Abstract
Using mixed-chain lipids, we have recorded cooling and heating curves of planar bilayer membranes while they passed the lipid phase transition range. With unmodified planar bilayers, spontaneous current fluctuations are observed near the lipid phase transition temperature (tc approximately 29 degrees C). This effect coincides with the expected and measured decrease in membrane capacitance. Carrier (valinomycin)-modified planar bilayers exhibit near tc an abrupt change from a high-conducting state above tc to the state of bare membrane conductance below tc. In contrast to this behavior, planar bilayers modified by pore-forming antibiotics (gramicidin A, alamethicin) do not show any peculiar effect at tc. However, at 22--23 degrees C a pronounced maximum in pore-induced conductance is seen. Whereas the gramicidin A pore abruptly stops stepwise fluctuations below approximately 16 degrees C, with alamethicin a few long-lasting pore and pore state fluctuations persist down to 10 degrees C. It is suggested that the carrier may freeze out into the membrane/water interface. The effects observed with pore-forming substances, on the other hand, are interpreted in terms of lateral phase separation into pure lipid and lipid/antibiotic domains.
Full text
PDF
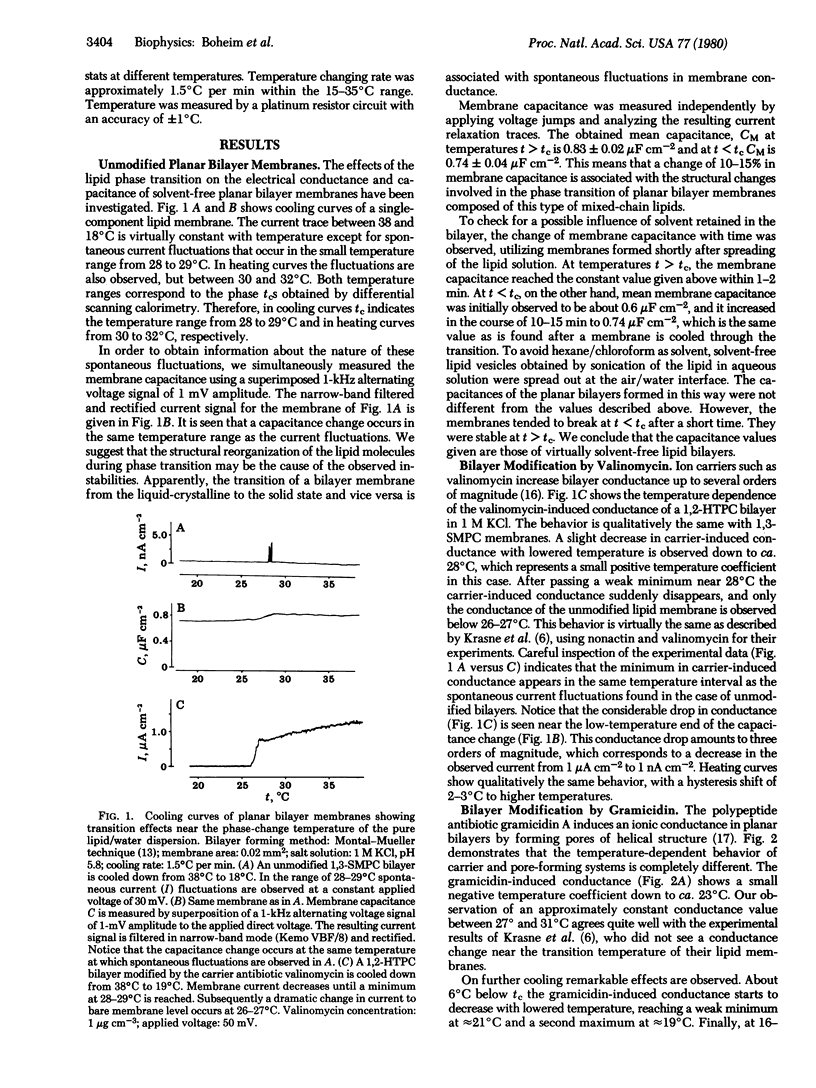


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonov V. F., Petrov V. V., Molnar A. A., Predvoditelev D. A., Ivanov A. S. The appearance of single-ion channels in unmodified lipid bilayer membranes at the phase transition temperature. Nature. 1980 Feb 7;283(5747):585–586. doi: 10.1038/283585a0. [DOI] [PubMed] [Google Scholar]
- Bamberg E., Läuger P. Channel formation kinetics of gramicidin A in lipid bilayer membranes. J Membr Biol. 1973;11(2):177–194. doi: 10.1007/BF01869820. [DOI] [PubMed] [Google Scholar]
- Bamberg E., Läuger P. Temperature-dependent properties of gramicidin A channels. Biochim Biophys Acta. 1974 Oct 29;367(2):127–133. doi: 10.1016/0005-2736(74)90037-6. [DOI] [PubMed] [Google Scholar]
- Chapman D., Cornell B. A., Ellasz A. W., Perry A. Interactions of helical polypepetide segments which span the hydrocarbon region of lipid bilayers. Studies of the gramicidin A lipid-water system. J Mol Biol. 1977 Jul 5;113(3):517–538. doi: 10.1016/0022-2836(77)90236-4. [DOI] [PubMed] [Google Scholar]
- Chapman D. Phase transitions and fluidity characteristics of lipids and cell membranes. Q Rev Biophys. 1975 May;8(2):185–235. doi: 10.1017/s0033583500001797. [DOI] [PubMed] [Google Scholar]
- Ehrenstein G., Lecar H. Electrically gated ionic channels in lipid bilayers. Q Rev Biophys. 1977 Feb;10(1):1–34. doi: 10.1017/s0033583500000123. [DOI] [PubMed] [Google Scholar]
