Abstract
This study used fMRI to measure brain activity during adult processing of cries of infants with autistic disorder (AD) compared to cries of typically developing (TD) infants. Using whole brain analysis, we found that cries of infants with AD compared to those of TD infants elicited enhanced activity in brain regions associated with verbal and prosodic processing, perhaps because altered acoustic patterns of AD cries render them especially difficult to interpret, and increased activity in brain regions associated with emotional processing, indicating that AD cries also elicit more negative feelings and may be perceived as more aversive and/or arousing. Perceived distress engendered by AD cries related to increased activation in brain regions associated with emotional processing. This study supports the hypothesis that cry is an early and meaningful anomaly displayed by children with AD. It could be that cries associated with AD alter parent-child interactions much earlier than the time that reliable AD diagnosis normally occurs.
Autistic Disorder (AD) is a neurodevelopmental disorder of children’s communicative and social skills as well as their motor repertoire. With an eye toward intervention and treatment, interest has now focused on the earliest stages of communication and social development in AD and how child characteristics altered by AD might influence children’s social relationships, especially with their caregivers.
Murdock, Cost, and Tieso (2007) suggested that alterations to communication in AD can vary from absence of verbal communication to pragmatic deficits in otherwise fluent speech. Because of the significance of AD in development, it is important to understand compromises to very early communication in AD; in infants, this means facial expression and vocalization. Some research has focused on the facial expressions of children with AD during the first years of life (Cassel et al., 2007), but fewer studies have been conducted on vocal development, especially crying, in children with AD (Bieberich and Morgan, 1998; Esposito and Venuti, 2008, 2010). Human infants display a range of affiliative behaviors to elicit care from mothers or other potential caregivers. Among the first channels of communication newborns and infants use to express their needs is to cry (Barr et al., 2000; Cecchini et al., 2007; Irwin, 2003; Gingras et al., 2005). Cry is a critical feature of infants’ first communications and is a vital early social signal in human development, but cry is also a well-researched physiological and psychopathological indicator (Acebo and Thoman, 1992, 1995; LaGasse et al., 2005; Rautava et al., 2007). Functionally, infant crying involves two agents: the infant who cries and the caregiver who listens to the crying, interprets it, and acts on that interpretation. Crying elicits physiological reactions in adults, such as increases in heart rate (Huffman et al., 1998) and endocrine responses (specifically, higher levels of prolactin; Fleming et al., 2002) that prompt care behaviors to reduce infant distress (Bowlby, 1969; Gustafson et al., 2000; LaGasse et al., 2005). Crying also activates the central nervous systems of both infant and caregiver, and this activation reinforces states of reciprocal attention. For these reasons crying is considered a core complex behavioral mechanism that regulates the infant-caregiver relationship, and has generated a large research literature on parents’ and others’ reactions.
Adult responses to infant cry reflect morphological characteristics of the cry itself (Gustafson and Green, 1989; Ziefman, 2003), caregiver characteristics (Frodi, 1995), and cultural norms (Barr et al., 1991). Here, we focused on specific prominent morphological characteristics.
Morphological characteristics, the acoustic features, of infant cry, include a variety of parameters that contribute to regulating adult responses (Gustafson and Green, 1989; Ziefman, 2003). However, cry pitch (related to frequency) has been identified as the most influential factor governing caregiver perceptions and responses (Lester and LaGasse, 2008); notably, higher-frequency cries are regularly perceived as more aversive and distressed than lower-frequency cries (Dessureau et al., 1998; Zeskind and Lester, 2001). Duration of pauses within an episode of cry is another important acoustic variable. Zeskind and colleagues (1992) modified the length of the expiratory phase and pauses in infant crying episodes. Listeners regarded shorter pauses as more activating and informative and also more distressed. Generally, the morphological characteristics of child cry elicit alertness, distress, and uneasiness (Zeskind et al., 1992).
Morphological characteristics of cries vary with child health and psychopathological status (Lester and LaGasse, 2008; Trevarthen and Daniel, 2005). For example, the cries of children with visceral colic are characterized by their high frequency and are typically perceived as more distressed. Neurological insult typically leads to overall higher levels of the fundamental frequency (f0). Most pertinent to the present study, analyses of acoustic features of cries of children with AD, compared to those of TD children, reveal a number of differences. Episodes of cry in children with AD have higher fundamental frequencies (Esposito and Venuti, 2008, 2010; Esposito et al., 2012). Atypical functioning of the brainstem and limbic system (both areas are compromised in children with AD; Amaral et al., 2008) has been invoked to explain anomalies found in the cry of children with AD (Bieberich and Morgan, 1998).
Previous behavioral research has focused on how parents perceive crying of children with AD compared to TD children (Venuti et al., 2004; Esposito and Venuti, 2008, 2009a, 2010a, b). In a clinical interview study, parents of children with AD often mentioned their negative feelings with respect to crying and that AD cries were not understandable in the sense that parents could not easily identify their cause (Esposito and Venuti, 2008). In a “Listen-and-Response” experiment designed to assess effects of the atypical structure of crying episodes characteristic of children with AD, adults also reported experiencing increasingly negative mental states (i.e., anxiety) when listening to AD cries and less negative mental states when listening to cries of TD children (Esposito and Venuti, 2008). A follow-up study confirmed that the higher fundamental frequencies acoustic characteristic of cries of children with AD accounted for the mental state uneasiness in listeners (Esposito and Venuti, 2010).
Given the evolutionary significance of infants’ cries, recent work has turned to investigate the loci of adult brain responses to cries of TD infants using neuroimaging techniques. Studies that compare cry to non-vocal stimuli, such as noise, have reported preferential activation of the thalamus, medial prefrontal cortex, right orbitofrontal cortex and cingulate cortex, midbrain, hypothalamus, dorsal and ventral striatum, and the lateral septal region (e.g., Loberbaum et al., 2002). Results of these studies are heterogeneous likely due to their wide methodological variation. When cry is compared to other emotional vocalizations, activity in the cingulate cortex, insula, amygdala, ventral prefrontal cortex, and temporo-parietal cortex is reported (e.g., Seifritz et al., 2003). Studies investigating responses to own versus other infant cry in parents have revealed preferential activation of midbrain, basal ganglia, cingulate, amygdala, and insula (Swain et al., 2003) as well as prefrontal cortex and hypothalamic regions (Swain et al., 2004). Overall, infant cries appear to activate brain areas associated with parenting care, processing of aversive and alarming stimulation, and empathy. Thus, neuroimaging data support the idea that cry is a key component of early parent-child transactions and a crucial infant communicative signal that activates a variety of care-related responses in adults (Sroufe, 2000; Trevarthen, 2003; Tronick, 2005).
