Abstract
Background
Exposure to traffic-related air pollutants, including polycyclic aromatic hydrocarbons (PAHs), can induce asthma. However, the effects of early repeated PAH exposure over time on different asthma phenotypes have not been examined.
Objective
To assess associations between repeated PAH exposure, measured from prenatal personal and residential indoor monitors in children's homes, and asthma in an inner-city cohort.
Methods
Prenatal exposure was assessed by personal air monitoring during 48 hours and exposure at 5 to 6 years of age by 2-week residential monitoring in the Columbia Center for Children's Environmental Health cohort. PAH was dichotomized into pyrene (representative semivolatile PAH) and the sum of 8 nonvolatile PAHs. High exposure to each was defined as measures above the median at both repeated time points. Asthma and wheeze were determined by validated questionnaires at ages 5 to 6 years. Children with specific IgE levels greater than 0.35 IU/mL to any of 5 indoor allergens were considered seroatopic.
Results
Among all 354 children, repeated high exposure to pyrene was associated with asthma (odds ratio [OR], 1.90; 95% confidence interval [CI], 1.13-3.20). Among 242 nonatopic children, but not those sensitized to indoor allergens (n = 87) or with elevated total IgE levels (n = 171), high pyrene levels were associated positively with asthma (OR, 2.89; 95% CI, 1.77-5.69), asthma medication use (OR, 2.28; 95% CI, 1.13-4.59), and emergency department visits for asthma (OR, 2.43; 95% CI, 1.20-4.91). Associations between the levels of the 8 nonvolatile PAHs and asthma were not observed, even when stratifying by seroatopy.
Conclusion
Nonatopic children may be more susceptible to the respiratory consequences of early pyrene exposures.
Introduction
Allergic sensitization is a well-established risk factor for the development of asthma,1 and early sensitization to indoor allergens in particular may be associated with asthma among urban children.2 The development of the atopic phenotype, as opposed to the nonatopic phenotype, depends on the child's genetic susceptibility and exposure to environmental allergens. Much of childhood asthma research has focused on those with the atopic phenotype, even though a substantial portion of childhood asthma (up to 31%-59%) is not attributed to allergic immune responses.3-5 In these cases, the environmental exposures that induce asthma are less evident.
Exposure to particulate matter less than 2.5 μm (PM2.5), a common air pollutant, has been linked to asthma exacerbations, the development of new wheeze, and decreased lung function.6,7 More recently, exposure to individual chemical components of PM2.5 has been associated with the development of asthma in young children, including the diesel soot fraction of PM2.5 (black carbon or elemental carbon),8 transition metals (eg, nickel and vanadium),9,10 and polycyclic aromatic hydrocarbons (PAHs).11,12
PAHs have 2 types of anthropogenic sources: petrogenic (eg, direct evaporation of petroleum products, such as gasoline and diesel fuel) and pyrogenic (eg, incomplete combustions of organic material, such as fuels, coal, wood, tobacco, and oil). Although lower-molecular-weight semivolatile PAHs, such as pyrene, are emitted from both petrogenic and pyrogenic sources, the higher-molecular-weight nonvolatile PAHs with 5 to 6 benzene rings are generated predominantly by pyrogenic emissions.13,14 Semivolatile PAHs also have prominent indoor sources, such as space heating, cooking, smoking, or burning incense or candles, whereas nonvolatile PAHs arise predominantly from traffic emissions and heating oil combustion.15 Experimental work suggests that pyrene in particular can induce either allergic or nonallergic immune responses, depending on the model.16-18 Our group at the Columbia Center for Children's Environmental Health (CCCEH) reported that higher prenatal exposure to the sum of 8 nonvolatile PAHs (Σ8PAHnonvolatile) combined with environmental tobacco smoke (ETS) was associated with wheeze and asthma in infants and young children.12,19 Although these studies highlighted the importance of the prenatal period of PAH exposure with ETS exposure on the later development of asthma in young children, the effects on asthma of repeated pyrene and Σ8PAHnonvolatile exposure over time are not well understood.
Because emerging evidence has suggested that exposure to traffic-related air pollutants (eg, PM) may trigger the development of nonatopic asthma in particular,5 we sought a priori to examine whether the associations between repeated early pyrene and Σ8PAHnonvolatile exposure and asthma would differ between nona-topic and atopic children in an inner-city cohort. Our approach was to study participants from the CCCEH cohort, a longitudinal birth cohort in Northern Manhattan and South Bronx, New York. We hypothesized that (1) early repeated exposure to pyrene and Σ8PAHnonvolatile measured from prenatal personal and childhood residential monitors in children's homes is associated with a greater risk of developing asthma and asthma-related symptoms at 5 to 6 years of age and (2) associations between pyrene and Σ8PAHnonvolatile exposure and asthma may vary by the development of seroatopy.
Methods
Study population
A total of 727 nonsmoking, healthy Dominican or African American women living in Northern Manhattan or the South Bronx were enrolled during pregnancy and their children were followed up prospectively.19 Questionnaires were administrated to the mother prenatally, every 3 months through 2 years of age, and every 6 months thereafter. Data were analyzed for those children (n=349) who completed airborne PAH measures at 2 time points (prenatal, 5-6 years of age) and the questionnaire given at 5 to 6 years of age. The study was approved by the Columbia University Institutional Review Board, and written informed consent was obtained from all study participants.
