Abstract
Background
The extent to which nutrient intake may influence bone structure and strength during maximum rates of skeletal growth remains uncertain.
Objective
To examine the relationship of dietary intake of micronutrients and bone macro-architectural structure in young girls.
Design
This cross-sectional analysis included baseline data from 363 4th and 6th grade girls enrolled in the Jump-In study. Nutrient intake was assessed using the Harvard Youth/Adolescent Food Frequency Questionnaire. Volumetric BMD (vBMD), bone geometry and strength were measured by peripheral quantitative computed tomography (pQCT). Correlations and regression modeling assessed relations between usual nutrient intake and bone parameters.
Results
In 4th grade girls, metaphyseal and diaphyseal area and circumferences, and diaphyseal strength were associated with vitamin C intake (r = 0.15–0.19; p<0.05). Zinc intake was correlated with diaphyseal vBMD (r = 0.15–0.16; p<0.05). Using multiple linear regression to adjust for important covariates, we observed significant independent associations for vitamin C and zinc with bone parameters. For every mg/d of vitamin C intake trabecular area increased by 11%, cortical strength improved by 14%; and periosteal and endosteal circumferences increased by 5% and 8.6%, respectively. For every mg/d of zinc intake, cortical vBMD increased by <1%. No significant associations were observed in 6th-grade girls.
Conclusion
Results of this study suggests that vitamin C and zinc intake are positively associated with objective measures of bone geometry, size and strength in 4th-grade girls. This indicates potential differences in micronutrient and bone associations at various age-associated stages of bone maturation perhaps indicative of competing hormonal influences.
Keywords: peripheral quantitative computed tomography (pQCT), preadolescence, bone mineral density (BMD), bone strength, micronutrient intake, vitamin C
INTRODUCTION
Osteoporosis is among the diseases proposed to have origins in childhood eating and physical activity habits. Optimizing bone strength during growth is imperative for bone health and may decrease the incidence of osteoporosis [1] and related fracture risk. Peak bone strength is determined by bone mass, and composition, and material properties, geometry and microstructure [2]. The accrual of bone mineral occurs gradually throughout childhood and adolescence until the 2 years surrounding peak linear growth, when acquisition peaks. It is during this adolescent growth spurt, also known as peak height velocity (PHV), that approximately 40–60% of peak bone mass (PBM) is achieved [3]. It is widely accepted that PBM and peak bone strength, obtained during this phase of rapid skeletal growth, are critical determinants of adult bone status [3–4]. Poor mineral accrual and lower PBM are key factors in the etiology of osteoporosis, which can be exacerbated by a deficiency of nutrients essential to bone metabolism and maintenance [5].
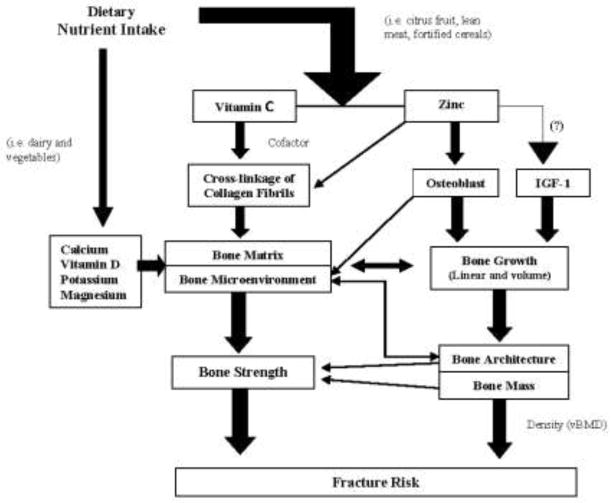
The roles of calcium and vitamin D in bone health are well established in adults and children [6–9] (Figure 1). Past research has demonstrated increased bone mineral content (BMC), bone mass and presumably greater PBM in children supplemented with 300–1200 mg additional calcium and 200 IU vitamin D/d [10–12]. Conversely, supplementation of this magnitude frequently produces no change or only modest increases in bone mineral density (BMD) at clinically relevant sites (hip, spine, total body) that are often transient and usually arise roughly two years after the onset of menarche [13]. As research focuses on maximizing PBM for reduction of osteoporotic fracture risk later in life, it is critical to study modifiable dietary factors beyond calcium and vitamin D that may affect bone development [14–18]. It is equally important to investigate associations using more precise measures of bone status, e.g., as assessed by peripheral quantitative computed tomography (pQCT), in addition to dual energy x-ray absorptiometry (DXA), which can measure volumetric density directly and other aspects of bone strength such as bone geometry.
Fig. 1.
Influences of select micronutrients on bone development and fracture risk
Other nutrients known to be important for adult skeletal health include magnesium, potassium, iron, zinc and vitamin C (Figure 1) [7, 19–20]. Vitamin C and zinc are required for collagen production and bone growth [5]. Vitamin C is an important cofactor in the cross-linking of collagen fibrils [21]. Collagen, a critical component of bone matrix, is vital to bone’s resistance to permanent deformation and fracture [13]. Its development is of particular concern during critical periods of rapid growth when longitudinal and circumferential skeletal growth outpace increases in mineralization (bone mass), leaving bones weak and more susceptible to fracture [4]. Zinc is essential for osteoblast activity [22] and may stimulate synthesis of insulin-like growth factor I (IGF-I) [22], a mediator of linear growth and a bone anabolic factor [12]. Perhaps surprisingly, little is known about the impact of the intake of these nutrients during important periods of maturation and growth [20]. The purpose of the present study was to examine associations between less frequently studied dietary intake of micronutrients [23], and bone material and structural (density and geometry) properties as components of bone strength using pQCT in pre and early pubertal girls.
