Abstract
B cells contribute to autoimmunity both as secretors of pathogenic antibodies and through the activation of autoreactive T cells. B cells and antibodies acquire higher affinity to self-antigen through a process known as immunoglobulin hypermutation or SHM. The contribution of SHM to pathogenic antibody development in lupus has been established in various autoimmune mouse models and by examining antibodies from patients. However, its role in the antibody-independent contribution of B cells to autoimmunity has not been examined. Herein, we generate lupus-prone MRL/lpr mice with a limited IgM-only B cell repertoire, no secreted antibodies and no SHM. This enabled us to isolate the role of somatic hypermutation in B cell-mediated autoimmunity and found that SHM-deficiency correlated with a reduction in autoreactive B cells, a decrease in T cell activation and in kidney lymphocytic infiltration. These data establish AID as an important contributor to the antibody-independent role of B cells in autoimmunity.
Keywords: somatic hypermutation, AID, B cells, T cells, lupus
Introduction
Somatic hypermutation (SHM) of immunoglobulin (Ig) genes in B cells is a process of deliberate hypermutation of the genes encoding Ig variable (V) regions that occurs in the germinal centers (GC) of secondary lymphoid tissues [1, 2]. Mutations of the Ig V regions can lead to amino acid replacements that alter the specificity of the Ig receptor. Coupled to a process of cellular selection for enhanced recognition and binding, SHM can lead to the formation of high affinity memory B cells to a foreign antigen [3–6]. There is strong evidence that these mutations occasionally generate or accentuate an autoreactive Ig receptor, leading to the formation of autoimmune memory B cells [7–12]. It is likely that there is a separate layer of negative selection in GC’s monitoring newly formed autoreactive B cells, and when this fails it can lead to the formation of autoreactive memory B cells [13–18]. A breakdown in tolerance checkpoint following SHM may lead to the formation of high affinity memory B cells to self-antigens leading to autoimmune disease.
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease characterized by the circulation of autoantibodies and immune complex deposition in various tissues, particularly the kidney glomeruli [19]. Hallmark autoantibodies of SLE recognize nuclear cellular components, in particular double-stranded DNA (dsDNA). SLE patients and MRL/lpr mice afflicted with a lupus-like syndrome, have high levels of these pathogenic autoreactive antibodies that originate from B cells with extensive mutations in their Ig V regions, with some enhancing autoreactivity [7–17]. This suggests that breakdown in tolerance checkpoints in GC’s contribute to the generation of pathogenic antibodies and that SHM may be an independent factor contributing to autoimmunity. Indeed, MRL/lpr mice with defective SHM have reduced levels of pathogenic antibodies [20, 21].
The role of SHM in autoimmunity may not only be reflected in the generation of autoreactive antibodies but also in the activation of autoreactive T cells. This is because B cells contribute to the lupus-like syndrome of MRL/lpr mice not only as secretors of pathogenic antibodies but also as activators of autoreactive T cells; MRL/lpr mice with B cells but lacking secreted antibodies display inflammatory cell infiltration in the kidneys not observed in MRL/lpr mice completely lacking B cells [22, 23]. A contribution by SHM to the antibody-independent role of B cells to autoimmunity is unknown because the importance of the specificity of the B cell receptor in this aspect of B-cell mediated autoimmunity is not understood. In order to examine this, we generated various MRL/lpr strains capable of making B cells but lacking secreted antibodies that differ in their ability to undergo SHM because of a defect in the gene encoding activation-induced deaminase (AID), a molecule required for SHM.
AID is the critical trigger for SHM and class switch recombination (CSR) of Ig genes, the process that generates antibodies of the IgG, IgA, or IgE isotype[24, 25]. Mice and humans with impaired AID function lack somatically mutated, isotype switched antibodies, and AID-deficient MRL/lpr mice are protected from lupus nephritis experiencing a dramatic increase in survival [20, 21, 26]. While SHM and CSR are independent from each other, they tend to co-occur making it difficult to isolate the role of SHM in autoimmunity from CSR. This is because the IgG isotype and its subclasses are known independent contributors to B-cell mediated autoimmune disease [27–31]. To circumvent this problem, we generated MRL/lpr mice with a defect in the Ig heavy chain locus (JhT), that results in loss of B cells but that was rescued by expression of a transgene encoding a single heavy chain specificity within membrane IgM, as done previously [22, 23]. We then crossed these mice to AID/MRL/lpr mice to generate MRL/lpr mice with no secreted antibodies but with B cells encoding a limited repertoire of IgM specificities incapable of undergoing SHM to compare to siblings without the AID defect and thus with intact SHM capabilities. The only way B cells from AID deficient mice can accomplish Ig diversity is through the pairing with light chains, whereas AID-wild type mice can also undergo SHM. This enabled us to compare the role of AID through SHM in the antibody-independent role of B cells in autoimmunity.
Material and methods
Generation of mice
Strains MRL/MpJ-Faslpr/J (MRL/lpr), B6.129P2-Igh-Jtm1Cgn/J (JHT), and NODCaj.Cg-Igh-6tm1Cgn Tg(Igh-VB1-8/Igh-6m)1Mjsk/FswJ (μMT.mIg) were purchased from The Jackson Laboratory (stock # 000485, 002438, and 005306, respectively). μMT.mIg was crossbred with MRL/lpr for 10~11 generations and the allele mIg was selected to generate strain mIg.MRL/lpr. JHT was bred with MRL/lpr for at least 9 generations to generate strain JHT.MRL/lpr. This novel strain was crossed with mIg.MRL/lpr and with our previously generated strain AID−/−MRL/lpr to finally generate strain JHT.mIg.MRL/lpr (which we will refer as JHT.mIg mice) and strain JHT.mIg.AID−/−MRL/lpr [21]. All the mice were housed in the specific pathogen-free animal facility of the National Institute of Environmental Health and Sciences, NIH.
