Abstract
Semaphorin 7A (Sema7A) is a membrane-associated/secreted protein that plays an essential role in connecting the vertebrate neuronal and immune systems. However, the role of Sema7A has not been elucidated in viral pathogenesis. In this study, we show that abrogation of Sema7A protects mice from lethal West Nile virus (WNV) infection. Mice lacking Sema7A showed increased survival, reduced viral burden, and less blood–brain barrier permeability upon WNV infection. Increased Sema7A levels were evident in murine tissues, as well as in murine cortical neurons and primary human macrophages upon WNV infection. Treatment with Sema7A Ab blocked WNV infection in both of these cell types. Furthermore, Sema7A positively regulates the production of TGF-β1 and Smad6 to facilitate WNV pathogenesis in mice. Collectively, these data elucidate the role of Sema7A in shared signaling pathways used by the immune and nervous systems during viral pathogenesis that may lead to the development of Sema7A-blocking therapies for WNV and possibly other flaviviral infections.
Introduction
West Nile virus (WNV) is a mosquito-borne flavivirus that can infect humans and cause fever, encephalitis, and death (1, 2). Treatment of WNV is supportive, because vaccines or therapeutics have not been approved for use in humans. A murine model characterized the kinetics of WNV, a neurotrophic virus that replicates in peripheral organs, crosses the blood–brain barrier (BBB), and infects the CNS, leading to death (3–6). Studies using genetically modified mice that lack specific immune molecules provided insight into the immunopathogenesis of WNV (7–13). However, studies that characterize molecules involved in cross-talk between the nervous and immune system upon WNV infection are lacking. Therefore, we undertook the current study to understand the molecular relationships between these two disparate systems to provide novel insights into WNV pathogenesis.
Semaphorins are a large family of phylogenetically conserved soluble and membrane-bound proteins that function in both the nervous and immune systems (14–18). Semaphorins are divided into eight classes based on sequence similarities and distinct structural features (19, 20). Class 1 and 2 semaphorins are found in invertebrates (14–18), class 3 to 7 semaphorins are found in vertebrates, and class 8 semaphorins are found in viruses (16, 19). The characteristic feature of all semaphorins is the presence of the ∼500-aa Sema domain at the N terminus (16, 19). The Sema domain is implicated in stimulating various signaling pathways in both nervous and immune systems (14–18). Numerous studies have elucidated that semaphorins act as axon-guidance molecules and are involved in functions ranging from axon pruning to synaptic formation, specificity, and plasticity (14, 19–22). Semaphorins are also implicated in the regulation of blood vessel development, modulation of the immune system, regulation of organogenesis, angiogenesis, apoptosis, and neoplasia (19, 20, 23–25). Abnormalities of semaphorins are associated with tumor progression and neurologic diseases (19, 20).
Semaphorin 7A (Sema7A), also called CDw108, was identified in a search for vertebrate homologs of viral semaphorins (16, 26, 27). Sema7A is expressed broadly by lymphoid cells, myeloid cells, bone cells, the nervous system, epidermal keratinocytes, fibroblasts, and endothelial cells of blood vessels (25, 28, 29). In addition, human T lymphocytes and NK cells express Sema7A at high levels (30). Sema7A is unique among the semaphorins for two reasons: it is the only member of the family that is GPI linked, and it enhances axonal growth and proper axon track formation during embryonic development (19, 20, 23, 27). Mice deficient in Sema7A show diminished axonal tracts, which can be restored by treatment with Sema7A to increase axonal growth (20, 31). Sema7A can induce monocyte chemotaxis and cytokine production (25) and is a negative regulator of T cell responses (23). The effects of Sema7A in both the nervous and immune system are believed to be mediated via at least two receptors: plexin C1 and β1 integrin (17, 20, 24). However, the significance of these interactions in various tissues is poorly understood.
DNA viruses that infect humans, such as vaccinia and alcelaphine herpesvirus, encode secreted semaphorin A39R and Alcelaphine herpes virus (AHV)-sema (16, 26, 27). These viral semaphorins show high similarity to vertebrate Sema7A (16, 26, 27). Similarly to Sema7A, these semaphorins bind plexin-like receptors (32). Upon vaccinia and AHV infection, A39R and AHV-sema are believed to play immunomodulatory role(s) by mimicking Sema7A (16, 26, 27). Indeed, A39R induces CD54 expression and stimulates production of IL-6 and IL-8 by cultured monocytes (16, 33). No study has reported the presence of Sema7A homologs in RNA viral genomes. Therefore, we postulated that RNA viruses may use vertebrate Sema7A during infection and assessed the role of vertebrate Sema7A using the murine model of WNV infection.
Materials and Methods
Murine infection and survival studies
All WNV-challenge experiments were performed with 6–8-wk-old female mice in a Biosafety level 3 animal facility, according to the regulations of Yale University. C57BL/6 wild-type mice were purchased from Charles River Laboratories, and Sema7A knockout mice (34) were a kind gift from the Dr. Jack Elias laboratory (Yale University) and were bred at the Yale Animal Resources Center, under pathogen-free conditions, to the C57BL/6 background by backcrossing for 10 successive generations. For each infection study, mice groups were rigorously matched for age and sex. Groups of 10–20 mice were inoculated i.p. with a lethal dose of 103 PFU wild-type WNV strain CT 2741 (provided by J.F.A.) in PBS with 1% gelatin. Upon WNV infection, mice typically died at 6–14 d postinfection (p.i.) as the result of CNS invasion by the virus. Mice were monitored daily for WNV-associated clinical signs (including lethargy, anorexia, and difficulty ambulating), as well as for survival until days 21–25 p.i., as described (35). Mice survival data summarize the results of two independent experiments. Surviving mice were either euthanized to end the experiment or, in selected experiments, mice were euthanized for harvesting of tissues (brain, blood, and spleen). For Ab-protection studies, mice were inoculated i.p. with 100 μg Sema7A mAb or the isotype-matched negative control rat IgG Ab (obtained from R&D Systems) 1 d prior to WNV challenge; a second dose of either of these Abs was given at 2 d p.i. All mice experiments were approved and performed in accordance with regulations of the Institutional Animal Care and Use Committee at Yale University.
Infection of in vitro cell cultures
Human HEK 293 and mouse cerebrospinal microvascular endothelial cell lines were purchased from the American Type Culture Collection and propagated according to their protocols. Primary cultures of cortical neurons were isolated and established from the brains of embryonic day-16 C57BL/6 or Sema7A-deficient mouse embryos (obtained from pregnant female mice). Primary cultures of neurons and other cell types were seeded on 6- or 12-well plates at cell densities of 2 × 105 or 105 cells/ml, respectively; incubated for 24 h at 37°C; and infected with WNV at multiplicity of infection (MOI) of 2. Cell lysates were collected for analysis by quantitative RT-PCR (Q-PCR). For Sema7A Ab in vitro cell line-protection assays, 2.5, 5, 10, or 20 μg Sema7A mAb or the isotype-matched negative control rat IgG Ab was treated for 2 h prior to WNV infection at an MOI of 1. Primary cultures of neurons or cerebrospinal microvascular cells were harvested in duplicates on days 1–3 for extraction of total RNA and Q-PCR. For silencing studies, small interfering RNAs (siRNAs) for TGF-β1, Smad6, or control (scrambled) were obtained from Santa Cruz Biotechnologies. Human HEK 293 cells were transfected with the respective siRNA, per the manufacturer’s instructions. Cells were infected with WNV at an MOI of 2 and collected in duplicate from days 1–3 for total RNA extraction, followed by Q-PCR and Western blot analysis.
Human macrophage isolation and Sema7A Ab-protection assays
Heparinized blood from healthy human donors (Subjects A–D), with no acute illness and not taking antibiotics or nonsteroidal anti-inflammatory drugs, was obtained from the blood bank in New York City, as described (36). Human PBMCs were isolated using Ficoll-Hypaque (GE Healthcare) density-gradient centrifugation and suspended in RPMI 1640 medium containing 10% FBS, 2 mM glutamine, 100 U/ml penicillin, and 1000 μg/ml streptomycin (Invitrogen). Cells were plated at 5 × 106 PBMCs/well in six-well plates (BD Falcon), and nonadherent cells were removed after 2 h. Adherent cells were cultured for 7 d and washed once with prewarmed fresh medium. Differentiated macrophages were treated with 2.5, 5, 10, or 20 μg Sema7A mAb or the isotype-matched negative control rat IgG Ab for 2 h prior to WNV infection at an MOI of 1. Macrophages were then harvested in duplicates on days 1–3 for extraction of total RNA, followed by Q-PCR.
