Abstract
Background
The incidence of and risk factors for falls in human immunodeficiency (HIV)-1-infected persons are unknown.
Methods
Fall history during the prior 12 months, medical diagnoses, and functional assessments were collected on HIV-infected persons 45 to 65 years of age receiving effective antiretroviral therapy. Fall risk was evaluated using univariate and multivariate regression analyses.
Results
Of 359 subjects, 250 persons (70%) reported no falls, 109 (30%) had ≥1 fall; 66 (18%) were recurrent fallers. Females, Caucasians, and smokers were more like to be recurrent fallers (p≤0.05). HIV-related characteristics including current and nadir CD4 T-cell count, estimated HIV duration, and Veterans Aging Cohort Study Index scores were not predictors of falls (all p≥0.09); didanosine recipients were more likely to be recurrent fallers (p=0.04). The odds of falling increased 1.7 for each comorbidity and 1.4 for each medication (p<0.001), and were higher in persons with cardiovascular disease, hypertension, dementia, neuropathy, arthritis, chronic pain, psychiatric disease, frailty or disability (all OR≥ 1.8; p≤0.05). Beta-blockers, antidepressants, anti-psychotics, sedatives, and opiates were independently associated with falling (all OR ≥2.7; p≤0.01). Female gender, diabetes, antidepressants, sedatives, opiates, didanosine, exhaustion, weight loss, and difficulty with balance were the most significant predictors of falls in logistic regression (all OR ≥2.5; p≤0.05).
Conclusion
Middle-aged HIV-infected adults have high fall risk. Multiple comorbidities, medications, and functional impairment were predictive of falls, but surrogate markers of HIV infection or an HIV-specific multimorbidity index were not. Fall risk should be assessed routinely as part the care of HIV-infected persons.
Keywords: HIV, aging, accidental falls
INTRODUCTION
Falls are common among community dwelling adults 65 years and older, with more than one-third of older adults sustaining a fall each year1,2. Falls are one of the most common causes of emergency room visits and loss of independence among aging adults, with a non-injurious fall increasing the risk of placement in a skilled nursing facility by more than three-fold3. As a geriatric syndrome, falls are the consequence of multiple interrelated factors including comorbidities (arthritis, diabetes, pain, depression among many others), physical impairments (vision, cognition, neuropathy, strength, gait), and polypharmacy (especially psychoactive medications)4.
Persons aging with human immunodeficiency (HIV)-1 infection are thought to manifest “accelerated aging” with an earlier than expected occurrence of many diseases of aging5. Similarly, persons with HIV infection have a high prevalence of several comorbidities and physical impairments associated with an elevated fall risk6. Approximately 75% of HIV-infected persons receive at least one prescription medication in addition to antiretroviral therapy, and prescriptions associated with high-fall risk (cardiovascular and psychoactive medications) are among the most common7.
Despite heightened awareness of aging complications in the HIV-infected population, the rate of falls and risk factors for falls among HIV-1-infected adults are unknown. We hypothesized that a greater number of fall risk factors would result in a higher than expected fall rate among middle-aged HIV-1-infected adults.
METHODS
Study population
All individuals who received care for HIV-1 infection within twelve months prior to February 2010 in the Infectious Diseases Group Practice clinic at the University of Colorado Hospital were evaluated for participation. Individuals meeting the following criteria were eligible: 1) 45 to 65 years of age; 2) able to consent and participate in study procedures; and 3) taking combination (two or more) antiretroviral therapy for at least six months with one undetectable plasma HIV-1 RNA (<48 copies/mL) and no plasma HIV-1 RNA >200 copies/mL in the prior six months. Eligible individuals were contacted in person, by telephone, or by letter to determine interest in study participation. Approval was obtained by from the Colorado Multiple Institutional Review Board, and informed consent was obtained from all participants. All participants completed a single study visit that included a medical record review, a standardized interview, and a physical function assessment.
Clinical Assessments
Fall was defined as unintentionally coming to rest on the ground or other lower level, not as a result of a major intrinsic event or external hazard 8. A history of one or more falls during the past twelve months was collected. Persons falling more than one time during the prior twelve months were considered recurrent fallers and were compared to non-fallers for the analyses. Recurrent fallers represent a higher risk group than those with a single fall, with significantly higher mortality and risk of admission to long-term care compared to single-fallers or non-fallers9–11.
