Abstract
Cigarette smoke (CS), the major cause of chronic obstructive pulmonary disease, contains a variety of oxidative components that were implicated in the regulation of Src homology domain 2-containing protein tyrosine phosphatase 2 (Shp2) activity. However, the contribution of Shp2 enzyme to chronic obstructive pulmonary disease pathogenesis remains unclear. We investigated the role of Shp2 enzyme in blockading CS-induced pulmonary inflammation. Shp2 levels were assessed in vivo and in vitro. Mice (C57BL/6) or pulmonary epithelial cells (NCI-H292) were exposed to CS or cigarette smoke extract (CSE) to induce acute injury and inflammation. Lungs of smoking mice showed increased levels of Shp2, compared with those of controls. Treatment of lung epithelial cells with CSE showed elevated levels of Shp2 associated with the increased release of IL-8. Selective inhibition or knockdown of Shp2 resulted in decreased IL-8 release in response to CSE treatment in pulmonary epithelial cells. In comparison with CS-exposed wild-type mice, selective inhibition or conditional knockout of Shp2 in lung epithelia reduced IL-8 release and pulmonary inflammation in CS-exposed mice. In vitro biochemical data correlate CSE-mediated IL-8 release with Shp2-regulated epidermal growth factor receptor/Grb-2–associated binders/MAPK signaling. Our data suggest an important role for Shp2 in the pathological alteration associated with CS-mediated inflammation. Shp2 may be a potential target for therapeutic intervention for inflammation in CS-induced pulmonary diseases.
Introduction
Chronic obstructive pulmonary disease (COPD) is an increasing global health problem. The airflow limitation in COPD is usually progressive and associated with an abnormal inflammatory response of the lung to noxious particles or gases (1). Early studies have proposed that its processes are frequently triggered by cigarette smoke (CS), which has been identified as the primary cause of COPD (2–4). The pulmonary epithelial barrier is the first innate defense system of the lungs that protects against CS (5). Within the barrier, the epithelial cells play an important role in defense; when induced by CS, these cells secrete inflammatory mediators, including TGF-β (6), TNF-α (7), and IL-8 (8). Pulmonary epithelial cells are an important source of IL-8, a chemoattractant for inflammatory cells, including neutrophils and lymphocytes (9), which in turn help to stimulate IL-8 secretion (8, 9). Furthermore, CS triggers macrophage recruitment and activation (10), and activated macrophages release IL-8 (11). Given the characteristics of chronic inflammatory responses in COPD, it is not surprising that IL-8 plays an important role. However, the underlying cellular and molecular mechanisms of chronic inflammatory response and airflow obstruction triggered by CS remain elusive.
Reversible tyrosine phosphorylation in proteins is important in maintaining normal cell signaling linked to cellular development and pathological processes. Protein tyrosine phosphorylation and dephosphorylation are governed by the balanced action of protein tyrosine kinases and protein tyrosine phosphatases (PTPs) (12–14). Src homology domain 2-containing protein tyrosine phosphatase 2 (Shp2) is an intracellular classical PTP (15). Recently, Shp2 has been shown to play an important role in a wide variety of diseases, including atherosclerosis (16), glioma (15), and gastric carcinoma (17). Our previous studies have shown that Shp2 is important in the control of proliferation, differentiation, and survival in stem cells (18), lymphocytes, and mammary glands (19, 20). Of interest, Shp2 is known to be universally expressed in the lungs (21, 22). However, the role of Shp2 in the pathogenesis of lung diseases remains unclear.
In this work, we hypothesized that Shp2 regulates CS-induced IL-8 production and inflammation in the lungs. This idea was tested using an in vivo pharmacological inhibitor (23) and transgenic strategy that specifically abolished Shp2 in the pulmonary epithelia. Our observation offers a novel insight into the pathogenesis of smoking-related lung diseases. Understanding the role of Shp2 in chemokine production in pulmonary epithelia could aid in developing therapies to target this currently untreatable disease.
Materials and Methods
Materials
Phenylhydrazonopyrazolone sulfonate 1 (PHPS1) was obtained from Sigma-Aldrich (St. Louis, MO). RPMI 1640, FBS, penicillin, and streptomycin were obtained from Thermo Fisher Scientific (Kalamazoo, MI). TRIzol reagents were purchased from Takara (Otsu, Shiga, Japan). ERK, p-ERK, p-Shp2, β-actin (Cell Signaling Technology, Danvers, MA), and Shp2 (Santa Cruz Biotechnology, Santa Cruz, CA) primary Abs were used in the immunoblotting analysis. Lipofectamine LTX (Invitrogen, Carlsbad, CA) was used in the small interfering RNA (siRNA) experiment.
Mice
C57BL/6 mice (Laboratory Animal Center of Zhejiang University, Hangzhou, China; certificate no. SCXK 2007-0029) weighing 20 ± 2 g were studied in all experiments. All animals were housed in Plexiglas cages, kept on a 12/12-h light–dark cycle and received food and water ad libitum in temperature- and humidity-controlled rooms. To investigate the treatment effects of PHPS1 on airway inflammation and remodeling, mice were pretreated with PHPS1 by an i.p. injection at concentrations of 0.3, 1, and 3 mg/kg dissolved in saline with 0.5% DMSO 0.5 h before CS exposure for 4 d. An equal volume of saline with 0.5% DMSO was substituted for the PHPS1 in the model group and control group, respectively. After the treatment, the animals were placed in a plastic box and exposed to CS.
Endogenous disruption of the Shp2 enzyme in lung epithelia was generated using a combined genetic strategy. Shp2f/f mice (C57BL/6 background), which are homozygous for a “floxed” Shp2 in the pulmonary epithelium, were selected. SP-C-rtTAtg/− and (tetO)7CMV-Cretg/− transgenic mice (C57BL/6 background), as previously described, mated with Shp2f/f mice to generate double-transgenic mice with SP-C-rtTA/Shp2f/f or (tetO)7-Cretg/−/Shp2f/f. These mice then mated to generate SP-C-rtTA/(tetO)7-Cre/Shp2f/f triple transgenic mice. To confirm the mice’s genotypes, genomic DNA was extracted from tails or lungs, and PCR was performed on the SP-C-rtTA, (tetO)7CMV-Cre, and Shp2f/f genes, using the following primers: SP-C-rtTA, 5′-GACACATATAGAGACCCTGGTCA-3′ and 5′-AAAATCTTGCCAGCTTTCCC-3′; (tetO)7CMV-Cre, 5′-TGDDACGACCAAGTGACAGCAATG-3′ and 5′-AGAGACGGAAATCCATCGCTCG-3′; and Shp2f/f, 5′-ACGTCATGATCCGCTGTCAG-3′ and 5′-ATGGGAGGGACAGTGCAGTG-3′.
