Abstract
Background
The impact of blood transfusion on the development of post-operative stroke after coronary artery bypass grafting (CABG) is not well established. We, therefore, investigated this issue.
Materials and methods.
Complete data on peri-operative blood transfusion were available for 2,226 patients who underwent CABG in three Finnish hospitals.
Results
Stroke occurred post-operatively in 53 patients (2.4%). Logistic regression showed that pre-operative creatinine (OR 1.003, 95% CI 1.000–1.006), extracardiac arteriopathy (OR 2.344, 95% CI 1.133–4.847), pre-operative atrial fibrillation (OR 2.409, 95% CI 1.149–5.052), and the number of packed red blood cell units transfused (OR 1.121, 95% CI 1.065–1.180) were significantly associated with post-operative stroke. When the various blood product transfusions instead of transfused units were included in the multivariable analysis, solvent/detergent treated plasma (Octaplas®) transfusion (OR 2.149, 95% CI 1.141–4.047), but not red blood cell transfusion, was significantly associated with postoperative stroke. Use of blood products ranging from no transfusion (stroke rate 1.6%) to combined transfusion of red blood cells, platelets and Octaplas® was associated with a significant increase in post-operative stroke incidence (6.6%, adjusted analysis: OR 1.727, 95% 1.350–2.209). Patients who received >2 units of red blood cells, >4 units of Octaplas® units and >8 units of platelets had the highest stroke rate of 21%. CART analysis showed that increasing amount of transfused Octaplas®, platelets and history of extracardiac arteriopathy were significantly associated with post-operative stroke.
Conclusions
Transfusion of blood products after CABG has a strong, dose-dependent association with the risk of stroke. The use of Octaplas® and platelet transfusions seem to have an even larger impact on the development of stroke than red blood cell transfusions.
Keywords: Coronary artery bypass surgery, stroke, blood transfusion, red blood cells
Introduction
A recent meta-analysis of the impact of re-exploration for excessive bleeding after cardiac surgery in adults showed a significantly increased risk of post-operative stroke which was consistent among the studies included1. We hypothesised that this negative prognostic effect of re-exploration for bleeding may be mediated by the use of blood products during the early post-operative period. There is some evidence that intra-operative anaemia is a determinant of post-operative stroke2,3, although whether this is due to anaemia-induced brain ischaemia or a possible prothrombotic status induced by blood transfusion is unclear. We investigated this issue in a consecutive series of patients who underwent coronary artery bypass grafting (CABG) in three Finnish hospitals.
Material and methods
This study is part of a wider protocol in progress to assess thrombotic and bleeding complications of cardiac procedures in western Finland4–6. The present study is based on a consecutive series of patients who underwent CABG between January 2008 and December 2010 at the Oulu University Hospital, Turku University Hospital and Vaasa Central Hospital, Finland. During the study period, 2,234 consecutive patients underwent CABG in these hospitals. Complete data on the peri-operative use of blood products such as red blood cells, platelets and solvent/detergent treated plasma (Octaplas®, Octapharma AG, Lachen, Switzerland) were available for 2,226 patients who are the subjects of the present analysis. The patients’ characteristics are summarised in Table I.
Table I.
Baseline characteristics and operative data for 2,226 patients who underwent coronary artery bypass surgery. Results of univariable and multivariable analyses for prediction of post-operative stroke are reported.
| N. of patients | Univariable analysis p-value | Multivariable analysis OR, 95% CI | |
|---|---|---|---|
| Age (years)* | 67.1±9.2 | 0.047 | |
| Females | 476 (21.4) | 0.31 | |
| Haemoglobin (g/L) | 137±16 | 0.98 | |
| Creatinine (μmol/L)* | 90±52 | <0.0001 | 1.003, 1.000–1.006 |
| INR | 1.1±0.7 | 0.88 | |
| Dialysis | 17 (0.8) | 0.060 | |
| Pulmonary disease | 213 (9.6) | 0.48 | |
| Diabetes | 594 (26.6) | 0.88 | |
| Hypertension | 1,557 (69.9) | 0.65 | |
| Atrial fibrillation* | 187 (8.4) | 0.011 | 2.409, 1.149–5.052 |
| Transient ischaemic attack | 67 (3.0) | 0.67 | |
| Stroke | 92 (4.1) | 1.00 | |
| Neurological dysfunction | 38 (1.7) | 0.060 | |
| Extracardiac arteriopathy* | 201 (9.0) | 0.011 | 2.342, 1.133–4.844 |
| Previous PCI | 288 (12.9) | 0.15 | |
| Previous cardiac surgery | 52 (2.3) | 0.63 | |
| Pre-operative clopidogrel* | 613 (27.5) | 0.030 | |
| Pre-operative aspirin | 2,109 (94.7) | 0.73 | |
| Pre-operative warfarin | 169 (7.6) | 0.10 | |
| Recent myocardial infarction | 831 (37.3) | 0.13 | |
| Left ventricular ejection fraction* | 0.037 | ||
| >50% | 1,651 (74.2) | 0.037 | |
| 30–50% | 449 (20.2) | ||
| <30 | 100 (4.5) | ||
| Systemic pulmonary pressure >60 mmHg | 35 (1.6) | 0.20 | |
| Unstable angina* | 329 (14.8) | 0.010 | |
| Critical pre-operative status | 130 (5.8) | 0.55 | |
| Emergency surgery | 201 (9.0) | 0.28 | |
| Epi-aortic ultrasound | 850 (38.2) | 0.64 | |
| Diseased ascending aorta | 111 (5.0) | 0.73 | |
| Off-pump coronary surgery | 804 (36.1) | 0.80 | |
| Intra-operative use of tranexamic acid | 1,641 (73.7) | 0.98 | |
| N. of distal anastomoses | 3.5±1.1 | 0.53 | |
| Logistic EuroSCORE (%) | 5.6±8.4 | 0.003 |
Legend: Continuous variables are reported as mean and standard deviation or counts (%); INR: International Normalised Ratio; PCI: percutaneous coronary intervention;
variable included in the logistic regression model.