Flow cytometry
Mouse splenocytes were prepared from spleens as described[21]. For flow cytometry analysis, 1 × 106 cells from each spleen were stained with following conjugated antibodies: anti-mouse B220 APC-Cy7, anti-mouse CD19 PE-Cy7, anti-mouse CD21 FITC, anti-mouse CD23 APC, anti-mouse CD3 PE-Cy7, anti-mouse CD8 APC-Cy7, anti-mouse CD4 PE, anti-mouse CD62L FITC, anti-mouse CD44 APC (all purchased from BD Pharmingen). Data were acquired with BD LSR II flow cytometer (BD Biosciences) and analyzed with FlowJo flow cytometry software (Tree Star).
To detect dsDNA-binding IgM+ B cells, salmon sperm DNA (Sigma) was treated with S1 nuclease (USB), fragmented with Hae III (Roche), and biotinylated with Photoprobe (Vectoras Laboratories) as previously reported[21]. Splenocytes were incubated with anti-mouse CD19 PE, anti-mouse IgM FITC, and biotinylated dsDNA/streptavidin APC (1ug each/106 cells) (all reagents except biotinylated dsDNA came from BD Pharmingen). DNA binding cells were examined by FACS as described above or sorted by BD FacsAria II Cell sorter (BD Biosciences. See below).
Sequencing of Vκ from splenic B cells
Spleens were collected from JHT.mIg.MRL/lpr and JHT.mIg.AID−/−MRL/lpr mice. Splenocytes were stained with anti-CD19 PE, anti-IgM FITC, and biotinylated dsDNA/streptavidin APC as mentioned previously. Under CD19+ gate, both IgM+dsDNA+ cells and IgM+dsDNA- cells were sorted using BD FacsAria II Cell sorter (BD Biosciences). The cells were lysed in 1ml Invitrogen’s TRIzol reagent, followed by purification of the aqueous phase using the Qiagen RNeasy mini kit to isolate RNA according to the manufacturer’s instructions. cDNA was generated using Invitrogen SuperScript III First-Strand Synthesis System for RT-PCR. The V region of kappa light chain was amplified as described with modification[32]. Briefly, Vκ was amplified using forward primer CCAGATGTGTGATGACCCAGACTCCA and reverse primer GTTGGTGCAGCATCAGC and the following PCR conditions: 94°C for 3 minutes; 35 cycles of 94°C for 15 seconds, 58°C for 1 minute, 68°C for 1 minute; and 68°C for 2 minutes, resulting in a 352 bp product. The amplified Vκ DNA fragments were purified using QIAquick Gel Extraction kit (Qiagen) and cloned into Invitrogen pCR2.1 vector. The clones were sequenced using Applied Biosystem’s BigDye Terminator v1.1 Cycle Sequencing kit. The sequences were BLASTed against the mouse immunoglobulin database (IgBLAST) at the National Center for Biotechnology Information (NCBI).
Histology evaluation
Formalin fixed, paraffin embedded kidney samples were prepared for hematoxylin and eosin (H&E) stain and for periodic acid–Schiff stain. Lymphocyte infiltration in the kidneys were examined by a pathologist and graded using a scale of 1–4, where 1 = minimal, 2 = mild, 3 = moderate, and 4 = marked, as reported previously[21].
For immunohistochemical stain, formalin fixed, paraffin embedded kidney sections were prepared and stained for F4/80 as described previously[26]. The same protocol was also used to stain PAX5 except using different optimal antibody dilutions. Primary antibody, PAX5 (Santa Cruz Biotechnology, Santa Cruz, CA) and Normal Goat Serum (negative control; Jackson Immunoresearch Laboratories, Inc., West Grove, PA) were incubated for 60 minutes at a 1:500 dilution. Secondary antibody of Horse anti-Goat (Vector Laboratories, Burlingame, CA) was incubated for 30 minutes at 1:1000 dilution. CD3 staining was performed on the Discovery XT™ Automated System (Ventana Medical Systems, Tucson, AZ) with the OmniMap anti-Rabbit Detection Kit. The CD3 antibody (Abcam, Cambridge, MA) was incubated at 1:250 for one hour without heat. The slides were treated with the anti-rabbit polymer for 16 minutes. Staining was visualized using 3-diaminobenzidine (DAB) chromagen from the kit, followed by a hematoxylin counterstain. A non-immune serum matching the primary antibody host was used at the protein concentration of the antibody as a negative control.
Statistical Analysis
All statistical analyses were done by SigmaPlot (Systat Software). Student’s t-test was performed where Normality Test (Shapiro-Wilk) and Equal Variance Test had been passed. Otherwise, Mann-Whitney Rank Sum Test was used instead.
Results
To examine the role of SHM in B cell-mediated autoimmunity, we generated several MRL/lpr mouse strains that lack secreted antibodies but differ in their ability to undergo hypermutation (Table 1). These mice lack a functional Ig heavy chain locus, but are able to make B cells through expression of a membrane IgM-encoding transgene (Supplemental Figure 1A). AID deficiency was introduced through breeding, and as a result, these mice (JHT.mIg.AID), which only express membrane IgM, can only acquire repertoire diversity through pairing with the light chain, unlike their AID wild type siblings that can also diversify or improve specificity of the Ig receptor to specific antigen through SHM. The number of splenic B and T cells in JHT.mIg.AID mice was similar to that of their AID-wild type counterparts, although the proportion of follicular B cells in AID deficient mice was increased (Supplemental Figure 1A–B, Supplemental Figure 2).