Air monitoring
Prenatal PAH (pyrene and Σ8PAHnonvolatile) exposure was measured from 48-hour personal air monitoring between 1998 and 2006, and PAH exposure at 5 to 6 years of age was measured from 2-week residential indoor monitoring between 2005 and 2011. Samples were analyzed for pyrene and Σ8PAHnonvolatile (benz[a]anthracene, chrysene/iso-chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, indeno[1,2,3-c,d]pyrene, dibenz[a,h]anthracene, ad benzo[g,h,i]perylene) as a single extract (gas and particle phase combined) at Southwest Research Institute as previously described.15,19 Seven other semivolatile PAHs (1-methylphenanthrene, 2-methylphenanthrene, 3-methylphenanthrene, 9-methylphenanthrene, 1,7-dimethylphenanthrene, 3,6-dimethylphenanthrene, and phenanthrene) were measured only at 5 to 6 years of age and not included in the main analysis. Prenatal and postnatal ETS exposure was assessed using report of any smoker in the home during pregnancy and when the child was 6 months or 1, 2, 3, or 5 to 6 years of age, respectively.
For a subset of study participants (n=102), prenatal residential indoor samples were collected continuously beginning during the 32nd week of pregnancy until delivery.20 This protocol permitted simultaneous comparisons of PAH levels measured by different sampling methods (48-hour personal vs concurrent 2-week residential indoor exposure) during the prenatal period.
Health outcomes
The International Study of Asthma and Allergies in Childhood questionnaire21 assessed ever wheeze and recent wheeze, the latter defined as any report of wheeze in the past 12 months at either 5 or 6 years of age. The Brief Respiratory Questionnaire, validated in a similar local urban population,22 was used to assess parental report of physician diagnosis of asthma and asthma medication use in the past 12 months at either age. Emergency room (ER) visits were defined as reporting at least one visit to the ER in the past 12 months due to breathing problems when queried by Brief Respiratory Questionnaire at 5 or 6 years of age.
Total serum IgE and specific IgE to cat, mouse, dog, Dermatophagoides farinae, and German cockroach at 5 years of age were measured in duplicate from serum samples using ImmunoCap (Phadia, Uppsala, Sweden).2,23 Children were considered sensitized to indoor allergens if they had a specific IgE level of 0.35 IU/mL or greater to any of the allergens tested. They were classified as having any sensitization if the total IgE level was 50 IU/mL or higher. Children were defined as seroatopic if they met either of these criteria.
Statistical analysis
Because of the different properties of semivolatile pyrene and the nonvolatile PAHs, especially in regards to volatility,24 sources,24 genotoxicity,25 reactivity with ambient oxidizing compounds,15 and their relatively minimal correlations with each other (r = 0.35; P < .001 for prenatal and r = 0.24; P<.001 for 5-6 years of age), pyrene and Σ8PAHnonvolatile were analyzed separately. Given high correlations between pyrene and other semivolatile PAHs measured at 5 to 6 years of age (r = 0.51-0.78; P<.001), pyrene was considered a marker for semivolatile PAH exposure.
As published previously,26 prenatal PAHs were dichotomized at the median to obtain a measure of high and low exposure (median, 2.61 ng/m3 and 2.08 ng/m3 for pyrene and Σ8PAHnonvolatile, respectively). Similarly, measures at 5 to 6 years of age were dichotomized at the median (median, 1.01 ng/m3 and 1.29 ng/m3 for pyrene and Σ8PAHnonvolatile, respectively). The repeated high PAH exposure group was defined as those with high prenatal and high 5 to 6 years of age measures (above median for each; high-high) and compared with the other groups (high-low, low-high, and low-low).
Respiratory outcomes were analyzed using 2 pollutant logistic regression models. Potential confounding variables used in the analyses included maternal ethnicity, sex, maternal asthma, maternal education, prenatal ETS exposure, postnatal ETS exposure, and cold/influenza season.9,12 Prenatal maternal demoralization score (range, 0-4), previously associated with childhood wheeze by 5 years of age in this cohort,27 was also included. One dichotomous variable was included in the model to indicate whether the participant had any change in address between the prenatal and 5 to 6 years of age exposure assessment. Another dichotomous variable indicated whether the residential monitoring was conducted before the 5 or 6 years of age questionnaire.
Sensitivity analyses were conducted as follows: (1) reanalysis after removing upper or lower 5% of measures at both time points and (2) reanalysis after eliminating the 5% of prenatal and childhood exposures immediately above and below the median. All statistical analyses were performed using SPSS statistical software, version 18 (SPSS Inc, Chicago, Illinois).
Results
Cohort characteristics
Children who underwent prenatal and 5 to 6 years of age air monitoring and completed questionnaires at 5 or 6 years of age did not differ demographically from CCCEH children excluded from the current analyses (ie, had not reached 5 years of age or were missing exposure or health outcome data), with the exception of a lower frequency of prenatal ETS exposure, lower prenatal maternal demoralization scores, and a higher prevalence of an elevated total IgE level (≥50 IU/mL) (Table 1). A total of 28% and 26% of children fell repeatedly in the high category of pyrene and Σ8PAHnonvolatile exposure, respectively.
Table 1.