Baseline and operative data were obtained from local institutional clinical registries in which information is prospectively collected in computerised databases. Furthermore, the full medical records of the eligible patients were reviewed in order to determine the peri-operative antithrombotic strategies and the incidence of major operative complications. Operative risk was assessed by the EuroSCORE risk scoring method7.
Peri-operative antithrombotic treatment
Aspirin 100 mg p.o. was discontinued preoperatively in most of the patients undergoing elective surgery and administered until the day of surgery in patients with acute coronary syndrome.
Heparin (2.5–4.0 mg/kg) was administered intravenously after sternotomy to maintain an activated clotting time of more than 400 seconds and it was neutralised at the end of the procedure by protamine sulphate (1.5–3.0 mg/kg). Further protamine was given in the case of bleeding during closure of the chest or within the first hour after surgery according to the activated coagulation time. Aprotinin was not used in any of these patients. Tranexamic acid was administered intra-operatively at the discretion of the anaesthetist. Octaplas® as well as platelets were transfused according to the amount of bleeding, International Normalised Ratio (INR) levels and platelet counts. Enoxaparin (40–80 mg once daily) was started in all patients on the evening of the day of surgery. Warfarin was started in the case of persistent atrial fibrillation. Clopidogrel was used postoperatively in these patients only in the case of allergy to aspirin or recent percutaneus coronary intervention.
Surgical techniques
Intermittent antegrade and retrograde cold blood cardioplegia was used during conventional CABG. Proximal anastomoses were sutured to the ascending aorta during cross clamping, when the latter was considered safe. Epi-aortic ultrasound was performed according to the surgeon’s preference. The ascending aorta was left untouched in the case of grade III diseased aorta8. An Octopus® stabiliser, intracoronary shunts and, in some instances, a Starfish® stabiliser (Medtronic, Minneapolis, MN, USA) were used in patients who underwent off-pump coronary surgery.
Outcome end-points
The primary outcome end-point of this study was stroke. Secondary outcome end-points were in-hospital death, new onset renal failure requiring dialysis, re-exploration for excessive bleeding, use of blood products and duration of stay in the intensive care unit.
Stroke was defined as a new neurological deficit following surgery lasting >24 hours accompanied by new structural changes detected by computed tomography or magnetic resonance imaging. In the case of absence of structural changes at imaging, the diagnosis of stroke was made clinically by a neurologist. Renal failure was defined as a postoperative renal failure requiring temporary or prolonged dialysis. Low cardiac output syndrome was defined as a post-operative cardiac index <2.0 L/min/m2. A composite outcome end-point included in-hospital death, low cardiac output syndrome, de novo dialysis, stroke, re-exploration for excessive bleeding, or intensive care unit stay ≥5 days. The outcome events were collected from the period of the index hospitalisation.
Ethical considerations
The study protocol was approved by the Institutional Review Boards of Turku and Oulu University Hospitals. This study was not financially supported.
Statistical analysis
Data were analysed with the use of PASW version 18 statistical software (IBM SPSS Inc., Chicago, IL, USA). Continuous variables are reported as the mean ± standard deviation. Pearson’s chi-square test, Fisher’s exact test and Mann-Whitney’s test were used for univariate analysis. Correlations between continuous variables were assessed by Spearman’s test. Logistic regression with the help of backward selection was used for multivariate analysis. Only variables with a p<0.050 at univariate analysis were included in the logistic regression model.
We estimated the prognostic impact of blood transfusion by defining a priori classes of increasing amount of transfused blood products according to the type of blood product (red blood cells units: 0, 1–2 units, or >2 units; platelet units: 0, 1–8 units, or >8 units; Octaplas® units: 0, 1–4 units, or >4 units). The sum of these classes was used to estimate the overall amount of blood products and to identify those patients who received large amount of blood products, i.e. red blood cells >2 units, Octaplas® >4 units and platelets >8 units. This was done assuming that only patients who received >2 units of red blood cells, >4 units of Octaplas® and/or >8 units of platelets were those at higher risk of transfusion-related adverse events. These cut-off values were later on confirmed by classification and regression tree analysis (CART).
CART analysis was performed to identify independent risk factors for post-operative stroke. Validation of the classification tree procedure was assessed by cross-validation through 25 folds. The minimum number of patients for the parent node was set at 30 and the minimum for the child node was 1. The maximum classification tree depth was 5. Gini’s method was used to measure impurity, which is the extent to which a node does not represent a homogenous subset of cases. A minimum change in improvement was set at 0.0001. Receiver operating characteristic (ROC) curve analysis was used to estimate the area under the curve of probabilities values estimated by the CART analysis model outcome.
We observed that patients who received all three types of blood products had a significantly higher risk of stroke compared to those who did not receive any blood transfusion. These two groups differed markedly with respect to pre-operative co-variables; such differences between the study groups, i.e. patients who did not receive any blood product and those who received red blood cells, Octaplas® and platelet transfusion, were accounted for by developing a propensity score for the treatment method. Propensity score analysis was used to control for all known patients’ factors that might be related to the decision to administer blood products, and thus potentially to the outcome of interest. The propensity score was calculated by logistic regression with backward selection by including clinical variables with a certain difference between the study groups as indicated by p<0.050 in univariable analysis. ROC curve analysis was used to estimate the area under the curve of the model, predicting the probability of being included in the study groups The calculated propensity score was employed only for one-to-one matching, because our purpose was to identify two study groups with similar characteristics in order to fulfil the criteria of comparability. One-to-one propensity score matching between study groups was done according to a difference in the logit of propensity score of less than 0.04 between each pair of patients in the study groups. Such a caliper width was equal to 0.2 of the standard deviation of the logit of the herein calculated propensity score9. Standardised differences were estimated to compare the means of continuous and binary variables between the study groups. Standardised differences less than 0.1 were taken to indicate a negligible difference in the mean or prevalence of covariates between the study groups9. The propensity score was used to adjust transfusion of all three types of blood products in predicting postoperative stroke. A p value <0.050 was considered statistically significant.
