Abstract
Background:
Hyaluronic acid dermal fillers are frequently used for lip augmentation, and a new filler has been developed with characteristics especially suited for the lips.
Methods:
Four European sites treated 60 subjects with Juvéderm® Volbella™ injectable gel in the perioral area, and subjects returned to the clinic at 1, 3, 6, 9, and 12 months for follow-up. The primary effectiveness endpoint established a priori was a Month 3 responder rate on the 4-point Lip Fullness Scale (LFS) of ≥40% and statistically > 0%, where responders improved ≥ 1 point from baseline on the investigator’s assessment of LFS. At follow-up, subjects assessed lip fullness goal achievement, the look and feel of their lips, and their satisfaction with the effects of treatment.
Results:
The Month 3 LFS responder rate was 93.2% (P < 0.0001), so the primary endpoint was met, and clinical effectiveness was demonstrated. The responder rate over time showed that 78.0% of subjects still had improved lip fullness at Month 9 and 48.3% at Month 12. After treatment 98.3% of subjects reported that their lip fullness goal had been achieved, and this was maintained at 86.4% at Month 9 and 56.9% at Month 12. At Month 1, 81.0% of subjects reported that their lips felt smooth, and 91.4% reported that their lips looked natural (scores of 7–10 on an 11-point scale, where 0 was an unfavorable outcome and 10 was a favorable outcome). Similarly, 96.6% of subjects reported being satisfied (scores between 7 and 10 on an 11-point scale where 0 = very dissatisfied, 10 = very satisfied) at Month 1, and by Month 12 more than 80% of subjects were still satisfied. There were no severe adverse events related to treatment.
Conclusion:
Juvéderm® Volbella™ injectable gel is well tolerated and has been demonstrated to provide a smooth and natural improvement in lip fullness that lasts for up to 1 year.
Keywords: dermal filler, hyaluronic acid, lips, patient satisfaction
Background
Non-permanent hyaluronic acid (HA)-based dermal fillers, such as Juvéderm® (Allergan, Inc, Santa Barbara, CA), Restylane® (Q-MED AB, Uppsala, Sweden), and others, have long been used in the lips and perioral areas to increase the overall volume of the lips, enhance the vermilion border, minimize perioral lines, and to sculpt and define the lips. The clinical effectiveness of these products may be assessed by various aesthetic parameters, such as symmetry, projection, fullness, softness, and nodularity.
Juvéderm® Volbella™ injectable gel (also sold as Juvéderm® Ultra Lip, Allergan, Inc, Pringy, France) is a smooth, non-particle, viscous HA gel developed specifically for the lip area. Its patented Vycross™ (Allergan, Inc, Irvine, CA) technology incorporates short chain HA together with long chain HA to provide more efficient crosslinking than Juvéderm® Ultra, which has only long chain HA. Inclusion of the short chains of HA allows more crosslinkers to attach to HA chains at both ends, which results in longer product duration than fillers that include only long chain HA. The more efficient crosslinking also produces a higher viscosity gel which, in turn, produces a greater lift capacity as the gel is better able to lift against the pressure of the skin.
The elastic modulus (G' or gel hardness) of ∼160 Pa is lower than that of other fillers;1 this provides a smoother and softer gel that is easy to inject, and that is ideal for spreading and molding in the lips. In addition, the HA concentration of 15 mg/mL (versus 20 mg/mL for Restylane® and 24 mg/mL for Juvéderm®)1 means that the gel is less hydrophilic and will absorb less water from surrounding tissue after injection. This is an important attribute for a lip filler to ensure that patients do not end up with an unnatural looking result.
There are numerous HA products on the market, particularly in Europe, and product development is now focused on improving the aesthetic outcome in terms of softness, a “natural” look and feel of the product in situ, as well as the longevity of the results.
This study was designed to assess the aesthetic outcome, longevity of the result, and patient satisfaction for Juvéderm® Volbella™ over the course of 12 months. Standardized scales were used to assess lip volume, as well as the severity of perioral lines and of the melomental folds (downturned mouth corners).
