Abstract
Multiple sclerosis (MS) is a demyelinating disease of the CNS that is presumably mediated by CD4+ autoimmune T cells. Although both Th1 and Th17 cells have the potential to cause inflammatory CNS pathology in rodents, the identity of pathogenic T cells remains unclear in human MS. Given that each Th cell subset preferentially expresses specific chemokine receptors, we were interested to know whether T cells defined by a particular chemokine receptor profile play an active role in the pathogenesis of MS. In this article, we report that CCR2+CCR5+ T cells constitute a unique population selectively enriched in the cerebrospinal fluid of MS patients during relapse but not in patients with other neurologic diseases. After polyclonal stimulation, the CCR2+CCR5+ T cells exhibited a distinct ability to produce matrix metalloproteinase-9 and osteopontin, which are involved in the CNS pathology of MS. Furthermore, after TCR stimulation, the CCR2+CCR5+ T cells showed a higher invasive potential across an in vitro blood–brain barrier model compared with other T cells. Of note, the CCR2+CCR5+ T cells from MS patients in relapse are reactive to myelin basic protein, as assessed by production of IFN-γ. We also demonstrated that the CCR6−, but not the CCR6+, population within CCR2+CCR5+ T cells was highly enriched in the cerebrospinal fluid during MS relapse (p < 0.0005) and expressed higher levels of IFN-γ and matrix metalloproteinase-9. Taken together, we propose that autoimmune CCR2+CCR5+CCR6− Th1 cells play a crucial role in the pathogenesis of MS.
Introduction
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the CNS that is presumably mediated by CD4+ T cells reactive to myelin Ag, such as myelin basic protein (MBP) (1). Approximately two thirds of patients with MS have relapsing-remitting MS (RR-MS), which is characterized by acute episodes of exacerbations followed by partial or complete recovery. Although there are periods of remission in the RR-MS stage, a proportion of patients enters a stage of secondary progressive MS decades after the onset of MS. There are no real periods of remission in secondary progressive MS, in which neurodegeneration can be the major cause of irreversible neurologic disability (2).
It is proposed that an initiation of relapse in RR-MS is preceded by activation of autoimmune CD4+ T cells in the peripheral lymphoid organs. These T cells that are potentially reactive to myelin Ag could be activated in response to cross-reactive Ag that are generated by microbial infections (3) or following exposure to proinflammatory factors, such as osteopontin (OPN) (4), thereby acquiring the ability to migrate and infiltrate into the CNS (5, 6). The study performed in experimental autoimmune encephalomyelitis (EAE) showed that activated MBP-specific T cells first reach subarachnoid spaces filled with the cerebrospinal fluid (CSF) after crossing the endothelial barrier. After encountering perivascular APC presenting myelin Ag, the autoimmune T cells are reactivated and produce proinflammatory cytokines, such as IFN-γ and IL-17, as well as proteases, including matrix metalloproteinase (MMP)-9 (7). The proteases degrade components of the basement membranes, leading to the disruption of the blood–brain barrier (BBB). The T cells may invade into the parenchyma through the disrupted area of the BBB and cause CNS inflammation (8).
Research on EAE demonstrated that both IFN-γ–producing Th1 and IL-17–producing Th17 cells could cause inflammatory pathology in the CNS (9, 10). Although characterization of pathogenic T cells in EAE has ignited a search for similar cells in humans, the identity of pathogenic T cells in MS has not been established (10). Recent studies showed the involvement of Th17 cells (11) and of T cells producing both IFN-γ and IL-17 in the pathology of MS (12). However, because the administration of IFN-γ worsened MS in a previous clinical trial (13), the role of Th1 cells in MS needs to be analyzed further. In addition, increasing evidence suggest a pathogenic role for cytotoxic effector T cells in MS (14, 15). Moreover, a recent clinical trial of anti–IL-12p40 Ab to block IL-12/IL-23 signaling failed to modulate MS (16), making it difficult to portray a complete picture of MS (9).
Chemokines are a family of secreted proteins that function as key regulators of cell migration via interaction with a subset of seven-transmembrane, G protein-coupled receptors (17, 18). Chemokines are known to be highly efficient and potent chemoattractants for inflammatory cells in EAE (19). In the Th cell-differentiation process, CD4+ T cells acquire the ability to produce sets of cytokines and to express chemokine receptors. Although Th1 cells preferentially express CCR5 and CXCR3, Th2 cells express CCR4 and CRTh2 (20, 21). The chemokine receptor expression pattern would confer to each Th subset a unique characteristic of migration to corresponding ligand chemokines (22). It was recently reported that human Th17 cells are enriched in CCR4+CCR6+, CCR2+CCR5−, and CCR6+ populations (23–25).
The present study using multicolor flow cytometry was initiated to address whether Th17 cells bearing Th17 phenotypes (CCR4+CCR6+, CCR2+CCR5−, or CCR6+) are increased in the CSF of patients with MS compared with the peripheral blood (PB). In contrast to our expectations, none of these populations was increased in the CSF of MS. Instead, we found that T cells expressing both CCR2 and CCR5 were selectively enriched in the CSF of patients with exacerbated MS but not in patients with other neurologic diseases. The CCR2+CCR5+ memory CD4+ T cells were shown to produce IFN-γ (24). Comparison with other memory T cell subpopulations revealed that the CCR2+CCR5+ T cells possessed a distinct ability to produce MMP-9 and OPN, which are critical for initiating and perpetuating the inflammatory pathology in the CNS (4, 7). Consistent with the increased production of MMP-9, which is capable of degrading basement membranes, the CCR2+CCR5+ T cells showed a greater potential to invade across an in vitro model of the glia limitans, the physiological barrier separating CSF from the CNS parenchyma. Furthermore, the CCR2+CCR5+ T cells in the PB of active MS contained MBP-reactive T cells producing IFN-γ. We further demonstrated that CCR6−, but not CCR6+, cells within CCR2+CCR5+ T cells were enriched in the CSF of patients with MS during relapse and expressed high levels of IFN-γ and MMP-9. These results suggest that CCR2+CCR5+CCR6− Th1 cells play a crucial role in the pathogenesis of MS.
Materials and Methods
Subjects
Thirty-four RR-MS patients were examined for the expression of chemokine receptors on T cells. As controls for MS, 11 sex- and age-matched healthy subjects (HS), 6 patients with noninflammatory neurologic disease (NIND), and 4 patients with other inflammatory neurologic disease (OIND) were enrolled in this study. All of the MS patients fulfilled the diagnostic criteria of McDonald et al. (26). Patients with serum aquaporin 4 Abs or with longitudinally extensive spinal cord lesions on the magnetic resonance imaging scan were excluded from this study. In this article, we define “MS in remission” as patients who have been clinically stable without i.v. corticosteroid pulse therapy for >1 mo; “MS in relapse” is defined as patients who have developed an apparent exacerbation within an interval of 1 wk. The detailed demographic characteristics of the cohorts are summarized in Table I. None of the above patients had received IFN-β, i.v. corticosteroids, other immunomodulatory drugs, plasma exchange, or i.v. Ig for ≥1 mo before blood sampling.
Table I. Patient summary.
| PB Analysis | MS in Remission | MS in Relapse | HS | NINDa |
|---|---|---|---|---|
| Males/females (n) | 3/8 | 5/6 | 5/6 | 2/4 |
| Age (y; mean ± SD) | 44 ± 12 | 42 ± 13 | 39 ± 5 | 64 ± 13 |
| PB/CSF Analysis |
MS in Relapseb |
NIND |
OINDc |
|
| Males/females (n) | 5/7 | 2/4 | 2/2 | |
| Age (y; mean ± SD) | 46 ± 15 | 64 ± 13 | 44 ± 14 |
NIND includes one patient with Parkinson’s disease, one patients with myasthenia gravis, three patients with normal pressure hydrocephalus, and one patient with multiple system atrophy.
Five MS patients were being treated with immunomodulatory drugs (one with IFN-β, two with oral corticosteroids, and two with an immunosuppressive drug) before their relapses.
OIND includes one patient with mumps meningitis, one patient with herpes encephalitis, and two patients with undiagnosed viral meningitis in acute phase.
CSF and PB pairs were obtained from 12 MS patients in relapse, 6 NIND patients, and 4 OIND patients (Table I). Although NIND patients were significantly older than the MS patient cohort, we confirmed that there was no correlation between age and the frequency of T cell subsets in the CSF of NIND patients. All MS patients were recruited from the National Center Hospital, National Center of Neurology and Psychiatry. OIND patients were recruited from the Yokohama City University Graduate School of Medicine. Written informed consent was obtained from all of the subjects. The National Center of Neurology and Psychiatry Ethics Committee approved this study.