Table 1.
Predictive phenotype of MRL/lpr derived mouse strains
| Strain | mature B cells | mIgM | sIgM | mIgG sIgG | SHM | CSR |
|---|---|---|---|---|---|---|
| MRL/lpr (WT) | yes | yes | yes | yes | yes | yes |
| JHT.MRL/lpr | no | no | no | no | no | no |
| JHT.mIg.MRL/lpr | yes | Limited repertoire | no | no | yes | no |
| JHT.mIg.AID−/−MRL/lpr | yes | Limited repertoire | no | no | no | no |
mIgM, membrane IgM; sIgM, secreted IgM; mIgG, membrane IgG; sIgG, secreted IgG; SHM, somatic hypermutation; CSR, class switching recombination.
AID deficiency was associated with a significant increase in naïve CD4+ and CD8+ T cells and a concomitant decrease in memory T cells
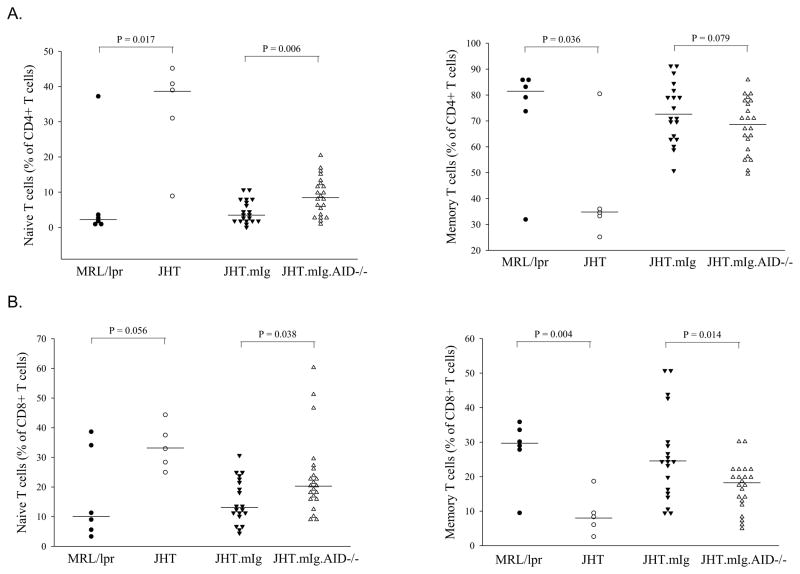
Previously, Shlomchik and colleagues demonstrated that B cells contribute to autoimmunity not just as secretors of pathogenic antibodies but also through the indirect or direct activation of autoreactive T cells [22, 23]. In the absence of any B cells, naïve T cells were elevated, an effect that was reversed with the introduction of a membrane IgM-encoding transgene that rescued normal B cells but not the secretion of antibodies. Similarly, in this study, JHT.mIg mice at 7–8 months of age with B cells but lacking antibodies had much lower levels of naïve CD4+ and CD8+ T cells than mice completely lacking B cells, supporting the previous findings for a role of B cells in activation of T cells that is independent of antibodies (Figure 1A–B). Furthermore, with AID deficiency, the levels of naïve CD4 and CD8 T cells increased significantly when compared to AID-wild type mice with the IgM transgene, with a concomitant decrease in memory T cells supporting a role for SHM in B-cell mediated activation of autoreactive T cells (Figure 1A–B). The increase in naïve T cells in AID-deficient mice was not as dramatic as in mice completely lacking B cells (JHT), suggesting light chain pairing also contributes to the B cell-mediated activation of T cells in these mice.
Figure 1.
AID deficiency correlated with increased naïve and reduced memory T cell populations in JHT.mIg.MRL/lpr mice. The number of naïve CD4 T cells (A) and CD8+ T cells (B) where increased with B cell deficiency (JHT mice), reduced with introduction of a membrane IgM transgene (JHT.mIg mice) that resulted in rescue of non-secreting B cells but increased again when rescued B cells lacked AID (JHT.mIg.AID mice). Each symbol represents a mouse of approximately 7–8 months of age. MRL/lpr is the conventional MRL/lpr strain. CD4+ and CD8+ T cells were defined as being naïve (CD44low, CD62high), activated (CD44high, CD62Lhigh), or memory (CD44high, CD62Llow). Student’s t-test was performed except following comparisons where Normality Test (Shapiro-Wilk) and/or Equal Variance Test failed and Mann-Whitney Rank Sum Test was used: naïve CD4+ T cells (JHT.mIg vs JHT.mIg.AID−/−), naïve and memory CD8+ T cells (JHT.mIg vs JHT.mIg.AID−/−).