With these several literatures in mind, notably evidence of atypical features of AD cry, and adult brain and behavior sensitivities to infant cry, the present study aimed to evaluate brain activity during adults’ processing of cries of infants with AD compared to cries of TD infants. Guided by this literature, we developed two main hypotheses. First, we expected increased activation of brain areas involved in auditory processing in response to cries of infants with AD compared to those of TD children; second, we expected increased activation in areas involved in processing of aversive and/or alarming stimuli in response to cries of infants with AD compared to those of TD children.
With respect to our first hypothesis, we expected increased activation at both primary and secondary processing levels. Specifically, in accord with AD cries’ atypical acoustic features, we expected cries of children with AD would activate the primary auditory cortex (BA41,42) more than cries of TD children as activation in this area is largely modulated by basic acoustic properties of the stimuli such as f0 (Woods et al., 2009). More importantly, we expected that, compared to cries of TD children, cries of children with AD would elicit enhanced activity in the secondary auditory cortex (BA 21, 22) which is involved in responding to behaviorally relevant complex sounds (Hart et al., 2003) and in bilateral inferior frontal areas that have been reported as relevant to processing emotional prosody (Wildgruber et al., 2006). These expectations reflect behavioral evidence from previous studies of the difficulties adults report in understanding and interpreting AD cry.
With respect to our second hypothesis, based on published behavioral studies showing that AD cries are perceived as more aversive and elicit more negative feelings compared to TD cries, we expected to find increased activity in brain structures involved in processing negative feelings typically induced by aversive and arousing stimuli, such as amygdala (Anderson and Phelps, 2001; Zald, 2003) and insula (Caria et al., 2010; Reiman et al., 1997; Wager et al., 2003). The increased activation in areas involved in processing of aversive and/or alarming stimuli in response to cries of children with AD compared to those of TD children would replicate and corroborate those of previous behavioral studies on subjective responses to cries of children with AD and would, for the first time, extend them to an objective neurophysiological level.
Before testing our two main hypotheses, AD cries were compared to white noise to verify that they elicited brain activation consistent with that reported in the literature about typical cry. Specifically, we expect that cries of children with AD activated temporo-parietal regions implicated in sound and voice processing as well as brain areas involved in parenting behaviors and, more generally, with processing of emotional stimuli, including thalamus, putamen, and insula (Swain et al. 2007).
Because crying is a fundamental biosocial phenomenon that reflects the status of the nervous system and influences early parent-child interactions (Lester, 1984; Lester et al., 1995, LaGasse et al., 2005), we hoped that the results of this study would shed light on the early communicative signals of children with AD, a developmental disorder of striking and increasing international incidence (Elsabbagh et al. 2012) whose initial ontogenetic stages are still largely unknown (Esposito et al. 2009a).
Methods
Participants
Twenty-one healthy adults (12 females: M age = 31.86 years, SD = 5.17; and 9 males: M age = 35.38 years, SD = 4.63; 11 primiparous parents of TD children (6F/5M), 1 non primiparous father and 9 non-parents (6F/3M; 20 right-handed, 1 left-handed) were recruited through the University of Trento webpage and local advertisements. Advertisement did not provide details of the study. Inclusion criteria were age between 18 and 40 years, ethnically homogeneous of European heritage, and, for parents, own children older than 3 years. Exclusion criteria were neurological or psychiatric disorders, substance abuse/dependence, psychotropic medications, and pregnancy. We planned to have an experimental group of about 20 participants as conventional fMRI studies require N = 16–20. Prior to the experiment, candidates were screened by a neurologist to check compatibility with MRI scan. Occupation and level of education varied in the sample, but most participants had attended (33%) or completed (38%) university. All participants gave written informed consent for participation, and experimental procedures were approved by the ethical committee for experiments involving humans at the University of Trento.
Acoustic Stimuli
A total of 20 acoustic excerpts of natural cry episodes from infants were used. Excerpts were extracted from 16 home videos of unedited cry bouts of 16 firstborns taken at 13 months of age. They belonged to one of two groups of children: autistic disorders (AD; n = 8, 4 boys/4 girls) and typically developing (TD; n = 8, 4 girls/4 boys). Children with AD received a clinical diagnosis at the age of ~ 3 years from a child psychiatrist according to DSM-IV-R criteria, confirmed by ADI-R, ADOS-G. Cries of infants with a diagnosis of Pervasive Developmental Disorder not otherwise specified (PDD-Nos) or Asperger Syndrome were not included. To prevent the presence in the stimulus sample of cases of autism in comorbidity with other psychopathologies, children with AD had to be free of other medical conditions (e.g., seizures, Fragile syndrome, etc.) and had no other known visual or hearing impairments. TD children were part of a longitudinal research project on typical development and did not present any significant medical or developmental concerns as confirmed by their normal scores on the Child Behavior Checklist (Achenbach and Rescorla, 2001). A research assistant who was unaware of the purposes of the study and blind to children’s group membership gleaned audio records of the two groups of 13-month-old children from a large pool of home videos. This set of videos was collected by the Observation and Functional Diagnosis Laboratory at University of Trento (Esposito and Venuti, 2008, 2009a, b, Esposito and Venuti, 2010, 2011). The home videos (e.g., segments from family play situations) did not vary systematically between groups.