Demographic characteristics for the study cohorta
| Characteristic | Patients included in the studyb (n = 379) | Patients excluded from the studyc (n = 348) | P valued |
|---|---|---|---|
| Maternal ethnicity | .48 | ||
| Dominican | 242/379 (64) | 232/348 (67) | |
| African American | 137/378 (36) | 116/348 (33) | |
| Girls | 201/378 (53) | 175/348 (50) | .46 |
| Maternal high school or greater degree | 238/378 (63) | 219/335 (65) | .56 |
| Maternal asthma | 96/379 (25) | 66/288 (23) | .05 |
| Prenatal ETSe | 115/374 (31) | 131/342 (38) | .03 |
| Postnatal ETSf | 161/379 (43) | 116/285 (41) | .71 |
| Sensitized to indoor allergensg | 97/351 (28) | 35/127 (28) | .71 |
| Total IgE ≥50 IU/mL | 150/330 (45) | 48/136 (35) | .04 |
| Asthmah | 123/379 (33) | 52/158 (33) | .92 |
| Prenatal demoralization,i mean ± SD | 1.12 ± 0.66 | 1.19 ± 0.60 | .05j |
Abbreviation: ETS, environmental tobacco smoke.
Data are presented as number/total number (percentage) of patients unless otherwise indicated.
Includes participants who underwent residential monitoring and had questionnaire data at 5 or 6 years of age.
Because of missing data, 313 Columbia Center for Children's Environmental Health patients did not undergo childhood air monitoring, 30 had invalid air measures either at prenatal or childhood monitoring periods, and 5 had missing questionnaire data.
Pearson χ2 test was performed. Demographic characteristics were similar between the children included when compared with those excluded (all P>.05) except for prenatal ETS, total IgE, and prenatal demoralization score.
Prenatal ETS exposure was defined as the report of any smoker in the house during pregnancy.
Postnatal ETS was defined as the report of any smoker in the house when the child was aged 6 months or 1, 2, 3, or 5 to 6 years.
At least one specific IgE level of 0.35 IU/mL or greater at 5 years of age.
Asthma defined as parental report of physician diagnosis of asthma at 5 to 6 years of age.
Maternal demoralization score (range, 0-4) was measured using the Psychiatric Epidemiology Research Instrument–Demoralization during pregnancy.
Mann-Whitney test was performed.
Repeated PAH measures and health outcomes
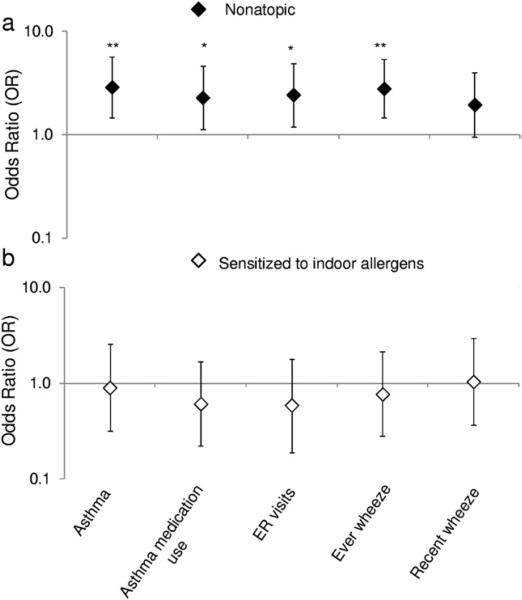
Overall, higher levels of pyrene were associated with report of asthma (odds ratio [OR], 1.90; 95% confidence interval [CI], 1.13-3.20; Table 2) but not other asthma symptom-related outcomes. Furthermore, 2 stratified models were built based on sensitization to indoor allergens at 5 years of age. Among the 242 nonatopic children (ie, lacking an elevated indoor IgE level), high pyrene levels were associated with asthma (OR, 2.89; 95% CI, 1.47-5.69), asthma medication use (OR, 2.28; 95% CI, 1.13-4.59), emergency department visits (OR, 2.43; 95% CI, 1.20-4.91), and ever wheeze (OR, 2.79; 95% CI, 1.46-5.34) (Fig 1 and Table 2). When nonatopic children were redefined as possessing a total IgE level less than 50 IU/mL (n = 171), the positive association between high pyrene and asthma remained, with a greater OR (OR, 3.59; 95% CI, 1.58-8.15). Also among nonatopic children, an inverse association was found between high Σ8PAHnonvolatile levels and recent wheeze (OR, 0.47; 95% CI, 0.22-1.00) but not asthma. Among the 87 children sensitized to indoor allergens, significant associations were not detected between pyrene or Σ8PAHnonvolatile and asthma and asthma-related outcomes (Fig 1 and Table 2).
Table 2.
