Hexahydropyrroloindoline alkaloids are a large class of natural products that are formally derived from two molecules of tryptophan.[1] A subset of this class, the C3–C3′ indole alkaloids, contain the 3a-(3-indolyl)-hexahydropyrrolo-[2,3-b]indole skeleton and include compounds such as gliocladin C,[2] gliocladine C,[3] leptosin D,[4] and the bionectins[5] (Figure 1). Aside from their interesting structural features, they exhibit a broad range of potent biological activities. For example, gliocladin C[2] and leptosin D[4] are cytotoxic against P-388 lymphocytic leukemia cell lines with ED50 values of 240 ng mL−1 and 86 ng mL−1, respectively, while bionectins A and B[5] exhibit antibacterial activity against MRSA (methicillin-resistant S. aureus) and QRSA (quinolone-resistant S. aureus) with MIC = 10–30 µm mL−1.
Figure 1.
Representative examples of cytotoxic and antibiotic C3–C3′ bisindole alkaloids.[6]
The structural complexity and high biological activities of hexahydropyrroloindoline alkaloids,[7] have gained the attention of several research groups, thus resulting in total syntheses of natural products that incorporate C3–C3′ pyrroloindoline dimers,[8, 9] including work by the research groups of Hino,[9a] Danishefsky,[9b] Overman,[9c] Movassaghi,[9d–g] de Lera,[9h,i] Sodeoka,[9j] and Baran.[9k] Elegant approaches to the synthesis of C3–C7′ and C3–N1′ bisindole alkaloids have also been achieved by Overman and Govek (C3–C7′),[10] Baran and co-workers,[11a,c] and Rainier and Espejo (C3–N1′).[11b] However, the total syntheses of gliocladin C by Overman and co-workers, starting from isatin in 2007[12a] and the subsequent second generation synthesis in 2011,[12b] remain the only completed nondimeric C3–C3′ bisindole alkaloid syntheses. Moreover, the strategy by Overman and co-workers illustrated the importance of gliocladin C as a key intermediate for the preparation of other C3–C3′ bisindole alkaloids. For example, bis-Boc-protected 1 can be effectively converted into gliocladine C (2) in six steps. Two additional reports by Crich et al.,[13] and Somei and co-workers[14] were aimed at the synthesis of the core.[15, 16] Herein, we report the total synthesis of gliocladin C (10 steps total) which was enabled by a highly efficient radical coupling reaction that is mediated by visible-light photoredox catalysis (Scheme 1).[17–24]
Scheme 1.
Retrosynthesis of gliocladin C (1; top) and visible-light-mediated C–C bond formation (bottom).
During our studies into the visible-light-mediated synthesis of indole alkaloid natural products using the photoredox catalyst tris(bipyridyl)ruthenium(II) chloride ([Ru-(bpy)3Cl2])[25] we serendipitously discovered an efficient method for the reductive dehalogenation of activated C–X bonds.[20a, 26] In the process, we were able to effectively access the tertiary benzylic radical 7 (Scheme 1) from bromopyrroloindolines 6 en route to the corresponding reduced compounds. By utilizing the method developed within our group, we envisioned that the trapping of 7 with an indole derivative would provide a direct approach to C3–C3′ bisindoles 8, and thus efficient access to an entire class of natural products.
The key step in our synthetic strategy was evaluated by exposing simple bromopyrrolindoline 9,[11b] which is derived from tryptamine in two steps on a large scale, to N-methylindole (10) under typical reductive quenching reaction conditions for photoredox catalysis (Scheme 2). As expected[27] only the C3–C2′ coupled product 11 was observed, with no detectable traces of products containing the desired C3–C3′ connectivity. However, by effectively blocking the indole C2′-position with a carboxylate group, we were able to direct the reactivity towards the preferred indole C3′-position. Indeed, coupling with methyl indole-2-carboxylate (12) led to 58% yield of the desired C3–C3′ coupling product 13 (Scheme 2). The visible-light-mediated coupling of indoles with bromopyrroloindolines now selectively enables the synthetic access to both the unnatural C3–C2′ and the natural C3–C3′ connectivity, depending on the indole substitution pattern.
Scheme 2.
Visible-light-mediated coupling of bromopyrroloindoline 9 with indoles enables selective access to both C2′- and C3′-substituted bisindoles. Boc = tert-butyloxycarbonyl.