From the pool of about 120 home videos, a different research assistant selected crying episodes with the best acoustic quality. To ensure that the cries used in this study were representative of the typical range of cry sounds for the two groups (AD and TD), the cry sounds were digitized and analyzed using the Praat acoustic analysis software (Boersma and Weenink, 2005). A long-term average spectrum (LTAS) was employed to provide spectral information for each cry episode. LTAS is used to discriminate cry characteristics of different categories of children (Lin and Green, 2007). For all episodes of crying, the First Spectral Peak (FSP) of the LTAS was obtained. FSP is the frequency value (in Hz) of the first amplitude peak across the LTAS. It is an estimate of the average fundamental frequency (f0) of the episode of crying (Lin and Green, 2007). The FSPs of cry episodes used in this study were for AD M = 637.87 (SD = 66.56) and for TD M = 526.87 (SD = 60.33), a significant difference, F(1,16)=18.8, p<.001. This result is consonant with previous findings that cries of children with AD at 13 months of age have higher f0 (e.g., Esposito and Venuti, 2010 a, b, 2011). Cries of children with AD at 13 months of age also have shorter pauses (AD M = 1.9 s, SD = 2.1; TD M= 4.7 s, SD = 1.3), F(1,15)=3.36, p ≤ .01). The durations of the original cry bouts across groups were analyzed as well; no difference emerged across groups (AD M = 27.3 s, SD = 12.9; TD M= 31.1 s, SD = 10.5), F(1,16)=2.06, ns. Audio clips of white noise (WN), controlled for f0 and duration, were used as additional comparison stimuli. All acoustic files were edited using computer software to normalize them for volume and duration (10 s) and to remove all background noise.
fMRI protocol
During functional scanning, participants passively listened to the acoustic stimuli presented binaurally at ~ 75 dB SPL using Serene Sound (Resonance Technologies, Northridge, CA) headphones, with stereo quality sound (40 Hz to 40KHz frequency response) and passive scanner noise attenuation (30 dB). Subjects underwent a single fMRI run in which stimuli were presented in a blocked design. Acoustic stimuli of each category (TD, AD and WN) lasted 10 sec, with an inter-stimulus interval of 14 sec during which no stimuli was presented. The stimulus categories were presented in the following fixed order: TD, WN, AD, WN repeated for a total of ten times. The ten cry stimuli in each category (10 TD and 10 AD) were different, and they were pseudo-randomized between participants. Each individual cry was presented once to reduce habituation effects.
fMRI data acquisition
Participants underwent MRI scanning at 4 Tesla in a MedSpec Biospin MR scanner (Bruker Ettlingen, Germany) and an 8-channel birdcage head coil. Mild external head restraint was used to minimize head movement during scanning. Before collecting functional images, a high-resolution T1 weighted image of the whole brain (MPRAGE: 176 slices, GRAPPA acquisition with an acceleration factor of 2, FOV=256×256 mm2, voxel size = 1×1×1 mm, TI = 1020 ms TE = 4.18 ms, TR = 2700 ms) was acquired for the purpose of spatial coregistration. Whole-brain functional data were acquired using echoplanar imaging, sensitive to BOLD contrast (34 slices, tilted 18° from intracommisural plane, FOV=192×192 mm, voxel size = 3×3×3 mm, slice gap = 15%, flip angle (FA), 73°, TE = 33 ms, TR = 2 s per volume). We performed an additional scan to measure the point-spread function (PSF) of the acquired sequence, which served for distortion correction that is expected with high-field imaging. The experimental session consisted of 489 whole brain images per participant; these included four dummy scans at the start of each time series to allow for T1 equilibration. The total experiment lasted about 20 min.
fMRI data analysis
To correct for distortions in geometry and intensity in the EPI images, we applied distortion correction on the basis of the PSF data acquired before the EPI scans (Zeng and Constable, 2002). The fMRI time series data were analyzed using SPM5 (http://www.fil.ion.ucl.ac.uk/spm/software/spm5/) on a Matlab platform (Mathworks Inc.). Echoplanar images were corrected for head movement, and the T1 image was coregistered to the mean of realigned images. Functional images were then normalized to the Montreal Neurological Institute (MNI) stereotaxic standard space and then spatially (9-mm full-width half-maximum Gaussian kernel) and temporally (cut-off period 256 s) smoothed. For each participant, an analytic design matrix was constructed, modeling onsets and duration of each trial as epochs convolved with a hemodynamic response function. The three stimulus types (AD, TD, WN) were modeled as separate regressors and interrogated to derive contrast images for second-level (group) mixed-effect analysis using a general linear model (GLM). For each participant, contrast images of AD cries versus WN and AD versus TD cries were created. These images were then entered into a second-level (random-effects) analysis to allow inferences across participants that generalize to the population. One sample t-tests on the contrast images imported from the first-level analysis were performed to assess group effects across all participants. The t-tests indicated whether observed differences between AD cry and WN, and AD cry and TD cry, differed significantly from zero (Holmes and Friston, 1998). Threshold significance for functional imaging data was set at p < 0.01, corrected using a cluster-wise (k = 10) false discovery rate (FDR) correction for multiple comparisons (Chumbley and Friston, 2009). A whole brain analysis was performed.
Results
fMRI data
AD cry versus WN comparison
AD cry compared to WN elicited activity in several cortical and subcortical regions of both hemispheres. Specifically, bilateral activity was detected in superior temporal gyri and poles, inferior frontal gyri (pars triangularis and opercularis), middle temporal gyri, thalamus, and precentral gyri. Additionally, we found preferential activity in the left Heschl gyrus, superior frontal gyrus, insula, putamen, and supplementary motor area (p < 0.01, FDR corrected; Table 1, Fig. 1).
Table 1.
AD cry > noise
| Location | Side | Coordinates(MNI) | Brodmann Area (BA) | t value | ||||
|---|---|---|---|---|---|---|---|---|
| Superior temporal gyrus | L | −51 | −6 | 0 | 22 | 20.02 | ||
| Superior temporal gyrus | R | 60 | −9 | −3 | 21 | 18.40 | ||
| Heschl gyrus | L | −39 | 27 | 12 | 41 | 14.55 | ||
| Superior temporal pole | R | 51 | −3 | −12 | 38 | 13.91 | ||
| Middle temporal gyrus | R | 63 | −36 | 3 | 22 | 13.38 | ||
| Superior temporal gyrus | R | 63 | −33 | 12 | 42 | 12.97 | ||
| Superior temporal pole | L | −45 | 6 | −12 | 38 | 10.85 | ||
| Inferior frontal gyrus (pars triangularis) | R | 51 | 27 | 3 | 45 | 10.55 | ||
| Inferior frontal gyrus (pars triangularis) | L | −48 | 27 | 9 | 45 | 8.16 | ||
| Inferior frontal gyrus (pars opercularis) | R | 54 | 15 | 18 | 44 | 7.61 | ||
| Middle temporal gyrus | L | −66 | −45 | 9 | 22 | 7.53 | ||
| Inferior frontal gyrus (pars opercularis) | L | −39 | 30 | −6 | 45 | 7.45 | ||
| Superior frontal gyrus | L | −3 | 57 | 39 | 9 | 7.41 | ||
| Insula | L | −45 | 18 | 0 | 47 | 7.33 | ||
| Putamen | L | −18 | 3 | 9 | 6.75 | |||
| Thalamus | R | 12 | −12 | 6 | 6.70 | |||
| Supplementary motor area | L | −3 | 15 | 66 | 6 | 6.60 | ||
| Precentral gyrus | L | 51 | −3 | 48 | 6 | 6.50 | ||
| Thalamus | L | −9 | −12 | 6 | 6.33 | |||
| Precentral gyrus | R | −48 | 3 | 54 | 6 | 5.66 | ||
Note. Significant BOLD activations during processing of cries of infants with AD compared to white noise
Figure 1. AD cry > Noise.