Transgenic mice and nondeleted littermate control mice were used for the experiments. To induce the activation of Cre recombinase in transgenic mice, as described previously, 4-wk-old mice were fed doxycycline (DOX; Sigma-Aldrich) in their drinking water (2 mg/ml) for 7 d. After the completion of doxycycline therapy, the mice were designated as Shp2 knockout (KO) mice. To detect Shp2 KO in the lung, a forward primer (5′-CAGTTGCAACTTTCTTACCTC-3′) in intron 3 and a reverse primer (5′-GCAGGAGACTGCAGCTCAGTGATG-3′) in intron 4 were used. For Shp2f/f and Shp2 WT, a Shp2f/f primer was used.
Animal exposures
The mice were exposed to whole-body CS generated from research-grade cigarettes (3R4F; University of Kentucky, Lexington, KY) in 5-l smoking chambers for 4 d, the method of which was modified according to previous research (24–26). Mice were exposed to 7 cigarettes (control mice were exposed to laboratory air) on the first day, 9 cigarettes on the second day, and 11 cigarettes on each of the third and fourth days. The lung tissue and bronchoalveolar lavage fluids (BALFs) were collected 18 h after the last CS exposure.
Assessment of lung inflammation and histology
With the mice under terminal anesthesia, the left lungs were removed, infused with 10% formalin, and immersed in the same solution before tissue processing in paraffin-embedded blocks. Sections were stained with H&E to evaluate general morphology.
Preparation of BALFs and cell count
At 18 h after the last CS exposure, mice were euthanized by i.p. pentobarbital injection of 6 g/kg urethane. BALFs were obtained by cannulating the trachea and lavaging with PBS containing 1% BSA and 5000 IU/l heparin. BALF cells were centrifuged once with PBS containing 2% FCS at 500 g for 10 min at 4°C. The pelleted BALF cells were resuspended in PBS, and the total number of leukocytes was counted by a Neubauer chamber. A total of 200 cells in a cytocentrifuged preparation of BALFs stained with Wright–Giemsa were differentiated under a light microscope according to classical cell morphology. The total number of each cell type was determined by multiplying the percentage by the total number of cells. The results were expressed as the number of each cell population in 1 ml BALFs.
Cell culture
NCI-H292 cells, a human pulmonary epithelial cell line, were obtained from the Cell Bank, Chinese Academy of Sciences. The cells were maintained in RPMI 1640 (HyClone, Logan, UT) containing 10% FBS (HyClone) at 37°C in the presence of 5% CO2.
Preparation of cigarette smoke extract
Research-grade cigarettes (3R4F) were obtained from the Kentucky Tobacco Research Council (University of Kentucky). The composition of 3R4F research-grade cigarettes was as follows: total particulate matter, 10.9 mg per cigarette; tar, 9.4 mg per cigarette; and nicotine, 0.726 mg per cigarette. Cigarette smoke extract (CSE) was prepared by bubbling smoke from three cigarettes into 30 ml PBS, modifying the method used in previous research. CSE was standardized by measuring the absorbance at a wavelength of 320 nm. After filtering through a 0.45-μM filter, CSE was frozen in aliquots and stored at −80°C. An aliquot of CSE was thawed immediately before use.
Shp2 siRNA preparation and transfection
Shp2-specific siRNA was obtained from Prof. Feng Gensheng at the Burnham Institute for Medical Research, La Jolla, CA. Sequences of the oligonucleotides are as follows: 5′-GAACAUCACGGCAAUUAAUU-3′; 5′-GAACACUGGUGAUUACUAUUU-3′. The cells were cultured in a 24-well plate for 24 h. Then the Shp2-specific or control siRNA was transfected into NCI-H292 cells using Lipofectamine LTX (Invitrogen) according to the manufacturer’s instructions. Immunoblotting analysis was used to examine Shp2 silencing by siRNA at 72 h after transfection.
RT-PCR and quantitative PCR
Total RNA was extracted with TRIzol reagent (Takara) according to the manufacturer’s instructions. The PCR primers were purchased from Shanghai Bioengineering (Shanghai, China). After PCR, the products were run on a 1.5% agarose gel electrophoresis and stained with ethidium bromide. All primers were checked against the basic local alignment search tool for selectivity. Real-time PCR cycling was carried out (7500 Real-Time PCR System; Applied Biosystems, Carlsbad, CA) under the following conditions: denaturation at 95°C for 10 s, annealing at 60°C for 15 s, and extension at 72°C for 30 s. An initial denaturation step at 95°C for 5 min and a final extension step at 72°C for 10 min were also included. PCR was performed for 40 cycles. β-Actin was amplified as an internal control. The mRNA levels were calculated using the comparative parameter threshold cycle (Ct) and normalized top-actin.
Measurement of IL-8 by ELISA
NCI-H292 cells were plated in a 24-well plate. Subconfluent monolayers of NCI-H292 cells were exposed to CSE in the presence or absence of PHPS1 for 24 h. Supernatants were then collected and stored at −80°C until assayed for IL-8 by ELISA (Boster, Wuhang, China). To investigate the effects of PHPS1 on cytokine expression in lung tissues, mice were treated with an i.p. injection of PHPS1 at concentrations of 3 mg/kg 0.5 h before CS exposure. At 18 h after CS-exposed mice were euthanized by injection of urethane (KC and MIP-2), the tissues were analyzed with ELISA kits (eBioscience, San Diego, CA), using paired matched Abs, according to the manufacturer's instructions.
Immunoblotting analysis
NCI-H292 cells were seeded into a six-well plate. After reaching the confluent, the cells were incubated in serum-free medium (RPMI 1640) overnight and then exposed to CSE in the presence or absence of PHPS1 for 15 min. After treatment, the cells were washed three times with ice-cold PBS and lysed in 100 μl radioimmunoprecipitation assay buffer with 10 mM PMSF (Beyotime, Haimen, China). The protein concentration was measured by the BCA Protein Assay Kit (cwbiotech, Beijing, China). A sample of protein (20–50 μg) from the cell lysates was separated by SDS-PAGE in 12% polyacrylamide gel and transferred to nitrocellulose membranes (Pall, Port Washington, NY), which were blocked with 5% fat-free milk (1 h at room temperature). The membranes were then incubated with p-ERK, ERK (Cell Signaling Technology), Shp2 (Santa Cruz Biotechnology), p-Shp2, and actin primary Abs (Bioworld, St. Louis Park, MN). Afterward, the membranes were rinsed with TBST and then probed with secondary Abs (Invitrogen) for 1 h at room temperature. Immunoreactive bands were visualized by a two-color infrared imaging system (Odyssey; LI-COR, Lincoln, NE).
Shp2 immunohistochemistry
For Shp2 immunohistochemistry, the lung tissues were obtained from C57BL/6 mice exposed to CS or laboratory air. Immunostaining for Shp2 was performed using Shp2 mAb. All specimens were stained at the same time.
Statistical analysis
Data were expressed as mean ± SEM. Statistical tests were performed using SPSS software (version 16.0; SPSS, Chicago, IL). One-way ANOVA followed by the Student–Newman–Keuls test was used to determine multiple comparisons. Statistical significance was accepted at p < 0.05.