Methods
Study design
The objective of this prospective, multicenter, open-label, post market study was to demonstrate the safety and effectiveness of Juvéderm® Volbella™ injectable gel for lip enhancement. Ethics Committee approval was obtained from Sheffield Research Ethics Committee (Leeds, UK), and the study was conducted at four European sites (three in the UK and one in Northern Ireland). The study was registered at http://www.clinicaltrials.gov (NCT 01176773), and all subjects provided written informed consent.
Following treatment with Juvéderm® Volbella™ injectable gel, subjects returned to the clinic for follow-up at 1, 3, 6, 9, and 12 months after initial treatment. An optional top-up treatment was allowed at 2 weeks, and safety follow-up via telephone occurred 3–5 days after each treatment. If at any time point at or after the 6-month visit the investigator determined that the subject’s Lip Fullness Scale (LFS) score had returned to baseline, the investigator could perform a repeat treatment prior to exiting the subject from the study.
Allowable treatment sites were the cutaneous and mucosal lips including the vermilion, vermilion borders, Cupid’s bow and philtral columns, perioral lines, and oral commissures, with a maximum volume of 2 mL. Subjects who desired lip enhancement, had a realistic lip fullness treatment goal, had lip fullness of minimal or mild on the 4-point LFS (minimal, mild, moderate, marked), and were at least 18 years old were eligible for study participation. Key exclusion criteria included cosmetic facial treatments within 6 months of study entry, or a history of semipermanent fillers or permanent implants in the lips.
Response measures
At baseline, both subjects and investigators used the validated photographic LFS to assess current lip fullness and to develop a lip fullness goal. LFS was then assessed by both subjects and investigators at Months 1, 3, 6, 9, and 12, and lip fullness goal achievement was assessed by investigators at Month 3 and by subjects at all of these visits. At baseline and at follow-up the investigators assessed the severity of perioral lines and oral commissures using validated 4-point photographic scales (none, mild, moderate, severe), and subjects rated the look and feel of their lips on various parameters using an 11-point scale, where 0 was an unfavorable outcome (eg, lumpy) and 10 was a favorable outcome (eg, smooth). Subjects also assessed their overall satisfaction with the effects of study treatment on an 11-point scale (0 = very dissatisfied, 10 = very satisfied).
The primary effectiveness endpoint was established a priori as a Month 3 LFS responder at a rate of ≥40% and statistically > 0%. The responder rate was the percentage of subjects who demonstrated an increase in fullness of ≥1 point since baseline on the investigator’s assessment of LFS. Statistical significance was calculated using a two-sided exact binomial test at a 0.05 significance level against a null hypothesis of 0. For effectiveness endpoints at Months 9 and 12, those subjects who had undergone repeat treatments were included in the denominator in order to avoid overstating the degree of effectiveness.
Adverse events were collected at all clinic visits and via telephone calls to subjects after each treatment.
Results
Baseline and treatment characteristics
The four investigators enrolled and treated 60 subjects between October 2010 and January 2011, and 59 of these subjects (98.3%) completed the study. All of the subjects were women, and the mean age was 50 years (range, 21–74). Most had lighter Fitzpatrick skin types, with 10% type I, 35% type II, 40% type III, and 15% type IV. Subjects reported a mean of 2.1 hours of sun exposure per day, and 18.3% were smokers.
Because the study product did not include lidocaine, all subjects received anesthetic in preparation for treatment, most commonly topical (56.7%, 34/60) and/or nerve block (43.3%, 26/60). One quarter of treatments (25.0%, 15/60) used local anesthetic, and none used ice. All injections were performed with a 30 G needle followed by a massage of the treatment area (96.7% gentle massage, 3.3% moderate massage). For 98.3% of treatments (59/60) investigators reported that the product was very easy to inject (mean of 1.8 on a scale where 0 is very easy and 10 is very difficult). Fifteen of the subjects (25%) received a top-up treatment at 2 weeks.
The mean injection volume was 1.2 mL (range, 0.5–2 mL) for initial treatment and 0.55 mL (range, 0.3–1 mL) for touch-up treatment, with a mean total of 1.33 mL (range, 0.5–2 mL) for initial plus top-up combined. Greater volumes were injected to the upper and lower lips, as well as to the oral commissures rather than to the perioral lines (Table 1).