Q-PCR analysis
To determine the viral burden and expression of Sema7A, TGF-β1, SMAD genes, and several cytokine mRNA levels, total RNA was extracted from either frozen tissues or cell cultures using an RNeasy Mini kit (QIAGEN). cDNA was synthesized using the iScript cDNA kit (Bio-Rad). Q-PCR was performed using previously published primers for WNV E, TNF-α, IL-6, IL-12p40, IFN-α, IFN-β, TGF-β1, and the SMAD genes (7, 34, 35). The mRNA levels of these genes were normalized to β-actin mRNA levels. The ratio of WNV E gene copy/β-actin gene copy was used as an index to determine the infection rate of each sample.
Western blotting
Total brain lysates or HEK 293 cells were resuspended in SDS sample buffer containing 2-ME, boiled for 5 min, and resolved on a 4–15%-gradient SDS-PAGE gel. Blots were probed with specific Abs (Sema7A Ab used in Fig. 1D was obtained from Abcam [Cambridge, MA] and Abs against SMAD 2 or 6 used in Fig. 6G were obtained from Cell Signaling Technologies), followed by the respective HRP-conjugated secondary Abs (obtained from Sigma, St. Louis, MO) and detected with an ECL system from GE Healthcare. Quantification of Western blots was performed as described (37).
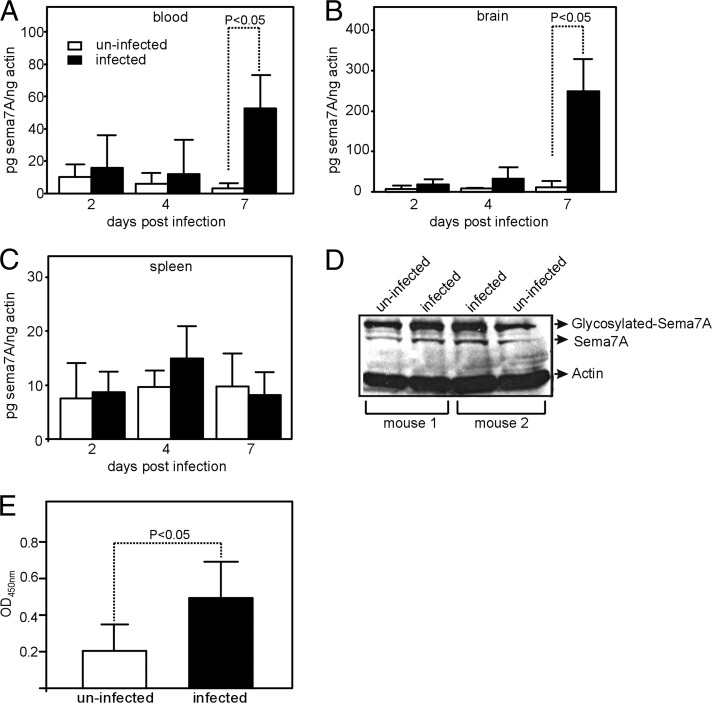
FIGURE 1.
WNV induces Sema7A expression in mice. Q-PCR results showing expression levels of sema7A mRNA in blood (A), brain (B), and spleen (C) on days 2, 4, and 7 in uninfected and WNV-infected mice (n = 5 mice per group and per data point). Levels of sema7A mRNA were normalized to the levels of actin mRNA. Error bars represent + SD from the mean value. Statistical significance was calculated using the Student t test. (D) Immunoblotting results showing expression of Sema7A in brain tissue lysates from uninfected and infected (day-7 p.i.) mice. Levels of total actin were used as loading control. Representative gel images from two independent experiments are shown. (E) ELISA to quantify total amount of Sema7A in uninfected and infected (day 7 p.i.) mice brain tissue lysates used in (D). Data from two independent experiments are shown in (A–C) and (E); Q-PCR was performed in triplicate.
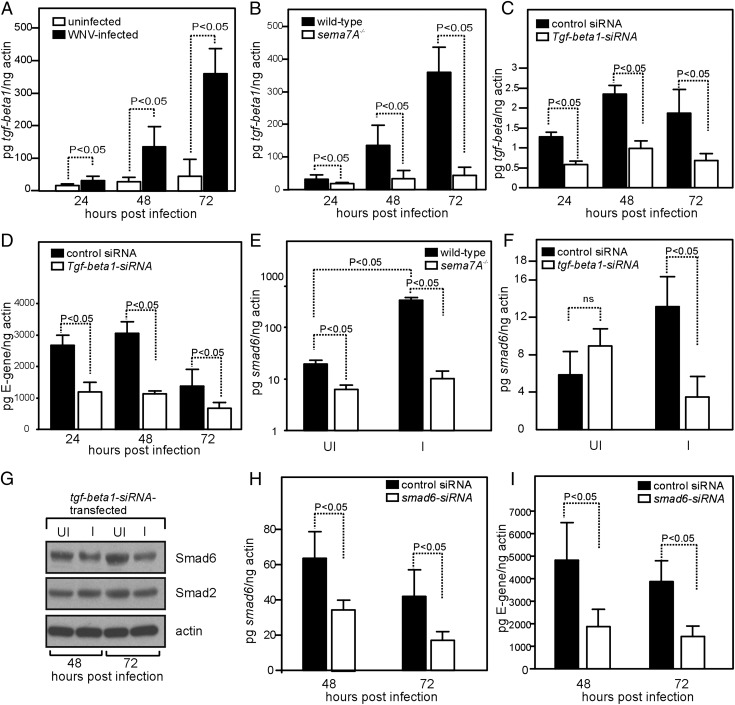
FIGURE 6.
Sema7A facilitates WNV pathogenesis through TGF-β1/Smad6 signaling. (A) Q-PCR results showing expression of tgf-beta1 mRNA levels at 24, 48, and 72 h p.i. with WNV in cortical neurons isolated from wild-type mice. Levels of tgf-beta1 mRNA were normalized to the levels of actin mRNA. (B) Expression of tgf-beta1 mRNA levels at 24, 48, and 72 h p.i. with WNV in cortical neurons isolated from wild-type or sema7A−/− mice. (C) Expression of tgf-beta1 mRNA at 24, 48, and 72 h p.i. with WNV in HEK 293 cells transfected with tgf-beta1 or control siRNA. (D) Kinetics of WNV infection in HEK 293 cells transfected with tgf-beta1 or control siRNA at 24, 48, and 72 h p.i. (E) Q-PCR results showing smad6 mRNA levels in cortical neurons isolated from wild-type or sema7A−/− mice at 48 h p.i. with WNV. (F) Q-PCR results showing expression of smad6 mRNA levels at 72 h p.i. with WNV in HEK 293 cells transfected with tgf-beta1 or control siRNA. (G) Immunoblot showing levels of Smad6 and Smad2 in HEK 293 cells transfected with tgf-beta1 siRNA at 48 and 72 h p.i. with WNV. Actin levels served as loading control. Representative gel images from two independent experiments are shown. Q-PCR results showing expression of smad6 mRNA (H) or the kinetics of WNV infection (I) at 48 and 72 h p.i. in HEK 293 cells transfected with smad6 or control siRNA. All data are representative of results obtained from two independent experiments, performed in triplicate. Error bars represent + SD from the mean value. Statistical significance was calculated using the Student t test.
Confocal microscopy
Cortical neurons were isolated from the brains of embryonic day-16 C57BL/6 mouse embryos and infected with WNV (MOI 2). Twenty-four hours p.i., neurons were fixed with 4% paraformaldehyde and processed for immunofluorescence using Sema7A Ab (Abcam), followed by treatment with FITC-conjugated secondary Ab (Sigma). WNV was detected with anti-WNV Ab (Chemicon, Billerica, MA), followed by treatment with tetramethyl rhodamine isothiocyanate-conjugated secondary Ab (Sigma). Confocal microscopy was performed, as described (35).