Results
Stroke occurred in 53 patients (2.4%). These strokes were regarded of ischaemic origin. Table II summarises other in-hospital post-operative adverse events observed in this series of patients.
Table II.
Adverse events during the immediate post-operative period after coronary artery bypass surgery. Results of univariable and multivariable analyses for prediction of post-operative stroke are reported.
| N. | Univariable analysis p-value | Multivariable analysis OR, 95% CI | |
|---|---|---|---|
| In-hospital mortality | 50 (2.2) | - | |
| Stroke | 53 (2.4) | - | |
| Low cardiac output syndrome | 324 (14.6) | 0.001 | |
| Post-operative blood loss (mL) | 858±590 | 0.16 | |
| Resternotomy for bleeding | 138 (6.2) | 0.57 | |
| “Surgical bleeding” | 83 (3.7) | 0.72 | |
| RBC transfusion | 1,134 (50.9) | 0.005 | |
| RBC units transfused* | 2.0±3.1 | <0.0001 | 1.121, 1.065–1.180 |
| Octaplas® transfusion | 486 (21.8) | <0.0001 | |
| Octaplas® units transfused* | 0.8±2.0 | <0.0001 | |
| Platelet transfusion | 458 (20.6) | <0.0001 | |
| Platelet units transfused* | 1.8±4.9 | <0.0001 | |
| De novo dialysis | 41 (1.8) | <0.0001 | |
| Atrial fibrillation* | 799 (35.9) | 0.043 | |
| ICU stay (days) | 2.2±3.1 | - | |
| ICU stay ≥5 days | 222 (10.0) | - | |
| Composite outcome end-point | 548 (24.6) | - |
Legend: Continuous variables are reported as mean ± standard deviation or counts (%); RBC: red blood cell; ICU: intensive care unit; Composite end-point: in-hospital death, low cardiac output syndrome, de novo dialysis, stroke, repeat sternotomy for excessive bleeding, or intensive care unit stay ≥5days;
variable included in the logistic regression model.
Prognostic impact of types of transfused blood products
Since there was a strong correlation between the amount of transfused red blood cell units and transfused platelet units (rho: 0.467, p<0.0001) as well as transfused Octaplas® units (rho: 0.423, p<0.0001), all these three risk factors were analysed further. The area under the ROC curve for predicting post-operative stroke was 0.653 (95% CI 0.570–0.736) for red blood cell units, 0.627 (95% CI 0.543–0.712) for Octaplas® units and 0.618 (95% CI 0.532–0.703) for platelet units.
Figure 1 summarises the unadjusted rate of post-operative stroke according to increasing amount of red blood cell units (0, 1–2 units, or >2 units), platelets units (0, 1–8 units, or >8 units) and Octaplas® units (0, 1–4 units, or >4 units) transfused (p<0.0001 adjusted for pre-operative haemoglobin and post-operative blood loss). When the use of all blood products was summed, a dose-dependent increase of post-operative stroke was observed (when adjusted for pre-operative haemoglobin and post-operative blood loss: OR 1.474, 95% CI 1.262–1.720, p<0.0001, Figure 2). Similarly, the increase in the use of blood product types ranging from no transfusion to combined transfusion of red blood cells, platelets and Octaplas® was associated with a significantly increased rate of post-operative stroke (when adjusted for pre-operative haemoglobin and post-operative blood loss: OR 1.727, 95% CI 1.350–2.209, p<0.0001, Figure 3).
Figure 1.
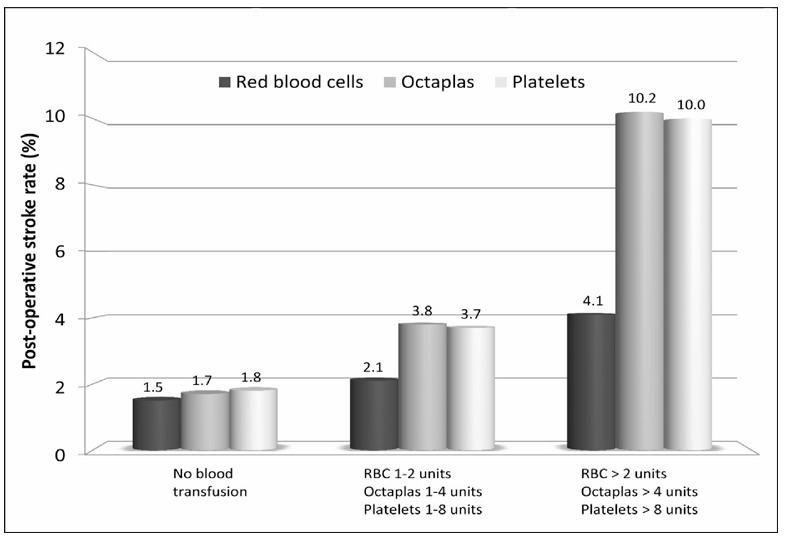
Post-operative stroke rates according to increasing amount of red blood cell (RBC) units (0=none, 1=1–2 units, 2=>2 units; p=0.002), platelets units (0=none, 1=1–8 units, 2=>8 units; p<0.0001) and Octaplas® units (0=none, 1=1–4 units, 2=>4 units; p<0.0001) transfused.
Figure 2.
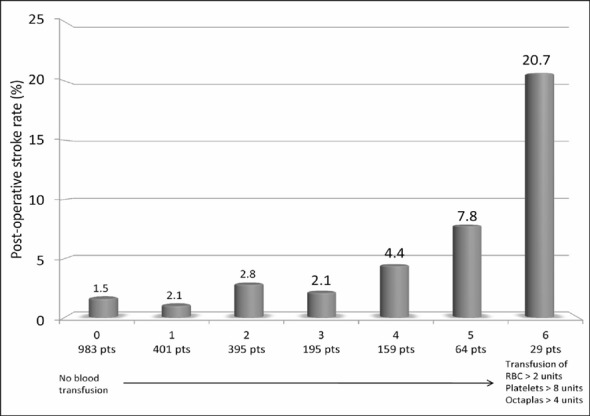
Post-operative stroke rates according to the sum of increasing amount of red blood cell (RBC) units (0=none, 1=1–2 units, 2=>2 units), platelets units (0=none, 1=1–8 units, 2=>8 units) and Octaplas® units (0=none, 1=1–4 units, 2=>4 units) transfused (p<0.0001).