Further model studies towards the total synthesis of gliocladin C were conducted with Boc-l-tryptophan-derived bromopyrroloindoline 14 [Eq. (1)]. We identified indole-2-carboxaldehyde (15) as the best coupling partner and the desired product 16 was obtained in 72% yield with only 1 mol% of [Ru(bpy)3Cl2] on up to a 2 g scale.[28] This strategy not only employs mild reaction conditions and low catalyst loading, but also provides rapid access to the C3–C3′ bisindole alkaloid core structure in high yield on a large scale.
 |
(1) |
After securing a rapid and scalable route to the core structure of the C3–C3′ bisindole alkaloid framework, we initiated our synthesis of 1 by using an orthogonal nitrogen protection of Boc-d-tryptophan methyl ester (17) with CbzCl (Scheme 3). Bromocyclization using NBS and PPTS[29] yielded bromopyrroloindoline 18 in 91% yield over the two steps. Methylamidation of 18 with aqueous MeNH2 in THF resulted in the formation of the corresponding methylamide 19 in 87%yield. Bromopyrroloindoline 19 was then subjected to the key indole coupling reaction using the previously optimized reaction conditions. Treatment of a mixture of amide 19 and aldehyde 15 (5.0 equiv)[30] with Et3N (2.0 equiv) in the presence of 1 mol% of [Ru(bpy)3Cl2] in DMF under blue-light[31, 32] irradiation, successfully provided the desired coupling product 20 in 82% yield. During further optimization studies, we found that the use of an amine with a lower vapor pressure instead of Et3N proved beneficial to the reaction conversion and the yield of the isolated product.[33] As a result, the use of nBu3N as the stoichiometric reducing agent resulted in the complete conversion of the starting material on a preparative scale (3.8 mmol) and provided 20 in 82% yield.
Scheme 3.
a) CbzCl, NaOH, Bu4NHSO4, CH2Cl2, 12 h; b) NBS, PPTS, CH2Cl2, 23°C, 12 h, 91% (two steps); c) MeNH2, THF, 23°C, 3 d, 87%; d) [Ru(bpy)3Cl2] (1.0 mol%), Bu3N (2 equiv), 15 (5 equiv), DMF, blue LEDs, 12 h, 82%; e) [Rh(Ph3P)3Cl] (1 equiv), xylenes, 140°C, 12 h, 86% or [Rh(CO)(Ph3P)2Cl] (20 mol%), dppp (44 mol%), DPPA (2 equiv), xylenes, 140°C, 85%; f) CbzCl, NaOH, Bu4NHSO4, CH2Cl2, 12 h, 98%; g) TMSI, CH3CN, 0°C, 1 h, 91%. Cbz = benzyloxycarbonyl; DMF = N,N′-dimethylformamide; DPPA = diphenylphosphoryl azide; dppp = 1,3-bis(diphenylphosphino)propane; LED = light-emitting diode; NBS = N-bromosuccinimide; PPTS = pyridinium p-toluenesulfonate; THF = tetrahydrofuran; TMS = trimethylsilyl.
With a scalable and highly efficient synthetic route to the core structure established, catalytic decarbonylation of the aldehyde at the C2′-position of the indole was explored. Initial attempts using [Rh(Ph3P)3Cl] (20 mol%) and DPPA (2.0 equiv)[34] provided 21 in an unsatisfactory yield of 60%. Hence, we opted to complete the synthesis using a stoichiometric decarbonylation reaction by heating compound 20 in xylenes (140 °C) in the presence of [Rh(Ph3P)3Cl] to achieve the desired decarbonylation in 86% yield. Subsequent re-evaluation of the catalytic decarbonylation conditions led to improved results using 20 mol% of [Rh(CO)(Ph3P)3Cl], dppp[35] (44 mol%), and DPPA (2.0 equiv) in xylenes at 140 °C, and provided 21 in 85% yield.