ELISA
ELISA was performed to detect Sema7A protein levels in the total lysates prepared from uninfected or WNV-infected wild-type mice brain tissue. Ten micrograms (100 μl) of total lysates from uninfected or WNV-infected wild-type mice brain tissue was coated onto ELISA plates (Nunc, Rochester, NY) and incubated at 4°C overnight. Following incubation, wells were blocked and incubated with Sema7A Ab diluted in PBS-Tween 20 (0.05%) buffer. Wells were then incubated with secondary Ab conjugated to HRP, followed by treatment with TMB Microwell Peroxidase (KPL) for color development. The reactions were stopped after 15 min using TMB Stop Solution (KPL), and OD was read at 450 nm.
Statistical analysis
Error bars represent mean (+ SD) values. We used a log-rank test (Prism 4; GraphPad Software) to assess the statistical differences between the survival rates. For calculating statistical significance between two means, nonpaired two-tailed Student t test was used. A p value < 0.05 was considered statistically significant in our analyses.
Results
Sema7A is upregulated upon WNV infection
Sema7A functions in the nervous and immune systems (14, 16–18). Therefore, we analyzed its role during neurotrophic WNV infection. We first determined whether WNV influences sema7A expression in mice. RNA samples were extracted from different tissues of mice infected with WNV on days 2, 4, and 7 p.i. Q-PCR showed that sema7A mRNA levels were significantly upregulated at days 6 and 7 p.i. in blood (p < 0.05) and brain (p < 0.05) compared with the uninfected controls (Fig. 1A–C, Supplemental Fig. 1A). Immunoblots further showed that Sema7A protein (∼75 kDa) levels in the brain were induced 2–3-fold at day 7 p.i. (Fig. 1D), the time point at which WNV invades the CNS. Higher levels of the glycosylated form of Sema7A protein (∼100 kDa) were also evident in WNV-infected brain samples in comparison with controls (Fig. 1D). ELISA confirmed a significant increase (p < 0.05, 2–3-fold) in Sema7A protein expression in the brain at day 7 p.i. (Fig. 1E). Together, these data show that Sema7A mRNA and protein levels are induced upon WNV infection.
Sema7A deficiency decreases murine lethality from WNV infection
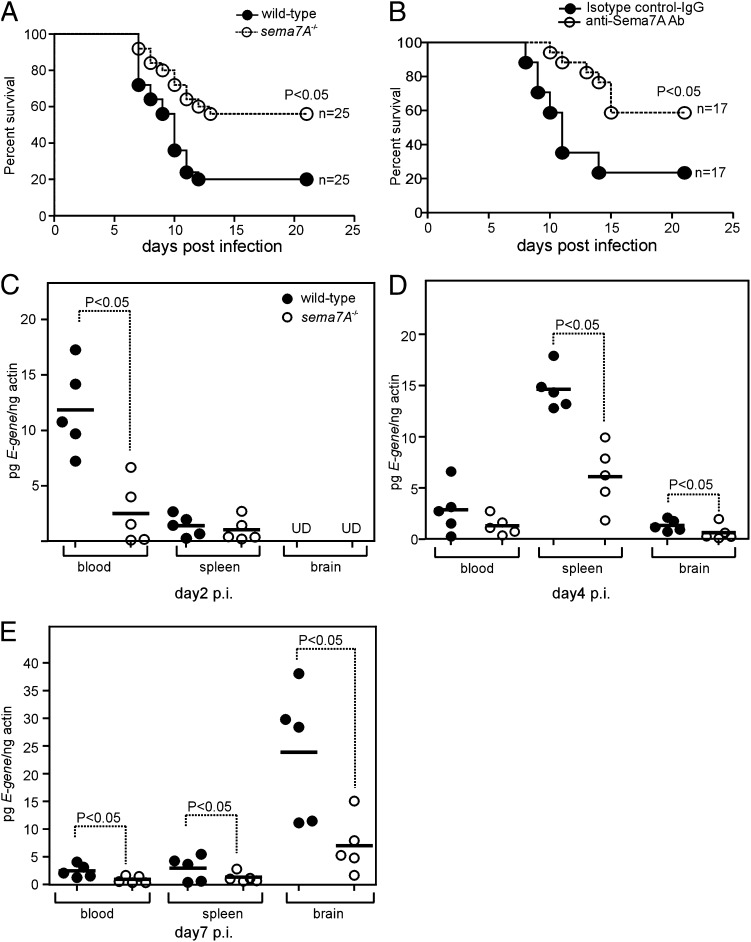
Because Sema7A expression was dramatically upregulated upon WNV infection, we characterized its role in vivo in the absence of Sema7A by infecting either Sema7A-deficient (sema7A−/−) or Sema7A Ab-treated mice. When animals were infected with 1000 PFU of WNV, survival rates of sema7A−/− mice were considerably higher compared with the wild-type controls (Fig. 2A). sema7A−/− mice were significantly more resistant to death caused by WNV than were wild-type animals (56 versus 20% survival rate, p < 0.05, log-rank test) (Fig. 2A). Similarly, Sema7A Ab-treated mice showed substantial resistance to the lethality caused by WNV compared with IgG isotype-matched negative control Ab-treated mice (60 versus 20% survival rate, p < 0.05) (Fig. 2B). Collectively, these results show that Sema7A is an important factor in murine resistance to WNV infection.
FIGURE 2.
Abrogation of Sema7A renders resistance to WNV-induced lethality in mice. (A) Survival of wild-type and sema7A−/− mice challenged i.p. with 103 PFU of wild-type WNV. Survival was recorded daily until day 21 p.i.; data were pooled from two independent experiments and represent a total of 25 animals/group. (B) Survival of C57BL/6 wild-type mice inoculated with WNV and treated with anti-Sema7A mAb or isotype-matched negative control Ab on days −1 and +2. Data are pooled from two independent experiments and represent a total of 17 animals/group. Statistical significance (p < 0.05) was calculated using Prism 4 software. WNV is less neuroinvasive in sema7A−/− mice than in wild-type mice. Viral loads in selected tissues (blood, spleen, and brain) of wild-type and sema7A−/− mice inoculated i.p. with 103 PFU of WNV. Each group contained fie animals examined at day 2 (C), day 4 (D), or day 7 (E) after viral challenge. Data are representative of results obtained in two independent experiments, performed in triplicate. Horizontal bars represent mean of the values. Statistical significance was calculated using the Student t test. UD, Undetectable.
Sema7A influences WNV neuroinvasion
During the initial stage of murine infection (days 2–4 p.i.), WNV replicates in peripheral organs (3–6). By day 5 p.i., WNV crosses the BBB and invades the CNS, leading to death of the animals starting at day 7 p.i (3–6, 38). Because Sema7A deficiency resulted in enhanced survival after WNV infection in mice, we analyzed the kinetics of WNV infection to determine whether this was due to reduced viral invasion into the brain. Indeed, when we quantified WNV E-gene transcripts normalized to murine β-actin by Q-PCR, the sema7A−/− mice had a significantly reduced viral burden in the blood at day 2 p.i. compared with the control mice (∼5-fold, p < 0.05; Fig. 2C). Decreased viral loads were also evident in both spleen (2–3-fold, p < 0.05) and brains (2–3-fold, p < 0.05) of sema7A−/− mice at day 4 p.i. compared with the controls (Fig. 2D). At days 6 and 7 p.i., sema7A−/− mice had a significantly reduced (2–3-fold, p < 0.05) viral burden compared with control mice in all three tissues (Fig. 2E, Supplemental Fig. 1B). These results suggest that the increased survival of sema7A−/− mice is due to both diminished viremia during early infection and decreased viral neuroinvasion.
