Abstract
Context:
Several studies have reported an association between low serum TSH, or subclinical hyperthyroidism (SH), and dementia, but little emphasis has been placed on this field because not all studies have demonstrated the same association. We performed a detailed systematic review to assess the evidence available to support the association between these two conditions.
Methods:
We performed a systematic search through the PubMed, Embase (1996 to 2012 wk 4), Cochrane Library, and Medline (1996 to January wk 4, 2012) electronic databases using key search terms encompassing subclinical hyperthyroidism, TSH, dementia, and cognitive impairment.
Results:
This review examines the 23 studies that provide information about the association between SH or lower serum TSH within the reference range and cognition. Fourteen of these studies, including several well-designed and well-powered cross-sectional and longitudinal analyses, have shown a consistent finding of an association between SH with cognitive impairment or dementia.
Conclusion:
There is a substantial body of evidence to support the association between SH and cognitive impairment, but there is no clear mechanistic explanation for these associations. Nor is there an indication that antithyroid treatment might ameliorate dementia. Larger and more detailed prospective longitudinal or randomized controlled trials are required to inform these important questions.
With ready access to sensitive hormone assays, the last few decades have witnessed a dramatic increase in serum thyroid function testing. This has raised many issues about the interpretation of minor deviations in thyroid function test results, particularly in individuals with little or no conventional clinical evidence of thyroid disease. The elderly are overrepresented among individuals with minor abnormalities in serum TSH and thyroid hormone concentration, and the clinical significance of these biochemical abnormalities in older people is the least clear. Overt hypothyroidism is well-established as a reversible cause of cognitive impairment, which may sometimes be profound. However, overt hyperthyroidism is also well known to be associated with impairment of concentration, mood changes, and alterations in perception. A more difficult, but nonetheless important question is whether there is evidence to support a relationship between more subtle alterations in thyroid function, in particular subclinical hyperthyroidism (SH) or low serum TSH concentration and cognitive impairment. However, the literature on this subject is heterogeneous with regard to study design and conclusions. In this review article, we aim to address the question of whether there is an association between low serum TSH and cognitive impairment and to explore possible underlying mechanisms.
Background to SH and the Aging Thyroid Axis
SH is defined as a serum TSH concentration below the reference range, with normal free T4 (FT4) and free T3 (FT3) levels. It can be divided into two groups; patients with a mildly reduced TSH (0.1–0.4 mU/liter) can be classified as having grade I SH, whereas those with a serum TSH of less than 0.1 mU/liter, including a completely suppressed TSH level (<0.01 mU/liter) are classified as having grade II SH (1). The prevalence of SH has been reported to range from 0.63 to 2.1% in epidemiological studies (2–4).
Most people with SH have no symptoms of hyperthyroidism, and relatively few develop specific complications attributable to SH: atrial fibrillation or osteoporosis (5). Prognostically, many people with SH have only a transient abnormality of thyroid function, with resolution of biochemical abnormalities found in 25–75% of those with grade I SH on repeat testing (6, 7). Thus, the majority of individuals with grade I SH do not have intrinsic thyroid disease, and the low TSH reflects nonthyroidal illness or drug effects. Nevertheless, approximately 1–2% of patients over the age of 60 with SH progress to overt hyperthyroidism, with progression most likely in those with grade II SH. This reflects the indolent nature of multinodular goiter as the dominant cause in this age group, with mild thyroid autonomy developing slowly. A small number of individuals with SH have early Graves' disease, and progression to overt hyperthyroidism is more probable in these cases (7). Conversely, 1–2% of elderly individuals have a persistent and stably low serum TSH that remains unexplained by any primary thyroid pathology. Thus, these alterations in thyroid function may reflect either altered physiology or pathology associated with advanced age.
The study of the physiological changes in the thyroid axis during aging is complicated by the presence of confounders such as medication, chronic illness, and increased risk of thyroid disease with age. Nevertheless, many observational studies in healthy older individuals, which took into account common confounders, have revealed an age-dependent decline in serum TSH and FT3 and an age-dependent increase in rT3 with maintenance of stable serum FT4 levels (8, 9). A further study demonstrated that thyroid function was well preserved until the eighth decade of life, with a decreased level of serum FT3 only observed in centenarians (10). However, the upper limit of serum TSH has also been shown to increase with age (11), leading to a wider spread of the TSH distribution with age, expanding both above and below the reference intervals for younger individuals. The mechanisms responsible for this age-related decline in serum TSH have not been fully examined. One hypothesis suggests an age-related reduction in the secretion of pituitary TSH and/or hypothalamic TRH, due to an increased pituitary thyrotrope sensitivity to peripheral T3/T4 negative feedback (12). Interestingly, the pattern of TSH pulsatility is also changed in the healthy elderly, with a blunted nocturnal TSH peak being observed, followed by a 1- to 1.5-h backward shift in the circadian rhythm of TSH secretion, leading to an earlier peak (13, 14). Equally compelling, a primary defect in thyroid hormone inactivation and disposal might explain the observation of unchanged serum T4 levels despite reduced tropic drive from lower pituitary TSH secretion. Therefore, reduced T4 and T3 degradation and clearance may lead to reduced throughput of hormone and a lower tone for the axis (15).
Brain and Thyroid Function
The population prevalence of dementia is about 7%, and this rises to more than 30% in those aged 85 yr and above (16, 17). Dementia can be classified as primary or secondary depending on its etiology. Alzheimer's disease (AD), frontal temporal lobe dementia, and Lewy body dementia are caused by primary degeneration of the brain, comprising about 70% of cases of primary dementia (18). Vascular dementia accounts for a further 15% of primary cases (19). The “cholinergic hypothesis” of AD pathogenesis is well accepted, with characteristic depletion of acetylcholine and presynaptic cholinergic markers in AD cortex and hippocampus (20, 21). Studies using single photon emission computed tomography or magnetic resonance spectroscopy have found similar characteristics in AD and in cognitively impaired patients with hyperthyroidism (22–24). Correspondingly, reduced levels of choline-related compounds in the brain have been demonstrated in hyperthyroidism, with a gradual normalization after antithyroid medication (22). Paradoxically, T4 administration led to an enhancement of rats' learning ability, along with increased cholinergic activity in the frontal cortex and hippocampus (25). Interestingly, TRH has also been shown to exert strong stimulant action on the cholinergic pathways of the cortex and hippocampus (26). Intraseptal injection and intracerebroventricular administration of TRH increased the turnover of acetylcholine in rat hippocampus and parietal cortex, respectively (27, 28). Hence, reduced TRH secretion could contribute to acetylcholine depletion, which is associated with the cognitive changes in AD.
The β amyloid plaque is the pathological hallmark of AD, and the potential pathways of β amyloid-mediated neurotoxicity include inhibition of acetylcholine activity in the cortex and hippocampus, oxidative stress from free radical damage, mitochondrial dysfunction, and ultimately apoptotic cell death (29–33). At the same time, thyroid function has been shown to influence systemic oxidative stress. Experimental and clinical studies have demonstrated increased reactive oxygen species and lipid peroxidases in the hyperthyroid state, resulting in diminishing antioxidative enzymes (34, 35). Furthermore, T3 has been shown to affect splicing of certain β-amyloid precursor protein isoforms, which are preferentially expressed in the AD brain (36). Therefore, thyroid hormones could also have a role in modulating the intracellular and extracellular contents of β-amyloid precursor protein isoforms and directly influence the pathogenesis of AD (36, 37).
Methods
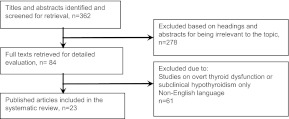
Search strategy and analysis (Fig. 1)
Fig. 1.
Literature triage for systematic review.
