Abstract
Environmental models, often applied to questions on the fate and transport of chemical hazards, have recently become important in tracing certain environmental pathogens to their upstream sources of contamination. These tools, such as first order decay models applied to contaminants in surface waters, offer promise for quantifying the fate and transport of pathogens with multiple environmental stages and/or multiple hosts, in addition to those pathogens whose environmental stages are entirely waterborne. Here we consider the fate and transport capabilities of the human schistosome Schistosoma japonicum, which exhibits two waterborne stages and is carried by an amphibious intermediate snail host. We present experimentally-derived dispersal estimates for the intermediate snail host and fate and transport estimates for the passive downstream diffusion of cercariae, the waterborne, human-infective parasite stage. Using a one dimensional advective transport model exhibiting first-order decay, we simulate the added spatial reach and relative increase in cercarial concentrations that dispersing snail hosts contribute to downstream sites. Simulation results suggest that snail dispersal can substantially increase the concentrations of cercariae reaching downstream locations, relative to no snail dispersal, effectively putting otherwise isolated downstream sites at increased risk of exposure to cercariae from upstream sources. The models developed here can be applied to other infectious diseases with multiple life-stages and hosts, and have important implications for targeted ecological control of disease spread.
Keywords: infectious disease, Schistosoma japonicum, fate and transport, migration, dispersal, spatial spread, environment, public health
INTRODUCTION
Waterborne and water-related infectious pathogens depend upon surface waters for their sustained transmission, persistence, and spread (Cairncross and Feacham 1999; Sharma, Sachdeva et al. 2003) and contribute substantially to the global burden of disease (Prüss, Kay et al. 2002). Overland flows are a dominant means by which waterborne pathogenic microorganisms are transported from land to surface waters and to downstream populations (Tyrrel and Quinton 2003). For instance, studies have found that surface water flows play a significant role in moving certain enteric pathogens from diffuse, non-point sources into channels (Mawdsley, Bardgett et al. 1995; Tate, Atwill et al. 2000), where they can be transported long distances from the source. One-dimensional advective transport models, or distance decay models, can be used to estimate the attenuation of an environmental hazard as it is transported away from an emission source (Briggs and Elliott 1995), and have been used to quantify the loss of pathogens as they move in surface waters, as well as to provide estimates of the spatial scale of risk resulting from a source of contamination (Kay and McDonald 1980). These models, however, and fate and transport models generally (Schnoor 1996), have rarely been used to estimate the spatial scale of risk stemming from pathogens that are not strictly waterborne or airborne, such as those with complex lifecycles.
Human schistosomes, for instance, have waterborne life-stages as well as stages that exist outside of the aquatic environment, where they are not subject to the downstream movement of overland flow. For instance, Schistosoma japonicum, the parasite that causes human schistosomiasis in East and Southeast Asia, is carried by amphibious intermediate host snails that disperse in both upstream and downstream directions both on land and in water. This characteristic presents a challenge to traditional distance decay models, as they must be extended to accommodate active dispersal of hosts, and in some analogous instances, vectors, along with passive transport in the aquatic medium. Even as some hydrologists have called for a better understanding of the overland spread of diffuse pathogens from animal waste (Ziemer, Bonner et al. 2010), and some have accounted for hydrological connectivity in the spread of infectious disease (Pringle 2003), the focus of such efforts has been limited to strictly aquatic pathogens. Here, we explore how such models can provide insight into the spatial dynamics of pathogens with multiple life-stages.
Schistosoma japonicum has two aquatic stages: a human-infective larval stage, called cercariae, and a stage that infects snails, termed miracidia. The juvenile stages of the intermediate snail host are aquatic, while the adult snail is amphibious and actively disperses outside of the water column. New acute schistosomiasis cases over the last decade in China have signaled that the disease may have re-emerged in some counties, which has led to investigations as to whether the new cases were locally transmitted or were imported from neighboring areas.
Recent theoretical work has shown that surface-water connections can maintain endemic schistosomiasis in a set of inter-connected villages, even when local, within-village transmission alone is insufficient to sustain disease in the absence of these connections (Gurarie and Seto 2008). Other work has shown that the degree of isolation (or connectedness) among communities, if quantified, can potentially be used to identify and target upstream sources of schistosome risk (Remais, Akullian et al. 2010). As of yet, however, empirically-derived estimates of village-to-village transport of these parasites are not available, and theoretical explorations of schistosome connectivity have ignored intermediate host dispersal as a contributor to that transport (Seto, Xu et al.; Gurarie and Seto 2008).
Here, we generate experimental estimates of parasite and snail movement, and spatially model the degree to which cercariae are imported by villages from beyond their boundaries. The fundamental question posed here is the degree to which downstream transport of these parasites, as measured by relative changes in cercarial concentrations at downstream exposure sites, is sensitive to the dispersal of intermediate hosts. We consider the specific mechanisms by which schistosomes, in their aquatic life stages and within intermediate hosts, move from one location to the next.