METHODS
Study design and participants
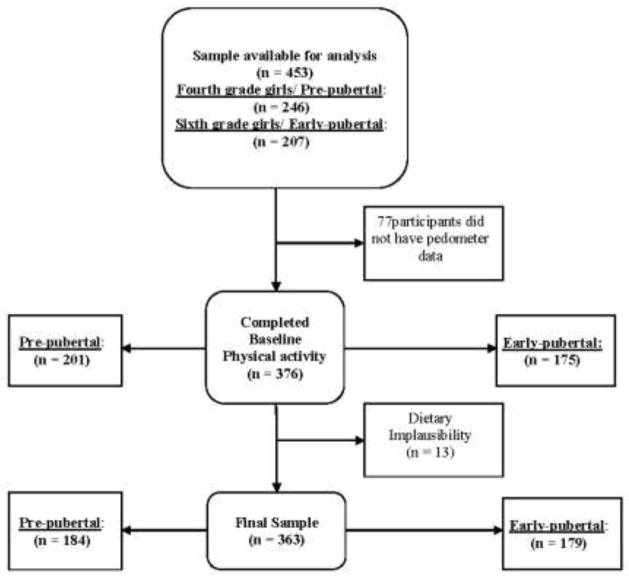
Baseline data from 453 healthy 4th and 6th grade girls, aged 8–12 years who were participating in the “Jump-In: Building Better Bones” study were considered for inclusion in this cross-sectional analysis (Figure 2). Details of the Jump-In study have been described elsewhere [24]. Briefly, Jump-In is a randomized, controlled trial of the effects of exercise on bone development in preadolescent girls. Participants were recruited from 14 elementary and 4 middle schools from two school districts in Tucson, Arizona. Schools were matched on school demographics (enrollment; mobility rates; socioeconomic status, reflected in percent enrollment qualifying for free and reduced lunch; race/ethnicity) and block randomized to the exercise intervention or control groups. Inclusion criteria were any female in school grade 4 or 6. Exclusion criteria included learning disabilities (identified by schools) that made it impossible to complete questionnaires or otherwise unable to comply with assessment protocols; medications, medical conditions, or a disability that limited participation in physical exercise [25]; and the inability to read and understand English. This study was approved by the University of Arizona Institutional Review Board; written child assent and parental consent were obtained for all participants.
Fig. 2.
Consort Diagram
Physical maturation
Although Tanner staging is common in developmental studies, its ability to accurately assess maturation is limited [26]. Therefore, an alternate index of maturation (maturity offset) was estimated using Mirwald’s gender specific prediction equations [27], based on six-year longitudinal data in boys and girls [28]. The algorithms incorporate interactions between height, weight, sitting height, leg length, and chronological age to predict where an individual is in relation to peak height velocity (PHV). As a benchmark of maximum growth rate during adolescence, age of PHV is a common indicator of maturity.
Dietary assessment
Energy and micronutrient intakes were assessed using the semi-quantitative Harvard Youth/Adolescent Food Frequency Questionnaire (YAQ). Acceptable validity and reproducibility of the YAQ have been established in children and adolescents [29]. Participants completed the YAQ with supervision and with the guidance of written instructions. YAQs were reviewed by trained study staff for completeness and were coded following standard coding procedures [29]. YAQs were then sent to Channing Laboratories (Boston, MA) for nutrient analysis.
Physical Activity (PA) assessment
PA was estimated from pedometer step counts [24]. Subsequent to laboratory testing, participants were instructed to wear an Omron HJ-720ITC (Bannockburn, IL) pedometer on their right hip for 7 contiguous days during all waking hours, except when in water [24]. Validity and reliability for this pedometer has been tested under prescribed and self-paced walking conditions [30]. Physical activity levels (PAL) were assigned as follows: sedentary < 7,000 steps/day (PAL = 1.2); low active 7,000–9,499 steps/day (PAL = 1.5); somewhat active 9,500–11,999 steps/day (PAL = 1.8); active 12,000–14,499 steps/day (PAL = 2.0); and highly active > 14,500 steps/day (PAL = 2.2) [31]. PALs were then used to estimate total energy expenditure (TEE).
Anthropometry
Anthropometric measures were completed according to standard protocols; the average of two measurements was used as the criterion measure [32]. Standing and sitting height (nearest 0.1 cm) were assessed at full inhalation using a stadiometer (Shorr Height Measuring Board, Olney, MD). Body mass was measured (nearest 0.1 kg) with a calibrated digital scale (Seca, Model 881; Hamburg, Germany). Body mass index (BMI) was calculated as weight (kg)/height (m2). Femur and tibia lengths were measured on the non-dominant (ND) leg (nearest mm). Femur length was measured from the base of the patella to the inguinal crease. Tibia length was measured from the proximal end of the medial border of the tibial plateau to the distal edge of the medial malleolus. Coefficients of variation (CVs) for femur and tibia lengths were 0.09% and 0.08%, respectively (n = 363).
Bone and body composition assessment
Dual energy x-ray absorptiometry
Whole body and regional (total hip and lumbar spine, LS), BMC (kg), areal BMD (aBMD, g/cm2) and soft tissue composition (lean soft tissue and fat masses (kg)) were assessed by DXA (GE/Lunar Radiation Corp; Madison, WI; PRODIGY). All scans were performed and analyzed by a certified technician following standard procedures. CVs for DXA (0.6% for spine phantom, BMD, L2–L4) and BMD precision (± 1.8% for lumbar spine, ± 2.4% for femoral neck and trochanter, and ± 0.8% for total body BMD) in our laboratory have been reported previously based on the repeat scans (approximately 1 week apart) of 261 women [21].
Peripheral quantitative computed tomography
pQCT was performed using the Stratec XCT 3000 scanner (STRATEC Medizintechnik GmbH, Pforzheim, Germany, Division of Orthometrix; White Plains, NY). Details regarding pQCT bone measurements and procedures have been published previously [24]. At baseline, volumetric BMD (vBMD), bone geometry (trabecular density, area and periosteal circumference, and cortical density, area, endosteal and periosteal circumferences) and indices of bone strength (bone strength index (BSI, mg2/mm4) and strength-strain index (SSI, mm3) were measured at the 4% and 20% femur and 4% and 66% tibia sites relative to the respective distal growth plates of the non-dominant limb. BSI, calculated as described by Kontulainen [33], is an estimate of the bone’s ability to withstand compression at metaphyseal sites. SSI, calculated as described by Macdonald [34], estimates the bone’s ability to resist torsion and bending forces at diaphyseal sites. Scout scans were performed at the distal growth plates of the femur and tibia, and measured sites were subsequently located based on tibia and femur lengths. The pQCT instrument was calibrated and quality assurance procedures were completed daily. All scans were performed by a single operator and were analyzed using Stratec software (Version 5.50) by a second trained technician. CVs (n = 29 per skeletal site) previously reported in our laboratory [24] were <1.1% for vBMD, bone geometry, and indices of bone strength (BSI, SSI).