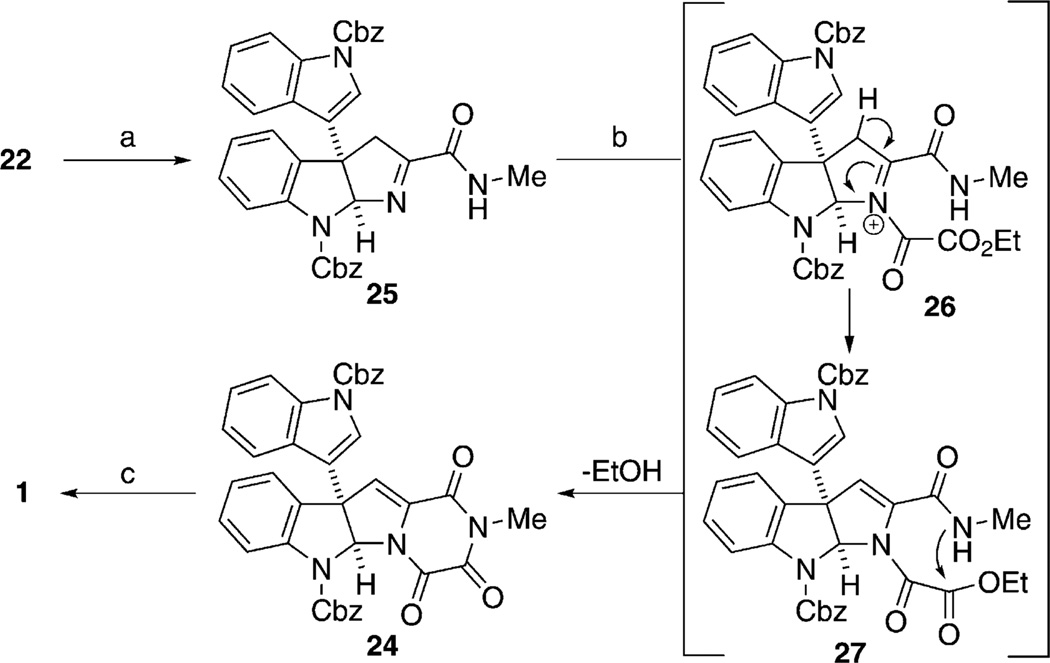
At this stage, two challenges remained to complete an efficient synthesis of gliocladin C: 1) the formation of the triketopiperazine moiety and 2) the introduction of the α,β-unsaturated imide. Several attempts to complete the synthesis, first by conversion of 21 into bis-Cbz-protected dihydrogliocladin C [23; N-acylation with ClCOCO2Et/Et3N then cyclization using hexamethyldisilazane (HMDS), 140 °C,[36] 56% yield over the two steps; Eq. (2)] followed by dehydrogenation to introduce the α,β-unsaturated imide (e.g. LiHMDS/NBS, DDQ) failed to provide 24 in an acceptable yield. Under unoptimized reaction conditions, treatment of 23 with Pd/C (20 mol%, toluene, reflux, 3 days) provided 24 in <50%yield.We reasoned that the orientation of the methine hydrogen at C11a inside the concave face of the ring prevented efficient conversion into 24. Attempts to epimerize C11a resulted only in the ring opening of the triketopiperazine.
 |
(2) |
Inspired by the historical approach by Woodward and Ling to unnatural cyclic amino acids by using the acylation/elimination of cyclic oxime ethers,[37] we chose to pursue a one-pot N-acyliminium ion promoted enamine formation/intramolecular amidation to introduce the triketopiperazine and the α,β-unsaturated imide in a single transformation (Scheme 4; 25→24).[38] Accordingly, the oxidation of the secondary amine by sequential treatment with NBS and DBU provided the requisite imine 25 in nearly quantitative yield. The acylation/cyclization step was then accomplished by microwave irradiation of a mixture of imine 25, ClCOCO2Et, and Et3N in toluene at 150°C. Presumably, the imine reacts with ClCOCO2Et to provide acyliminium intermediate 26, which upon deprotonation forms enamine 27. Intramolecular amidation occurs by attack of the amide nitrogen atom on the ester carbonyl to close the ring, thus providing the desired triketopiperazine 24 in 76% yield (after one recycle). As a final step, global Cbz removal using BCl3 in CH2Cl2 (−78°C to 23°C) provided gliocladin C (1) in 80% yield. Spectroscopic and optical rotation data for synthetic 1 were in agreement with the data reported for the natural sample.[39]
Scheme 4.
a) NBS, CH2Cl2, DBU, 23°C, 99%; b) ClCOCO2Et, Et3N, 150°C, microwaves, 0.5 h, 76% (90% conversion, two cycles); c) BCl3, CH2Cl2, −78 °C to 23°C, 12 h, 80%. DBU = 1,8-diazabicyclo-[5.4.0]undec-7-ene.