Reagents
Anti–CCR2-biotin, anti–CCR5-FITC, anti–CCR6-FITC, and anti–CCR7-FITC mAb were purchased from R&D Systems (Minneapolis, MN). Streptavidin-PE, streptavidin–energy-coupled dye (ECD), anti–CD45RA-ECD, and mouse IgG1-FITC mAb were purchased from Beckman Coulter (Brea, CA). Anti–CD4-PerCP-Cy5.5, anti–CCR4-PE-Cy7, anti–CCR5-allophycocyanin, anti–CCR4-PE, and anti–CCR6-biotin mAb were purchased from BD Biosciences (San Jose, CA). Human MBP was prepared as described previously (27). For cell culture medium, we used RPMI 1640 (Invitrogen, La Jolla, CA) supplemented with 0.05 mM 2-ME, 2 mM l-glutamine, 100 U/ml penicillin/streptomycin, and 10% FBS.
Cell preparation
PBMC were freshly isolated by density-gradient centrifugation using Ficoll-Paque Plus (GE Healthcare, Oakville, ON, Canada). We used a Memory CD4+ T cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) to purify memory CD4+ T cells from PBMC. Briefly, PBMC were labeled with a mixture of biotin-conjugated mAb directed against nonmemory CD4+ T cells and then reacted with magnetic microbead-conjugated anti-biotin mAb. The magnetically labeled nonmemory CD4+ T cells were depleted with auto-MACS (Miltenyi Biotec), which yielded >80% purity of memory CD4+ T cells, as assessed by flow cytometry.
To further separate memory CD4+ T cells according to CCR2, CCR5, CCR4, and CCR6 expression, the cells were labeled with anti–CCR2-biotin, anti–CCR5-allophycocyanin, anti–CCR4-PE-Cy7, and anti–CCR6-FITC mAb and streptavidin-PE, in addition to CD4-PerCP-Cy5.5 and CD45RA-ECD. The stained cells were separated by a flow cytometric cell sorter (FACSAria; BD Biosciences). To measure Ag-specific responses, memory CD4+ T cells were separated into CCR2+CCR5+ T cells and those depleted of CCR2+CCR5+ T cells by the cell sorter FACSAria II (BD Biosciences). To prepare APC, PBMC depleted of memory CD4+ T cells were stained with anti–CD3-allophycocyanin-Cy7 and anti–CD56-PE mAb. Subsequently, CD3−CD56− cells were sorted by FACSAria II and used as APC. This procedure yielded >95% purity of the cells.
Flow cytometric analysis of chemokine receptors
To evaluate expression of chemokine receptors on memory CD4+ T cells, PBMC were first labeled with magnetic microbead-conjugated anti-CD14 mAb, and the labeled CD14+ cells were depleted with auto-MACS, which yielded >95% purity of non-CD14+ PBMC. CD14+ cell-depleted PBMC were stained with anti–CD4-PerCP-Cy5.5, anti–CD45RA-ECD, anti–CCR2-biotin, anti–CCR5-allophycocyanin, anti–CCR4-PE-Cy7, and anti–CCR6-FITC mAb, as well as streptavidin-PE. anti–CCR7-FITC, anti–CCR4-PE, and anti–CCR6-biotin mAb and streptavidin-ECD were used for the staining of CCR7. CSF cells were stained directly with the above-mentioned Abs without depleting CD14+ cells. An isotype control of each Ab was used as a negative control. At the end of the incubation, cells were washed and resuspended in PBS supplemented with 0.5% BSA and immediately analyzed by FACSAria.
Cell culture and cytokine measurements by ELISA
Purified memory CD4+ T cell subsets were suspended at 5 × 105 cells/ml and stimulated with PMA (50 ng/ml) and ionomycin (500 ng/ml) in 96-well U-bottom plates for 24 h. The concentrations of IFN-γ, IL-17, and OPN in the supernatants were measured by Human IFN-γ ELISA Set (BD Biosciences), Human IL-17 DuoSet (R&D Systems), and Human Osteopontin DuoSet (R&D Systems). The procedures were performed according to the manufacturers’ instructions.
Intracellular cytokine staining of IL-17 and IFN-γ
Purified memory CD4+CCR2+CCR5+ and CD4+CCR2−CCR5+ T cells were stimulated with PMA and ionomycin in the presence of monensin for 18 h, fixed in PBS containing 2% paraformaldehyde, and permeabilized with 0.1% saponin. Subsequently, the cells were stained with anti–IL-17-Alexa Fluor 488 and anti–IFN-γ-PE-Cy7 mAb (eBioscience, San Diego, CA). Mouse IgG1-Alexa Fluor 488 and Mouse IgG1-PE-Cy7 were used as isotype control Abs.
T cell stimulation with MBP
To assess the presence of memory MBP-reactive T cells in the purified T cell subsets, FACS-sorted T cell subsets (2 × 104 cells/well) were cocultured with the irradiated (3500 rad) APC (2 × 105 cells/well), in the presence or absence of MBP (10 μg/ml) or OVA (10 μg/ml), in 96-well flat-bottom plates for 5 d. rIL-2 (20 IU/ml) was added to support the growth of T cells. Cytokine concentrations in the culture supernatants were measured by ELISA.
Real-time RT-PCR
FACS-sorted cells were stimulated with PMA and ionomycin for 12 h, as described above. Total RNA was extracted from cultured cells with an RNeasy Mini Kit (QIAGEN, Tokyo, Japan), according to the manufacturer’s instructions. cDNA was synthesized with a PrimeScript RT-PCR kit using oligo-dT Primers (Takara Bio, Otsu, Shiga, Japan). Gene expression was quantified by LightCycler (Roche Diagnostics, Indianapolis, IN) with SYBR Premix Ex Taq (Takara Bio). All procedures were performed according to the manufacturers’ protocols. mRNA levels were normalized to endogenous β-actin (ACTB) in each sample. The specific primers used in this study are listed in Table III.
Table III. Primers used in this study.
| Primer | 5′–3′ |
|---|---|
| MMP1 forward | GATGAAGCAGCCCAGATGTGG |
| MMP1 reverse | GGAGAGTTGTCCCGATGATC |
| MMP9 forward | AGCGAGGTGGACCGGATGTTCC |
| MMP9 reverse | GAGCCCTAGTCCTCAGGGCA |
| MMP10 forward | GTGTGGAGTTCCTGACGTTGG |
| MMP10 reverse | GCATCTCTTGGCAAATCTGG |
| MMP19 forward | CAAGATGTCTCCTGGCTTCC |
| MMP19 reverse | CGGAGCCCTTAAAGAGGAACAC |
| MMP28 forward | TGCAGCTGCTACTGTGGGGCCA |
| MMP28 reverse | TCCAACACGCCGCTGACAGGTAGC |
| OPN forward | GGCAACGGGGATGGCCTTGT |
| OPN reverse | TTTTCCACGGACCTGCCAGCAAC |
| β-actin forward | CACTCTTCCAGCCTTCCTTCC |
| β-actin reverse | GCGTACAGGTCTTTGCGGATG |
| IFN-γ forward | CAGGTCATTCAGATGTAGCG |
| IFN-γ reverse | GCTTTTCGAAGTCATCTCG |
| IL-17 forward | CCAGGATGCCCAAATTCTGAGGAC |
| IL-17 reverse | CAAGGTGAGGTGGATCGGTTGTAG |
| RORC forward | AGAAGGACAGGGAGCCAAGG |
| RORC reverse | GTGATAACCCCGTAGTGGATC |
| T-bet forward | TCAGGGAAAGGACTCACCTG |
| T-bet reverse | AATAGCCTCCCCCATTCAAA |
Zymography
MMP-9 activity was determined as previously reported (28). Briefly, SDS-polyacrylamide gels were copolymerized with 1 mg/ml type A gelatin derived from porcine skin (Sigma-Aldrich, St. Louis, MO). CCR2+CCR5+ T cells and CD4+ T cells depleted of CCR2+CCR5+ T cells were stimulated with PMA and ionomycin, and 20 μl the culture supernatant and recombinant MMP-9 were electrophoresed. The gels were washed twice in 2% Triton X-100 for 30 min and incubated for 18 h at 37°C in buffer (150 mM NaCl, 50 mM Tris-HCl, 5 mM CaCl2, and 0.02% NaN3, [pH 7.5]). After fixing with methanol containing acetic acid, the gels were stained with 0.1% Coomassie blue R-250 (Nakarai Tesque, Kyoto, Japan). The gels were scanned with a UV transilluminator (BioDoc-It Imaging System, UVP, Upland, CA) in grayscale mode, and the image was inverted by Adobe Photoshop (Adobe Systems, Mountain View, CA). Recombinant MMP-9 (GE Healthcare) was used as a positive control.