Statistical analysis
Descriptive statistics (means, SDs, and ranges) were calculated and the distributions of all nutrient variables were examined for outliers and normality. Skewed nutrient intakes (vitamin D, vitamin C and iron) were normalized using log transformations; transformed data were used in all subsequent analyses. An independent t-test was used to test each nutrient and all bone variables for significant differences between 4th and 6th grade girls. Paired t-tests were used to assess significant differences between reported micronutrient intakes estimated with and without reported supplement use (calcium, vitamin D, potassium, magnesium, vitamin C, zinc, and iron). Pearson correlation coefficients were computed to assess bivariate relationships between bone-related micronutrient intakes and bone measures.
Multiple linear regression was used to examine independent associations of bone parameters and micronutrient intakes while controlling with covariates known to influence bone during growth (i.e. age, height, weight, fat and lean masses, tibia or femur length, and maturation). Biological impacts on bone and statistical implications were considered for each covariate. To protect against colinearity from highly correlated covariates (i.e. height and leg lengths (r >0.90), and weight and fat mass (r=0.90)), and overcorrecting our model, only lean mass (from DXA) and maturation (maturity offset) were included in the model (Model 1). This simplified model retained maximum predictability (adjusted R2 ≥ 0.584 for all bone sites) and minimized probable biological and statistical noise that may mask the less prominent impact of nutrient intake. Regression analyses were repeated adjusting the basic model for EI and PA (Model 2) to account for the potentially confounding influence of these factors. Analyses were repeated substituting Tanner Stage for maturity offset (data not shown). All data were analyzed using SPSS for Windows statistical software, Version 18.0 (Chicago, IL, USA).
RESULTS
Subject characteristics
The descriptive characteristics the participants (n=363 4th and 6th graders) are shown in Table 1. Sample ethnicity (4th and 6th grade, respectively) was 22% and 21% Hispanic, and 78% and 79% non-Hispanic, Sample race was 85% and 89% white, 10% and 4% Asian, 3% black or African American, 1% and 2% Latino, 1% Native Hawaiian or other Pacific Islander, and 0.5% and 0.6% others in 4th and 6th graders, respectively. Significant differences between 4th and 6th grade girls were evident for all characteristics including bone measurements. Based on the Centers for Disease Control and Prevention (CDC) percentiles for BMI [35]: 3.8% (4th grade) and 4.5% (6th grade) of the girls were underweight (BMI < 5th percentile), 77.2% and 70.4% were healthy weight (BMI 5th–85th percentile), 13% and 17.3% were overweight (BMI 85th–95th percentile), and 6% and 7.8% were obese (BMI > 95th percentile) in 4th and 6th graders, respectively. Maturity offset values indicated that 40% of 6th grade girls had reached PHV and 34.6% were estimated to be within 6 months of it. None of the 4th grade girls had reached PHV; 95.1% were one or more years pre-PHV.
Table 1.
Descriptive characteristics, maturation status and bone measurements of 363 young girls
| 4th Grade (N = 184) | 6th Grade (N = 179) | |||
|---|---|---|---|---|
|
| ||||
| Mean ± SD | Range | Mean ± SD | Range | |
| Age, y | 9.8 ± 0.5 | 8.8 – 11.5 | 11.7 ± 0.5 | 10.2 – 12.8 |
| Height, cm | 138.8 ± 6.6 | 120.3 – 155.3 | 152.2 ± 7.6 | 131.2 – 171.9 |
| Weight, kg | 34.1 ± 7.2 | 19.3 – 55.9 | 45.1 ± 9.9 | 22.7 – 71.3 |
| BMI, kg/m2 | 17.6 ± 2.7 | 12.4 – 27.3 | 19.4 ± 3.5 | 13.0 – 32.1 |
| Lean Mass, kg | 23.2 ± 3.3 | 15.8 – 33.1 | 30.2 ± 4.7 | 17.4 – 43.8 |
| Fat Mass, kg | 9.5 ± 4.8 | 1.6 – 27.6 | 13.6 ± 6.8 | 3.7 – 37.7 |
| Tibia Length, cm | 31.6 ± 2.1 | 25.5 – 36.9 | 35.2 ± 2.3 | 29.2 – 40.7 |
| Femur Length, cm | 32.5 ± 2.2 | 24.4 – 37.3 | 36.4 ± 2.5 | 29.5 –43.1 |
| Maturation Status | ||||
| a Maturity Offset, y | −1.9 ± 0.5 | −3.2 - (−0.6) | −0.1 ± 0.6 | −1.9 – (1.2) |
| pQCT bone measurements | ||||
| Femur 4% sites | ||||
| Trabecular Density, mg/ccm | 232.2 ± 32.6 | 149.6 – 309.9 | 242.1 ± 32.7 | 153.9 – 335.0 |
| Trabecular Area, mm2 | 935.0 ± 162.2 | 545.5 – 1564.6 | 1190.8 ± 203.8 | 709.1 – 2028.1 |
| Periosteal Circumference, mm | 118.9 ± 9.5 | 93.9 – 152.3 | 133.6 ± 10.5 | 104.3 – 172.4 |
| b BSI, mg2/mm4 | 84.3 ± 21.9 | 42.0 – 154.5 | 109.9 ± 29.2 | 53.5 – 215.6 |
| Femur 20% sites | ||||
| Cortical Density, mg/ccm | 1040.9 ± 22.2 | 983.0 – 1088.4 | 1049.1 ± 24.3 | 976.8 – 1107.6 |