Effect of sensitization to indoor allergen on the associations between repeated polycyclic aromatic hydrocarbon measures and asthma symptoms at 5 to 6 years of agea
| Measure | Cases, No. (%)b | OR (95% CI) |
|
|---|---|---|---|
| Pyrene | Σ8PAHnonvolatile | ||
| Overall (N = 354) | |||
| Asthmac | 115(32.5) | 1.90h (1.13-3.20) | 0.90 (0.52-1.56) |
| Asthma medication used | 107 (30.2) | 1.35 (0.80-2.29) | 0.89 (0.51-1.53) |
| ED visitse | 97 (27.4) | 1.21 (0.71-2.09) | 0.82 (0.46-1.45) |
| Ever wheezef | 159 (44.9) | 1.53 (0.93-2.51) | 0.86 (0.52-1.42) |
| Recent wheezeg | 110 (31.1) | 1.39 (0.81-2.37) | 0.62 (0.35-1.11) |
| Nonatopic children (n = 242) | |||
| Asthmac | 70 (28.9) | 2.89i (1.47-5.69) | 0.67 (0.33-1.36) |
| Asthma medication used | 62 (25.6) | 2.28h (1.13-4.59) | 0.62 (0.30-1.28) |
| ED visitse | 59 (24.4) | 2.43h (1.20-4.91) | 0.67 (0.32-1.40) |
| Ever wheezef | 103 (42.6) | 2.79i (1.46-5.34) | 0.65 (0.35-1.23) |
| Recent wheezeg | 67 (27.7) | 1.95 (0.95-3.97) | 0.47h (0.22-1.00) |
| Sensitized to indoor allergens (n = 87) | |||
| Asthmac | 40 (46.0) | 0.90 (0.32-2.56) | 0.74 (0.22-2.47) |
| Asthma medication used | 41 (47.1) | 0.61 (0.22-1.68) | 1.41 (0.44-4.53) |
| ED visitse | 36 (41.4) | 0.59 (0.19-1.78) | 0.90 (0.26-3.18) |
| Ever wheezef | 51 (56.0) | 0.77 (0.28-2.15) | 0.92 (0.27-3.12) |
| Recent wheezeg | 39 (44.8) | 1.04 (0.37-2.96) | 0.52 (0.18-2.15) |
Abbreviations: Σ8PAHnonvolatile, sum of 8 nonvolatile polycyclic aromatic hydrocarbons; CI, confidence interval; ED, emergency department; OR, odds ratio.
Model was adjusted for ethnicity, sex, maternal educational level, maternal asthma, prenatal environmental tobacco smoke, postnatal environmental tobacco smoke, cold/flu season, prenatal maternal demoralization, ever moved, and residential monitoring conducted before the 5 or 6 years of age questionnaires. Sensitized to indoor allergens if the specific IgE level was 0.35 IU/mL or higher to any of indoor allergen tested at 5 years of age.
Cases of symptom outcomes are presented.
Asthma defined as parental report of physician diagnosis of asthma.
Medication use defined as taking medication due to wheeze or whistling in the past 12 months.
ED visits defined as a report of ED visit at 5 or 6 years of age.
Wheeze ever defined as any report of wheeze in the past.
Recent wheeze defined as any report of wheeze in the past 12 months.
P<.05.
P<.01.
Fig. 1.
Effect of sensitization to indoor allergens on the associations between pyrene exposure and asthma symptoms at 5 to 6 years of age: (A) nonatopic children (n = 242) and (B) children sensitized to indoor allergens (n = 87). The data points and error bars indicate the odds ratios and 95% confidence intervals, respectively, for an increase in respiratory outcomes at 5 to 6 years of age associated with repeated polycyclic aromatic hydrocarbon exposure, adjusting for ethnicity, sex, maternal educational level, maternal asthma, prenatal ETS exposure, postnatal ETS exposure, cold/flu season, prenatal maternal demoralization, ever moved, and residential monitoring conducted before the 5 or 6 years of age questionnaires; *P<.05 and **P < .01.
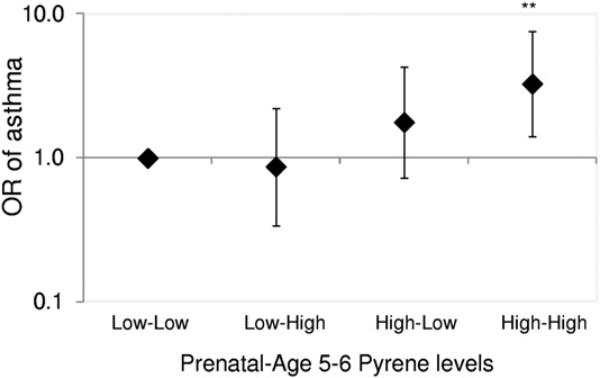
In secondary analyses, adjusted logistic models were repeated with multilevels of pyrene exposure (prenatal to 5-6 years of age: high-high, high-low, and low-high vs low-low as reference). Consistent with the main findings, among nonatopic children, but not children sensitized to indoor allergens, a positive association was found between persistently high-high pyrene exposure and asthma (OR, 3.28; 95% CI, 1.40-7.66; Fig 2). In contrast, no significant associations were found between asthma and low-high or high-low pyrene exposure or Σ8PAHnonvolatile exposure (Fig 2).
Fig. 2.
Relationship of repeatedly elevated levels of prenatal and 5 to 6 years of age pyrene levels with asthma among nonatopic children (n = 242). The data points and error bars indicate the odds ratios (ORs) and 95% confidence intervals, respectively, for an increase in asthma at 5 to 6 years of age associated with each combination of prenatal and age 5 to 6 years pyrene levels (low-high, high-low, and high-high vs low-low), adjusting for race/ethnicity, sex, maternal educational level, maternal asthma, prenatal ETS exposure, postnatal ETS exposure, cold/flu season, prenatal maternal demoralization, ever moved, and residential monitoring conducted before the 5 or 6 years of age questionnaires; **P < .01.
Sensitivity analysis
Models displayed in Figure 1A were robust to the exclusion of the upper or lower 5% of pyrene measures (Table 3). After removing the 5% of prenatal and 5 to 6 years of age measures immediately above and below the median, the association between high pyrene and asthma remained, with a greater OR (OR, 3.43; 95% CI, 1.46-8.27).
Table 3.