Migration assay
Migration assays were performed with 24-well Transwell membrane inserts (Corning, Wilkes-Barre, PA). The upper sides of Transwell membrane inserts (8 μm; Corning) were coated with 10 μg/ml laminin-1 (Sigma) or 20 μg/ml laminin-2 (Bio Lamina, Stockholm, Sweden). After aspirating the laminin solutions, the membrane inserts were turned upside down, and normal human astrocytes (NHA; Takara Bio) were seeded on the lower sides of the membrane inserts (2 × 105/well). After 18 h, astrocytes formed a confluent monolayer, as confirmed by Diff-Quick staining. Then the membranes were washed twice with RPMI 1640 medium supplemented with 10% FBS and settled in a 24-well plate. PBMC from HS were sorted into memory CD4+CCR2+CCR5+ T cells, memory CD4+ T cells depleted of CCR2+CCR5+ T cells, and memory CD4+ T cells by flow cytometry. These T cells were stimulated with plate-bound anti-CD3/CD28 mAb for 60 h. Then the cells were harvested, suspended in the fresh medium, and seeded onto the upper chambers at 1 × 105 cells/ well, and 600 μl the medium was added to the lower chambers. After 8 h, 500 μl cell suspension was collected from the lower chambers after careful pipetting, and absolute numbers of migrated cells were counted by flow cytometry using Trucount tubes (BD Bioscience).
Statistics
A one-way ANOVA test was used to compare the frequency of chemokine receptor expression within each group of patients or HS. A paired Student t test was used to evaluate the difference in the percentage inhibition of migration and in the frequency of chemokine receptor expression between PB and CSF from the same patients. For statistical analysis of other data, an unpaired Student t test or one-way ANOVA was used. The p values < 0.05 were considered statistically significant.
Results
CCR2+CCR5+ T cells are enriched in the CSF of MS patients in relapse
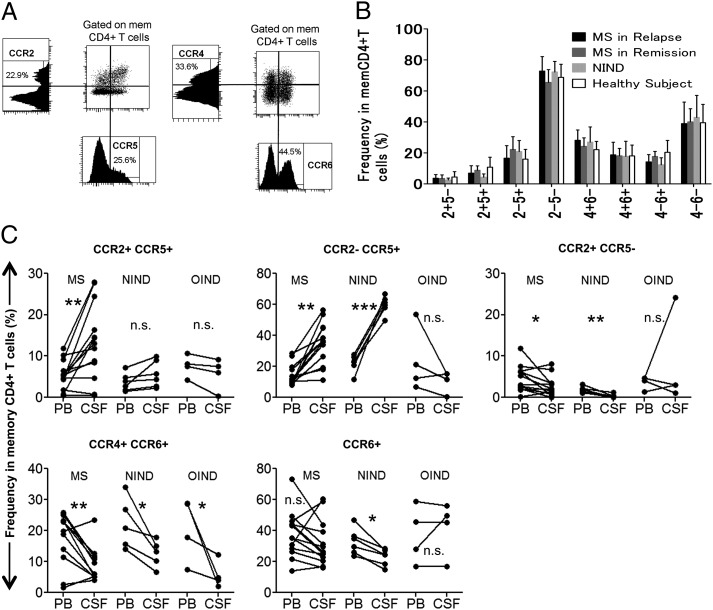
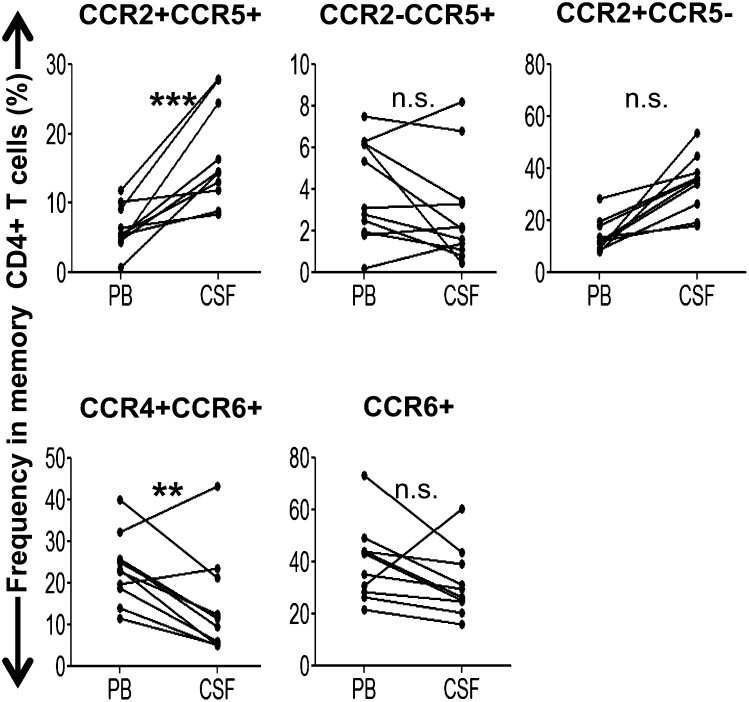
First, we analyzed the chemokine receptor-expression profile of memory CD4+ T cells in the PB of MS patients (Table I) compared with HS and those with NIND. Multicolor flow cytometric analysis was performed on PBMC after staining with differentially labeled anti-CCR2, -CCR4, -CCR5, and -CCR6 mAb. Patterns of coexpression for four chemokine receptors are summarized in Supplemental Fig. 1 and Supplemental Table I. When the memory T cells in PB were grouped based on CCR2 versus CCR5 or CCR4 versus CCR6 expression (Fig. 1A), no particular population was found to be altered in MS patients compared with HS or NIND patients, irrespective of whether the MS patients were in relapse or in remission (Fig. 1B). We next analyzed sets of CSF and PB samples from individual patients with MS, OIND, or NIND. As shown in Fig. 1C, CCR2−CCR5+ T cells formed the predominant T cell population in the CSF of patients with NIND or MS during relapse, suggesting that this population, which was previously shown to be enriched for Th1 cells (24), is allowed to enter the CSF spaces in the patients with MS and NIND. It was reported that human IL-17–producing T cells or Th17 cells are enriched in CCR2+CCR5−, CCR4+CCR6+, or CCR6+ cells (23–25). We were initially interested in knowing whether examination of the chemokine receptor profile could reveal an increase in Th17 cells in the CSF of MS patients. However, the frequencies of CCR2+CCR5−, CCR4+CCR6+, and CCR6+ cells were lower, rather than higher, in the CSF compared with the PB of patients with MS or NIND. In contrast, the frequency of CCR2+CCR5+ T cells in the CSF of patients with MS was significantly higher than in the PB (Fig. 1C). Of note, this increase was specific for MS and was not found in the patients with other neurologic diseases, indicating that cells of this subset are selectively recruited to autoimmune inflammatory lesions or would expand in the CSF during relapse of MS. In addition, if we separate the MS patients by disease duration (<10 y [n = 8] or >10 y [n = 4]), the higher frequency of CCR2+CCR5+ T cells in CSF compared with PB was evident (p < 0.0005) in those with the shorter history of MS (<10 y) (Fig. 2, Table II), but not in those with longer history (data not shown). We also noted that enrichment for CCR2−CCR5+ T cells in the CSF was not detected in the patients with the shorter history of MS. In contrast, the proportion of CCR4+CCR6+ T cells was significantly lower (p < 0.005) in CSF compared with PB of these MS patients. These results indicate that selective enrichment of CCR2+CCR5+ T cells in the CSF is detected in relatively early stages of MS.
FIGURE 1.
CCR2+CCR5+ T cells are enriched in the CSF of MS patients in relapse. (A) PBMC depleted of CD14+ cells were stained with differentially labeled anti-CD4, -CD45RA, -CCR2, -CCR5, -CCR4, and -CCR6 mAb simultaneously. The CD4+CD45RA− population was analyzed for expression of CCR2 and CCR5 (left panels) or CCR4 and CCR6 (right panels). Graphs of the corresponding parameters are also shown. Numbers (%) indicate the percentage of the positive population in the graphs. (B) Cells were stained, as described in (A), and frequencies of T cell subsets in memory CD4+ T cells of 11 MS patients in relapse, 11 MS patients in remission, 6 NIND patients, and 11 HS were calculated. For brevity, “CCR” is omitted from the figure (e.g., 2+5− represents CCR2+CCR5−). (C) Comparison of the frequencies of the T cell subsets in the CSF and PB from 12 MS patients in relapse, 6 patients with NIND, and 4 patients with OIND. Lines connect data for paired CSF and PB samples from the same patients. *p < 0.05, **p < 0.005, ***p < 0.0005. n.s., Not significant.