Table 1.
Injection volume by treatment area
| Treatment area |
Initial treatment
|
Top-up treatment
|
Initial and top-up combined
|
|||
|---|---|---|---|---|---|---|
| N | Mean (mL) | N | Mean (mL) | N | Mean (mL) | |
| Overall | 60 | 1.20 | 15 | 0.55 | 60 | 1.33 |
| Upper lip | 57 | 0.41 | 10 | 0.27 | 57 | 0.46 |
| Lower lip | 58 | 0.32 | 11 | 0.27 | 59 | 0.37 |
| Oral commissures | 49 | 0.51 | 5 | 0.37 | 51 | 0.52 |
| Perioral lines | 25 | 0.19 | 4 | 0.18 | 26 | 0.21 |
The vermilion border was the most common treatment site, and the majority of subjects were treated in the upper and lower lips as well as the oral commissures (Table 2). The retrograde tunneling technique was used for 75% of subjects, and fanning and crosshatching were used for approximately half of the subjects (Table 2), though primarily for the oral commissures.
Table 2.
Treatment areas and techniques
| Initial treatment (N = 60) % (n/N) | Top-up treatment (N = 15) % (n/N) | |
|---|---|---|
| Treatment areas | ||
| Upper lip | 95.0% (57/60) | 66.7% (10/15) |
| Vermilion border | 85.0% (51/60) | 53.3% (8/15) |
| Vermilion mucosa | 46.7% (28/60) | 13.3% (2/15) |
| Cupid’s bow | 48.3% (29/60) | 13.3% (2/15) |
| Philtral columns | 8.3% (5/60) | 6.7% (1/15) |
| Lower lip | 96.7% (58/60) | 73.3% (11/15) |
| Vermilion border | 91.7% (55/60) | 60.0% (9/15) |
| Vermilion mucosa | 35.0% (21/60) | 26.7% (4/15) |
| Oral commissures | 81.7% (49/60) | 33.3% (5/15) |
| Perioral lines | 41.7% (25/60) | 26.7% (4/15) |
| Treatment techniques | ||
| Tunneling | 100.0% (60/60) | 93.3% (14/15) |
| Retrograde | 75.0% (45/60) | 60.0% (9/15) |
| Antegrade | 26.7% (16/60) | 33.3% (5/15) |
| Serial puncture | 25.0% (15/60) | 20.0% (3/15) |
| Fanning | 50.0% (30/60) | 13.3% (2/15) |
| Crosshatching | 46.7% (28/60) | 6.7% (1/15) |
Effectiveness
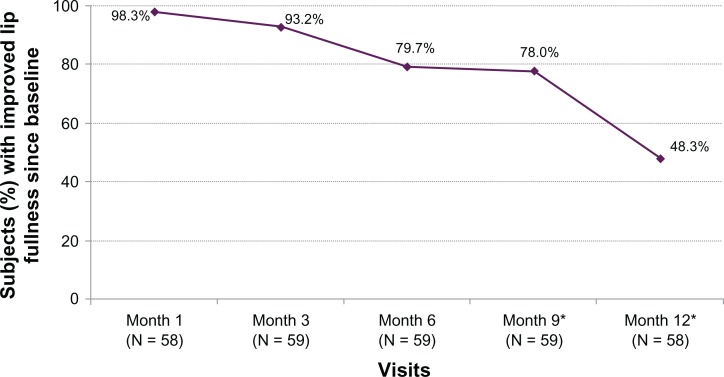
The Month 3 LFS responder rate based on investigator assessments was 93.2% (P < 0.0001), so the primary endpoint was achieved, and clinical effectiveness was demonstrated. The responder rate over time showed that more than three-quarters of subjects still had improved lip fullness at Month 9 and almost half had improved fullness at Month 12 (Figure 1). Similar LFS results were obtained from subject assessments. Prior to treatment, investigators rated 16.7% of subjects as having minimal lip fullness and 83.3% of subjects with mild fullness. After treatment they rated 0% as minimal, 5.2% as mild, 86.2% as moderate, and 8.6% as marked, with the mean lip fullness scores improving from 1.8 (mild) to 3.0 (moderate).