METHODS
Intermediate host dispersal
The amphibious, freshwater molluscan host for Schistosoma japonicum is Oncomelania hupensis robertsoni, a mountain snail endemic to certain areas of Sichuan province. O.h. robertsoni is confined to the mountainous regions of Central China, above the Three Gorges Dam, where it is protected from seasonal flooding of the Yangtze river (Wilke, Davis et al. 2000). The snail inhabits a complex network of small irrigation channels within agricultural regions of Western China, and displays a well-mixed and fairly stable population genetic structure due to its geographic position away from major drainage systems (Wilke, Davis et al. 2000).
An ecological hallmark of O.h. robertsoni behavior is its persistent upstream and uphill movement, especially following heavy rains (Davis, Wilke et al. 1999; Davis, Wu et al. 2006). Lab observations of O. hupensis have confirmed that snails actively move against an elevation gradient when threatened by simulated flooding (Ritchie 1955). What is more, Oncomelania has been observed to spread via irrigation systems that link one village to the next and are also commonly observed along perennially wet slopes that are not connected to irrigation systems (Xu, Wei et al. 2000; Davis, Wu et al. 2006). Even marginal snail habitat can support sufficient snails for human transmission to occur, especially if human and animal waste reaches these sites on a continual basis (Davis, Wu et al. 2006).
Quantifying intermediate host dispersal
Direct estimates of intermediate host dispersal are rare, often based on observations of just a few individuals, and have generally been limited to dispersal in the downstream direction (Chlyeh, Dodet et al. 2006). In this study, mass mark release (MMR), a common technique for studying invertebrate dispersal (Osborne, Loxdale et al. 2002), was used to estimate the distribution of distances dispersed by individuals from a source population per unit time (McCallum 2000). The MMR experiment was conducted in Gongqiao village, near the city of Deyang (pop. 4 million; 31° 8′ 0″ N, 104° 24′ 0″ E) in the Chengdu plain, a highly irrigated setting that provides ideal habitat for O. hupensis. The topology of the irrigation network in Gongqiao is highly representative of mountainous villages in this region, in which one major regional channel (termed ‘A’ channel; channel dimensions approximately 5 m wide, 2 m deep) carries water from an upstream reservoir to several outlets in the village that feed smaller ‘B’ channels (1–2 m wide, 1 m deep), which in turn feed more minor ‘C’ (0.5 – 1 m wide, 0.5 m deep) and ‘D’ (0.25 m wide, 0.25 m deep) channels that carry water into agricultural fields. C and D channels are sometimes concrete lined, but are more commonly dug-out mud ditches that support snail host populations.
The MMR was carried out in a C channel chosen based on the presence of mud substrate, high soil moisture, vegetative banks and easy access for release and recapture. A total of 1,440 O. h. robertsoni snails were collected from a nearby site of high snail density and brought back to the lab to be cleaned, marked with colored varnish, and set to dry according to methods outlined elsewhere (Woolhouse 1988; Remais, Hubbard et al. 2007). Snails were divided randomly into five colored marking groups as in previous work, which found no differential survival or behavior among marked and un-marked O. h. robertsoni snails (Remais, Hubbard et al. 2007). All live, marked snails were returned to the study channel the next day. Each color group (n=288 snails) was released at one of five locations spaced equidistant from one another along the channel so as to replicate the experiment at different locations (Figure 1A in Appendix). At each release site, snails were released evenly between two 0.11 m2 frames (a traditional Chinese unit of area termed kuang) placed over the mud substrate on either side of the channel. On day 8 the entire channel was exhaustively searched by visual inspection such that all marked and unmarked snails were recaptured along the length of the channel, the distance moved from original release site was noted, and all snails were subsequently destroyed. Distances moved from each of the five locations were aggregated to generate a distribution of dispersal distances per unit time. Non-linear regression was used to fit inverse power functions for both upstream and downstream dispersal, following previous work estimating dispersal of aquatic invertebrates (Elliott 2003). The proportion of individuals dispersing to a given distance is expressed as Pdisp = a(d)b, where d is distance in meters, and a and b are fit parameters.
Measuring parasite fate and transport
Asexual reproduction of S. japonicum within the snail produces cercariae, the free swimming aquatic stage that is infective to humans. Irrigation canals are hypothesized to be important conduits for cercarial transport (Watts and Katsha 1997), and cercariae have been found to travel long distances in flowing water (Jordan 1972). While cercariae are actively motile in still water, moving up to 3 meters over the course of their life-time (Upatham, 1973), their geographic range is determined almost entirely by their passive transport in flowing water. In fact, their surface-seeking behavior tends to concentrate cercariae within the zone of the water column (at the meniscus) where advective flows are the strongest, and thus their active movement behaviors may predispose them to downstream transport. This work follows previous modeling where microorganisms are treated as particles to estimate their fate and transport (Tyrrel and Quinton 2003). We assume cercariae behave similarly to suspended particles subject to passive transport in advective flow in channels, as others have assumed for schistosoma japonicum cercariae (Maszle, Whitehead et al. 1998; Xu, Gong et al. 2006) and for other similarly sized pathogens (Ferguson, Husman et al. 2003).