FIGURE 2.
Frequencies of the T cell subsets in the CSF and PB from eight MS patients with disease duration <10 y. **p < 0.005, ***p < 0.0005. n.s., Not significant.
Table II. CCR2+CCR5+ T cells are involved in early stages of MS.
| Disease History |
||
|---|---|---|
| Clinical Parameter | <10 y | >10 y |
| Disease duration (y; mean ± SD) | 4.8 ± 3.8 | 15.5 ± 4.4 |
| No. of patients | 8a | 4b |
| Age (y; mean ± SD) | 42 ± 14 | 54 ± 15 |
| Relapse rate (times/y; mean ± SD) | 1.8 ± 0.9 | 2.0 ± 14 |
| EDSS (mean ± SD) | 3.1 ± 0.7 | 4.0 ± 1.2 |
| No. of patients with more CCR2+CCR5+ T cells in CSF than in PB | 8* | 0 |
Three patients were treated with IFN-β (n = 1), oral steroid (n = 1), or immunosuppressant (n = 1).
Two patients were treated with oral steroid.
p < 0.0005.
CCR2+CCR5+ T cells in the PB contain both central and effector memory cells and produce both IFN-γ and IL-17
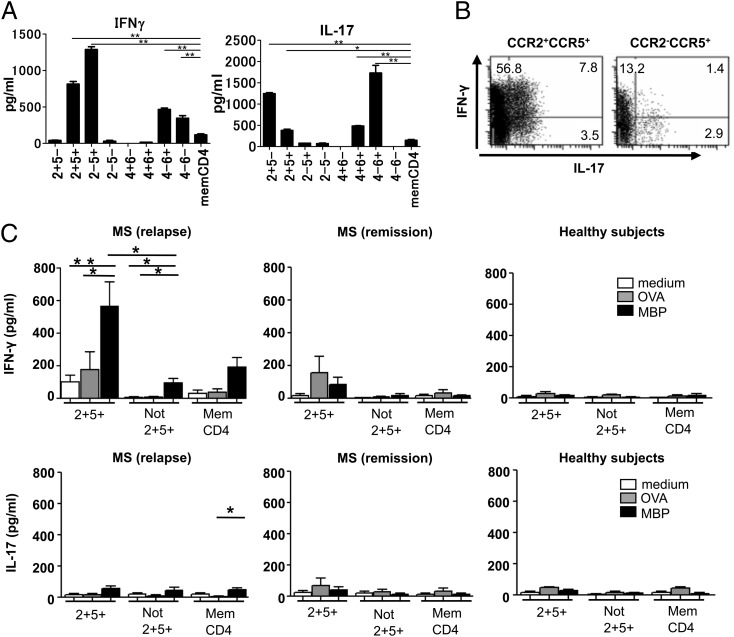
Memory CD4+ T cells are divided into CCR7+ central memory and CCR7− effector memory subsets, which are differentially endowed with effector functions (29). The staining of CCR7, together with CCR2/5 or CCR4/6, revealed a higher effector memory/central memory ratio in CCR2+CCR5+ T cells and CCR2−CCR5+ T cells (Supplemental Fig. 2). We next analyzed cytokine production by each T cell population bearing a distinct chemokine receptor profile. The cells of interest were separated from PB of HS and were stimulated with PMA and ionomycin. Compared with unfractionated memory CD4+ T cells, CCR2+CCR5+ and CCR2−CCR5+ T cells produced a larger quantity of IFN-γ (Fig. 3A). Although CCR2+CCR5+ T cells produced a significant amount of IL-17, production of IL-17 from CCR2−CCR5+ T cells was only marginal. CCR2+CCR5− T cells and CCR4+CCR6+ T cells selectively produced IL-17, whereas CCR4−CCR6− T cells selectively produced IFN-γ. These results were consistent with the results of previous studies (23, 24). Because T cells expressing both IFN-γ and IL-17 are reportedly present in highly infiltrated lesions of MS brain sections (12), it was of interest to know whether similar T cells producing both IFN-γ and IL-17 are present in CCR2+CCR5+ T cells. By conducting intracellular cytokine staining, we revealed that the CCR2+CCR5+ T cells, as well as CCR2−CCR5+ T cells, are composed of IFN-γ+IL-17− cells, IFN-γ+IL-17+ cells, and IFN-γ−IL-17+ cells (Fig. 3B). In both T cell populations, IFN-γ+IL-17− cells were a major subset of IFN-γ production.
FIGURE 3.
Cytokine production and reactivity to MBP by CCR2+CCR5+ T cells in the PB. (A) Memory CD4+ T cell subsets purified from PBMC of HS by flow cytometry were stimulated with PMA and ionomycin. Concentrations of IFN-γ and IL-17 in the supernatants were measured by ELISA. Data represent mean ± SD of three HS. (B) Purified CCR2+CCR5+ T cells (left panel) and CCR2−CCR5+ T cells (right panel) were stimulated with PMA and ionomycin for 18 h, and the production of IL-17 and IFN-γ was assessed by intracellular cytokine staining. Numbers indicate the frequency (%) of cells in each quadrant. One representative experiment from three independent experiments with PBMC from HS is shown. (C) Purified memory CD4+ T cell subsets were cultured in duplicate with irradiated APC in the presence of MBP (10 μg/ml) or OVA (100 μg/ml) for 5 d. Concentrations of IFN-γ and IL-17 in the supernatants were measured by ELISA. Data represent mean ± SD of six MS patients in relapse, three MS patients in remission, and three HS. *p < 0.05, **p < 0.005.
CCR2+CCR5+ T cells in the PB from MS patients during relapse are reactive to MBP
Given that the CCR2+CCR5+ T cells are proportionally higher in CSF than PB of MS during relapse, we were interested to know whether the CCR2+CCR5+ T cells are enriched in autoimmune, pathogenic T cells. We therefore examined if the CCR2+CCR5+ T cells might react to MBP, a putative autoantigen for MS. We isolated memory CD4+CCR2+CCR5+ T cells and memory CD4+ T cells depleted of CCR2+CCR5+ T cells from the PB of MS in relapse, MS in remission and HS. We stimulated these cells with MBP or OVA in the presence of autologous APC and measured the levels of IFN-γ and IL-17 in the supernatants after culture (Fig. 3C). The T cell populations separated from HS did not show any significant response to MBP or OVA in this assay. A marginal IFN-γ response to MBP and OVA was noted in CCR2+CCR5+ T cells from MS in remission. Strikingly, the CCR2+CCR5+ T cells from MS in relapse selectively and significantly responded to MBP by producing a large amount of IFN-γ, whereas those depleted of CCR2+CCR5+ T cells or total memory CD4+ T cells showed a much smaller response. These results suggest that MBP-specific IFN-γ-producing cells might be enriched in CCR2+CCR5+ T cells during relapse of MS.
