Abstract
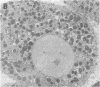
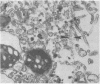
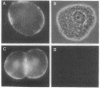
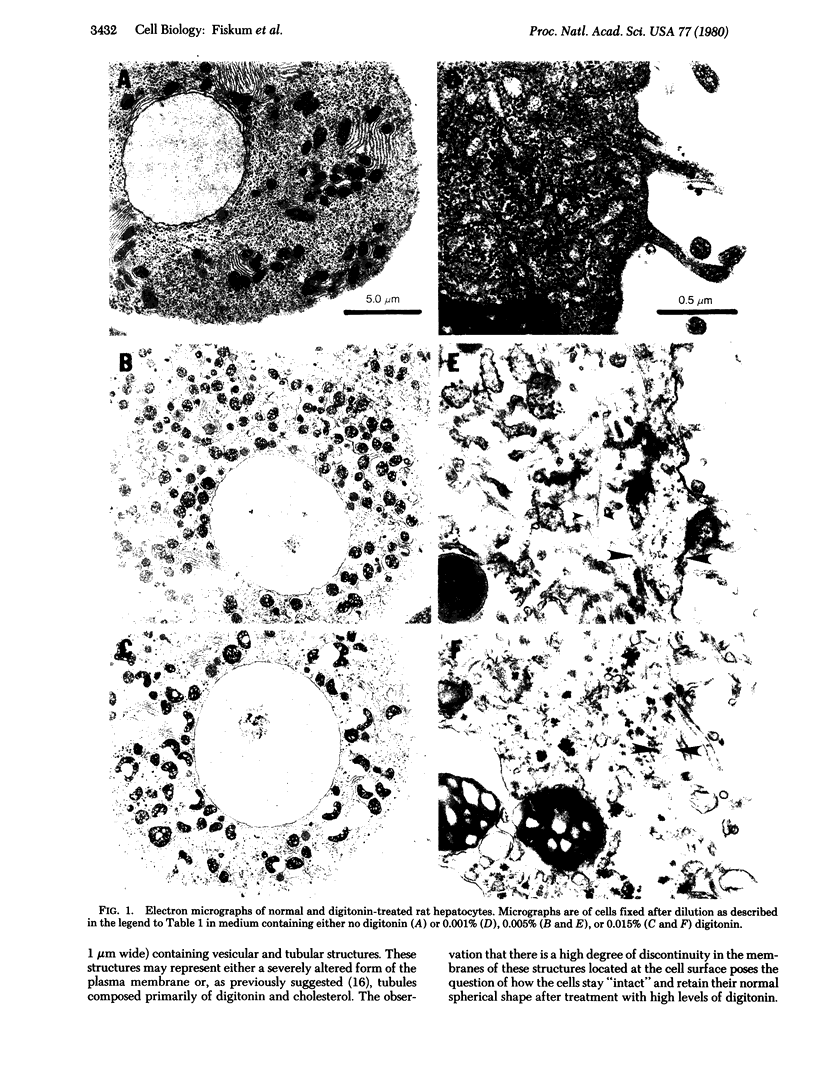
Treatment of isolated rat hepatocptes with low concentrations of digitonin increases the permeability of the plsma membrane to cytosolic proteins without causing release of organelles such as mitochondria into the surrounding medium. Electron microscopy showed that treatment of the cells with increasing concentations of digitonin results in a progressive loss in the continuity of the plasma membrane, while most other aspects of cellular morphology remain normal. Depletion of background staining material from the cytosol by digitonin treatment of the cells greatly enhances the visualization of the cytoskeleton. The use of this technique, together with immunofluorescent light microscopy, has verified the presence of an actin-containing filamentous network at the hepatocyte cortex as well as intermediate filaments distributed throughout the cell. Digitonin is thus useful both for selectively permeabilizing the plasma membrane and for intensifying the appearance of intracellular structures such as microfilaments that are normally difficult to observe in cells such as hepatocytes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adair W. S., Jurivich D., Goodenough U. W. Localization of cellular antigens in sodium dodecyl sulfate-polyacrylamide gels. J Cell Biol. 1978 Oct;79(1):281–285. doi: 10.1083/jcb.79.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon D. L. The identification of myosin in rabbit hepatocytes. Eur J Biochem. 1976 May 17;65(1):139–146. doi: 10.1111/j.1432-1033.1976.tb10398.x. [DOI] [PubMed] [Google Scholar]
- Colbeau A., Nachbaur J., Vignais P. M. Enzymic characterization and lipid composition of rat liver subcellular membranes. Biochim Biophys Acta. 1971 Dec 3;249(2):462–492. doi: 10.1016/0005-2736(71)90123-4. [DOI] [PubMed] [Google Scholar]
- Cornell N. W., Lund P., Hems R., Krebs H. A. Acceleration of gluconeogenesis from lactate by lysine (Short Communication). Biochem J. 1973 Jun;134(2):671–672. doi: 10.1042/bj1340671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig S. W., Lancashire C. L. Comparison of intestinal brush-border 95-Kdalton polypeptide and alpha-actinins. J Cell Biol. 1980 Mar;84(3):655–667. doi: 10.1083/jcb.84.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig S. W., Pardo J. V. alpha-Actinin localization in the junctional complex of intestinal epithelial cells. J Cell Biol. 1979 Jan;80(1):203–210. doi: 10.1083/jcb.80.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker G. L., Lennarz W. J. Sperm binding and fertilization envelope formation in a cell surface complex isolated from sea urchin eggs. J Cell Biol. 1979 Apr;81(1):92–103. doi: 10.1083/jcb.81.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubinsky W. P., Cockrell R. S. Ca2+ transport across plasma and mitochondrial membranes of isolated hepatocytes. FEBS Lett. 1975 Nov 1;59(1):39–43. doi: 10.1016/0014-5793(75)80336-x. [DOI] [PubMed] [Google Scholar]