Results
CS induces the elevated level of Shp2 in the mouse model in vivo and pulmonary epithelial cells in vitro
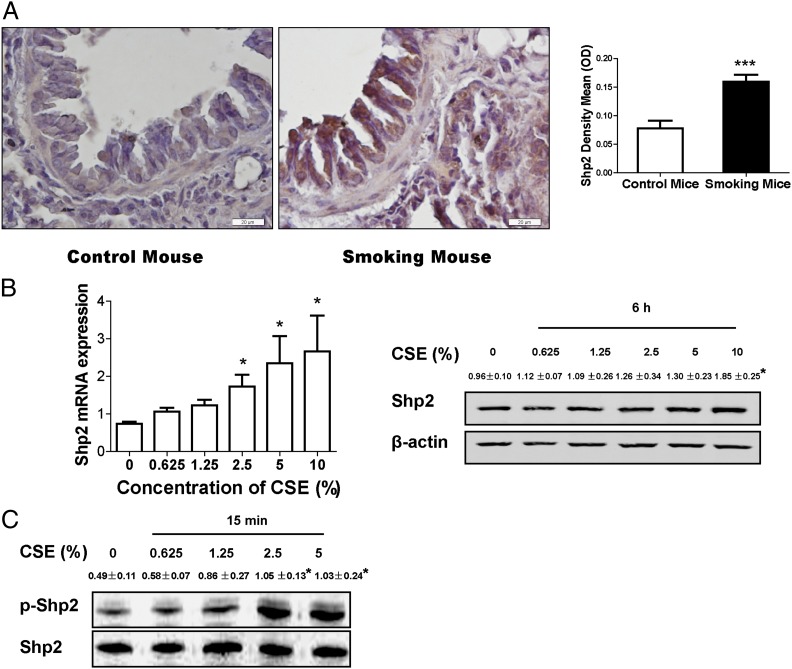
Tyrosine phosphatase Shp2 is known to modulate multiple signaling involved in inflammatory responses (27–29). We investigated the effect of cigarette smoking on Shp2 levels in mouse lungs. As shown in Fig. 1A, cigarette smoking elevated Shp2 expression in vivo. To investigate CS-induced Shp2 expression in inflammatory cells, total inflammatory cells in BALFs were collected from smoke-exposed mice 18 h after the last CS exposure, and Shp2 levels were measured. The results (Supplemental Fig. 2) indicate no significant change in Shp2 gene expression in the inflammatory cells compared with the controls, suggesting that CS-induced inflammation in the lungs does not affect Shp2 expression in inflammatory cells. In addition, we found that the increased Shp2 level was triggered by CSE in pulmonary epithelial cells in vitro. The pulmonary epithelial cells (NCI-H292 cells) were exposed to CSE for 6 h. As shown in Fig. 1B, CSE, in a concentration-dependent fashion, triggered Shp2 production in the early stage of CSE exposure. We also measured the activity of Shp2 enzyme in lung epithelial cells in response to CSE stimulation in vitro. We noticed enhanced levels of the phosphor site (Y-542) of Shp2 in a concentration-dependent manner (Fig. 1C). To corroborate the acute effects of CS on Shp2 expression, we determined the change of Shp2 levels in mouse lungs after smoke cessation. We found that an increased level of Shp2 persisted over 7 d and then gradually decreased to baseline by 30 d post CS exposure (Supplemental Fig. 4). These data underscore the importance of Shp2 in CS-mediated acute inflammatory response.
FIGURE 1.
CS induces the increased level of Shp2 in the mouse model and pulmonary epithelial cells in vitro. (A) Shp2 immunohistochemistry was assessed in lung tissues from mice exposed to CS or laboratory air. Shp2 immunohistochemistry in mice showed that cigarette smoking elevated Shp2 expression. Data were expressed as mean ± SEM (n = 9 per group) of three independent experiments. Scale bar, 20 μm. ***p < 0.001, compared with control. (B) The pulmonary epithelial cells (NCI-H292 cells) were treated with CSE for 6 h. After treatment, cells were harvested to measure Shp2 gene expression by real-time PCR (n = 9 per group) and Shp2 protein production by immunoblotting (n = 3 per group). (C) Cells were exposed to CSE for 15 min. In a concentration-dependent manner, CSE activated Shp2. Total Shp2 and phosphorylated Shp2 were examined by immunoblotting, as described above (n = 3 per group). Data were shown as mean ± SEM of three independent experiments. *p < 0.05 compared with no treatment with CSE.
CSE triggers IL-8 release in pulmonary epithelial cells
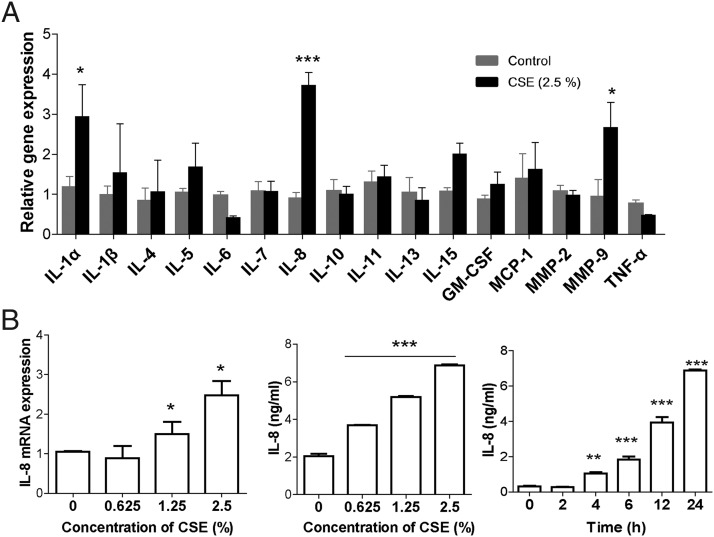
To examine the effects of CSE on cytokine production in lung epithelia, pulmonary epithelial cells were treated with CSE, followed by analysis of a panel of chemokines and cytokines. We found a remarkable elevation of IL-8 in lung epithelial cells in vitro (Fig. 2A, Supplemental Fig. 3). Further studies suggest that IL-8 release is induced by CSE in a concentration- and time-dependent manner (Fig. 2B). Our data are consistent with recent studies, which have shown increased levels of IL-8 in lung epithelia as a potent chemoattractant for recruiting inflammatory cells (30, 31).
FIGURE 2.
CS induces IL-8 expression and release in pulmonary epithelial cells in vitro. (A) NCI-H292 cells were treated with CSE for 6 h. A panel of 16 chemokines and cytokines were measured by real-time PCR (n = 9 per group). (B) The cells were treated with different concentrations of CSE for 24 h. Then the cells were exposed to 2.5% CSE for different times (2–24 h). The mRNA and protein of IL-8 were separately assessed by RT-PCR and ELISA (n = 9 per group). Data were expressed as mean ± SEM of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, compared with control.