Activation of B cells in young mice reveals decreased levels of memory T cells in AID-deficient JHT.mig mice
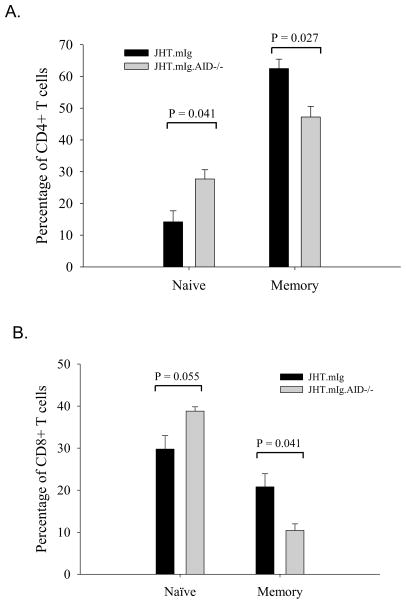
The differences seen in older JHT.mIg and JHT.mIg.AID mice in the number of naïve and memory T cells was not obvious in mice less than 10 weeks of age. We speculated that this might be due to a lack of accumulation of mutations from a lack of B cell activation in these young mice. Since these mice express the Ig Vh186.2, known to participate in the (4-hydroxy-3-nitro-phenyl) acetyl (NP) response, we immunized young mice and examined the fraction of naïve and activated splenic T cells. Following immunization with NP-CGG, the fraction of naïve T cells in JHT.mIg mice was significantly lower than that in the AID-deficient counterparts (Figure 2A–B). A corresponding increase in memory T cells in comparison to AID-deficient mice was also observed in AID-wild type mice. As with older mice, these differences were seen with both, CD8+ and CD4+ T cells.
Figure 2.
Following immunization, young JHT.mIg mice with AID deficiency also exhibited an increased in naïve T cells. JHT.mIg.MRL/lpr and its AID-deficient counterpart (3 mice/group) were immunized with NP at 3 months of age and euthanized 2 weeks later. The impact of AID deficiency on the fraction of naïve and memory T cells in CD4+ (A) and CD8+ T (B) cells was similar to that seen in older mice. CD4+ and CD8+ T cells were defined as being naïve (CD44low, CD62high), activated (CD44high, CD62Lhigh), or memory (CD44high, CD62Llow). The error bars represent standard errors. Student’s t-test was performed.
With AID deficiency there was a decrease in infiltration of the renal interstitium by T cells
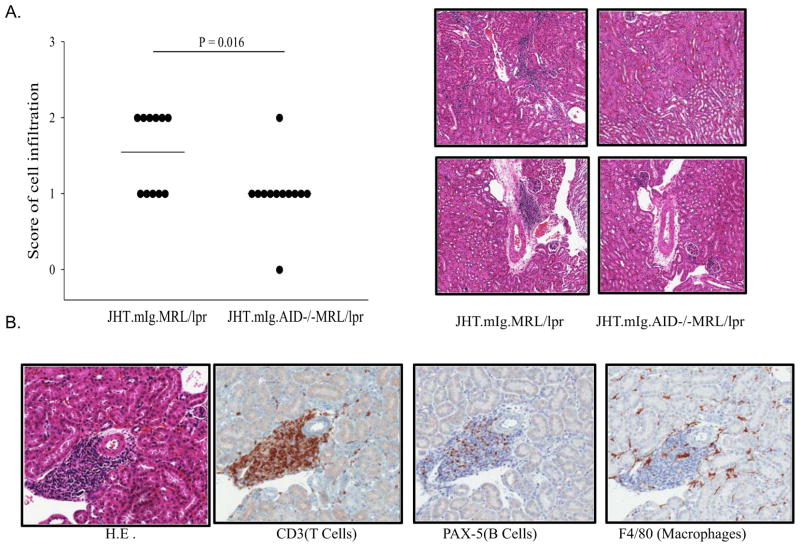
Next, we examined whether the decrease in activation of splenic T cells in autoimmune mice correlated with kidney pathology as evidenced by an increased in inflammatory cell infiltration and glomerulonephritis. There was little evidence for glomerulonephritis in either group (data not shown). This is consistent with previous results in mice lacking secreted antibodies [22, 23]. However, inflammatory cell infiltration was significantly more pronounced in the AID-wild type mice (Figure 3A). The inflammatory cells infiltrating the kidneys of AID-wild type mice were T cells (Figure 3B). This is consistent with the increased activation of CD4+and CD8+ T cells in these mice.
Figure 3.
AID deficiency correlated with a decrease in interstitial T cell infiltration in the kidneys. (A) AID deficiency correlated with a reduction in infiltration of inflammatory cells in the renal interstitium. Each dot represents data from one mouse. JHT.mIg.MRL/lpr mice (N= 11) and JHT.mIg.AID−/−MRL/lpr mice (N = 12) at 5~6 months of age were used. The H&E stain of kidney sections were from representative JHT.mIg.MRL/lpr and AID-deficient counterparts. B. The majority of Infiltrated cells in the renal interstitium were T cells, with some B cells and a few macrophages. The stain of H&E, CD3, PAX5, and F4/80 in kidney sections were from a representative JHT.mIg.MRL/lpr mouse from the same study in (A). Mann-Whitney Rank Sum Test was performed.