Sensitivity analysis with asthma outcome among nonatopic childrena
| Exclusion | No. of children | OR (95% CI) for pyrene |
|---|---|---|
| Upper 5% of prenatal and childhood PAHs | 196 | 2.51b (1.20-5.26) |
| Lower 5% of prenatal and childhood PAHs | 198 | 3.23c (1.54-6.76) |
| 5% of exposures above and below the median | 173 | 3.43c (1.46-8.27) |
Abbreviations: CI, confidence interval; OR, odds ratio; PAHs, polycyclic aromatic hydrocarbons.
Model adjusted for ethnicity, sex, maternal educational level, maternal asthma, prenatal environmental tobacco smoke, postnatal environmental tobacco smoke, cold/flu season, prenatal maternal demoralization, ever moved, and residential monitoring conducted before the 5 or 6 years of age questionnaires.
P<.05.
P<.01.
Discussion
Our objective was to assess associations between repeated PAH exposure, measured from prenatal personal and residential indoor monitors in children's homes, and asthma. We found that repeated high exposure to semivolatile pyrene, but not Σ8PAHnonvolatile, was associated positively with the development of asthma, ever wheeze, asthma medication use, and emergency department visits for asthma. The positive association with repeated exposure to pyrene detected here is novel and may indicate that pyrene, used as a representative maker for semivolatile PAH exposure, may be a key component responsible for adverse respiratory effects. Furthermore, we observed that these associations were greatest among nonatopic children.
Among the few cohort studies that focused on environmental triggers of nonatopic asthma, it was reported that exposure to air pollution (eg, PM and ETS) may induce or exacerbate the disease.5,28 Nonatopic asthmatic children also have been found to exhibit greater bronchial reactivity after methacholine challenges than atopic children, suggesting that nonatopic children may be more susceptible to airway irritants, such as ambient air pollution.29 In this urban CCCEH cohort, in which 62% of asthmatic children are not sensitized to indoor allergens at 5 years of age, we found that these children and those without elevated total IgE levels may be more susceptible to the effects of pyrene exposure on asthma and asthma symptoms.
Growing evidence suggests that long-term air pollution exposure may contribute to the development of new asthma and adverse effects on lung growth. For example, early long-term exposure to ambient PM and black carbon was associated with the development of asthma and new wheeze among young children30,31 and reduction of lung growth in preadolescent children in urban cohorts.32 The latter was supported by experimental findings that found that mice exposed in the long term to urban levels of PM2.5 during the prenatal and postnatal period developed alterations of alveolar structure and lung elastic properties, reducing lung growth.33 A prospective study in Krakow, Poland, found that ambient prenatal measures of 9 PAHs (pyrene and Σ8PAHnonvolatile) were associated with higher risks of transient wheeze and cough at 1 to 2 years of age.11,34 One study conducted in California reported that higher 1-year averaged particulate PAH exposure, estimated by a land use regression model, was associated with decreased forced expiratory volume in 1 second in asthmatic children.35 Previously, our group at CCCEH reported that higher prenatal Σ8PAHnonvolatile levels, only when combined with exposure to ETS, were associated with respiratory symptoms at 1 to 2 years of age and asthma at 5 to 6 years of age.12,19 However, the interaction of repeated PAHs with ETS exposure on asthma was not significant in these analyses (data not shown). To our knowledge, this is the first study in which the associations of repeated measures of PAHs during pregnancy and 5 to 6 years of age were assessed on young children, particularly nonatopic children in an inner-city birth cohort.
We suspect that the volatility associated with pyrene, as opposed to the higher-molecular-weight Σ8PAHnonvolatile, may facilitate inhalation into the distal airways and be absorbed more efficiently by the respiratory tract.36 In addition to experimental support for pyrene's role in the induction of several types of inflammation,16-18 another semivolatile PAH, phenanthrene, also has been shown to upregulate B-cell and IgE production in vitro37 and act in vivo as an adjuvant for allergen specific IgE production in the human upper airways.38 Although these latter results mostly support the role of semivolatile PAHs on asthma through allergic immune mechanisms, there is also evidence that nonallergic mechanisms may be important. Fluoranthene, a structural isomer of pyrene, induced airway surface acidification through impaired cyclic adenosine monophosphate–mediated HCO3– secretion and potentiated Cl– secretion.39 Airway acidification is associated with airway inflammation and bronchoconstriction.40 Alternately, it is possible that observed positive associations between PAH levels and asthma in previous studies was driven by pyrene or fluoranthene, generally the most dominant compounds among the measured PAHs.15 Previous studies, conducted in Krakow, Poland, and Fresno, California, assessed PAHs as either Σ8PAHnonvolatile combined with pyrene or the sum of Σ8PAHnonvolatile and fluoranthene.
Sensitivity analyses revealed the associations with pyrene to be robust. The positive association of pyrene with asthma also was replicated when a total IgE level less than 50 IU/mL was used to define those who were nonseroatopic. Furthermore, analyses of multilevel PAH exposure confirmed the positive association between asthma and persistently high exposure to pyrene (high-high) but not other exposure categories (high-low or low-high) prenatally and at 5 to 6 years of age.
We observed an unexpected inverse association of Σ8PAHnonvolatile with recent wheeze. Although it is intuitively improbable, there are several plausible mechanisms by which Σ8PAHnonvolatile levels could be associated with lower odds of recent wheeze, such as impairment of T-cell and/or B-cell function.41-44 In addition, studies have shown that exposure to diesel exhaust particles containing nonvolatile PAHs may induce a low level of oxidative stress that increases antioxidant defense activities and thus protects against airway inflammation and asthma.45Alternatively, nonvolatile PAHs can be degraded by oxidizing compounds (hydroxyl radicals, nitrate radicals, and ozone) in the ambient air and further transformed into unmeasured and possibly toxic oxygenated or nitrated PAHs,46 underestimating their true exposure levels. Nevertheless, our use of indoor PAH measures, which have significantly lower ozone levels compared with the outdoors, makes this possibility less likely.47 The inverse relationship with Σ8PAHnonvolatile may be an interesting pattern; however, it needs to be studied further and interpreted in the context of other cohort research on PAHs.
