Abstract
Metaplasticity, the adaptive changes of long-term potentiation (LTP) and long-term depression (LTD) in response to fluctuations in neural activity is well documented in visual cortex, where dark rearing shifts the frequency threshold for the induction of LTP and LTD. Here we studied metaplasticity affecting spike-timing-dependent plasticity, in which the polarity of plasticity is determined not by the stimulation frequency, but by the temporal relationship between near-coincidental presynaptic and postsynaptic firing. We found that in mouse visual cortex the same regime of deprivation that restricts the frequency range for inducing rate-dependent LTD extends the integration window for inducing timing-dependent LTD, enabling LTD induction with random presynaptic and postsynaptic firing. Notably, the underlying mechanism for the changes in both rate-dependent and time-dependent LTD appears to be an increase of NR2b-containing NMDAR at the synapse. Thus, the rules of metaplasticity might manifest in opposite directions, depending on the plasticity-induction paradigms.
Introduction
Mechanisms of activity-dependent synaptic modifications, such as NMDAR-dependent long-term potentiation (LTP) and long-term depression (LTD) are considered essential for the sculpting of visual cortex by visual experience. Traditionally, the selective induction of LTP and LTD has been achieved by varying the presynaptic firing rate or the postsynaptic potential during conditioning stimuli (Malenka and Bear, 2004). Synaptic plasticity can also be induced by near-coincidental presynaptic and postsynaptic firing: presynaptic firing preceding postsynaptic firing induces LTP, whereas the reverse order induces LTD. Since the polarity and magnitude of the synaptic changes are solely specified by the timing between presynaptic and postsynaptic firing, spike-timing-dependent plasticity (STDP) has become an attractive paradigm to model naturally occurring plasticity (Caporale and Dan, 2008; Richards et al., 2010).
Theoretical considerations on the stability of plastic synaptic networks (Bienenstock et al., 1982) and experimental results from deprivation studies (Smith et al., 2009) indicate that the mechanisms controlling the gain and polarity of synaptic plasticity need to adapt to changes in neural activity. Experimental evidence for this plasticity of synaptic plasticity, termed metaplasticity (Abraham and Bear, 1996), comes from studies showing that the frequency threshold for inducing LTP and LTD with rate-dependent paradigms shifts toward lower values in animals reared in the dark (Kirkwood et al., 1996; Philpot et al., 2003). As a result, the frequency range for LTP induction expands at the expense of LTD, which is thought to constitute a homeostatic mechanism to restore neural activity in the deprived cortex. The possibility that deprivation also induces adaptive metaplasticity of STDP has not been explored.
The consensus on the underlying mechanisms of LTP and LTD is that a moderate NMDAR activation leads to LTD, while stronger activation leads to LTP (Malenka and Bear, 2004). Consistent with that, the sliding of the thresholds for rate-dependent LTP and LTD to lower values appears to result from an increase in the fraction of NR2b-containing (or GluN2b-containing) NMDARs, and the concomitant prolongation of the duration of the NMDAR-mediated synaptic response (Quinlan et al., 1999). Interestingly, according to current models of STDP, LTP will occur at those synapses in which the NMDARs have bound glutamate at the arrival of the backpropagating action potential (Shouval et al., 2010). Thus, the temporal window for the coincidence of presynaptic and postsynaptic activation to elicit LTP is restricted by the duration of the NMDAR activation. These observations prompted us to examine the effects of dark exposure on the induction of STDP. We found that dark exposure expanded the temporal integration window for the induction of both timing-dependent LTP and LTD, yet at the same time it restricted the induction of LTD with rate-dependent and voltage-dependent paradigms.
Materials and Methods
Visual cortical slices.
Visual cortical slices (300–400 micrometers) from 3–4-week-old C57BL/6 mice of either sex reared in normal light/dark 12 h cycles or in the dark for 2 d with care provided under infrared illumination were cut as described previously (Seol et al., 2007) in ice-cold dissection buffer containing the following (in mm): 212.7 sucrose, 5 KCl, 1.25 NaH2PO4, 10 MgCl2, 0.5 CaCl2, 26 NaHCO3, 10 dextrose, bubbled with 95% O2/5% CO2, pH 7.4. Slices were transferred to normal artificial CSF (ACSF) for at least 1 h before recording. Normal ACSF was similar to the dissection buffer except that sucrose was replaced by 124 mm NaCl, MgCl2 was lowered to 1 mm, and CaCl2 was raised to 2 mm.
Visualized whole-cell current-clamp recordings.
Visualized whole-cell current-clamp recordings were made from layer II/III regular-spiking pyramidal cells with glass pipettes (4–6 MΩ) filled with intracellular solution containing the following (in mm): 130 K-gluconate, 10 KCl, 0.2 EGTA, 10 HEPES, 4 Mg-ATP, 0.5 Na-GTP, and 10 Na-phosphocreatine, pH adjusted to 7.25 with KOH, 280–290 mOsm. Only cells with membrane potentials more negative than −65 mV, series resistance <20 MΩ (8–18 MΩ), and input resistance >100 MΩ were studied. Cells were excluded if input resistance changed >15% over experiment. Data were filtered at 2 kHz and digitized at 5 kHz using Igor Pro (WaveMetrics).
Synaptic responses.
Synaptic responses were evoked every 20 s by stimulating layer IV with 0.2 ms pulses delivered through two concentric bipolar stimulating electrodes (diameter, 125 μm; FHC) placed ∼900 μm apart in the middle of the cortical thickness. Intensity was adjusted to evoke 4–6 mV responses. Input independence was confirmed by linear summation and the absence of paired pulse interactions, and synaptic strength was quantified as the initial slope (the first 2 ms) of the EPSP.
Induction of STDP.
Spike-timing-dependent LTP (tLTP) and spike-timing-dependent LTD (tLTD) were induced by pairing presynaptic stimulation in one pathway or the two pathways with a burst of four action potentials (100 Hz) evoked by passing suprathreshold depolarizing current steps through the recording electrode (∼1 nA, 2 ms). We chose to stimulate with burst over single action potentials because it yields larger magnitude of STDP (Wittenberg and Wang, 2006; Seol et al., 2007). Associative pairing consisted of 200 pairing epochs delivered at 1 Hz. One cell per slice was used.
Rate-dependent synaptic plasticity.
In these experiments, layer II/III field potentials (FPs) were recorded in 400 μm slices with a recording pipette filled with ACSF placed in layer II/III and evoked by stimulation of the underlying layer IV. Baselines were acquired at stimulus intensities evoking an FP approximately half of maximal amplitude (∼0.7–1.5 mV). Synaptic plasticity was evaluated with tetanus of different frequencies as described previously (Kirkwood et al., 1996). As a model for high-frequency stimulation, we used theta burst stimulation consisting of four trains of 10 bursts (4 stimuli at 100 Hz) delivered at 5 Hz. The trains were delivered every 10 s. For medium frequencies, we used 120 pulses delivered at 40 Hz. For low-frequency stimulation, we delivered 900 pulses at 1 Hz.
Voltage-dependent synaptic plasticity.
These experiments were done in pyramidal cells recorded as described in the main section. To induce plasticity, the recording mode was switched from current-clamp to voltage-clamp as described previously (Huang et al., 2012). Pairing consisted of 150 epochs (0.75 Hz), during which the holding potential Vh was alternated between two target values (666 ms for each value). To assess LTP, the Vh alternated between −70 and −10 mV; for LTD, the Vh alternated between −70 and −40 mV. In each pairing epoch, the synaptic stimulation pulse was delivered 100 ms after the onset of depolarization.