We performed a systematic search through the PubMed, Embase (1996 to 2012 wk 4), Cochrane Library, and Medline (1996 to January wk 4, 2012) electronic databases. The key search terms were “subclinical hyperthyroidism,” “subclinical thyroid disorders or dysfunction,” “cognitive decline or *impairment,” “dementia,” and “Alzheimer's disease.” The fields evaluated were “treatment, epidemiology, complication and mortality.” We included all cross-sectional or longitudinal studies published or translated into the English language. Meta-analysis has not been performed in light of the heterogeneity in study design, statistical analysis methods, and outcome measures among the studies.
Results
Systematic review of the relationship between SH and cognitive function
Eighty-four studies were identified that investigated the relationship between thyroid dysfunction or serum thyroid parameters and cognitive impairment. We excluded investigations that involved participants with overt thyroid disorders and only included published papers that examined the relationship between SH, or variations of thyroid function within reference intervals, with cognitive function. Twenty-three studies published from 1996 to January 2012, evaluating a total of 31,482 patients, fulfilled the inclusion criteria. Fifteen studies indicated the correlation between thyroid function and dementia as the primary study objective. Eight studies included other entities such as cardiovascular or osteoporosis risk or imaging changes as the primary outcome, with the correlation of thyroid function and cognition as a secondary objective.
Of the 23 studies, 13 were case-control or cross-sectional single-phase trials, and the remaining 10 studies had a prospective longitudinal population-based design. To date, there have been no randomized interventional studies examining the effects of antithyroid treatment on cognition in SH.
Cross-sectional/case-control studies (38–50)
Categorical analysis of subclinical thyroid disease vs. euthyroidism; or quantiles of serum TSH concentration (38–43)
Six studies made a categorical analysis, either of SH vs. euthyroidism or of quantiles of serum TSH and thyroid hormone concentration in relation to cognitive function (Table 1). The Sao Paulo Ageing and Health Study, a large cross-sectional study comprising 1119 community-dwelling participants aged 65 yr and over, found an association between SH and dementia after multivariate adjustment (38). The effect was strongest for vascular dementia [odds ratio (OR) for all types of dementia, 4.9; 95% confidence interval (CI), 1.5–15.7; vascular dementia OR, 5.8; 95% CI, 1.4–33.1]. This was confirmed using an analysis of quintiles of TSH; those with the lowest serum TSH quintile showed more than a 3-fold increase in risk for all types of dementia [age-adjusted OR for dementia, 3.6 (95% CI, 1.4–8.9); vascular dementia, 9.3 (95% CI, 1.1–75.5)]. Similarly, the InCHIANTI Study, a population-based study of 916 Italians aged 65 yr and older, demonstrated a significantly lower Mini-Mental State Examination (MMSE) score among those with SH compared with the euthyroid group (22.61 ± 6.88 vs. 24.72 ± 4.52; P < 0.03) (39). Furthermore, SH was associated with more than a 2-fold risk of scoring less than 24 out of 30 on the MMSE; a standard threshold for significant dementia [hazard ratio (HR) = 2.26 (95% CI, 1.32–3.91); P = 0.003]. These two large studies were well designed with more than a 90% participant response rate, and both carefully excluded patients with overt thyroid dysfunction as well as those taking thyroid medication. Two smaller studies [van Osch et al. (40) and Dobert et al. (41)], involving 469 and 119 participants, respectively, supported these findings. van Osch et al. (40) investigated a cognitively intact group and an AD cohort aged greater than 52 yr. Interestingly, the euthyroid participants with a TSH level in the lowest tertile (TSH, 0.5–1.3 mU/liter) had more than a 2-fold increase in AD prevalence compared with those with serum TSH in the highest tertile (2.1–6.0 mU/liter) [OR, 2.04 (95% CI, 1.18–3.53); P = 0.01]. Similarly, Dobert et al. (41) demonstrated a significantly lower TSH level in participants with both AD and vascular dementia (P < 0.01) compared with controls without cognitive impairment.
Table 1.
Cross-sectional and case control studies on the relationship between SH and cognitive decline (1996–2011)
| First author, year (Ref.) | Study size (n) | Mean age (range) | Thyroid status | Thyroid function indicators (normal range) | Objective cognitive measures | Study outcomes | Covariates | Exclusion criteria |
|---|---|---|---|---|---|---|---|---|
| Categorical analysis of subclinical thyroid disease vs. euthyroidism; or quantiles of serum T4 concentration | ||||||||
| Benseñor, 2010 (38) (Sao Paulo study) | 1119 | Patients with dementia: 78.5 (8) Non-dementia, 71.9 (6.1) |
SH and euthyroid | TSH, FT4; TSH (0.4–4.0 mIU/liter) FT4 (0.8–1.9 mg/liter) |
CSI-D (Community Screening Instrument for Dementia), Geriatric Mental State (GMS), HAS-DDS (History and Aetiology Schedule Dementia Diagnosis and Subtype) | SH increased the risk of developing dementia, especially vascular dementia All type of dementia (OR, 4.9; 95% CI, 1.5–15.7) Vascular dementia (OR, 5.8; 95% CI, 1.4–33.1) AD (OR, 2.5; 95% CI, 0.3–20.8; P, NS) Age-adjusted OR for dementia, 3.6 (95% CI, 1.4–8.9); vascular dementia OR, 9.3 (95% CI, 1.1–75.5) |
Age, gender, BMI, education, smoking, history of alcohol abuse, hypertension | Overt thyroid dysfunction Patients on thyroid medications |
| Ceresini, 2009 (39) (InCHIANTI study) | 916 | >65 | SH and euthyroid | TSH, FT3, FT4 TSH (0.46–4.68 mU/liter) FT4 (0.77–2.19 ng/dl) |
MMSE | MMSE was significantly lower in SH group compared to euthyroid group (22.61 ± 6.88 vs. 24.72 ± 4.52; P < 0.03) HR, 2.26 (95% CI, 1.32–3.91); P = 0.003 |
Age, sex, smoking, chronic heart failure, DM, hypertension, Parkinson's disease, BMI, physical activity | Participants on thyroid medications, amiodarone, lithium; dementia |
| Van Osch, 2004 (40) | 469 | >52 | Euthyroid only | TSH (0.5–6 mU/liter) | CAMCOG (Cambridge Examinations for Mental Disorders of the Elderly), MMSE | Lowest tertile of TSH was associated with a more than 2-fold increased risk of AD, compared to the highest tertile (OR, 2.04; 95% CI, 1.18–3.53; P = 0.01) | Smoking, hypertension, stroke, DM, CVD, alcohol. APOEϵ4 genotype, total homocysteine level, depression | TSH outside normal range; patients on thyroid medications |
| Dobert, 2003 (41) | 119 | 69.8 ± 14 | SH and euthyroid | TSH, FT4, FT3 TSH (0.5–4.0 mU/liter) FT4 (11–24 pmol/liter) |
MMSE, CERAD, Short Cognitive Performance test, MRI, PDG-PET | Patients with dementia showed 3-fold increased probability of having decreased or borderline TSH values (29%) vs. control (10%) | Age, sex | History of overt thyroid disease; patients on thyroid medications or contrast medium 4 wk before the laboratory testing |
| Roberts, 2006 (42) | 5868 | (65–98) | SH and euthyroid | TSH (0.4–5.5 mU/liter), FT4 (9–20 pmol/liter) FT3 (3.5–6.5 pmol/liter) |
MMSE, MEAMS (Middlesex Elderly Assessment of Mental State) | No association between subclinical thyroid dysfunction and cognition or mood | Dementia, CVD. RA, PVD, psychiatry diseases, osteoporosis, nonspecific thyroid diseases, DM, pulmonary or gastrointestinal diseases, medications (amiodarone, lithium, β-blocker, antiepileptics, antidepressants, kelp, tranquilizers, steroid, morphine) | Overt thyroid diseases or taking thyroid medications |