Some attempts have been made to quantify the fate and transport of schistosomes in controlled irrigation canals (Wang, Cain et al. 1960; Upatham 1974; Lowe, Xi et al. 2005), though the low recovery of parasites has limited these experiments to small scales, e.g. cercariae traveled and infected mice up to 390 meters from a release point in a controlled flow experiment (Lowe, Xi et al. 2005). To estimate cercarial transport at longer scales, a tracer study was conducted by releasing Rhodamine WT dye (Rho-WT) in two B concrete irrigation channels selected to be typical of B channels of average water velocities based on previous work (Maszle, Whitehead et al. 1998). A known concentration of dye was injected at each upstream site, and water samples were collected repeatedly at two downstream sites until the breakthrough curve was observed at each location. Injections of Rho-WT followed safety parameters listed in Rho-WT material safety data sheets. Water samples were collected and analyzed according to methods described elsewhere (Hubbard 1982), with sample fluorescence determined by an Aquafluor field fluorometer (Turner Designs; Sunnyvale, CA). The results (in ppb) were used to create a dye-dilution curve over time for each site, and the peak fluorescence level at each sampling site, and the corresponding sampling time, were used to calculate the exponential decay rate of Rho-WT dye in each irrigation channel (Vasudevan, Fimmen et al. 2001).
We consider Rho-WT to be a highly conservative surrogate for a parasite distance decay parameter in the spatial model based on the transport characteristics of S. japonicum at the scale of interest for this study (up to 2 km). The transport of cercariae was assumed to be analogous to the transport of suspended constituents; indeed, S. japonicum cercariae have a long window of viability and have been observed to be transported distances as long as 1000 m (Wang, Cai et al. 1960; Lowe, Xi et al. 2005). Rho-WT was selected as a tracer given its widespread use to trace both solute (Laenen and Bencala 2001) and suspended (Yotsukura, Cory et al. 1972) constituents in flowing water, as well as its benign environmental impact, low cost, high detection sensitivity and local availability of detection equipment in China.
Geospatial analysis of coupled intermediate host and parasite transport
To explore the implications of coupled intermediate host dispersal and parasite transport in water on relative changes in the proportion of cercariae successfully reaching a downstream site, a simple geospatial model was constructed and parameterized using the experimental snail dispersal estimates and the dye experiment estimate of cercarial transport. A source (upstream) village and a target (downstream) village were selected to explore the marginal increase in the proportion of cercariae reaching the target community at four varying snail dispersal rates from the upstream source. Dispersal rates were calculated based on the minimum distance at which 25%, 50%, and 75% of migrating snails would likely disperse— 100% would indicate the furthest distance reached by at least one snail—based on results from the MMR experiment, as follows.
First, the absolute snail population inhabiting the source village, Ns, was estimated based on an average snail density of 2.5 snails per kuang (0.11 m2) observed in the region (Gong, Xu et al. 2006) multiplied by the total area of snail habitat (m2) in the source village estimated in previous work (Spear, Seto et al. 2004). From this source population (Ns=1.87×105), the inverse power function fit to the MMR data was used to simulate snail dispersal to the target at four dispersal rates: no dispersal, 25th, 50th, and 75th percentile dispersal distances (meters/day). Each level of snail dispersal reflects a spatially expanded snail population from which the release of cercariae was simulated in order to estimate the spatial extent of cercarial concentrations with distance.
The fundamental question posed here is the degree to which cercarial concentration at a downstream location is sensitive to the dispersal of hosts. The goal here is not to estimate absolute concentrations of cercariae remaining with distance from release, for which considerable additional data would be necessary. Rather, the spatial shift of the relative proportion of cercariae remaining is evaluated as snail dispersal is varied at levels observed in the MMR experiment. While a source and target village (2 km apart) were selected to explore the sensitivity of cercarial diffusion to different levels of snail dispersal, the results were generalized to communities separated by varying distances. Locational data were georectified to the Universal Transverse Mercator (UTM) zone 48N projection, 1984 datum, and geospatial modeling was done using Spatial Analyst in ArcGIS 9.3 (ESRI 2008).