NMDAR-mediated EPSCs.
Pharmacologically isolated NMDAR-mediated EPSCs (NMDAR-EPSCs) were recorded at +40 mV in the presence 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 25 μm) and gabazine (2.5 μm), with 4 mm Ca2+ and 4 mm Mg2+ in the ACSF to reduce recruitment of polysynaptic responses.
To evaluate the decay kinetics of the NMDAR-EPSCs in a given condition, 30–50 traces were first averaged and normalized. Then the decay was fitted with two exponentials, one fast and one slow, using Igor. As a measure of the decay, we calculated a weighted time constant (τw) according to the following equation as described previously (Rumbaugh and Vicini, 1999; Philpot et al., 2001): τw = τf [If /(If + Is)] + τs [Is /(If + Is)], where τf and τs are the time constant for the fast and slow components, while If and Is are their respective amplitudes.
To record the AMPA/NMDA ratio, isolated glutamatergic (AMPA/NMDA) currents were evoked in the presence of gabazine (2.5 μm), 4 mm Ca2+, and 4 mm Mg2+ in the ACSF. NMDAR-EPSCs and AMPAR-EPSCs were discriminated based on their kinetics and voltage dependence. NMDAR-EPSCs were taken as the amplitude at Vh = +40 mV, 70 ms after the response onset, whereas the AMPAR-EPSCs were taken as the peak amplitude response recorded at Vh = −80 mV.
Most drugs, including methoxamine (Mtx), isoproterenol (Iso), and CNQX were purchased from Sigma-Aldrich. Gabazine and AM251 were purchased from Tocris Bioscience. Iso was applied with 10 μm sodium ascorbate to prevent oxidation of the drug.
Statistical analysis.
Group plots are presented as average ± SEM. The magnitude of plasticity was taken as the average of the last 10 min of recording, beginning 20 min after conditioning stimulation. The significance of LTP and LTD was assessed using the paired Student's t test. Other comparisons were done using Student's t test or the ANOVA test.
Results
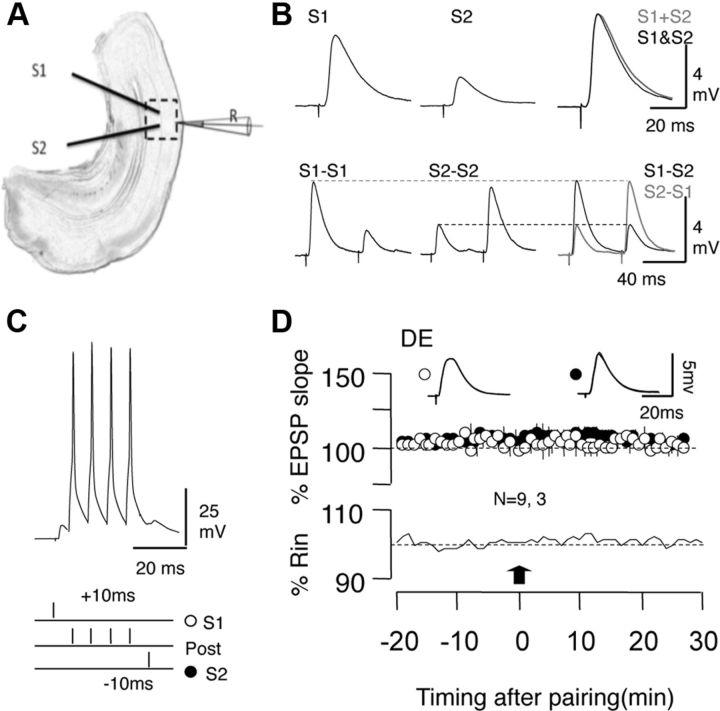
We studied the effects of 2 d exposure to dark on the induction of STDP in visual cortical slices from C57BL/6 mice. We chose the brief exposure over dark rearing since birth to avoid potential developmental issues, and because that duration is sufficient to induce other forms of homeostatic plasticity (Goel and Lee, 2007; Gao et al., 2010). STDP was tested in layer II/III pyramidal cells with associative pairings consisting of presynaptic stimulation followed by a burst of postsynaptic firing to induce LTP, or in the reverse order to induce LTD (see Materials and Methods; Fig. 1A–C) as described previously (Seol et al., 2007; Huang et al., 2012). We previously showed that, under standard experimental conditions, the induction of STDP in layer II/III pyramidal cells requires “priming” the synapses by stimulation of neuromodulatory receptors that phosphorylate AMPA receptors at sites specific for LTP and LTD (Seol et al., 2007). Since dark exposure promotes AMPA receptor phosphorylation at some of these sites (Goel et al., 2006), we tested whether it also removes the need for neuromodulators in STDP induction. It does not: in slices for dark-exposed (DE) mice, the associative conditioning did not elicit either tLTP (100.1 ± 3.4% of baseline at 30 min, n = 9 slices, 3 mice; paired t test, p = 0.95) or tLTD (100.7 ± 3.5%, n = 9 slices, 3 mice; paired t test, p = 0.834; Fig. 1D). Therefore, in all subsequent experiments the associative conditioning was delivered in conjunction with a 10 min bath of either the β-adrenergic agonist Iso (10 mm) to enable tLTP, the α1-adrenergic agonist Mtx (5 mm) to enable tLTD, or the coapplication of both Iso and Mtx to enable bidirectional STDP (Seol et al., 2007).
Figure 1.
Induction of STDP in visual cortex. A, EPSPs were recorded in layer II/III pyramidal cells and evoked by stimulating the underlying layer IV at two sites (S1 and S2). B, Independence of the two pathways was judged by the linear summation of the responses (top) and by the absence of paired pulse interactions (bottom). C, Conditioning paradigm to induce STDP. During each pairing epoch (200 at 1 Hz), stimulation of one or both pathways was paired with a postsynaptic burst of four action potentials (100 Hz). D, In control ACSF, the pairing paradigm (arrow) does not affect the EPSPs in slices prepared from DE mice. Plotted data are average ± SEM. Superimposed traces are averages of 10 consecutive responses recorded right before (thin line) and 27–30 min after conditioning.