| Van der Cammen, 2003 (43) | 829 | 78.2 | All thyroid status (TSH) | TSH (no normal range provided) | Diagnostic and Statistical Manual of Mental Disorders to diagnose AD | No differences in TSH level between AD patients and those without dementia | No information available | No exclusion criteria (patients with overt thyroid disease were included) |
| Multivariate analysis with thyroid function markers or cognitive performance as continuous variables | ||||||||
| Wahlin, 1998 (44) | 200 | (75–96) | Euthyroid only | TSH, FT4 TSH (0.4–5 mU/liter) FT4 (12–25 pmol/liter) |
Two-letter fluency tasks; Block design test with WAIS-R; Trail Making Test; Episodic memory tests | Positive association between low normal TSH and worse episodic memory (P < 0.01) | Age, education, mood symptoms | Overt thyroid dysfunction, patients on neuroleptic or antithyroid medications, TFT outside the normal range, psychiatric illness |
| Stuerenburg, 2006 (45) | 227 | 71.6 | All thyroid status (mean TSH 17.3 ± 3.2) | FT4, TSH, FT3, TT4, TT3 Lowest FT4 quartile <15.1 Highest FT4 quartile >19.0 |
MMSE | Significant inverse correlation between plasma FT4 and MMSE score (Spearman rank correlation = −0.14; P = 0.04) | Smoking, hypertension, LDL, HDL, APOEϵ4 allele, depression | No exclusion criteria |
| De Jongh, 2011 (46) | 1219 (34-SH) | 75.5 (68.9–82.1) | Euthyroid, SH and subclinical hypothyroidism | TSH, FT4, FT3 TSH (0.3–4.5 mU/liter) |
MMSE, the Raven'S colored progressive matrices (RCPM), the coding task and the audiotry verbal learning test | Subclinical hyper- or hypothyroidism was not associated with impaired global cognitive function | Age, sex, alcohol use, smoking, educational level, mean arterial pressure, BMI, heart rate, total cholesterol, and physical activity | Antithyroid or T4 medications |
| Patterson, 2010 (47) | 409 | 76.9 (52–94) | Euthyroid only | TSH, FT4 No normal range provided |
NART (National Adult Reading Test); MMSE; HVLT (Hopkins Verbal Learning test) | No relationship between cognitive function and thyroid hormones. Higher FT4 was associated with worse functional independence (P < 0.001) |
Age, sex, mood | No exclusion criteria |
| van Boxtel, 2004 (48) | 120 (healthy volunteers) | >45 | All thyroid status | TSH No normal range provided |
MAAS test battery (memory, sensorimotor speed, information processing, cognitive flexibility | Higher TSH was associated with poorer memory (P < 0.05), but this association disappeared after correction for depression score | Depression, education, overt thyroid diseases | Dementia, PD, CVD, epilepsy, chronic psychotropic drug usage, CNS tumor |
| Quinlan, 2010 (49) | 69 | 60.9–66.8 | All thyroid status: | TSH, FT4, TT4, TT3 Only TT3 level given (1.4–1.6 nmol/liter) |
Trail Making Test; RAVLT delayed recall; Block design; Token test; Boston Naming test, Stroop test etc | MCI group with higher TT3 showed more cognitive impairment in episodic memory (P = 0.0080), language (P = 0.001), and executive function (P = 0.012) | Age, sex, BMI, total cholesterol, HDL, LDL, BP, use of T4, β-blocker and estrogen | Psychiatric disorders, depression, systemic illness, diabetes, cerebral tumor, CNS infection, chronic alcoholism, patients on steroid treatments, patients with dementia |
| Prinz, 1999 (50) | 44 | 72 | All thyroid status | TT3, TT4, TSH; no normal range provided | MMSE; CATMEAN (category fluency), FASMEAN (verbal fluency) etc | Higher TT4 was significantly associated with better WAIS score (P < 0.05) and global cognitive performance (P < 0.01) | Age, education | Overt thyroid diseases, DM, dementia (MMSE score <27) depression, MI, BMI >18 or <33 kg/m2, hypertension, CNS medication used (including 2 wk prior to testing), head/trauma or infection, other systemic illness, neurological, alcohol, sleep disorder |
NS, Nonsignificant; MMSE, Mini-Mental State Examination; CERAD, Consortium to Establish a Registry for Alzheimer's disease; BMI, body mass index; DM, diabetes mellitus; CVD, cardiovascular disease; RA, rheumatoid arthritis; PVD, peripheral vascular disease; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TFT, thyroid function test; APOEε4, apolipoprotein Eε4; BP, blood pressure; PD, Parkinson's disease; MI, myocardial infarction.
In contrast, the largest cross-sectional population-based study, involving 5868 participants, showed no association between SH and cognition (42). However, this study invited participants by mail, and the response rate was poor at 38%. Although the responders were representative of the regional population with respect to demographic characteristics, the average MMSE score was above 27 in all subgroups, suggesting that the responder population was skewed toward a cognitively intact group. Another cross-sectional observational study, involving 829 consecutive unselected referrals to a hospital geriatric clinic, did not demonstrate any difference in TSH level between the group with AD and the cohort without dementia (43). However, comorbidities and medication use were not described, so the results may be confounded by nonthyroidal illness, thyroid medication, or overt thyroid dysfunction.
Serum thyroid function markers (TSH/FT4/TT3) or cognitive performance as continuous variables (44–50)
An additional seven studies performed multivariate analyses to explore the relationship between cognition and thyroid function. Wahlin et al. (44) were the first to suggest an association between poorer cognitive performance and lower serum TSH concentrations within the reference range. A significant positive correlation was found between a lower episodic memory score and serum TSH concentration (P < 0.01) in 200 healthy, euthyroid, community-dwelling participants aged over 75 yr. In keeping with these findings, Stuerenburg et al. (45) demonstrated an inverse correlation (P = 0.04) between serum FT4 level and MMSE score in 227 patients aged 49–91 yr with mild to moderate AD.
In contrast, the remaining five studies of this design failed to demonstrate any association between cognition and low serum TSH or high serum FT4 level (46–50). In a cross-sectional study involving 1219 community-dwelling participants, global cognitive impairment was not increased in those with SH (n = 34) (46). In a hospital-based study involving 409 euthyroid patients aged 52–94 yr diagnosed with probable AD, no association was found between serum TSH or FT4 and dementia (47). Two smaller studies involving 120 healthy volunteers and 69 hospital-based participants, respectively, showed similar findings (48, 49). Paradoxically, a study of 44 healthy, community-dwelling male volunteers with a mean age of 72 yr observed a significant association between higher total T4 (TT4) levels and better cognition [Verbal performance in Wechsler Adult Intelligence Scale (P < 0.05) and global cognitive performance (P < 0.01)] but failed to reproduce this trend with TT3 and FT4 (50). It is worth noting that almost all of these studies have relatively small sample sizes, that each made a single measurement of thyroid function, and they used different cognitive performance tests, which limits the generalizability of these results.