Pharmacological suppression of Shp2 alleviates IL-8 release and inflammatory responses in CS-exposed mice
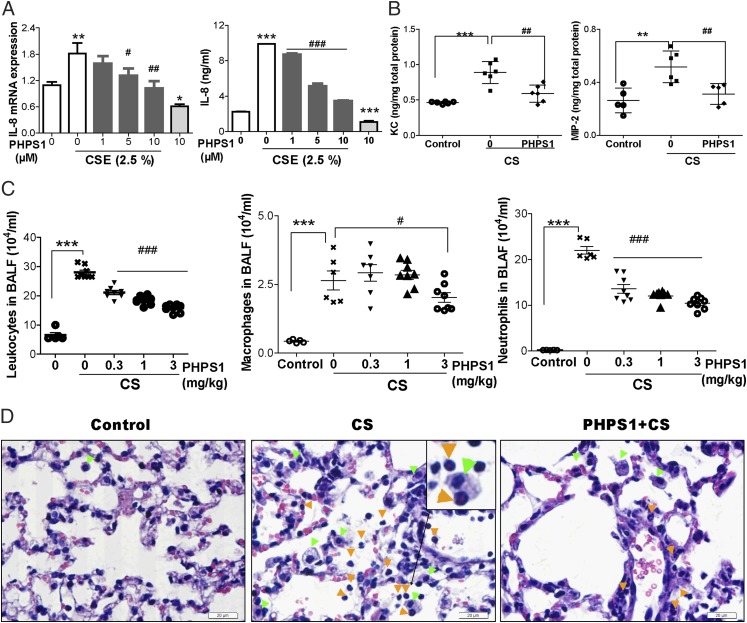
Next, we asked whether Shp2 activity correlates with IL-8–mediated lung injuries in CS-exposed mice. To assess the potential role of Shp2 in regulating inflammation, we examined the effects of CSE on IL-8 gene expression and production in pulmonary epithelial cells with the administration of PHPS1, a Shp2 inhibitor. As shown in Fig. 3A, pharmacological inhibition of Shp2 results in marked reduction of IL-8 in both mRNA and protein levels. To further probe the effect of Shp2 in CS exposure in vivo, C57BL/6 mice were exposed to CS or laboratory air for 4 d consecutively. CS-model mice were i.p. injected with increasing doses of PHPS1, from 0.3 to 3 mg/kg, 30 min before quotidian CS exposure. Lung tissues were harvested 18 h after the last CS exposure. Our findings suggested that IL-8 (MIP-2 and KC) release was significantly reduced in PHPS1-pretreated mice (Fig. 3B). The total inflammatory cell count in BALFs collected from mice suggested a remarkable reduction of macrophages and neutrophils in the group treated with PHPS1 compared with controls (Fig. 3C). Lung sections were further analyzed with histological studies. As illustrated in Fig. 3D, H&E staining revealed that PHPS1 administration, compared with CS exposure alone, alleviated the influx of inflammatory cells, characterized as a significant infiltration of macrophages and neutrophils into alveolar spaces. These observations reveal a potential role for Shp2 in IL-8–modulated recruitment of inflammatory cells in acute mouse models of CS.
FIGURE 3.
PHPS1 suppresses CSE-induced IL-8 release in pulmonary epithelial cells and inflammatory responses in CS mouse models. (A) NCI-H292 cells were pretreated with PHPS1 (1–10 μM) for 15 min; then, the cells were stimulated with 2.5% CSE for 24 h. After stimulation with CSE, cell culture media were collected to measure IL-8 protein by ELISA, and the cells were harvested for IL-8 gene expression by real-time PCR (n = 8 per group). (B) C57BL/6 mice were repeatedly exposed to CS for 4 d. Mice were treated with PHPS1 (3 mg/kg) by i.p. injection at 30 min before daily CS exposure. At 18 h after the last CS exposure, BALFs were harvested to measure KC and MIP-2 release by ELISA. (C) The total inflammatory cells in BALFs were counted, and a minimum of 200 cells were used to classify inflammatory cells as macrophages or neutrophils. (D) Lung tissues were stained with H&E, representative of three independent experiments. The inflammatory cells were observed under microscopes. Mice with air exposure had no infiltration of inflammatory cells. Mice with CS exposure showed an influx of macrophages (green arrowheads) and neutrophils (orange arrowheads) into the alveolar spaces. The macrophage is mononuclear, and its cytoplasm contains vacuoles and granules. The neutrophil contains a nucleus divided into two to five lobes (the separated lobes are connected by chromatin), and the cytoplasm stains neutral pink. Treatment with PHPS1 attenuated the inflammatory cells’ infiltration. Data were expressed as mean ± SEM of three independent experiments. Scale bar, 20 μm. *p < 0.05. **p < 0.01, ***p < 0.001 compared with control. #p < 0.05, ##p < 0.01, ###p < 0.001 compared with CSE-stimulated cells or mice with CS exposure.
Endogenous inactivation of Shp2 exhibits decreased inflammation in CS-induced lung injuries
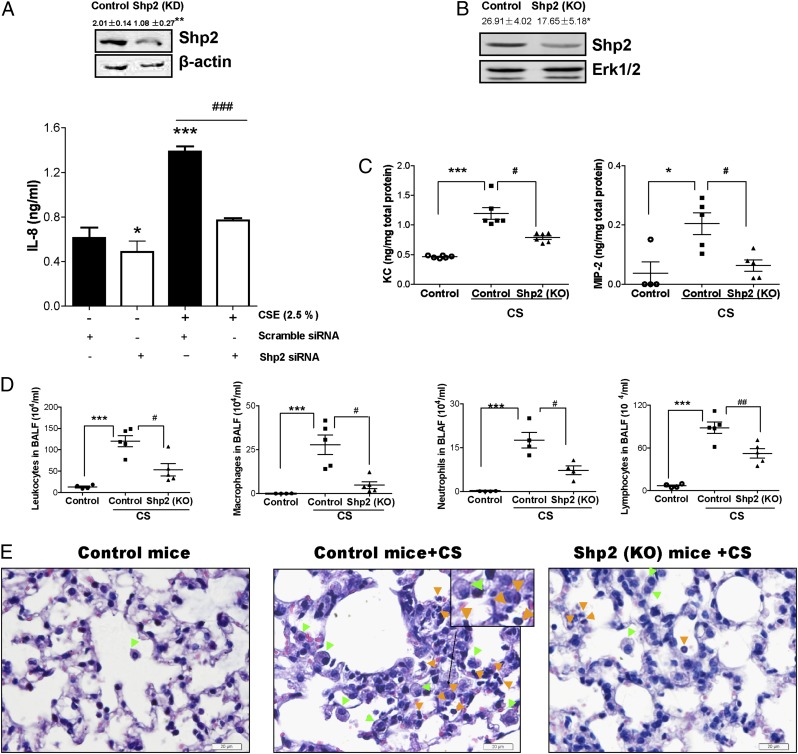
The observation that PHPS1 alleviated inflammation in CS-induced lung injury was further investigated by endogenous inactivation of Shp2 in lung epithelial cells and in vivo transgenic mice. Similar experiments were conducted to examine the contribution of Shp2 to CS-induced lung inflammation. Using siRNA-mediated knockdown of Shp2 in pulmonary epithelial cells, we found that inactivation of Shp2 decreased CSE-induced IL-8 release (Fig. 4A). We next generated lung-specific Shp2 KO mice. The specificity of the Shp2 deletion in the lungs was confirmed by immunoblotting analysis (Fig. 4B, Supplemental Fig. 1D). Shp2 KO mice displayed levels of MIP-2 and KC similar to those in control mice upon exposure to laboratory air (Supplemental Fig. 1). However, as we observed, control mice exposed to CS for 4 consecutive days showed a remarkable elevation of MIP-2 and KC in controls, but not in Shp2 KO mice (Fig. 4C). In addition, inflammatory cells—especially macrophages, neutrophils, and lymphocytes—were decreased in Shp2 KO mice (Fig. 4D). Pathological analysis of the lungs using H&E staining revealed a pronounced decrease in the infiltration of macrophages and neutrophils into alveolar spaces, compared with controls (Fig. 4E). Consistent with findings from inhibitor treatment, these data further suggest the in vivo importance of Shp2 in regulating the inflammatory response in acute CS mouse models.