Sema7A-deficient mice infected with WNV have less inflammation
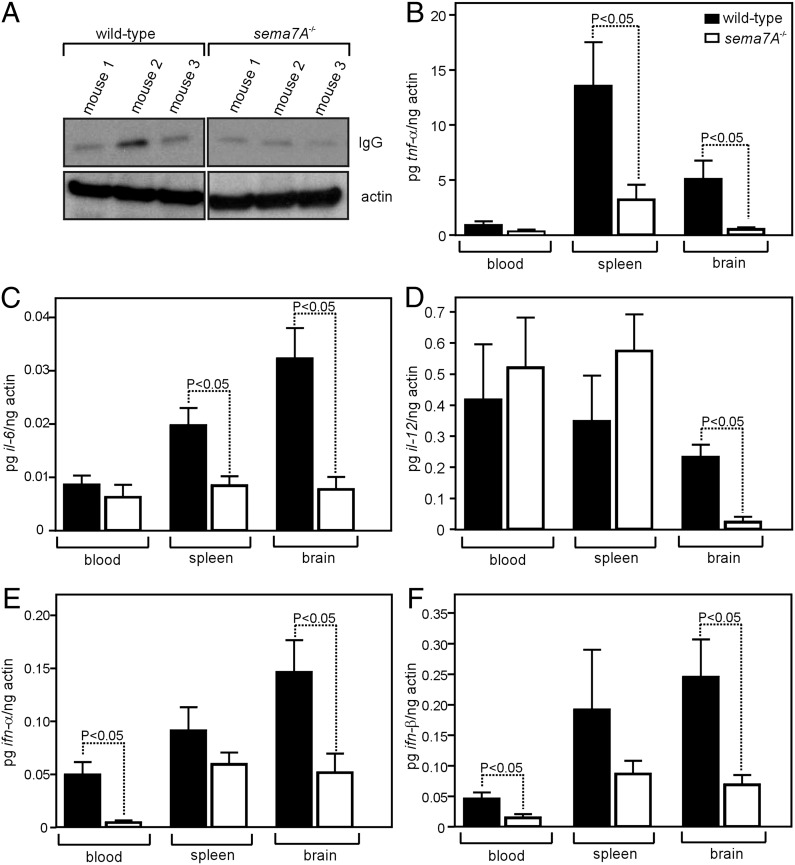
To identify a mechanism for reduced neuroinvasion of WNV in sema7A−/− mice, we assessed BBB permeability by quantifying IgG levels (indicative of BBB permeability) in the brain tissue. Immunoblots showed that sema7A−/− mice had less IgG in the brain compared with the control mice, indicating less BBB permeability (Fig. 3A, Supplemental Table I). Increased production of inflammatory and antiviral cytokines, such as TNF-α, IL-6, IL-12, IFN-α, and IFN-β, facilitate WNV neuroinvasion into the CNS by altering the BBB permeability (3, 7). Therefore, we measured mRNA levels of these cytokines in sema7A−/− mice compared with the controls. Consistent with the measurement of viral loads (Fig. 2E) and BBB permeability (Fig. 3A), mRNA levels of TNF-α, IL-6, IL-12, IFN-α, and IFN-β cytokines were significantly decreased in brain tissue of sema7A−/− mice at day 7 p.i. compared with the control mice (3–8-fold, p < 0.05, Fig. 3B–F). In addition, blood from sema7A−/− mice showed reduced mRNA levels of IFN-α (9-fold, p < 0.05) and IFN-β (3-fold, p < 0.05) compared with controls at day 7 p.i (Fig. 3E, 3F), suggesting an important role for Sema7A in facilitating the WNV infection from blood to brain. Furthermore, at day 7 p.i., TNF-α and IL-6 levels were significantly diminished (4- and 2-fold, respectively, p < 0.05) in spleens of sema7A−/−mice (Fig. 3B, 3C). Overall, these results show that Sema7A contributes to cytokine responses that are associated with inflammation during WNV infection.
FIGURE 3.
sema7A−/− mice have reduced cytokine responses that are associated with inflammation during WNV infection. (A) Immunoblot of IgG (H chain) in WNV-infected wild-type or sema7A−/− mice brain tissue lysates. Actin served as the loading control. Representative gel images from two independent experiments are shown. TNF-α (B)), IL-6 (C), IL-12 (D), IFN-α (E), and IFN-β (F) mRNA levels in the blood, spleen, and brain of WNV-infected wild-type or sema7A−/− mice at day 7 p.i. Both groups of mice were infected with 103 PFU of WNV. Results are from five mice/group and two independent experiments, performed in triplicate. Statistical significance (p < 0.05) was calculated using the Student t test. Error bars represent + SD from the mean value.
Abrogation of Sema7A blocks WNV infection of cortical neurons in vitro
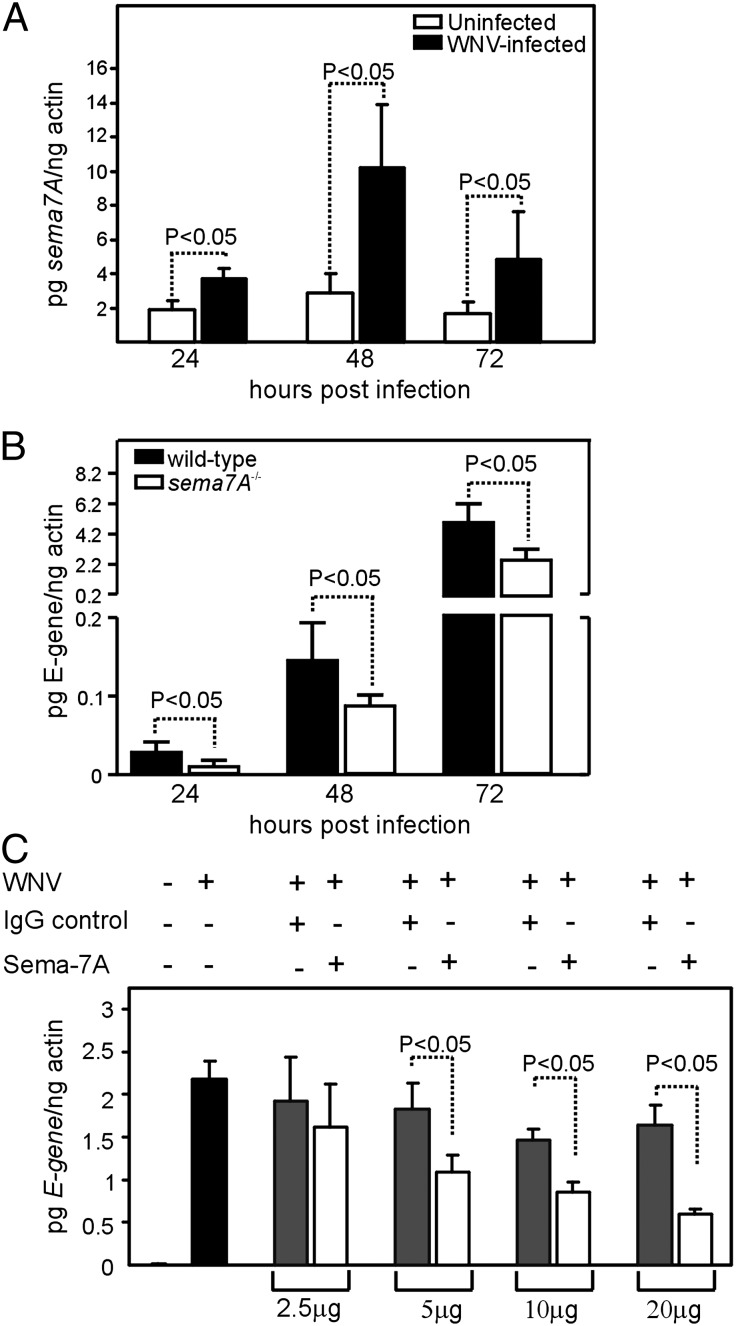
To assess the role of sema7A in viral infection of neural tissue, we assessed viral replication in cortical neurons, which is a pathogenic feature of WNV during CNS invasion (3–6, 39). We analyzed whether abrogation of Sema7A leads to altered viral replication by determining the sema7A mRNA levels in cortical neurons infected with WNV (35). Q-PCR results showed that sema7A mRNA levels were significantly elevated in WNV-infected cortical neurons compared with uninfected controls at all time points (24, 48, and 72 h) p.i. (2–5-fold, p < 0.05, Fig. 4A). Coassociation of Sema7A with WNV was also evident in cultured cortical neurons (Supplemental Fig. 1C). We then determined whether the absence of Sema7A has any impact on viral infection in cortical neurons. Cortical neurons from wild-type and sema7A−/− mice were isolated, and WNV infection kinetics were analyzed at different time points (24, 48, and 72 h) p.i. (Fig. 4B). The results showed a significantly reduced viral burden in cortical neurons isolated from sema7A−/− mice compared with cortical neurons isolated from wild-type mice at all time points (p < 0.05, Fig. 4B). We next determined whether treatment of cortical neurons with Sema7A Ab leads to reduced viral replication. Uninfected cortical neurons isolated from wild-type mice were treated with either Sema7A or isotype-matched negative control Abs for 2 h, followed by infection with WNV. Viral replication was analyzed 48 h p.i. Q-PCR showed that neurons treated with 5–20 μg of Sema7A mAb showed significantly (p < 0.05) reduced WNV E-gene transcripts compared with the control (Fig. 4C). Similar results were obtained when we performed experiments with a mouse cerebral microvascular endothelial in vitro cell line (Supplemental Fig. 1D). These results support a role for Sema7A in neuroinvasion and replication of WNV in the CNS.
