Abstract
Yellow fever (YF) is a serious public health problem in Bolivia since at least the 19th century. Surprisingly, very limited information has been made available to date regarding the genetic characterisation and epidemiology of Bolivian YF virus (YFV) strains. Here, we conducted the genetic characterization of 12 human isolates of YFV collected in Bolivia between 1999 and 2008, by sequencing and analysis of two regions of the viral genome: a fragment encoding structural proteins “PrM” (premembrane and envelope) and a distal region “EMF,” spanning the end of the virus genome. Our study reveals a high genetic diversity of YFV strains circulating in Bolivia during the last decade: we identified not only “Peruvian-like” genotype II viruses (related to previously characterized Bolivian strains), but also, for the fist time, “Brazilian-like” genotype I viruses. During the complete period of the study, only cases of “jungle” YF were detected (i.e., circulation of YFV via a sylvatic cycle) with no cluster of urban cases. However, the very significant spread of the Aedes aegypti mosquito across Bolivian cities threatens the country with the reappearance of an urban YFV transmission cycle and thus is required a sustained epidemiological surveillance.
Key Words: Aedes, Epidemiology, Yellow fever
Introduction
Despite the availability of a safe and effective vaccine (17D), yellow fever (YF) remains a public health concern in tropical areas of Africa and South America. The virus responsible, YF virus (YFV), is a mosquito-borne flavivirus transmitted between susceptible vertebrate hosts by infected mosquitoes in the genera Aedes, Haemagogus, or Sabethes (Mutebi et al. 2001). In Africa, YFV is maintained endemically in equatorial moist forest and savannahs via sylvatic vectors. Transmitted by the urban vector Aedes aegypti, it may periodically emerge in human populations, causing severe outbreaks in urban zones of Africa. In South America, transmission of YFV is currently occurring mostly via a sylvatic cycle involving forest mosquitoes of Haemagogus and Sabethes species (Bryant et al. 2003, Mutebi et al. 2004) and presumably nonhuman primates such as Alouatta sp. monkeys. More than 200 cases of jungle YF are reported from South America each year (Barrett and Monath 2003). Most of them take place in Brazil, Peru, and Bolivia and involve most frequently men aged 15–45 years who are agricultural and forest workers. YF usually occurs from November to May (referred to as the humid season) and peaks during the first 3 months of the year, when populations of Haemagogus mosquitoes are highest during the rainy season (Barrett and Monath 2003).
Limited information has been available to date regarding the epidemiology of YF in Bolivia and the genetic characterization of Bolivian YF strains. Here, we performed a retrospective molecular characterization of human isolates from 1999 to 2008 collected by the CENETROP Microbiology Diagnostics Department. Phylogenetic reconstructions and analysis of available epidemiologic data were combined to further characterize YF distribution, evolution, and epidemiology during the study period.
Materials and Methods
Material studied
The CENETROP Microbiology Diagnostics Department received during the period 1999–2008 a large number of human clinical samples for suspected cases of YF.
They were tested for the presence of specific immunoglobulin M (IgM) to YFV by a standard Mac Elisa technique. Viral antigen was prepared from a lysate of Vero cells infected at high multiplicity by the Bolivian YFV strain JR35/99. A negative control was prepared similarly by using a lysate of noninfected Vero cells.
Amongst 427 patients with IgM antibody to YFV, a number of isolates could be made by direct inoculation of the serum onto C6/36 HT cells (Roche et al. 2000) in Eagle's Minimum Essential Medium (EMEM) medium at 34°C; culture supernatant was collected at day 5 postinfection, aliquoted, and conserved at −80°C.
Here, 12 selected YFV isolates made at CENETROP between 1999 and 2008 were studied (Table 1). Viral RNA was extracted from 200 μL of frozen material using the QIAamp viral RNA minikit (Qiagen) according to the manufacturer's recommendations.
Table 1.