In summary, gliocladin C (1) was synthesized in 10 steps from commercially available Boc-d-tryptophan methyl ester in 30% overall yield. This study highlights photoredox catalysis not only as a viable method in the context of this particular synthesis, but also as a general, mild, and robust means to potentially access a wide variety of complex molecules. With the route to gliocladin C by photoredox catalysis established, we anticipate using imine 25 as a common intermediate for the preparation of other members of this important class of indole alkaloids by using the imine annulation sequence outlined in Scheme 4.
Footnotes
Financial support for this research from the NIH-NIGMS (R01-GM096129), Boston University, and Boehringer Ingelheim is gratefully acknowledged. C.R.J.S. is a fellow of the Alfred P. Sloan Foundation. L.F. thanks the Novartis Institutes for BioMedical Research for a graduate fellowship. NMR (CHE-0619339) and MS (CHE-0443618) facilities at BU are supported by the NSF.
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201103145.
References
- 1.a) Bisindole Alkaloids“: Cordell GA, Saxton JE. In: The Alkaloids: Chemistry and Physiology. Manske RHF, Rodrigo RGA, editors. Vol. 20. New York: Academic Press; 1981. pp. 3–294. b) ”Chemistry and Reactions of Cyclic Tautomers of Tryptamines and Tryptophans“: Hino T, Nakagawa M. In: The Alkaloids: Chemistry and Pharmacology. Brossi A, editor. Vol. 34. New York: Academic Press; 1989. pp. 1–75. c) ”Alkaloids from the Medicinal Plants of New Caledonia“: Sévenet T, Pusset J. In: The Alkaloids: Chemistry and Pharmacology. Cordell GA, editor. Vol. 48. New York: Academic Press; 1996. pp. 58–59.
- 2.Usami Y, Yamaguchi J, Numata A. Heterocycles. 2004;63:1123. [Google Scholar]
- 3.Dong J-Y, He H-P, Shen Y-M, Zhang K-Q. J. Nat. Prod. 2005;68:1510. doi: 10.1021/np0502241. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi C, Numata A, Matsumura E, Minoura K, Eto H, Shingu T, Ito T, Hasegawa T. J. Antibiot. 1994;47:1242. doi: 10.7164/antibiotics.47.1242. [DOI] [PubMed] [Google Scholar]
- 5.Zheng CJ, Kim CJ, Bae KS, Kim YH, Kim WG. J. Nat. Prod. 2006;69:1816. doi: 10.1021/np060348t. [DOI] [PubMed] [Google Scholar]
- 6.Structures are labeled according to the numbering scheme illustrated in Ref. [11b].
- 7.For a review on pyrrolindoline alkaloids, see: Crich D, Banerjee A. Acc. Chem. Res. 2007;40:151. doi: 10.1021/ar050175j.
- 8.For excellent reviews on the synthesis of dimeric pyrroloindolines, see: Steven A, Overman LE. Angew. Chem. 2007;119:5584. doi: 10.1002/anie.200700612. Angew. Chem. Int. Ed.2007, 46, 5488; Schmidt A, Movassaghi M. Synlett. 2008:313.