We acknowledge several limitations to the study. First, the panel of PAHs studied was not completely representative of all PAHs known to comprise urban air. Levels of additional semivolatile PAHs beyond pyrene, such as phenanthrene, a predominant traffic-related PAH, were not included in these analyses because they were not measured in prenatal air samples. However, they did correlate strongly in the 5 to 6 years of age residential air samples. Second, prenatal and 5 to 6 years of age PAHs were collected using different sampling methods. However, our previous study found that the personal and residential PAH samples collected simultaneously during the prenatal period correlated significantly with each other.48 When also analyzed as dichotomized measures (high vs low), the results were mostly similar regardless of sampling methods (data not shown). This latter result indicates that most of the study participants who were exposed to high prenatal personal PAH levels also would be exposed to high prenatal residential PAH levels, especially because people spend most of their time indoors.49 Third, high PAH exposure was assessed only by 2 repeated measures 5 to 6 years apart, leading to the possibility of exposure misclassification. Fourth, reliance on the parental reports of wheeze could result in both false-positive and false-negative responses,50 although this would be expected to bias toward the null and therefore is unlikely to affect the positive associations observed between pyrene levels and asthma. Lastly, we were not able to compare directly high exposure level (eg, above the median levels of prenatal personal and 5-6 years of age residential PAHs) with that reported in other cohort studies because they were measured by different sampling and analytical methods.
To our knowledge, this prospective study provides the first evidence of early repeated exposure to pyrene during pregnancy and at 5 to 6 years of age being associated with nonatopic asthma among young, urban children. This positive association was not apparent with atopic asthma or the nonvolatile PAHs. These results suggest that exposure to semivolatile pyrene, mostly generated from indoor sources beyond traffic emission, may be a key component responsible for adverse respiratory effects, and children not already sensitized to allergens may be more susceptible. Given the high prevalence of nonatopic asthma, these results indicate that many children may be at significant risk of asthma-related morbidity due to early pyrene exposure. Specific interventions aimed at reducing PAH exposure by reducing sources of indoor emissions, such as cooking, space heating, burning incense and candle, and ETS, may help to diminish the risk of developing asthma, especially for nonatopic, young children.
Acknowledgments
Funding Sources: This work was supported by National Institutes of Health grants R01ES013163, P50ES015905, P01ES09600, R01ES08977, and P30ES09089; Environmental Protection Agency grants R827027, RD832141, and RD834509; the Educational Foundation of America; the John & Wendy Neu Family Foundation; the New York Community Trust; and the Trustees of the Blanchette Hooker Rockefeller Fund.
Footnotes
Disclosures: Authors have nothing to disclose.
References
- 1.Lau S, Illi S, Sommerfeld C, et al. Early exposure to house-dust mite and cat allergens and development of childhood asthma: a cohort study. Lancet. 2000;356:1392–1397. doi: 10.1016/s0140-6736(00)02842-7. [DOI] [PubMed] [Google Scholar]
- 2.Donohue KM, Al-alem U, Perzanowski MS, et al. Anti-cockroach and anti-mouse IgE are associated with early wheeze and atopy in an inner-city birth cohort. J Allergy Clin Immunol. 2008;122:914–920. doi: 10.1016/j.jaci.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barreto ML, Cunha SS, Fiaccone R, et al. Poverty, dirt, infections and non-atopic wheezing in children from a Brazilian urban center. Respir Res. 2010;11:167–177. doi: 10.1186/1465-9921-11-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castro-Rodriguez JA, Ramirez AM, Toche P, et al. Clinical, functional, and epidemiological differences between atopic and nonatopic asthmatic children from a tertiary care hospital in a developing country. Ann Allergy Asthma Immunol. 2007;98:239–244. doi: 10.1016/S1081-1206(10)60712-0. [DOI] [PubMed] [Google Scholar]
- 5.McCormack MC, Breysse PN, Matsui EC, et al. Indoor particulate matter increases asthma morbidity in children with non-atopic and atopic asthma. Ann Allergy Asthma Immunol. 2011;106:308–315. doi: 10.1016/j.anai.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pope C, III, Dockery D. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc. 2006;56:709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- 7.Jung KH, Hsu SI, Yan B, et al. Childhood exposure to fine particulate matter and black carbon and the development of new wheeze between ages 5 and 7 in an urban prospective cohort. Environ Int. 2012;45:44–50. doi: 10.1016/j.envint.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spira-Cohen A, Chen L, Kendall M, Lall R, Thurston G. Personal exposures to traffic-related air pollution and acute respiratory health among Bronx school children with asthma. Environ Health Perspect. 