Main Characteristics of Yellow Fever Virus Isolates Studied
| South American genotype | Isolate name | Month | Year | Sex | Department | City | Elevationa | Ecozone | Climate |
|---|---|---|---|---|---|---|---|---|---|
| Genotype I | Bolivia PrM 1266/02 2002b | June | 2002 | M | Santa Cruz | San Mathias | 100 | Plain | Tropical humid and hot |
| Bolivia PrM 2288/07 2007 | March | 2007 | M | La Paz | Alto Beni | 1200 | Wooded high plateau | Tropical humid and hot | |
| Genotype II, group A | Bolivia PrM 28/99 1999 | January | 1999 | M | Santa Cruz | Cabezas | 450 | Mountainous valley | Subtropical humid |
| Bolivia PrM 92/99 1999 | February | 1999 | F | Santa Cruz | Vallegrande | 2030 | Mountainous valley | Subtropical dry | |
| Bolivia PrM 323/99 1999 | May | 1999 | M | Santa Cruz | El Torno | 580 | Wooded plain | Tropical humid and hot | |
| Bolivia PrM JR35/99 1999 | March | 1999 | F | Santa Cruz | Bermejo | 400 | Mountainous valley | Subtropical dry | |
| Bolivia PrM 47 1/06 2006b | February | 2006 | M | Cochabamba | Villa Tunari | 300 | Wooded valley | Subtropical humid | |
| Genotype II, group B | Bolivia PrM 322/99 1999 | May | 1999 | M | Santa Cruz | Ciudad | 400 | Plain | Tropical humid and hot |
| Bolivia PrM 1026/02 2002 | May | 2002 | M | La Paz | Viacha | 3800 | High plateau | Subtropical dry and cold | |
| Bolivia PrM 452/05 2005 | March | 2005 | M | Cochabamba | Eterazama | 2500 | Wooded valley | Subtropical humid | |
| Bolivia PrM FBV115/05 2005b | April | 2005 | M | Cochabamba | Eterazama | 2500 | Wooded valley | Subtropical humid | |
| Bolivia PrM 408/06 2006 | February | 2006 | M | Cochabamba | Chipiriri | 2500 | Wooded valley | Subtropical humid |
Elevation: meters above sea level.
Persons deceased following YFV infection.
YFV, yellow fever virus.
Clinical and epidemiological data regarding YF infections in Bolivia from 1999 to 2008 were obtained from the CENETROP database.
Molecular characterization
Reverse transcription was performed using random hexamers and the TaqMan reverse transcription reagents kit (Applied Biosystems). Amplicons were generated using the Triplemaster PCR system kit (Eppendorf ) under standard polymerase chain reaction (PCR) amplification conditions. The first set of analyses involved amplification of a 716-bp fragment comprising the 3′ 108 nucleotides of the premembrane (PrM) protein gene, and the 5′ 359 nucleotides of the envelope (E) protein-coding gene. The amplicons were obtained using the genomic-sense primer YFV-PrM-S1 [TCAATGGARTACAAYTGTCC] and the genomic-complementary primer YFV-PrM-R1 [GTRAAYTTRGCRCASGCCACAAT] (this study). The second set of analyses involved amplification of a 694-bp fragment comprising the 3′ 300 nucleotides of NS5 at the end of the genomic ORF and the first 394 bp of the 3′ noncoding region. The primers used to amplify this region were the genomic-sense primer YFV-EMF-S2 [GGRARAGGRGAGTGGATGACCAC] (this study) and the genomic-complementary primer VD8-R [GGGTCTCCTCTAACCTCTAG] (Bryant and Barrett 2003). Amplified PCR products were visualized on electrophoresis gel and purified using the Qiagen PCR extraction kit.
Amplicons were subsequently sequenced using the amplification primers. Viral nucleotide sequences of each YF isolate were aligned using ClustalX (Thompson et al. 1997) together with relevant sequences retrieved from GenBank. Phylogenetic analyses were performed using two different methods. First, analyses were conducted with MEGA version 4.1 (Tamura et al. 2007) using the uncorrected p-distance for distance calculation, and neighbor-joining and 500 bootstrap replicates for construction of unrooted trees. Second, maximum likelihood (ML) analyses were carried out. The model of nucleotide substitution and parameter values were selected via Modeltest (Posada and Crandall 1998), and was used to estimate maximum liklihood (ML) phylogenetic trees in PAUP (Swofford 2000). Values for the substitution matrix, base composition, gamma distribution among site-rate variation (G) and the proportion of invariate sites (I) are available from the authors upon request (see contact information at the end of this article). A bootstrap resampling analysis was conducted using 1000 replicate neighbor-joining trees based on the ML substitution matrix described earlier, in PAUP (Swofford 2000) (Supplemental Figs. S1 and S2, are available upon request from the corresponding author.).