Dark exposure extends the temporal window for the induction of STDP
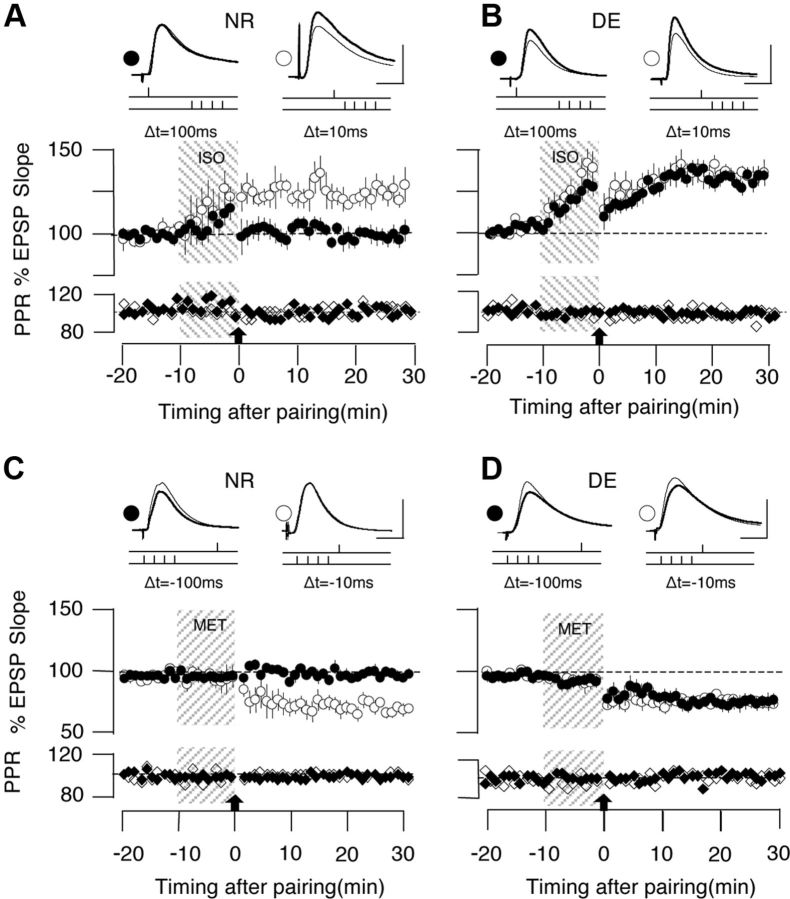
To evaluate how dark exposure affects the temporal window for STDP, we first tested two delays (10 and 100 ms) between presynaptic and postsynaptic stimulation.
In cells from normal-reared (NR) mice, the prepairing then postpairing at the end of the Iso application resulted in tLTP when the delay was 10 ms (124.1 ± 3.9%; n = 9 slices, 7 mice) but not when it was 100 ms (100.4 ± 3.3%, n = 8 slices, 7 mice; p < 0.001; Fig. 2A). In contrast, in cells from DE mice, both delays resulted in comparable tLTP (+10 ms: 131.8 ± 3.7%, n = 10 slices, 8 mice; +100 ms: 128.5 ± 2.6%, n = 10 slices, 8 mice; p = 0.570; Fig. 2B). Concerning tLTD, we expected a reduction because previous studies showed a reduction of LTD induced by low-frequency stimulation in dark-reared rodents. Notably, however, dark exposure also extended the window for tLTD. In NR mice, the postpairing then prepairing delivered at the end of a Mtx application resulted in tLTD when the delay was −10 ms (75.5 ± 3.5%, n = 8 slices, 4 mice), but not when it was −100 ms (102.1 ± 1.7%, n = 9 slices, 4 mice; p < 0.001; Fig. 2C); whereas in the DE mice, pairing with the two delay-induced tLTD of comparable magnitude (−100 ms: 77.4 ± 5.5%, n = 8 slices, 5 mice; −10 ms: 78.1 ± 3.4%, n = 9 slices, 5 mice; p = 0.918; Fig. 2D).
Figure 2.
Dark exposure extends the integration time for the induction tLTP and tLTD with spike-timing pairings. A, In cells from NR mice, the β-adrenergic agonist Iso (10 μm, 10 min, gray bar) promotes the induction of tLTP when the delay between presynaptic stimulation and postsynaptic spike is 10 ms (open circles), but not when it is 100 ms (filled circles). B, In cells from DE mice, both delays elicit equally robust tLTP. C, In cells from NR mice, the α1 adrenergic agonist Mtx (5 μm, 10 min, gray bar) promotes tLTD when the pre-post delay is −10 ms (open circles), but not when it is −100 ms (filled circles). D, In cells from DE mice, tLTD can be induced with both delays. Traces are averages of 10 EPSPs recorded before (thin line) and 30 min after (thick line) induction of plasticity. Scale bars: 2.5 mV, 5 ms. Note the absence of changes in the normalized paired-pulse ratio (PPR) and membrane resistance (Rin), which are displayed below each group plot. Plotted data are average ± SEM.
The robust induction of tLTD with the −100 ms delay raised the possibility that dark exposure might have reactivated a previously described form of tLTD that has a long integration window (Feldman, 2000), that is only transiently expressed earlier during postnatal development, and that does not contribute to tLTD after postnatal day 21 in visual cortex (Corlew et al., 2007; Seol et al., 2007). This early form of tLTD is expressed presynaptically and requires the activation of CB1-cannabinoid receptors (Sjöström et al., 2003; Bender et al., 2006; Nevian and Sakmann, 2006; Min and Nevian, 2012). We found, however, that the CB1R antagonist AM251 (10 mm) did not affect the induction of tLTD with −100 ms delays in cells from DE mice (AM251: 80.8 ± 3.7%, n = 9 slices, 5 mice; control DMSO, 77.5 ± 5.5%, n = 8 slices, 5 mice; p = 0.61; data not shown). In addition, the paired pulse ratio, a crude estimate of presynaptic release, did not change significantly after tLTP or tLTD (paired t test, p > 0.15 in all cases), which is consistent with a postsynaptic mechanism of expression of STDP (Seol et al., 2007).
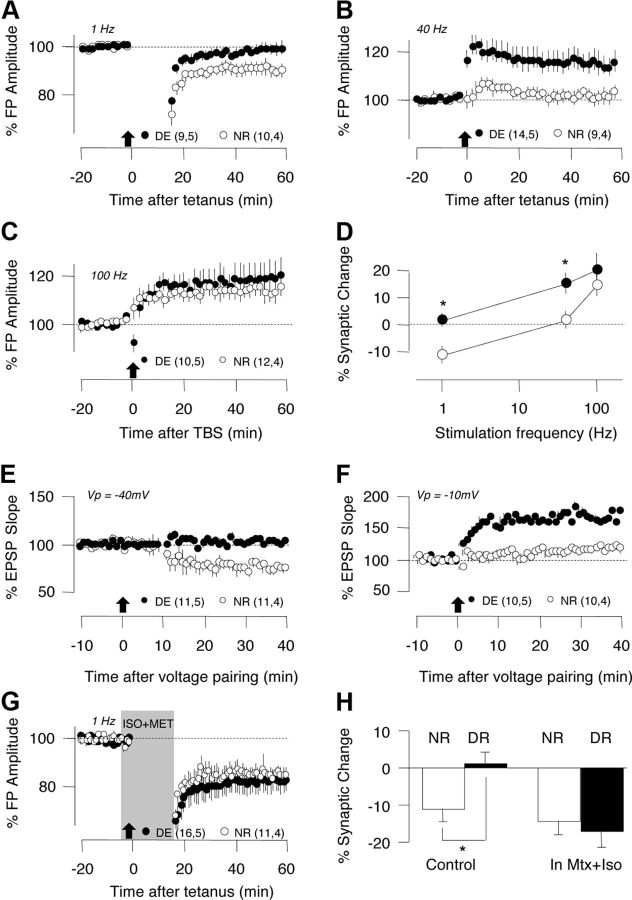
The extended temporal window for tLTD in DE mice contrasts with the reduced window of frequencies for inducting rate-dependent LTD observed after dark rearing from birth (Kirkwood et al., 1996; Philpot et al., 2003). To determine whether the duration of visual deprivation made the difference, we tested the effects of dark exposure (2 d) on LTP and LTD induced with rate-dependent and voltage-dependent paradigms (see Materials and Methods). In slices from DE mice, the normally optimal 1 Hz low-frequency stimulation (Kirkwood et al., 1996; Philpot et al., 2003) induced minimal LTD (DE: 101.1 ± 3.4%, n = 9 slices, 4 mice; NR: 88.9 ± 4.0%, n = 10 slices, 4 mice; p = 0.034) and the normally neutral 40 Hz stimulation (Kirkwood et al., 1996; Philpot et al., 2003) induced robust LTP (DE: 114.7 ± 4.6%, n = 14 slices, 4 mice; NR: 101.3 ± 3.4%, n = 9 slices, 4 mice; p = 0.022; Fig. 3A–D). Similarly, dark exposure reduced LTD induced by pairing synaptic stimulation with the depolarization at −40 mV (Fig. 3E; p < 0.001), which is normally optimal for LTD induction (Choi et al., 2005), and enabled LTP (Fig. 3F; p = 0.03) induction by pairing at −20 mV, normally subthreshold for LTP (Choi et al., 2005; Huang et al., 2012). Altogether, these results are consistent with the established notion that dark exposure shifts the optimal stimulation for LTD induced with rate-dependent and voltage-dependent paradigms while allowing LTP induction with suboptimal parameters. However, it is important to note that, unlike STDP induction, rate-dependent synaptic plasticity does not require exogenous neuromodulators. Interestingly, bath application of Iso and Mtx during the 1 Hz conditioning stimulation allowed the induction of LTD in slices from DE mice (Fig. 3G,H) of comparable magnitude to that obtained in control NR mice (t test, p = 0.630). These results suggest that some aspects of the expression of metaplasticity depends on the neuromodulatory tone.