RESULTS
Snail dispersal and tracer experiments
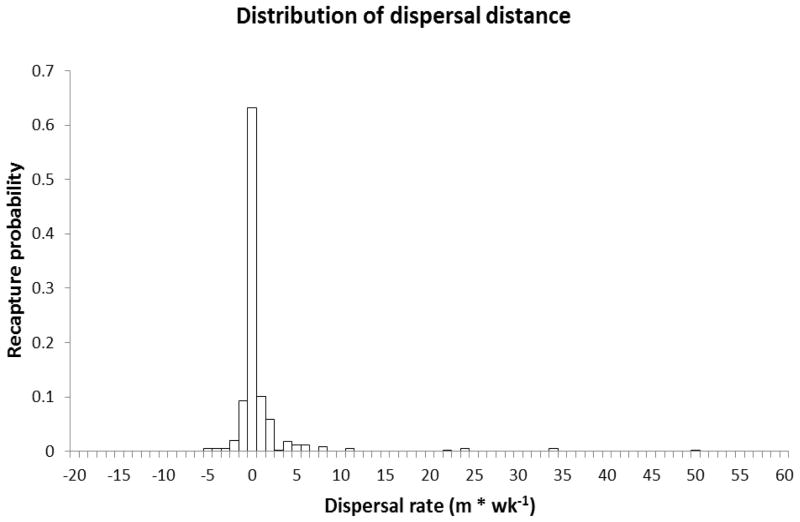
Of 1,440 initially tagged and released snails in the MMR experiment, 336 (23%) were recovered during recapture, and Figure 1 displays the recapture probability with bidirectional distance for all snails recovered. The mean dispersal distance of those moving downstream over the course of the experiment was 6.53 m (0.82 m/day), and 1.19 m (0.15 m/day) for those who moved upstream. Maximum dispersal over the course of the study was 5 m (0.63 m/day) in the upstream direction and 50 m (6.25m/day) in the downstream direction. Marked Oncomelania moved, on average, 6 times further downstream than upstream over the course of the observation period. The relationship between distance travelled and the proportion of individual Oncomelania dispersing was well described by an inverse power function for both upstream and downstream dispersal (Table 1).
Figure 1.
Recapture probability for 1,440 marked Oncomelania hupensis robertsoni snails with distance (m) from release. Negative values indicate distance upstream.
Table 1.
Parameter values for the inverse power function fit to upstream and downstream dispersal distances for the intermediate host.
| Dispersal direction | Parameter values
|
R2 | |
|---|---|---|---|
| a (95% CI) | b (95% CI) | ||
| Upstream | 0.704 (0.644, 0.764) | −2.158 (−2.587, −1.729) | 0.99 |
| Downstream | 0.044 (0.016, 0.120) | −0.733 (−1.156, −0.311) | 0.60 |
Water samples collected at both sampling sites in the dye experiment incorporated the entire passage of the dye cloud. The fluorescence levels of dye in samples are plotted against time (seconds after dye injection) for each study channel (Figure 2A in Appendix). Rhodamine WT dye’s exponential decay in the study channels is calculated from the peak fluorescence levels at the sampling sites. The decay coefficient for dye transport, averaged across the two concrete channels in which the tracer was injected, was 1.3 × 10−3 (SD = 8.5 × 10−4). This parameter value was used in the spatial fate and transport modeling to simulate cercarial decay.
Geospatial cercarial transport model
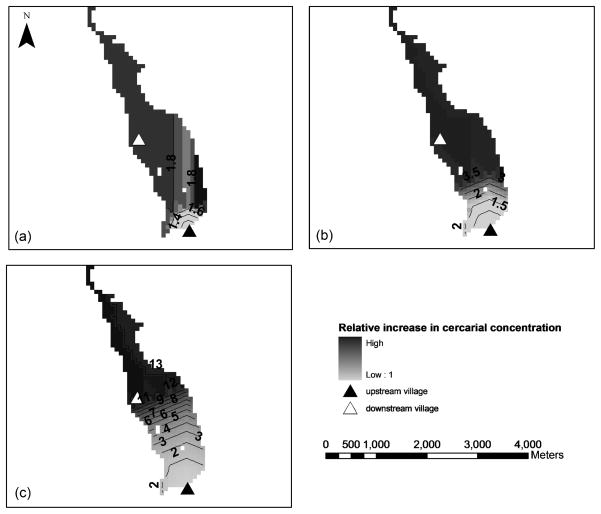
Simulations of snails originating in the upstream village, dispersing at rates in the MMR experiment, and reaching the target 2 km downstream indicated that 20 – 30 snails reach the target village (Figure 3A in Appendix). Baseline simulations were run of cercariae released from non-migrating snails at the upstream village and subjected to distance decay. The relative increase, above this baseline, in the simulated proportion of cercariae reaching the downstream target village is shown spatially at three levels of snail dispersal defined by increasing percentiles of dispersal distance (Figure 2). Snail dispersal at or above the 75th percentile dispersal distance resulted in a twelve-fold increase (relative to the no dispersal case) in the proportion of cercariae reaching the target village compared to a 3.5-fold increase at the 50th percentile distance and 1.8-fold increase at the 25th percentile distance.