- Eibl H., Arnold D., Weltzien H. U., Westphal O. Zur Synthese von alpha- und beta-Lecithinen und ihren Atheranaloga. Justus Liebigs Ann Chem. 1967;709:226–230. doi: 10.1002/jlac.19677090124. [DOI] [PubMed] [Google Scholar]
- Eibl H., Lands W. E. Phosphorylation of 1-alkenyl-2-acylglycerol and preparation of 2-acylphosphoglycerides. Biochemistry. 1970 Jan 20;9(2):423–428. doi: 10.1021/bi00804a033. [DOI] [PubMed] [Google Scholar]
- Eibl H. Phospholipid synthesis: Oxazaphospholanes and dioxaphospholanes as intermediates. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4074–4077. doi: 10.1073/pnas.75.9.4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg M., Hall J. E., Mead C. A. The nature of the voltage-dependent conductance induced by alamethicin in black lipid membranes. J Membr Biol. 1973 Dec 31;14(2):143–176. doi: 10.1007/BF01868075. [DOI] [PubMed] [Google Scholar]
- Engelman D. M. Lipid bilayer structure in the membrane of Mycoplasma laidlawii. J Mol Biol. 1971 May 28;58(1):153–165. doi: 10.1016/0022-2836(71)90238-5. [DOI] [PubMed] [Google Scholar]
- Fringeli U. P., Fringeli M. Pore formation in lipid membranes by alamethicin. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3852–3856. doi: 10.1073/pnas.76.8.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon L. G., Haydon D. A. Potential-dependent conductances in lipid membranes containing alamethicin. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):433–447. doi: 10.1098/rstb.1975.0021. [DOI] [PubMed] [Google Scholar]
- Hartmann W., Galla H. J., Sackmann E. Polymyxin binding to charged lipid membranes. An example of cooperative lipid-protein interaction. Biochim Biophys Acta. 1978 Jun 16;510(1):124–139. doi: 10.1016/0005-2736(78)90135-9. [DOI] [PubMed] [Google Scholar]
- Haydon D. A., Hladky S. B. Ion transport across thin lipid membranes: a critical discussion of mechanisms in selected systems. Q Rev Biophys. 1972 May;5(2):187–282. doi: 10.1017/s0033583500000883. [DOI] [PubMed] [Google Scholar]
- Hsu M. C., Chan S. I. Nuclear magnetic resonance studies of the interaction of valinomycin with unsonicated lecithin bilayers. Biochemistry. 1973 Sep 25;12(20):3872–3876. doi: 10.1021/bi00744a012. [DOI] [PubMed] [Google Scholar]
- Keough K. M., Davis P. J. Gel to liquid-crystalline phase transitions in water dispersions of saturated mixed-acid phosphatidylcholines. Biochemistry. 1979 Apr 17;18(8):1453–1459. doi: 10.1021/bi00575a011. [DOI] [PubMed] [Google Scholar]
- Krasne S., Eisenman G., Szabo G. Freezing and melting of lipid bilayers and the mode of action of nonactin, valinomycin, and gramicidin. Science. 1971 Oct 22;174(4007):412–415. doi: 10.1126/science.174.4007.412. [DOI] [PubMed] [Google Scholar]
- Lau A. L., Chan S. I. Nuclear magnetic resonance studies of the interaction of alamethicin with lecithin bilayers. Biochemistry. 1974 Nov 19;13(24):4942–4948. doi: 10.1021/bi00721a010. [DOI] [PubMed] [Google Scholar]
