. 5.
The effect of 0.02 mg/kg SCH 23390 (SCH) on the disruptive effects of 1 mg/kg amphetamine (Amph) on latent inhibition. Mean suppression ratios to the light conditioned stimulus (CS, ±s.e.m.) for non-pre-exposed (control, white bars) and pre-exposed (PE, light grey bars) rats that had previously been treated with saline, Amph, SCH or Amph+SCH. * Indicates significant difference from similarly treated PE group (p<0.05).
Expt 4: assessment of locomotor activity
Data for 12 of the rats (six Amph and six Amph+SCH 23390) were lost due to computer failure so that there were 18 animals in each of the drug groups. Analysis of the total beam breaks revealed that animals that had previously been treated with Amph+SCH 23390 (at pre-exposure and conditioning) showed lower levels of activity in response to the amphetamine challenge relative to animals that had previously been treated with amphetamine only (F1,34=15.1, p<0.001) [total mean beam breaks (±s.e.m.): Amph group=2297.8 (±85.2); Amph+SCH 23390 group=1812.1(±91.5)].
Discussion
Because of the dose-related reduction in LI (but not overshadowing) after treatment with the D1 agonist SKF 81297, the follow-up experiment with the D1 antagonist SCH 23390 tested for increased selective learning using the LI procedure (only) and with procedural variants standard to test for enhancement of the LI effect (weak pre-exposure). Accordingly, expt 2a used 10 presentations of a 5 s light CS and expt 2b used 10 presentations of a 5 s noise CS. Expt 3 reverted to the standard number of pre-exposures (30) and the same light CS as was used in expt 1 because these parameters yield robust LI in normal animals that is abolished by amphetamine (e.g. Nelson et al. 2011a). The objective of expt 3 was to test for D1-mediated reversal of amphetamine effects on LI. Thus, the current experiments examined the role of D1 receptors in LI and overshadowing. In expt 1, although LI was somewhat reduced by SKF 81297, we found no evidence that activation of D1 receptors was sufficient to clearly abolish LI or overshadowing (O'Tuathaigh & Moran, 2002, 2004). In expt 2, following up on the attenuation of LI under 0.4 mg/kg SKF 81297, antagonism of D1 receptors failed to enhance LI. However, expt 3 provided evidence for D1 receptor involvement in the disruptive effects of amphetamine on LI, in that these were reversed by SCH 23390. Expt 4 suggested a mechanism in that the locomotor activity data are consistent with the conjecture that treatment with SCH 23390 can prevent sensitized DA release.
Previously, the partial D1 agonist SKF 38393 has been shown to have dissociable effects on LI and overshadowing: LI is spared but overshadowing can be abolished by activation of D1 receptors (Feldon et al. 1991; Loskutova et al. 2010; O'Tuathaigh & Moran, 2002; Zelikowsky & Fanselow, 2010). Here we found that at a dose of 0.8 mg/kg the full D1 agonist SKF 81297 led to an overall increase in the level of conditioning to the CS but this effect was found in all three conditioning groups irrespective of the animal's experience of the CS (i.e. whether novel, pre-exposed or conditioned in compound). Thus, despite higher levels of conditioning to the CS relative to saline-injected animals, the SKF 81297 pre-exposed and overshadowed animals nonetheless showed less conditioned suppression to the CS relative to controls treated with SKF 81297. At the lower dose of 0.4 mg/kg, there was also some evidence that SKF 81297 attenuated LI relative to saline-injected animals but there was still differential conditioning to the CS between pre-exposed and non-pre-exposed animals treated with SKF 81297. Given that both LI and overshadowing manifest behaviourally as a reduction in conditioning relative to control animals that have not been pre-exposed to the CS or conditioned to the CS in compound with a more intense CS, it is not the absolute magnitude of conditioning to the CS that is the critical determinant of the actions of a drug on LI and overshadowing, but rather the level of conditioning relative to control animals treated with that drug. On this basis, SKF 81297 cannot be said to have abolished either LI or overshadowing in the current experiments.