- 9.a) Nakagawa M, Sugumi H, Kodato S, Hino T. Tetrahedron Lett. 1981;22:5323. [Google Scholar]; b) Depew KM, Marsden SP, Zatorska D, Zatorski A, Bornmann WG, Danishefsky SJ. J. Am. Chem. Soc. 1999;121:11953. [Google Scholar]; c) Overman LE, Paone DV. J. Am. Chem. Soc. 2001;123:9465. doi: 10.1021/ja0166141. [DOI] [PubMed] [Google Scholar]; d) Movassaghi M, Schmidt MA. Angew. Chem. 2007;119:3799. doi: 10.1002/anie.200700705. Angew. Chem. Int. Ed.2007, 46, 3725; [DOI] [PubMed] [Google Scholar]; e) Movassaghi M, Schmidt MA, Ashenhurst JA. Angew. Chem. 2008;120:1507. doi: 10.1002/anie.200704960. Angew. Chem. Int. Ed.2008, 47, 1485; [DOI] [PubMed] [Google Scholar]; f) Kim J, Ashenhurst JA, Movassaghi M. Science. 2009;324:238. doi: 10.1126/science.1170777. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Kim J, Movassaghi M. J. Am. Chem. Soc. 2010;132:14376. doi: 10.1021/ja106869s. [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Pérez-Balado C, de Lera AR. Org. Lett. 2008;10:3701. doi: 10.1021/ol8013073. [DOI] [PubMed] [Google Scholar]; i) Pérez-Balado C, Rodriguez-Grana P, de Lera AR. Chem. Eur. J. 2009;15:9928. doi: 10.1002/chem.200901056. [DOI] [PubMed] [Google Scholar]; j) Iwasa E, Hamashima Y, Fugishiro S, Higuchi E, Ito A, Yoshida M, Sodeoka M. J. Am. Chem. Soc. 2010;132:4078. doi: 10.1021/ja101280p. [DOI] [PubMed] [Google Scholar]; k) Foo K, Newhouse T, Mori I, Takayama H, Baran PS. Angew. Chem. 2011;123:2768. doi: 10.1002/anie.201008048. [DOI] [PMC free article] [PubMed] [Google Scholar]; Takayama H, Baran PS. Angew. Chem. 2011;123:2768. doi: 10.1002/anie.201008048. Angew. Chem. Int. Ed.2011, 50, 2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.For the synthesis of C3 –C7′ linked pyrroloindolines, see: Govek SP, Overman LE. J. Am. Chem. Soc. 2001;123:9468. doi: 10.1016/j.tet.2007.05.127.
- 11.For syntheses of C3–N1′-linked pyrroloindolines, see: Newhouse T, Baran PS. J. Am. Chem. Soc. 2008;130:10886. doi: 10.1021/ja8042307. Espejo VR, Rainier JD. J. Am. Chem. Soc. 2008;130:12894. doi: 10.1021/ja8061908. Newhouse T, Lewis CA, Baran PS. J. Am. Chem. Soc. 2009;131:6360. doi: 10.1021/ja901573x.
- 12.a) Overman LE, Shin Y. Org. Lett. 2007;9:339. doi: 10.1021/ol062801y. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) DeLorbe JE, Jabri SY, Mennen SM, Overman LE, Zhang F-L. J. Am. Chem. Soc. 2011;133:6549. doi: 10.1021/ja201789v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crich D, Fredette E, Flosi WJ. Heterocycles. 1998;48:545. [Google Scholar]
- 14.Yamada F, Goto A, Somei M. Heterocycles. 2000;53:1255. [Google Scholar]
- 15.For alkylative dearomatization approaches to pyrroloindoline natural products and scaffolds, see: Austin JF, Kim SG, Sinz CJ, Xiao WJ, MacMillan DWC. Proc. Natl. Acad. Sci. USA. 2004;101:5482. doi: 10.1073/pnas.0308177101. Lucarini S, Bartoccini F, Battistoni F, Diamantini G, Piersanti G, Righi M, Spadoni G. Org. Lett. 2010;12:3844. doi: 10.1021/ol101527j. Repka LM, Ni J, Reisman SE. J. Am. Chem. Soc. 2010;132:14418. doi: 10.1021/ja107328g.
- 16.For an approach to pyrroloindolines involving an interrupted Fischer indole synthesis, see: Boal BW, Schammel AW, Garg NK. Org. Lett. 2009;11:3458. doi: 10.1021/ol901383j. Schammel AW, Boal BW, Zu L, Mesganaw T, Garg NK. Tetrahedron. 2010;66:4687. doi: 10.1016/j.tet.2010.02.050.
- 17.For reviews on photoredox catalysis and its applications in organic synthesis, see: Narayanam JMR, Stephenson CRJ. Chem. Soc. Rev. 2011;40:102. doi: 10.1039/b913880n. Yoon TP, Ischay MA, Du J. Nat. Chem. 2010;2:527. doi: 10.1038/nchem.687. Zeitler K. Angew. Chem. 2009;121:9969. Angew. Chem. Int. Ed.2009, 48, 9785.