Figure 3.
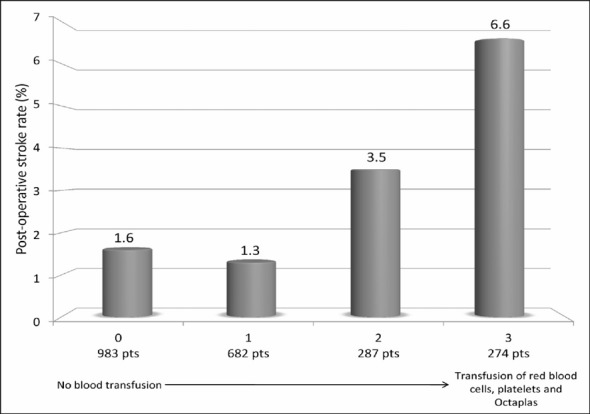
Post-operative stroke rates according to the sum of any type of transfusion (ranging from 0=no blood transfusion to 3=transfusion of red blood cells, platelets and Octaplas®) (p<0.0001).
Logistic regression
Logistic regression including the risk factors indicated in Tables I and II showed that pre-operative creatinine (p=0.040, OR 1.003, 95% CI 1.000–1.006), extracardiac arteriopathy (p=0.022, OR 2.344, 95% CI 1.133–4.847), pre-operative atrial fibrillation (p=0.020, OR 2.409, 95% CI 1.149–5.052), and the amount of red blood cell units transfused (p<0.0001, OR 1.121, 95% CI 1.065–1.180) (Hosmer-Lemeshow’s test: p=0.433) were significantly associated with post-operative stroke. When type of blood product instead of their specific amount was included in the multivariable analysis, Octaplas® transfusion (p=0.018, OR 2.149, 95% CI 1.141–4.047), but not red blood cell transfusion, was significantly associated with post-operative stroke along with pre-operative creatinine, pre-operative atrial fibrillation and extracardiac arteriopathy. Stroke rate was not significantly different between the three hospitals (p-values ranging from 0.186 to 0.820) when the hospitals were in the regression models.
Classification and regression tree analysis
Renal failure, pre-operative atrial fibrillation and extracardiac arteriopathy were included in the CART model along with classes of increasing amount of transfused red blood cells, Octaplas® and platelets. This model showed that increasing amount of transfused Octaplas®, platelets and history of extracardiac arteriopathy were significantly associated with post-operative stroke (area under the ROC curve for this predictive model: 0.631, 95% CI 0.546–0.716) (Figure 4).
Figure 4.
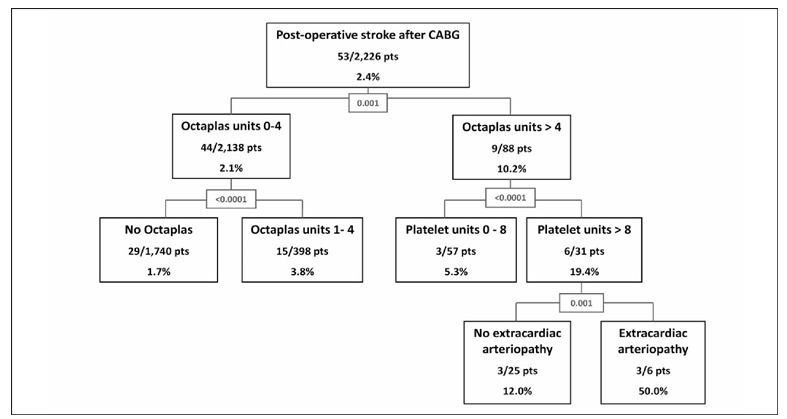
Classification and regression tree analysis of post-operative stroke after CABG. Percentages refer to post-operative stroke rates. Numerical values at splitting nodes are improvement values.
Propensity score analysis
The propensity score was calculated to estimate the risk of receiving transfusion of all types of blood products (red blood cells, Octaplas® and platelets). Logistic regression showed that patient’s age, pre-operative haemoglobin, pre-operative exposure to clopidogrel, chronic use of warfarin, previous cardiac surgery, recent myocardial infarction, emergency operation, critical operative status, no diabetes and no intra-operative use of tranexamic acid were significantly associated with peri-operative use of all types of blood products (Hosmer-Lemeshow’s test p=0.731). The area under the ROC curve for this predictive model was 0.820 (95% CI 0.791–0.848).
When use of all three types of blood products was adjusted for the propensity score, the former was significantly associated with post-operative stroke (OR 4.832, 95% CI 2.370–9.851).
One-to-one propensity score matching allowed us to get 210 pairs of patients who did not receive any blood transfusion and patients who received transfusion of red blood cells, Octaplas® and platelets (Table III). It is noteworthy that despite the optimal area under the ROC curve of the propensity score as well as adequate goodness of fit for this logistic regression model, there were still a few differences between the study groups as denoted by standardised differences (Table III). Patients who did not receive blood products had a post-operative stroke rate of 1.0%, whereas it was 6.7% in patients who received all three types of blood products (p=0.004, Table IV). Logistic regression showed that, when adjusted for different institutions (p-values ranging from 0.277 to 0.537), transfusion of all three blood products was associated with a rather high post-operative stroke risk (OR 9.497, 95% CI 1.949–46.265). Overall, transfusion of all three types of blood products was associated with a remarkably higher risk of all major adverse postoperative end-points (Table IV) except in-hospital mortality, for which the difference did not reach statistical significance (2.4% vs. 5.7%, p=0.08).
Table III.