Figure 1.
Percent of subjects with improved lip fullness score from baseline based on investigator assessments.
Note: *Denominator includes subjects who received repeat treatment.
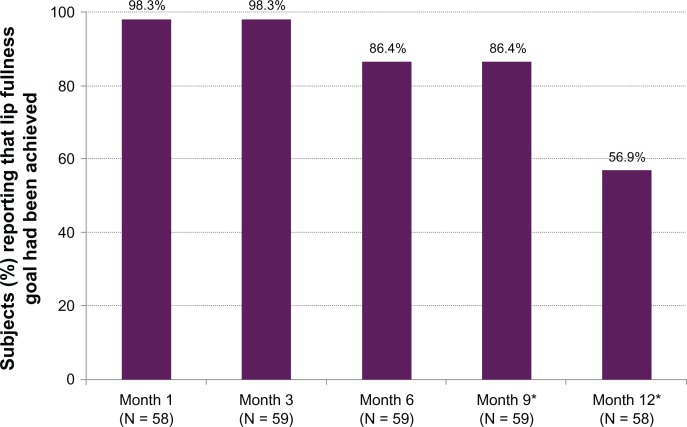
According to investigator ratings, the lip fullness goal was achieved for 100% of subjects at Month 3, and all but one subject (98.3%) also reported that their lip fullness goal had been achieved at Months 1 and 3. By Month 12 the majority of subjects still reported that their goal was being met (Figure 2).
Figure 2.
Percent of subjects reporting that their lip fullness goal had been achieved.
Notes: *Numerator includes subjects without repeat treatment; denominator includes subjects with repeat treatment.
For the 52 subjects who received treatment in oral commissures, investigators rated them as predominantly moderate (50.0%, 26/52) or severe (26.9%, 14/52) at baseline. After treatment, 70.0% of subjects (35/50) showed improvement in their oral commissures. Similarly, investigators assessed subjects’ perioral lines as being predominantly moderate (50.0%, 13/26) or severe (34.6%, 9/26) for those who received treatment in that area, and 84.0% showed improvement in perioral lines after treatment.
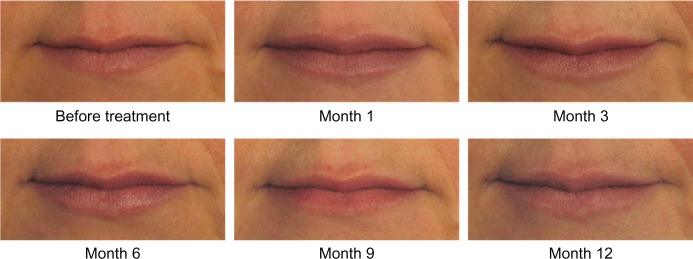
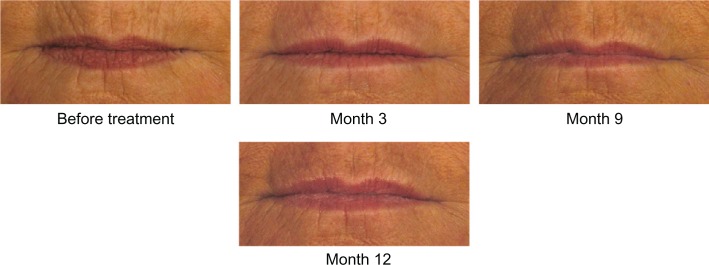
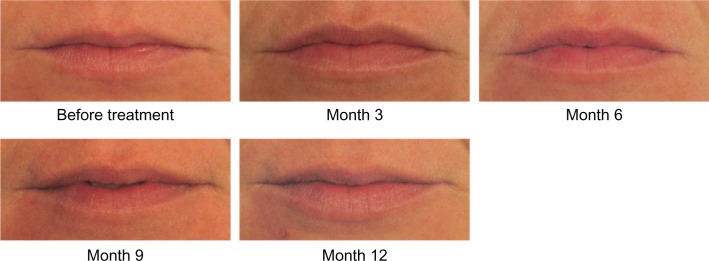
On the 11-point look and feel questionnaire where 0 is lumpy and 10 is smooth, 81.0% of subjects had ratings of 7–10 at Month 1, indicating that they thought their lips felt smooth. On similar questions at Month 1, 67.2% of subjects reported that their lips felt soft, 91.4% reported their lips felt natural, 91.4% reported their lips looked natural, and 84.5% reported their lips looked even. High ratings on all of these outcomes were maintained throughout the course of the study. Photographic examples of smooth and natural outcomes for study subjects are provided in Figures 3–5.