FIGURE 4.
Genetic ablation of Shp2 reduces the levels of IL-8 triggered by CSE in NCI-H292 cells and the inflammatory response induced by CS in mice. (A) The pulmonary epithelial cells were treated with transfection agent Lipofectamine LTX and transfected with either Shp2 siRNA (100 nM) or nonspecific control siRNA (100 nM). After 24 h, the cultured cells were treated with or without CSE (2.5%) for 24 h. The cells were collected to assess the levels of Shp2 production by immunoblotting assay, and the culture medium was used to measure IL-8 by ELISA. Data were expressed as mean ± SEM (n = 9 per group) of three independent experiments. *p < 0.05; **p < 0.01, ***p < 0.001 compared with cells (scramble siRNA) without CSE exposure. ###p < 0.001 compared with cells (Shp2 siRNA) with CSE exposure. (B) The Shp2 protein levels of Shp2 KO mice were evaluated by immunoblotting assays. Results were expressed as mean ± SEM of three independent experiments. *p < 0.05 compared with control mice. (C) Shp2 KO mice and control mice were repeatedly exposed to CS or laboratory air for 4 d. At 18 h after the last CS exposure, the BALFs were collected to evaluate the levels of KC and MIP-2 by ELISA. (D) The total leukocytes, macrophages, neutrophils, and lymphocytes were determined in BALFs. (E) Lung sections were stained with H&E, representative of three independent experiments. Mice with CS exposure, compared with air-exposed mice, showed macrophage (green arrowheads) and neutrophil (orange arrowheads) influx into the alveolar spaces. Shp2 KO mice exhibited a decrease in inflammatory cell infiltration. Results were expressed as mean ± SEM of three independent experiments. Scale bar, 20 μm. *p < 0.05, ***p < 0.001 compared with control mice with air exposure. #p < 0.05, ##p < 0.01 compared with control mice with CS exposure.
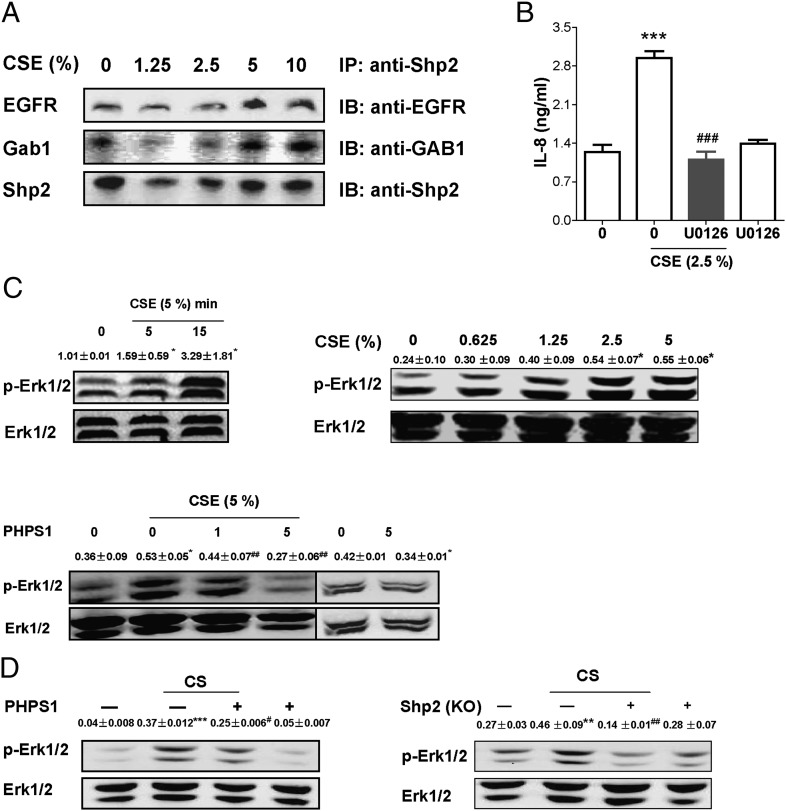
Shp2 regulates CSE-induced epidermal growth factor receptor/ Grb-2-associated binders/MAPK axis, contributing to IL-8 release
In previous studies, Shp2 has been demonstrated to mediate epidermal growth factor (EGF) signaling events in various cells and tissues (23). In this study, we asked whether Shp2 is involved in EGF-induced inflammation in lung epithelia. In NCI-H292 cells, we found that, upon CSE exposure, Shp2 was recruited to an epidermal growth factor receptor (EGFR)-dependent complex that involves Grb-2–associated binder 1 (Gab1) docking proteins (Fig. 5A). Our data suggest that direct interaction between cytoplasmic Shp2 and docking protein Gab1 may increase activation of EGFR initiated by CSE. This idea has been further supported by increased levels of active ERK following CSE treatment (Fig. 5C). Pharmacological inhibition of both Shp2 and ERK decreased CSE-mediated IL-8 production (Fig. 3A, Fig. 5B); meanwhile, wild-type mice pretreated with PHPS1 and Shp2 KO mice revealed decreased activation of Erk1/2 induced by CS (Fig. 5D). Taken together, our results identify the importance of Shp2 in regulating IL-8 release in lung epithelia in response to CS exposure via EGFR/Gabs/MAPK and thereby potentially contribute to the development of a novel therapeutic target for lung inflammatory diseases.
FIGURE 5.