| Cortical Area, mm2 | 157.0 ± 23.8 | 109.6 – 226.6 | 197.3 ± 31.2 | 128.9 – 275.0 |
| Periosteal Circumference, mm | 73.2 ± 6.8 | 55.4 – 97.1 | 83.1 ± 7.4 | 64.3 – 103.5 |
| Endosteal Circumference, mm | 58.1 ± 7.4 | 39.0 – 81.4 | 66.5 ± 7.8 | 46.5 – 88.2 |
| c SSI, mm3 | 1119.7 ± 257.5 | 565.5 – 2076.6 | 1625.7 ± 377.3 | 881.4 – 2822.3 |
| Tibia 4% sites | ||||
| Trabecular Density, mg/ccm | 218.9 ± 26.8 | 155.1 – 306.1 | 224.6 ± 27.6 | 154.1 – 301.4 |
| Trabecular Area, mm2 | 422.9 ± 75.6 | 174.5 – 644.1 | 537.2 ± 100.2 | 231.7 – 869.1 |
| Periosteal Circumference, mm | 81.9 ± 6.4 | 59.4 – 98.9 | 91.9 ± 7.7 | 68.6 – 114.9 |
| b BSI, mg2/mm4 | 46.1 ± 11.3 | 23.1 – 80.8 | 58.4 ± 15.2 | 28.2 – 106.8 |
| Tibia 66% sites | ||||
| Cortical Density, mg/ccm | 1017.9 ± 29.5 | 886.3 – 1098.6 | 1041.1 ± 31.8 | 953.4 – 1121.0 |
| Cortical Area, mm2 | 172.2 ± 28.9 | 78.6 – 250.7 | 212.1 ± 32.9 | 123.8 – 306.1 |
| Periosteal Circumference, mm | 68.6 ± 5.8 | 55.4 – 87.7 | 75.7 ± 6.1 | 54.8 – 92.8 |
| Endosteal Circumference, mm | 50.4 ± 6.1 | 34.2 – 70.3 | 55.3 ± 6.7 | 38.0 – 73.4 |
| c SSI, mm3 | 999.9 ± 247.8 | 320.7 – 1795.8 | 1391.3 ± 313.9 | 583.8 – 2225.1 |
| DXA bone measurements | ||||
| d TBBMC, kg | 1.2 ± 0.2 | 0.8 – 2.0 | 1.7 ± 0.4 | 0.8 – 2.7 |
| e TBBMD, m/kg | 0.9 ± 0.1 | 0.7 – 1.0 | 1.0 ± 0.1 | 0.8 – 1.1 |
| f ND Total Hip, m/kg | 0.8 ± 0.1 | 0.5 – 1.1 | 0.9 ± 0.1 | 0.6 – 1.2 |
| g L2–L4 Spine, m/kg | 0.8 ± 0.1 | 0.6 – 1.1 | 0.9 ± 0.1 | 0.6 – 1.3 |
All characteristics and bone measures are significantly different between 4th and 6th grade girls
Maturity Offset = years from peak height velocity (PHV). Maturity offset values can include zero and the range may include both negative and positive integers where a value of 0 = at PHV, < 0 = number of years before PHV, and > 0 = number of years after PHV.
BSI = bone strength index
SSI = Strength-strain index
TBBMC = total body bone mineral content
TBBMD = total body areal bone mineral density
ND Total Hip = non-dominant total hip areal bone mineral density
L2–L4 Spine = Lumbar 2 - Lumbar 4 Spine areal bone mineral density
Dietary intake and energy expenditure
Mean reported daily intake of total energy, macro and micronutrients, and estimated TEE are shown in Table 2. Fourth grade girls reported higher intakes of total energy, all macronutrients, and more PA than 6th grade girls. Fourteen (7.6%) 4th grade and 3 (1.7%) 6th grade girls achieved the recommended 12,000 steps/d (females aged 6–12 yrs). The portion of 4th and 6th grade girls respectively who met the RDA for the bone-related nutrients were as follows: calcium (27%, 18%), vitamin D (55%, 51%), potassium (AI, 1%, 0%), magnesium (51%, 35%), vitamin C (87%, 89%), zinc (78%, 69%), iron (84%, 87%), total energy (59%, 47%), and protein (91%, 93%). Paired t-tests showed significant differences in reported intake of calcium, iron, zinc, magnesium, and vitamins C and D estimated with and without supplements (Table 3).
Table 2.
Mean daily dietary intake and estimated energy expenditure of 363 young girls
| 4th Grade | 6th Grade | ||||
|---|---|---|---|---|---|
|
| |||||
| Mean ± SD | Range | Mean ± SD | Range | RDA | |
| Total Energy, Kcals (MJ) | 1730 ± 573 (7.2 ± 2.4) | 552 – 2927 | 1569 ± 493† (6.6 ± 2.1) | 623 – 2918 | 1600–2000 |
| Protein, g | 70 ± 26 | 14 – 132 | 62 ± 22† | 18 – 123 | 34 |
| Fat, g | 60.9 ± 22.8 | 14 – 114 | 54 ± 18‡ | 15 – 100 | 62–85 |
| Carbohydrate, g | 232 ± 79 | 74 – 460 | 215 ± 73* | 71 – 436 | 130 |
| Fiber, g | 14 ± 6 | 4 – 38 | 13 ± 6 | 4 – 36 | 23–28 |
| Calcium, mga | 1019 ± 458 | 252 – 2386 | 933 ± 387 | 273 – 2022 | 1300 |
| Potassium, mg | 2372 ± 882 | 580 – 4677 | 2190 ± 775* | 733 – 4393 | 4500 |
| Iron, mga | 16 ± 8 | 4 – 45 | 15 ± 8 | 5 – 50 | 8 |
| Magnesium, mga | 245 ± 88 | 76 – 484 | 225 ± 77* | 71 – 499 | 240 |
| Zinc, mga | 13 ± 6 | 3 – 30 | 12 ± 6* | 4 – 34 | 8 |
| Vitamin D, IUa | 284 ± 186 | 22 – 926 | 261 ± 173 | 23 – 953 | 600 |
| Vitamin C, mga | 109 ± 62 | 11 – 284 | 107 ± 68 | 21 – 339 | 45 |
| BMR, MJ/24 h (Kcals) | 4.9 ± 0.5 (1171 ± 120) | 3.7 – 6.8 | 5.4 ± 0.6 (1291 ± 143) | 4.2 – 6.9 | NA |
| Avg. Total Steps/d | 8930 ± 2429 | 3799 – 16579 | 8014 ± 1770† | 3603 – 12883 | 12000 b |
| PAL | 1.6 ± 0.3 | 1.2 – 2.2 | 1.5 ± 0.2† | 1.2 – 2 | NA |
Value includes supplements
Significant differences between 4th and 6th grade girls:
p < .05,
p < .01,
p < .001
RDA for carbohydrate = minimum
BMR = 0.085 wt(kg) + 2.033 (for females 3–10 y); BMR = 0.056 wt(kg) + 2.898 (for females 10–18 y)
Recommended number of steps per day for females aged 6–12 y
PAL = physical activity level category
NA = guideline not established
Table 3.