Baseline characteristics and operative data on 210 propensity score matched pairs who underwent coronary artery bypass surgery who did not receive any blood transfusion and those who received all three types of blood products peri-operatively.
| No blood transfusion 210 patients | Transfusion of RBC, Octaplas and platelets 210 patients | Standardised difference | p-value | |
|---|---|---|---|---|
| Age (years) | 68.7±8.8 | 68.3±8.9 | 0.05 | 0.733 |
| Females | 42 (20.0) | 43 (20.5) | 0.01 | 0.903 |
| Haemoglobin (gr/L) | 135±14 | 136±15 | 0.07 | 0.543 |
| Creatinine (μmol/L) | 89±61 | 96±77 | 0.1 | 0.455 |
| INR | 1.2±0.3 | 1.2±0.3 | 0.32 | 0.010 |
| Dialysis | 0 (0) | 0 (0) | - | - |
| Pulmonary disease | 20 (9.5) | 23 (11.0) | 0.05 | 0.629 |
| Diabetes | 36 (17.1) | 38 (18.1) | 0.03 | 0.798 |
| Hypertension | 139 (66.2) | 126 (60.0) | 0.13 | 0.189 |
| Atrial fibrillation | 22 (10.5) | 22 (10.5) | 0 | 1.000 |
| Transient ischaemic attack | 5 (2.4) | 13 (6.2) | 0.19 | 0.089 |
| Stroke | 17 (8.1) | 6 (2.9) | 0.23 | 0.018 |
| Neurological dysfunction | 4 (1.9) | 4 (1.9) | 0 | 1.000 |
| Extracardiac arteriopathy | 23 (11.0) | 27 (12.9) | 0.06 | 0.547 |
| Previous PCI | 35 (16.7) | 21 (10.0) | 0.20 | 0.044 |
| Previous cardiac surgery | 9 (4.3) | 8 (3.8) | 0.03 | 0.804 |
| Pre-operative clopidogrel | 84 (40.0) | 89 (42.4) | 0.05 | 0.620 |
| Pre-operative aspirin | 197 (93.8) | 201 (95.7) | 0.09 | 0.381 |
| Pre-operative warfarin | 20 (9.5) | 18 (8.6) | 0.03 | 0.734 |
| Recent myocardial infarction | 90 (42.9) | 97 (46.2) | 0.07 | 0.492 |
| Left ventricular ejection fraction | 0.017 | |||
| >50% | 163 (77.6) | 144 (71.6) | 0.14 | |
| 30–50% | 45 (21.4) | 45 (22.4) | 0.02 | |
| <30% | 2 (1.0) | 12 (6.0) | 0.27 | |
| Systemic pulmonary pressure >60 mmHg | 1 (0.5) | 4 (1.9) | 0.13 | 0.372 |
| Unstable angina | 30 (14.3) | 46 (21.9) | 0.20 | 0.043 |
| Critical pre-operative status | 12 (5.7) | 17 (8.1) | 0.09 | 0.336 |
| Emergency surgery | 19 (9.0) | 22 (10.5) | 0.05 | 0.622 |
| Epi-aortic ultrasound | 92 (44.9) | 74 (35.2) | 0.20 | 0.045 |
| Diseased ascending aorta | 15 (8.9) | 10 (5.5) | 0.13 | 0.224 |
| Off-pump coronary surgery | 95 (45.2) | 68 (32.4) | 0.26 | 0.007 |
| Intra-operative use of tranexamic acid | 139 (66.2) | 143 (68.1) | 0.04 | 0.678 |
| N. of distal anastomoses | 3.3±1.1 | 3.9±1.1 | 0.51 | <0.0001 |
| Logistic EuroSCORE (%) | 5.7±7.3 | 7.0±9.8 | 0.15 | 0.090 |
Legend: Continuous variables are reported as mean and standard deviation or counts (%); INR: International Normalised Ratio; PCI: percutaneous coronary intervention; RBC: red blood cells.
Table IV.
Adverse events during the immediate post-operative period after coronary artery bypass surgery in 210 propensity score matched pairs of patients who did not receive any blood transfusion and those who received all three types of blood products. Results of univariable and multivariable analyses for prediction of post-operative stroke are reported.
| No blood transfusion 210 patients | Transfusion of RBC, Octaplas and platelets 210 patients | p-value | |
|---|---|---|---|
| In-hospital mortality | 5 (2.4) | 12 (5.7) | 0.08 |
| Stroke | 2 (1.0) | 14 (6.7) | 0.004 |
| Low cardiac output syndrome | 14 (6.7) | 46 (21.9) | <0.0001 |
| Post-operative blood loss (mL) | 683±347 | 1385±910 | <0.0001 |
| Re-sternotomy for bleeding | 3 (1.4) | 57 (27.1) | <0.0001 |
| “Surgical bleeding” | 2 (1.0) | 41 (19.5) | <0.0001 |
| De novo dialysis | 1 (0.5) | 12 (5.7) | 0.003 |
| Atrial fibrillation | 64 (30.6) | 92 (43.8) | 0.005 |
| ICU stay (days) | 1.1±3.0 | 3.2±4.1 | <0.0001 |
| ICU stay ≥5 days | 4 (1.9) | 36 (17.1) | <0.0001 |
| Composite outcome end-point | 20 (9.5) | 108 (51.4) | <0.0001 |
Legend: Continuous variable are reported as mean ± standard deviation or counts (%); RBC: red blood cells; ICU: intensive care unit; Composite end-point: in-hospital death, low cardiac output syndrome, de novo dialysis, stroke, re-sternotomy for excessive bleeding, or intensive care unit stay ≥5 days.
Post-operative outcome according to the EuroSCORE
Since certain differences persisted between study groups in the propensity score matching analysis, we assessed the immediate post-operative outcome in patients with increasing operative risk as estimated by additive EuroSCORE classes (additive EuroSCORE 0–5, 6–9 and >9 points). This sensitivity analysis confirmed the major impact of transfusion of all three types of blood products on the development of stroke as well as other major adverse events (Table V).
Table V.