2011;119:559–565. doi: 10.1289/ehp.1002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel M, Hoepner L, Garfinkel R, et al. Ambient metals, elemental carbon, and wheeze and cough in New York City children through age 24 months. Am J Respir Crit Care Med. 2009;180:1107–1113. doi: 10.1164/rccm.200901-0122OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bell ML, Ebisu K, Peng RD, Samet JM, Dominici F. Hospital admissions and chemical composition of fine particle air pollution. Am J Respir Crit Care Med. 2009;179:1115. doi: 10.1164/rccm.200808-1240OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jedrychowski W, Perera F, Maugeri U, et al. Intrauterine exposure to polycyclic aromatic hydrocarbons, fine particulate matter and early wheeze: prospective birth cohort study in 4 year olds. Pediatr Allergy Immunol. 2010;21:e723–e732. doi: 10.1111/j.1399-3038.2010.01034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller RL, Garfinkel R, Horton M, et al. Polycyclic aromatic hydrocarbons, environmental tobacco smoke, and respiratory symptoms in an Inner-city Birth Cohort. Chest. 2004;126:1071–1078. doi: 10.1378/chest.126.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Page D, Boehm P, Douglas G, Bence A, Burns W, Mankiewicz P. Pyrogenic polycyclic aromatic hydrocarbons in sediments record past human activity: a case study in Prince William Sound, Alaska. Mar Pollut Bull. 1999;38:247–260. [Google Scholar]
- 14.Fischer P, Hoek G, Van Reeuwijk H, et al. Traffic-related differences in outdoor and indoor concentrations of particles and volatile organic compounds in Amsterdam. Atmos Environ. 2000;34:3713–3722. [Google Scholar]
- 15.Jung KH, Patel MM, Moors K, et al. Effects of heating season on residential indoor and outdoor polycyclic aromatic hydrocarbons, black carbon, and particulate matter in an urban birth cohort. Atmos Environ. 2010;44:4545–4552. doi: 10.1016/j.atmosenv.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bömmel H, Li-Weber M, Serfling E, Duschl A. The environmental pollutant pyrene induces the production of IL-4. J Allergy Clin Immunol. 2000;105:796–802. doi: 10.1067/mai.2000.105124. [DOI] [PubMed] [Google Scholar]
- 17.Bommel H, Haake M, Luft P, et al. The diesel exhaust component pyrene induces expression of IL-8 but not of eotaxin. Int Immunopharmacol. 2003;3:1371–1379. doi: 10.1016/S1567-5769(03)00135-8. [DOI] [PubMed] [Google Scholar]
- 18.Kimata H, Yoshida A, Ishioka C, Lindley I, Mikawa H. Interleukin 8 (IL-8) selectively inhibits immunoglobulin E production induced by IL-4 in human B cells. J Exp Med. 1992;176:1227–1231. doi: 10.1084/jem.176.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosa MJ, Jung KH, Perzanowski MS, et al. Prenatal exposure to polycyclic aromatic hydrocarbons, environmental tobacco smoke and asthma. Respir Med. 2011;105:869–876. doi: 10.1016/j.rmed.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whyatt RM, Garfinkel R, Hoepner LA, et al. Within-and between-home variability in indoor-air insecticide levels during pregnancy among an inner-city cohort from New York City. Environ Health Perspect. 2007;115:383–389. doi: 10.1289/ehp.9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenkins MA, Clarke JR, Carlin JB, et al. Validation of questionnaire and bronchial hyperresponsiveness against respiratory physician assessment in the diagnosis of asthma. Int J Epidemiol. 1996;25:609–616. doi: 10.1093/ije/25.3.609. [DOI] [PubMed] [Google Scholar]
- 22.Bonner S, Matte T, Rubin M, et al. Validating an asthma case detection instrument in a Head Start sample. J Sch Health. 2006;76:471–478. doi: 10.1111/j.1746-1561.2006.00144.x. [DOI] [PubMed] [Google Scholar]
- 23.Chang C, Gauvey-Kern K, Johnson A, et al. Cord blood versus age 5 mononuclear cell proliferation on IgE and asthma. Clin Mol Allergy. 2010;8:11–18. doi: 10.1186/1476-7961-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baek S, Field R, Goldstone M, Kirk P, Lester J, Perry R. A review of atmospheric polycyclic aromatic hydrocarbons: sources, fate and behavior. Water Air Soil Poll. 1991;60:279–300. [Google Scholar]
- 25.Boffetta P. Cancer risk from occupational and environmental exposure to polycylic aromatic hydrocarbons. Cancer Causes Control. 1997;8:442–472. doi: 10.1023/a:1018465507029. [DOI] [PubMed] [Google Scholar]
- 26.Perera F, Li Z, Whyatt R, et al. Prenatal airborne polycyclic aromatic hydrocarbon exposure and child IQ at age 5 years. Pediatrics. 2009;124:e195–e202. doi: 10.1542/peds.2008-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reyes M, Perzanowski MS, Whyatt RM, et al. Relationship between maternal demoralization, wheeze, and immunoglobulin E among inner-city children. Ann Allergy Asthma Immunol. 2011;107:42–49. doi: 10.1016/j.anai.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.García Marcos L, Castro Rodríguez JA, Suarez Varela MM, et al. A different pattern of risk factors for atopic and non atopic wheezing in 9–12 year old children. Pediatr Allergy Immunol. 2005;16:471–477. doi: 10.1111/j.1399-3038.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 29.Mochizuki H, Shigeta M, Tokuyama K, Morikawa A. Difference in airway reactivity in children with atopic vs nonatopic asthma. Chest. 1999;116:619–624. doi: 10.1378/chest.116.3.619. [DOI] [PubMed] [Google Scholar]