Paired t-test for micronutrient intakes estimated with and without supplement use (N = 363)
| Micronutrient | Mean | Standard Deviation | p-value (2-tailed) | |
|---|---|---|---|---|
| Pair 1 | Calcium, mg | 976.3 | 425.9 | .000 |
| Calcium, mg (without supplements) | 948.7 | 418.0 | ||
| Pair 2 | Magnesium, mg | 234.9 | 83.3 | .000 |
| Magnesium, mg (without supplements) | 228.1 | 81.1 | ||
| Pair 3 | Potassium, mg | 2282.5a | 834.5 | N/A |
| Potassium, mg (without supplements) | 2282.5a | 834.5 | ||
| Pair 4 | Zinc, mg | 12.3 | 5.7 | .000 |
| Zinc, mg (without supplements) | 9.6 | 3.5 | ||
| Pair 5 | Iron, mg | 15.4 | 7.7 | .000 |
| Iron, mg (without supplements) | 12.0 | 5.1 | ||
| Pair 6 | Vitamin C, mg | 107.9 | 64.6 | .000 |
| Vitamin C, mg (without supplements) | 94.2 | 58.6 | ||
| Pair 7 | Vitamin D, IU | 272.8 | 179.7 | .000 |
| Vitamin D, IU (without supplements) | 203.9 | 132.1 |
The correlation and t cannot be computed because the standard error of the difference is 0.
Associations between bone measurements and bone related micronutrients
Bone geometry, size and density from pQCT were significantly correlated with calcium, vitamin C and zinc intake in 4th grade girls (Table 4). There were no significant associations between the intakes of any of the evaluated micronutrients and bone measures in 6th grade girls (Table 4).
Table 4.
Pearson correlation coefficients between pQCT bone parameters and mean dietary intake of select bone related nutrients
| Calcium | Vitamin D | Vitamin C | Zinc | Iron | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| 4th | 6th | 4th | 6th | 4th | 6th | 4th | 6th | 4th | 6th | |
| Femur 4% sites | ||||||||||
| Trabecular Area, mm2 | −0.15* | 0.07 | −0.11 | 0.02 | 0.11 | 0.07 | −0.09 | 0.01 | −0.06 | 0.00 |
| Periosteal Circ, mm | −0.16* | 0.05 | −0.10 | 0.10 | 0.11 | 0.08 | −0.08 | 0.00 | −0.05 | 0.00 |
| Femur 20% sites | ||||||||||
| Cortical Density, mg/mm | 0.12 | −0.02 | 0.09 | −0.08 | 0.13 | 0.03 | 0.16* | −0.05 | 0.10 | −0.05 |
| Periosteal Circ, mm | −0.09 | 0.02 | −0.02 | −0.04 | 0.17* | 0.07 | 0.03 | −0.02 | 0.06 | 0.00 |
| Endosteal Circ, mm | −0.09 | 0.04 | −0.05 | 0.00 | 0.17* | 0.08 | 0.01 | 0.02 | 0.05 | 0.04 |
| SSI, mm3 | −0.03 | −0.01 | 0.06 | −0.11 | 0.18* | 0.02 | 0.09 | −0.10 | 0.08 | −0.07 |
| Tibia 4% sites | ||||||||||
| Trabecular Area, mm2 | −0.02 | −0.01 | 0.00 | −0.05 | 0.18* | 0.01 | 0.08 | −0.02 | 0.07 | −0.02 |
| Periosteal Circ, mm | −0.03 | −0.03 | 0.01 | −0.07 | 0.19† | 0.02 | 0.07 | −0.04 | 0.07 | −0.04 |
| Tibia 66% sites | ||||||||||
| Cortical Density, mg/mm | 0.11 | −0.05 | 0.12 | −0.07 | 0.12 | −0.03 | 0.15* | −0.05 | 0.12 | −0.05 |
| Cortical Area, mm2 | −0.07 | −0.01 | 0.03 | −0.09 | 0.15* | 0.02 | 0.05 | −0.11 | 0.06 | −0.08 |
| a SSI, mm3 | −0.06 | −0.01 | 0.04 | −0.10 | 0.18* | 0.02 | 0.08 | −0.11 | 0.08 | −0.08 |
p < 0.05
p < 0.01
SSI = strength-strain index (mm3)
Multiple linear regression analysis
In 4th grade girls, vitamin C was independently associated with femoral trabecular area, periosteal and endosteal circumferences, and tibial SSI (Table 5). Zinc had a positive independent association with femoral cortical density and iron was negatively associated with femoral cortical area, and tibial SSI (Table 5). The regression analysis (M1) did not reveal significant independent associations for bone-related nutrients at any of the femur sites in 6th grade girls. After adjustment (M2), significant linear relationships were found between magnesium, tibial trabecular area (β = −0.46, P < 0.05) and periosteal circumference (β = −0.42, P < 0.05). Zinc had an independent negative association with tibial cortical area (β = −0.35, P < 0.05) and TBBMC (β = −0.26, P = 0.01). Analyses with Tanner stage substituted for maturity offset gave similar results and did not change the magnitude or direction of the observed relationships between dietary nutrient intake and bone measures.
Table 5.