Figure 2.
Relative increase in the proportion of cercariae reaching downstream locations when released from snail populations having dispersed to the 25th (a), 50th (b) and 75th (c) percentile dispersal distances. Contours display values of the relative increases in cercarial concentrations with distance.
Discussion
Our simulations of snail dispersal beyond the boundaries of a source village provide theoretical support for an increase in the spatial reach of infectious cercariae in the presence of host movements. Snails dispersed further, on average, downstream than upstream over the course of the MMR experiment. Thus, even slow-moving flows in the small irrigation channels studied were observed to facilitate snail dispersal in the downstream direction. No other study, to our knowledge, has directly measured the influence of flow direction on snail movements. Furthermore, while Oncomelania snails have been observed to disperse passively over several kilometers in large river systems (Davis, Wu et al. 2006), no study has measured downstream dispersal in minor irrigation networks, which provide key hydrological links between villages in many mountainous and hilly endemic regions in China (Gurarie and Seto 2008). There was also a significant movement of snails upstream during the observation period, a phenomenon that may have important implications for moving parasites across hydrologically disconnected drainages.
Animal models have documented a strong dose-response relationship between exposure to cercariae and the intensity of transmission, with the number of cercariae infecting a host observed to rise proportionately with increasing cercarial concentrations above a threshold (Upatham 1974). Consistent with animal studies, infection intensity in humans is assumed to be an increasing function of exposure to cercariae, where exposure is defined as the product of duration of water contact, a time-weighted cercarial concentration, and water contact intensity by activity (Spear, Zhong et al. 2004). An almost two-fold increase in the number of cercariae able to reach a downstream exposure site, as for example could be the case according to simulated snail dispersal at the 25th percentile dispersal distance relative to no dispersal, could potentially double an individual’s exposure to cercariae originating at a site 2 km upstream.
To our knowledge, only one study has examined cercarial viability with distance (Lowe, Xi et al. 2005), but was limited in its experimental design to cercarial transport at short, intra village scales (distances <400 meters). Our study extends the consideration of cercarial transport in flowing water to the inter-village scale (~1–2 km), which provides a basis for estimating the potential exchange of risk between communities as they are commonly situated in the Sichuan S. japonicum transmission environment. Our approach necessarily differed from the direct measurements made by Lowe et al. (2005) because estimating cercarial numbers at distances further downstream of their experimental zone (where the concentration of cercariae is likely to be low) would have required a prohibitively large number of mice (as bioassays) and a very large experimental emission of miracidia, which carries its own risks (Lowe, Xi et al. 2005). Thus, the dye technique used in the current study was selected to explore the tail of the transport distance distribution, a region that is unobservable using animal assays.
The use of Rho-WT presents certain limitations as a proxy for parasite fate and transport. The decay of Rho-WT may differ from that of suspended particles (Yotsukura, Cory et al. 1972), including cercariae. Furthermore, the distance decay approximation provided by Rho- WT does not incorporate particle loss due to obstruction by physical barriers such as vegetation and sediment, nor mortality or decline in infectivity of schistosome larvae. Alternatives to Rho-WT, such as particle tracers, might prove yet more effective for estimating cercarial transport and should be considered in future work.
Our results indicate that villages separated by over 2 km in downstream distance have the potential to “import” parasites from upstream sources via mobile hosts and overland flows, with important consequences for disease spread. Long distance dispersal of mobile hosts can potentially place otherwise isolated villages within reach of parasite transport, and thus transmission. Long range dispersal of even a few individuals has been shown to have important implications in other ecological processes, such as the establishment of an invasive organism in new geographic areas (Kot, Lewis et al. 1996) and the spread of plant pathogens (Nagarajan and Singh 1990). Frequent long-distance dispersal events—modeled as stochastic, long-range movement of humans—have been shown to be important in the spread of influenza via global air travel (Colizza, Barrat et al. 2007). For diseases with multiple modes of transport, such as hosts, vectors and free-living stages, the spatial process of disease spread depends on the interaction between, and sequence of, a number of dynamic movement behaviors, a subset of which were explored here.
The degree to which transmission in one village results from cercariae shed from beyond the village boundary has important implications for disease control. Environmental management and land-use change have proven effective in reducing snail population sizes in schistosome endemic areas with high snail density (Zhou, Yang et al. 2011). Information on snail dispersal and parasite transport can be used to identify locations in the hydrological network where such interventions might be targeted. Snail dispersal may shift the area of risk such that communities previously considered isolated are in fact connected to potential upstream sources of contamination. Simulations of the sort conducted in the present work could be used to direct snail control efforts that focus not just on a single village, but on multiple sites in the downstream hydrologic network that potentially import pathogens. Likewise, such future simulations may be useful in a disease surveillance context to delineate areas surrounding an index village where surveillance should be extended in the situation where a case is observed at the index.