FIGURE 4.
Abrogation of Sema7A blocks WNV infection of cortical neurons in vitro. (A) Q-PCR analysis showing expression of sema7A mRNA levels at 24, 48, and 72 h p.i. with WNV in cortical neurons isolated from wild-type mice. Uninfected cortical neurons were used as controls. Levels of sema7A mRNA were normalized to the levels of actin mRNA. (B) Kinetics of WNV infection in cortical neuronal cells isolated from wild-type or sema7A−/− mice at 24, 48, and 72 h p.i. (C) Q-PCR analysis showing WNV burden in Sema7A mAb-treated or isotype-matched negative control Ab-treated cortical neurons at 48 p.i. All results are from three independent experiments, performed in triplicate. Error bars represent + SD from the mean value. Statistical significance was calculated using the Student t test.
Abrogation of Sema7A blocks WNV infection of human macrophages in vitro
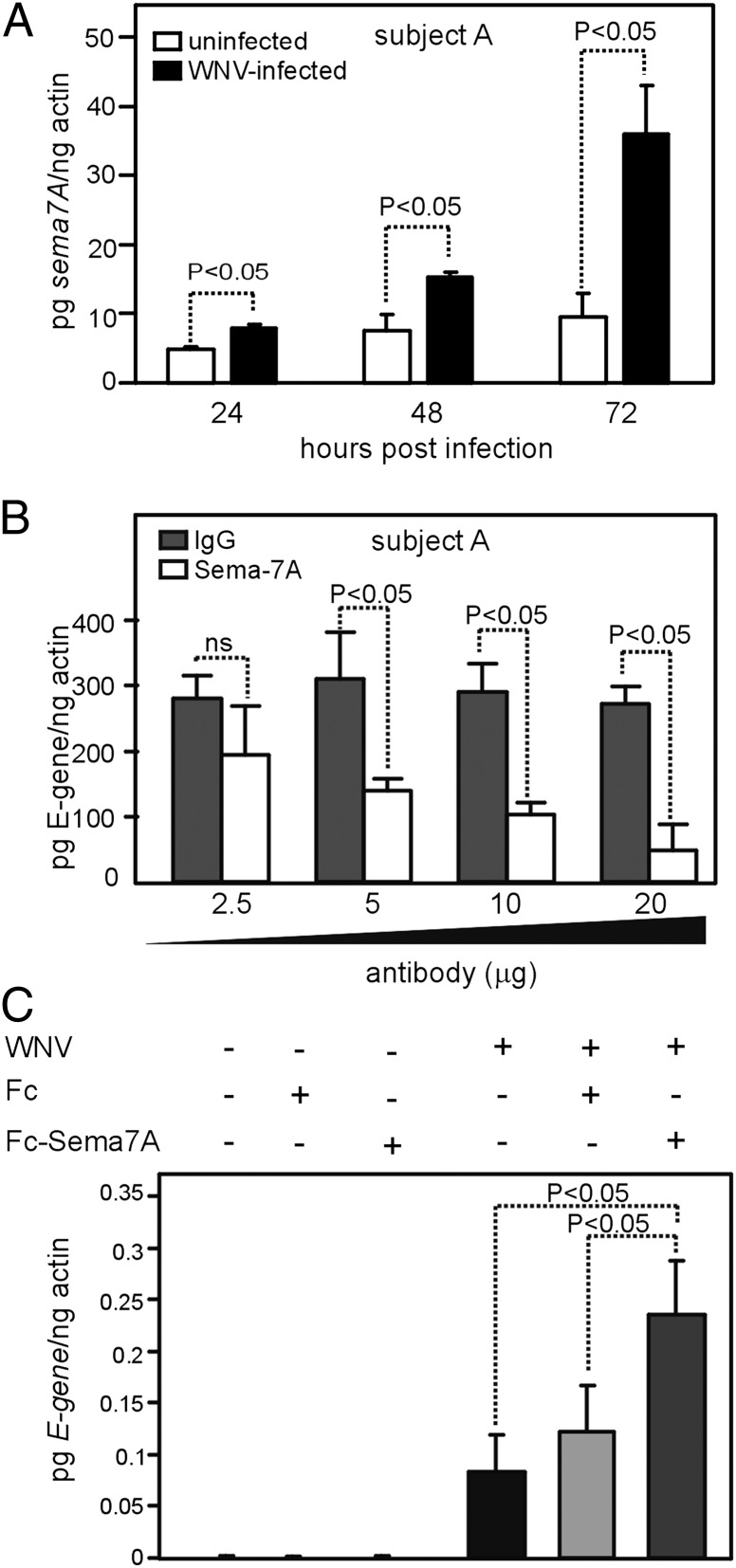
In addition to the role for Sema7A in enhancing WNV replication in neurons, we analyzed whether it is involved in immune-mediated pathways. Therefore, we investigated whether abrogation of Sema7A in human macrophages also influences WNV replication. PBMCs were isolated from healthy human volunteers and allowed to differentiate into macrophages (36). We first analyzed sema7A mRNA levels in macrophages infected with WNV and compared the expression levels with uninfected controls. We found that sema7A mRNA levels were significantly elevated in WNV-infected human macrophages isolated from all four volunteers compared with uninfected controls at all time points (24, 48, and 72 h) p.i. (3–8-fold, p < 0.05, Fig. 5A, Supplemental Fig. 2A–C). We determined whether treatment of human macrophages with Sema7A Ab has any influence on viral replication. Uninfected human macrophages were treated with either Sema7A mAb or isotype-matched negative control Ab for 2 h, followed by infection with WNV. The Q-PCR results showed that macrophages from all four volunteers treated with 10–20 μg of Sema7A Ab showed significantly reduced WNV E-gene transcripts compared with the controls at 48 h p.i. (p < 0.05, Fig. 5B, Supplemental Fig. 2D–F). We next determined whether overexpression of Sema7A influences WNV replication in an in vitro human cell line. We used HEK 293 cells because of their extreme transfection efficiency. Constructs carrying Sema7A-Fc and Fc alone, which was used previously (23), were first transfected into HEK 293 cells and infected with WNV. At 24 h post-WNV infection, HEK 293 cells transfected with Sema7A-Fc showed significantly increased WNV E-gene transcripts compared with cells transfected with Fc control (2–3-fold, p < 0.05, Fig. 5C). Taken together, these results further validate the role of Sema7A as a host factor that enhances viral replication and supports the development of Sema7A as a therapeutic target for WNV infection.
FIGURE 5.
Abrogation of Sema7A blocks WNV infection of human macrophages. (A) Q-PCR analysis showing expression of sema7A mRNA levels at 24, 48, and 72 h p.i. with WNV in macrophages isolated from a representative healthy human volunteer (Subject A). Uninfected macrophages were used as controls. Levels of sema7A mRNA were normalized to the levels of actin mRNA. (B) Q-PCR analysis showing WNV burden in Sema7A mAb-treated or isotype-matched negative control Ab-treated human macrophages from Subject A at 48 h p.i. (C) Q-PCR analysis showing viral burden in Fc or Fc-Sema7A–transfected HEK 293 cells. Infected, but untransfected, cells were used as controls. Data are representative of results performed in triplicates. Error bars represent + SD from the mean value. Statistical significance was calculated using the Student t test.