Prospective longitudinal studies (51–60)
Categorical analysis of subclinical thyroid disease vs. euthyroidism; or quantiles of serum TSH concentration (51–56)
Six studies performed categorical analyses, either of SH vs. euthyroidism or quantiles of serum thyroid hormone concentration in relation to cognitive function (Table 2). The Rotterdam study was the first longitudinal study to examine the relationship between SH and dementia in 1893 population-based subjects (mean age, 69 yr) (51). Over a 2-yr follow-up, a 3-fold increased risk of dementia from all causes [relative risk (RR), 3.5; 95% CI, 1.2–10] and AD (RR, 3.5; 95% CI, 1.1–11) was found among those with a low baseline TSH level (<0.4 mU/liter). This study was limited by the small number of the SH group with dementia (n = 25) and a short follow-up period. Nevertheless, the positive findings in this study are supported by two well-conducted, large population-based studies with a longer follow-up period. The first study, a community-based observational study, was carried out over a period of 12 yr by Tan et al. (52) in 1864 patients who were free of dementia for 3 yr at recruitment. The patients were then divided into three tertiles according to baseline TSH: T1, less than 1.0 mU/liter; T2, 1.0–2.1 mU/liter; and T3, more than 2.1 mU/liter. A positive correlation in women with a serum TSH level in the lowest (T1) tertile (HR, 2.26; 95% CI, 1.36–3.77; P = 0.002) and the highest (T3) tertile (HR, 1.84; 95% CI, 1.10–3.08; P = 0.003) with AD risk was found. This relationship was not found in men, but other indices of thyroid function were not studied. However, the two aforementioned large prospective studies involved only a single measurement of thyroid function. This limitation has recently been addressed by the largest observational study involving 12,115 participants (2,004 with SH; 10,111 euthyroid individuals) with a mean age of 66.5 yr and a median follow-up period of 5.6 yr (53). This well-designed, population-based study had a carefully selected SH cohort, including only individuals with two confirmatory measurements of serum TSH at least 4 months apart. Patients whose TSH normalized or who developed overt thyroid disease during the course of follow- up were excluded. A positive association between SH and dementia was observed (adjusted HR, 1.64; 95% CI, 1.20–2.25). Interestingly, when patients were divided into two groups according to the TSH level (0.1–0.4 vs. <0.1 mU/liter), no relationship could be established between suppressed TSH (<0.1 mU/liter) and dementia, but a significant association remained for the group with TSH at 0.1–0.4 mU/liter.
Table 2.
Longitudinal populational-based studies on the relationship between SH and cognitive decline (1996–2011)
| Author and year of publication | Study size (n) and setting | Mean age (range) | Follow-up interval (yr) | Participants' thyroid status | Thyroid function indicators (normal range) | Objective cognitive measures | Study outcomes | Covariates | Exclusion criteria |
|---|---|---|---|---|---|---|---|---|---|
| Categorical analysis of subclinical thyroid disease vs. euthyroidism; or quantiles of serum T4 concentration | |||||||||
| Kalmijn, 2000 (51) | 1893 community | 68.8 (54–94) | 2–4 | SH and euthyroid | TSH, FT4, TT4 TSH (0.4–4.0 mU/liter) FT4 (11–25 pmol/liter) |
MMSE GMS-A CAMDEX |
SH increased the risk of dementia and AD 3-fold after a 2-yr follow-up (RR, 3.5; 95% CI, 1.2–10) | Age, sex, education, smoking status, atrial fibrillation, depression | Dementia at baseline, patients on amiodarone, β-blocker or thyroid medications |
| Tan, 2008 (52) | 1864 community | 71 | 12.7 | SH and euthyroid | TSH (0.5–5.0 mU/liter) | MMSE | Positive association between women with serum TSH in the lowest (<1.0 mIU/liter) and highest (>2.1 mIU/liter) and increased risk of AD | Age, plasma homocysteine levels, BMI, education, APOEϵ4 allele, stroke, atrial fibrillation | Dementia at baseline Risk of AD for: lowest TSH tertile (HR, 2.26; 95% CI, 1.36–3.77); highest TSH tertile (HR, 1.84; 95% CI, 1.10–3.08) |
| Vadiveloo, 2011 (53) | 12,115 community; SH- 2004; euthyroid, 10,111 | 66.5 ± 15.9 | Median, 5.6 yr | SH and euthyroid | TSH (0.4–4.0 mU/liter); FT4 (10–25 pmol/liter); FT3 (0.9–2.6 nmol/liter) (at least 2 measurements of TSH, minimally 4 months apart) | Diagnosis of dementia, ICD9 and 10 | Positive association between SH and dementia. Adjusted HR 1.64 (95% CI, 1.20–2.25) No relationship established between TSH concentration (0.1–0.4) vs. <0.1 mU/liter and dementia (limited by small number of patients with TSH <0.1) |
Age, gender, history of dementia and psychiatry disease | Age <18 yr, patients treated with antithyroid medications/RAI/ thyroidectomy before and during the first year after the first abnormal TFT, patient on amiodarone, T4 replacement during the study period, pregnancy. Patient on long-term steroid replacement/ pituitary disease |
| de Jong, 2009 (54) | 615 community | 77.3–78.6 | 5 | SH and euthyroid | TSH, FT4, TT4. TSH (0.4–4.3 mIU/liter); FT4 (0.85–1.94 ng/dl) | CASI (100 point Cognitive Ability Screening Instruments) | Higher TT4 and FT4 were associated with dementia (HR 1.21; 95% CI, 1.04–1.40); AD (HR, 1.31; 95% CI, 1.14–1.51) and neuropathology | Age, education level, depression, albumin level, BMI, cholesterol , DM, hypertension, smoking, patients on T4, β-blocker or other cardiac antiarrhythmic drugs | T4 level out of normal range |
| de Jong, 2006 (55) | 1,025 community | 72.3 (60–90) | 5.5 | Euthyroid only | TSH, FT4, FT3. TSH (0.4–4.3 mU/liter); FT4 (0.85–1.94 ng/dl) | MMSE, GMS-A, CAMDEX | No association between increased risk of dementia or AD with TSH or thyroid hormone Higher FT4 associated with greater atrophy at hippocampus and amygdala on MRI |
Sex, educational level, smoking, depression, medication use (amiodarone, β-blocker, steroids), BMI, cholesterol, homocysteine, smoking, creatinine APOEϵ4 genotype, diabetes, atrial fibrillation | Blindness, dementia, contraindication to MRI, patients on thyroid medications |
| Volpato, 2002 (56) | 464 community | 77.5 | 3 | Euthyroid only | TSH, FT4. TSH (0.3–5.0 mU/liter); FT4 (4.5–12.5 ng/dl) | MMSE | Low T4 within the normal range was associated with cognitive impairment over a 3-yr period (RR, 1.97; 95% CI, 1.10–3.5) | Age, race, educational level, coronary heart disease, hypertension, stroke, diabetes, PVD, depression, cancer | Nil |
| Multivariate analysis with thyroid function markers or cognitive performance as continuous variables | |||||||||
| Hogervorst, 2008 (57) | 1,047 community | (64–94) | 2 | Euthyroid only | TSH, FT4. TSH (0.3–4.8 mU/liter); FT4 (13–23 pmol/liter) | MMSE; AGECAT (Automated Geriatric Examination and Computer Assisted Taxonomy) | High normal FT4 had a negative association with baseline MMSE and accelerated cognitive decline after 2 yr (P = 0.03) | Age, sex, education, MMSE at baseline, mood, vascular risk factor (smoking, hypertension, heart disease, DM, stroke) | MMSE <18 |
| Gussekloo, 2004 (58) | 558 community | 85 | 3.7 | All thyroid status | TSH, FT4, FT3. TSH (0.3–4.8 mU/liter); FT4 (13–23 pmol/liter) | MMSE; Stroop test; Letter Digit Coding test; Word Learning test | Increased level of TSH was associated with better memory on follow-up (P = 0.03), when participants on T4 were excluded | Age, education | No exclusion criteria |
| Wahlin, 2005 (59) | 200 community | 75–96 | 3, then 6 yr | Euthyroid only | TSH, FT4. TSH (0.4–5 mU/liter); FT4 (12–25 pmol/liter) | Two-letter fluency tasks; Block design test with WAIS-R; Trail Making Test; Episodic memory tests | Positive association between decreased level of TSH with increased episodic recall deficits at 6-yr follow-up (β 0.290; P < 0.05) No association found in 3-yr follow-up |
Age, education, mood symptoms | Over thyroid diseases, thyroid medications, psychiatric illness (but dementia is not excluded due to small sample size) |
| Annerbo, 2006 (60) | 93 hospital-based | Men, 64.7; women, 65.4 | 5 | All thyroid status | TSH (no normal range provided) | MMSE | Low TSH predicted risk of developing AD after controlled for other risk factors (OR for square root of TSH, 0.287; 95% CI, 0.088–0.931) | Stroke, cardiovascular disease, T4 treatment | No exclusion criteria |
GMS-A, Geriatric Mental State schedule; CAMDEX, Cambridge examination for disorders of the elderly; BMI, body mass index; APOEε4, apolipoprotein Eε4; DM, diabetes mellitus; PVD, peripheral vascular disease; RAI, radioiodine therapy; TFT, thyroid function test.