With regard to LI, these results are consistent with what has previously been found with the partial D1 agonist SKF 38393 (Feldon et al. 1991; Loskutova et al. 2010) and provide yet further evidence that preferential stimulation of D1 receptors does not abolish LI. It remains possible, however, that as both the direct D2/3 agonist quinpirole as well as the direct D1/2 agonist apomorphine are equally without effect on LI (Feldon et al. 1991; Loskutova et al. 2010), the failure of SKF 81297 to abolish LI may not be entirely attributable to the DA receptor specificity of this agent but rather to its being a direct agonist. It appears that LI is only affected by impulse-dependent DA release whereas the DA release elicited by direct agonists is not coupled to impulse flow and, as such, LI is spared following treatment with direct agonists (Gray et al. 1997; Weiner, 2003; Young et al. 2005).
There are reports from two independent laboratories of abolished overshadowing following treatment with the partial D1 agonist SKF 38393 (O'Tuathaigh & Moran, 2002; Zelikowsky & Fanselow, 2010); a finding that we have failed to replicate here with the full agonist SKF 81297. This apparent dissociation in the effects of SKF 81297 and SKF 38393 might be taken as evidence to suggest that overshadowing is differentially sensitive to partial and full D1 agonists. However, in our hands, overshadowing is similarly insensitive to treatment with low dose amphetamine (Nelson et al. 2011a). Thus, it would appear that dose and, in particular, strain of rat may be a critical determinant of the sensitivity of overshadowing to pharmacological manipulations of forebrain DA systems.
Consistent with the failure of the D1 agonists to abolish LI, treatment with the D1 antagonist SCH 23390 did not facilitate LI under conditions of weak pre-exposure, which do not yield LI in controls. Moreover, in expt 3, which used standard LI parameters suitable for testing for amphetamine-induced abolition (30 CS pre-exposures), there was no evidence of a pre-exposure effect in animals treated with SCH 23390. One possible explanation for the inability of SCH 23390 to enhance LI is that, as well as being a potent D1 antagonist, it also acts as an agonist at 5-HT2c receptors (e.g. Briggs et al. 1991; Millan et al. 2001). Although this additional action cannot completely be ruled out as a potential explanation of the failure of SCH 23390 to enhance LI, increased 5-HT neurotransmission has been shown to facilitate LI. For example, treatment with the 5-HT reuptake inhibitor sertraline has been found to potentiate LI (Loskutova et al. 1990).
Alternatively, it could be argued that the failure to demonstrate LI enhancement in animals treated with SCH 23390 stems from a general learning impairment, in that animals in both in pre-exposed and non-pre-exposed conditions exhibited relatively high suppression ratios (i.e. poor learning). Thus, a floor effect could potentially explain the lack of a drug effect and mask differences between the two pre-exposure groups. However, there was no statistical evidence that non-pre-exposed animals differed by drug and, subsequently, in expt 2b it was shown that – even with more robust levels of conditioning – SCH 23390 did not enhance LI. Furthermore, the current finding replicates previous reports that the selective D1 antagonists NNC 01-0112 and SCH 39166 similarly do not potentiate LI with weak CS pre-exposure (Trimble et al. 2002). Taken together, these results provide compelling evidence that blockade of D1 receptors does not facilitate LI. This proposition is entirely consistent with the well-established finding that antipsychotics facilitate LI, an effect that is widely attributed to the high affinity of such drugs for D2 receptors (e.g. Feldon & Weiner, 1991; Shadach et al. 2000; Weiner, 2003; Weiner & Feldon, 1987).
