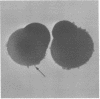
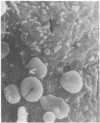
Abstract
Superficial cells from early amphibian embryos display regional specializations of their cell surfaces. That portion of the plasma membrane facing the perivitelline space (apical surface) is nonadhesive, whereas, in the same cell, the lateral and basal portions of the plasma membrane will adhere to other cells. These adhesive differences are maintained on single cells that have been dissociated from the intact embryo. Extracts of cleavage-stage Rana pipiens embryos are capable of agglutinating formalinized sheep erythrocytes. The hemagglutination activity can be blocked by a yeast mannan and a family of glycoproteins containing high levels of mannose, indicating the presence of a lectin with oligomannosyl specificity. The cell surface location of this carbohydrate-binding component can be demonstrated by the ability of the formalinized sheep erythrocytes to form rosettes with living dissociated embryonic superficial cells. Rosette formation is blocked by the same inhibitors that are effective in blocking the activity of the crude extracts. The formalinized sheep erythrocytes form rosettes only to those cell surface regions of the superficial cells that are capable of adhering to other amphibian embryo cells. Receptors for concanavalin A, a lectin that binds D-mannose and D-glucose residues, have also been shown to be present exclusively over the adhesive regions of the superficial cells. The involvement of a carbohydrate-binding component with oligomannosyl specificity in the adhesive mechanisms of these cells is suggested by this restriction of both the embryonic amphibian lectin and its possible receptors (concanavalin A receptors) to adhesive regions of the cell surface.
Keywords: nonadhesiveness, cell recognition, endogenous lectins, concanavalin A, membrane domains
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTLER W. T. HEMAGGLUTINATION STUDIES WITH FORMALINIZED ERYTHROCYTES. EFFECT OF BIS-DIAZO-BENZIDINE AND TANNIC ACID TREATMENT ON SENSITIZATION BY SOLUBLE ANTIGEN. J Immunol. 1963 May;90:663–671. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Dazzo F. B., Yanke W. E., Brill W. J. Trifolin: a Rhizobium recognition protein from white clover. Biochim Biophys Acta. 1978 Mar 20;539(3):276–286. doi: 10.1016/0304-4165(78)90032-6. [DOI] [PubMed] [Google Scholar]
- Gartner T. K., Williams D. C., Phillips D. R. Platelet plasma membrane lectin activity. Biochem Biophys Res Commun. 1977 Nov 21;79(2):592–599. doi: 10.1016/0006-291x(77)90198-x. [DOI] [PubMed] [Google Scholar]
- Goldstein I. J., So L. L. Protein-carbonhydrate interaction. 3. Agar gel-diffusion studies on the interaction of Concanavalin A, a lectin isolated from jack bean, with polysaccharides. Arch Biochem Biophys. 1965 Aug;111(2):407–414. doi: 10.1016/0003-9861(65)90203-1. [DOI] [PubMed] [Google Scholar]
- Grabel L. B., Rosen S. D., Martin G. R. Teratocarcinoma stem cells have a cell surface carbohydrate-binding component implicated in cell-cell adhesion. Cell. 1979 Jul;17(3):477–484. doi: 10.1016/0092-8674(79)90255-1. [DOI] [PubMed] [Google Scholar]
- Kobiler D., Beyer E. C., Barondes S. H. Developmentally regulated lectins from chick muscle, brain, and liver have similar chemical and immunological properties. Dev Biol. 1978 Jun;64(2):265–272. doi: 10.1016/0012-1606(78)90077-5. [DOI] [PubMed] [Google Scholar]
- Ofek I., Mirelman D., Sharon N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature. 1977 Feb 17;265(5595):623–625. doi: 10.1038/265623a0. [DOI] [PubMed] [Google Scholar]
- Roberson M. M., Armstrong P. B. Regional segregation of ConA receptors on dissociated amphibian embryo cells. Exp Cell Res. 1979 Aug;122(1):23–29. doi: 10.1016/0014-4827(79)90556-1. [DOI] [PubMed] [Google Scholar]
- Rosen S. D., Chang C. M., Barondes S. H. Intercellular adhesion in the cellular slime mold Polysphondylium pallidum inhibited by interaction of asialofetuin or specific univalent antibody with endogenous cell surface lectin. Dev Biol. 1977 Dec;61(2):202–213. doi: 10.1016/0012-1606(77)90292-5. [DOI] [PubMed] [Google Scholar]
- Rosen S. D., Simpson D. L., Rose J. E., Barondes S. H. Carbohydrate-binding protein from Polysphondylium pallidum implicated in intercellular adhesion. Nature. 1974 Nov 8;252(5479):128, 149-50. doi: 10.1038/252128a0. [DOI] [PubMed] [Google Scholar]
- Stahl P. D., Rodman J. S., Miller M. J., Schlesinger P. H. Evidence for receptor-mediated binding of glycoproteins, glycoconjugates, and lysosomal glycosidases by alveolar macrophages. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1399–1403. doi: 10.1073/pnas.75.3.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarentino A. L., Plummer T. H., Jr, Maley F. The release of intact oligosaccharides from specific glycoproteins by endo-beta-N-acetylglucosaminidase H. J Biol Chem. 1974 Feb 10;249(3):818–824. [PubMed] [Google Scholar]
- WRIGHT P. A., FLATHERS A. R. Facilitation of pituitary-induced frog ovulation by progesterone in early fall. Proc Soc Exp Biol Med. 1961 Feb;106:346–347. doi: 10.3181/00379727-106-26333. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Yamada S. S., Pastan I. The major cell surface glycoprotein of chick embryo fibroblasts is an agglutinin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3158–3162. doi: 10.1073/pnas.72.8.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]