Regression coefficients for selected nutrients regressed on pQCT bone measurements in 4th grade girls
| Calcium | Vitamin D | Vitamin C | Zinc | Iron | |
|---|---|---|---|---|---|
| Femur Metaphyseal (4%) | |||||
| Trabecular Area, mm2 | 0.04 | −0.01 | 0.18* | −0.08 | −0.11 |
| Periosteal Circ, mm | 0.03 | −0.01 | 0.17* | −0.09 | −0.11 |
| Femur Diaphyseal (20%) | |||||
| Cortical Density, mg/ccm | 0.17 | −0.23 | 0.09 | 0.55* | −0.33 |
| Cortical Area, mm2 | −0.06 | 0.12 | 0.02 | 0.25a | −0.31* |
| Endosteal Circ, mm | 0.06 | −0.08 | 0.15* | 0.08 | −0.08 |
| Tibia Diaphyseal (66%) | |||||
| Cortical Area, mm2 | −0.09 | 0.08 | 0.13 | 0.14a | −0.27a |
| Periosteal Circ, mm | 0.17 | −0.22 | 0.12a | 0.17 | −0.13 |
| b SSI, mm3 | 0.00 | −0.03 | 0.15* | 0.31a | −0.34* |
Results were derived using regression model 2: intercept + lean mass (kg) + maturity offset (y) + PA (avg. steps/d) + EI (Kcals) + nutrients
Values are standardized β coefficients
P < 0.05
β coefficient approaches significance at p = .05
SSI = strength-strain index (mm3)
DISCUSSION
We examined the relationships between mean intakes of dietary micronutrients and bone status, with adjustments for the most influential and potentially confounding factors including maturation, lean body mass, total EI and PA [36] in preadolescent girls. The finding of a positive association between vitamin C intake and metaphyseal geometry as well as diaphyseal strength in prepubescent girls is novel, since most past studies have used 2-dimensional DXA measures. This work expands upon the prior literature in several ways including the use of pQCT along with DXA to evaluate micronutrient-bone health associations in preadolescent females undergoing skeletal maturation. Our ability to assess associations at different benchmarks of maturation was another novel aspect of the research.
The nutritional factors that were studied are thought to be of particular importance during periods of rapid skeletal growth due to their involvement in bone formation, quality and status overall [5, 26, 37]. Nevertheless, relationships between intakes of these nutrients, bone development and attainment of bone strength have not been fully investigated. Consequently, apriori we hypothesized that all of these nutrients would be significant predictors of strength and structural parameters, as well as (as shown in previous studies) mass and density. Interestingly, only vitamin C and zinc intake were positively associated with bone measures in the current analysis and most consistently in the younger (4th grade) girls. Negligible relationships between intake of micronutrients and bone status observed in 6th graders may underscore the increasing and predominant influence of hormones (i.e. Oestrogen, etc.) on bone accretion as girls approach maturity. Around age 10y, hormonal changes occur more rapidly and likely begin to overshadow the influence of micronutrient intakes on skeletal growth. Similarly, the potential for micronutrient intakes to influence bone may vary during critical phases of bone apposition throughout maturation. With key roles in collagen production, vitamin C and zinc may be more influential during periods when collagen production is prominent. In general, the divergence in maturation between the two groups may explain the differential results in 4th compared to 6th grade girls.
Previous studies in children and adults have reported a positive influence of vitamin C on aBMD of the hip, the heel and BMC of the LS [14–15, 17]. In this study, using DXA, the association was positive as hypothesized and approached significance (total hip (p = 0.07) and LS (p = 0.09)), although only in 4th grade girls. Using pQCT, our data showed that vitamin C is positively related to trabecular geometry and cortical strength in 4th grade girls. Importantly, vitamin C intake in the current study was more than twice the RDA in both groups.
Despite reported associations between zinc intake and BMD in premenopausal women [17] and with bone formation in animal studies [22], a study in healthy 12-year old girls did not show an effect of zinc supplementation on bone metabolism [22]. In the current study, zinc was positively correlated with femoral and tibial cortical vBMD and tibial cortical strength in 4th grade girls, whereas iron was negatively associated with cortical strength and area. Inconsistent relationships for iron and zinc suggest a stronger influence of the covariates in the regression models on bone status cross-sectionally than the intake of these specific nutrients. The negative relation of iron to bone measures in this study is novel given that iron is thought to be beneficial for bone [5]. The impact of iron intake and menstruation on iron status combined with the multifactorial relationship of iron to lean body mass during the adolescent growth spurt in girls, are grounds for cautious interpretation. Unfortunately, the variability in these longitudinal factors could not be assessed in this cross-sectional study. However, these new observations may underscore the importance of pQCT in the study of rapidly changing bone.
Previous studies in adolescents (7–17 y) have reported positive effects of supplementation with calcium and vitamin D on BMD and BMC [12]. The lack of significant associations between calcium and vitamin D intake and bone parameters in our sample may be attributable to intake below those reported in “supplemented” intervention studies and current recommendations. Additionally, dietary sources of vitamin D intakes are limited and are not commonly consumed by girls in this age range, and thus contribute minimally to overall vitamin D status in young girls residing in Arizona who generally experience generous daily UV sunlight exposure.
Although DXA is frequently used to assess skeletal status in research involving children [14–16, 19, 37–41], it is confounded by the accelerated changes experienced during growth, an important limitation of past work in this area. Unlike DXA, pQCT provides 3-dimensional measures of true volumetric density and geometrical features of the bone and it is possible to differentiate between cortical and trabecular BMD compartments [42–43]. To date, few studies have used pQCT [44–45] to investigate bone-micronutrient relationships, and none have examined the diversity of micronutrient intake assessed in our sample. Continued and broader use of pQCT should help clarify the relationship between dietary intake and bone parameters, particularly among children. Replication of our findings related to vitamin C and pQCT are necessary.
This study is limited by the cross-sectional design; causal relationships cannot be inferred. The lack of racial diversity in this sample is also problematic, limiting generalizability. The fact that our data show a consistent positive influence of vitamin C and a potential for zinc and iron to be related to bone status in prepubescent, but not early-pubescent girls is novel and warrants further investigation. The findings also demonstrate that nutrients other than calcium and vitamin D play vital roles in early bone development. Longitudinal analyses are warranted.
CONCLUSION
In summary, intakes of vitamin C and zinc are related positively to bone size, geometry and strength in preadolescent girls as measured by pQCT. Differential micronutrient-bone relationships in 4th and 6th grade girls demonstrate that the influence of nutrient intake on bone may vary through the peri-pubertal years. Our data are consistent with the recommendation that girls consume adequate vitamin C and zinc, from foods like citrus fruit, lean meat, poultry and fortified cereals to maximize bone development.
Acknowledgments
We appreciate the participation and support of the principals, teachers, parents and students from the schools in the Catalina Foothills and Marana School Districts. We would also like to thank the radiation technicians, program coordinators, and all members of the Jump-In Study team for their contributions. The project described was supported by Award Number HD-050775 (SG) from the National Institute of Child Health and Human Development. We also wish to acknowledge contributions by Dr. Kathryn Schmitz (The University of Pennsylvania) to the preparation of this manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Child Health and Human Development or the National Institutes of Health.
Footnotes
DISCLOSURES: NONE
None of the authors had a personal or financial competing interest.