In addition to these three large population-based studies, de Jong et al. (54) carried out a smaller prospective study among 615 Japanese-American men in the Honolulu Heart Program. The cohort had a mean age of 77.5 yr, and the mean duration of follow-up was 5 yr. This study observed a 20 and 30% increased risk for dementia and AD, respectively, for each sd increase in serum FT4 (HR for dementia, 1.21; 95% CI, 1.04–1.40; and HR for AD, 1.31; 95% CI, 1.14–1.51). In addition, neuropathological examinations from the autopsy program instituted as part of this study demonstrated a higher neocortical neurofibrillary tangle count per sd increase in TT4 (0.25; 95% CI, 0.05–0.46).
In contrast with the studies discussed so far, the Rotterdam Scan study, another prospective community-based study involving 1025 subjects aged 60–90 yr with a mean follow-up interval of 5.5 yr, showed no association between dementia and serum TSH or thyroid hormone levels (55). Nevertheless, they found a positive association between a higher FT4 and rT3 and greater atrophy at the hippocampus and amygdala on magnetic resonance imaging (MRI), in keeping with the finding in the Honolulu aging study. Although the Rotterdam Scan study had greater power than the original Rotterdam study, due to a larger-sized dementia cohort (n = 60 vs. 25) and a longer follow-up period, it is limited by single measurements of thyroid function and a small number of dementia cases with low TSH (n = 7).
Finally, a community-based study involving 464 euthyroid women aged 65 yr or older found an association between lower FT4 concentrations within the reference range and cognitive impairment over a 3-yr period (RR, 1.97; 95% CI, 1.10–3.5) (56). However, the same pattern was not exhibited in those with a frankly low TSH or an elevated FT4 level. There was a 14% dropout rate during follow-up, largely due to missing MMSE scores, which might have introduced a significant bias.
Serum thyroid function markers (TSH/FT4/TT3) or cognitive performance as continuous variables (57–60)
Four studies made multivariate analyses using thyroid function parameters or cognitive function (MMSE) as continuous variables. Hogervorst et al. (57) conducted a large prospective study involving 1047 community-dwelling patients aged 64–94 yr with MMSE scores of at least 25 in the United Kingdom and Wales. Higher serum FT4 concentrations within the reference range were associated with lower baseline MMSE scores and accelerated cognitive decline after 2 yr [OR, 1.13 (95% CI, 1.03–1.23); P = 0.006]. This study was limited by a single measurement of thyroid function. Prospective follow-up of 558 individuals aged 85 living in Leiden, The Netherlands, demonstrated an association between higher serum TSH and better memory function (β, 0.13; P = 0.03) (58). Nevertheless, this study had a small sample size and a 41.5% loss of follow-up due to death or refusal to continue participation.
After the cross-sectional study in 1998 (44), Wahlin et al. (59) conducted a follow-up survey on the same cohort and found an association between low serum TSH levels within the reference range and decreased verbal memory or episodic recall deficits at the 6-yr follow-up (β, 0.290; P < 0.05). Similarly, in a small hospital-based study, Annerbo et al. (60) demonstrated that for each unit decrease in serum TSH, the OR of developing dementia increased by 3.5.
Summary of observational studies
Twenty-three studies that met our criteria have examined the association between SH and cognition. Fourteen of these studies, including several well-designed and well-powered cross-sectional and longitudinal analyses, have shown a consistent finding of an association of SH, or low serum TSH within the reference interval, with cognitive impairment or dementia. In particular, this association was seen in more than three fourths of the prospective longitudinal studies, providing reliable evidence from robust studies. Several of the studies that did not confirm this result are potentially compromised by small samples sizes, recruitment from hospital environments, or the challenging nature of working with participants with cognitive problems (e.g. failure to return questionnaires).
Discussion
Given the weight of information suggesting an association of SH and lower serum TSH concentrations with cognitive impairment summarized above, we need to consider several explanations that could explain these findings (Table 3). A conventional explanation (explanation A) might be that mild endogenous SH reflects true thyroid overactivity causing excessive thyroid hormone action on the central nervous system. “Toxic” effects of thyroid hormones on the brain could be mediated by increased brain oxidative stress caused by the mild hyperthyroidism, which promotes reactive oxygen species production (35). Alternatively, thyroid hormone effects on the heart could mediate vascular dementia via thromboembolism from a combination of atrial fibrillation, myocardial and endothelial dysfunction, and hypercoagulability (5). However, we believe this explanation to be unlikely for several reasons. First, only about 20% of individuals with SH have a suppressed TSH (grade II SH), and so most don't have true endogenous hyperthyroidism. Clearly, neither do those people with serum TSH concentrations in the lower centiles of the healthy reference range. Indeed, the low but not suppressed TSH in the 0.1–0.4 mU/liter range is most commonly seen as a manifestation of nonthyroidal illness and is associated with a lowering of circulating FT3, rather than thyroid hormone excess. Second, for this explanation to be true, we would expect to see a biological gradient with cognitive defects being worse in individuals with the lowest TSH. In fact, this was not observed in the study of Vadiveloo et al. (53) where, if anything, the cognitive effects were most marked in the grade I SH group.
Table 3.
Possible mechanism for association of cognitive impairment with SH or low serum TSH
| A. Excess circulating thyroid hormone resulting in neuronal loss. |
| B. Primary neurodegeneration causes reduced central nervous system TRH secretion, hence lower TSH. |
| C. SH and low TSH are biomarkers for age, and so are associated with other diseases of advanced age including dementias. |
| D. Subjects with cognitive impairment have a high burden of comorbidity, and association is due to nonthyroidal illness and drug effects on serum TSH. |
An alternative explanation (explanation B) is that the organic brain diseases causing cognitive impairment also reduce TRH secretion from the hypothalamus and other brain areas. This would have a “knock-on” effect leading to lower TSH secretion and hence reduced thyroid hormone turnover (production and excretion)—in effect, a state of mild central hypothyroidism. There is little evidence to either support or refute this contention. It is known that there are projections from many areas of the brain to the paraventricular nucleus containing the TRH-secreting neurons, such as the C1–3 adrenergic neurons of the brainstem, the hypothalamic arcuate nucleus, and the neurons of the hypothalamic dorsomedial nucleus, which exert different effects on the hypophysiotropic TRH neurons (61–66). Furthermore, TRH is not only released from the paraventricular nucleus of hypothalamus but is also localized in neurons of the septal nuclei, preoptic area, raphe nuclei of the medulla oblongata, and spinal cord (67–69). Thus, TRH has a more generalized role as a central nervous system (CNS) neurotransmitter, and the brain involution of the dementia process may lead to a widespread perturbation of neurotransmitters, including TRH. If we accept this explanation as being plausible, then paradoxically, a study of thyroid hormone supplementation in dementia might be warranted.