Figure 3.
Dark exposure shifts the thresholds for the induction of LTP and LTD with rate-dependent paradigms (A–D) and depolarization-pairing paradigms (E, F). A–D, Effects of dark exposure on the induction of synaptic plasticity of layer II/III FPs with rate-dependent paradigms (see Materials and Methods). A, Dark exposure reduces LTD of the FP amplitude induced with a 1 Hz tetanus (900 pulses). B, Dark exposure enables the induction of LTP of the FP amplitude with a 40 Hz tetanus (120 pulses). C, Dark exposure does not affect the induction of LTP with theta burst stimulation (TBS; 120 pulses in patterned bursts of 100 Hz; see Materials and Methods). D, Summary of the effects of dark exposure on the induction of plasticity at different stimulation frequencies. Asterisks denote p < 0.05. E, F, Effects of dark exposure on the induction of synaptic plasticity with voltage-pairing paradigms. G, Dark exposure reduces LTD of the EPSPs induced by pairing 120 stimulation pulses with postsynaptic depolarization to −40 mV. H, Dark exposure enables LTP of the EPSPs induced by pairing synaptic stimulation with postsynaptic depolarization to −10 mV. Plotted data are average ± SEM. G, Dark exposure does not affect LTD induced with 1 Hz in the presence of adrenergic agonists (10 μm Iso and 5 μm Mtx, gray bar). H, Summary of the effects of dark exposure on the induction of LTD with 1 Hz stimulation.
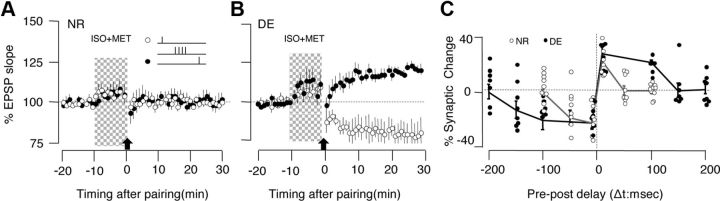
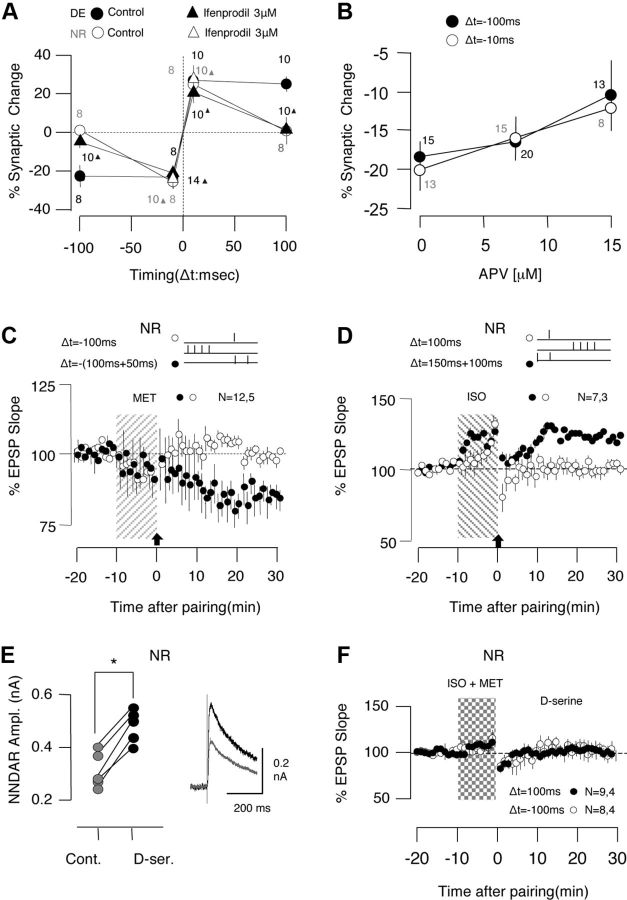
To better characterize the changes in STDP after dark exposure, we systematically varied the pre-post delay from 200 to −200 ms, and induced tLTP and tLTD under the same conditions by coapplying the agonists 10 μm Iso and 5 μm Mtx. We confirmed that with agonist mixture the pairing delays of 100 and −100 ms did not elicit any changes in cells from NR mice (100 ms: p > 0.960; −100 ms: p = 0.780; Fig. 4A), but robust tLTP (p < 0.001) and tLTD (p = 0.022) in cells from DE mice (Fig. 4B). The summary results, shown in Figure 4C, indicate that dark exposure does not change the peak magnitude of tLTP (at 10 ms) or tLTD (at −10 ms) but extends the integration window for tLTP from <50 ms to more that 100 ms, and the window for tLTD from <−100 ms to >−150 ms. A two-way ANOVA (F(3,66) = 22.66, p < 0.001) confirmed the significance of these findings. Altogether, the results indicate that dark exposure increases the integration window for postsynaptic tLTP and tLTD.
Figure 4.
Dark exposure extends the integration time for the induction for STDP. A, B, tLTP and tLTD were induced simultaneously in two pathways in the presence of an agonist mixture (10 μm Iso and 5 μm Mtx, 10 min). A, In cells from NR mice, no changes were induced when the delay between presynaptic and postsynaptic stimulation was 100 ms (filled circles) or −100 ms (open circles). B, The same pairing delays induced tLTP (open circles, 100 ms delay) and tLTD (filled circles, −100 ms delay) in cells from DE mice. C, Summary of changes in the EPSPs (30 min after conditioning) elicited by pre-post pairing with different time delays. Individual experiments are represented as circles (NR, open circles; DE, filled circles). Some data points are laterally displaced for viewing purposes. The lines connect the average changes for each delay from cells of NR mice (gray) and DE mice (filled circle). Plotted data are average ± SEM.