The authors have stated that they have no conflict of interest.
References
- 1.Ondrak KS, Morgan DW. Physical activity, calcium intake and bone health in children and adolescents. Sports Med. 2007;37(7):587–600. doi: 10.2165/00007256-200737070-00003. [DOI] [PubMed] [Google Scholar]
- 2.Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3:S131–S139. doi: 10.2215/CJN.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartman C, Hochberg Z, Shamir R. Osteoporosis in pediatrics. Reviews. IMAJ. 2003;5:509–515. [PubMed] [Google Scholar]
- 4.Cooper C, Westlake S, Harvey N, Dennison E. CHP 16: Developmental Origins of Osteoporotic Fracture. Southampton; UK: 2009. Breast-Feeding: Early Influences on Later Health; pp. 217–236. [DOI] [PubMed] [Google Scholar]
- 5.Palacios C. The role of nutrients in bone health, from A to Z. Critical Reviews in Food Science and Nutrition. 2006;46:621–628. doi: 10.1080/10408390500466174. [DOI] [PubMed] [Google Scholar]
- 6.Brannon PM, Yetley EA, Bailey RL, et al. Overview of the conference ‘Vitamin D and health in the 21st century: an update’. Am J Clin Nutr. 2008;88:483S–90S. doi: 10.1093/ajcn/88.2.483S. [DOI] [PubMed] [Google Scholar]
- 7.Bergman C, Gray-Scott D, Chen JJ, Meacham S. What is next for the dietary reference intakes for bone metabolism related nutrients beyond calcium: phosphorus, magnesium, vitamin D, and fluoride? Critical Reviews in Food Science and Nutrition. 2009;49(2):136–144. doi: 10.1080/10408390701764468. [DOI] [PubMed] [Google Scholar]
- 8.Dawson-Hughes B. Calcium supplementation and bone loss: a review of controlled clinical trials. Am J Clin Nutr. 1991;54:274S–80S. doi: 10.1093/ajcn/54.1.274S. [DOI] [PubMed] [Google Scholar]
- 9.Rafferty K, Heaney RP. Nutrient effects on the calcium economy: emphasizing the potassium controversy. J Nutr. 2008;138:166S–171S. doi: 10.1093/jn/138.1.166S. [DOI] [PubMed] [Google Scholar]
- 10.Fiorito LM, Mitchell DC, Smiciklas-Wright H, Birch LL. Girls’ calcium intake is associated with bone mineral content during middle childhood. J Nutr. 2006;136:1281–1286. doi: 10.1093/jn/136.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonjour J-P, Carrie AL, Ferrari S, Clavien H, Slosman D, Theintz G, Rizzoli R. Calcium-enriched food and bone mass growth in prepubertal girls: a randomized, double-blind, placebo-controlled trial. J Clin Invest. 1997;99:1287–1294. doi: 10.1172/JCI119287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rizzoli R, Bianchi ML, Garabedian M, Mckay HA, Moreno LA. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone. 2010;46:294–305. doi: 10.1016/j.bone.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Borer KT. Physical activity in the prevention and amelioration of osteoporosis in women. Sports Med. 2005;35(9):779–830. doi: 10.2165/00007256-200535090-00004. [DOI] [PubMed] [Google Scholar]
- 14.Prynne CJ, Mishra GD, O’Connell MA, Muniz G, Laskey MA, Van L, Prentice A, Ginty F. Fruit and vegetable intakes and bone mineral status: a cross-sectional study in 5 age and sex cohorts. Am J Clin Nutr. 2006;83:1420–1428. doi: 10.1093/ajcn/83.6.1420. [DOI] [PubMed] [Google Scholar]
- 15.McGartland CP, Robson PJ, Murray LJ, et al. Fruit and vegetable consumption and bone mineral density: the Northern Ireland Young Hearts Project. Am J Clin Nutr. 2004;80:1019–1023. doi: 10.1093/ajcn/80.4.1019. [DOI] [PubMed] [Google Scholar]
- 16.Tylavsky FA, Holliday K, Danish R, Womack C, Norwood J, Carbone L. Fruit and vegetable intakes are an independent predictor of bone size in early pubertal children. Am J Clin Nutr. 2004;79:311–317. doi: 10.1093/ajcn/79.2.311. [DOI] [PubMed] [Google Scholar]
- 17.New SA. Nutritional influences on bone mineral density: a cross-sectional study in premenopausal women. Am J Clin Nutr. 1997;65:1831–1839. doi: 10.1093/ajcn/65.6.1831. [DOI] [PubMed] [Google Scholar]
- 18.New SA. Dietary influences on bone mass and bone metabolism: further evidence of a positive link between fruit and vegetable consumption and bone health? Am J Clin Nutr. 2000;71:142–151. doi: 10.1093/ajcn/71.1.142. [DOI] [PubMed] [Google Scholar]
- 19.Vatanparast H, Baxter-Jones A, Faulkner RA, Bailey DA, Whiting SJ. Positive effects of vegetable and fruit consumption and calcium intake on bone mineral accrual in boys during growth from childhood to adolescence: the University of Saskatchewan Pediatric Bone Mineral Accrual Study. Am J Clin Nutr. 2005;82:700–706. doi: 10.1093/ajcn.82.3.700. [DOI] [PubMed] [Google Scholar]
- 20.Prentice A, Schoenmakers I, Laskey MA, de Bono1 S, Ginty F, Goldberg GR. Symposium on ‘Nutrition and health in children and adolescents’ Session 1: Nutrition in growth and development: Nutrition and bone growth and development. Proc Nutr Soc. 2006;65(4):348–360. doi: 10.1017/s0029665106005192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Going S, Lohman T, Houtkooper L, et al. Effects of exercise on bone mineral density in calcium-replete postmenopausal women with and without hormone replacement therapy. Osteoporos Int. 2003;14:637–643. doi: 10.1007/s00198-003-1436-x. [DOI] [PubMed] [Google Scholar]
- 22.Hall SL, Dimai HP, Farley JR. Effects of zinc on human skeletal alkaline phosphatase activity in vitro. Calcif Tissue Int. 1999;6:163–172. doi: 10.1007/s002239900597. [DOI] [PubMed] [Google Scholar]