Fried’s frailty score was assessed as previously described 12. Shrinking was defined as unintentional weight loss of ≥ 10 pounds, or decrease of 5% of body weight in the last year (self-reported and verified by records when available). Exhaustion was defined by three to four times per week of feeling “everything I do is an effort” or “sometimes I just cannot get going”. Low activity was defined as self-report of being “limited a lot” in vigorous physical activities 13,14. Weakness was assessed by the average of three dominant hand grip measurements using a Lafayette dynamometer, applying previously defined gender and body mass index (BMI) cut-offs 12. Slowness was defined by 4.5-m walk time: men ≤173 cm and women ≤159 cm in height requiring ≥7 seconds or men >173 cm and women >159 cm requiring ≥6 seconds met a criterion 12. One point was given for each abnormality; a score of three or more was considered frail.
The Short Physical Performance Battery (SPPB) assessed tandem stand by ability to stand heel-to-toe for ten seconds, walking speed by the faster of two 4-m walks at usual pace, and sit-stand test time by five repetitions of sit-to-stand without use of the arms 15. On each task, zero points indicated inability to complete, two or less points was considered “difficulty”, and three or four points was considered performance within the expected range. A total score of 9 or less was considered disability 16. 400-m walk time was measured on a set walking course by asking the participant to walk the distance as quickly as possible 17.
The presence or absence of the following comorbidities were determined by medical record review and included in a comorbidity count: seizure disorder, dementia, stroke, neuropathy, psychiatric disease, arthritis, osteopenia or osteoporosis (prior stress fracture or T-score < −1 on bone densitometry scan), diabetes, kidney disease (calculated creatinine clearance <30 mL/min by Cockcroft-Gault, SI units 0.5mL/s/m2), malignancy (excluding non-melanoma skin cancer), solid organ transplant, lung disease, hypertension, cardiovascular disease, viral hepatitis (hepatitis B or C), and chronic liver disease. Current medication usage was determined by medical record and self-report; laboratory values were the most recent values available in the medical record. Current/prior alcohol use was defined as drinking > 7 drinks per week or a self-reported history of abuse; debilitating pain was defined as responding “moderately”, “quite a bit”, or “extremely” to the question “during the past four weeks, how much did pain interfere with your normal work (including both work outside the home and housework)?”. The Veterans Aging Cohort Study (VACS) Index score for predicted mortality was calculated for each subject as previously described 18.
Statistical Analysis
Data were collected and managed with Research Electronic Data Capture (REDCap) tools hosted at the University of Colorado 19. Demographic characteristics were summarized with mean and standard error (SE) for continuous outcomes, and frequency with percentage for categorical variables. Relationships between comorbid conditions, medications, functional assessment and odds of recurrent falls versus no falls were described by odds ratios (OR) and 95% confidence intervals (CI), and tested with Wald Chi-square. A sensitivity analysis was performed to evaluate the effect of the potential confounders of age >50, female gender, and current CD4+ lymphocyte count <200 cells/µL on demographics, comorbidities, medications, and functional assessment components. Odds ratios for medications were additionally adjusted for underlying treatment indication. To jointly assess demographics, comorbid conditions, and individual components of functional testing on the odds of falling, a logistic model was estimated. Comorbid conditions, medications, and individual components of functional testing with univariate-screen p value ≤ 0.05 were used to evaluate odds of falling in a logistic regression model. To create a model that could be implemented at the bedside without specialized equipment, grip strength was excluded. Model selection involved removal of suspected co-linear terms where concordance index was lower than 0.6 in univariate analysis, followed by terms with p>0.05 from the full model. The predictive power of the final model was described with receiver-operator-characteristic area-under-the-curve (ROC-AUC). Analyses were performed in SAS v9.2 (SAS Institute Inc., Cary, NC).
RESULTS
A total of 359 subjects completed the study visit, of whom 85% were male, 74% self-reported Caucasian, 18% Hispanic or Latino, 65% were men having sex with men, 21% with a history of intravenous drug use, and less than one percent reporting current intravenous drug use. The mean age was 52 ± 0.3 years, the mean CD4+ lymphocyte count was 594 ± 16 cells/µL, and 95% had plasma HIV-1 RNA below the limits of detection.