- Läuger P. Carrier-mediated ion transport. Science. 1972 Oct 6;178(4056):24–30. doi: 10.1126/science.178.4056.24. [DOI] [PubMed] [Google Scholar]
- Marcelja S., Wolfe J. Properties of bilayer membranes in the phase transition or phase separation region. Biochim Biophys Acta. 1979 Oct 19;557(1):24–31. doi: 10.1016/0005-2736(79)90086-5. [DOI] [PubMed] [Google Scholar]
- Marsh D., Watts A., Knowles P. F. Evidence for phase boundary lipid. Permeability of Tempo-choline into dimyristoylphosphatidylcholine vesicles at the phase transition. Biochemistry. 1976 Aug 10;15(16):3570–3578. doi: 10.1021/bi00661a027. [DOI] [PubMed] [Google Scholar]
- Montal M., Mueller P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3561–3566. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller P., Rudin D. O. Action potentials induced in biomolecular lipid membranes. Nature. 1968 Feb 24;217(5130):713–719. doi: 10.1038/217713a0. [DOI] [PubMed] [Google Scholar]
- Pagano R. E., Cherry R. J., Chapman D. Phase transitions and heterogeneity in lipid bilayers. Science. 1973 Aug 10;181(4099):557–559. doi: 10.1126/science.181.4099.557. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Jacobson K., Nir S., Isac T. Phase transitions in phospholipid vesicles. Fluorescence polarization and permeability measurements concerning the effect of temperature and cholesterol. Biochim Biophys Acta. 1973 Jul 6;311(3):330–348. doi: 10.1016/0005-2736(73)90314-3. [DOI] [PubMed] [Google Scholar]
- Pohl G. W., Knoll W., Gisin B. F., Stark G. Optical and electrical studies on dansyllysine-valinomycin in thin lipid membranes. Biophys Struct Mech. 1976 Aug 23;2(2):119–137. doi: 10.1007/BF00863705. [DOI] [PubMed] [Google Scholar]
- Schindler H., Rosenbusch J. P. Matrix protein from Escherichia coli outer membranes forms voltage-controlled channels in lipid bilayers. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3751–3755. doi: 10.1073/pnas.75.8.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Träuble H., Eibl H. Electrostatic effects on lipid phase transitions: membrane structure and ionic environment. Proc Natl Acad Sci U S A. 1974 Jan;71(1):214–219. doi: 10.1073/pnas.71.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Träuble H. Phasenumwandlungen in Lipiden. Mögliche Schaltprozesse in biologischen Membranen. Naturwissenschaften. 1971 Jun;58(6):277–284. doi: 10.1007/BF00624732. [DOI] [PubMed] [Google Scholar]
- Veatch W. R., Mathies R., Eisenberg M., Stryer L. Simultaneous fluorescence and conductance studies of planar bilayer membranes containing a highly active and fluorescent analog of gramicidin A. J Mol Biol. 1975 Nov 25;99(1):75–92. doi: 10.1016/s0022-2836(75)80160-4. [DOI] [PubMed] [Google Scholar]