Few Bolivian YFV sequences were available on public databases: four in PrM and two in EMF, all belonging to South American genotype II (sequences in PrM: OBS7687, OBS7549, OBS7937, OBS8026 and sequences in EMF: OBS7687, OBS8026).
Results
Phylogenetic reconstructions
Recently, nucleotide sequencing studies of different genome regions delineated seven genotypes of YFV worldwide: five genotypes in Africa and two in South America (Wang et al. 1996, Mutebi et al. 2001, Bryant and Barrett 2003). The Brazilian and Peruvian YFVs represent the two major South American YFV genotypes I and II, respectively.
Here, we examined two regions of YFV genome, a fragment encoding structural proteins “PrM” (premembrane and envelope genes) and a distal region “EMF,” spanning the end of NS5 and a part of 3′UTR (Bryant and Barrett 2003), from Bolivian isolates.
Analysis of the PrM region
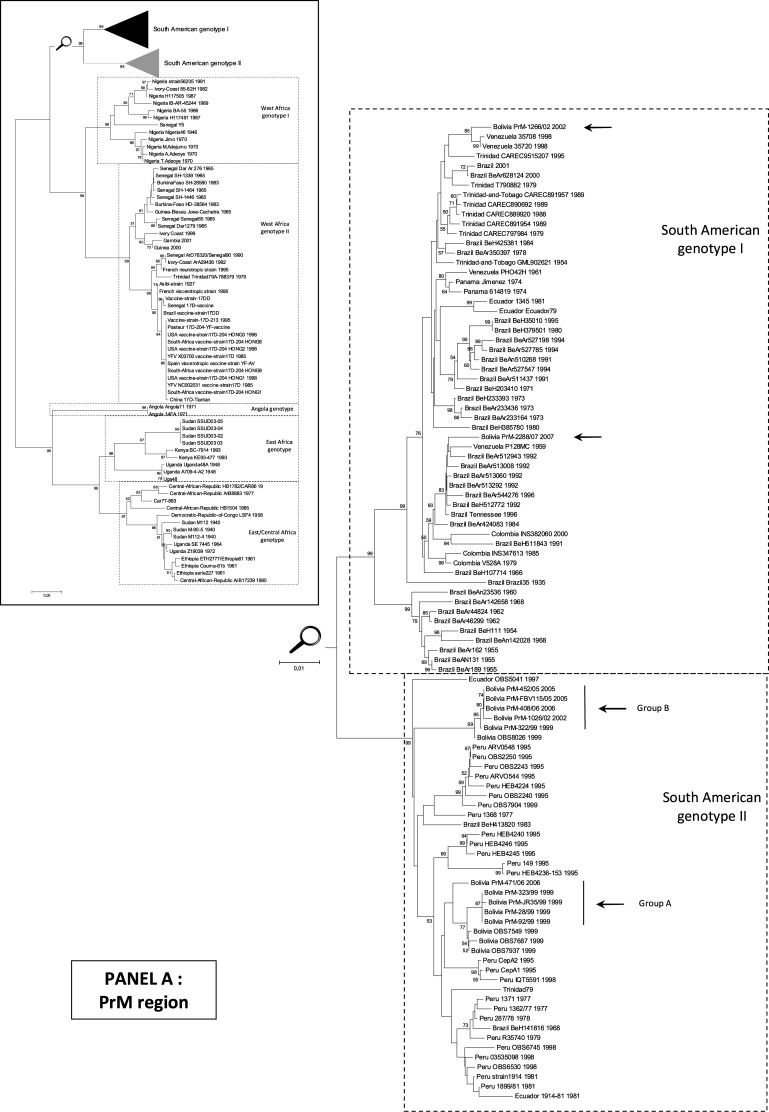
Phylogenetic analysis was performed, based on a 716-nt sequence in the PrM region and revealed (Fig. 1) that 10 (of the 12 Bolivian isolates studied) belonged to South American genotype II, in agreement with previous studies (Bryant et al. 2005). Within this genotype, Bolivian isolates were distributed into two distinct clusters (genetic distance between clusters: ∼0.04): one gathering isolates from 1999 and 2006, namely group A, and a second, group B, with isolates from 1999, 2002, 2005, and 2006 (Fig. 1). In both groups, the newly reported isolates were closely related (p-distances <0.002) and similar to Bolivian sequences previously reported and available in GenBank (p-distance <0.008). However, within group A, isolate 471/06 (2006) was more distantly related to other isolates (p-distance ∼0.015). Its sequence included in the N-terminal region of the PrM gene several synonymous nucleotide substitutions similar to Bolivian strains of group B. This isolate seems to represent a distinct evolutionary lineage inside group A rather than an evolution from 1999 isolates (92/99, JR35/99, 323/99, 28/99, OBS7549, OBS7687, OBS7937). Within group B, isolates had been collected in 1999, 2002, 2005, and 2006. Over this 7-year period, the genetic divergence observed was ∼0.2% and included synonymous mutations only.