Thirty-percent (109 subjects) reported at least one fall during the year prior to study visit. Of the fallers, 43 (39%) reported one fall and 66 (61%) were recurrent fallers. Females and tobacco users were more likely to be recurrent fallers, while persons using illicit substances (majority marijuana) were more likely to be non-fallers (Table 1). Age, ethnicity, and alcohol use were not significant predictors of falls (p ≥ 0.30). In a sensitivity analysis, odds of falling predicted by demographic characteristics did not differ by more than 10% after adjusting for age, gender, and CD4 lymphocyte count with the exception of a 13% increase in the odds of falling among Caucasian persons.
Table 1.
Odds of recurrent falling by demographic characteristics
| Demographic | Non-Fallers N= 250 (%) |
Single Fallers N=43 (%) |
Recurrent Fallers N= 66 (%) |
Odds Ratio (95% CI)* |
|---|---|---|---|---|
| Age in years (mean ± SE) | 52.0 ± 0.3 | 51.8 ± 0.7 | 52.1 ± 0.5 | 1.0 (0.96, 1.1) |
| Female gender | 31 (12) | 6 (14) | 17 (26) | 2.5 (1.3, 4.8) |
| Caucasian | 177 (71) | 35 (81) | 53 (80) | 1.7 (0.9, 3.3) |
| Hispanic ethnicity | 45 (18) | 5 (12) | 15 (23) | 1.3 (0.7, 2.6) |
| Current tobacco use | 74 (30) | 18 (42) | 31 (47) | 2.1 (1.2, 3.7) |
| Alcohol use > 7 drinks/week | 11 (4) | 3 (7) | 1 (2) | 0.3 (0.04, 2.7) |
| Current illicit drugs | 80 (32) | 11 (26) | 12 (18) | 0.5 (0.2, 0.9) |
| Years since HIV diagnosis (mean ± SE) | 14.0 ± 0.5 | 16.8 ± 1.1 | 15.8 ± 0.9 | 1.0 (1.0, 1.1)† |
| Current CD4 count (mean ± SE) | 595 ± 20 | 586 ± 28 | 599 ± 38 | 1.0 (1.0, 1.1)‡ |
| Nadir CD4 count (mean ± SE) | 168 ± 10 | 169 ± 27 | 164 ± 17 | 1.0 (0.9, 1.1)‡ |
| HIV-1 RNA below detection | 238 (95) | 40 (93) | 64 (97) | 1.6 (0.4, 7.4) |
| VACS Index Score (mean ± SE) | 17.6 ± 0.9 | 17.9 ± 2.4 | 20.7 ± 1.8 | 1.0 (1.0, 1.03)† |
CI, confidence interval; SE, standard error; VACS, Veterans Aging Cohort Study,
Odds ratio comparing non-fallers and recurrent fallers
HIV-Related Factors and Fall Risk
Although recurrent fallers had a longer time since HIV diagnosis compared to non-fallers, nadir CD4+ lymphocyte count, current CD4+ lymphocyte count, and persons with HIV-1 RNA below detection (Table 1, all p ≥ 0.54) were not predictive of falling. VACS Index score was not a significant predictor of falls (Table 1, p = 0.13). In a sensitivity analysis, odds of falling predicted by HIV-related characteristics did not differ by more than 10% after adjusting for age, gender, and CD4 lymphocyte count with the exception of a 12% decrease in the odds of falling among persons with HIV-1 RNA below detection.
Comorbidity and Fall Risk
Comorbidity was associated with an increased odds of recurrent falls, with each additional comorbid condition associated with 1.7 greater odds of falls (95% CI 1.5, 2.1; p <0.001). The odds of falls with each individual comorbid condition are shown in Table 2. Hemoglobin was lower among recurrent fallers (14.7 ± 0.2 g/dL) than non-fallers (15.3 ± 0.1 g/dL; p=0.007). Blood pressure, heart rate, and body mass index were not significant predictors of recurrent falls (all p >0.4).
Table 2.