However, as abolished and enhanced LI may depend on dissociable neural substrates (e.g. Gal et al. 2005; Nelson et al. 2010; Weiner & Arad, 2009), the demonstration that D1 antagonists do not enhance LI does not preclude their involvement in LI (Bay-Richter et al. 2009; O'Callaghan et al. 2010). Thus, in expt 3, we tested the ability of SCH 23390 to reverse amphetamine-induced disruption of LI using experimental parameters explicitly designed to test for LI abolition (30 pre-exposures). We found that, although on its own SCH 23390 did not enhance LI, it blocked amphetamine-induced disruption of LI such that animals treated with both amphetamine and SCH 23390 exhibited LI. Previously, both typical and atypical antipsychotics have been shown to reverse the disruptive effects of amphetamine on LI (e.g. Gosselin et al. 1996; Moran et al. 1996; Warburton et al. 1994; Weiner et al. 1996) indicating amphetamine effects on LI are mediated in part by its actions at D2 receptors. The current findings suggest that amphetamine also exerts its disruptive effects on LI via actions at D1 receptors and concurrent antagonism of D1 receptors is sufficient to reverse amphetamine-induced disruption of LI. This finding is entirely consistent with other evidence demonstrating that the behavioural effects of amphetamine can depend on co-activation of both D1 and D2 receptors in a variety of behavioural paradigms (e.g. Ranaldi & Beninger, 1993; St Onge & Floresco, 2009). Moreover, the disruptive effects of nicotine on LI, which are thought to be modulated by activation of the mesolimbic DA system (Joseph et al. 1993), are similarly blocked by concurrent administration of SCH 23390 (Young et al. 2005). Recently, it has been shown that, when tested with standard LI experimental parameters (i.e. low number of conditioning trials, high number of pre-exposures) haloperidol paradoxically attenuates LI but this effect is blocked by concurrent treatment with the D1 agonist SKF 38393 (Loskutova et al. 2010). These data point to the importance of interactions between D1 and D2 receptors in regulating the behavioural processes underlying LI. Interestingly, antagonism of D1 receptors blocks the development of sensitization to the locomotor activating effects of amphetamine (Vezina, 1996; Vezina & Stewart, 1989). Here we also found that animals previously treated with SCH 23390 and amphetamine showed less locomotor activity in response to a subsequent amphetamine challenge relative to animals that had been treated with amphetamine alone. As sensitized DA release by amphetamine is required to abolish LI (Joseph et al. 2000), one possible mechanism by which SCH 23390 may reverse amphetamine effects on LI is by preventing sensitized DA release.
A further and somewhat unexpected finding in expt 3 was the demonstration that SCH 23390 administered alone appeared to disrupt LI, in that there was no difference in the level of conditioning to the CS between the PE- and NPE-group animals. It should be noted that this paradoxical disruption of LI by SCH 23390 is qualitatively different from the abolition produced by amphetamine. Amphetamine-induced disruption of LI manifests as a selective increase in conditioning to the CS in the PE group (i.e. reduced LI), whereas the apparent disruption seen after SCH 23390 is mediated by a decrease in conditioning to the CS in non-pre-exposed animals. To exclude non-specific drug effects on learning per se, drug-induced disruption of LI is only reliably demonstrated when drug actions occur in the pre-exposed condition (as is the case with amphetamine; for a full discussion of these issues, see Weiner & Arad, 2009). As the lack of difference in between the NPE and PE animals cannot be attributed to an action of the drug on conditioning in the PE group but rather to reduced conditioning in the NPE group, the effect of SCH 23390 more likely reflects a non-specific effect of SCH 23390 on conditioning. Conversely, in expt 1, the D1 agonist SKF 81297 generally increased conditioning (at 0.8 but not at 0.4 mg/kg). This proposition is broadly consistent with other evidence suggesting that D1 receptor antagonists can disrupt Pavlovian conditioning (e.g. Eyny & Horvitz, 2003). In the present study, such an effect is controlled for by the comparison of PE and NPE groups; despite its effect on the baseline level of fear conditioning, treatment with SCH 23390 nonetheless restored LI in amphetamine-treated rats.
Conclusions
These experiments provide novel insights into DA mechanisms in LI. The finding that neither stimulation nor blockade of D1 receptors had any unambiguous effect on LI suggests that in isolation D1 receptors do not play a role in the modulation of LI by DA. Nonetheless, the demonstration that antagonism of D1 receptors restored normal LI in amphetamine-treated rats indicates that activity at D1 receptors, perhaps through interactive effects with D2 receptors, contributes to the dopaminergic modulation of the behavioural processes underlying LI (Loskutova et al. 2010).
Acknowledgements
This work was supported by the Wellcome Trust (ref. 082940). We thank Matthew Nolan and Thomas Woolfe for their assistance with the running of expt 3.
Statement of Interest
None.