CSE activates EGFR/Shp2/MAPK signaling in NCI-H292 cells. (A) The cells were incubated with different concentrations of CSE for 15 h. Cell lysates were subjected to immunoprecipitation with anti-Shp2 Ab and immunoblotting with anti-EGFR and anti-Gab1 Abs. Similar data were obtained in two independent experiments. (B) NCI-H292 cells were pretreated with U0126 (10 μM) for 15 min; then cells were treated with CSE (2.5%) for 24 h. The culture supernatants were measured for IL-8 release by ELISA. (C) NCI-H292 cells were exposed to CSE (5%) for different lengths of time (0–15 min), or the cells were treated with various concentrations of CSE for 15 min. The activity of Erk1/2 was determined in the indicated cell line by immunoblotting. CSE induced Erk1/2 activation in a concentration- and time-dependent manner. Then the cells were pretreated with PHPS1 for 15 min, followed by CSE (5%) treatment for 15 min. Pretreatment of PHPS1 attenuated the activity of Erk1/2 induced by CSE. Data were expressed as mean ± SEM (n = 3 per group) of three independent experiments. *p < 0.05, ***p < 0.001 compared with control. ##p < 0.01, ###p < 0.001 compared with treatment with CSE alone. (D) Lungs obtained from Shp2 inhibition or Shp2 (KO) mice were harvested to measure the activity of Erk1/2. Results were expressed as mean ± SEM (n = 3 per group) of three independent experiments. **p < 0.001, ***p < 0.001 compared with control. #p < 0.05, ##p < 0.01 compared with mice with CS exposure. IB, immunoblotting; IP, immunoprecipitation.
Discussion
In this article, we report that inhibition of the tyrosine phosphatase Shp2 in pulmonary epithelia prevents the inflammatory response induced by CS via the EGFR/MAPK axis. These findings enable us to understand a novel biological role for the Shp2 enzyme in the pathogenesis of chronic pulmonary inflammation.
Cigarette smoking is a leading cause of COPD, which is associated with a persistent inflammatory response (32, 33). The lung inflammatory response to CS exposure is more complex than the neutrophil accumulation. However, targeting the acute effects of CS-mediated pulmonary inflammation by interfering with specific molecules, such as Shp2, may lead to the development of novel anti-COPD agents. Shp2, a member of a subfamily of PTPs, has been recently linked to cell growth and chemotactic responses (18, 34). However, no study has provided evidence on the effects of Shp2 on the CS-induced inflammatory response in COPD.
Our research has focused on the role of Shp2 in acute inflammatory lung responses triggered by CS. Considering that the acute lung response to CS in mice may differ depending on whether the mice have been previously exposed to CS, we studied the acute response to a few days of CS exposure in mice that have never been exposed to smoke, rather than the response to repeated smoke exposure periods of weeks or months. We found increased levels of Shp2 in mice with CS-induced lung inflammation. Similarly, CSE elevated Shp2 expression and activated the Shp2 enzyme in pulmonary epithelial cells in vitro. These findings suggest that Shp2 may be involved in the pathogenesis of pulmonary inflammation induced by CS. Consistent with a previous report (35), we observed a greater elevation of IL-8 expression than of other proinflammatory cytokines and chemokines in the pulmonary epithelial cells and mouse model of acute CS. Pulmonary epithelia play a key role in the early defense against CSE. The increased IL-8 levels in epithelia recruit inflammatory cells in the lungs, which are involved in predominant inflammatory responses. Therefore, it is possible that Shp2 may regulate the CS-evoked inflammatory response, particularly IL-8 expression and release.
In this article, we showed that inhibiting Shp2 prevented smoke-induced pulmonary inflammation by reducing macrophage and neutrophil infiltration as well as the release of proinflammatory cytokines, such as MIP-2 and KC, found in BALFs in vivo. Similarly, inhibition of Shp2 significantly reduced CSE-stimulated levels of IL-8 gene expression and protein production in human pulmonary epithelial cells in vitro. Thus, we provide novel evidence that Shp2 regulates the lung inflammatory responses induced by CS. This finding is further supported by studies of pulmonary epithelia-specific Shp2 KO mice in vivo and the genetic ablation of Shp2 on NCI-H292 cells in vitro.
We hypothesized that Shp2 could regulate smoke-induced lung inflammation via signaling pathways such as the MAPK pathway, because Shp2 positively regulates cell proliferation, differentiation, and survival by activating the MAPK pathway (36, 37). It has been suggested that Shp2 regulates MAPK signaling in response to EGF, hepatocyte growth factor, and other growth factors and is required for the binding of the docking protein Gab1 (36, 38). In addition, Shp2 is considered an important downstream protein of EGFR (23). It has been reported that CS activates EGFR in human epithelial cells (39, 40). In response to stimulation, the activated EGFR recruits and activates a docking protein Gab1, which is a substrate of Shp2 and involved in regulating Erk1/2 activation (38). As a result, the activated Gab1 attracts and activates Shp2 (36, 38). In our studies, we observed that CSE, independent of concentration, induced the binding of Shp2 to EGFR and Gab1. We also found that CSE, in a concentration-dependent manner, increases Shp2 expression and activates phosphorylation of Shp2 protein. Therefore, it is possible that regulation of Shp2 in the smoke-induced IL-8 release relates to the involvement of Gab1 and EGFR. Previous research has shown that Shp2 acts on the upstream portion of the MAPK pathways (23, 41). Moreover, PHPS1 significantly inhibits Erk1/2 activation (23). Therefore, we assumed that Shp2 regulates acute pulmonary inflammation induced by CS, at least partially, through the Erk1/2 MAPKs pathway. To prove this hypothesis, a pharmacological inhibitor of Erk1/2, 1,4-diamino-2,3-dicyano-1,4-bis[2-aminophenylthio]butadiene (U0126), was used in the experiment. We found that U0126 blocks the release of IL-8 induced by CSE in NCI-H292 cells. Involvement of the Erk1/2 pathway is further demonstrated by experiments detecting Erk1/2 phosphorylation. Key findings show that activation of Erk1/2 by CS was inhibited by PHPS1 or endogenous inactivation of Shp2 in vitro and in vivo. Moreover, these findings are consistent with recent research indicating that CSE induces IL-8 release through an Erk1/2-dependent pathway in human lung fibroblasts cells and small airway epithelial cells (42, 43). Recent studies have shown that the formation of the Shp2 and Gab1 complex plays an essential role in EGFR/MAPK signaling (44). Therefore, MAPK pathways are involved in Shp2-regulated IL-8 production by CSE.
In conclusion, we performed pioneering work on the effects of Shp2 in regulating the pulmonary inflammatory response triggered by CS. Stimulating NCI-H292 cells with CSE resulted in the elevation of IL-8 gene expression and protein release, which was alleviated by inhibiting the Shp2 enzyme or by the endogenous inactivation of Shp2. These processes act through Shp2/MAPK pathways. In addition, either the inhibition of the Shp2 enzyme or the genetic ablation of Shp2 led to an attenuated inflammatory response induced by CS in mouse lungs. Considering the role of Shp2 in regulating release of the proinflammatory mediator IL-8 as well as inflammatory cell recruitment and influx into the lungs, we presume that involvement of Shp2 in regulating acute inflammatory responses mediated by CS would have affected processes leading to COPD pathogenesis. Further research is required to assess whether Shp2 can regulate other inflammatory factors released by CS and their molecular mechanisms and to examine the role of Shp2 in the regulation of chronic inflammatory response to long-term CS exposure. Enhanced understanding of the impact of the Shp2 enzyme on the release of inflammatory mediators improves our ability to develop novel and targeted therapeutic interventions for smoking-mediated lung diseases.