Note. SPM t- maps of adults brain activations in response to cries of AD infants compared to noise. (p< 0.001, uncorrected for visualization). SFG = Superior frontal gyrus. SMA = Supplementary motor area. Th = Thalamus. IFG/AI = inferior frontal gyrus/anterior insula. STG = Superior temporal gyrus
AD cry versus TD cry comparison
AD cries activated the superior temporal and inferior frontal gyri bilaterally (BA 21, 22, 41, 42). Moreover, activity in the right superior frontal gyrus (BA 9) was observed. The maximum activation was observed in a cluster with a peak in the left superior temporal gyrus extending to the supramarginal gyrus (BA 40). The cluster of activity in the left inferior frontal gyrus included the anterior insula (p < 0.01, FDR corrected; Table 2, Fig. 2). No clusters were significantly activated by TD compared to AD cries. Assessments of participant gender and parental status showed no differences when comparing male and female participants, or parents and non-parents, in the contrasts of interest (using a p- value corrected for multiple comparisons).
Table 2.
AD cry > TD cry
| Location | Side | Coordinates(MNI) | BroNdmann Area (BA) | t value | |||
|---|---|---|---|---|---|---|---|
| Superior temporal gyrus | L | −42 | −30 | 3 | 21 | 7.43 | |
| Superior temporal gyrus | R | 45 | −33 | 9 | 41 | 6.84 | |
| Superior frontal gyrus | R | −3 | 54 | 39 | 9 | 6.62 | |
| Superior temporal gyrus | R | 48 | −18 | −9 | 22 | 6.38 | |
| Superior temporal gyrus | R | 57 | −36 | 18 | 42 | 6.01 | |
| Supramarginal gyrus | L | −54 | −36 | 27 | 40 | 5.93 | |
| Inferior frontal gyrus (pars triangularis) | L | −54 | 18 | 24 | 44 | 5.86 | |
| Inferior frontal gyrus (pars orbitalis) | R | 54 | 33 | −6 | 47 | 5.62 | |
| Inferior frontal gyrus (pars orbitalis)/anterior insula | L | −39 | 27 | −6 | 47 | 4.41 | |
Note. Significant BOLD activations during processing of cries of infants with AD compared to cries of TD infants.
Figure 2. AD cry > TD cry.
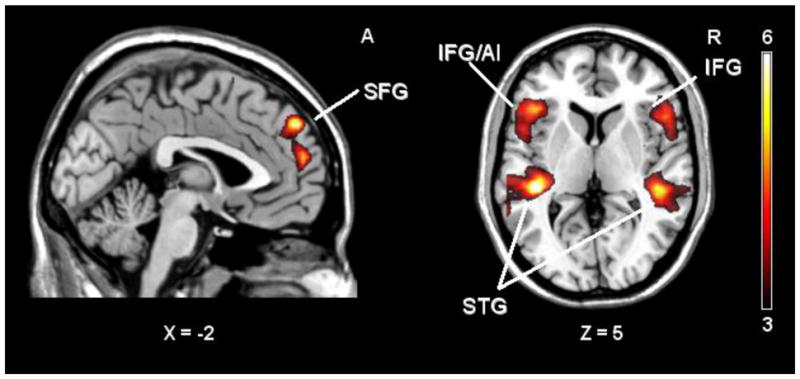
Note. SPM t- maps of adults brain activations in response to cries of AD infants compared to TD infants. (p< 0.001, uncorrected for visualization). SFG = Superior frontal gyrus. IFG/AI = inferior frontal gyrus/anterior insula. IFG = inferior frontal gyrus. STG = Superior temporal gyrus
Behavioral data
To further test our hypothesis that AD cries compared to TD cries elicit increased activity in brain structures involved in processing aversive or arousing stimuli, following the scanning session participants rated on a 4-point Likert-type scale (ranging from not at all to extremely) their feeling of distress when hearing each cry. Repeated-measures ANOVA (RM-ANOVA) on these scale scores used cry type (AD vs. TD) as a repeated factor. A main effect of cry type emerged. Participants reported feeling more distress when they heard AD cries (M = 2.77, SD = .59) compared to TD cries (M = 2.07, SD = .59), F(1, 19) = 29.43. We then computed for each participant: (1) the change of activation in the left inferior frontal gyrus/anterior (MNI coordinates: −39, 27, −6) during AD cries versus TD cries (AD minus TD BOLD percent signal change) and (2) the mean difference in ratings of distress for the two types of cries (AD minus TD distress rating). The Pearson correlation between these two measures was positive and significant(r=0.51, df= 19, p=0.01; Fig. 3).
Figure 3. Correlation of distress rating and activity in IFG/AI.
Note. The inter-individual significant positive Pearson correlation between (1) the change of activation in the left inferior frontal gyrus/anterior insula (MNI coordinates: −39, 27, −6) during AD versus TD cries and (2) the mean difference in the rating of distress for AD versus TD cries (r=0.51, p=0.01).
Additionally, participants rated all cries on a similar Likert-type scale for their desire to care for the child. With this rating we wanted to assess whether AD and TD cries elicit different care responses. No RM-ANOVA main effect emerged for the desire-to-care response to AD cry (M = 2.63, SD = .62) compared to TD cry (M = 2.62, SD = .67), F(1, 19) =.01, ns. Both cry types elicited equivalent ratings of participants’ desire to care for a crying infant.