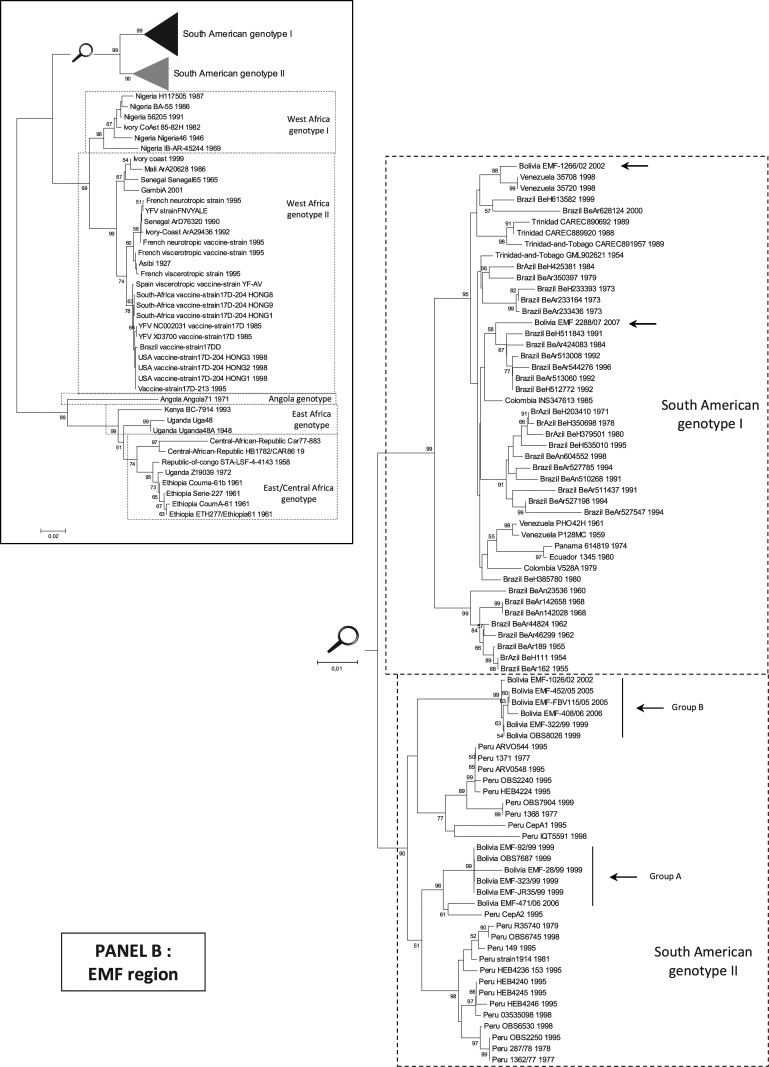
FIG. 1.
Phylogenetic analyses of YFV isolates in the “PrM” and “EMF” regions (nucleotide sequences). All trees presented were obtained using p-distance parameter algorithm for distance calculation, neighbor-joining, and 500 bootstrap replicates (values greater than 50% are indicated on major nodes branches). Horizontal bars are proportional to genetic distances. Identical topologies were obtained using the maximum likelihood methodologies (Supplemental Figs. S1 and S2). Panel A: PrM region. Phylogenetic tree reconstructed using YFV PrM-E gene sequences (nt 1–666 referring to the numbering of strain Venezuela_35708_1998, GenBank accession no. AY540490). To improve legibility, the reconstruction for the South American genotype I and II is presented after magnification. Panel B: EMF region. Phylogenetic tree reconstructed using YFV NS5-3′UTR gene sequences (nt 10097–10690 referring to the numbering of strain Asibi 1927, GenBank accession no. AY640589). To improve legibility, the reconstruction for the South American genotypes I and II is presented after magnification. YFV, yellow fever virus.