Supplementary Material
Acknowledgments
We thank G.S. Feng (University of California, San Diego, La Jolla, CA) for Shp2F/F mice and J.A. Whitsett (College of Medicine, University of Cincinnati, Cincinnati, OH) and Ning Wen (Nankai University, Tianjin, China) for the SP-C-rtTAtg/− and (tetO)7CMV-Cretg/− transgenic mice.
This work was supported by grants from the National Science Foundation of China (30973542, 30871291, and 30971504) and the National Basic Research Program of China (2009CB522103).
The online version of this article contains supplemental material.
- BALF
- bronchoalveolar lavage fluid
- COPD
- chronic obstructive pulmonary disease
- CS
- cigarette smoke
- CSE
- cigarette smoke extract
- EGF
- epidermal growth factor
- EGFR
- epidermal growth factor receptor
- Gab1
- Grb-2–associated binder 1
- KO
- knockout
- PHPS1
- phenylhydrazonopyrazolone sulfonate 1
- PTP
- protein tyrosine phosphatase
- Shp2
- Src homology domain 2-containing protein tyrosine phosphatase 2
- siRNA
- small interfering RNA
- U0126
- 1,4-diamino-2,3-dicyano-1,4-bis[2-aminophenylthio]butadiene.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Rabe K. F., Hurd S., Anzueto A., Barnes P. J., Buist S. A., Calverley P., Fukuchi Y., Jenkins C., Rodriguez-Roisin R., van Weel C., Zielinski J., Global Initiative for Chronic Obstructive Lung Disease 2007. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 176: 532–555 [DOI] [PubMed] [Google Scholar]
- 2.Wewers M. E., Munzer A., Ewart G. 2010. Tackling a root cause of chronic lung disease: the ATS, FDA, and tobacco control. Am. J. Respir. Crit. Care Med. 181: 1281–1282 [DOI] [PubMed] [Google Scholar]
- 3.Rojas M., Mora A. L. 2011. Age and smoke: a risky combination. Am. J. Respir. Crit. Care Med. 183: 423–424 [DOI] [PubMed] [Google Scholar]
- 4.Barreiro E., Peinado V. I., Galdiz J. B., Ferrer E., Marin-Corral J., Sánchez F., Gea J., Barberà J. A., ENIGMA in COPD Project 2010. Cigarette smoke-induced oxidative stress: a role in chronic obstructive pulmonary disease skeletal muscle dysfunction. Am. J. Respir. Crit. Care Med. 182: 477–488 [DOI] [PubMed] [Google Scholar]
- 5.Crosby L. M., Waters C. M. 2010. Epithelial repair mechanisms in the lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 298: L715–L731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takizawa H., Tanaka M., Takami K., Ohtoshi T., Ito K., Satoh M., Okada Y., Yamasawa F., Nakahara K., Umeda A. 2001. Increased expression of transforming growth factor-beta1 in small airway epithelium from tobacco smokers and patients with chronic obstructive pulmonary disease (COPD). Am. J. Respir. Crit. Care Med. 163: 1476–1483 [DOI] [PubMed] [Google Scholar]
- 7.Sapey E., Wood A. M., Ahmad A., Stockley R. A. 2010. Tumor necrosis factor-alpha rs361525 polymorphism is associated with increased local production and downstream inflammation in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 182: 192–199 [DOI] [PubMed] [Google Scholar]
- 8.Mio T., Romberger D. J., Thompson A. B., Robbins R. A., Heires A., Rennard S. I. 1997. Cigarette smoke induces interleukin-8 release from human bronchial epithelial cells. Am. J. Respir. Crit. Care Med. 155: 1770–1776 [DOI] [PubMed] [Google Scholar]
- 9.Pease J. E., Sabroe I. 2002. The role of interleukin-8 and its receptors in inflammatory lung disease: implications for therapy. Am. J. Respir. Med. 1: 19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirkham P. A., Spooner G., Ffoulkes-Jones C., Calvez R. 2003. Cigarette smoke triggers macrophage adhesion and activation: role of lipid peroxidation products and scavenger receptor. Free Radic. Biol. Med. 35: 697–710 [DOI] [PubMed] [Google Scholar]
- 11.Teranishi A., Akada S., Saito S., Hatake K., Morikawa H. 2002. Macrophage colony-stimulating factor restored chemotherapy-induced granulocyte dysfunctions: role of IL-8 production by monocytes. Int. Immunopharmacol. 2: 83–94 [DOI] [PubMed] [Google Scholar]
- 12.Tiganis T., Bennett A. M. 2007. Protein tyrosine phosphatase function: the substrate perspective. Biochem. J. 402: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chong Z. Z., Maiese K. 2007. The Src homology 2 domain tyrosine phosphatases SHP-1 and SHP-2: diversified control of cell growth, inflammation, and injury. Histol. Histopathol. 22: 1251–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.López-Ruiz P., Rodriguez-Ubreva J., Cariaga A. E., Cortes M. A., Colás B. 2011. SHP-1 in cell-cycle regulation. Anticancer. Agents Med. Chem. 11: 89–98 [DOI] [PubMed] [Google Scholar]
- 15.Navis A. C., van den Eijnden M., Schepens J. T., Hooft van Huijsduijnen R., Wesseling P., Hendriks W. J. 2010. Protein tyrosine phosphatases in glioma biology. Acta Neuropathol. 119: 157–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Won K. J., Lee H. M., Lee C. K., Lin H. Y., Na H., Lim K. W., Roh H. Y., Sim S., Song H., Choi W. S., et al. 2011. Protein tyrosine phosphatase SHP-2 is positively involved in platelet-derived growth factor-signaling in vascular neointima formation via the reactive oxygen species-related pathway. J. Pharmacol. Sci. 115: 164–175 [DOI] [PubMed] [Google Scholar]
- 17.Higashi H., Tsutsumi R., Muto S., Sugiyama T., Azuma T., Asaka M., Hatakeyama M. 2002. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori Cag. Science 295: 683–686 [DOI] [PubMed] [Google Scholar]
- 18.Ke Y., Zhang E. E., Hagihara K., Wu D., Pang Y., Klein R., Curran T., Ranscht B., Feng G. S. 2007. Deletion of Shp2 in the brain leads to defective proliferation and differentiation in neural stem cells and early postnatal lethality. Mol. Cell. Biol. 27: 6706–6717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen T. V., Ke Y., Zhang E. E., Feng G. S. 2006. Conditional deletion of Shp2 tyrosine phosphatase in thymocytes suppresses both pre-TCR and TCR signals. J. Immunol. 177: 5990–5996 [DOI] [PubMed] [Google Scholar]
- 20.Ke Y., Lesperance J., Zhang E. E., Bard-Chapeau E. A., Oshima R. G., Muller W. J., Feng G. S. 2006. Conditional deletion of Shp2 in the mammary gland leads to impaired lobulo-alveolar outgrowth and attenuated Stat5 activation. J. Biol. Chem. 281: 34374–34380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tefft D., De Langhe S. P., Del Moral P. M., Sala F., Shi W., Bellusci S., Warburton D. 2005. A novel function for the protein tyrosine phosphatase Shp2 during lung branching morphogenesis. Dev. Biol. 282: 422–431 [DOI] [PubMed] [Google Scholar]