AID deficiency resulted in a reduction in the proportion of dsDNA-binding B cells
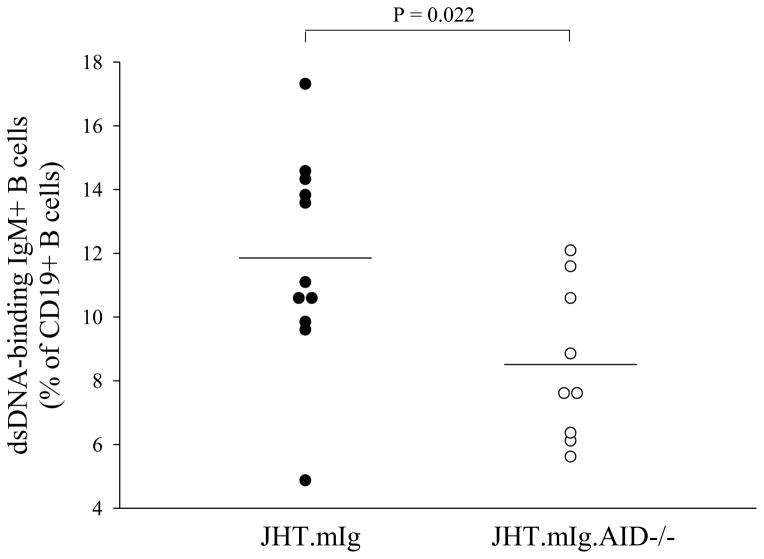
The results predict that JHT.mIg.AID mice lacking functional AID, have a decrease in the number of autoreactive B cells, as they lack the ability to increase the affinity to self-antigen through SHM, which in turn may result in decreased activation of autoreactive T cells. Since the transgenic heavy chain used in this mouse model to rescue the B cell defect is the VH186.2, a VH not normally associated with autoreactivity, it is also possible that SHM could generate new autoreactive B cells by increasing the diversity of the repertoire in an autoimmune (thus non-tolerant) background. Indeed, JHT.mIg.AID displayed lower levels of anti-dsDNA binding splenic B cells than their AID wild type counterparts (Figure 4).
Figure 4.
Reduction in the number of dsDNA-binding IgM+ B cells in AID-deficient JHT.mIg.MRL/lpr mice. Splenocytes from each strain were stained for CD19, IgM and dsDNA binding as described in the Materials and Methods. Each symbol represents a mouse at about 6 months of age. Student’s t-test was performed.
Next, we examined whether mutations in the VH186.2 gene of JHT.mIg mice could explain the higher frequency of anti-dsDNA binding cells in these mice. The transgene was not effectively targeted for SHM, as following immunization with NP, the VH186.2 from B cell clones derived from germinal center (GL7+) B cells from JHT.mIg mice were not mutated (Data not shown). These data indicated that the SHM-generated autoreactivity may have originated from the V regions of Ig light chains, λ or κ. Ig λ light chain V regions examined from GL7+ B cells of immunized mice, revealed no evidence of SHM (data not shown). Mutation was also examined in Vκ genes from dsDNA binding B cells. To establish a background error rate or changes that may be attributed to germline Vκ genes yet not identified in MRL/lpr mice, we also established mutation frequency for JHT.mIg.AID mice (with AID deficiency). JHT.mIg mice Ig Vκ genes displayed a 4-fold increase in amino acid differences in V κ genes over that seen in JHT.mIg.AID (0.002 mutations/bp vs 0.0005 mutations/bps respectively). The few mutations observed in AID deficient mice were likely Vκ polymorphisms in the MRL/lpr strain. Amino acid differences from AID-wild type mice demonstrated evidence of selection-driven mutation as 46% of the mutations fell within complementarity determining regions (CDR’s), as compared to 20% in AID-deficient mice. Nearly a quarter of the mutations in JHT.mIg mice were amino acid replacements to arginine, with over 80% of these falling within the CDR’s, among DNA-binding cells. Arginines in the CDR’s are residues frequently associated with autoreactive Ig VH chains [13]. These data are consistent with SHM-driven generation of autoreactive Ig receptors by modification of the Ig Vκ light chains.
Limited repertoire of Ig kappa light chains in dsDNA-binding B cells from JHT.mIg.AID mice
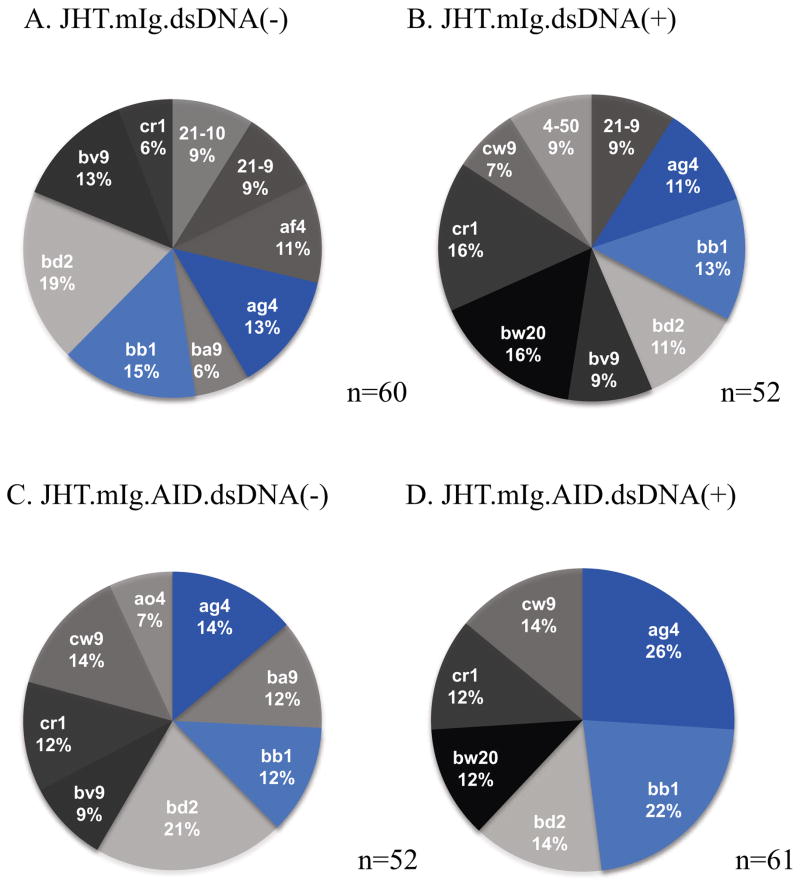
If in fact SHM helps generate autoreractive B cell receptors through the introduction of residues that enhance autoreactivity, a limited repertoire of autoreactive B cells would be predicted in the absence of SHM, as only those autoreactive from light chain pairing in germline configuration would contribute to self-binding. To test this, we examined the Ig Vκ light chains, as the VH in these mice can only originate from the transgene bearing VH186.2, and thus the heavy chain locus cannot contribute to repertoire diversity. Ig Vκ light chains from dsDNA-binding B cells from the AID-deficient mice originated from fewer V genes than those from JHT.mIg mice with normal AID and SHM. Indeed, close to 50% of the Vκ light chains originated from previously identified clones Ag4 and bb1 (using Ig blast nomenclature; http://www.ncbi.nlm.nih.gov/igblast) in AID deficient mice (Figure 5). Non-dsDNA binding B cells from JHT.mIg.AID mice did not display this reduction making it unlikely that the low repertoire of dsDNA binding B cells in these mice was the result of an overall reduction in the repertoire with AID deficiency. Interestingly, Ig kappa bw20 was only seen in the clones isolated from dsDNA binding cells in both JHT.mIg.AID and JHT.mIg mice, suggesting this V gene has a propensity towards autoreactivity in the germline configuration (Figure 5).