- Elias P. M., Goerke J., Friend D. S., Brown B. E. Freeze-fracture identification of sterol-digitonin complexes in cell and liposome membranes. J Cell Biol. 1978 Aug;78(2):577–596. doi: 10.1083/jcb.78.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackenbrock C. R. Ultrastructural bases for metabolically linked mechanical activity in mitochondria. I. Reversible ultrastructural changes with change in metabolic steady state in isolated liver mitochondria. J Cell Biol. 1966 Aug;30(2):269–297. doi: 10.1083/jcb.30.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lux S. E. Dissecting the red cell membrane skeleton. Nature. 1979 Oct 11;281(5731):426–429. doi: 10.1038/281426a0. [DOI] [PubMed] [Google Scholar]
- Mackall J., Meredith M., Lane M. D. A mild procedure for the rapid release of cytoplasmic enzymes from cultured animal cells. Anal Biochem. 1979 May;95(1):270–274. doi: 10.1016/0003-2697(79)90216-1. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. T., Burgess D. R. SDS microslab linear gradient polyacrylamide gel electrophoresis. Anal Biochem. 1978 Jul 1;87(2):386–396. doi: 10.1016/0003-2697(78)90688-7. [DOI] [PubMed] [Google Scholar]
- Oda M., Price V. M., Fisher M. M., Phillips M. J. Ultrastructure of bile canaliculi, with special reference to the surface coat and the pericanalicular web. Lab Invest. 1974 Oct;31(4):314–323. [PubMed] [Google Scholar]
- PESCE A., MCKAY R. H., STOLZENBACH F., CAHN R. D., KAPLAN N. O. THE COMPARATIVE ENZYMOLOGY OF LACTIC DEHYDROGENASES. I. PROPERTIES OF THE CRYSTALLINE BEEF AND CHICKEN ENZYMES. J Biol Chem. 1964 Jun;239:1753–1761. [PubMed] [Google Scholar]
- Pollard T. D., Korn E. D. Electron microscopic identification of actin associated with isolated amoeba plasma membranes. J Biol Chem. 1973 Jan 25;248(2):448–450. [PubMed] [Google Scholar]
- Prentki M., Chaponnier C., Jeanrenaud B., Gabbiani G. Actin microfilaments, cell shape, and secretory processes in isolated rat hepatocytes. Effect of phalloidin and cytochalasin D. J Cell Biol. 1979 Jun;81(3):592–607. doi: 10.1083/jcb.81.3.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven E. P., Axline S. G. Subplasmalemmal microfilaments and microtubules in resting and phagocytizing cultivated macrophages. J Cell Biol. 1973 Oct;59(1):12–27. doi: 10.1083/jcb.59.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZARKOWSKA L., KLINGENBERG M. ON THE ROLE OF UBIQUINONE IN MITOCHONDRIA. SPECTROPHOTOMETRIC AND CHEMICAL MEASUREMENTS OF ITS REDOX REACTIONS. Biochem Z. 1963;338:674–697. [PubMed] [Google Scholar]
- Scallen T. J., Dietert S. E. The quantitative retention of cholesterol in mouse liver prepared for electron microscopy by fixation in a digitonin-containing aldehyde solution. J Cell Biol. 1969 Mar;40(3):802–813. doi: 10.1083/jcb.40.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siess E. A., Wieland O. H. Phosphorylation state of cytosolic and mitochondrial adenine nucleotides and of pyruvate dehydrogenase in isolated rat liver cells. Biochem J. 1976 Apr 15;156(1):91–102. doi: 10.1042/bj1560091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R., Rosenberg M., Estensen R. Endocytosis in Chang liver cells. Quantitation by sucrose- 3 H uptake and inhibition by cytochalasin B. J Cell Biol. 1971 Sep;50(3):804–817. doi: 10.1083/jcb.50.3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]