Odds of recurrent falling by comorbidity and medications
| Comorbidities | Non- Fallers N=250 |
Single Fallers N=43 |
Recurrent Fallers N=66 |
Fall Rate (any fall) |
Odds Ratio (95% confidence interval) |
|
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted* | |||||
| CVD | 17 (7) | 5 (12) | 12 (18) | 50% | 3.0 (1.4, 6.8) | 3.4 (1.5, 7.8) |
| Hypertension | 95 (38) | 20 (47) | 33 (50) | 36% | 1.6 (0.95, 2.8) | 1.8 (1.0, 3.1) |
| Diabetes | 15 (6) | 5 (12) | 17 (27) | 60% | 5.6 (2.6, 12.1) | 6.2 (2.8, 13.7) |
| Stroke | 7 (3) | 2 (5) | 4 (6) | 46% | 2.2 (0.6, 7.9) | 1.9 (0.5, 7.0) |
| Lung disease | 30 (12) | 9 (20) | 10 (15) | 39% | 1.1 (0.5, 2.4) | 1.1 (0.5, 2.4) |
| Hepatitis | 59 (24) | 11(26) | 19 (28) | 34% | 1.3 (0.7, 2.4) | 1.3 (0.7, 2.4) |
| Dementia | 3 (1) | 2 (5) | 6 (9) | 73% | 10.5 (2.5, 43.7) | 10.5 (2.5, 43.7) |
| Neuropathy | 78 (31) | 19 (44) | 39 (59) | 43% | 3.3 (1.9, 5.9) | 3.3 (1.9, 5.9) |
| Arthritis | 75 (30) | 17 (40) | 38 (58) | 42% | 4.8 (2.7, 8.7) | 3.0 (1.7, 5.4) |
| Chronic Pain | 49 (20) | 12 (28) | 35 (53) | 49% | 4.8 (2.7, 8.7) | 4.8 (2.7, 8.7) |
| Psychiatric disease | 125 (50) | 30 (70) | 52 (79) | 40% | 3.6 (1.9, 6.8) | 3.6 (1.9, 6.8) |
| Medications | ||||||
| Beta-blocker | 22 (9) | 3 (7) | 17 (26) | 48% | 3.6 (1.8, 7.3) | 3.4 (1.5, 7.6)† |
| Calcium channel blocker | 13 (5) | 5 (12) | 8 (12) | 50% | 2.5 (1.0, 6.4) | 1.9 (0.7, 5.3)† |
| Diabetes therapy (any) | 13 (5) | 5 (12) | 13 (20) | 59% | 4.5 (2.0, 10.2) | 0.8 (0.1, 5.7)‡ |
| Testosterone (men) | 33 (15) | 7 (19) | 8 (16) | 31% | 1.1 (0.5, 2.6) | 1.1 (0.5, 2.6) |
| Vitamin D | 41 (16) | 8 (19) | 16 (24) | 37% | 1.4 (0.8, 2.5) | 1.5 (0.8, 3.0) |
| Bladder/prostate | 16 (6) | 3 (7) | 8 (19) | 41% | 1.6 (0.7, 3.7) | 2.0 (0.8, 5.0) |
| Antidepressant | 52 (21) | 15 (35) | 36 (55) | 50% | 4.6 (2.6, 8.1) | 3.5 (1.8, 6.9)§ |
| Benzodiazepine | 32 (13) | 10 (23) | 19 (29) | 48% | 2.5 (1.4, 4.3) | 1.9 (0.9, 3.8)§ |
| Antipsychotic | 19 (8) | 8 (19) | 16 (24) | 56% | 3.4 (1.8, 6.6) | 2.7 (1.2, 5.8)§ |
| Sedative/hypnotic | 34 (14) | 6 (14) | 25 (38) | 48% | 2.5 (1.5, 4.4) | 3.2 (1.7, 6.1)§ |
| Anticonvulsant | 13 (6) | 5 (14) | 11 (18) | 55% | 3.2 (1.5, 6.9) | 2.3 (0.9, 5.6)§ |
| Gabapentin/pregabalin | 22 (9) | 5 (12) | 14 (21) | 46% | 2.2 (1.1, 4.2) | 1.8 (0.8, 4.0)‖ |
| Opiate | 45 (18) | 10 (23) | 36 (55) | 51% | 3.3 (2.0, 5.5) | 3.4 (1.4, 8.1)‖ |
| Any didanosine | 57 (23) | 10 (23) | 24 (36) | 37% | 1.5 (0.9, 2.5) | 1.9 (1.03, 3.5)¶ |
| Any stavudine | 93 (37) | 22 (51) | 33 (50) | 37% | 1.7 (1.1, 2.7) | 1.6 (0.9, 2.9)¶ |
| Efavirenz | 86 (34) | 10 (23) | 22 (33) | 27% | 0.8 (0.5, 1.3) | 1.0 (0.5, 1.8) |
CVD, cardiovascular disease;
Adjusted for age, gender, CD4+ lymphocyte count; Adjusted for underlying treatment indication in addition to age, gender, CD4+ lymphocyte count:
hypertension;
diabetes,
psychiatric disease,
chronic pain, or
neuropathy (result of therapy rather than treatment indication)
Medications and Fall Risk
Polypharmacy was associated with an increased odds of falls, with each additional prescribed medication associated with an incremental increase of 1.4 in the odds of falls (CI 1.3, 1.6; p <0.001). Odds of falling with the use of individual medications are shown in Table 2. To examine the relationship of medications with falls, when adjusted for the underlying comorbid condition, the respective comorbidity being treated was added to each model. Didanosine and stavudine were adjusted for the comorbidity (neuropathy) presumed to be resultant from current or prior use of the medication (Table 2). Use of beta-blockers, opiates, antidepressants, antipsychotics, sedatives, and didanosine remained significantly more common among fallers than non-fallers after adjusting for the comorbidity being treated. In further sensitivity analysis, after adjusting for age, gender, and CD4 lymphocyte count, the odds of falling did not differ by more than 10% with the exception of a 12% increase with any stavudine use.