Figure 5.
Vκ gene family usage among dsDNA-binding and non-dsDNA-binding IgM+ B cells in JHT.mIg.MRL/lpr mice and their AID-deficient counterparts. The Vκ gene was determined by sequencing of amplified clones from cDNA from sorted dsDNA-binding and non-dsDNA-binding IgM+ B cells cDNA. NCBI IgBLAST was used to determine which V gene family each clone matched to. The pie chart percentages only depicts V genes that occurred in at least 5% of the clones. The sample size (n) depicts the number of clones examined. Ten mice (5 mice per strain) were examined.
Discussion and Conclusions
The role of SHM in the generation of pathogenic antibodies is evident in the fact that a majority of autoantibodies in SLE and other autoimmune diseases originate from B cells having undergone this process and, in some cases, affinity maturation for increased autoreactivity following SHM [10, 11, 14, 17, 33–36]. Therefore, it is likely that SHM alongside CSR is pivotal for the generation of highly pathogenic autoantibodies in B cell-mediated autoimmune diseases. Here we ask whether SHM also plays a role in the antibody-independent role of B cells in autoimmunity. Previous work by Shlomchik and colleagues demonstrated that B cells contribute to autoimmunity not only as secretors of pathogenic antibodies, but also as activators of autoreactive T cells; likely as self-antigen presenting cells [22, 23]. This was demonstrated by generating autoimmune mice with B cells but incapable of secreting any antibodies. Here, we generated JHT.mIg.AID in the MRL/lpr mice that have B cells with a single Vh specificity, Vh186.2, incapable of secreting antibodies, and their B cells are not capable of undergoing SHM. These mice had an increase in the number of naïve CD4+ and CD8+ T cells, suggesting that SHM also contributes to the B cell-mediated activation of T cells in autoimmunity. Interestingly, the difference between AID-wild type and AID-deficient JHT.mIg young mice became obvious only after following activation of B cells through immunization consistent with a role for SHM, an antigen-activated process. Recent work by Qin and colleagues [37] suggest for the first time that there is expression of AID in a small subset of CD4+ T cells that may be linked to T cell activation. It is possible that the decrease in T cell activation we see in this study is from AID deficiency in T cells, and not through a defect in SHM by B cells. However, in that study, AID expression was rare in CD8+ T cells, while we see an effect on both CD4+ and CD8+ T cells that is similar in magnitude and even more significant in memory CD8+ T cells. The most likely explanation for our results is that activation of autoreactive T cells by B cells is limited in animals with a limited B cell repertoire and no SHM. These results also distinguish the role of SHM from CSR in autoimmunity, previously, a difficult task, as these mechanisms tend to co-occur. Diversity from light chain pairing also contributes to autoreactivity as the increase in naïve T cells was not as dramatic with AID deficiency as it was with B cell deficiency, although it is likely that the affinity of autoreactive B cells to self-antigens is higher with SHM and this may exacerbate pathology presumably through activation of highly autoreactive T cells.
While neither group displayed any significant evidence of glomerulonephritis, the increased activation of autoreactive T cells in the AID wild type JHT.mIg mice, resulted in an increase in T cell infiltration of the kidney. These results make sense as lupus-mediated glomerulonephritis is caused by the deposition of antibody-mediated immune complexes and these mice lack secreted antibodies regardless of AID status. On the other hand, these results suggest that in the presence of AID, there is increased kidney infiltration by autoreactive T cells. The increased levels of autoreactive inflammatory cells in the kidney, can eventually lead to cell-mediated pathology through podocyte dysfunction and glomerulosclerosis [38].