Role of NR2b-containing NMDARs in the expansion of the integration window
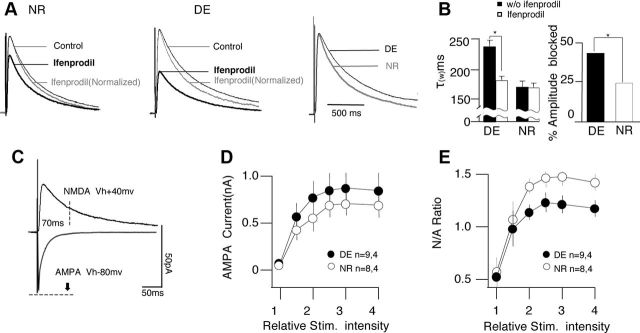
Previous studies indicate that brief dark exposure promotes the translation of the NR2b subunit of the NMDAR (Chen and Bear, 2007). A longer duration of NMDAR responses due to an increased NR2b/NR2a fraction (Philpot et al., 2001) is an obvious mechanism to account for the extended integration window for tLTP. To examine this possibility, we first confirmed that a brief 2 d dark exposure increases NR2b functionality at the synapse by testing the effects of the NR2b-specific antagonist ifenprodil (3 mm) on pharmacologically isolated NMDAR-synaptic currents (NMDAR-EPSCs). Dark exposure increased the fraction of the NMDAR-EPSCs blocked by ifenprodil (DE: 43.0 ± 3.9%, n = 8 slices, 4 mice; NR: 24.2 ± 3.9%, n = 8 slices, 4 mice; t test, p = 0.004; Fig. 5A,B). The duration of the NMDAR-EPSCs, quantified as a τw of two exponentials (see Materials and Methods), was also longer and more sensitive to ifenprodil in cells from DE mice (DEcontrol τw: 231.6 ± 13.7 ms; DEifenprodil τw: 179.1 ± 13.6 ms; n = 8 slices, 4 mice; NR control τw: 168.8 ± 6.7 ms; NRifenprodil τw: 167.9 ± 9.1 ms, n = 8 slices, 4 mice; 2-way ANOVA, p = 0.023 for rearing condition, p = 0.029 for the interaction with the drug; Fig. 5A,B). In addition, the ratio of the NMDAR-EPSCs and AMPAR-EPSCs was increased in the DE cells (2-way ANOVA, F(1,90) = 4.95, p = 0.029; Fig. 5E), which is consistent with the notion that dark exposure promotes the synaptic incorporation of NR2b-containing NMDARs. We also detected a small, yet significant increase in the input–output relationship for the AMPAR-EPSCs, possibly reflecting synaptic scaling induced by the exposure to dark (Goel and Lee, 2007).
Figure 5.
Dark exposure increases the NR2b component of the NMDAR-mediated response. A, B, Dark exposure increases the duration and fraction of the NMDAR-mediated response blocked by the NR2b antagonist ifenprodil (3 μm). A, Pharmacologically isolated NMDAR-mediated EPSCs (see text) recorded before (black lines) and after bath application of ifenprodil (3 mm; thick black line) in cells from NR mice (left) and DE mice (center). The normalized responses after ifenprodil are also included (gray lines). Right, Control responses from NR and DE mice are compared. The traces are normalized to the peak amplitude in control ACSF and are the average of all cells recorded in each rearing condition (15–20 responses per cell). B, Average changes in the decay constant (left) and the response fraction blocked by ifenprodil (right) induced by dark exposure. C–E, Dark exposure increases the magnitude of the NMDAR-mediated response. C, Diagram illustrating the measurements of the AMPAR-mediated and the NMDAR-mediated components of the EPSCs. D, Effects of dark exposure (NR, open circles; DE, filled circles) on input–output (I/O) curve for the AMPAR-component. Relative stimulus intensity is normalized to the threshold. E, Effects of dark exposure (NR, open circles; DE, filled circles) on the input–output (I/O) curve for the NMDAR/AMPAR ratio. Asterisks denote p < 0.05. Plotted data are average ± SEM.
Next we tested the effects of bath application of ifenprodil in the induction of STDP in DE cells. The antagonist prevented the induction of tLTP and tLTD with long delays (+100 ms, −100 ms), but not with short delays (+10 ms, −10 ms), rendering the DE STDP profile similar to the NR STPD profile (Fig. 6A). A two-way ANOVA followed by a Fisher post hoc test confirmed the significance of these findings. On the other hand, ifenprodil did not affect the induction of tLTP and tLTD with short delays in NR cells (F(1,32) = 0.076, p = 0.784; Fig. 6A, open triangles). The results indicate that the specific blockade of NR2b-containing NMDARs abolishes the STDP differences between cells from DE and NR mice. To further determine whether this is due to specific block of NR2b or a general reduction in NMDAR function, we examined whether the nonspecific antagonist dl-APV, expected to equally block NR2b and NR2a function, would convert the DE STDP profile into a NR STDP profile. We focused on the induction of tLTD with suboptimal doses of dl-APV (7.5 and 15 μm) in DE cells. Unlike ifenprodil, APV reduced the magnitude of tLTD induced with both delays (−10 and −100 ms) (F(2,78) = 0.140, p = 0.869) (Fig. 6B). Altogether, the specific effects of ifenprodil, along with the unspecific effects of APV, support the idea that the increased NR2b functionality extends the STDP integration window in DE mice.
Figure 6.
Involvement of NR2b-containing NMDARs in the expansion of the integration window. A, Changes in the EPSPs (30 min after conditioning) elicited by pairing at different delays in cells from NR mice (open circles) and DE mice in the presence (filled triangles) or absence of the NR2b-specific antagonist ifenprodil (filled circles). The number of experiments included is indicated on the side of each data point. B, Changes in the EPSPs (30 min after conditioning) elicited by pairing at −10 ms (open circles) and −100 ms (filled circles) in cells from DE mice in the presence of the nonspecific antagonist dl-APV at the indicated concentrations. The number of experiments included is indicated on the side of each data point. C, D, In cells from NR mice, paired pulse synaptic stimulation (50 ms interval) during the associative pairing enables the induction of tLTD (C) and tLTP (D) with long delays (C, −100 ms; D, 100 ms). Paired pulses, filled circles; single pulses, open circles. E, F, In cells from NR mice, d-serine (100 μm) enhances the magnitude (E) of the NMDAR-mediated synaptic currents (control responses, gray; after 5 min in d-serine, black), but does not enable the induction of STDP (F) with long delays (100 ms, filled circles; −100 ms, open circles). Asterisks denote p < 0.05. Plotted data are average ± SEM.
Subsequently, we examined whether the increased NMDAR function is sufficient to account for the extended integration window in the DE mice. To that end, in cells from NR mice we stimulated with pairs of pulses separated by a 50 ms interval, which was expected to increase the duration and magnitude of the NMDAR-mediated responses during the pairings. In these experiments, one pathway was conditioned with the paired pulses, while the other pathway was conditioned with a single pulse at the same long pre-post delay (+100 or −100 ms) to serve as a control. The paired pulse stimulation (PPS) enabled the induction of tLTP with +100 ms delays (PPS: 120.6 ± 6.2%; control: 99.5 ± 2.2%, n = 7 slices, 3 mice; paired t test, p = 0.008) and the induction of tLTD with −100 ms delays (PPS: 85.1 ± 3.9%; control: 98.7 ± 1.0%; n = 12 slices, 5 mice; p = 0.009) in cells from NR mice (Fig. 6C,D). Thus, pairing with paired pulses is sufficient to expand the integration window in NR mice.