Adverse events during the immediate post-operative period after coronary artery bypass surgery in patients who did not receive any blood transfusion and those who received all three types of blood products. Results are according to increasing additive EuroSCORE classes.
| Additive EuroSCORE 0–5 | Additive EuroSCORE 6–9 | Additive EuroSCORE >9 | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| No blood transfusion 804 patients | Transfusion of RBC, Octaplas and platelets 117 patients | No blood transfusion 142 patients | Transfusion of RBC, Octaplas and platelets 91 patients | No blood transfusion 22 patients | Transfusion of RBC, Octaplas and platelets 54 patients | |
| In-hospital mortality | 1 (0.1) | 2 (1.7)* | 1 (0.7) | 6 (6.6)* | 4 (18.2) | 13 (24.1) |
| Stroke | 13 (1.6) | 6 (5.1)* | 3 (2.1) | 7 (7.7)* | 0 (0) | 4 (7.4) |
| Low cardiac output syndrome | 32 (4.0) | 18 (15.4)* | 10 (7.0) | 23 (25.3)* | 8 (36.4) | 24 (44.4) |
| Post-opertative blood loss (mL) | 723±395 | 1,546±970* | 650±366 | 1,138±820* | 663±267 | 975±682* |
| Re-sternotomy for bleeding | 7 (0.9) | 37 (31.6)* | 0 (0) | 17 (18.7)* | 1 (4.5) | 7 (13.0) |
| “Surgical bleeding” | 4 (0.5) | 27 (23.1)* | 0 (0) | 11 (12.1)* | 0 (0) | 5 (9.3) |
| De novo dialysis | 3 (0.4) | 2 (1.7) | 1 (0.7) | 7 (7.7)* | 2 (9.1) | 8 (14.8) |
| Atrial fibrillation | 221 (27.5) | 43 (36.8)* | 48 (33.8) | 47 (51.6)* | 11 (50.0) | 31 (57.4) |
| ICU stay (days) | 1.4±2.1 | 2.5±3.9* | 1.4±1.2 | 3.4±3.6* | 2.0±3.0 | 5.3±4.9 |
| ICU stay ≥5 days | 18 (2.2) | 11 (9.4)* | 4 (2.8) | 20 (22.0)* | 2 (9.1) | 23 (42.6)* |
| Composite outcome end-point | 58 (7.2) | 53 (45.3)* | 14 (9.9) | 47 (51.6)* | 10 (45.5) | 44 (81.5* |
Legend: Continuous variable are reported as mean ± standard deviation or counts (%); RBC: red blood cells; ICU: intensive care unit; Composite end-point: in-hospital death, low cardiac output syndrome, de novo dialysis, stroke, re-sternotomy for excessive bleeding, or intensive care unit stay ≥5 days;
p<0.050.
Logistic regression including additive EuroSCORE classes, amount of post-operative bleeding, different institutions and transfusion of all three blood products, showed that the last variable was the only independent predictor of post-operative stroke (p<0.0001, OR 4.212, 95% CI 2.053–8.643).
Discussion
Stroke is one of the most severe complications occurring after cardiac surgery. It has a significant impact on patient’s immediate and late outcome and quality of life10,11. The aetiopathogenesis of stroke is complex, multifactorial and related to a large number of peri-operative factors and patient comorbidities12. Intra-operative embolism is considered the most important mechanism of post-operative stroke, but the role of various contributing factors is difficult to ascertain or investigate. Use of off-pump surgery and a no-touch aorta technique in the case of diseased ascending aorta8,13, improving cardiopulmonary bypass techniques and materials12,14, as well as reducing cardiopulmonary bypass time15, avoidance of peri-operative arrhythmias16, hypoperfusion17 and haemodilutional anaemia3 are measures which may significantly reduce the risk of post-operative stroke.
Excessive bleeding requiring re-exploration has recently been shown to be associated with a high risk of post-operative stroke1, an observation which confirms that acute surgical anaemia is a major determinant of neurological events2,3. Indeed, a study by Kulier and colleagues18 also provided evidence of an increased risk of post-operative stroke after CABG among patients with pre-operative anaemia, but this finding was not confirmed by the present or other previous studies2,19,20 probably because pre-operative anaemia is now actively corrected before or at the start of surgery. However, severe peri-operative haemodilutional anaemia may certainly induce significant cerebral ischaemia. Experimental data indicate that under moderate or profound hypothermic conditions, severe cerebral ischaemia develops when the haematocrit sinks below 10–15%21,22. In accordance, clinical data indicate that the risk of post-operative neurological events increases in cardiac surgery in adults with haematocrit levels <20–22%3,23, thereby confirming the importance of avoiding excessive bleeding during cardiac surgery. However, these findings do not take into consideration the amount of postoperative bleeding and immediate post-operative anaemia, which can be even more profound than intra-operatively.
On the other hand, the use of blood products to correct anaemia and restore coagulation may also contribute to the development of neurological events2,24. Recently, Barhainwala and colleagues2 provided strong evidence about the independent prognostic impact of both post-operative haemoglobin levels and the need for intra-operative red blood cell transfusion on the risk of post-operative stroke. In the present study, logistic regression showed the amount of transfused packed red blood cells was an independent predictor of stroke, but the detailed analysis of type of transfused blood products demonstrated that need for Octaplas® transfusion was an independent predictor of stroke, whereas that of red blood cells was not. This finding was confirmed also by CART analysis, which showed the significant impact of transfusion of Octaplas® >4 units and platelets >8 units (stroke rate, 19.4%). Our findings are in agreement with the results of the study by Whitson and colleagues2, which indicated that the risk of stroke as well as of mortality and other important adverse events is increased by the need for excessive blood product transfusion rather than minimal need of transfusion. Similarly, Figures 2 and 3 show that, in the present series, transfusion of all three types of blood products was dose-dependently associated with stroke risk. Importantly, this risk was evident when Octaplas® >4 units and platelets >8 units were transfused but to a much less extent when red blood cell >2 units were transfused (Figure 1). In the absence of data on peri-operative haemoglobin and haematocrit nadir, we may speculate that the need for transfusion of all three types of blood products certainly indicates significant bleeding, even if the amount of post-operative blood loss was not associated with post-operative stroke.