A third explanation refers back to our understanding of thyroid hormone metabolism in older age. With this mechanism, one has to consider that low serum TSH (and indeed higher FT4) may be a marker of biological age, reflecting reduced hepatic clearance of thyroid hormones (including reduced type 1 deiodinase activity) and a consequent reduction in thyroid axis turnover. The well-established observation that older people with hypothyroidism require less levothyroxine replacement is the clinical correlate of this phenomenon (15, 70). In effect, the epidemiological studies reviewed above identify a group of individuals within a cohort who have more advanced biological age, using TSH as the biomarker (1). These individuals therefore have an excess of the degenerative disorders of advanced age, which includes cognitive impairment and dementia. Compellingly, SH is also associated with excess rates of atrial fibrillation, vascular events, low bone mineral density, fracture, and reduced muscle strength (71–74). Thus, dementia can be viewed as another noncausal association of low TSH/SH (1).
Individuals with cognitive impairment and dementias have a high burden of comorbidity and associated medication use. This may include many episodic intercurrent illnesses, such as transient infection, as well as more chronic degenerative conditions. All of these comorbidities can lead to reduced serum TSH, consequent to the well-described changes in serum thyroid hormones associated with nonthyroidal illness, known as “euthyroid sick syndrome.” Similarly, a wide variety of medications for conditions other than thyroid diseases including glucocorticoids, opiates, L-DOPA, amiodarone, and metformin are known to reduce serum TSH concentrations (1, 75). Thus, the combination of excess comorbidity and the consequent additional medications that may be prescribed for this may also explain a noncausal association of SH/low TSH with cognitive impairment. The fact that most of the studies performed in this field have relied upon a single TSH measurement makes these data particularly vulnerable to this interpretation. Nevertheless, although it is possible that this explanation contributes to some of the association between SH and dementia, it is unlikely to be the dominant factor. The large study of Vadiveloo et al. (53) involving 12,115 patients showed that this association persists after stringent ascertainment of a cohort categorized as having SH after repeated serum TSH measurement.
At the current time, we believe that there are insufficient data to allow discrimination between the various mechanistic possibilities outlined above (Table 3). Indeed, it is likely that there may be contributions from several of the above factors to the observed association between SH or low serum TSH and cognitive impairment. Until such time as more information becomes available, this remains an open question.
Conclusion
We have performed the first detailed review of a large and heterogeneous literature relating to the association between low serum TSH and cognitive impairment in older people. Overall, taking into account the largest and most robustly designed studies, there is a strong body of evidence to support the association between SH and cognitive impairment. However, there is no clear mechanistic explanation for these associations, nor is there any evidence to support the use of antithyroid measures to prevent or improve cognitive decline in this patient group. Larger and more detailed prospective longitudinal or randomized controlled trials are required to inform these important questions.
Supplementary Material
Acknowledgments
We thank Professor John O'Brien, Professor of Old Age Psychiatry in the Institute for Aging and Health (Newcastle University), for his kindness in providing helpful comments about this manuscript.
The preparation of this manuscript was supported by the Medical Research Council (Grant G0500783).
Disclosure Summary: S.H.S.P. has accepted speaker fees from Merck Serono. E.H.G. has nothing to declare.
Footnotes
- AD
- Alzheimer's disease
- CI
- confidence interval
- CNS
- central nervous system
- FT3
- free T3
- FT4
- free T4
- HR
- hazard ratio
- MRI
- magnetic resonance imaging
- OR
- odds ratio
- RR
- relative risk
- SH
- subclinical hyperthyroidism
- TT3
- total T3
- TT4
- total T4.
References
- 1. Mitchell AL, Pearce SH. 2010. How should we treat patients with low serum thyrotropin concentrations? Clin Endocrinol (Oxf) 72:292–296 [DOI] [PubMed] [Google Scholar]
- 2. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. 2000. The Colorado Thyroid Disease Prevalence Study. Arch Intern Med 160:526–534 [DOI] [PubMed] [Google Scholar]
- 3. Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. 2002. Serum TSH, T4 and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 87:489–499 [DOI] [PubMed] [Google Scholar]
- 4. Vadiveloo T, Donnan PT, Cochrane L, Leese GP. 2011. The Thyroid Epidemiology, Audit, and research Study (TEARS): the natural history of endogenous subclinical hyperthyroidism. J Clin Endocrinol Metab 96:E1–E8 [DOI] [PubMed] [Google Scholar]
- 5. Biondi B, Cooper DS. 2008. The clinical significance of subclinical thyroid dysfunction. Endocr Rev 29:76–131 [DOI] [PubMed] [Google Scholar]
- 6. Parle JV, Franklyn JA, Cross KW, Jones SC, Sheppard MC. 1991. Prevalence and follow-up of abnormal thyrotrophin (TSH) concentrations in the elderly in the United Kingdom. Clin Endocrinol (Oxf) 34:77–83 [DOI] [PubMed] [Google Scholar]
- 7. Rosario PW. 2010. Natural history of subclinical hyperthyroidism in elderly patients with TSH between 0.1 and 0.4 mIU/l: a prospective study. Clin Endocrinol (Oxf) 72:685–688 [DOI] [PubMed] [Google Scholar]
- 8. van den Beld AW, Visser TJ, Feelders RA, Grobbee DE, Lamberts SWJ. 2005. Thyroid hormone concentration, disease, physical function and mortality in elderly men. J Clin Endocrinol Metab 90:6403–6409 [DOI] [PubMed] [Google Scholar]
- 9. van Coevorden A, Laurent E, Decoster C, Kerkhofs M, Neve P, van Cauter E, Mockel J. 1989. Decreased basal and stimulated thyrotropin secretion in healthy elderly men. J Clin Endocrinol Metab 69:177–185 [DOI] [PubMed] [Google Scholar]
- 10. Mariotti S, Barbesino G, Caturegli P, Bartalena L, Sansoni P, Fagnoni F, Monti D, Fagiolo U, Franceschi C, Pinchera A. 1993. Complex alteration of thyroid function in healthy centenarians. J Clin Endocrinol Metab 77:1130–1134 [DOI] [PubMed] [Google Scholar]
- 11. Atzmon G, Barzilai N, Hollowell JG, Surks MI, Gabriely I. 2009. Extreme longevity is associated with increased serum thyrotropin. J Clin Endocrinol Metab 94:1251–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lewis GF, Alessi CA, Imperial JG, Refetoff S. 1991. Low serum free thyroxine index in ambulating elderly is due to a resetting of the threshold of thyrotropin feedback suppression. J Clin Endocrinol Metab 73:843–849 [DOI] [PubMed] [Google Scholar]
- 13. van Coevorden A, Mockel J, Laurent E, Kerkhofs M, L'Hermite-Balériaux M, Decoster C, Nève P, Van Cauter E. 1991. Neuroendocrine rhythms and sleep in aging men. Am J Physiol 260:E651–E661 [DOI] [PubMed] [Google Scholar]