Discussion
The present study aimed to assess whether cries of infants later diagnosed with AD elicit specific brain responses in adults compared to otherwise matched cries of TD infants. Cries of children with AD have specific acoustic features and represent one of the earliest anomalies that specifically characterize Autistic Disorder (Esposito and Venuti, 2010). Based on previous behavioral studies indicating that adults report comprehension difficulties and more negative emotional experiences when listening to cries of children with AD compared to those of TD children (Esposito and Venuti, 2008), we hypothesized, first, that cries of children with AD would elicit activity in brain areas implicated in auditory processing and interpretation of the human voice and, second, in brain regions mediating emotional responses to aversive and arousing stimuli. To test these hypotheses we applied fMRI during passive listening of 10 s of continuous cry episodes of infants later diagnosed with AD and cries of TD children and with white noise. Prior to the fMRI experiment we analyzed the acoustic characteristics of the selected cry episodes and verified that AD cries possess atypical features, such as higher fundamental frequency and shorter pauses. Studies of neurological responses to infant cry are of moment for several reasons. infant crying facilitates bonding, and this bonding is instrumental to infant survival. Infant survival is facilitated because, when hearing the infant cry, the caregiver is alerted and can respond to the infant’s primary needs (Murray, 1979) as well as the infant’s affective, social, and cognitive requirements. Moreover, caregivers’ behavioral responsiveness to infant cry has historically been conceived to play an important role in affective, social, cognitive, and language development in the child (Ainsworth et al., 1978; Bowlby, 1969).
Compared to white noise, cries of infants with AD elicited brain responses in line with previous neuroimaging studies conducted on cries of TD children (Lorberbaum et al., 1999, 2002; Seifritz et al., 2003, Swain et al., 2003). Specifically, cries of children with AD activated temporo-parietal regions implicated in sound and voice processing as well as brain areas involved in parenting behaviors and, more generally, with processing of emotional stimuli, including thalamus, putamen, and insula (Swain, Lorberbaum, Kose, and Strathearn, 2007). Additionally, activity in supplementary motor cortex and precentral gyri emerged, suggesting preparation to care behaviors (Nachev et al., 2008).
Supporting our first main hypothesis, we found that cries of children with AD compared to those of TD children specifically activate brain regions critical for second level acoustic processing in addition to regions associated with the analysis of basic acoustic features. Cries of children with AD preferentially activated the primary auditory cortex (BA 41, 42) as well as the secondary auditory cortex (BA 21, 22 including Wernicke’s area), together with the inferior frontal gyrus bilaterally (including Broca’s area) and the left supramarginal gyrus. Whereas activity in the primary auditory cortex is largely activated by basic acoustic properties of stimuli (such as frequency), the secondary auditory cortex responds to behaviorally relevant complex sounds (Hart et al., 2003). Moreover, activation of the superior temporal cortex and IFG in the right and left hemispheres has been found to subserve comprehension of emotional prosody (Leitman, Wolf, Ragland, Laukka, Loughead, Valdez, et al., 2010; Wildgruber, Ackermann, Kreifelts, and Ethofer, 2006). In particular, the right IFG plays a critical role in processing emotional information and evaluating the affective salience of speech (Rota et al., 2009; Friederici and Alter, 2004). Moreover, the bilateral superior temporal cortex is specifically associated with voice discrimination during changing auditory conditions which require sustained auditory attention to target stimuli (Ikeda et al., 2010). The left supramarginal gyrus is also implicated in voice processing; specifically, preferential activity of the left supramarginal gyrus occurs when speech comprehension is made more difficult at the syntactic, semantic, or perceptual levels (Price, 2010) and during reading.
Cries of infants later diagnosed with AD compared to white noise also activated the right superior frontal gyrus (dorso-medial prefrontal cortex, BA9) that is implicated in cognitive abilities such as executive function (Cohen et al., 1996) and working memory (Townsend et al., 2010). Nakai and colleagues (2005) reported that discrimination of human speech is associated with activation in BA9 together with Wernicke’s area and Broca’s area. Preferential activation in this area during listening to AD cries may reflect efforts to attend to a vocal communicative signal with atypical features.
In short, it seems that listening to the cries of children with AD calls for deeper and more effortful auditory attention and comprehension, and in particular comprehension of “emotional content” which may be compromised in the cries of infants with AD. The augmented recruitment of brain areas involved in human voice processing may be attributable to altered acoustic patterns that typify the cries of children with AD and render them difficult to interpret. These findings accord with behavioral studies that have reported difficulty in decoding the causes of AD cries vis-à-vis TD cries by healthy parents (Esposito and Venuti, 2008). Cries of children with AD have been described as “inexplicable” by parents (Esposito and Venuti, 2008).
Consistent with our second hypothesis, cries of children with AD compared to those of TD children activated the left inferior frontal gyrus/anterior insula which have been found to mediate brain responses to aversive and arousing stimuli. Our participants subjectively rated cries of children with AD as more distressing than those of TD children. The anterior insular region has also been found to activate during awareness (Singer et al., 2004, Singer et al., 2006) or viewing (Jackson et al., 2006; Lamm et al., 2007; Morrison et al., 2004) of another individual’s pain. Previous neuroimaging studies of TD children’s cries revealed that the insula is activated by child cry in general and by own children’s cry in particular (Lorberbaum, 2002; Seifritz, 2003; Swain et al., 2003, 2004). More generally, the insula is associated with subjective perceptions of emotional states (Craig, 2002, 2003) and awareness of emotionally salient stimuli (Craig, 2009; Critchley et al., 2004). Further evidence of the role of the left insula in mediating negative emotions comes from an investigation by Caria and colleagues (2010) who, using neurofeedback, demonstrated that increased activity of insula led to perceiving aversive stimuli as more unpleasant. Additionally, the insular cortex has been suggested as being an arousal center, participating in emotional responses to potentially distressing cognitive or interoceptive sensory stimuli (Reiman et al., 1997). The insular cortex is also implicated in sound detection, non-verbal processing, and auditory temporal processing (Bamiou et al., 2003; Levitin et al., 2003; Lorberbaum et al., 2002; Seifritz et al., 2003, Swain et al., 2003, 2004).
In short, it appears that, compared to cries of TD infants, cries of infants later diagnosed with AD elicit more negative feelings and are perceived as more aversive and/or arousing. Behavioral data acquired after the fMRI experiment help to confirm this interpretation. The altered acoustic characteristics of cries of AD infants, and especially their high fundamental frequencies and shorter pauses, may help to account for participants’ negative emotional reactions, as higher-frequency cries are often perceived as more aversive and distressed than lower-frequency cries (Lester and LaGasse, 2008). Moreover, the difficulty in interpreting AD cries may engender feelings of uneasiness. That said, participants’ subjective ratings indicated that cries of children’s with AD and TD elicited equivalent levels of desire to care for the crying child. Although atypical and more distressing, AD cries appear to activate adults’ intentions to reduce infant distress with the same intensity as TD cries.