CCR2+CCR5+ T cells in the PB produce MMP-9 and OPN
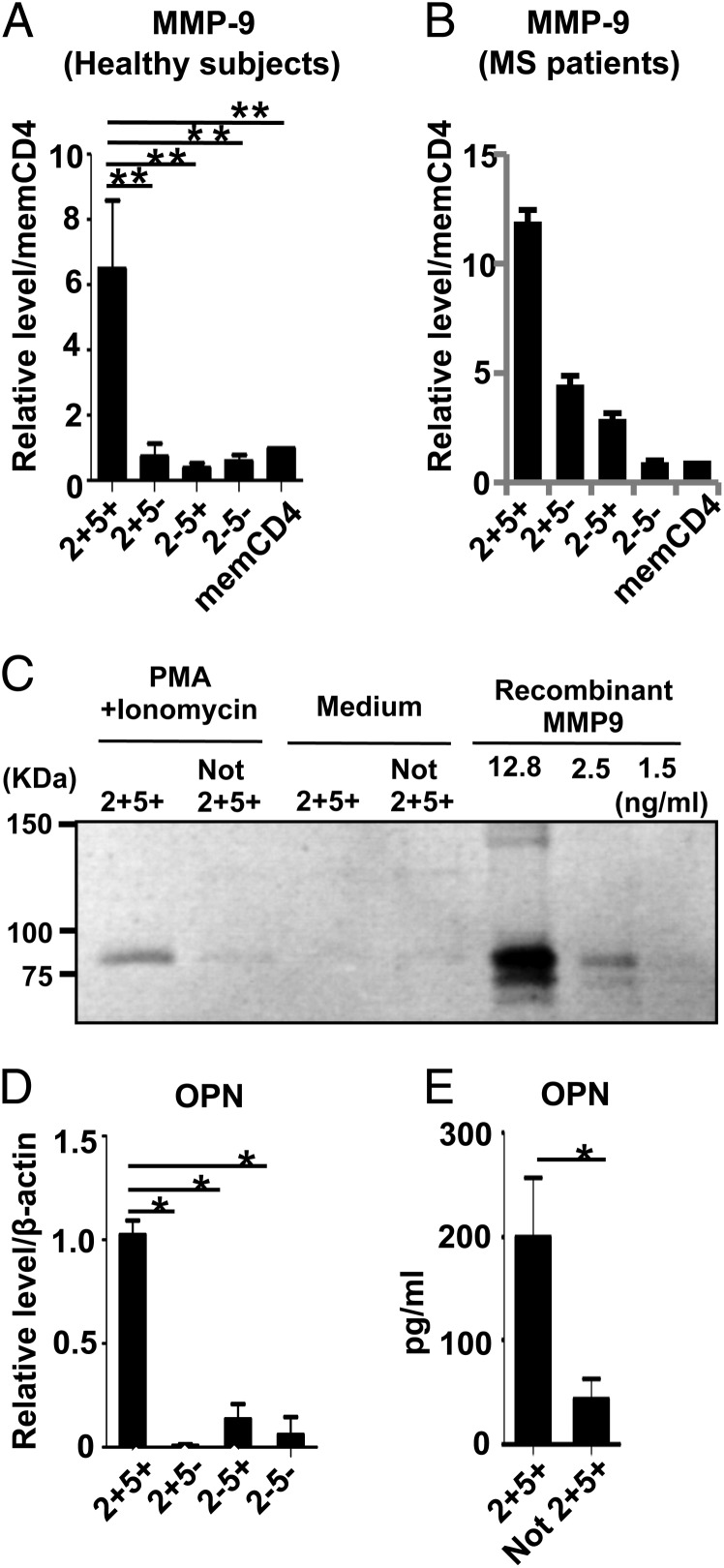
Lymphocyte migration/infiltration is a critical step for the development of autoimmune pathology in the CNS, and two physical barriers protect the CNS parenchyma from entry of the immune cells: the vascular endothelium barrier and the glia limitans barrier made up of extending astrocyte foot processes (8, 30). Each barrier possesses its own basement membrane, and the CSF circulates in the perivascular space between the two membranes. Thus, initiation of CNS inflammation requires immune cells that are capable of disrupting these physical barriers. Because type IV collagenase MMP-9 is selectively elevated in the CSF in MS, MMP-9 is assumed to play a role in disrupting the BBB in MS (28, 31, 32). Speculating that CCR2+CCR5+ T cells may have a distinct ability to initiate the processes of CNS inflammation, we examined whether CCR2+CCR5+ T cells are able to produce MMP-9. Strikingly, quantitative RT-PCR analysis of whole and CCR2/CCR5 fractions of memory T cells from HS and MS showed that expression of MMP-9 was mainly restricted to CCR2+CCR5+ T cells (Fig. 4A, 4B, Table III). The expression of MMP-1 and MMP-19, which also possess the potential to degrade the basement membrane, was highest in CCR2+CCR5+ T cells, whereas all T cell populations similarly expressed MMP-10 and MMP-28 (Supplemental Fig. 3). We also measured MMP-2, -7, -14, -15, -23, and -25, but none of these was detected. Using zymography, we further examined MMP-9 enzymatic activity in the culture supernatants of activated CCR2+CCR5+ T cells. As shown in Fig. 4C, supernatants from CCR2+CCR5+ T cells exhibited MMP-9 activity, but those from T cells depleted of the CCR2+CCR5+ population did not.
FIGURE 4.
CCR2+CCR5+ T cells in the PB have the potential to produce MMP-9 and OPN. Each memory CD4+ T cell subset was isolated from the PBMC of HS (A) or MS (B) and was stimulated with PMA and ionomycin. Expression levels of MMP-9 mRNA were determined by quantitative RT-PCR. Results were normalized based on the values in unfractionated memory CD4+ T cells. Data represent mean ± SD of four HS or three MS patients. (C) CCR2+CCR5+ T cells and CD4+ T cells depleted of CCR2+CCR5+ T cells from HS were stimulated with PMA and ionomycin, and 20 μl of the culture supernatant and recombinant MMP-9 were electrophoresed. Shown are CCR2+CCR5+ T cells activated with PMA and ionomycin (lane 1), memory CD4+ T cells depleted of CCR2+CCR5+ T cells activated with PMA and ionomycin (lane 2), CCR2+CCR5+ T cells without activation (lane 3), memory CD4+ T cells depleted of CCR2+CCR5+ T cells without activation (lane 4), and serial dilution of recombinant MMP-9 (lanes 5–7). The results shown are representative of three independent experiments. (D) Purified memory CD4+ T cell subsets from HS were stimulated with PMA and ionomycin. Expression levels of OPN mRNA were determined by quantitative RT-PCR. Data were normalized to the amount of β-actin mRNA. Data represent mean ± SD of four independent experiments. (E) Purified memory CD4+ T cell subsets were stimulated with PMA and ionomycin. OPN concentrations in the supernatants were measured by ELISA. Data represent mean ± SD of four different HS. *p < 0.05, **p < 0.005.
Recent studies suggested that OPN, which is also expressed by T cells (33, 34), might be involved in the pathogenesis of MS. Although OPN-deficient mice were resistant to relapse of EAE (4, 33), administration of recombinant OPN to OPN-deficient mice reversed the ongoing remission of the disease and induced progressive exacerbation of the clinical symptoms (4). These findings prompted us to examine whether OPN is overexpressed in CCR2+CCR5+ T cells after stimulation with PMA and ionomycin. As shown in Fig. 4D and 4E, CCR2+CCR5+ T cells expressed a much higher level of OPN than did the other memory T cell populations at both the mRNA and the protein levels.
CCR2+CCR5+ T cells are superior to other T cells in the ability to invade the CNS
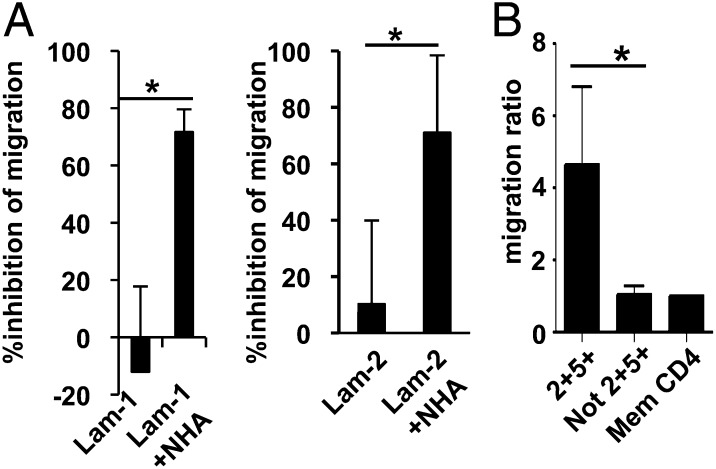
Although activated T cells are able to cross the endothelial barrier and enter the CSF compartment relatively easily, the parenchymal basement membrane and the glia limitans would hamper the further entry of the T cells into the CNS parenchyma. Although the endothelial cell basement membrane contains laminin-8 and -10, the parenchymal basal lamina is composed of laminin-1 or -2 (35). It was suggested that leukocyte penetration through the glia limitans requires MMP, such as MMP-2 and MMP-9 (36). After demonstrating that CCR2+CCR5+ T cells have the potential to produce MMP-9, we explored whether CCR2+CCR5+ T cells efficiently invade the CNS parenchyma across the basal lamina and glia limitans. To recapitulate the glia limitans layered with parenchymal basal lamina experimentally, we coated the upper sides of Transwell membrane inserts with laminin-1 or -2 and seeded NHA on the lower sides of the membrane inserts, as described in Materials and Methods. When we applied activated T cells to the upper chamber, their migration across the NHA layered with laminin-1 or -2 was less efficient compared with the migration across the untreated membrane or the membrane treated with laminin alone, as assessed by the number of migrated activated T cells collected from the lower chamber (Fig. 5A). Therefore, we assumed that this model would exhibit barrier functions against the penetration of activated T cells. Moreover, we applied CCR2+CCR5+ T cells and memory CD4+ T cells depleted of CCR2+CCR5+ T cells and showed that CCR2+CCR5+ T cells more efficiently penetrated and migrated to the lower chamber compared with the other T cells (Fig. 5B). These results indicate that CCR2+CCR5+ T cells capable of producing MMP-9 and OPN have a greater potential to invade the brain parenchyma.