- 30.Clark NA, Demers PA, Karr CJ, et al. Effect of early life exposure to air pollution on development of childhood asthma. Environ Health Perspect. 2010;118:284–290. doi: 10.1289/ehp.0900916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jung KH, Hsu S, Yan B, et al. Childhood exposure to find particulate matter and black carbon and the development of new wheeze between ages 5 and 7 in an urban prospective cohort. Environ Int. 2012;45:44–50. doi: 10.1016/j.envint.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gauderman WJ, Avol E, Gilliland F, et al. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med. 2004;351:1057–1067. doi: 10.1056/NEJMoa040610. [DOI] [PubMed] [Google Scholar]
- 33.Mauad T, Rivero DHRF, Carvalho de Oliveira R, et al. Chronic exposure to ambient levels of urban particles affects mouse lung development. Am J Respir Crit Care Med. 2008;178:721–728. doi: 10.1164/rccm.200803-436OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jedrychowski W, Galas A, Pac A, et al. Prenatal ambient air exposure to polycyclic aromatic hydrocarbons and the occurrence of respiratory symptoms over the first year of life. Eur J Epidemiol. 2005;20:775–782. doi: 10.1007/s10654-005-1048-1. [DOI] [PubMed] [Google Scholar]
- 35.Nadeau K, McDonald-Hyman C, Noth EM, et al. Ambient air pollution impairs regulatory T-cell function in asthma. J Allergy Clin Immunol. 2010;126:845–852. doi: 10.1016/j.jaci.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 36.International Agency for Research on Cancer . IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. International Agency for Research on Cancer; Lyon, France: 2002. [PMC free article] [PubMed] [Google Scholar]
- 37.Tsien A, Diaz-Sanchez D, Ma J, Saxon A. The organic component of diesel exhaust particles and phenanthrene, a major polyaromatic hydrocarbon constituent, enhances IgE production by IgE-secreting EBV-transformed human B cells in vitro. Toxicol Appl Pharmacol. 1997;142:256–263. doi: 10.1006/taap.1996.8063. [DOI] [PubMed] [Google Scholar]
- 38.Saxon A, Diaz-Sanchez D. Diesel exhaust as a model xenobiotic in allergic inflammation. Immunopharmacology. 2000;48:325–327. doi: 10.1016/s0162-3109(00)00234-4. [DOI] [PubMed] [Google Scholar]
- 39.Ito Y, Son M, Sato S, et al. Effects of fluoranthene, a polycyclic aromatic hydrocarbon, on cAMP-dependent anion secretion in human airway epithelia. J Pharmacol Exp Ther. 2004;308:651–657. doi: 10.1124/jpet.103.059089. [DOI] [PubMed] [Google Scholar]
- 40.Fischer H, Widdicombe JH. Mechanisms of acid and base secretion by the airway epithelium. J Membr Biol. 2006;211:139–150. doi: 10.1007/s00232-006-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davila DR, Romero DL, Burchiel SW. Human T cells are highly sensitive to suppression of mitogenesis by polycyclic aromatic hydrocarbons and this effect is differentially reversed by [alpha]-naphthoflavone. Toxicol Appl Pharmacol. 1996;139:333–341. doi: 10.1006/taap.1996.0173. [DOI] [PubMed] [Google Scholar]
- 42.Lutz CT, Browne G, Petzold CR. Methylcholanthrene causes increased thymocyte apoptosis. Toxicology. 1998;128:151–167. doi: 10.1016/s0300-483x(98)00043-2. [DOI] [PubMed] [Google Scholar]
- 43.De Jong WH, Kroese ED, Vos JG, Van Loveren H. Detection of immunotoxicity of benzo [a] pyrene in a subacute toxicity study after oral exposure in rats. Toxicol Sci. 1999;50:214–220. doi: 10.1093/toxsci/50.2.214. [DOI] [PubMed] [Google Scholar]
- 44.Vorderstrasse BA, Steppan LB, Silverstone AE, Kerkvliet NI. Aryl hydrocarbon receptor-deficient mice generate normal immune responses to model antigens and are resistant to TCDD-induced immune suppression. Toxicol Appl Pharmacol. 2001;171:157–164. doi: 10.1006/taap.2000.9122. [DOI] [PubMed] [Google Scholar]
- 45.Xiao GG, Wang M, Li N, Loo JA, Nel AE. Use of proteomics to demonstrate a hierarchical oxidative stress response to diesel exhaust particle chemicals in a macrophage cell line. J Biol Chem. 2003;278:50781–50790. doi: 10.1074/jbc.M306423200. [DOI] [PubMed] [Google Scholar]
- 46.Yu H. Environmental carcinogenic polycyclic aromatic hydrocarbons: photo-chemistry and phototoxicity. J Environ Sci Health. 2002;20:149–183. doi: 10.1081/GNC-120016203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J, Lioy PJ. Ozone in residential air: concentrations, I/O ratios, indoor chemistry, and exposures. Indoor Air. 1994;4:95–105. [Google Scholar]
- 48.Rundle A, Hoepner L, Hassoun A, et al. Association of childhood obesity with maternal exposure to ambient air polycyclic aromatic hydrocarbons during pregnancy. Am J Epidemiol. 2012;175:1163–1172. doi: 10.1093/aje/kwr455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jenkins PL, Phillips TJ, Mulberg EJ, Hui SP. Activity patterns of Californians: use of and proximity to indoor pollutant sources. Atmos Environ. 1992;26:2141–2148. [Google Scholar]
- 50.Cane R, Ranganathan S, McKenzie S. What do parents of wheezy children understand by “wheeze”? Arch Dis Child. 2000;82:327–332. doi: 10.1136/adc.82.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]