- 14. Barreca T, Franceschini R, Messina V, Bottaro L, Rolandi E. 1985. 24-Hour thyroid stimulating hormone secretory pattern in elderly men. Gerontology 31:119–123 [DOI] [PubMed] [Google Scholar]
- 15. Gregerman RI, Gaffney GW, Shock NW, Crowder SE. 1962. Thyroxine turnover in euthyroid man with special reference to changes with age. J Clin Invest 41:2065–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ott A, Breteler MM, van Harskamp F, Claus JJ, van der Cammen TJ, Grobbee DE, Hofman A. 1995. Prevalence of Alzheimer's disease and vascular dementia: association with education. The Rotterdam Study. BMJ 310:970–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Adelman S, Blanchard M, Rait G, Leavey G, Livingston G. 2011. Prevalence of dementia in African-Caribbean compared with UK-born White older people: two-stage cross-sectional study. Br J Psychiatry 199:119–125 [DOI] [PubMed] [Google Scholar]
- 18. Brookmeyer R, Gray S, Kawas C. 1998. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health 88:1337–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miller JW. 1999. Homocysteine and Alzheimer's disease. Nutr Rev 57:126–129 [DOI] [PubMed] [Google Scholar]
- 20. Coyle JT, Price DL, DeLong MR. 1983. Alzheimer's disease: a disorder of cortical cholinergic innervations. Science 219:1184–1190 [DOI] [PubMed] [Google Scholar]
- 21. Davies P. 1988. Neurochemical studies: an update on Alzheimer's disease. J Clin Psychiatry 49(Suppl):23–28 [PubMed] [Google Scholar]
- 22. Bhatara VS, Tripathi RP, Sankar R, Gupta A, Khushu S. 1998. Frontal lobe proton magnetic-resonance spectroscopy in Graves' disease: a pilot study. Psychoneuroendocrinology 23:605–612 [DOI] [PubMed] [Google Scholar]
- 23. Fukui T, Hasegawa Y, Takenaka H. 2001. Hyperthyroid dementia: clinicoradiological findings and response to treatment. J Neurol Sci 184:81–88 [DOI] [PubMed] [Google Scholar]
- 24. Kantarci K, Lowe V, Przybelski SA, Senjem ML, Weigand SD, Ivnik RJ, Roberts R, Geda YE, Boeve BF, Knopman DS, Petersen RC, Jack CR., Jr 2011. Magnetic resonance spectroscopy, β-amyloid load, and cognition in a population-based sample of cognitively normal older adults. Neurology 77:951–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smith JW, Evans AT, Costall B, Smythe JW. 2002. Thyroid hormones, brain function and cognition: a brief review. Neurosci Biobehav Rev 26:45–60 [DOI] [PubMed] [Google Scholar]
- 26. Giovannini MG, Casamenti F, Nistri A, Paoli F, Pepeu G. 1991. Effect of thyrotropin releasing hormone (TRH) on acetylcholine release from different brain areas investigated by microdialysis. Br J Pharmacol 102:363–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brunello N, Cheney DL. 1981. The septal-hippocampal cholinergic pathway: role in antagonism of pentobarbital anesthesia and regulation by various afferents. J Pharmacol Exp Ther 219:489–495 [PubMed] [Google Scholar]
- 28. Malthe-Sorenssen D, Wood PL, Cheney DL, Costa E. 1978. Modulation of the turnover rate of acetylcholine in rat brain by intraventricular injections of thyrotropin-releasing hormone, somatostatin, neurotensin and angiotensin II. J Neurochem 31:685–691 [DOI] [PubMed] [Google Scholar]
- 29. Fu AL, Dong ZH, Sun MJ. 2006. Protective effect of N-acetyl-L-cysteine on amyloid β-peptide-induced learning and memory deficits in mice. Brain Res 1109:201–206 [DOI] [PubMed] [Google Scholar]
- 30. Maurice T, Lockhart BP, Privat A. 1996. Amnesia induced in mice by centrally administered β-amyloid peptides involves cholinergic dysfunction. Brain Res 706:181–193 [DOI] [PubMed] [Google Scholar]
- 31. Stéphan A, Laroche S, Davis S. 2001. Generation of aggregated β-amyloid in the rat hippocampus impairs synaptic transmission and plasticity and causes memory deficits. J Neurosci 21:5703–5714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tsai KJ, Tsai YC, Shen CK. 2007. G-CSF rescues the memory impairment of animal models of Alzheimer's disease. J Exp Med 204:1273–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hardy J, Selkoe DJ. 2002. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297:353–356 [DOI] [PubMed] [Google Scholar]
- 34. Aslan M, Cosar N, Celik H, Aksoy N, Dulger AC, Begenik H, Soyoral YU, Kucukoglu ME, Selek S. 2011. Evaluation of oxidative status in patients with hyperthyroidism. Endocrine 40:285–289 [DOI] [PubMed] [Google Scholar]
- 35. Mayer L, Romiæ Z, Skreb F, Baciæ-Vrca V, Cepelak I, Zaniæ-Grubisiæ T, Kirin M. 2004. Antioxidants in patients with hyperthyroidism. Clin Chem Lab Med 42:154–158 [DOI] [PubMed] [Google Scholar]
- 36. Latasa MJ, Belandia B, Pascual A. 1998. Thyroid hormones regulate β-amyloid gene splicing and protein secretion in neuroblastoma cells. Endocrinology 139:2692–2698 [DOI] [PubMed] [Google Scholar]
- 37. Belandia B, Latasa MJ, Villa A, Pascual A. 1998. Thyroid hormone negatively regulates the transcriptional activity of the β-amyloid precursor protein gene. J Biol Chem 273:30366–30371 [DOI] [PubMed] [Google Scholar]
- 38. Benseñor IM, Lotufo PA, Menezes PR, Scazufca M. 2010. Subclinical hyperthyroidism and dementia: the Sao Paulo Ageing, Health Study (SPAH). BMC Public Health 10:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ceresini G, Lauretani F, Maggio M, Ceda GP, Morganti S, Usberti E, Chezzi C, Valcavi R, Bandinelli S, Guralnik JM, Cappola AR, Valenti G, Ferrucci L. 2009. Thyroid function abnormalities and cognitive impairment in elderly people: results of the Invecchiare in Chianti study. J Am Geriatr Soc 57:89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van Osch LA, Hogervorst E, Combrinck M, Smith AD. 2004. Low thyroid-stimulating hormone as an independent risk factor for Alzheimer disease. Neurology 62:1967–1971 [DOI] [PubMed] [Google Scholar]
- 41. Döbert N, Hamscho N, Menzel C, Peters J, Frölich L, Tsolakis A, Zaplatnikov K, Kratzsch T, Diener J, Maurer K, Grünwald F. 2003. Subclinical hyperthyroidism in dementia and correlation of the metabolic index in FDG-PET. Acta Med Austriaca 30:130–133 [PubMed] [Google Scholar]
- 42. Roberts LM, Pattison H, Roalfe A, Franklyn J, Wilson S, Hobbs FD, Parle JV. 2006. Is subclinical thyroid dysfunction in the elderly associated with depression or cognitive dysfunction? Ann Intern Med 145:573–581 [DOI] [PubMed] [Google Scholar]
- 43. van der Cammen TJ, Mattace-Raso F, van Harskamp F, de Jager MC. 2003. Lack of association between thyroid disorders and Alzheimer's disease in older persons: a cross-sectional observational study in a geriatric outpatient population. J Am Geriatr Soc 51:884. [DOI] [PubMed] [Google Scholar]
- 44. Wahlin A, Wahlin TB, Small BJ, Bäckman L. 1998. Influences of thyroid stimulating hormone on cognitive functioning in very old age. J Gerontol B Psychol Sci Soc Sci 53:P234–P239 [DOI] [PubMed] [Google Scholar]
- 45. Stuerenburg HJ, Arlt S, Mueller-Thomsen T. 2006. Free thyroxine, cognitive decline and depression in Alzheimer's disease. Neuro Endocrinol Lett 27:535–537 [PubMed] [Google Scholar]
- 46. de Jongh RT, Lips P, van Schoor NM, Rijs KJ, Deeg DJ, Comijs HC, Kramer MH, Vandenbroucke JP, Dekkers OM. 2011. Endogenous subclinical thyroid disorders, physical and cognitive function, depression and mortality in older individuals. Eur J Endocrinol 165:545–554 [DOI] [PubMed] [Google Scholar]