FIGURE 5.
T cell migration across an in vitro glia limitans model. (A) The upper sides of Transwell membrane inserts were coated with laminin-1 (Lam-1; left panel) or laminin-2 (Lam-2; right panel), and NHA were seeded on the lower sides of the membrane inserts, as described in Materials and Methods. Unfractionated T cells isolated from PBMC were stimulated with PMA and ionomycin for 18 h and seeded onto the upper chambers. Eight hours later, absolute numbers of migrated cells were counted by flow cytometry. Data shown are the percentage inhibition of the migration, calculated as follows: [(migrated cell number through uncoated membrane) − (migrated cell number through membrane coated with laminin alone or laminin and NHA)] × 100/(migrated cell number through uncoated membrane). Data represent mean ± SD of four independent experiments. (B) PBMC from HS were sorted into memory CD4+CCR2+CCR5+ T cells (2+5+), memory CD4+ T cells depleted of CCR2+CCR5+ T cells (Not 2+5+), and unfractionated memory CD4+ T cells (Mem CD4) by flow cytometry. The cells were stimulated with plate-bound anti-CD3/CD28 mAb for 60 h and seeded onto the upper chambers, whose membranes were coated with laminin-2 and NHA, as described in (A). Eight hours later, absolute numbers of migrated cells were counted by flow cytometry. To normalize individual variance, data are expressed as migration ratio of the number of migrated cells/number of migrated unfractionated memory CD4+ T cells. Data represent mean ± SD of four independent experiments. *p < 0.05.
CCR2+CCR5+CCR6− subset producing IFN-γ and MMP-9 is selectively enriched in the CSF in MS
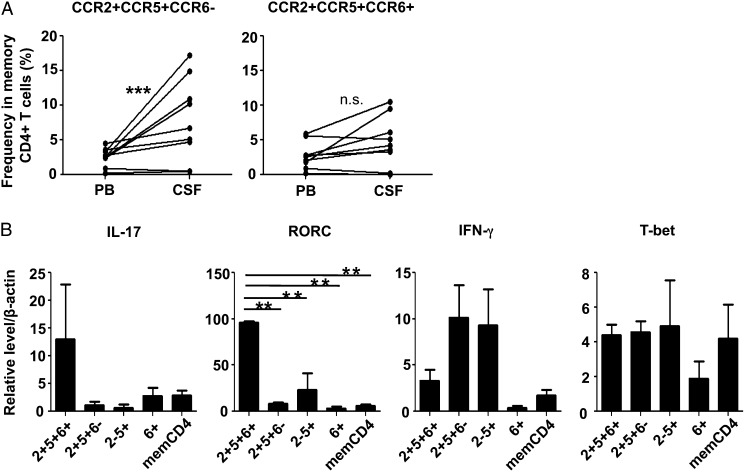
We noticed that CCR2+CCR5+ T cells consist of CCR6+ and CCR6− subset (Supplemental Fig. 1). Because Th17 cells appear to be enriched in CCR6+ T cells, we were interested to know whether CCR2+CCR5+ T cells could be functionally divided based on the expression of CCR6. When we compared the frequency of CCR2+CCR5+CCR6+ and CCR2+CCR5+CCR6− T cells between PB and CSF, the frequency of CCR6− subset was much higher in the CSF of MS in relapse than in PB (p < 0.0005), whereas the CCR6+ subset was not (Fig. 6A). Further analyses revealed that expression levels of IFN-γ in the CCR6− subset were higher than those in the CCR6+ subset, as assessed by RT-PCR (Fig. 6B). In contrast, the CCR6+ subset expressed much higher levels of IL-17 and RORC compared with the CCR6− subset. We also measured expression levels of MMP-9 mRNA; a much higher level of MMP-9 mRNA was found in the CCR6− subset (individual relative expression level from two samples = 1.0257 and 0.1127306) compared with the CCR6+ subset (individual relative expression level = 0.0185 and 0.00345). Taken together, we postulate that CCR2+CCR5+CCR6− T cells producing IFN-γ, but not CCR2+CCR5+CCR6+ T cells, play a crucial role in triggering the relapse of MS and expand in the CSF during relapse.
FIGURE 6.
CCR2+CCR5+CCR6− T cells, but not CCR2+CCR5+CCR6+ T cells, were enriched in the CSF of MS in relapse. (A) Cells were stained, as described in Fig. 1C, and frequencies of CCR2+CCR5+CCR6− and CCR2+CCR5+CCR6− T cells in the CSF and PB from nine MS patients in relapse were examined. Lines connect data from paired CSF and PB samples from the same patients. (B) Each memory CD4+ T cell subset was isolated from the PBMC of HS and stimulated with PMA and ionomycin for 18 h. Expression levels of IL-17, IFN-γ, RORC, and T-bet mRNA were determined by quantitative RT-PCR. Data represent mean ± SD of three independent experiments. **p < 0.005, ***p < 0.0005. n.s., Not significant.
Discussion
Chemokines are a family of small chemotactic cytokines, which is a key to understanding the immune homeostasis, self-defense, and inflammation. Interactions between chemokines and their receptors are crucial for the migration of lymphocyte populations, such as T cells, macrophages, dendritic cells, and neutrophils, in autoimmune diseases, allergy, and cancer (37). Although chemokine receptor expression by the CSF lymphocytes or by brain-infiltrating T cells has been repeatedly investigated with regard to the pathogenesis of MS (38), most of the previous studies did not analyze the proportional changes of T cells simultaneously expressing more than two chemokine receptors. We showed that memory CD4+ T cells expressing both CCR2 and CCR5 are selectively enriched in the CSF in MS during relapse but not in NIND or OIND.
Both CCR2 and CCR5 belong to the CC family of chemokines, which have two adjacent cysteines close to their N terminus. CCR2 binds CCL2 (MCP-1), CCL7 (MCP-3), CCL11 (eotaxin), CCL13 (MCP-4), and CCL16 (LEC), whereas CCR5 binds CCL3 (MIP-1α), CCL4 (MIP-1β), CCL5 (RANTES), CCL8, CCL11, CCL13, and CCL14. Among these chemokines, CCL5 was increased in the CSF in MS during acute relapses (39), and overexpression of CCL3 was detected in the brain tissues from MS (40). In contrast, CCL2 was decreased in the CSF in MS during relapses (38, 41), and this could be the result of consumption by CCR2+ cells (42). Moreover, the presence of CCL2 and CCL5 was recently demonstrated in endothelial cells in brain samples from MS (43). CCL2 and CCL5 appear to play a critical role in adhesions of the encephalitogenic T cells to brain endothelial cells in a model of EAE (44). More recently, CCL2–CCR2 pairs were shown to play a critical role in the transendothelial migration of effector CD4+ T cells (45), suggesting the importance of CCR2 expression for BBB transmigration. Taking these into consideration, we postulate that the chemokine gradient would facilitate the adherence of CCR2+CCR5+ T cells to the endothelial cells, as well as T cell entry into the CNS parenchyma during relapses of MS. Interestingly, CCR2−CCR5+ T cells, which produce a large quantity of IFN-γ, were also enriched in the CSF in MS. However, it was not specific for MS but was also present in the patients with NIND (Fig. 1C), indicating that only CCR2+CCR5+ T cells are specifically involved in the autoimmune pathology of MS. We subsequently found that the CCR2+CCR5+ T cells have an exceptional ability to produce MMP-9, an enzyme that is capable of disrupting the glia limitans, which led us to speculate that they have the potential to destroy the integrity of the BBB and trigger the cascade of inflammatory pathology. The MMP-9–producing CCR2+CCR5+ T cells were indeed superior to the other T cells in their ability to cross the in vitro model of glia limitans layered by laminin-1 or -2.
MMP-9 appears to play a major role in EAE by disrupting the glia limitans, and a specific substrate of MMP-9 was shown to be dystroglycan, anchoring astrocyte end feet to parenchymal basement membrane via interaction with laminin-1 and -2 (36). Laminin-1 and -2 constitute the major laminin isoforms present in the CNS parenchymal basal lamina (35). Taken together, we postulate that the distinguished ability to produce MMP-9 would license the CCR2+CCR5+ T cells to serve as early invaders into the CNS parenchyma during relapses of MS. The CCR2+CCR5+ T cells also produce a large amount of OPN, an integrin-binding protein abundantly expressed in active MS lesions (33). OPN is a pleiotropic protein that interacts with various integrins. In addition to its function as an adhesion molecule, OPN promotes the survival of activated T cells and the production of proinflammatory cytokines by APC (46). It is very likely that paracrine OPN produced by the CCR2+CCR5+ T cells would promote the survival of these MMP-9–producing T cells in the CNS, which leads to further enrichment of the CCR2+CCR5+ T cells in the CSF.