Functional Capacity and Fall Risk
In functional assessments, both frailty and disability were associated with falls (p<0.001). A one point worsening on Fried’s frailty score increased the odds of falls by 3.1 (CI 2.3, 4.2; p <0.001), while a one point worsening on the SPPB increased the odds by 1.4 (CI 1.3, 1.8; p <0.001). With the exception of the short walk components, all of the frailty and SPPB components were associated with recurrent falls (Table 3). Recurrent fallers had a significantly slower pace on the 400-m walk (1.33 ± 0.04 m/sec) than non-fallers (1.52 ± 0.02 m/sec, p <0.001).
Table 3.
Odds of recurrent falls by functional assessment tools and individual components
| Functional Component | Non- Fallers N=250 (%) |
Single Fallers N=43 (%) |
Recurrent Fallers N=66 (%) |
Odds Ratio* (95% confidence interval) |
|
|---|---|---|---|---|---|
| Unadjusted | Adjusted† | ||||
| Frailty (≥3 points) | 7 (3) | 6 (14) | 14 (21) | 9.4 (3.6, 24.3) | 9.5 (3.6, 25.1) |
| Weakness | 16 (6) | 5 (12) | 16 (24) | 4.7 (2.2, 10.0) | 4.6 (2.2, 10.0) |
| Slowness | 2 (1) | 3 (7) | 3 (5) | 5.9 (1.0, 36.1) | 3.3 (0.7, 28.9) |
| Exhaustion | 54 (22) | 17 (40) | 39 (59) | 5.2 (3.0, 9.3) | 5.4 (3.0, 9.9) |
| Shrinking | 17 (7) | 8 (19) | 10 (15) | 2.5 (1.1, 5.6) | 2.4 (1.0, 5.6) |
| Low activity | 159 (64) | 30 (71) | 60 (91) | 5.7 (2.4, 13.8) | 6.0 (2.4, 14.5) |
| Disability (SPPB ≤ 9 points) | 17 (7) | 12 (28) | 16 (24) | 4.4 (2.1, 9.3) | 4.2 (2.0, 9.0) |
| Tandem difficulty‡ | 4 (2) | 3 (7) | 12 (18) | 13.7 (4.2, 44.0) | 15.6 (4.6, 53.1) |
| Sit-stand difficulty‡ | 27 (11) | 14 (33) | 16 (24) | 2.6 (1.3, 5.3) | 2.6 (1.3, 5.3) |
| 4-m walk difficulty‡ | 3 (1) | 3 (7) | 1 (2) | 1.3 (0.1, 12.4) | 0.9 (0.1, 9.7) |
| 400-m pace (mean m/sec ± SE) | 1.52 ± 0.02 | 1.44 ± 0.06 | 1.33 ± 0.04 | 14.3 (4.8, 50.0) | 10.0 (4.3, 50.0) |