To determine whether the amplitude increase after dark exposure was sufficient, we applied the allosteric NMDAR agonist d-serine, which increases the NMDAR responses in pyramidal cells (Martina et al., 2003; Li and Han, 2008). Bath application of 100 μm d-serine, which in separate experiments increased the NMDAR-mediated response (142.5 ± 12.6%; n = 5 slices, 3 mice; paired t test, p = 0.002; Fig. 6E), did not enable the induction of tLTP (100.5 ± 4.5%; n = 9 slices, 4 mice; paired t test, p = 0.824) or tLTD (101.6 ± 4.6%; n = 8 slices, 4 mice; p = 0.743) with long delays in cells from NR mice (Fig. 6B). Thus, increasing the NMDAR response magnitude was insufficient to expand the integration window in NR mice This suggests that the increase in duration of the NMDAR response, alone or in conjunction with the magnitude increase, is the substrate for the metaplasticity of STDP.
Dark exposure enables induction of associative LTD with random presynaptic and postsynaptic spiking
In a final set of experiments, we set out to examine the possible functional consequences on the changes in STDP induced by dark exposure. Dark exposure increases spontaneous activity in visual cortex (from ∼2.5 to 3.5 Hz under isofluorane anesthesia; E. Quinlan, personal communication; see also Benevento et al., 1992; Gianfranceschi et al., 2003) and it has been hypothesized that random presynaptic and postsynaptic activity can lead to a net tLTD, which might serve as a homeostatic mechanism (Song and Abbott, 2001). In that context, it was of interest to evaluate how dark exposure affects associative plasticity induced with irregular stimulation patterns. STDP rules obtained with repeated associative pairings of fixed delays are insufficient to predict the outcome in irregular presynaptic and postsynaptic activity because multiple presynaptic and postsynaptic events occurring within a short time can interact in complex nonlinear ways (for review, see Froemke et al., 2010a). Therefore, we directly evaluated associative plasticity with trains of presynaptic and postsynaptic stimulation with random interstimulus intervals (Poisson distribution; mean frequency, 0.5–10 Hz) (Robinson et al., 1993). In these experiments, a random stimulation train (200 stimulation pulses) of a given frequency was delivered to one pathway while eliciting a random train of 200 postsynaptic action potentials of the same average frequency. The second pathway was unstimulated and served as a control for possible heterosynaptic effects. In cells from NR mice, the random train pairings had no effect at low frequencies (0.5 Hz: 100.2 ± 2.8%, n = 6 slices, 5 mice; paired t test, p = 0.884; 3 Hz: 98.3 ± 0.9%, n = 6 slices, 5 mice; paired t test, p = 0.884) and elicited LTP, but not LTD, at frequencies >6 Hz (Fig. 7). Dark exposure enabled the induction of LTD with low-frequency random trains pairing (0.5 Hz: 83.9 ± 4,7%, n = 6 slices, 5 mice; paired t test, p = 0.0.007) and lowered the frequency threshold for LTP (3 Hz: 121.1 ± 3.6%, n = 7 slices, 5 mice; paired t test, p = 0.001). A two-way ANOVA confirmed the significance of the results (F(4,50) = 3.962, p = 0.007). No lasting changes were detected in the unstimulated pathways (p > 0.7 in all cases). Thus, dark exposure enables the bidirectional induction of plasticity with Poisson pairing at frequencies within the range of spontaneous activity.
Figure 7.
Dark exposure enhances induction of bidirectional plasticity with random pairing of presynaptic and postsynaptic stimulation. A, Pairing trains of synaptic stimulation with random interstimulus intervals (Poisson: 200 pulses, 0.5 Hz) with random postsynaptic firing (Poisson: 200 pulses, 0.5 Hz) induced homosynaptic LTD in cells from DE mice (filled circles, top graph), but not from NR mice (open circles, bottom graph). Lines in both graphs represent responses in the control, nonstimulated pathway. Lines in both graphs represent responses in the control, nonstimulated pathway. B, The same type of random pairing delivered at a higher frequency (3 Hz) induced homosynaptic LTP in cells from DE mice (filled circles, top graph), but not from NR mice (open circles, bottom graph). C, Changes in the EPSPs (30 min after conditioning) elicited by random pairing delivered at different frequencies in cells from NR mice (open circles) and DE mice (filled circle). The number of experiments included is indicated close to each data point. Asterisks denote p < 0.0 5. Plotted data are average ± SEM.
Discussion
Previous studies on metaplasticity established that visual deprivation shifts the threshold for the induction of LTP and LTD with rate-dependent paradigms favoring LTP at the expense of LTD (Kirkwood et al., 1996; Philpot et al., 2003). Our present investigation with spike-timing-dependent paradigms showed that brief dark exposure extends the integration temporal window for the induction of both tLTP and tLTD. In addition, it enables the induction of associative of LTD with random stimulation at frequencies within the range of the spontaneous firing rates. Importantly, consistent with previous studies showing the changes in the frequency threshold for LTP and LTD (Philpot et al., 2003), the changes in the integration temporal window results from the incorporation of NR2b-containing NMDARs and the consequent prolongation of the NMDAR-mediated response. We propose that sensory deprivation has contrasting effects on NMDAR-dependent LTD, depending on the induction conditions: it expands the temporal window for inducing tLTD, but prevents the induction of LTD with low-frequency stimulation or pairing paradigms.
Several lines of evidence indicate that an increase in NR2b-containing NMDARs at the synapse mediates the expanded integration window for STDP after dark exposure. First, we showed that dark exposure increases the magnitude and duration of the NMDAR-mediated response, and their susceptibility to block by ifenprodil. These changes in electrophysiological measures of synaptic NR2b-NMDAR are consistent with reports of increased translation of NR2b after brief dark exposure (Chen and Bear, 2007) or synaptic inactivity (Lee et al., 2010) and rapid synaptic incorporation of NR2b-containing receptors after reduced input activity (Storey et al., 2011). Second, the selective block of NR2b-NMDAR abolishes the changes in STDP elicited by dark exposure. Finally, mimicking the increase in response duration caused by dark exposure extends the integration window in NR mice. In sum, we propose that an increase in synaptic NR2b, which is known to reduce the frequency window for rate-dependent LTD (Kirkwood et al., 1996; Choi et al., 2002; Philpot et al., 2003; Chen and Bear, 2007; Kanold et al., 2009), expands the window for tLTD and tLTP.
The extended integration window for tLTP in NR2b-NMDAR-“enriched” synapses dovetails with the prevailing view that the backpropagating action potential serves to relieve the voltage-dependent Mg2+ block in NMDARs bound to glutamate. The extended window for postsynaptic tLTD, on the other hand, is harder to interpret because multiple models have been proposed to explain how action potentials followed by synaptic activation might generate an LTD-inducing Ca2+ signal (Shouval et al., 2010) as, for example, after depolarization from the action potential (Shouval et al., 2002) and partial suppression of the NMDAR response (Froemke et al., 2005). It should be noticed, however, that the after-depolarization model predicts a modest increase in the window for tLTD if the duration of the NMDAR response is increased (Shouval et al., 2002). Regardless of the particular model, our findings indicate that the duration of the NMDAR response is a determining factor of integration window for tLTD that needs to be considered. We propose that an increase in NR2b-NMDAR at the synapse after dark exposure is sufficient to account for the expanded integration window for STDP induction. We cannot rule out, however, that changes in somatic and dendritic excitability (Froemke et al., 2010b) and processes related to the anchoring of NR2b-NMDARs (Barria and Malinow, 2005) might also contribute to shape STDP in the DE mice.