Figure 3.
A 45-year-old female before and after treatment with 1.0 mL of Juvéderm® Volbella™: 0.6 mL in the upper lip, 0.2 mL in the lower lip, and 0.2 mL in the oral commissures.
Figure 5.
A 64-year-old female before and after treatment with 1.8 mL of Juvéderm® Volbella™: 0.8 mL in the upper lip, 0.4 mL in the lower lip, 0.2 mL in the oral commissures, and 0.4 mL in the perioral lines.
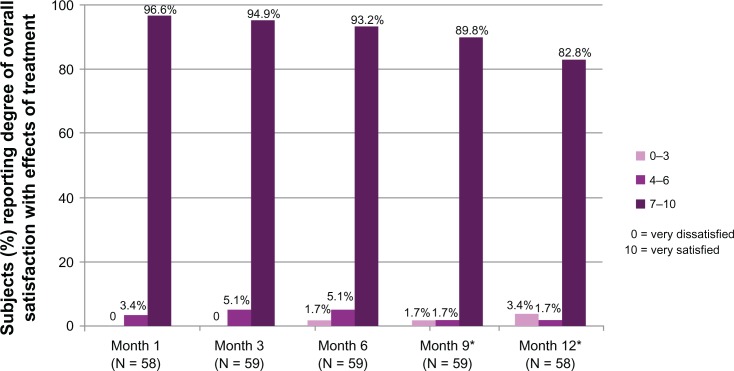
In terms of overall satisfaction with the effects of treatment, 96.6% of subjects reported being satisfied (scores of 7–10 on the 11-point scale) at Month 1, and by Month 12 more than 80% of subjects were still satisfied (Figure 6). The mean satisfaction score at Month 1 was 9.3, and it remained at 9.0 or better at every subsequent visit.
Figure 6.
Percent of subjects reporting their degree of overall satisfaction with the effects of study treatment.
Note: *Denominator includes subjects who received repeat treatment.
Safety
There were 34 subjects with 72 adverse events related to the study injection or device, and the most frequent was injection site bruising (51.7%, 31/60 subjects). Injection site swelling and lumps each occurred at much lower rates (8.3%, 5/60 subjects), and all other adverse events occurred in less than 4% of subjects. Three-quarters of the events (75.0%) were mild, and the remainder (25.0%) were of moderate severity. Two-thirds of the events (66.7%) resolved within 1 week, and the vast majority resolved without sequelae (98.6%) and did not require treatment (97.2%). One event of an injection site mass (lump) was ongoing. There were no severe or serious events related to treatment.
Discussion
While this was an open-label study and, thus, subject to reporting bias, it is a common study design for initial studies of dermal fillers in the lips,2,3 as opposed to the nasolabial folds, which provide a ready-made comparator for a split-face study design. Also helping to overcome this limitation are the study’s numerous scales, which cover different aspects of the changes that occur in lip appearance over time as assessed by both investigators and subjects.
Perhaps most remarkable is that a lower volume of Juvéderm® Volbella™ injectable gel produced effects with better duration than that seen in lip studies of the market-leading lip fillers. An open-label US study of 50 subjects injected with Juvéderm® Ultra (Allergan, Inc, Santa Barbara, CA) had a median injection volume of 1.6 mL for initial treatment and 0.6 mL for top-up treatment,2 and the pivotal study for a US lip indication for Restylane® (Medicis Pharmaceutical Corporation, Scottsdale, AZ) had a mean injection volume of 2.85 mL for initial and top-up treatment combined.4 The mean injection volume of 1.33 mL (1.20 for initial and 0.55 for top-up) for Volbella™ compares favorably to the volumes that were used of these other hyaluronic acid fillers.