This study has two notable limitations. First, a stronger test of the specificity of brain responses to AD cries might involve comparison of cries of children with other developmental disorders. However, previous behavioral studies have shown that acoustic features (high pitch sounds, aspiration/expiration, pause) of cries of children with some developmental delays do not differ from those of cries of TD children but do differ from those of children with AD (Esposito and Venuti, 2009b). To limit the duration of the participants in the scanner in present experiment, and to reduce risks associated with habituation effects, we did not include additional types of child cry. Although we cannot draw conclusions about the specificity to AD, we believe that the results deepen our understanding of communicative behavior in a disorder whose earliest developmental stages are still largely unknown. Second, our sample included a mix of females and males, parents and non-parents, and this sample composition modifies the generalizability of our findings. On the one hand, we found no gender or parenthood status effects, and therefore believe that our findings may be broadly generalizable. In this first study of neurobiological responses to cries of children with AD, we aimed to include both adults with and without previous parenting experience because they represent the range of caregivers of children with AD. On the other hand, Seifritz and colleagues (2003) showed that parenthood modulated amygdala response to cry of unfamiliar TD children but did not influence activity in other brain areas involved in cry processing. Clearly, larger numbers of participants may modify these conclusions.
In terms of future work, several other possible paths are laid out by these initial findings. Endocrine responses are often activated while listening to child cries (higher levels of prolactin, for example, in Fleming et al. 2002. We have not considered them in this study. Theoretically, we would expect a role of endocrine responses on brain activation. For this reason we aim to include assessments of different hormones in association with brain area activation in future studies of typical and atypical cry perception.
In conclusion, the present study identifies brain regions activated by cries of AD infants. These regions may underlie why adults perceive this crucial infant communication signal as less intelligible and more distressing. The differential neurobiological responses in adults activated by cries of infants with AD versus TD may also underpin adult interactions with infants with AD much earlier than reliable AD diagnosis.
Research Highlights.
Cries of infants with autistic disorder compared to those of typically developping infants elicite enhanced activity in brain regions involved in verbal and prosodic processing.
Cries of children with autistic disorder elicite increased activity in the left inferior frontal gyrus/anterior insula.
Autistic cries may elicit more negative feelings and may be perceived as more aversive and/or arousing.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, NICHD. The fMRI experiment was performed at the Center for Mind/Brain Sciences (CiMeC) of the University of Trento. We thank Paola Rigo for assistance.
References
- Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nature Reviews Genetics. 2008;9:341–55. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acebo C, Thoman EB. Crying as a social behaviour. Infant Mental Health Journal. 1992;13:67–82. [Google Scholar]
- Acebo C, Thoman EB. Role of infant crying in the early mother–infant dialogue. Physiological Behaviour. 1995;57:541–547. doi: 10.1016/0031-9384(94)00345-6. [DOI] [PubMed] [Google Scholar]
- Achenbach TM, Rescorla LA. Manual for the ASEBA Preschool forms and Profiles. Burlington, VT: University of Vermon; 2000. [Google Scholar]
- Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends in Neurosciences. 2008;31:137–45. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Bamiou D, Musiek FE, Luxon LM. The insula (Island of Reil) and its role in auditory processing. Literature review. Brain Research Reviews. 2003;42:143–154. doi: 10.1016/s0165-0173(03)00172-3. [DOI] [PubMed] [Google Scholar]
- Barr RG, Konner M, Bakeman R, Adamson L. Crying in Kung San infants: A test of cultural specificity hypothesis. Developmental Medicine and Child Neurolology. 1991;33:601–10. doi: 10.1111/j.1469-8749.1991.tb14930.x. [DOI] [PubMed] [Google Scholar]
- Bieberich A, Morgan SB. Brief report: affective expression in children with autism or Down’s syndrome. Journal of Autism and Developmental Disorders. 1998;28:333–8. doi: 10.1023/a:1026016804357. [DOI] [PubMed] [Google Scholar]
- Bowlby J. Attachment. Vol. 1. London: Hogarth Press; 1969. Attachment and Loss. [Google Scholar]
- Caria A, Sitaram R, Veit R, Begliomini C, Birbaumer N. Volitional Control of Anterior Insula Activity Modulates the Response to Aversive Stimuli. A Real- Time Functional Magnetic Resonance Imaging Study. Biological Psychiatry. 2010;68:425–432. doi: 10.1016/j.biopsych.2010.04.020. [DOI] [PubMed] [Google Scholar]
- Cassel TD, Messinger DS, Ibanez LV, Haltigan JD, Acosta SI, Buchman AC. Early Social and Emotional Communication in the Infant Siblings of Children with Autism Spectrum Disorders: An Examination of the Broad Phenotype. Journal of Autism and Developmental Disorders. 2007;37:122–132. doi: 10.1007/s10803-006-0337-1. [DOI] [PubMed] [Google Scholar]
- Cecchini M, Lai C, Langher V. Communication and crying in newborns. Infant Behavior and Development. 2007;30(4):655–65. doi: 10.1016/j.infbeh.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Chumbley JR, Friston KJ. False discovery rate revisited: FDR and topological inference using Gaussian random fields. Neuroimage. 2009;44:62. doi: 10.1016/j.neuroimage.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Cohen MS, Kosslyn SM, Breiter HC, DiGirolamo GJ, Thompson WL, Anderson AK, Bookheimer SY, Rosen BR, Belliveau JW. Changes in cortical activity during mental rotation: A mapping study using functional MRI. Brain. 1996;119:89–10. doi: 10.1093/brain/119.1.89. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: The sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: The sense of the physiological condition of the body. Current Opinion in Neurobiology. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel now? The anterior insula and human awareness. Nature Review Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Dessureau BK, Hiatt FL. A reassessment of the role of pitch and duration in adults responses to infant crying. Infant Behavior and Development. 1998;21:366–371. [Google Scholar]