We have confirmed that, under our experimental conditions, STDP induction depends crucially on neuromodulators (Seol et al., 2007). This requirement for neuromodulators is intriguing, but not surprising. Indeed, the induction of LTP and LTD with voltage-pairing protocols do depend on endogenous levels of neuromodulators to the point that antagonists of Gq-coupled receptors block pairing-induced LTD (Choi et al., 2005), while antagonists against Gs-coupled receptors block pairing-induced LTP (Huang et al., 2012). In addition, it is worth noting that studies reporting robust cortical STDP in the absence of added neuromodulators (Sjöström et al., 2001; Bender et al., 2006; Nevian and Sakmann, 2006) are typically performed at a younger age (2–3 weeks old), when plasticity of layer II/III synapses is still maturing (Goel and Lee, 2007; Jiang et al., 2007). Whether the endogenous levels of neuromodulators are higher in the immature cortex remains to be determined.
A central finding of this study is the contrasting effect of dark exposure on the induction of LTD with rate-dependent and timing-dependent paradigms: it prevented LTD induced with 1 Hz stimulation while enabled tLTD with −100 ms delay pairings. These different consequences for LTD induction might relate to the conditions used to evoke plasticity. In our studies, STDP was induced in the presence of neuromodulators that increase the gain of plasticity downstream for the NMDAR activation. Hence the magnitude of the NMDAR-EPSC is not the sole determinant of the polarity of plasticity (Kirkwood et al., 1999; Seol et al., 2007, Huang et al., 2012). On the other hand, rate-dependent plasticity is typically done without added neuromodulators, and the threshold for the induction of LTP and LTD is determined mainly by the NMDAR-EPSC magnitude (Malenka and Bear, 2004). Indeed, adding neuromodulators to the bath restored the induction of rate-dependent LTD in slices from DE mice. Altogether, our findings suggest a scenario in which the outcome of metaplasticity would depend on the neuromodulatory tone. Sensory deprivation would promote time-dependent LTD when the neuromodulatory tone is high and restrict rate-dependent LTD when it is low. One must bear in mind, however, that these two induction modes likely represent two extremes of a continuum, and that the relative contribution of rate-dependent and time-dependent LTD to cortical remodeling is yet to be determined.
The functional consequences of metaplasticity of STDP remain to be explored. A shift of the threshold for rate-dependent LTD and LTP in favor of LTP is thought to help restore firing levels in the deprived cortex. Such adaptive function is unlikely to be subserved by an expanded temporal window for tLTD. Indeed, the increased width of the tLTD might serve to compensate for the increased tLTP window and prevent saturation of plasticity (Song and Abbott, 2001). However, an expanded integration window for STDP and the concomitant loss of temporal precision, along with increased spontaneous firing rates, might degrade the synaptic organization of the deprived cortex (Freeman et al., 1981). On the other hand, the increased window of opportunities for both tLTP and tLTD caused by a high NR2b/NR2a ratio might increase the contrast between deprived and nondeprived inputs. Thus, enhanced STDP could be an attractive candidate mechanism to account for the accelerated remodeling of adult visual cortical circuits observed after interventions that increase the NR2b content, such as local cortical lesions (Huemmeke et al., 2004) or dark exposure (He et al., 2006).
Footnotes
This work was supported by National Institutes of Health Grant R01 EY012124 to A.K., Natural Science Foundation of China Grant 30730099 to K.Z., a Howard Hughes Medical Institute Undergraduate Research Fellowship to K.M., and National Institutes of Health Grant R01 EY014882 to H.-K.L.
References
- Abraham WC, Bear MF. Metaplasticity: The plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Bender VA, Bender KJ, Brasier DJ, Feldman DE. Two coincidence detectors for spike timing-dependent plasticity in somatosensory cortex. J Neurosci. 2006;26:4166–4177. doi: 10.1523/JNEUROSCI.0176-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benevento LA, Bakkum BW, Port JD, Cohen RS. The effects of dark rearing on the electrophysiology of the rat visual cortex. Brain Res. 1992;572:198–207. doi: 10.1016/0006-8993(92)90470-t. [DOI] [PubMed] [Google Scholar]
- Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporale N, Dan Y. Spike timing-dependent plasticity: a Hebbian learning rule. Annu Rev Neurosci. 2008;31:25–46. doi: 10.1146/annurev.neuro.31.060407.125639. [DOI] [PubMed] [Google Scholar]
- Chen WS, Bear MF. Activity-dependent regulation of NR2B translation contributes to metaplasticity in mouse visual cortex. Neuropharmacology. 2007;52:200–214. doi: 10.1016/j.neuropharm.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Choi SY, Morales B, Lee HK, Kirkwood A. Absence of long-term depression in the visual cortex of glutamic acid decarboxylase-65 knock-out mice. J Neurosci. 2002;22:5271–5276. doi: 10.1523/JNEUROSCI.22-13-05271.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SY, Chang J, Jiang B, Seol GH, Min SS, Han JS, Shin HS, Gallagher M, Kirkwood A. Multiple receptors coupled to PLC gate LTD in visual cortex. J Neurosci. 2005;25:11433–11443. doi: 10.1523/JNEUROSCI.4084-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlew R, Wang Y, Ghermazien H, Erisir A, Philpot BD. Developmental switch in the contribution of presynaptic and postsynaptic NMDA receptors to long-term depression. J Neurosci. 2007;27:9835–9845. doi: 10.1523/JNEUROSCI.5494-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE. Timing-based LTP and LTD at vertical inputs to layer II/III pyramidal cells in rat barrel cortex. Neuron. 2000;27:45–56. doi: 10.1016/s0896-6273(00)00008-8. [DOI] [PubMed] [Google Scholar]
- Freeman RD, Mallach R, Hartley S. Responsivity of normal kitten striate cortex deteriorates after brief binocular deprivation. J Neurophysiol. 1981;45:1074–1084. doi: 10.1152/jn.1981.45.6.1074. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Poo MM, Dan Y. Spike-timing-dependent synaptic plasticity depends on dendritic location. Nature. 2005;434:221–225. doi: 10.1038/nature03366. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Debanne D, Bi GQ. Temporal modulation of spike-timing-dependent plasticity. Front Synaptic Neurosci. 2010a;2:19. doi: 10.3389/fnsyn.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, Letzkus JJ, Kampa BM, Hang GB, Stuart GJ. Dendritic synapse location and neocortical spike-timing-dependent plasticity. Front Synaptic Neurosci. 2010b;2:29. doi: 10.3389/fnsyn.2010.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Sossa K, Song L, Errington L, Cummings L, Hwang H, Kuhl D, Worley P, Lee HK. A specific requirement of Arc/Arg3.1 for visual experience-induced homeostatic synaptic plasticity in mouse primary visual cortex. J Neurosci. 