- 18.a) Nicewicz DA, MacMillan DWC. Science. 2008;322:77. doi: 10.1126/science.1161976. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Nagib DA, Scott ME, MacMillan DWC. J. Am. Chem. Soc. 2009;131:10875. doi: 10.1021/ja9053338. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Shih H, Vander Wal MN, Grange RL, MacMillan DWC. J. Am. Chem. Soc. 2010;132:13600. doi: 10.1021/ja106593m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.a) Ischay MA, Anzovino ME, Du J, Yoon TP. J. Am. Chem. Soc. 2008;130:12886. doi: 10.1021/ja805387f. [DOI] [PubMed] [Google Scholar]; b) Du J, Yoon TP. J. Am. Chem. Soc. 2009;131:14604. doi: 10.1021/ja903732v. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Ischay MA, Lu Z, Yoon TP. J. Am. Chem. Soc. 2010;132:8572. doi: 10.1021/ja103934y. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Lu Z, Shen M, Yoon TP. J. Am. Chem. Soc. 2011;133:1162. doi: 10.1021/ja107849y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.For recent applications of photoredox catalysis from our research group, see: Narayanam JMR, Tucker JW, Stephenson CRJ. J. Am. Chem. Soc. 2009;131:8756. doi: 10.1021/ja9033582. Tucker JW, Narayanam JMR, Krabbe SW, Stephenson CRJ. Org. Lett. 2010;12:368. doi: 10.1021/ol902703k. Condie AG, González-Gómez JC, Stephenson CRJ. J. Am. Chem. Soc. 2010;132:1464. doi: 10.1021/ja909145y. Tucker JW, Nguyen JD, Narayanam JMR, Krabbe SW, Stephenson CRJ. Chem. Commun. 2010;46:4985. doi: 10.1039/c0cc00981d. Furst L, Matsuura BS, Narayanam JMR, Tucker JW, Stephenson CRJ. Org. Lett. 2010;12:3104. doi: 10.1021/ol101146f. Dai C, Narayanam JMR, Stephenson CRJ. Nat. Chem. 2011;3:140. doi: 10.1038/nchem.949. Nguyen JD, Tucker JW, Konieczynska MD, Stephenson CRJ. J. Am. Chem. Soc. 2011;133:4160. doi: 10.1021/ja108560e. Tucker JW, Narayanam JMR, Shah PS, Stephenson CRJ. Chem. Commun. 2011;47:5040. doi: 10.1039/c1cc10827a.
- 21.For additional examples of photoredox catalysis, see: Koike T, Akita M. Chem. Lett. 2009;38:166. DeClue MS, Monnard PA, Bailey JA, Maurer SE, Collis GE, Ziock HJ, Rasmussen S, Boncella JM. J. Am. Chem. Soc. 2009;131:931. doi: 10.1021/ja808200n. Borak JB, Falvey DE. J. Org. Chem. 2009;74:3894. doi: 10.1021/jo900182x. Andrews SR, Becker JJ, Gagné MR. Angew. Chem. 2010;122:7432. Angew. Chem. Int. Ed.2010, 49, 7274; Rueping M, Villa C, Koenigs RM, Poscharny K, Fabry DC. Chem. Commun. 2011;47:2360. doi: 10.1039/c0cc04539j. Neumann M, Fuldner S, Konig B, Zeitler K. Angew. Chem. 2011;123:981. doi: 10.1002/anie.201002992. Angew. Chem. Int. Ed.2011, 50, 951; Chen Y, Damlet AS, Steinman JB, Liu DR. Nat. Chem. 2011;3:146. doi: 10.1038/nchem.932. Andrews SR, Becker JJ, Gagné MR. Org. Lett. 2011;13:2406. doi: 10.1021/ol200644w. Larraufie M-H, Pellet R, Fensterbank L, Goddard J-P, Lacôte E, Malacria M, Ollivier C. Angew. Chem. 2011;123:4555. doi: 10.1002/anie.201007571. Angew. Chem. Int. Ed.2011, 50, 4463.
- 22.For reviews on photoinduced electron transfer (UV), see: Julliard M, Chanon M. Chem. Rev. 1983;83:425. Cossy J. Bull. Soc. Chim. Fr. 1994;131:344.
- 23.For a review on photochemical reactions that are used as key steps in natural product synthesis, see: Bach T, Hehn JP. Angew. Chem. 2011;123:1032. doi: 10.1002/anie.201002845. Angew. Chem. Int. Ed.2011, 50, 1000.
- 24.For selected examples of photoinduced electron transfer (UV), see: Jeon YT, Lee C-P, Mariano PS. J. Am. Chem. Soc. 1991;113:8847. Bertrand S, Hoffmann N, Pete J-P. Eur. J. Org. Chem. 2000:2227. doi: 10.1021/jo001166l. Bauer A, Westkämper F, Grimme S, Bach T. Nature. 2005;436:1139. doi: 10.1038/nature03955.