- Elsabbagh M, Divan G, Koh YJ, Kim YS, Kauchali S, Marcín C, Montiel-Nava C, Patel V, Paula CS, Wang C, Yasamy MT, Fombonne E. Global Prevalence of Autism and Other Pervasive Developmental Disorders. Autism Research. 2012 doi: 10.1002/aur.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G, Venuti P. How is crying perceived in children with Autistic Spectrum Disorder? Research in Autism Spectrum Disorders. 2008;2:371–84. [Google Scholar]
- Esposito G, Venuti P, Maestro S, Muratori F. Movement in infants with Autism Spectrum Disorder: the analysis of lying. Brain and Development. 2009a;31:131–8. doi: 10.1016/j.braindev.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Esposito G, Venuti P. Comparative analysis of crying in children with Autism, Developmental Delays, and Typical Development. Focus on Autism and Other Developmental Disabilities, and Development. 2009b;24(4):240–247. [Google Scholar]
- Esposito G, Venuti P. Understanding early communication signals in autism: a study of the perception of infants’ cry. Journal of Intellectual Disability Research. 2010a;54(3):216–23. doi: 10.1111/j.1365-2788.2010.01252.x. [DOI] [PubMed] [Google Scholar]
- Esposito G, Venuti P. Developmental changes in the fundamental frequency (f0) of infants’ cries: a study of children with Autism Spectrum Disorder. Early Child Development and Care. 2010b;180(8):1093–1102. [Google Scholar]
- Esposito G, Venuti P, Bornstein MH. Assessment of Distress in Young Children: A Comparison of Autistic Disorder, Developmental Delay, and Typical Development. Research in Autism Spectrum Disorders. 2011;5:1510–1516. doi: 10.1016/j.rasd.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G, Nakazawa J, Venuti P, Bornstein MH. Perceptions of distress in young children with autism compared to typically developing children: a cultural comparison between Japan and Italy. Research in Developmental Disability. 2012 doi: 10.1016/j.ridd.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming AS, Corter C, Stallings J, Steiner M. Testosterone and prolactin are associated with emotional responses to infant cries in new fathers. Hormones and Behavior. 2002;42:399–413. doi: 10.1006/hbeh.2002.1840. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Alter K. Lateralization of auditory language functions: A dynamic dual pathway model. Brain and Language. 2004;89:267–276. doi: 10.1016/S0093-934X(03)00351-1. [DOI] [PubMed] [Google Scholar]
- Frodi A. When emphathy fails. Avversative infant crying and child abuse. In: Lester BM, Boukydis CFZ, editors. Infant crying: theoretical and research perspectives. New York: Plenum; 1995. pp. 263–77. [Google Scholar]
- Gingras JL, Mitchell EA, Grattan KE. Fetal homologue of infant crying. Archives of Disease in Childhood- Fetal and Neonatal Edition. 2005;90:415–418. doi: 10.1136/adc.2004.062257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub HL. A physioacoustic model of the infant cry. In: Lester MB, Boukydis CFZ, editors. Infant crying: Theoretical and research perspectives. New York: Plenum; 1989. pp. 59–82. [Google Scholar]
- Gustafson G, Wood R, Green J. Can we hear the causes of infants’ crying? In: Barr R, Hopkins B, Green J, editors. Crying as a sign, a signal, and a symptom. London: Mac Keith; 2000. pp. 8–22. [Google Scholar]
- Gustafson GE, Green JA. On the importance of fundamental frequency and other acoustic features in cry perception and infant development. Child Development. 1989;60:772–80. [PubMed] [Google Scholar]
- Holmes AP, Friston KJ. Generalisability, random effects and population inference. Neuroimage. 1998;7:S754–70. [Google Scholar]
- Huffman LC, Bryan YE, del Carmen R, Pedersen FA, Doussard-Roosevelt JA, Porges SW. Infant temperament and cardiac vagal tone: assessments at twelve weeks of age. Child Development. 1998;69:624–35. [PubMed] [Google Scholar]
- Irwin JR. Parent and non-parent perception of the multimodal infant cry. Infancy. 2003;4:503–516. [Google Scholar]
- Iverson MJ, Wozniak RH. Variation in vocal-motor development of infant siblings of children with autism. Journal of Autism and Developmental Disorders. 2007;37:158–170. doi: 10.1007/s10803-006-0339-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Yahata N, Takahashi H, Koeda M, Asai K, Okubo Y, Suzuki H. Cerebral activation associated with speech sound discrimination during the diotic listening task: An fMRI study. Neuroscience Research. 2010;67:65–71. doi: 10.1016/j.neures.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Meltzoff AN, Decety J. How do we perceive the pain of others: A window into the neural processes involved in empathy. Neuroimage. 2005;24:771–779. doi: 10.1016/j.neuroimage.2004.09.006. [DOI] [PubMed] [Google Scholar]
- LaGasse LL, Neal AR, Lester BM. Assessment of infant cry: acoustic cry analysis and parental perception. Mental Retardation and Developmental Disabilities. 2005;11:83–93. doi: 10.1002/mrdd.20050. [DOI] [PubMed] [Google Scholar]
- Lamm C, Batson CD, Decety J. The neural substrate of human empathy: Effects of perspective-taking and cognitive appraisal. Journal of Cognitive Neuroscience. 2007;19:42–58. doi: 10.1162/jocn.2007.19.1.42. [DOI] [PubMed] [Google Scholar]
- Leitman DI, Wolf DH, Ragland JD, Laukka P, Loughead J, Valdez JN, et al. “It’s Not What You Say, But How You Say it”: A reciprocal temporo-frontal network for affective prosody. Frontiers in Human Neuroscience. 2010;4:19. doi: 10.3389/fnhum.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitin DJ, Menon V. Musical structure is processed in “language” areas of the brain: a possible role for Brodmann Area 47 in temporal coherence. NeuroImage. 2003;20:2142–2152. doi: 10.1016/j.neuroimage.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Lorberbaum JP, Newman JD, Dubno JR, Horwitz AR, Nahas Z, Teneback CC, Bloomer CW, Bohning DE, Vincent D, Johnson MR, Emmanuel N, Brawman-Mintzer O, Book SW, Lydiard RB, Ballenger JC, George MS. Feasibility of using fMRI to study mothers responding to infant cries. Depression and Anxiety. 1999;10:99–104. doi: 10.1002/(sici)1520-6394(1999)10:3<99::aid-da2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Lorberbaum JP, Newman JD, Horwitz AR, Dubno JR, Lydiard RB, Hamner MB, Bohning DE, George MS. A potential role for thalamocingulate circuitry in human maternal behavior. Biological Psychiatry. 2002;51:431–445. doi: 10.1016/s0006-3223(01)01284-7. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Pickles A, Rutter M. Autism Diagnostic Observation Schedule - Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Morrison I, Lloyd D, di Pellegrino G, Roberts N. Vicarious responses to pain in anterior cingulate cortex: Is empathy a multisensory issue? Cognitive, Affective and Behavioral Neuroscience. 2004;4:270–278. doi: 10.3758/cabn.4.2.270. [DOI] [PubMed] [Google Scholar]