In the 180-subject Restylane® study, 70% were responders on the 5-point Medicis LFS at week 24 (the last time point before repeat treatment) compared with 80% of responders in the Volbella™ study.5 The longer term outcomes are even more impressive, with Volbella™ responder rates of 78% at Month 9 and 48% at Month 12 versus 40% at 36 weeks and 18% at 48 weeks in the Juvéderm® Ultra study, which used the same 4-point LFS.2
The high subject satisfaction rates for Volbella™ eclipsed those seen for Restylane® in a Brazilian study6 of 1446 consecutive patients treated in up to four areas of the face. Of the 685 patients who received Restylane® for lip augmentation, 77.8% were satisfied at 3 months (38.3% very satisfied, 39.6% satisfied, and 22.2% unsatisfied), 50.8% were satisfied at 6 months (8.0% very satisfied, 42.8% satisfied, and 49.2% unsatisfied), and 36.6% were satisfied at 9 months (4.7% very satisfied, 32.0% satisfied, and 63.4% unsatisfied). This contrasts sharply with the satisfaction rates of 94.9%, 93.2%, and 89.8% seen at those same time points in the Volbella™ study.6
Study results showed that Volbella™ provides an aesthetic treatment for the lips that offers benefits to both the patient and physician. With long-lasting duration and ease of injection and massage, along with a good safety profile, Volbella™ delivers a smooth, natural-looking result, which is important when treating the lips and perioral area. In addition, the marketed product will contain lidocaine for patient comfort during treatment.
Conclusion
Juvéderm® Volbella™ injectable gel is well tolerated and has been demonstrated to provide a smooth and natural improvement in lip fullness that lasts for up to 1 year.
Figure 4.
A 29-year-old female before and after treatment with 0.5 mL of Juvéderm® Volbella™: 0.3 mL in the upper lip and 0.2 mL in the lower lip.
Acknowledgments
Allergan designed and funded the study, and statistical analysis of the data was performed by Nate Bennett, PhD, of Allergan. The study investigators included Mark Hamilton (Belfast, Ireland), Dalvi Humzah (West Midlands, UK), and Roy Saleh (Cheshire, UK).
Footnotes
Disclosure
The authors report no conflict of interest in this work. Dr Eccleston is a consultant for Allergan and received research support for conducting this study. Ms Murphy is an Allergan employee and stockholder.
References
- 1.Borrell M, Leslie DB, Tezel A. Lift capabilities of hyaluronic acid fillers. J Cosmet Laser Ther. 2011;13(1):21–27. doi: 10.3109/14764172.2011.552609. [DOI] [PubMed] [Google Scholar]
- 2.Fagien S, Maas C, Murphy DK, Thomas JA, Beddingfield FC, for the Juvéderm Lips Study Group Juvéderm® Ultra for lip enhancement: an open-label, multi-center study. Aesththetic Surgery Journal. 2012 doi: 10.1177/1090820X13478609. In press; [DOI] [PubMed] [Google Scholar]
- 3.Solish N, Swift A. An open-label, pilot study to assess the effectiveness and safety of hyaluronic acid gel in the restoration of soft tissue fullness of the lips. J Drugs Dermatol. 2011;10(2):145–149. [PubMed] [Google Scholar]
- 4.Restylane® instructions for use [package insert] Scottsdale, AZ: Medicis Aesthetics Inc; 2011. [Google Scholar]
- 5.Glogau RG, Bank D, Brandt F, et al. A randomized, evaluator-blinded, controlled study of the effectiveness and safety of small gel particle hyaluronic acid for lip augmentation. Dermatol Surg. 2012;38(7 Pt 2):1180–1192. doi: 10.1111/j.1524-4725.2012.02473.x. [DOI] [PubMed] [Google Scholar]
- 6.Bosniak S, Cantisano-Zilkha M, Glavas IP. Nonanimal stabilized hyaluronic acid for lip augmentation and facial rhytid ablation. Arch Facial Plast Surg. 2004;6(6):379–383. doi: 10.1001/archfaci.6.6.379. [DOI] [PubMed] [Google Scholar]