Sema7A facilitates WNV pathogenesis through TGF-β1/Smad6 signaling
To dissect the mechanisms underlying Sema7A’s enhancement of viral replication, we assessed signaling pathways involved in Sema7A activity. Because Sema7A signaling in both the nervous and immune systems is believed to be mediated via plexin C1 (32), we first analyzed whether infection of WNV induces plexin C1 mRNA expression in wild-type mice. We found no significant increase in plexin C1 mRNA expression upon WNV infection (Supplemental Fig. 3A) and found no differences in plexin C1 levels from sema7A−/− mice (Supplemental Fig. 3B). A recent study showed that Sema7A plays a critical role in TGF-β1–induced pulmonary fibrosis (34). To gain further insight into the involvement of Sema7A in WNV pathogenesis, we tested the hypothesis that Sema7A uses TGF-β1 signaling to mediate WNV pathogenesis. When we analyzed tgf-beta1 mRNA levels in cortical neurons p.i. with WNV, tgf-beta1 mRNA levels were significantly elevated in WNV-infected cortical neurons compared with uninfected controls at all time points (24, 48, and 72 h) p.i. (Fig. 6A). We then determined whether the absence of Sema7A has any impact on tgf-beta1 mRNA levels upon WNV infection in cortical neurons. The results showed significantly reduced levels of tgf-beta1 mRNA in cortical neurons isolated from sema7A−/− mice compared with controls (Fig. 6B). Because these results suggested involvement of TGF-β1 in Sema7A-mediated WNV pathogenesis, we analyzed the kinetics of WNV infection upon silencing of tgf-beta1 expression using HEK 293 cells. The expression of tgf-beta1 mRNA was also induced upon WNV infection in HEK 293 cells (Supplemental Fig. 3C) and was significantly reduced in tgf-beta1 siRNA-transfected cells compared with control siRNA (scrambled)-transfected cells at different time points (Fig. 6C). In addition, tgf-beta1–silenced cells showed a significant reduction in viral burden that was also evident at all time points (24, 48, and 72 h) p.i. in tgf-beta1–silenced cells compared with the control siRNA-transfected cells (Fig. 6D, Supplemental Fig. 3D). These studies highlight a previously unrecognized role for TGF-β1 in enhancing WNV infection.
TGF-β1 signals through Smad transcription factors that mediate various cellular events (40, 41). Therefore, we determined mRNA levels of several smads upon WNV infection in cortical neurons. Only smad6 was significantly elevated upon WNV infection in cortical neurons isolated from wild-type animals (Fig. 6E). We found no Sema7A-dependent differences in the expression of other smads upon WNV infection (Supplemental Fig. 3E). To test whether Sema7A mediates signaling through smad6 during WNV infection, we analyzed smad6 expression in cortical neurons from sema7A−/− mice. Results showed that, in the absence of Sema7A, no induction of smad6 was observed in sema7A−/− cortical neurons infected with WNV at 48 h p.i. (Fig. 6E). Reduced levels of smad6 mRNA were also evident in HEK 293 cells transfected with tgf-beta1 siRNA at 72 h p.i. with WNV compared with the control siRNA-transfected cells (Fig. 6F). In addition, we found that HEK 293 cells transfected with tgf-beta1 siRNA showed reduced Smad6 protein levels upon WNV infection compared with the uninfected controls at both 48 and 72 h p.i. (Fig. 6G, Supplemental Table I). To examine whether Smad6 controls viral replication, we analyzed WNV infection upon silencing of smad6 expression in HEK 293 cells. Q-PCR analysis showed a significant reduction in smad6 mRNA levels in smad6 siRNA-transfected cells (Fig. 6H). A significant reduction in viral burden was also evident at tested time points (48 and 72 h p.i.) in smad6-silenced cells compared with the control siRNA-transfected cells (Fig. 6I). Taken together, these results suggest that Sema7A contributes to WNV pathogenesis through the TGF-β1/Smad6–signaling pathway.
Discussion
Viruses, such as vaccinia and alcelaphine herpesvirus, encode semaphorin A39R and AHV-sema, which achieve immunomodulatory functions by mimicking vertebrate Sema7A (16, 26, 27, 33). The role of vertebrate Sema7A in viral pathogenesis has not been directly addressed. In this article, we describe a crucial role for Sema7A in WNV pathogenesis. Our finding that abrogation of Sema7A provides resistance to WNV infection in mice is supported by several lines of evidence. First, the upregulation of Sema7A upon WNV infection in blood and brain tissues suggests its role both in the peripheral organs and CNS. Second, mice that lack Sema7A had a reduced viral burden in the periphery during early infection and reduced levels in the brain during late infection, suggesting a role for Sema7A both in the early and late stages of WNV pathogenesis. Third, the level of TNF-α, which is important for WNV crossing the BBB, was significantly reduced in mice that lack Sema7A. Fourth, in the absence of Sema7A, IgG leakage into the brain was reduced upon WNV infection, implicating a role for Sema7A in protecting BBB permeability. Last, in the absence of Sema7A, viral replication in cortical neurons was significantly reduced, suggesting a role for Sema7A in brain cells.
Sema7A is a potent stimulator of monocytes and neutrophils (25). Studies demonstrated that concentrations as low as 1 pM of Sema7A can stimulate monocyte cytokine production and chemotaxis. Indeed, the addition of Sema7A influenced monocyte chemotaxis 1000 times more efficiently than did MCP-1, and it influenced neutrophil chemotaxis 100 times better than did IL-8 stimulation (25). Sema7A is abundantly expressed in monocytes, neutrophils, and other lymphoid cells (25, 28, 29). A recent study showed a biphasic response of PMNs to WNV infection; they serve as a reservoir for WNV replication and dissemination in the early stages of infection and later contribute to WNV clearance (42). The reduced viremia in the early stage of WNV infection in Sema7A-deficient mice (Fig. 2) suggests that Sema7A may participate in the replication of WNV at early stages in these cells and at later stages in the dissemination of WNV to the peripheral tissues and brain. It is possible that the absence of Sema7A results in slower recruitment of neutrophils to the site of WNV infection, which may affect PMN-dependent viral replication and dissemination. Peripheral blood monocytes are relatively immature precursor cells that can differentiate into macrophage or dendritic cells based on environmental stimuli (43). Sema7A induces the maturation of monocytes toward dendritic cell morphology (43). Studies show that TLR-3 binding to double-stranded viral RNA inside infected dendritic cells leads to the production of inflammatory cytokines, such as TNF-α (7). Our findings that Sema7A-deficient mice infected with WNV have lower production of TNF-α and less BBB permeability (Fig. 3) support our hypothesis that, in the absence of Sema7A, there might be one possible antiviral mechanism that reduces maturation of monocytes to dendritic cells. The reduction in dendritic cell maturation may lead to decreased TLR-3–mediated recognition of viral RNA and production of TNF-α that subsequently leads to less WNV crossing the BBB. We propose that Sema7A acts upstream of the host inflammatory reaction that results from WNV invasion.
TGF-β1 is synthesized as precursor that is complexed with latent TGF-β–binding proteins and is activated by a variety of mechanisms that include integrin binding (41, 44). Activated TGF-β1 binds to heterodimeric receptors containing types I and II receptor components that have tyrosine kinase activity (41, 44). Our studies demonstrate that mice lacking Sema7A exhibited a significant reduction in TGF-β1 mRNA levels. The reduced TGF-β1 mRNA levels correlated with reduced viral infection in cortical neurons isolated from Sema7A-deficient animals (Fig. 6). Kang et al. (34) showed that TGF-β1 induces the expression of Sema7A in a pulmonary fibrosis model. A more conservative interpretation of the possible mechanisms is warranted, because our study proposes that Sema7A contributes to WNV infection through TGF-β1/Smad–signaling pathways. It is possible that Sema7A induces TGF-β1, which, in turn, may induce Sema7A expression during WNV infection. A feedback mechanism was identified for semaphorin 3E signaling (45). We found that sema7A and tgf-β1 mRNA levels are coordinately induced in mouse cortical neurons during WNV infection (Figs. 4A, 6A). Although, we found that Sema7A deficiency abrogates tgf-β1 mRNA levels, we did not observe any changes in sema7A mRNA expression upon TGF-β1 silencing during WNV infection (data not shown). This role of Sema7A in TGF-β1 signaling and in virus infection suggests that these are two mechanisms that can both contribute to WNV pathogenesis. TGF-β1 signals via Smads, where Smad2 and Smad3 are phosphorylated by TGF-βR1 kinase. The phosphorylated Smad2/3 complex binds to Smad4 and translocates Smad4 to the nucleus (40, 41, 46). However, we did not observe any changes in the mRNA expression of either of these Smads upon WNV infection in murine cortical neurons (Supplemental Fig. 3E) or in WNV-infected mice lacking Sema7A (data not shown). There is increasing evidence for Smad2/3-independent pathways that mediate the effects of TGF-β1 (34, 40, 41, 46). Overexpression studies in mammalian cells elucidated that Smad6 associates with TGF-β1 type I receptor and inhibits Smad2 and Smad1 phosphorylation (46). This regulatory function of Smad6 may allow more precise regulation of TGF-β1–mediated Smad signaling (46). Based on our findings that WNV induces Smad6 expression and that the absence of Sema7A or TGF-β1 affected this induction, we hypothesize that Sema7A functions via TGF-β1/Smad6 signaling to contribute to WNV pathogenesis. The reduced WNV infection in Smad6-silenced cells further supports this hypothesis. Collectively, we propose that Sema7A deficiency may affect the ability of the TGF-β1/Smad6 pathway to influence cytokine production, antiproteases, transcription factors, and receptor components that contribute to WNV pathogenesis.