Close analysis of autoreactive B cells in JHT.mIg mice, revealed an increase in the number of dsDNA-binding B cells in AID-wild type mice. This suggested that only SHM contributed to the generation of autoreactive B cells in this model, as CSR could not occur in the transgene bearing an IgM transmembrane. Not surprisingly, Vh186.2 transgene was a poor target of SHM, as following immunization with NP, mutations could not be detected above background levels. On the other hand, there was significant mutation in the Vκ light chains of AID-wild type JHT.mIg. Close to half of the mutations in JHT.mIg mice were in the CDR’s, and a quarter of those were amino acid replacements to arginines, a residue commonly associated with autoreactivity in Vh genes [13]. In addition, the repertoire of Vκ light chains from dsDNA binding B cells in the AID-deficient JHT.mIg mice was limited compared to dsDNA-binding B cells from their AID wild type counterparts. Indeed, close to half of the Vκ light chains from these mice originated from just two Vκ genes. In contrast, over 9 Vk genes were used with similar frequency in dsDNA-binding B cells from JHT.mIg mice. Combined, these data suggest that SHM increases the number of Vκ light chains capable of recognizing dsDNA, most likely through the generation of autoreactive amino acids in the antigen binding pockets. Overall, these studies prove a definitive role for SHM in the B cell-mediated activation of autoreactive T cells in an autoimmune mouse model and suggest that an autoreactive immunoglobulin receptor in B cells correlates with the presentation of self-antigens directly or indirectly to T cells, causing their activation.
Supplementary Material
Acknowledgments
We are grateful to Michael Fessler and Donald Cook for helpful suggestions and critical reading of the manuscript. Special thanks to Ronald Herbert, Natasha Clayton, and Tiwanda Masinde, for histology, and to Maria Sifre and Carl Bortner for assistance with flow cytometry.
Footnotes
Declaration of Interest section
The authors declare no conflict of interest. This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences. Project Z01 ES101603.
References
- 1.Weigert MG, Cesari IM, Yonkovich SJ, Cohn M. Variability in the lambda light chain sequences of mouse antibody. Nature. 1970;228:1045–1047. doi: 10.1038/2281045a0. [DOI] [PubMed] [Google Scholar]
- 2.Berek C, Berger A, Apel M. Maturation of the immune response in germinal centers. Cell. 1991;67:1121–1129. doi: 10.1016/0092-8674(91)90289-b. [DOI] [PubMed] [Google Scholar]
- 3.Eisen HN, Siskind GW. Variations in affinities of antibodies during the immune response. Biochemistry. 1964;3:996–1008. doi: 10.1021/bi00895a027. [DOI] [PubMed] [Google Scholar]
- 4.Klinman NR. The mechanism of antigenic stimulation of primary and secondary clonal precursor cells. J Exp Med. 1972;136:241–260. doi: 10.1084/jem.136.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke SH, Huppi K, Ruezinsky D, Staudt L, Gerhard W, Weigert M. Inter-and intraclonal diversity in the antibody response to influenza hemagglutinin. J Exp Med. 1985;161:687–704. doi: 10.1084/jem.161.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crews S, Griffin J, Huang H, Calame K, Hood L. A single VH gene segment encodes the immune response to phosphorylcholine: somatic mutation is correlated with the class of the antibody. Cell. 1981;25:59–66. doi: 10.1016/0092-8674(81)90231-2. [DOI] [PubMed] [Google Scholar]
- 7.Shlomchik MJ, Marshak-Rothstein A, Wolfowicz CB, Rothstein TL, Weigert MG. The role of clonal selection and somatic mutation in autoimmunity. Nature. 1987;328:805–811. doi: 10.1038/328805a0. [DOI] [PubMed] [Google Scholar]
- 8.Radic MZ, Mascelli MA, Erikson J, Shan H, Shlomchik M, Weigert M. Structural patterns in anti-DNA antibodies from MRL/lpr mice. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 2):933–946. doi: 10.1101/sqb.1989.054.01.108. [DOI] [PubMed] [Google Scholar]
- 9.Shlomchik M, Mascelli M, Shan H, Radic MZ, Pisetsky D, Marshak-Rothstein A, et al. Anti-DNA antibodies from autoimmune mice arise by clonal expansion and somatic mutation. J Exp Med. 1990;171:265–292. doi: 10.1084/jem.171.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Es JH, Gmelig Meyling FH, van de Akker WR, Aanstoot H, Derksen RH, Logtenberg T. Somatic mutations in the variable regions of a human IgG anti-double-stranded DNA autoantibody suggest a role for antigen in the induction of systemic lupus erythematosus. J Exp Med. 1991;173:461–470. doi: 10.1084/jem.173.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winkler TH, Fehr H, Kalden JR. Analysis of immunoglobulin variable region genes from human IgG anti-DNA hybridomas. Eur J Immunol. 1992;22:1719–1728. doi: 10.1002/eji.1830220709. [DOI] [PubMed] [Google Scholar]
- 12.Radic MZ, Mascelli MA, Erikson J, Shan H, Weigert M. Ig H and L chain contributions to autoimmune specificities. J Immunol. 1991;146:176–182. [PubMed] [Google Scholar]
- 13.Radic MZ, Mackle J, Erikson J, Mol C, Anderson WF, Weigert M. Residues that mediate DNA binding of autoimmune antibodies. J Immunol. 1993;150:4966–4977. [PubMed] [Google Scholar]