The evidence of an increased risk of stroke after cardiac surgery in patients who received recombinant activated factor VII25 suggests that the beneficial haemostatic effects of coagulation-promoting drugs could be counterbalanced by severe atherothrombotic events. Although this may be purely speculative, large amounts of transfused blood products may also induce a prothrombotic status. There are no specific data about the clinical impact of Octaplas® transfusion in patients undergoing cardiac surgery, but De Maistre and colleagues26 observed an increased risk of venous thromboembolism after transfusion of fresh-frozen plasma in patients who underwent abdominal aortic surgery. The reported lower level of protein S in Octaplas® compared with in fresh-frozen plasma is of concern27. Administration of solvent/detergent-treated plasma has been shown to be associated with decreased protein S activity28,29, which may be detrimental in patients with congenital or acquired protein S deficiency. We, therefore, suspect that a prothrombotic status may be further enhanced by solvent/detergent-treated plasma in patients undergoing major surgery.
There are limited data indicating that platelet transfusion may be associated with an increased risk of thromboembolism30. A propensity score matched analysis by McGrath and colleagues31 did not demonstrate an increased morbidity risk with the use of platelet transfusion in cardiac surgery in adults. However, the authors did not take into account the amount of platelet units transfused and, therefore, no data on patients who received large numbers of platelet units were reported. However, it is known that platelet storage is associated with microparticles32 and the possible association of such microparticles with thrombotic risk is a very active area of investigation.
Our findings could have been affected by a number of factors. First, this is a retrospective analysis of data from three hospitals with different peri-operative approaches. Second, we do not have data about intra- and post-operative levels of haemoglobin and haematocrit, which would have been useful to confirm the independent impact of blood products on post-operative neurological events. Third, we do not have data on the possible use of procoagulant drugs other than tranexamic acid. However, potent procoagulants such as recombinant factor VII are only rarely used in our Institutions, mainly in the treatment of severe bleeding occurring after valve and aortic surgery. Fourth, the impact of patient’s comorbidities as well as operative technique are not easily assessable because of the complexity of the aetiopathogenesis of stroke and the interaction of variables resulting in excessive bleeding and at the same time in a prothrombotic state. However, Table IV shows that the rate of post-operative stroke and other major adverse events after transfusion of all three types of blood products is so high that patients’ comorbidities and peri-operative anaemia cannot be the only determinants of these complications. This is further confirmed by the sensitivity analysis of different additive EuroSCORE classes (Table V).
In conclusion, the present results indicate that transfusion of large amounts of blood products after CABG is associated with a very high risk of postoperative stroke. It is of particular interest that use of Octaplas® and platelet transfusions seems to have an even larger impact on the development of stroke than transfusion of red blood cells.
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Biancari F, Mikkola R, Heikkinen J, et al. Estimating the risk of complications related to re-exploration for bleeding after adult cardiac surgery: a systematic review and meta-analysis. Eur J Cardiothorac Surg. 2011 doi: 10.1016/j.ejcts.2011.04.023. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bahrainwala ZS, Grega MA, Hogue CW, et al. Intraoperative hemoglobin levels and transfusion independently predict stroke after cardiac operations. Ann Thorac Surg. 2011;91:1113–8. doi: 10.1016/j.athoracsur.2010.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karkouti K, Djaiani G, Borger MA, et al. Low hematocrit during cardiopulmonary bypass is associated with increased risk of perioperative stroke in cardiac surgery. Ann Thorac Surg. 2005;80:1381–7. doi: 10.1016/j.athoracsur.2005.03.137. [DOI] [PubMed] [Google Scholar]
- 4.Airaksinen KE, Biancari F, Karjalainen P, et al. Safety of coronary artery bypass surgery during therapeutic oral anticoagulation. Thromb Res. 2011;128:435–9. doi: 10.1016/j.thromres.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 5.Biancari F, Myllylä M, Lepojärvi S, et al. Preoperative warfarin treatment and outcome of coronary artery bypass graft surgery. Ann Thorac Surg. 2010;89:1139–45. doi: 10.1016/j.athoracsur.2009.12.072. [DOI] [PubMed] [Google Scholar]
- 6.Biancari F, Myllylä M, Porela P, et al. Postoperative stroke in patients on oral anticoagulation undergoing coronary artery bypass surgery. Scand Cardiovasc J. 2011;45:360–8. doi: 10.3109/14017431.2011.585403. [DOI] [PubMed] [Google Scholar]
- 7.Nashef SA, Roques F, Michel P, et al. European system for cardiac operative risk evaluation (EuroSCORE) Eur J Cardiothorac Surg. 1999;16:9–13. doi: 10.1016/s1010-7940(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 8.Biancari F, Yli-Pyky S. Meta-analysis on the use of the Heartstring anastomotic device to prevent stroke in patients undergoing off-pump coronary artery bypass grafting. Eur J Cardiothorac Surg. 2011;40:1236–40. doi: 10.1016/j.ejcts.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 9.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150–61. doi: 10.1002/pst.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filsoufi F, Rahmanian PB, Castillo JG, et al. Incidence, imaging analysis, and early and late outcomes of stroke after cardiac valve operation. Am J Cardiol. 2008;101:1472–8. doi: 10.1016/j.amjcard.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 11.Tarakji KG, Sabik JF, 3rd, Bhudia SK, et al. Temporal onset, risk factors, and outcomes associated with stroke after coronary artery bypass grafting. JAMA. 2011;305:381–90. doi: 10.1001/jama.2011.37. [DOI] [PubMed] [Google Scholar]