Vector, host or larval control Interventions that integrate factors such as geographic distance, hydrologic flows, topographic gradients, and habitat constraints to optimize reductions in pathogen export or minimize import are underway in some contexts. For example, malaria elimination efforts differ between areas isolated from recolonization and those connected by host movements (Tatem and Smith 2010). Methods that allow a quantitative exploration of connectivity are essential: the spread of infectious disease in networks consisting of smaller sub-networks that are well connected internally but have few cross-community connections might be best controlled by targeting individuals that bridge communities, for example, rather than highly connected individuals in a given community (Salathe and Jones 2010).
There are several limitations to using the methods presented here to improve our understanding of infectious disease transmission in connected landscapes. First, extrapolating observed dispersal and transport parameters from controlled experiments to a regional context involves a number of assumptions about how dispersal and distance decay processes occur, and scale, in complex landscapes. For the intermediate host examined here, the model assumes a continuous migration rate over time over the lifetime of the host, not subject to temporal or spatial variation in dispersal. Second, the model presented considers only topography and distance between villages in calculating connectivity, and does not take into consideration other environmental features between villages that are likely to facilitate or impede host dispersal and cercarial transport. Where land cover and land use information are available, these could be integrated with digital elevation model (DEM) data to consider how important landscape features mediate the dispersal of hosts and aquatic stages. DEM data has been found to be a sufficient approximation of the dominant flow direction when observations are made at the watershed scale (Turcotte, Fortin et al. 2001).
The dispersal parameters estimated here provide a first set of estimates on parasite fate and transport assuming a simple model based on distance and flow direction alone. Further modeling of other pathogen transport systems can expand on this basic model to explore the influence of varying hydrological conditions, such as flow rate, channel morphology and residence time, as well as introduce mammalian host movements. Thus, the model presented here provides an important starting point for an improved understanding of how certain landscape features modify dispersal and transport relationships, which is essential for advancing our ability to respond to environmentally-mediated infectious diseases (Remais and Eisenberg 2012).
Supplementary Material
Acknowledgments
This work was supported in part by the NSF/NIH Ecology of Infectious Disease Program (grant 0622743), by the National Institute for Allergy and Infectious Disease (grant nos. K01AI091864 and R01AI068854), and by the Emory University Global Health Institute Faculty Distinction Fund. The authors wish to thank our colleagues at the Sichuan Centers for Disease Control and Prevention (Chengdu, People’s Republic of China), and our colleagues at the Anxian, Zhongjiang and Jinyang County Anti-Schistosomiasis Stations for their continued support and collaboration. We would also like to thank Yiliu Chen for her contributions to the cercarial transport model and Kenneth Bencala for providing expert advice on modeling hydrological transport processes.
Contributor Information
Adam N. Akullian, Email: akullian@u.washington.edu.
Ding Lu, Email: arcadia_83@163.com.
George M. Davis, Email: georgedavis99@hotmail.com.
Robert C. Spear, Email: spear@berkeley.edu.
Justin V. Remais, Email: justin.remais@emory.edu.
References
- Briggs D, Elliott P. The use of geographical information systems in studies on environment and health. World Health Statistics Quarterly. 1995;48:85–94. [PubMed] [Google Scholar]
- Cairncross S, Feacham R. Environmental Health Engineering in the Tropics. Sussex, England: John Wiley & Sons; 1999. [Google Scholar]
- Chlyeh G, Dodet M, et al. Spatio-temporal distribution of freshwater snail species in relation to migration and environmental factors in an irrigated area from Morocco. Hydrobiologia. 2006;553:129–142. [Google Scholar]
- Colizza V, Barrat A, et al. Modeling the Worldwide Spread of Pandemic Influenza: Baseline Case and Containment Interventions. PLoS Med. 2007;4(1):e13. doi: 10.1371/journal.pmed.0040013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis G, Wu W, et al. Ecogenetics of shell sculpture in Oncomelanis (Gastropoda) in canals of Hubei, China, and relevance for schistosome transmission. Malacologia. 2006;48(1–2):253–264. [Google Scholar]