SPPB, short physical performance battery; SE, standard error;
Odds ratio comparing non-fallers and recurrent fallers;
Adjusted for age, gender, and CD4 lymphocyte count;
2 or less of 4 possible points
Multi-component Fall Risk
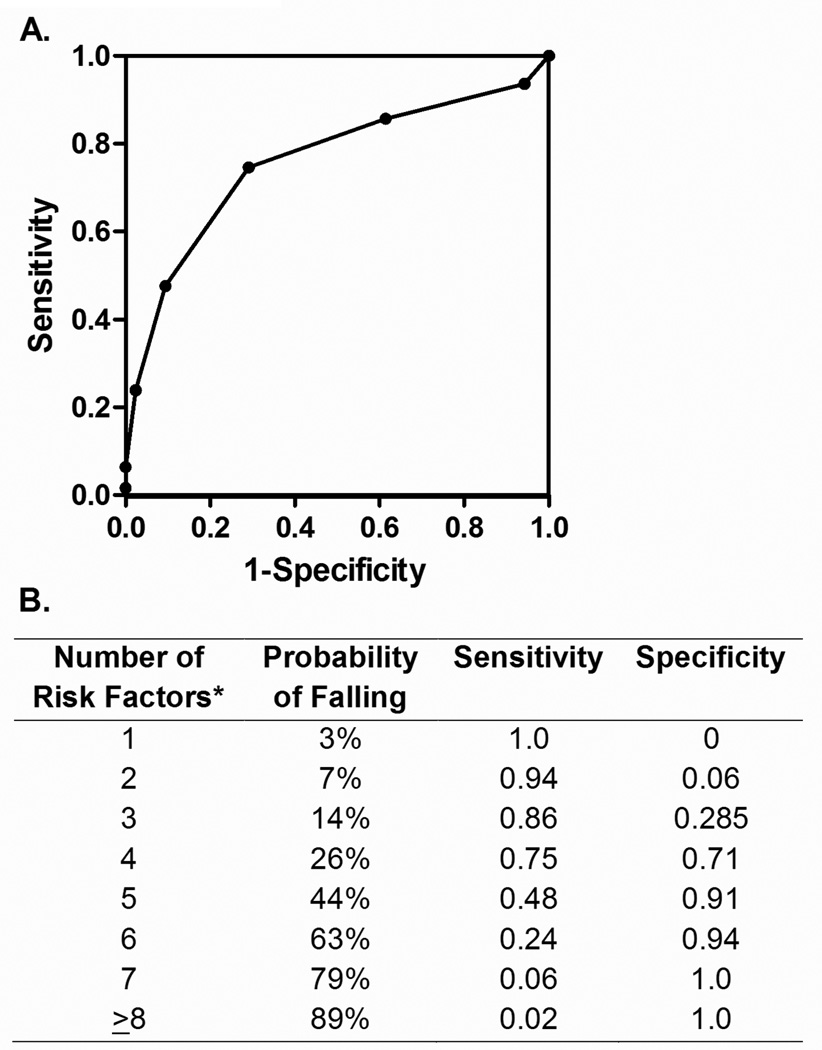
In a multivariate analyses, nine predictors of fall risk remained in a logistic model (Table 4). An ROC curve to assess the ability of the model to predict fall risk had an area under the curve of 0.866 (Figure 1A). The incremental odds of falling for each additional condition was 2.2 (CI 1.7, 2.8), with four risk factors having a sensitivity of 75% and specificity of 71% in predicting recurrent falls (Figure 1B).
Table 4.
Risk factors for recurrent falls from multivariate logistic regression models.
| Parameter | Odds Ratio (95% CI) |
|---|---|
| Difficulty with tandem stand* | 13.5 (3.0, 60.5) |
| Antidepressant use | 3.7 (1.8, 7.7) |
| Exhaustion† | 3.7 (1.8, 7.7) |
| Diabetes | 3.6 (1.4, 9.4) |
| Female gender | 3.5 (1.4, 8.8) |
| Shrinking‡ | 3.4 (1.2, 10.1) |
| Opiate use | 3.1 (1.5, 6.5) |
| Current/prior didanosine | 2.6 (1.2, 5.4) |
| Sedative use | 2.5 (1.1, 5.5) |
Inability to stand heel-to-toe for ≥ 3 seconds;
Three to four times/week of feeling “everything I do is an effort” or “sometimes I just cannot get going”;
Unintentional weight loss of ≥ 10 pounds or decrease of 5% body weight in the last year
Figure 1.
Risk of recurrent falls estimated by regression model. A) Fall risk shown by area under the receiver-operator-characteristic (ROC) curve, as estimated from the logistic regression model. B) Probability, sensitivity, and specificity of each additional fall risk factor. The risk factors identified through regression analyses were: difficulty with tandem stand, antidepressant use, exhaustion, diabetes, female gender, shrinking, opiate use, current/prior didanosine, and sedative use.