Seeing the specific increase in the CCR2+CCR5+ T cells in the CSF in MS, we were very curious to know whether this T cell population is enriched with autoimmune T cells critical for the initiation of MS pathology. By stimulating the PB CCR2+CCR5+ T cells with MBP, we showed that, in patients with MS relapse, this T cell subset produces a large quantity of IFN-γ and some IL-17 in response to MBP, a representative autoantigen for MS (Fig. 3C). In contrast, the cells from MS in remission or from healthy controls did not respond significantly. Although we did not examine the CSF T cells’ response to MBP because of a technical difficulty, it is likely that the CCR2+CCR5+ T cells in the CSF in MS during relapse are enriched with MBP-reactive autoimmune T cells as well.
Of note, Zhang et al. (47) recently reported that CCR2+CCR5+ cells are highly differentiated, yet stable, effector memory CD4+ T cells equipped for provoking rapid recall response. They showed evidence that CCR2+CCR5+ T cells should have undergone reactivation and subsequent proliferation more often than other memory T cell subsets and are resistant to apoptosis. Thus, it is likely that autoreactive T cells are enriched in CCR2+CCR5+ T cells that have survived following repeated reactivation over a long period of time. We assume that once autoreactive T cells differentiate into stable effector memory T cells expressing CCR2 and CCR5, they might persist and trigger relapse repeatedly. We further revealed that the CCR6−, but not the CCR6+, subset of CCR2+CCR5+ T cells was significantly enriched in the CSF of MS patients during relapse.
Reboldi et al. (48) reported that CCR6+ T cells are more enriched in CSF than in the PB of clinically isolated syndrome (CIS). Diagnosis of CIS can be made when patients developed a single attack of neurologic disability that is consistent with demyelinating pathology and that may turn out to be the first episode of MS (49). However, in our Japanese patients having clinically definite MS, we did not detect enrichment of CCR6+ T cells in the CSF. Rather, T cells bearing Th17 phenotypes appeared to be prohibited from entry into the CNS in MS. The difference between the results in CIS and MS could be explained by the premise that autoimmune pathology may be premature at the CIS stage. In contrast, the increase in CCR2+CCR5+ T cells in the CSF was not detected in the patients who had MS for >10 y. These observations are in accordance with the postulate that acquired and innate immune components, as well as neurodegenerative components, differentially contribute to the different stages of MS (50).
In summary, we identified a unique CCR2+CCR5+CCR6− T cell population that is enriched in the CSF of patients with exacerbated MS. Our data suggest that targeting this population may be a novel therapeutic approach for MS.
Supplementary Material
Acknowledgments
We thank Dr. Britta Engelhardt for valuable comments and Hiromi Yamaguchi and Ryoko Saga for excellent technical assistance.
This work was supported by a Research Grant on Super Special Consortia for Supporting the Development of Cutting-Edge Medical Care from Cabinet Office, Government of Japan; a Grant-in-Aid for Scientific Research (S) from the Japan Society for the Promotion of Science (18189009 to T.Y.); and Research Grants on Psychiatric and Neurological Diseases and Mental Health and a Health and Labor Sciences Research Grant on Intractable Diseases (Neuroimmunological Diseases) from the Ministry of Health, Labor and Welfare of Japan.
The online version of this article contains supplemental material.
- BBB
- blood–brain barrier
- CIS
- clinically isolated syndrome
- CSF
- cerebrospinal fluid
- EAE
- experimental autoimmune encephalomyelitis
- ECD
- energy-coupled dye
- HS
- healthy subject
- MBP
- myelin basic protein
- MMP
- matrix metalloproteinase
- MS
- multiple sclerosis
- NHA
- normal human astrocyte
- NIND
- noninflammatory neurologic disease
- OIND
- other inflammatory neurologic disease
- OPN
- osteopontin
- PB
- peripheral blood
- RR-MS
- relapsing-remitting multiple sclerosis.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.McFarland H. F., Martin R. 2007. Multiple sclerosis: a complicated picture of autoimmunity. Nat. Immunol. 8: 913–919 [DOI] [PubMed] [Google Scholar]
- 2.Trapp B. D., Nave K. A. 2008. Multiple sclerosis: an immune or neurodegenerative disorder? Annu. Rev. Neurosci. 31: 247–269 [DOI] [PubMed] [Google Scholar]
- 3.Wucherpfennig K. W., Strominger J. L. 1995. Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell 80: 695–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hur E. M., Youssef S., Haws M. E., Zhang S. Y., Sobel R. A., Steinman L. 2007. Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nat. Immunol. 8: 74–83 [DOI] [PubMed] [Google Scholar]
- 5.Ransohoff R. M., Kivisäkk P., Kidd G. 2003. Three or more routes for leukocyte migration into the central nervous system. Nat. Rev. Immunol. 3: 569–581 [DOI] [PubMed] [Google Scholar]
- 6.Bartholomäus I., Kawakami N., Odoardi F., Schläger C., Miljkovic D., Ellwart J. W., Klinkert W. E., Flügel-Koch C., Issekutz T. B., Wekerle H., Flügel A. 2009. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature 462: 94–98 [DOI] [PubMed] [Google Scholar]
- 7.Gijbels K., Galardy R. E., Steinman L. 1994. Reversal of experimental autoimmune encephalomyelitis with a hydroxamate inhibitor of matrix metalloproteases. J. Clin. Invest. 94: 2177–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engelhardt B. 2010. T cell migration into the central nervous system during health and disease: Different molecular keys allow access to different central nervous system compartments. Clin. Exp. Neuroimmunol. 1: 79–93 [Google Scholar]
- 9.Steinman L. 2009. Shifting therapeutic attention in MS to osteopontin, type 1 and type 2 IFN. Eur. J. Immunol. 39: 2358–2360 [DOI] [PubMed] [Google Scholar]
- 10.Aranami T., Yamamura T. 2008. Th17 Cells and autoimmune encephalomyelitis (EAE/MS). Allergol. Int. 57: 115–120 [DOI] [PubMed] [Google Scholar]
- 11.Tzartos J. S., Friese M. A., Craner M. J., Palace J., Newcombe J., Esiri M. M., Fugger L. 2008. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am. J. Pathol. 172: 146–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kebir H., Ifergan I., Alvarez J. I., Bernard M., Poirier J., Arbour N., Duquette P., Prat A. 2009. Preferential recruitment of interferon-gamma-expressing TH17 cells in multiple sclerosis. Ann. Neurol. 66: 390–402 [DOI] [PubMed] [Google Scholar]
- 13.Panitch H. S., Hirsch R. L., Haley A. S., Johnson K. P. 1987. Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet 1: 893–895 [DOI] [PubMed] [Google Scholar]
- 14.Broux B., Pannemans K., Zhang X., Markovic-Plese S., Broekmans T., Eijnde B. O., Van Wijmeersch B., Somers V., Geusens P., van der Pol S., et al. 2012. CX(3)CR1 drives cytotoxic CD4(+)CD28(−) T cells into the brain of multiple sclerosis patients. J. Autoimmun. 38: 10–19 [DOI] [PubMed] [Google Scholar]
- 15.Saxena A., Martin-Blondel G., Mars L. T., Liblau R. S. 2011. Role of CD8 T cell subsets in the pathogenesis of multiple sclerosis. FEBS Lett. 585: 3758–3763 [DOI] [PubMed] [Google Scholar]
- 16.Segal B. M., Constantinescu C. S., Raychaudhuri A., Kim L., Fidelus-Gort R., Kasper L. H., Ustekinumab MS Investigators 2008. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol. 7: 796–804 [DOI] [PubMed] [Google Scholar]
- 17.Mackay C. R. 2001. Chemokines: immunology’s high impact factors. Nat. Immunol. 2: 95–101 [DOI] [PubMed] [Google Scholar]
- 18.Proudfoot A. E. 2002. Chemokine receptors: multifaceted therapeutic targets. Nat. Rev. Immunol. 2: 106–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Izikson L., Klein R. S., Charo I. F., Weiner H. L., Luster A. D. 2000. Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2. J. Exp. Med. 192: 1075–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sallusto F., Lanzavecchia A. 2000. Understanding dendritic cell and T-lymphocyte traffic through the analysis of chemokine receptor expression. Immunol. Rev. 177: 134–140 [DOI] [PubMed] [Google Scholar]