- Davis GM, Wilke T, et al. Snail-Schistosoma, Paragonimus interactions in China: Population ecology, genetic diversity, coevolution and emerging diseases. Malacologia. 1999;41(2):355–377. [Google Scholar]
- Elliott JM. A comparative study of the dispersal of 10 species of stream invertebrates. Freshwater Biology. 2003;48(9):1652–1668. [Google Scholar]
- ESRI. ArcGIS Model Builder. Redlands, CA: 2008. [Google Scholar]
- Ferguson C, Husman AMdR, et al. Fate and Transport of Surface Water Pathogens in Watersheds. Critical Reviews in Environmental Science and Technology. 2003;33(3):299–361. [Google Scholar]
- Gong P, Xu B, et al. Remote sensing and geographic information systems in the spatial temporal dynamics modeling of infectious diseases. Science in China Series C: Life Sciences. 2006;49(6):573–582. doi: 10.1007/s11427-006-2015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurarie D, Seto EY. Connectivity sustains disease transmission in environments with low potential for endemicity: modelling schistosomiasis with hydrologic and social connectivities. J R Soc Interface. 2008 doi: 10.1098/rsif.2008.0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard E. Techniques of Water-Resources Investigations of the United States Geological Survey. 1982. Measurement of time of travel and dispersion in streams by dye tracing. [Google Scholar]
- Jordan P. Epidemiology and Control of Schistosomiasis. British Medical Bulletin. 1972;28(1):55. doi: 10.1093/oxfordjournals.bmb.a070894. [DOI] [PubMed] [Google Scholar]
- Kay D, McDonald A. Reduction of coliform bacteria in two upland reservoirs: The significance of distance decay relationships. Water Research. 1980;14(4):305–318. [Google Scholar]
- Kot M, Lewis MA, et al. Dispersal Data and the Spread of Invading Organisms. Ecology. 1996;77(7):2027–2042. [Google Scholar]
- Laenen A, Bencala KE. TRANSIENT STORAGE ASSESSMENTS OF DYE-TRACER INJECTIONS IN RIVERS OF THE WILLAMETTE BASIN, OREGON1. JAWRA Journal of the American Water Resources Association. 2001;37(2):367–377. [Google Scholar]
- Lowe D, Xi J, et al. Transport of Schistosoma japonicum cercariae and the feasibility of niclosamide for cercariae control. Parasitol Int. 2005;54(1):83–89. doi: 10.1016/j.parint.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Maszle DR, Whitehead PG, et al. Hydrological studies of schistosomiasis transport in Sichuan Province, China. Science of The Total Environment. 1998;216(3):193–203. doi: 10.1016/s0048-9697(98)00152-1. [DOI] [PubMed] [Google Scholar]
- Mawdsley JL, Bardgett RD, et al. Pathogens in Livestock Waste, Their Potential for Movement through Soil and Environmental-Pollution. Applied Soil Ecology. 1995;2(1):1–15. doi: 10.1016/0929-1393(94)00039-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum H. Population Parameters: Estimation for Ecological Models. Oxford: Blackwell Science Ltd; 2000. [Google Scholar]
- Nagarajan S, Singh DV. Long-Distance Dispersion of Rust Pathogens. 1990;28:139–153. doi: 10.1146/annurev.py.28.090190.001035. [DOI] [PubMed] [Google Scholar]
- Osborne J, Loxdale H, et al. Monitoring insect dispersal. In: Bullock J, Kenward R, editors. Dispersal Ecology. Malden: Blackwell Sciences; 2002. [Google Scholar]
- Pringle C. The need for a more predictive understanding of hydrologic connectivity. Aquatic Conservation: Marine and Freshwater Ecosystems. 2003;13(6):467–471. [Google Scholar]
- Prüss A, Kay D, et al. Estimating the burden of disease from water, sanitation, and hygiene at a global level. Environmental Health Perspectives. 2002;110:537–542. doi: 10.1289/ehp.110-1240845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remais J, Akullian A, et al. Analytical methods for quantifying environmental connectivity for the control and surveillance of infectious disease spread. J R Soc Interface. 2010 doi: 10.1098/rsif.2009.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remais J, Hubbard A, et al. Weather-driven dynamics of an intermediate host: mechanistic and statistical population modelling of Oncomelania hupensis. Journal of Applied Ecology. 2007;44(4):781–791. [Google Scholar]
- Remais JV, Eisenberg JNS. Balance between clinical and environmental responses to infectious diseases. The Lancet. 2012;379(9824):1457–1459. doi: 10.1016/S0140-6736(11)61227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie LS. The Biology and Control of the Amphibious Snails That Serve as Intermediate Hosts for Schistosoma Japonicum. The American Journal of Tropical Medicine and Hygiene. 1955;4(3):426–441. doi: 10.4269/ajtmh.1955.4.426. [DOI] [PubMed] [Google Scholar]