- 12.Carrascal Y, Guerrero AL. Neurological damage related to cardiac surgery. Pathophysiology, diagnostic tools and prevention strategies. Using actual knowledge for planning the future. Neurologist. 2010;16:152–64. doi: 10.1097/NRL.0b013e3181bd602b. [DOI] [PubMed] [Google Scholar]
- 13.Biancari F, Mosorin M, Rasinaho E, et al. Postoperative stroke after off-pump versus on-pump coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2007;133:169–73. doi: 10.1016/j.jtcvs.2006.06.052. [DOI] [PubMed] [Google Scholar]
- 14.Biancari F, Rimpiläinen R. Meta-analysis of randomised trials comparing the effectiveness of miniaturised versus conventional cardiopulmonary bypass in adult cardiac surgery. Heart. 2009;95:964–9. doi: 10.1136/hrt.2008.158709. [DOI] [PubMed] [Google Scholar]
- 15.Nissinen J, Biancari F, Wistbacka JO, et al. Safe time limits of aortic cross-clamping and cardiopulmonary bypass in adult cardiac surgery. Perfusion. 2009;24:297–305. doi: 10.1177/0267659109354656. [DOI] [PubMed] [Google Scholar]
- 16.Lahtinen J, Biancari F, Salmela E, et al. Postoperative atrial fibrillation is a major cause of stroke after on-pump coronary artery bypass surgery. Ann Thorac Surg. 2004;77:1241–4. doi: 10.1016/j.athoracsur.2003.09.077. [DOI] [PubMed] [Google Scholar]
- 17.Haugen O, Farstad M, Myklebust R, et al. Low perfusion pressure during CPB may induce cerebral metabolic and ultrastructural changes. Scand Cardiovasc J. 2007;41:331–8. doi: 10.1080/14017430701393218. [DOI] [PubMed] [Google Scholar]
- 18.Kulier A, Levin J, Moser R, et al. Investigators of the Multicenter Study of Perioperative Ischemia Research Group. Ischemia Research and Education Foundation Impact of preoperative anemia on outcome in patients undergoing coronary artery bypass graft surgery. Circulation. 2007;116:471–9. doi: 10.1161/CIRCULATIONAHA.106.653501. [DOI] [PubMed] [Google Scholar]
- 19.Bell ML, Grunwald GK, Baltz JH, et al. Does preoperative hemoglobin independently predict short-term outcomes after coronary artery bypass graft surgery? Ann Thorac Surg. 2008;86:1415–23. doi: 10.1016/j.athoracsur.2008.07.088. [DOI] [PubMed] [Google Scholar]
- 20.Boening A, Boedeker RH, Scheibelhut C, et al. Anemia before coronary artery bypass surgery as additional risk factor increases the perioperative risk. Ann Thorac Surg. 2011;92:805–10. doi: 10.1016/j.athoracsur.2011.02.076. [DOI] [PubMed] [Google Scholar]
- 21.Duebener LF, Sakamoto T, Hatsuoka S, et al. Effects of hematocrit on cerebral microcirculation and tissue oxygenation during deep hypothermic bypass. Circulation. 2001;104(12 Suppl. 1):I260–4. doi: 10.1161/hc37t1.094912. [DOI] [PubMed] [Google Scholar]
- 22.Miura T, Sakamoto T, Kobayashi M, et al. Hemodilutional anemia impairs neurologic outcome after cardiopulmonary bypass in a piglet model. J Thorac Cardiovasc Surg. 2007;133:29–36. doi: 10.1016/j.jtcvs.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 23.Habib RH, Zacharias A, Schwann TA, et al. Adverse effects of low hematocrit during cardiopulmonary bypass in the adult: should current practice be changed? J Thorac Cardiovasc Surg. 2003;125:1438–50. doi: 10.1016/s0022-5223(02)73291-1. [DOI] [PubMed] [Google Scholar]
- 24.Whitson BA, Huddleston SJ, Savik K, Shumway SJ. Bloodless cardiac surgery is associated with decreased morbidity and mortality. J Card Surg. 2007;22:373–8. doi: 10.1111/j.1540-8191.2007.00428.x. [DOI] [PubMed] [Google Scholar]
- 25.Ponschab M, Landoni G, Biondi-Zoccai G, et al. Recombinant activated factor VII increases stroke in cardiac surgery: a meta-analysis. J Cardiothorac Vasc Anesth. 2011;25:804–10. doi: 10.1053/j.jvca.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 26.de Maistre E, Terriat B, Lesne-Padieu AS, et al. High incidence of venous thrombosis after surgery for abdominal aortic aneurysm. J Vasc Surg. 2009;49:596–601. doi: 10.1016/j.jvs.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Buchta C, Felfernig M, Höcker P, et al. Stability of coagulation factors in thawed, solvent/detergent-treated plasma during storage at 4 degrees C for 6 days. Vox Sang. 2004;87:182–6. doi: 10.1111/j.1423-0410.2004.00552.x. [DOI] [PubMed] [Google Scholar]
- 28.Haubelt H, Blome M, Kiessling AH, et al. Effects of solvent/detergent-treated plasma and fresh-frozen plasma on haemostasis and fibrinolysis in complex coagulopathy following open-heart surgery. Vox Sang. 2002;82:9–14. doi: 10.1046/j.1423-0410.2002.00129.x. [DOI] [PubMed] [Google Scholar]
- 29.De Silvestro G, Bagatella P, Tison T, et al. Virus-inactivated plasma - Plasmasafe: a one-year experience. Blood Transfus. 2007;5:134–42. doi: 10.2450/2007.0004-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khorana AA, Francis CW, Blumberg N, et al. Blood transfusions, thrombosis, and mortality in hospitalized patients with cancer. Arch Intern Med. 2008;168:2377–81. doi: 10.1001/archinte.168.21.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGrath T, Koch CG, Xu M, et al. Platelet transfusion in cardiac surgery does not confer increased risk for adverse morbid outcomes. Ann Thorac Surg. 2008;86:543–53. doi: 10.1016/j.athoracsur.2008.04.051. [DOI] [PubMed] [Google Scholar]
- 32.Simak J, Gelderman MP. Cell membrane microparticles in blood and blood products: potentially pathogenic agents and diagnostic markers. Transfus Med Rev. 2006;20:1–26. doi: 10.1016/j.tmrv.2005.08.001. [DOI] [PubMed] [Google Scholar]