Several GPI-anchored proteins perform diverse roles in immunoregulation (47). GPI-anchored proteins occur in microdomains or “rafts” on the cell surface that can also be cleaved off from the cell surface by proteolytic cleavage, resulting in a secreted form of the proteins (48). Sema7A is a GPI-anchored protein that is released from the cell surface by proteolysis, suggesting its role in autocrine function (30, 49). Sema7A functions both as a cell surface and secreted molecule. Also, lipid rafts have been implicated in flavivirus entry into cells by the activation of PI3K/Akt signaling during the early stages of Japanese encephalitis virus infection in neural stem/progenitor cells (50). Sema7A plays a central role in a PI3K/PKB/AKT-dependent pathway that contributes to TGF-β1–induced fibrosis and modeling (34, 50). It is possible that initiation and propagation of the signaling events taking place during WNV pathogenesis occur in these specialized Sema7A-containing membrane regions. We postulate that, in its GPI-anchored form, Sema7A may facilitate viral entry on the cell surface (when it is linked to lipid rafts) either by directly binding to the virus or indirectly by forming a complex with other cell surface receptors. The fact that WNV infection in vitro is inhibited by the blockage of Sema7A further supports that it may be a receptor or coreceptor for WNV. In its secreted form, Sema7A may trigger downstream signaling in modulating the expression of TGF-β1/Smad6 and other inflammatory cytokines that contribute to WNV pathogenesis. With any of these models, the finding that abrogation of Sema7A results in increased resistance to WNV infection supports the role of Sema7A as a potential therapeutic target for disease caused by WNV.
Collectively, these data provide direct evidence for the involvement of Sema7A in WNV pathogenesis. Our results emphasize molecular mechanisms that WNV uses to establish and invade the brain, leading to the cause of lethal encephalitis. Because treatment with Sema7A Ab is efficacious in mice and provides protection in microvascular endothelial cells, murine cortical neurons, and human macrophages, our study opens up a promising area for the development of Sema7A-blocking therapies for WNV and possibly other flaviviral infections.
Supplementary Material
Acknowledgments
We thank Drs. Jack Elias and Bing Ma for providing sema7A−/− mice. We also thank Deborah Beck and Lida Yuan for technical assistance.
This work was funded by National Institutes of Health Grants AI 50031 and AI 070343.
The online version of this article contains supplemental material.
- AHV
- Alcelaphine herpes virus
- BBB
- blood–brain barrier
- MOI
- multiplicity of infection
- p.i.
- postinfection
- Q-PCR
- quantitative RT-PCR
- Sema7A
- semaphorin 7A
- siRNA
- small interfering RNA
- WNV
- West Nile virus.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Klee A. L., Maidin B., Edwin B., Poshni I., Mostashari F., Fine A., Layton M., Nash D. 2004. Long-term prognosis for clinical West Nile virus infection. Emerg. Infect. Dis. 10: 1405–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petersen L. R., Marfin A. A. 2002. West Nile virus: a primer for the clinician. Ann. Intern. Med. 137: 173–179 [DOI] [PubMed] [Google Scholar]
- 3.Diamond M. S., Klein R. S. 2004. West Nile virus: crossing the blood-brain barrier. Nat. Med. 10: 1294–1295 [DOI] [PubMed] [Google Scholar]
- 4.Lustig S., Olshevsky U., Ben-Nathan D., Lachmi B. E., Malkinson M., Kobiler D., Halevy M. 2000. A live attenuated West Nile virus strain as a potential veterinary vaccine. Viral Immunol. 13: 401–410 [DOI] [PubMed] [Google Scholar]
- 5.Samuel M. A., Diamond M. S. 2006. Pathogenesis of West Nile Virus infection: a balance between virulence, innate and adaptive immunity, and viral evasion. J. Virol. 80: 9349–9360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shrestha B., Gottlieb D., Diamond M. S. 2003. Infection and injury of neurons by West Nile encephalitis virus. J. Virol. 77: 13203–13213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang T., Town T., Alexopoulou L., Anderson J. F., Fikrig E., Flavell R. A. 2004. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat. Med. 10: 1366–1373 [DOI] [PubMed] [Google Scholar]
- 8.Arjona A., Foellmer H. G., Town T., Leng L., McDonald C., Wang T., Wong S. J., Montgomery R. R., Fikrig E., Bucala R. 2007. Abrogation of macrophage migration inhibitory factor decreases West Nile virus lethality by limiting viral neuroinvasion. J. Clin. Invest. 117: 3059–3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang P., Dai J., Bai F., Kong K. F., Wong S. J., Montgomery R. R., Madri J. A., Fikrig E. 2008. Matrix metalloproteinase 9 facilitates West Nile virus entry into the brain. J. Virol. 82: 8978–8985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brinton M. A. 2001. Host factors involved in West Nile virus replication. Ann. N. Y. Acad. Sci. 951: 207–219 [DOI] [PubMed] [Google Scholar]
- 11.Mashimo T., Lucas M., Simon-Chazottes D., Frenkiel M. P., Montagutelli X., Ceccaldi P. E., Deubel V., Guenet J. L., Despres P. 2002. A nonsense mutation in the gene encoding 2′-5′-oligoadenylate synthetase/L1 isoform is associated with West Nile virus susceptibility in laboratory mice. Proc. Natl. Acad. Sci. USA 99: 11311–11316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai J., Wang P., Bai F., Town T., Fikrig E. 2008. Icam-1 participates in the entry of west nile virus into the central nervous system. J. Virol. 82: 4164–4168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bai F., Town T., Qian F., Wang P., Kamanaka M., Connolly T. M., Gate D., Montgomery R. R., Flavell R. A., Fikrig E. 2009. IL-10 signaling blockade controls murine West Nile virus infection. PLoS Pathog. 5: e1000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolodkin A. L., Matthes D. J., O’Connor T. P., Patel N. H., Admon A., Bentley D., Goodman C. S. 1992. Fasciclin IV: sequence, expression, and function during growth cone guidance in the grasshopper embryo. Neuron 9: 831–845 [DOI] [PubMed] [Google Scholar]
- 15.Hall K. T., Boumsell L., Schultze J. L., Boussiotis V. A., Dorfman D. M., Cardoso A. A., Bensussan A., Nadler L. M., Freeman G. J. 1996. Human CD100, a novel leukocyte semaphorin that promotes B-cell aggregation and differentiation. Proc. Natl. Acad. Sci. USA 93: 11780–11785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lange C., Liehr T., Goen M., Gebhart E., Fleckenstein B., Ensser A. 1998. New eukaryotic semaphorins with close homology to semaphorins of DNA viruses. Genomics 51: 340–350 [DOI] [PubMed] [Google Scholar]
- 17.Suzuki K., Okuno T., Yamamoto M., Pasterkamp R. J., Takegahara N., Takamatsu H., Kitao T., Takagi J., Rennert P. D., Kolodkin A. L., et al. 2007. Semaphorin 7A initiates T-cell-mediated inflammatory responses through alpha1beta1 integrin. Nature 446: 680–684 [DOI] [PubMed] [Google Scholar]
- 18.Spriggs M. K. 1999. Shared resources between the neural and immune systems: semaphorins join the ranks. Curr. Opin. Immunol. 11: 387–391 [DOI] [PubMed] [Google Scholar]