- 25.For excellent reviews on the photophysical properties of [Ru-(bpy)3Cl2], see: Kalyanasundaram K. Coord. Chem. Rev. 1982;46:159. Juris A, Balzani V, Barigelletti F, Campagna S, Belser P, von Zelewsky A. Coord. Chem. Rev. 1988;84:85.
- 26.a) Fukuzumi S, Mochizuki S, Tanaka T. J. Phys. Chem. 1990;94:722. [Google Scholar]; b) Maji T, Karmakar A, Reiser O. J. Org. Chem. 2011;76:736. doi: 10.1021/jo102239x. [DOI] [PubMed] [Google Scholar]
- 27.Radical additions to indoles are known to occur preferentially at the C2-position. For selected examples, see: Baciocchi E, Muraglia E. J. Org. Chem. 1993;58:7610. Byers JH, DeWitt A, Nasveschuk CG, Swigor JE. Tetrahedron Lett. 2004;45:6587. Guadarrama-Morales O, Méndez F, Miranda LD. Tetrahedron Lett. 2007;48:4515. Lindsay KB, Ferrando F, Christensen KL, Overgaard J, Roca T, Bennasar M, Skrydstrup T. J. Org. Chem. 2007;72:4181. doi: 10.1021/jo070273d.
- 28.We have not had occasion to test the limits of the scalability of this transformation, however, no noticeable difference in reaction rate has been observed on changing from a 50 mg to a 2 g scale. Further investigations into the generality of this radical cross-coupling reaction will be reported in due course.
- 29.López CS, Balado CP, Grana PR, de Lera AR. Org. Lett. 2008;10:77. doi: 10.1021/ol702732j. [DOI] [PubMed] [Google Scholar]
- 30.The use of excess 10 was necessary to preclude competitive reductive dehalogenation (see Ref. [20a]). Unreacted 10 was quantitatively recovered during reaction purification and subsequently reused.
- 31.Blue LED’s were purchased at http//www.creativelightings.com (λmax = 435 nm, 1 W).
- 32.The reaction temperature does not exceed 30°C upon irradiation with blue LEDs.
- 33.We observed that the headspace volume of the reaction flask correlated inversely with the reaction conversion when using Et3N as the reductive quencher (stoichiometric reducing agent), wherein lower conversion was observed when there was a larger headspace volume. We hypothesized that the reductive quencher (Et3N) was partitioning into the headspace of the reaction flask, thereby impeding the catalytic cycle. Switching to a trialkylamine with a lower vapor pressure, eg. nBu3N (0.3 mm Hg, 20°C) compared with Et3N (51.8 mm Hg, 20°C) recovered the reactivity observed on a smaller scale.
- 34.O’Connor JM, Ma J. J. Org. Chem. 1992;57:5075. [Google Scholar]
- 35.Meyer MD, Kruse LI. J. Org. Chem. 1984;49:3195. [Google Scholar]
- 36.These reaction conditions were used by Overman and Shin for a related compound in the 2007 synthesis of 1 (see Ref. [12a]).
- 37.Ling VJ, Woodward RB. J. Org. Chem. 1979;44:2487. [Google Scholar]
- 38.Alternative mechanisms are also possible, such as initial imide formation with the subsequent attack of the imine. However, based on observations made on similar systems, both in our studies and in the literature (e.g. Ref. [12a]), we believe imine acylation precedes imidation.
- 39.The measured optical rotation for synthetic 1 in two solvents ([α]D = +128 deg cm3g−1dm−1 (c = 0.04 g/100 mL, CHCl3); +125 (c = 0.2 g/100 mL, C5H5N)) correlates well to the reported data for the natural product ([α]D = +131.4 deg cm3g−1dm−1 (c = 0.07 g/100 mL, CHCl3))[2] as well as to that reported by Overman and Shin in 2007[12a] ([a]D = +116.4 deg cm3g−1dm−1 (c = 0.02 g/100 mL, CHCl3)). In their most recent synthesis,[12b] Overman and co-workers observed poor solubility of crystalline gliocladin C in chloroform ([α]D = +113 deg cm3g−1dm−1 (c = 0.0093 g/100 mL, CHCl3)) and optical rotation data was also reported in pyridine ([α]D = +127 deg cm3g−1dm−1 (c = 0.23 g/100 mL, C5H5N)).