- Salathe M, Jones JH. Dynamics and control of diseases in networks with community structure. PLoS Comput Biol. 2010;6(4):e1000736. doi: 10.1371/journal.pcbi.1000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoor JL. Environmental modeling: fate and transport of pollutants in water, air, and soil. New York: John Wiley and Sons; 1996. [Google Scholar]
- Seto E, Xu B, et al. The use of remote sensing for predictive modeling of schistosomiasis in China. Photogrammetric Engineering and Remote Sensing. 68(2):167–174. [Google Scholar]
- Sharma S, Sachdeva P, et al. Emerging water-borne pathogens. Applied Microbiology and Biotechnology. 2003;61(5):424–428. doi: 10.1007/s00253-003-1302-y. [DOI] [PubMed] [Google Scholar]
- Spear RC, Seto E, et al. Factors influencing the transmission of schistosoma japonicum in the mountains of Sichuan Province of China. Am J Trop Med Hyg. 2004;70(1):48–56. [PubMed] [Google Scholar]
- Spear RC, Zhong B, et al. Spatial and temporal variability in schistosome cercarial density detected by mouse bioassays in village irrigation ditches in Sichuan, China. American Journal of Tropical Medicine and Hygiene. 2004;71(5):554–557. [PubMed] [Google Scholar]
- Tate KW, Atwill ER, et al. Cryptosporidium parvum transport from cattle fecal deposits on California rangelands. Journal of Range Management. 2000;53(3):295–299. [Google Scholar]
- Tatem AJ, Smith DL. International population movements and regional Plasmodium falciparum malaria elimination strategies. Proc Natl Acad Sci U S A. 2010;107(27):12222–12227. doi: 10.1073/pnas.1002971107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte R, Fortin JP, et al. Determination of the drainage structure of a watershed using a digital elevation model and a digital river and lake network. Journal of Hydrology. 2001;240(3–4):225–242. [Google Scholar]
- Tyrrel SF, Quinton JN. Overland flow transport of pathogens from agricultural land receiving faecal wastes. Journal of Applied Microbiology. 2003;94:87S–93S. doi: 10.1046/j.1365-2672.94.s1.10.x. [DOI] [PubMed] [Google Scholar]
- Upatham ES. Dispersion of St. Lu cian Schistosoma mansoni cercariae in natural standing and running waters determined by cercaria counts and mouse exposure. Ann Trop Med Parasitol. 1974;68(3):343–352. doi: 10.1080/00034983.1974.11686957. [DOI] [PubMed] [Google Scholar]
- Upatham ES. Studies on the effects of cercarial concentration and length of exposure on the infection of mice by St Lucian Schistosoma mansoni cercariae in a natural running-water habitat. Cambridge Journals Online. 1974;68:155–159. [PubMed] [Google Scholar]
- Vasudevan D, Fimmen RL, et al. Tracer-Grade Rhodamine WT: Structure of Constituent Isomers and Their Sorption Behavior. Environmental Science & Technology. 2001;35(20):4089–4096. doi: 10.1021/es010880x. [DOI] [PubMed] [Google Scholar]
- Wang MS, Cai SC, et al. The relationship between snail distribution and schistosomiasis infection in lake region. J Epidemiol. 1960;3:180–183. [Google Scholar]
- Watts S, Katsha SE. Irrigation, farming and schistosomiasis: a case study in the Nile delta. International Journal of Environmental Health Research. 1997;7(2):101–113. [Google Scholar]
- Wilke T, Davis GM, et al. Oncomelania hupensis (Gastropoda: Rissooidea) in eastern China: molecular phylogeny, population structure, and ecology. Acta Tropica. 2000;77(2):215–227. doi: 10.1016/s0001-706x(00)00143-1. [DOI] [PubMed] [Google Scholar]
- Woolhouse ME. A mark-recapture method for ecological studies of schistosomiasis vector snail populations. Ann Trop Med Parasitol. 1988;82(5):485–497. doi: 10.1080/00034983.1988.11812281. [DOI] [PubMed] [Google Scholar]
- Xu B, Gong P, et al. A Spatial-Temporal Model for Assessing the Effects of Intervillage Connectivity in Schistosomiasis Transmission. Annals of the Association of American Geographers. 2006;96(1):31–46. [Google Scholar]
- Xu XJ, Wei FH, et al. Possible effects of the Three Gorges dam on the transmission of Schistosoma japonicum on the Jiang Han plain, China. Ann Trop Med Parasitol. 2000;94(4):333–341. doi: 10.1080/00034983.2000.11813548. [DOI] [PubMed] [Google Scholar]
- Yotsukura N, Cory RL, et al. A Tracer Simulation of Waste Transport in the Muddy Creek-Rhode River Estuary, Maryland. Chesapeake Science. 1972;13(2):101–109. [Google Scholar]
- Zhou YB, Yang MX, et al. Effect of habitat fragmentation on the schistosome-transmitting snail Oncomelania hupensis in a mountainous area of China. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2011;105(4):189–196. doi: 10.1016/j.trstmh.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Ziemer CJ, Bonner JM, et al. Fate and transport of zoonotic, bacterial, viral, and parasitic pathogens during swine manure treatment, storage, and land application. Journal of Animal Science. 2010;88(13_electronic_suppl):E84–94. doi: 10.2527/jas.2009-2331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.