- 19.Pasterkamp R. J., Kolodkin A. L. 2003. Semaphorin junction: making tracks toward neural connectivity. Curr. Opin. Neurobiol. 13: 79–89 [DOI] [PubMed] [Google Scholar]
- 20.Pasterkamp R. J., Peschon J. J., Spriggs M. K., Kolodkin A. L. 2003. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature 424: 398–405 [DOI] [PubMed] [Google Scholar]
- 21.Kolodkin A. L., Matthes D. J., Goodman C. S. 1993. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell 75: 1389–1399 [DOI] [PubMed] [Google Scholar]
- 22.Luo Y., Raible D., Raper J. A. 1993. Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell 75: 217–227 [DOI] [PubMed] [Google Scholar]
- 23.Czopik A. K., Bynoe M. S., Palm N., Raine C. S., Medzhitov R. 2006. Semaphorin 7A is a negative regulator of T cell responses. Immunity 24: 591–600 [DOI] [PubMed] [Google Scholar]
- 24.Delorme G., Saltel F., Bonnelye E., Jurdic P., Machuca-Gayet I. 2005. Expression and function of semaphorin 7A in bone cells. Biol. Cell 97: 589–597 [DOI] [PubMed] [Google Scholar]
- 25.Holmes S., Downs A. M., Fosberry A., Hayes P. D., Michalovich D., Murdoch P., Moores K., Fox J., Deen K., Pettman G., et al. 2002. Sema7A is a potent monocyte stimulator. Scand. J. Immunol. 56: 270–275 [DOI] [PubMed] [Google Scholar]
- 26.Comeau M. R., Johnson R., DuBose R. F., Petersen M., Gearing P., VandenBos T., Park L., Farrah T., Buller R. M., Cohen J. I., et al. 1998. A poxvirus-encoded semaphorin induces cytokine production from monocytes and binds to a novel cellular semaphorin receptor, VESPR. Immunity 8: 473–482 [DOI] [PubMed] [Google Scholar]
- 27.Xu X., Ng S., Wu Z. L., Nguyen D., Homburger S., Seidel-Dugan C., Ebens A., Luo Y. 1998. Human semaphorin K1 is glycosylphosphatidylinositol-linked and defines a new subfamily of viral-related semaphorins. J. Biol. Chem. 273: 22428–22434 [DOI] [PubMed] [Google Scholar]
- 28.Sato Y., Takahashi H. 1998. Molecular cloning and expression of murine homologue of semaphorin K1 gene. Biochim. Biophys. Acta 1443: 419–422 [DOI] [PubMed] [Google Scholar]
- 29.Mine T., Harada K., Matsumoto T., Yamana H., Shirouzu K., Itoh K., Yamada A. 2000. CDw108 expression during T-cell development. Tissue Antigens 55: 429–436 [DOI] [PubMed] [Google Scholar]
- 30.Angelisová P., Drbal K., Cerny J., Hilgert I., Horejsí V. 1999. Characterization of the human leukocyte GPI-anchored glycoprotein CDw108 and its relation to other similar molecules. Immunobiology 200: 234–245 [DOI] [PubMed] [Google Scholar]
- 31.Pendergast A. M. 2002. The Abl family kinases: mechanisms of regulation and signaling. Adv. Cancer Res. 85: 51–100 [DOI] [PubMed] [Google Scholar]
- 32.Tamagnone L., Artigiani S., Chen H., He Z., Ming G. I., Song H., Chedotal A., Winberg M. L., Goodman C. S., Poo M., et al. 1999. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell 99: 71–80 [DOI] [PubMed] [Google Scholar]
- 33.Smith G. L., Symons J. A., Khanna A., Vanderplasschen A., Alcamí A. 1997. Vaccinia virus immune evasion. Immunol. Rev. 159: 137–154 [DOI] [PubMed] [Google Scholar]
- 34.Kang H. R., Lee C. G., Homer R. J., Elias J. A. 2007. Semaphorin 7A plays a critical role in TGF-beta1-induced pulmonary fibrosis. J. Exp. Med. 204: 1083–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sultana H., Foellmer H. G., Neelakanta G., Oliphant T., Engle M., Ledizet M., Krishnan M. N., Bonafé N., Anthony K. G., Marasco W. A., et al. 2009. Fusion loop peptide of the West Nile virus envelope protein is essential for pathogenesis and is recognized by a therapeutic cross-reactive human monoclonal antibody. J. Immunol. 183: 650–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qian F., Wang X., Zhang L., Lin A., Zhao H., Fikrig E., Montgomery R. R. 2011. Impaired interferon signaling in dendritic cells from older donors infected in vitro with West Nile virus. J. Infect. Dis. 203: 1415–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sultana H., Neelakanta G., Kantor F. S., Malawista S. E., Fish D., Montgomery R. R., Fikrig E. 2010. Anaplasma phagocytophilum induces actin phosphorylation to selectively regulate gene transcription in Ixodes scapularis ticks. J. Exp. Med. 207: 1727–1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Town T., Bai F., Wang T., Kaplan A. T., Qian F., Montgomery R. R., Anderson J. F., Flavell R. A., Fikrig E. 2009. Toll-like receptor 7 mitigates lethal West Nile encephalitis via interleukin 23-dependent immune cell infiltration and homing. Immunity 30: 242–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samuel M. A., Wang H., Siddharthan V., Morrey J. D., Diamond M. S. 2007. Axonal transport mediates West Nile virus entry into the central nervous system and induces acute flaccid paralysis. Proc. Natl. Acad. Sci. USA 104: 17140–17145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moustakas A., Souchelnytskyi S., Heldin C. H. 2001. Smad regulation in TGF-beta signal transduction. J. Cell Sci. 114: 4359–4369 [DOI] [PubMed] [Google Scholar]
- 41.Leask A., Abraham D. J. 2004. TGF-beta signaling and the fibrotic response. FASEB J. 18: 816–827 [DOI] [PubMed] [Google Scholar]
- 42.Bai F., Kong K. F., Dai J., Qian F., Zhang L., Brown C. R., Fikrig E., Montgomery R. R. 2010. A paradoxical role for neutrophils in the pathogenesis of West Nile virus. J. Infect. Dis. 202: 1804–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Peters. J. H., R. Giessler, B. Thiele, and F. Steinbach. 1996. Dendritic cells: from ontogenic orphans to myelomonocytic descendants. Immunol. Today 17: 273–278. [DOI] [PubMed]
- 44.Annes J. P., Munger J. S., Rifkin D. B. 2003. Making sense of latent TGFbeta activation. J. Cell Sci. 116: 217–224 [DOI] [PubMed] [Google Scholar]
- 45.Kim J., Oh W. J., Gaiano N., Yoshida Y., Gu C. 2011. Semaphorin 3E-Plexin-D1 signaling regulates VEGF function in developmental angiogenesis via a feedback mechanism. Genes Dev. 25: 1399–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kretzschmar M., Massagué J. 1998. SMADs: mediators and regulators of TGF-beta signaling. Curr. Opin. Genet. Dev. 8: 103–111 [DOI] [PubMed] [Google Scholar]
- 47.Horejsí V., Drbal K., Cebecauer M., Cerny J., Brdicka T., Angelisová P., Stockinger H. 1999. GPI-microdomains: a role in signalling via immunoreceptors. Immunol. Today 20: 356–361 [DOI] [PubMed] [Google Scholar]
- 48.Friedrichson T., Kurzchalia T. V. 1998. Microdomains of GPI-anchored proteins in living cells revealed by crosslinking. Nature 394: 802–805 [DOI] [PubMed] [Google Scholar]
- 49.Suzuki K., Kumanogoh A., Kikutani H. 2008. Semaphorins and their receptors in immune cell interactions. Nat. Immunol. 9: 17–23 [DOI] [PubMed] [Google Scholar]
- 50.Das S., Chakraborty S., Basu A. 2010. Critical role of lipid rafts in virus entry and activation of phosphoinositide 3′ kinase/Akt signaling during early stages of Japanese encephalitis virus infection in neural stem/progenitor cells. J. Neurochem. 115: 537–549 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.