- 22.Qu C. K., Yu W. M., Azzarelli B., Feng G. S. 1999. Genetic evidence that Shp-2 tyrosine phosphatase is a signal enhancer of the epidermal growth factor receptor in mammals. Proc. Natl. Acad. Sci. USA 96: 8528–8533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hellmuth K., Grosskopf S., Lum C. T., Würtele M., Röder N., von Kries J. P., Rosario M., Rademann J., Birchmeier W. 2008. Specific inhibitors of the protein tyrosine phosphatase Shp2 identified by high-throughput docking. Proc. Natl. Acad. Sci. USA 105: 7275–7280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wollin L., Pieper M. P. 2010. Tiotropium bromide exerts anti-inflammatory activity in a cigarette smoke mouse model of COPD. Pulm. Pharmacol. Ther. 23: 345–354 [DOI] [PubMed] [Google Scholar]
- 25.Yang S. R., Valvo S., Yao H., Kode A., Rajendrasozhan S., Edirisinghe I., Caito S., Adenuga D., Henry R., Fromm G., et al. 2008. IKK alpha causes chromatin modification on pro-inflammatory genes by cigarette smoke in mouse lung. Am. J. Respir. Cell Mol. Biol. 38: 689–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghio A. J., Hilborn E. D., Stonehuerner J. G., Dailey L. A., Carter J. D., Richards J. H., Crissman K. M., Foronjy R. F., Uyeminami D. L., Pinkerton K. E. 2008. Particulate matter in cigarette smoke alters iron homeostasis to produce a biological effect. Am. J. Respir. Crit. Care Med. 178: 1130–1138 [DOI] [PubMed] [Google Scholar]
- 27.Peters K. G., Kontos C. D., Lin P. C., Wong A. L., Rao P., Huang L., Dewhirst M. W., Sankar S. 2004. Functional significance of Tie2 signaling in the adult vasculature. Recent Prog. Horm. Res. 59: 51–71 [DOI] [PubMed] [Google Scholar]
- 28.Hirano T. 2010. Interleukin 6 in autoimmune and inflammatory diseases: a personal memoir. Proc. Jpn. Acad., Ser. B, Phys. Biol. Sci. 86: 717–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Necchi V., Sommi P., Ricci V., Solcia E. 2010. In vivo accumulation of Helicobacter pylori products, NOD1, ubiquitinated proteins and proteasome in a novel cytoplasmic structure. PLoS ONE 5: e9716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li B., Dong C., Wang G., Zheng H., Wang X., Bai C. 2011. Pulmonary epithelial CCR3 promotes LPS-induced lung inflammation by mediating release of IL-8. J. Cell. Physiol. 226: 2398–2405 [DOI] [PubMed] [Google Scholar]
- 31.Carpagnano G. E., Palladino G. P., Lacedonia D., Koutelou A., Orlando S., Foschino-Barbaro M. P. 2011. Neutrophilic airways inflammation in lung cancer: the role of exhaled LTB-4 and IL-8. BMC Cancer 11: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida T., Tuder R. M. 2007. Pathobiology of cigarette smoke-induced chronic obstructive pulmonary disease. Physiol. Rev. 87: 1047–1082 [DOI] [PubMed] [Google Scholar]
- 33.Goldkorn T., Filosto S. 2010. Lung injury and cancer: mechanistic insights into ceramide and EGFR signaling under cigarette smoke. Am. J. Respir. Cell Mol. Biol. 43: 259–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yi T., Mui A. L., Krystal G., Ihle J. N. 1993. Hematopoietic cell phosphatase associates with the interleukin-3 (IL-3) receptor beta chain and down-regulates IL-3-induced tyrosine phosphorylation and mitogenesis. Mol. Cell. Biol. 13: 7577–7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang G. J., Wang H. Y., Wang J. Y., Lee C. C., Tseng H. W., Wu Y. L., Shyue S. K., Lee T. S., Kou Y. R. 2011. Novel role of AMP-activated protein kinase signaling in cigarette smoke induction of IL-8 in human lung epithelial cells and lung inflammation in mice. Free Radic. Biol. Med. 50: 1492–1502 [DOI] [PubMed] [Google Scholar]
- 36.Matozaki T., Murata Y., Saito Y., Okazawa H., Ohnishi H. 2009. Protein tyrosine phosphatase SHP-2: a proto-oncogene product that promotes Ras activation. Cancer Sci. 100: 1786–1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajendrasozhan S., Yang S. R., Kinnula V. L., Rahman I. 2008. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 177: 861–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montagner A., Yart A., Dance M., Perret B., Salles J. P., Raynal P. 2005. A novel role for Gab1 and SHP2 in epidermal growth factor-induced Ras activation. J. Biol. Chem. 280: 5350–5360 [DOI] [PubMed] [Google Scholar]
- 39.Baginski T. K., Dabbagh K., Satjawatcharaphong C., Swinney D. C. 2006. Cigarette smoke synergistically enhances respiratory mucin induction by proinflammatory stimuli. Am. J. Respir. Cell Mol. Biol. 35: 165–174 [DOI] [PubMed] [Google Scholar]
- 40.Deshmukh H. S., Case L. M., Wesselkamper S. C., Borchers M. T., Martin L. D., Shertzer H. G., Nadel J. A., Leikauf G. D. 2005. Metalloproteinases mediate mucin 5AC expression by epidermal growth factor receptor activation. Am. J. Respir. Crit. Care Med. 171: 305–314 [DOI] [PubMed] [Google Scholar]
- 41.Gutch M. J., Flint A. J., Keller J., Tonks N. K., Hengartner M. O. 1998. The Caenorhabditis elegans SH2 domain-containing protein tyrosine phosphatase PTP-2 participates in signal transduction during oogenesis and vulval development. Genes Dev. 12: 571–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mortaz E., Rad M. V., Johnson M., Raats D., Nijkamp F. P., Folkerts G. 2008. Salmeterol with fluticasone enhances the suppression of IL-8 release and increases the translocation of glucocorticoid receptor by human neutrophils stimulated with cigarette smoke. J. Mol. Med. 86: 1045–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moretto N., Facchinetti F., Southworth T., Civelli M., Singh D., Patacchini R. 2009. alpha,beta-Unsaturated aldehydes contained in cigarette smoke elicit IL-8 release in pulmonary cells through mitogen-activated protein kinases. Am. J. Physiol. Lung Cell. Mol. Physiol. 296: L839–L848 [DOI] [PubMed] [Google Scholar]
- 44.Kapoor G. S., Zhan Y., Johnson G. R., O’Rourke D. M. 2004. Distinct domains in the SHP-2 phosphatase differentially regulate epidermal growth factor receptor/NF-kappaB activation through Gab1 in glioblastoma cells. Mol. Cell. Biol. 24: 823–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.