- 21.Nagata K., Tanaka K., Ogawa K., Kemmotsu K., Imai T., Yoshie O., Abe H., Tada K., Nakamura M., Sugamura K., Takano S. 1999. Selective expression of a novel surface molecule by human Th2 cells in vivo. J. Immunol. 162: 1278–1286 [PubMed] [Google Scholar]
- 22.Ransohoff R. M. 2009. Chemokines and chemokine receptors: standing at the crossroads of immunobiology and neurobiology. Immunity 31: 711–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Acosta-Rodriguez E. V., Rivino L., Geginat J., Jarrossay D., Gattorno M., Lanzavecchia A., Sallusto F., Napolitani G. 2007. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 8: 639–646 [DOI] [PubMed] [Google Scholar]
- 24.Sato W., Aranami T., Yamamura T. 2007. Cutting edge: Human Th17 cells are identified as bearing CCR2+CCR5− phenotype. J. Immunol. 178: 7525–7529 [DOI] [PubMed] [Google Scholar]
- 25.Singh S. P., Zhang H. H., Foley J. F., Hedrick M. N., Farber J. M. 2008. Human T cells that are able to produce IL-17 express the chemokine receptor CCR6. J. Immunol. 180: 214–221 [DOI] [PubMed] [Google Scholar]
- 26.McDonald W. I., Compston A., Edan G., Goodkin D., Hartung H. P., Lublin F. D., McFarland H. F., Paty D. W., Polman C. H., Reingold S. C., et al. 2001. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann. Neurol. 50: 121–127 [DOI] [PubMed] [Google Scholar]
- 27.Takahashi K., Aranami T., Endoh M., Miyake S., Yamamura T. 2004. The regulatory role of natural killer cells in multiple sclerosis. Brain 127: 1917–1927 [DOI] [PubMed] [Google Scholar]
- 28.Leppert D., Ford J., Stabler G., Grygar C., Lienert C., Huber S., Miller K. M., Hauser S. L., Kappos L. 1998. Matrix metalloproteinase-9 (gelatinase B) is selectively elevated in CSF during relapses and stable phases of multiple sclerosis. Brain 121: 2327–2334 [DOI] [PubMed] [Google Scholar]
- 29.Sallusto F., Geginat J., Lanzavecchia A. 2004. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 22: 745–763 [DOI] [PubMed] [Google Scholar]
- 30.Toft-Hansen H., Buist R., Sun X. J., Schellenberg A., Peeling J., Owens T. 2006. Metalloproteinases control brain inflammation induced by pertussis toxin in mice overexpressing the chemokine CCL2 in the central nervous system. J. Immunol. 177: 7242–7249 [DOI] [PubMed] [Google Scholar]
- 31.Stüve O., Dooley N. P., Uhm J. H., Antel J. P., Francis G. S., Williams G., Yong V. W. 1996. Interferon beta-1b decreases the migration of T lymphocytes in vitro: effects on matrix metalloproteinase-9. Ann. Neurol. 40: 853–863 [DOI] [PubMed] [Google Scholar]
- 32.Bar-Or A., Nuttall R. K., Duddy M., Alter A., Kim H. J., Ifergan I., Pennington C. J., Bourgoin P., Edwards D. R., Yong V. W. 2003. Analyses of all matrix metalloproteinase members in leukocytes emphasize monocytes as major inflammatory mediators in multiple sclerosis. Brain 126: 2738–2749 [DOI] [PubMed] [Google Scholar]
- 33.Chabas D., Baranzini S. E., Mitchell D., Bernard C. C., Rittling S. R., Denhardt D. T., Sobel R. A., Lock C., Karpuj M., Pedotti R., et al. 2001. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science 294: 1731–1735 [DOI] [PubMed] [Google Scholar]
- 34.Shinohara M. L., Jansson M., Hwang E. S., Werneck M. B., Glimcher L. H., Cantor H. 2005. T-bet-dependent expression of osteopontin contributes to T cell polarization. Proc. Natl. Acad. Sci. USA 102: 17101–17106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sixt M., Engelhardt B., Pausch F., Hallmann R., Wendler O., Sorokin L. M. 2001. Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood-brain barrier in experimental autoimmune encephalomyelitis. J. Cell Biol. 153: 933–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agrawal S., Anderson P., Durbeej M., van Rooijen N., Ivars F., Opdenakker G., Sorokin L. M. 2006. Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J. Exp. Med. 203: 1007–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moser B., Loetscher P. 2001. Lymphocyte traffic control by chemokines. Nat. Immunol. 2: 123–128 [DOI] [PubMed] [Google Scholar]
- 38.Sørensen T. L., Tani M., Jensen J., Pierce V., Lucchinetti C., Folcik V. A., Qin S., Rottman J., Sellebjerg F., Strieter R. M., et al. 1999. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J. Clin. Invest. 103: 807–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kivisäkk P., Trebst C., Liu Z., Tucky B. H., Sørensen T. L., Rudick R. A., Mack M., Ransohoff R. M. 2002. T-cells in the cerebrospinal fluid express a similar repertoire of inflammatory chemokine receptors in the absence or presence of CNS inflammation: implications for CNS trafficking. Clin. Exp. Immunol. 129: 510–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balashov K. E., Rottman J. B., Weiner H. L., Hancock W. W. 1999. CCR5(+) and CXCR3(+) T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions. Proc. Natl. Acad. Sci. USA 96: 6873–6878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franciotta D., Martino G., Zardini E., Furlan R., Bergamaschi R., Andreoni L., Cosi V. 2001. Serum and CSF levels of MCP-1 and IP-10 in multiple sclerosis patients with acute and stable disease and undergoing immunomodulatory therapies. J. Neuroimmunol. 115: 192–198 [DOI] [PubMed] [Google Scholar]
- 42.Mahad D., Callahan M. K., Williams K. A., Ubogu E. E., Kivisäkk P., Tucky B., Kidd G., Kingsbury G. A., Chang A., Fox R. J., et al. 2006. Modulating CCR2 and CCL2 at the blood-brain barrier: relevance for multiple sclerosis pathogenesis. Brain 129: 212–223 [DOI] [PubMed] [Google Scholar]
- 43.Subileau E. A., Rezaie P., Davies H. A., Colyer F. M., Greenwood J., Male D. K., Romero I. A. 2009. Expression of chemokines and their receptors by human brain endothelium: implications for multiple sclerosis. J. Neuropathol. Exp. Neurol. 68: 227–240 [DOI] [PubMed] [Google Scholar]
- 44.dos Santos A. C., Barsante M. M., Arantes R. M., Bernard C. C., Teixeira M. M., Carvalho-Tavares J. 2005. CCL2 and CCL5 mediate leukocyte adhesion in experimental autoimmune encephalomyelitis—an intravital microscopy study. J. Neuroimmunol. 162: 122–129 [DOI] [PubMed] [Google Scholar]
- 45.Shulman Z., Cohen S. J., Roediger B., Kalchenko V., Jain R., Grabovsky V., Klein E., Shinder V., Stoler-Barak L., Feigelson S. W., et al. 2012. Transendothelial migration of lymphocytes mediated by intraendothelial vesicle stores rather than by extracellular chemokine depots. Nat. Immunol. 13: 67–76 [DOI] [PubMed] [Google Scholar]
- 46.Denhardt D. T., Noda M., O’Regan A. W., Pavlin D., Berman J. S. 2001. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J. Clin. Invest. 107: 1055–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang H. H., Song K., Rabin R. L., Hill B. J., Perfetto S. P., Roederer M., Douek D. C., Siegel R. M., Farber J. M. 2010. CCR2 identifies a stable population of human effector memory CD4+ T cells equipped for rapid recall response. J. Immunol. 185: 6646–6663 [DOI] [PubMed] [Google Scholar]
- 48.Reboldi A., Coisne C., Baumjohann D., Benvenuto F., Bottinelli D., Lira S., Uccelli A., Lanzavecchia A., Engelhardt B., Sallusto F. 2009. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat. Immunol. 10: 514–523 [DOI] [PubMed] [Google Scholar]
- 49.Miller D., Barkhof F., Montalban X., Thompson A., Filippi M. 2005. Clinically isolated syndromes suggestive of multiple sclerosis, part I: natural history, pathogenesis, diagnosis, and prognosis. Lancet Neurol. 4: 281–288 [DOI] [PubMed] [Google Scholar]
- 50.Weiner H. L. 2008. A shift from adaptive to innate immunity: a potential mechanism of disease progression in multiple sclerosis. J. Neurol. 255(Suppl. 1): 3–11 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.