DISCUSSION
Although fall rates and risk factors for falls are well described in community dwelling and institutionalized elders, no prior studies have evaluated the rate or risk factors for falls among HIV-1-infected adults. We found that the fall rate in middle-aged adults (mean age 52.0 years) with HIV-1 infection is as common as in uninfected persons aged 65 years older 2. Falls in our cohort were associated with several previously reported risk factors such as hypertension, diabetes, impaired balance, and pain, as well as medications used in the treatment of these comorbidities1,4. A trend towards longer estimated duration of HIV-infection and a significant association with current or prior didanosine may reflect a greater number of accumulated comorbidities over time with HIV treatment. Other HIV-related risk factors were not seen, and, surprisingly, an HIV-specific multimorbidity index (the VACS Index) did not predict fall risk. Ultimately, the best predictors of fall risk were those factors known to be associated with fall risk in geriatric populations.
Our inclusion criteria provide a closer look at the fall rate and risk factors among middle-aged persons adherent to antiretroviral therapy. Although our study sample is reflective of the majority of persons engaged in HIV care20, our results may not be applicable to all HIV-infected populations. We expect that our fall rates may underestimate the fall rate among HIV-infected persons less compliant with antiretroviral therapy, with advanced immunodeficiency, or with greater intravenous drug abuse. Reliance on subject recall of fall history may even further underestimate the fall risk in our study population21.
Similar to other geriatric syndromes, such as frailty, multiple factors increase fall risk. Thus, understanding the etiology of falls requires a syndromic approach that assesses the multitude of potential factors demonstrated among recurrent fallers in our cohort, including tobacco use, comorbidities, medications, and functional status. Given that multiple factors lead to an increased fall risk, it is expected that successful interventions to reduce falls in HIV-infected persons will require a multipronged approach including medication adjustment, behavioral modifications, vitamin D supplementation, physical therapy, and exercise or balance programs22,23.
Available data suggest that HIV-infected persons have low bone density, increased fracture risk, and premature frailty5,6,13,14,24,25. Thus, in addition to increased risk of falls, aging HIV-infected persons are likely to be at increased risk of morbidity when falls occur. Providers caring for HIV-infected persons should routinely inquire about falls, assess fall risk factors in those at risk for falling, and when high fall risk is identified, intervene to reduce risk. Interventions such as discontinuation of psychotropic or other high risk medications, balance training, home safety evaluations, and exercise programs decrease fall risk in elderly, non-HIV-infected persons2,22,23. Future research is needed to investigate the effectiveness of interventions to reduce fall risk in middle-aged and older HIV-infected persons.
Acknowledgments
SOURCES OF FUNDING:
This work was supported by the National Institutes of Health [Colorado CTSI UL1 RR025780, T32 AI007447]; the Hartford Foundation Center of Excellence in Aging; and the GlaxoSmithKline HIV Collaborative Investigator Research Award. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views. The sponsors had no role in the design, execution, analysis or interpretation of data, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Prior Presentation:
This study was presented in part at the 2nd International Workshop on HIV and Aging, 27 October 2011, Baltimore, MD; Abstract O_05.
CONFLICTS OF INTEREST:
The authors report no conflicts of interest.