- 14.Wellmann U, Letz M, Herrmann M, Angermuller S, Kalden JR, Winkler TH. The evolution of human anti-double-stranded DNA autoantibodies. Proc Natl Acad Sci U S A. 2005;102:9258–9263. doi: 10.1073/pnas.0500132102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shlomchik MJ, Aucoin AH, Pisetsky DS, Weigert MG. Structure and function of anti-DNA autoantibodies derived from a single autoimmune mouse. Proc Natl Acad Sci U S A. 1987;84:9150–9154. doi: 10.1073/pnas.84.24.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vuyyuru R, Mohan C, Manser T, Rahman ZS. The lupus susceptibility locus Sle1 breaches peripheral B cell tolerance at the antibody-forming cell and germinal center checkpoints. J Immunol. 2009;183:5716–5727. doi: 10.4049/jimmunol.0804215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo W, Smith D, Aviszus K, Detanico T, Heiser RA, Wysocki LJ. Somatic hypermutation as a generator of antinuclear antibodies in a murine model of systemic autoimmunity. J Exp Med. 2010;207:2225–2237. doi: 10.1084/jem.20092712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ait-Azzouzene D, Kono DH, Gonzalez-Quintial R, McHeyzer-Williams LJ, Lim M, Wickramarachchi D, et al. Deletion of IgG-switched autoreactive B cells and defects in Fas(lpr) lupus mice. J Immunol. 2010;185:1015–1027. doi: 10.4049/jimmunol.1000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenberg R. Why can’t we find a new treatment for SLE? J Autoimmun. 2009;32:223–230. doi: 10.1016/j.jaut.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang C, Zhao ML, Diaz M. Activation-induced deaminase heterozygous MRL/lpr mice are delayed in the production of high-affinity pathogenic antibodies and in the development of lupus nephritis. Immunology. 2009;126:102–113. doi: 10.1111/j.1365-2567.2008.02882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang C, Foley J, Clayton N, Kissling G, Jokinen M, Herbert R, et al. Abrogation of lupus nephritis in activation-induced deaminase-deficient MRL/lpr mice. J Immunol. 2007;178:7422–7431. doi: 10.4049/jimmunol.178.11.7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan O, Shlomchik MJ. A new role for B cells in systemic autoimmunity: B cells promote spontaneous T cell activation in MRL-lpr/lpr mice. J Immunol. 1998;160:51–59. [PubMed] [Google Scholar]
- 23.Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med. 1999;189:1639–1648. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 25.Revy P, Muto T, Levy Y, Geissmann F, Plebani A, Sanal O, et al. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2) Cell. 2000;102:565–575. doi: 10.1016/s0092-8674(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 26.Jiang C, Zhao ML, Scearce RM, Diaz M. Activation-induced deaminase-deficient MRL/lpr mice secrete high levels of protective antibodies against lupus nephritis. Arthritis Rheum. 2011;63:1086–1096. doi: 10.1002/art.30230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hazenbos WL, Gessner JE, Hofhuis FM, Kuipers H, Meyer D, Heijnen IA, et al. Impaired IgG-dependent anaphylaxis and Arthus reaction in Fc gamma RIII (CD16) deficient mice. Immunity. 1996;5:181–188. doi: 10.1016/s1074-7613(00)80494-x. [DOI] [PubMed] [Google Scholar]
- 28.Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature. 1996;379:346–349. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- 29.Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 30.Boes M, Schmidt T, Linkemann K, Beaudette BC, Marshak-Rothstein A, Chen J. Accelerated development of IgG autoantibodies and autoimmune disease in the absence of secreted IgM. Proc Natl Acad Sci U S A. 2000;97:1184–1189. doi: 10.1073/pnas.97.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melamed D, Miri E, Leider N, Nemazee D. Unexpected autoantibody production in membrane Ig-mu-deficient/lpr mice. J Immunol. 2000;165:4353–4358. doi: 10.4049/jimmunol.165.8.4353. [DOI] [PubMed] [Google Scholar]
- 32.Liang Z, Xie C, Chen C, Kreska D, Hsu K, Li L, et al. Pathogenic profiles and molecular signatures of antinuclear autoantibodies rescued from NZM2410 lupus mice. J Exp Med. 2004;199:381–398. doi: 10.1084/jem.20030132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sims GP, Shiono H, Willcox N, Stott DI. Somatic hypermutation and selection of B cells in thymic germinal centers responding to acetylcholine receptor in myasthenia gravis. J Immunol. 2001;167:1935–1944. doi: 10.4049/jimmunol.167.4.1935. [DOI] [PubMed] [Google Scholar]
- 34.Stott DI, Hiepe F, Hummel M, Steinhauser G, Berek C. Antigen-driven clonal proliferation of B cells within the target tissue of an autoimmune disease. The salivary glands of patients with Sjogren’s syndrome. J Clin Invest. 1998;102:938–946. doi: 10.1172/JCI3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diamond B, Katz JB, Paul E, Aranow C, Lustgarten D, Scharff MD. The Role of Somatic Mutation in the Pathogenic Anti-DNA Response. Annual Review of Immunology. 1992;10:731–757. doi: 10.1146/annurev.iy.10.040192.003503. [DOI] [PubMed] [Google Scholar]
- 36.Li Z, Schettino EW, Padlan EA, Ikematsu H, Casali P. Structure-function analysis of a lupus anti-DNA autoantibody: central role of the heavy chain complementarity-determining region 3 Arg in binding of double- and single-stranded DNA. Eur J Immunol. 2000;30:2015–2026. doi: 10.1002/1521-4141(200007)30:7<2015::AID-IMMU2015>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qin H, Suzuki K, Nakata M, Chikuma S, Izumi N, et al. Activation-Induced Cytidine Deaminase Expression in CD4+ T Cells is Associated with a Unique IL-10-Producing Subset that Increases with Age. PLoS ONE. 2011;6:e29141. doi: 10.1371/journal.pone.0029141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee SB, Kalluri R. Mechanistic connection between inflammation and fibrosis. Kidney Int Suppl. 2010:S22–26. doi: 10.1038/ki.2010.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.