References
- Bay-Richter C, O'Tuathaigh CM, O'Sullivan G, Heery DM. et al. Enhanced latent inhibition in dopamine receptor-deficient mice is sex-specific for the D1 but not D2 receptor subtype: implications for antipsychotic drug action. International Journal of Neuropsychopharmacology. 2009;12:403–414. doi: 10.1017/S1461145708009656. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleuler E. Dementia Praecox or the Group of Schizophrenias. New York: International Universities Press; 1911. ( . Translated 1950. [Google Scholar]
- Briggs CA, Pollock NJ, Frail DE, Paxson CL. et al. Activation of the 5-HT1C receptor expressed in Xenopus oocytes by the benzazepines SCH 23390 and SKF 38393. British Journal of Pharmacology. 1991;104:1038–1044. doi: 10.1111/j.1476-5381.1991.tb12546.x. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassaday HJ, Moran PM. Lubow RE, Weiner I. Latent Inhibition: Cognition, Neuroscience, Applications and Schizophrenia. Cambridge, UK: Cambridge University Press; 2010. Latent inhibition and other salience modulation effects: same neural substrates? ( (Eds), [Google Scholar]
- Cheung THC, Bezzine G, Hampson CL, Body S. et al. Evidence for the sensitivity of operant timing behaviour to stimulation of D1 receptors. Psychopharmacology. 2007;195:213–222. doi: 10.1007/s00213-007-0892-y. ( [DOI] [PubMed] [Google Scholar]
- Crider A, Solomon PR, McMahon MA. Disruption of selective attention in the rat following chronic D-amphetamine administration: relationship to schizophrenic attention disorder. Biological Psychiatry. 1982;17:351–361. ( [PubMed] [Google Scholar]
- Enyny YS, Horvitz JC. Opposing roles of D1 and D2 receptors in appetitive conditioning. Journal of Neuroscience. 2003;23:1584–1587. doi: 10.1523/JNEUROSCI.23-05-01584.2003. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldon J, Shofel A, Weiner I. Latent inhibition is unaffected by direct dopamine agonists. Pharmacology Biochemistry and Behavior. 1991;38:309–314. doi: 10.1016/0091-3057(91)90283-8. ( [DOI] [PubMed] [Google Scholar]
- Feldon J, Weiner I. The latent inhibition model of schizophrenic attention disorder. Haloperidol and sulpiride enhance rats' ability to ignore irrelevant stimuli. Biological Psychiatry. 1991;29:635–646. doi: 10.1016/0006-3223(91)90133-7. ( [DOI] [PubMed] [Google Scholar]
- Gal G, Schiller D, Weiner I. Latent inhibition is disrupted by nucleus accumbens shell lesion but is abnormally persistent following entire nucleus accumbens lesion: the neural site controlling the expression and disruption of the stimulus pre-exposure effect. Behavioral Brain Research. 2005;162:246–255. doi: 10.1016/j.bbr.2005.03.019. ( [DOI] [PubMed] [Google Scholar]
- Gosselin G, Oberling P, Di Scala G. Antagonism of amphetamine-induced disruption of latent inhibition by the atypical antipsychotic olanzapine in rats. Behavioural Pharmacology. 1996;7:820–826. ( [PubMed] [Google Scholar]
- Gray JA, Feldon J, Rawlins JNP, Hemsley DR. et al. The neuropsychology of schizophrenia. Behavioral and Brain Sciences. 1991;14:1–20. ( [Google Scholar]
- Gray JA, Kumari V, Lawrence N, Young AMJ. Functions of the dopaminergic innervation of the nucleus accumbens. Psychobiology. 1999;27:225–235. ( [Google Scholar]
- Gray JA, Moran PM, Grigoryan G, Peters S. et al. Latent inhibition: the nucleus accumbens connection revisited. Behavioral Brain Research. 1997;88:27–35. doi: 10.1016/s0166-4328(97)02313-9. ( [DOI] [PubMed] [Google Scholar]
- Joseph MH, Peters SL, Gray JA. Nicotine blocks latent inhibition in rats: evidence for a critical role of increased functional activity of dopamine in the mesolimbic system at conditioning rather than pre-exposure. Psychopharmacology. 1993;110:187–192. doi: 10.1007/BF02246971. ( [DOI] [PubMed] [Google Scholar]