- 47. Patterson M, Lonie J, Starr JM. 2010. Thyroid function, cognition, functional independence and behavioural and psychological symptoms of dementia in Alzheimer's disease. Int J Geriatr Psychiatry 25:1196–1197 [DOI] [PubMed] [Google Scholar]
- 48. van Boxtel MP, Menheere PP, Bekers O, Hogervorst E, Jolles J. 2004. Thyroid function, depressed mood, and cognitive performance in older individuals: the Maastricht Aging Study. Psychoneuroendocrinology 29:891–898 [DOI] [PubMed] [Google Scholar]
- 49. Quinlan P, Nordlund A, Lind K, Gustafson D, Edman A, Wallin A. 2010. Thyroid hormones are associated with poorer cognition in mild cognitive impairment. Dement Geriatr Cogn Disord 30:205–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Prinz PN, Scanlan JM, Vitaliano PP, Moe KE, Borson S, Toivola B, Merriam GR, Larsen LH, Reed HL. 1999. Thyroid hormones: positive relationships with cognition in healthy, euthyroid older men. J Gerontol A Biol Sci Med Sci 54:M111–M116 [DOI] [PubMed] [Google Scholar]
- 51. Kalmijn S, Mehta KM, Pols HA, Hofman A, Drexhage HA, Breteler MM. 2000. Subclinical hyperthyroidism and the risk of dementia. The Rotterdam study. Clin Endocrinol (Oxf) 53:733–737 [DOI] [PubMed] [Google Scholar]
- 52. Tan ZS, Beiser A, Vasan RS, Au R, Auerbach S, Kiel DP, Wolf PA, Seshadri S. 2008. Thyroid function and the risk of Alzheimer disease: the Framingham Study. Arch Intern Med 168:1514–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vadiveloo T, Donnan PT, Cochrane L, Leese GP. 2011. The Thyroid Epidemiology, Audit, and Research Study (TEARS): morbidity in patients with endogenous subclinical hyperthyroidism. J Clin Endocrinol Metab 96:1344–1351 [DOI] [PubMed] [Google Scholar]
- 54. de Jong FJ, Masaki K, Chen H, Remaley AT, Breteler MM, Petrovitch H, White LR, Launer LJ. 2009. Thyroid function, the risk of dementia and neuropathologic changes: the Honolulu Asia Aging Study. Neurobiol Aging 30:600–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. de Jong FJ, den Heijer T, Visser TJ, de Rijke YB, Drexhage HA, Hofman A, Breteler MM. 2006. Thyroid hormones, dementia, and atrophy of the medial temporal lobe. J Clin Endocrinol Metab 91:2569–2573 [DOI] [PubMed] [Google Scholar]
- 56. Volpato S, Guralnik JM, Fried LP, Remaley AT, Cappola AR, Launer LJ. 2002. Serum thyroxine level and cognitive decline in euthyroid older women. Neurology 58:1055–1061 [DOI] [PubMed] [Google Scholar]
- 57. Hogervorst E, Huppert F, Matthews FE, Brayne C. 2008. Thyroid function and cognitive decline in the MRC Cognitive Function and Ageing Study. Psychoneuroendocrinology 33:1013–1022 [DOI] [PubMed] [Google Scholar]
- 58. Gussekloo J, van Exel E, de Craen AJ, Meinders AE, Frölich M, Westendorp RG. 2004. Thyroid status, disability and cognitive function, and survival in old age. JAMA 292:2591–2599 [DOI] [PubMed] [Google Scholar]
- 59. Wahlin A, Bunce D, Wahlin TB. 2005. Longitudinal evidence of the impact of normal thyroid stimulating hormone variations on cognitive functioning in very old age. Psychoneuroendocrinology 30:625–637 [DOI] [PubMed] [Google Scholar]
- 60. Annerbo S, Wahlund LO, Lökk J. 2006. The significance of thyroid-stimulating hormone and homocysteine in the development of Alzheimer's disease in mild cognitive impairment: a 6-year follow-up study. Am J Alzheimers Dis Other Demen 21:182–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Liposits Z, Paull WK, Wu P, Jackson IM, Lechan RM. 1987. Hypophysiotrophic thyrotropin releasing hormone (TRH) synthesizing neurons. Ultrastructure, adrenergic innervation and putative transmitter action. Histochemistry 88:1–10 [DOI] [PubMed] [Google Scholar]
- 62. Fekete C, Légrádi G, Mihály E, Huang QH, Tatro JB, Rand WM, Emerson CH, Lechan RM. 2000. α-Melanocyte-stimulating hormone is contained in nerve terminals innervating thyrotropin-releasing hormone-synthesizing neurons in the hypothalamic paraventricular nucleus and prevents fasting-induced suppression of prothyrotropin-releasing hormone gene expression. J Neurosci 20:1550–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Légrádi G, Lechan RM. 1999. Agouti-related protein containing nerve terminals innervate thyrotropin-releasing hormone neurons in the hypothalamic paraventricular nucleus. Endocrinology 140:3643–3652 [DOI] [PubMed] [Google Scholar]
- 64. Toni R, Jackson IM, Lechan RM. 1990. Thyrotropin-releasing-hormone-immunoreactive innervation of thyrotropin-releasing-hormone-tuberoinfundibular neurons in rat hypothalamus: anatomical basis to suggest ultrashort feedback regulation. Neuroendocrinology 52:422–428 [DOI] [PubMed] [Google Scholar]
- 65. Wittmann G, Sarkar S, Hrabovszky E, Liposits Z, Lechan RM, Fekete C. 2004. Galanin- but not galanin-like peptide-containing axon terminals innervate hypophysiotropic TRH-synthesizing neurons in the hypothalamic paraventricular nucleus. Brain Res 1002:43–50 [DOI] [PubMed] [Google Scholar]
- 66. Liao N, Vaudry H, Pelletier G. 1992. Neuroanatomical connections between corticotropin-releasing factor (CRF) and somatostatin (SRIF) nerve endings and thyrotropin-releasing hormone (TRH) neurons in the paraventricular nucleus of rat hypothalamus. Peptides 13:677–680 [DOI] [PubMed] [Google Scholar]
- 67. Brownstein MJ, Palkovits M, Saavedra JM, Bassiri RM, Utiger RD. 1974. Thyrotropin-releasing hormone in specific nuclei of rat brain. Science 185:267–269 [DOI] [PubMed] [Google Scholar]
- 68. Harkness DH, Brownfield MS. 1985. Intra and intersegmental distribution of thyrotropin releasing hormone (TRH) in rat spinal cord: topographical variation and presence in the dorsal horn. Soc Neurosci Abstr 11:349 [Google Scholar]
- 69. Palkovits M. 1984. Topography of chemically identified neurons in the central nervous system: progress in 1981–1983. In: Muller EE, McLeod RM, eds. Neuroendocrine perspectives. Amsterdam: Elsevier Science Publishers; 1–69 [Google Scholar]
- 70. Sawin CT, Geller A, Hershman JM, Castelli W, Bacharach P. 1989. The aging thyroid. The use of thyroid hormone in older persons. JAMA 261:2653–2655 [DOI] [PubMed] [Google Scholar]
- 71. Sawin CT, Geller A, Wolf PA, Belanger AJ, Baker E, Bacharach P, Wilson PW, Benjamin EJ, D'Agostino RB. 1994. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med 331:1249–1252 [DOI] [PubMed] [Google Scholar]
- 72. Parle JV, Maisonneuve P, Sheppard MC, Boyle P, Franklyn JA. 2001. Prediction of all-cause and cardiovascular mortality in elderly people from one low serum thyrotropin result: a 10-year cohort study. Lancet 358:861–865 [DOI] [PubMed] [Google Scholar]
- 73. Bauer DC, Ettinger B, Nevitt MC, Stone KL. 2001. Risk for fracture in women with low thyroid-stimulating hormone. Ann Intern Med 134:561–568 [DOI] [PubMed] [Google Scholar]
- 74. Brennan MD, Powell C, Kaufman KR, Sun PC, Bahn RS, Nair KS. 2006. The impact of overt and subclinical hyperthyroidism on skeletal muscle. Thyroid 16:375–380 [DOI] [PubMed] [Google Scholar]
- 75. Haugen BR. 2009. Drugs that suppress TSH or cause central hypothyroidism. Best Pract Res Clin Endocrinol Metab 23:793–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.