- 23.Medeiros DM, Plattner A, Jennings D, Stoecker B. Bone morphology, strength and density are compromised in iron-deficient rats and exacerbated by calcium restriction. J Nutr. 2002;132:3135–3141. doi: 10.1093/jn/131.10.3135. [DOI] [PubMed] [Google Scholar]
- 24.Farr JN, Lee VR, Blew RM, Lohman TG, Going SB. Quantifying bone-relevant activity and its relation to bone strength in girls. Med Sci Sports Exerc. 2011;43(3):476–483. doi: 10.1249/MSS.0b013e3181eeb2f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American Academy of Pediatrics. Medical conditions affecting sports participation. Pediatrics. 2001;107(5):1205–1209. doi: 10.1542/peds.107.5.1205. [DOI] [PubMed] [Google Scholar]
- 26.Sherar LB, Baxter-Jones AD, Mirwald RL. Limitations to the use of secondary sex characteristics for gender comparisons. Ann Hum Biol. 2004;31(5):586–593. doi: 10.1080/03014460400001222. [DOI] [PubMed] [Google Scholar]
- 27.Mirwald RL, Baxter-Jones AD, Bailey DA, Beunen GP. An assessment of maturity from anthropometric measurements. Med Sci Sports Exerc. 2002;34(4):689–694. doi: 10.1097/00005768-200204000-00020. [DOI] [PubMed] [Google Scholar]
- 28.Bailey DA, McKay HA, Mirwald RL, Crocker PR, Faulkner RA. A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: The University of Saskatchewan Bone Mineral Accrual Study. J Bone Miner Res. 1999;14:1672–1679. doi: 10.1359/jbmr.1999.14.10.1672. [DOI] [PubMed] [Google Scholar]
- 29.Rockett H, Breitenbach M, Frazier AL, et al. Validation of a Youth/Adolescent Food Frequency Questionnaire. Preventive Medicine. 1997;26:808–816. doi: 10.1006/pmed.1997.0200. [DOI] [PubMed] [Google Scholar]
- 30.Holbrook EA, Barreira TV, Kang M. Validity and reliability of Omron pedometers for prescribed and self-paced walking. Med Sci Sports Exerc. 2009;41(3):670–674. doi: 10.1249/MSS.0b013e3181886095. [DOI] [PubMed] [Google Scholar]
- 31.Tudor-Locke C, Pangrazi RP, Corbin CB, et al. BMI-referenced standards for recommended pedometer-determined steps/day in children. Prev Med. 2004;38:857–64. doi: 10.1016/j.ypmed.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 32.Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Human Kinetics; Champaign, Illinois: 1988. pp. 3–16. [Google Scholar]
- 33.Kontulainen SA, Johnston JD, Liu D, et al. Strength indices from pQCT imaging predict up to 85% of variance in bone failure properties at tibial epiphysis and diaphysis. J Musculoskelet Neuronal Interact. 2008;8:401–409. [PubMed] [Google Scholar]
- 34.Macdonald HM, Kontulainen SA, Khan KM, McKay HA. Is a school-based physical activity intervention effective for increasing tibial bone strength in boys and girls? J Bone Miner Res. 2007;22:434–446. doi: 10.1359/jbmr.061205. [DOI] [PubMed] [Google Scholar]
- 35.Kuczmarski RJ, Ogden CL, Guo SS, et al. CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;11(246):1–190. [PubMed] [Google Scholar]
- 36.Moyer-Mileur L, Xie B, Ball S, Bainbridge C, Stadler D, Jee WS. Predictors of bone mass by peripheral quantitative computed tomography in early adolescent girls. J Clin Densitom. 2001;4:313–23. doi: 10.1385/jcd:4:4:313. [DOI] [PubMed] [Google Scholar]
- 37.Matkovic V, Goel PK, Badenhop-Stevens NE, et al. Calcium supplementation and bone mineral density in females from childhood to young adulthood: a randomized controlled trial. Am J Clin Nutr. 2005;81:175–88. doi: 10.1093/ajcn/81.1.175. [DOI] [PubMed] [Google Scholar]
- 38.Lanou AJ, Berkow SE, Barnard ND. Calcium, dairy products, and bone health in children and young adults: a reevaluation of the evidence. Pediatrics. 2005;115:736–743. doi: 10.1542/peds.2004–0548. [DOI] [PubMed] [Google Scholar]
- 39.Welch JM, Weaver CM. Calcium and exercise affect the growing skeleton. Nutr Rev. 2005;63(11):361–373. doi: 10.1301/nr.2005.nov.361–373. [DOI] [PubMed] [Google Scholar]
- 40.Carter LM, Whiting SJ, Drinkwater DT, Zello GA, Faulkner RA, Bailey DA. Self-reported calcium intake and bone mineral content in children and adolescents. J Am Coll Nutr. 2001;20(5):502–509. doi: 10.1080/07315724.2001.10719059. [DOI] [PubMed] [Google Scholar]
- 41.Burrows M, Baxter-Jones A, Mirwald R, Macdonald H, McKay H. Bone mineral accrual across growth in a mixed-ethnic group of children: are Asian children disadvantaged from an early age? Calcif Tissue Int. 2009;84:366–378. doi: 10.1007/s00223-009-9236-8. [DOI] [PubMed] [Google Scholar]
- 42.Leonard MB, Shults J, Elliott DM, Stallings VA, Zemel BS. Interpretation of whole body dual energy x-ray absorptiometry measures in children: comparison with peripheral quantitative computed tomography. Bone. 2004;34:1044–1052. doi: 10.1016/j.bone.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 43.Baroncelli GI, Bertelloni S, Sodini F, Saggese G. Osteoporosis in children and adolescents: Etiology and management. Pediatr Drugs. 2005;7:295–323. doi: 10.2165/00148581-200507050-00003. [DOI] [PubMed] [Google Scholar]
- 44.Moyer-Mileur LJ, Xie B, Ball SD, Pratt T. Bone mass and density response to a 12-month trial of calcium and vitamin D supplement in preadolescent girls. J Musculoskel Neuron Interact. 2003;3:63–70. [PubMed] [Google Scholar]
- 45.Specker B, Binkley T. Randomized trial of physical activity and calcium supplementation on bone mineral content in 3- to 5-year old children. J Bone Miner Res. 2003;18:885–892. doi: 10.1359/jbmr.2003.18.5.885. [DOI] [PubMed] [Google Scholar]