For the first time, two Bolivian isolates belonging to South American genotype I were identified. One isolate from 2002 was very similar to Venezuelan strains (p-distance <0.011). One isolate from 2007 was very similar to Brazilian and Venezuelan strains (p-distance 0.013–0.021). These two Bolivian isolates are distantly related (p-distance ∼0.036).
Sequence analysis confirmed that, within the PrM fragment, most of the sequences of genotype I (except BEH 107714 and BEH 511843) retained at position 178 the ancestral residue N (by homology with African YF strains) (Bryant and Barrett 2003). Within genotype II, an N to H substitution at this site was observed within group A, whereas group B retained the ancestral N residue (Table 2).
Table 2.
Genetic Ancestral Conservation of Yellow Fever Virus Strains
| |
|
G II amino acid |
|
|---|---|---|---|
| AA positiona | G I amino acid | G II, group A | G II, group B |
| 178 | N | H | N |
Amino acid conservation of ancestral African residue (in PrM region), within South American genotypes I and II, of YFV strains in this study (Bolivian strains studied included).
Based on PrM coding region, 680 nt.
G I, genotype I; G II, genotype II.
Notably, in the same year (2002), two Bolivian isolates belonging to two different genotypes (South American genotypes I and II) had circulated simultaneously, but in different areas.
Analysis of the EMF region
Phylogenetic tree of Bolivian YFV isolates based on the region encoding the EMF suggested a similar topology as those based on the region coding for the PrM. Isolates were distributed in the South American genotype II group A (92/99, JR35/99, 323/99, 28/99, 471/06, OBS7687) and B (322/99, 1026/02, FBV115/05, 452/05, 408/06, OBS8026) and in the South American genotype I (2288/07, 1266/02).
Geographical distribution of YF
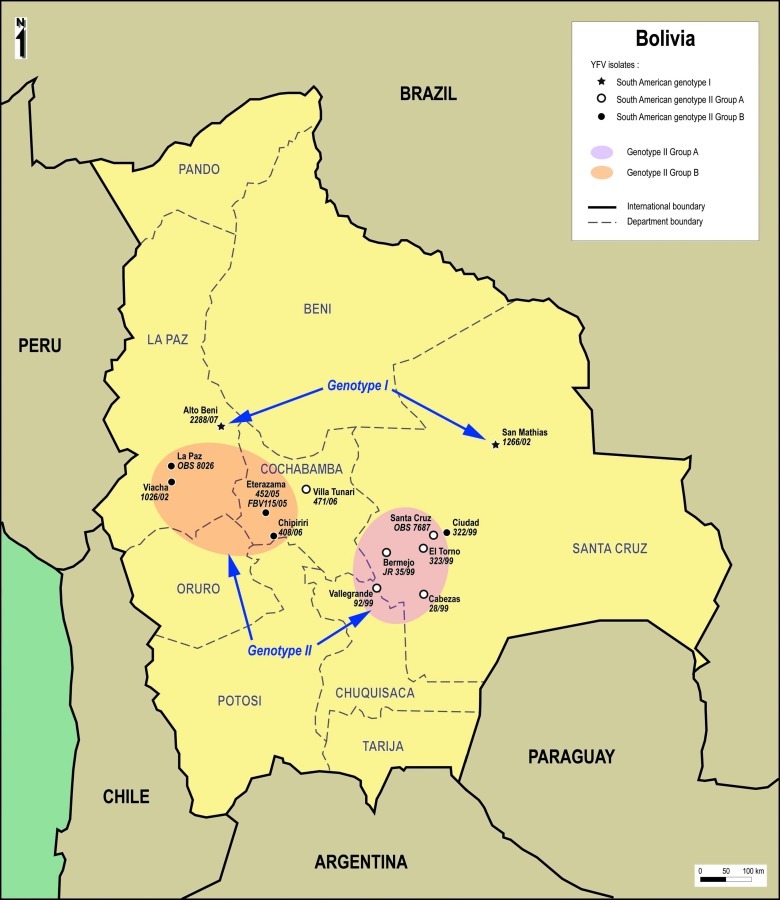
South American genotype I is mostly represented by Brazilian isolates, whereas genotype II contains principally isolates from Peru. Strategically situated between Brazil and Peru, Bolivia is a unique zone where viruses of the two genotypes can meet. Figure 2 shows the distribution of all the Bolivian isolates studied.