2010;30:7168–7178. doi: 10.1523/JNEUROSCI.1067-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianfranceschi L, Siciliano R, Walls J, Morales B, Kirkwood A, Huang ZJ, Tonegawa S, Maffei L. Visual cortex is rescued from the effects of dark rearing by overexpression of BDNF. Proc Natl Acad Sci U S A. 2003;100:12486–12491. doi: 10.1073/pnas.1934836100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A, Lee HK. Persistence of experience-induced homeostatic synaptic plasticity through adulthood in superficial layers of mouse visual cortex. J Neurosci. 2007;27:6692–6700. doi: 10.1523/JNEUROSCI.5038-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A, Jiang B, Xu LW, Song L, Kirkwood A, Lee HK. Cross-modal regulation of synaptic AMPA receptors in primary sensory cortices by visual experience. Nat Neurosci. 2006;9:1001–1003. doi: 10.1038/nn1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He HY, Hodos W, Quinlan EM. Visual deprivation reactivates rapid ocular dominance plasticity in adult visual cortex. J Neurosci. 2006;26:2951–2955. doi: 10.1523/JNEUROSCI.5554-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Treviño M, He K, Ardiles A, Pasquale Rd, Guo Y, Palacios A, Huganir R, Kirkwood A. Pull-push neuromodulation of LTP and LTD enables bidirectional experience-induced synaptic scaling in visual cortex. Neuron. 2012;73:497–510. doi: 10.1016/j.neuron.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huemmeke M, Eysel UT, Mittmann T. Lesion-induced enhancement of LTP in rat visual cortex is mediated by NMDA receptors containing the NR2B subunit. J Physiol. 2004;559:875–882. doi: 10.1113/jphysiol.2004.069534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Treviño M, Kirkwood A. Sequential development of long-term potentiation and depression in different layers of the mouse visual cortex. J Neurosci. 2007;27:9648–9652. doi: 10.1523/JNEUROSCI.2655-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanold PO, Kim YA, GrandPre T, Shatz CJ. Co-regulation of ocular dominance plasticity and NMDA receptor subunit expression in glutamic acid decarboxylase-65 knock-out mice. J Physiol. 2009;587:2857–2867. doi: 10.1113/jphysiol.2009.171215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A, Rioult MC, Bear MF. Experience-dependent modification of synaptic plasticity in visual cortex. Nature. 1996;381:526–528. doi: 10.1038/381526a0. [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Rozas C, Kirkwood J, Perez F, Bear MF. Modulation of long-term synaptic depression in visual cortex by acetylcholine and norepinephrine. J Neurosci. 1999;19:1599–1609. doi: 10.1523/JNEUROSCI.19-05-01599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MC, Yasuda R, Ehlers MD. Metaplasticity at single glutamatergic synapses. Neuron. 2010;66:859–870. doi: 10.1016/j.neuron.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YH, Han TZ. Glycine modulates synaptic NR2A- and NR2B-containing NMDA receptor-mediated responses in the rat visual cortex. Brain Res. 2008;1190:49–55. doi: 10.1016/j.brainres.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Martina M, Krasteniakov NV, Bergeron R. D-Serine differently modulates NMDA receptor function in rat CA1 hippocampal pyramidal cells and interneurons. J Physiol. 2003;548:411–423. doi: 10.1113/jphysiol.2002.037127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min R, Nevian T. Astrocyte signaling controls spike timing-dependent depression at neocortical synapses. Nat Neurosci. 2012;15:746–753. doi: 10.1038/nn.3075. [DOI] [PubMed] [Google Scholar]
- Nevian T, Sakmann B. Spine Ca2+ signaling in spike-timing-dependent plasticity. J Neurosci. 2006;26:11001–11013. doi: 10.1523/JNEUROSCI.1749-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot BD, Sekhar AK, Shouval HZ, Bear MF. Visual experience and deprivation bidirectionally modify the composition and function of NMDA receptors in visual cortex. Neuron. 2001;29:157–169. doi: 10.1016/s0896-6273(01)00187-8. [DOI] [PubMed] [Google Scholar]
- Philpot BD, Espinosa JS, Bear MF. Evidence for altered NMDA receptor function as a basis for metaplasticity in visual cortex. J Neurosci. 2003;23:5583–5588. doi: 10.1523/JNEUROSCI.23-13-05583.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan EM, Philpot BD, Huganir RL, Bear MF. Rapid, experience-dependent expression of synaptic NMDA receptors in visual cortex in vivo. Nat Neurosci. 1999;2:352–357. doi: 10.1038/7263. [DOI] [PubMed] [Google Scholar]
- Richards BA, Aizenman CD, Akerman CJ. In vivo spike-timing-dependent plasticity in the optic tectum of Xenopus laevis. Front Synaptic Neurosci. 2010;2:7. doi: 10.3389/fnsyn.2010.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson GB, Fluharty SJ, Zigmond MJ, Sclabassi RJ, Berger TW. Recovery of hippocampal dentate granule cell responsiveness to entorhinal cortical input following norepinephrine depletion. Brain Res. 1993;614:21–28. doi: 10.1016/0006-8993(93)91013-i. [DOI] [PubMed] [Google Scholar]
- Rumbaugh G, Vicini S. Distinct synaptic and extrasynaptic NMDA receptors in developing cerebellar granule neurons. J Neurosci. 1999;19:10603–10610. doi: 10.1523/JNEUROSCI.19-24-10603.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seol GH, Ziburkus J, Huang S, Song L, Kim IT, Takamiya K, Huganir RL, Lee HK, Kirkwood A. Neuromodulators control the polarity of spike-timing-dependent synaptic plasticity. Neuron. 2007;55:919–929. doi: 10.1016/j.neuron.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shouval HZ, Bear MF, Cooper LN. A unified model of NMDA receptor-dependent bidirectional synaptic plasticity. Proc Natl Acad Sci U S A. 2002;99:10831–10836. doi: 10.1073/pnas.152343099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shouval HZ, Wang SS, Wittenberg GM. Spike timing dependent plasticity: a consequence of more fundamental learning rules. Front Comput Neurosci. 2010;4:19. doi: 10.3389/fncom.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström PJ, Turrigiano GG, Nelson SB. Rate, timing, and cooperativity jointly determine cortical synaptic plasticity. Neuron. 2001;32:1149–1164. doi: 10.1016/s0896-6273(01)00542-6. [DOI] [PubMed] [Google Scholar]
- Sjöström PJ, Turrigiano GG, Nelson SB. Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron. 2003;39:641–654. doi: 10.1016/s0896-6273(03)00476-8. [DOI] [PubMed] [Google Scholar]
- Smith GB, Heynen AJ, Bear MF. Bidirectional synaptic mechanisms of ocular dominance plasticity in visual cortex. Philos Trans R Soc Lond B Biol Sci. 2009;364:357–367. doi: 10.1098/rstb.2008.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Abbott LF. Cortical development and remapping through spike timing-dependent plasticity. Neuron. 2001;32:339–350. doi: 10.1016/s0896-6273(01)00451-2. [DOI] [PubMed] [Google Scholar]
- Storey GP, Opitz-Araya X, Barria A. Molecular determinants controlling NMDA receptor synaptic incorporation. J Neurosci. 2011;31:6311–6316. doi: 10.1523/JNEUROSCI.5553-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg GM, Wang SS. Malleability of spike-timing-dependent plasticity at the CA3-CA1 synapse. J Neurosci. 2006;26:6610–6617. doi: 10.1523/JNEUROSCI.5388-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]