- Murdock LC, Cost HC, Tieso C. Measurement of social communication skills of children with autism spectrum disorders during interactions with typical peers. Focus on Autism and Other Developmental Disabilites. 2007;22:160–172. [Google Scholar]
- Murray AD. Infant crying as elicitor of parental behaviour: An examination of two models. Psychological Bullettin. 1979;86:191–215. [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nature Reviews Neuroscience. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Nakai T, Kato C, Matsuo K. An fMRI study to investigate auditory attention: a model of the cocktail party phenomenon. Magnetic Resonance in Medical Science. 2005;4:75–82. doi: 10.2463/mrms.4.75. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Region of interest analysis for fMR. Scan. 2007;2:67–70. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Annals of the New York Academy Of Sciences. 2010;1191:6268. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Rautava L, Lempinen A, Ojala S, Parkkola R, Rikalainen H, Lapinleimu H, et al. the PIPARI Study Group. Acoustic quality of cry in very-lowbirth- weight infants at the age of 1 1/2 years. Early Human Development. 2007;83:5–12. doi: 10.1016/j.earlhumdev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Lane RD, Ahern GL, Schwartz GE, Davidson RJ, Friston KJ, et al. Neuroanatomical correlates of externally and internally generated human emotion. America Journal of Psychiatry. 1997;154:918–925. doi: 10.1176/ajp.154.7.918. [DOI] [PubMed] [Google Scholar]
- Rota G, Sitaram R, Veit R, Erb M, Weiskopf N, Dogil G, Birbaumer N. Self-regulation of regional cortical activity using real-time fMRI: The right inferior frontal gyrus and linguistic processing. Human Brain Mapping. 2009;30:1605–1614. doi: 10.1002/hbm.20621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifritz E, Esposito F, Neuhoff JG, Luthi A, Mustovic H, Dammann G, von Bardeleben U, Radue EW, Cirillo S, Tedeschi G, Di Salle F. Differential sex-independent amygdala response to infant crying and laughing in parents versus nonparents. Biological Psychiatry. 2003;54:1367–1375. doi: 10.1016/s0006-3223(03)00697-8. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty JP, Stephan KE, Dolan RJ, Frith CD. Empathic neural responses are modulated by the perceived fairness of others. Nature. 2006;439:466–469. doi: 10.1038/nature04271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sroufe AL. Early relationships and the development of children. Infant Mental Health Journal. 2000;21:67–74. [Google Scholar]
- Swain JE, Leckman JF, Mayes LC, Feldman R, Constable RT, Schultz RT. The neural circuitry of parent-infant attachment in the early postpartum. Proceedings of the American College of Neuropsychopharmacology; Puerto Rico. 2003. [Google Scholar]
- Swain JE, Leckman JF, Mayes LC, Feldman R, Constable RT, Schultz RT. Neural substrates and psychology of human parent-infant attachment in the postpartum. Biological Psychiatry. 2004;55:153S. [Google Scholar]
- Swain JE, Lorberbaum JP, Kose S, Strathearn L. Brain basis of early parent-infant interactions: psychology, physiology, and in vivo functional neuroimaging studies. Journal of Child Psychology and Psychiatry. 2007;48:262–287. doi: 10.1111/j.1469-7610.2007.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend J, Bookheimer SY, Foland-Ross LC, Sugar CA, Altshuler LL. fMRI abnormalities in dorsolateral prefrontal cortex during a working memory task in manic, euthymic and depressed bipolar subjects. Psychiatry Research. 2010;182:22–9. doi: 10.1016/j.pscychresns.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevarthen C. Conversations with a two month-old. In: Raphael-Leff J, editor. Parent-infant psychodynamics: Wild things, mirrors and ghosts. Philadelphia: Whurr Publishers; 2003. pp. 25–34. [Google Scholar]
- Trevarthen C, Daniel S. Disorganised rhythm and synchrony: Early signs of autism and Rett syndrome. Brain and Development. 2005;27:25–34. doi: 10.1016/j.braindev.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Tronick EZ. Why is connection with others so critical? The formation of dyadic states of consciousness and the expansion of individuals’ states of consciousness: coherence governed selection and the co-creation of meaning out of messy meaning making. In: Nadel J, Muir D, editors. Emotional development: Recent research advances. New York: Oxford University Press; 2005. pp. 293–315. [Google Scholar]
- Venuti P, Esposito G, Giusti Z. A qualitative analysis of crying and vocal distress in children with autism. Journal of Intellectual Disability Research. 2004;48:338. [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: A meta-analysis of findings from neuroimaging. Neuroimage. 2003;19:513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Walter M, Matthiä C, Wiebking C, Rotte M, Tempelmann C, Bogerts B, Heinze HJ, Northoff G. Preceding attention and the dorsomedial prefrontal cortex: process specificity versus domain dependence. Human Brain Mapping. 2009;30:312–26. doi: 10.1002/hbm.20506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildgruber D, Ackermann H, Kreifelts B, Ethofer T. Cerebral processing of linguistic and emotional prosody: fMRI studies. Progress in Brain Research. 2006;156:249–268. doi: 10.1016/S0079-6123(06)56013-3. [DOI] [PubMed] [Google Scholar]
- Zeng H, Constable RT. Image distortion correction in EPI: Comparison of field mapping with point spread function mapping. Magnetic Resonance Medicine. 2002;48:137–146. doi: 10.1002/mrm.10200. [DOI] [PubMed] [Google Scholar]
- Zeskind PS, Klein L, Marshall TR. Adult’s perceptions of experimental modifications of durations of pauses and expiratory sounds in infant crying. Child Development. 1992;59:193–6. [Google Scholar]
- Zeskind PS, Lester BM. Analysis of infant crying. In: Singer LT, Zeskind PS, editors. Biobehavioral assessment of the infant. New York: Guilford Publications Inc; 2001. pp. 149–166. [Google Scholar]
- Ziefman DM. Predicting adult responses to infant distress: adult characteristics associated with perceptions, emotional reactions, and timing of intervention. Infant Mental Health Journal. 2003;24:597–612. [Google Scholar]