- Joseph MH, Peters SL, Moran PM, Grigoryan GA. et al. Modulation of latent inhibition in the rat by altered dopamine transmission in the nucleus accumbens at the time of conditioning. Neuroscience. 2000;101:921–930. doi: 10.1016/s0306-4522(00)00437-1. ( [DOI] [PubMed] [Google Scholar]
- Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. American Journal of Psychiatry. 2003;160:3. doi: 10.1176/appi.ajp.160.1.13. ( [DOI] [PubMed] [Google Scholar]
- Kapur S. How antipsychotics become anti-‘psychotic’ – from dopamine to salience to psychosis. Trends in Pharmacological Sciences. 2004;25:402–406. doi: 10.1016/j.tips.2004.06.005. ( [DOI] [PubMed] [Google Scholar]
- Loskutova LV, Kostyunina NV, Dubrovina NI. Involvement of different types of dopamine receptors in the formation of latent inhibition of a conditioned passive avoidance reaction in rats. Neuroscience and Behavioral Physiology. 2010;40:483–487. doi: 10.1007/s11055-010-9285-5. ( [DOI] [PubMed] [Google Scholar]
- Loskutova LV, Luk'yanenko FYA, Il'yuchenok RYU. Modeling of latent inhibition in rats by activation of the central serotoninergic system. Neuroscience and Behavioral Physiology. 1990;20:541–542. doi: 10.1007/BF01237281. ( [DOI] [PubMed] [Google Scholar]
- Lubow RE, Moore AU. Latent inhibition: the effect of non-reinforced preexposure to the conditional stimulus. Journal of Comparative and Physiological Psychology. 1959;52:415–419. doi: 10.1037/h0046700. ( [DOI] [PubMed] [Google Scholar]
- Millan MJ, Newman-Tancredi A, Quentric Y, Cussac D. The “selective” dopamine D1 receptor antagonist, SCH23390, is a potent and high efficacy agonist at cloned human serotonin2C receptors. Psychopharmacology. 2001;156:58–62. doi: 10.1007/s002130100742. ( [DOI] [PubMed] [Google Scholar]
- Moran PM, Fischer JM, Hitchcock JM, Moser PC. Effects of clozapine on latent inhibition in the rat. Behavioural Pharmacology. 1996;7:42–48. ( [PubMed] [Google Scholar]
- Nelson AJD, Thur KE, Marsden CA, Cassaday HJ. Catecholaminergic depletion within the prelimbic medial prefrontal cortex enhances latent inhibition. Neuroscience. 2010;170:99. doi: 10.1016/j.neuroscience.2010.06.066. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AJD Thur KE Marsden CA Cassaday HJ (2011aDopamine in nucleus accumbens: salience modulation in latent inhibition and overshadowing Journal of Psychopharmacology. Published online: 24 January 2011. doi:10.1177/0269881110389211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AJD Thur KE Horsley RR Spicer C et al. (2011bReduced dopamine function within the medial shell of the nucleus accumbens enhances latent inhibition Pharmacology Biochemistry and Behavior 981–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan M, Bay-Richter C, Mathur N, Fone KCF. et al. Latent inhibition in D1 dopamine receptor knock out mice. Journal of Psychopharmacology. 2010;24:A41. ( (Suppl.), [Google Scholar]
- O'Tuathaigh CMP, Moran PM. Evidence for dopamine D1 receptor involvement in the stimulus selection task overshadowing in the rat. Psychopharmacology. 2002;162:225–231. doi: 10.1007/s00213-002-1107-1. ( [DOI] [PubMed] [Google Scholar]
- O'Tuathaigh CMP, Moran PM. The effect of sulpiride on amphetamine induced disruption of overshadowing in the rat. Progress in Neuro-psychopharmacology and Biological Psychiatry. 2004;28:1249–1253. doi: 10.1016/j.pnpbp.2004.06.009. ( [DOI] [PubMed] [Google Scholar]
- O'Tuathaigh CMP, Salum C, Young AMJ, Pickering AD. et al. The effect of amphetamine disruption on Kamin blocking and overshadowing. Behavioural Pharmacology. 2003;14:315–322. doi: 10.1097/01.fbp.0000080416.18561.3e. ( [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned Reflexes (G. V. Anrep, Trans.) Oxford, UK: Oxford University Press; 1927. ( [Google Scholar]