REFERENCES
- 1.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319:1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 2.Tinetti ME. Clinical practice. Preventing falls in elderly persons. N Engl J Med. 2003;348:42–49. doi: 10.1056/NEJMcp020719. [DOI] [PubMed] [Google Scholar]
- 3.Tinetti ME, Williams CS. Falls, injuries due to falls, and the risk of admission to a nursing home. N Engl J Med. 1997;337:1279–1284. doi: 10.1056/NEJM199710303371806. [DOI] [PubMed] [Google Scholar]
- 4.Deandrea S, Lucenteforte E, Bravi F, Foschi R, La Vecchia C, Negri E. Risk factors for falls in community-dwelling older people: a systematic review and meta-analysis. Epidemiology. 2010;21:658–668. doi: 10.1097/EDE.0b013e3181e89905. [DOI] [PubMed] [Google Scholar]
- 5.Deeks SG. Immune dysfunction, inflammation, and accelerated aging in patients on antiretroviral therapy. Top HIV Med. 2009;17:118–123. [PubMed] [Google Scholar]
- 6.Effros RB, Fletcher CV, Gebo K, et al. Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clin Infect Dis. 2008;47:542–553. doi: 10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marzolini C, Elzi L, Gibbons S, et al. Prevalence of comedications and effect of potential drug-drug interactions in the Swiss HIV Cohort Study. Antivir Ther. 2010;15:413–423. doi: 10.3851/IMP1540. [DOI] [PubMed] [Google Scholar]
- 8.The prevention of falls in later life. A report of the Kellogg International Work Group on the Prevention of Falls by the Elderly. Danish medical bulletin. 1987;34(Suppl 4):1–24. [PubMed] [Google Scholar]
- 9.Gangavati A, Hajjar I, Quach L, et al. Hypertension, orthostatic hypotension, and the risk of falls in a community-dwelling elderly population: the maintenance of balance, independent living, intellect, and zest in the elderly of Boston study. J Am Geriatr Soc. 2011;59:383–389. doi: 10.1111/j.1532-5415.2011.03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donald IP, Bulpitt CJ. The prognosis of falls in elderly people living at home. Age Ageing. 1999;28:121–125. doi: 10.1093/ageing/28.2.121. [DOI] [PubMed] [Google Scholar]
- 11.Delbaere K, Close JC, Heim J, et al. A multifactorial approach to understanding fall risk in older people. J Am Geriatr Soc. 2010;58:1679–1685. doi: 10.1111/j.1532-5415.2010.03017.x. [DOI] [PubMed] [Google Scholar]
- 12.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 13.Desquilbet L, Jacobson LP, Fried LP, et al. HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. J Gerontol A Biol Sci Med Sci. 2007;62:1279–1286. doi: 10.1093/gerona/62.11.1279. [DOI] [PubMed] [Google Scholar]
- 14.Onen NF, Agbebi A, Shacham E, Stamm KE, Onen AR, Overton ET. Frailty among HIV-infected persons in an urban outpatient care setting. J Infect. 2009;59:346–352. doi: 10.1016/j.jinf.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 16.Rejeski WJ, Fielding RA, Blair SN, et al. The lifestyle interventions and independence for elders (LIFE) pilot study: design and methods. Contemporary clinical trials. 2005;26:141–154. doi: 10.1016/j.cct.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Simonsick EM, Montgomery PS, Newman AB, Bauer DC, Harris T. Measuring fitness in healthy older adults: the Health ABC Long Distance Corridor Walk. J Am Geriatr Soc. 2001;49:1544–1548. doi: 10.1046/j.1532-5415.2001.4911247.x. [DOI] [PubMed] [Google Scholar]
- 18.Tate JP, Justice AC. Veterans Aging Cohort Study and VACS Risk Index. 2011 Aug 9; Available at: http://www.vacohort.org/74621_VACS_Index_Handout_19Nov10.pdf.
- 19.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Das M, Chu PL, Santos GM, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One. 2010;5:e11068. doi: 10.1371/journal.pone.0011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hannan MT, Gagnon MM, Aneja J, et al. Optimizing the tracking of falls in studies of older participants: comparison of quarterly telephone recall with monthly falls calendars in the MOBILIZE Boston Study. Am J Epidemiol. 2010;171:1031–1036. doi: 10.1093/aje/kwq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tinetti ME. Prevention of falls and fall injuries in elderly persons: a research agenda. Preventive medicine. 1994;23:756–762. doi: 10.1006/pmed.1994.1130. [DOI] [PubMed] [Google Scholar]
- 23.Michael YL, Whitlock EP, Lin JS, Fu R, O'Connor EA, Gold R. Primary care-relevant interventions to prevent falling in older adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153:815–825. doi: 10.7326/0003-4819-153-12-201012210-00008. [DOI] [PubMed] [Google Scholar]
- 24.Terzian AS, Holman S, Nathwani N, et al. Factors associated with preclinical disability and frailty among HIV-infected and HIV-uninfected women in the era of cART. J Womens Health (Larchmt) 2009;18:1965–1974. doi: 10.1089/jwh.2008.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McComsey GA, Tebas P, Shane E, et al. Bone disease in HIV infection: a practical review and recommendations for HIV care providers. Clin Infect Dis. 2010;51:937–946. doi: 10.1086/656412. [DOI] [PMC free article] [PubMed] [Google Scholar]