- Ranaldi R, Beninger RJ. Dopamine D1 and D2 antagonists attenuate amphetamine-produced enhancement of responding for conditioned reward in rats. Psychopharmacology. 1993;113:110–118. doi: 10.1007/BF02244342. ( [DOI] [PubMed] [Google Scholar]
- Shadach E, Gaisler I, Schiller D, Weiner I. The latent inhibition model dissociates between clozapine, haloperidol, and ritanserin. Neuropsychopharmacology. 2000;23:151–161. doi: 10.1016/S0893-133X(00)00096-8. ( [DOI] [PubMed] [Google Scholar]
- Solomon PR, Crider A, Winkelman JW, Turi A. et al. Disrupted latent inhibition in the rat with chronic amphetamine or haloperidol-induced supersensitivity: relationship to schizophrenic attention disorder. Biological Psychiatry. 1981;16:519–537. ( [PubMed] [Google Scholar]
- St Onge JR, Floresco SB. Dopaminergic modulation of risk-based decision making. Neuropsychopharmacology. 2009;34:681–697. doi: 10.1038/npp.2008.121. ( [DOI] [PubMed] [Google Scholar]
- Trimble KM, Bell R, King DJ. Effects of the selective dopamine D(1) antagonists NNC 01-0112 and SCH 39166 on latent inhibition in the rat. Physiology and Behavior. 2002;77:115–123. doi: 10.1016/s0031-9384(02)00814-4. ( [DOI] [PubMed] [Google Scholar]
- Vezina P. D1 dopamine receptor activation is necessary for the induction of sensitization by amphetamine in the ventral tegmental area. Journal of Neuroscience. 1996;16:2411–2420. doi: 10.1523/JNEUROSCI.16-07-02411.1996. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P, Stewart J. The effect of dopamine receptor blockade on the development of sensitization to the locomotor activating effects of amphetamine and morphine. Brain Research. 1989;499:108–120. doi: 10.1016/0006-8993(89)91140-2. ( [DOI] [PubMed] [Google Scholar]
- Warburton EC, Joseph MH, Feldon J, Weiner I. et al. Antagonism of amphetamine-induced disruption of latent inhibition in rats by haloperidol and ondansetron – implications for a possible antipsychotic action of ondansetron. Psychopharmacology. 1994;114:657–664. doi: 10.1007/BF02244998. ( [DOI] [PubMed] [Google Scholar]
- Weiner I. Neural substrates of latent inhibition: the switching model. Psychological Bulletin. 1990;108:442–461. doi: 10.1037/0033-2909.108.3.442. ( [DOI] [PubMed] [Google Scholar]
- Weiner I. The ‘two-headed’ latent inhibition model of schizophrenia: modelling positive and negative symptoms and their treatment. Psychopharmacology. 2003;169:257–297. doi: 10.1007/s00213-002-1313-x. ( [DOI] [PubMed] [Google Scholar]
- Weiner I, Arad M. Using the pharmacology of latent inhibition to model domains of pathology in schizophrenia and their treatment. Behavioural Brain Research. 2009;204:369–386. doi: 10.1016/j.bbr.2009.05.004. ( [DOI] [PubMed] [Google Scholar]
- Weiner I, Feldon J. Facilitation of latent inhibition by haloperidol in rats. Psychopharmacology. 1987;91:248–253. doi: 10.1007/BF00217073. ( [DOI] [PubMed] [Google Scholar]
- Weiner I, Lubow RE, Feldon J. Abolition of the expression but not the acquisition of latent inhibition by chronic amphetamine in rats. Psychopharmacology. 1984;83:194–199. doi: 10.1007/BF00429734. ( [DOI] [PubMed] [Google Scholar]
- Weiner I, Shadach E, Tarrasch R, Kidron R. et al. The latent inhibition model of schizophrenia: further validation using the atypical neuroleptic, clozapine. Biological Psychiatry. 1996;40:834–843. doi: 10.1016/0006-3223(95)00573-0. ( [DOI] [PubMed] [Google Scholar]
- Young AM, Moran PM, Joseph MH. The role of dopamine in conditioning and latent inhibition: what, when, where and how? Neuroscience and Biobehavioral Reviews. 2005;29:963–976. doi: 10.1016/j.neubiorev.2005.02.004. ( [DOI] [PubMed] [Google Scholar]
- Zelikowsky M, Fanselow MS. Opioid regulation of Pavlovian overshadowing in fear conditioning. Behavioral Neuroscience. 2010;124:510–519. doi: 10.1037/a0020083. ( [DOI] [PMC free article] [PubMed] [Google Scholar]