FIG. 2.
Geographical distribution of the Bolivian isolates studied.
Regarding South American genotype II (that includes all Bolivian YFV isolates previously identified), four group A isolates (all from 1999) grouped together geographically toward the eastern side of the country in the Department of Santa Cruz. The last group A isolate (471/06, 2006) was genetically distantly related from the other Bolivian group A isolates and originated from a region located more toward the west (Department of Cochabamba). Group B isolates were mostly located on the west side of the country in the Cochabamba and La Paz Departments, with one exception 322/99 outlying at the east (in the Department of Santa Cruz).
Regarding the two South American genotype I isolates (1266/02, 2288/07), both were located north of genotype II isolates, in the Santa Cruz and La Paz Departments, respectively.
Table 1 summarizes the geographical information available for each isolate studied. It shows that cases were observed for patient living in a broad range of altitudes (i.e., from 100 to 3800 m).
Incidence by month
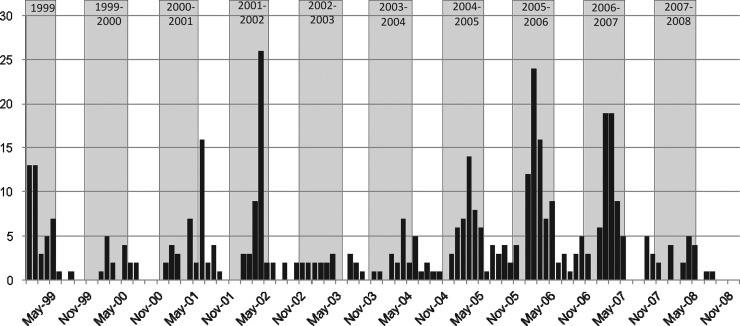
Figure 3 shows the distribution of positive IgM to YF tested by the CENETROP Microbiology Diagnostics Department between January 1999 and December 2008.
FIG. 3.
Incidence by month of confirmed cases of YF in Bolivia, 1999–2008.
It can be observed that a majority of cases occurred in the November to May period (i.e., during the humid season), with a peak during the first months of the year.
Discussion
History and ecological cycles
In this study, no evidence for urban transmission of YF in Bolivia over the period 1999–2008 was identified. No significant clusters of cases were reported during that period and a majority of infections occurred in young adult males (sex ratio m/f = 2.85, median age = 24), presumably reflecting greater outdoor exposure of boys.
This warrants further analysis since in the history of YF in Bolivia (with reports of presumed YF outbreaks as far back as 1867) (Bevier et al. 1953), both urban and jungle epidemic cycles of viral transmission have been reported. A. aegypti was the major vector incriminated in urban epidemics in South America. However, by 1943, A. aegypti had been virtually eliminated from all Bolivian territory and this led to the effective control of urban epidemics of YF (Bevier et al. 1953, Gubler 2001). Despite limited episodes of reinfestation by A. aegypti [e.g., 1948 (Bevier et al. 1953)], YF was therefore maintained in an enzootic forest cycle involving a jungle mosquito, of the genus Haemagogus (Bevier et al. 1953).
With the relaxation of control measures, A. aegypti reappeared in Santa Cruz de la Sierra in 1980 and was implicated in epidemics of dengue fever. The extension of dengue fever to a large part of the Bolivian territory [Departments of Santa Cruz, La Paz, Tarija, Cochabamba and Beni, (Roca et al. 2009)] indicates that A. aegypti has now spread broadly across Bolivia. Despite the presence of this potential urban vector of YF, no large urban YF outbreak occurred in recent years. Urban transmission of the virus remained limited in space and time (Van der Stuyft et al. 1998) and the virus seems to be confined to the sylvatic cycle.
Evidence to elucidate the absence of urban YF transmission is limited. Different geographical populations of mosquitoes have been shown to vary in their susceptibility to YFV (Gubler 1982), and thus it might be speculated that the mosquito populations that reinfested the Bolivian urban zones are poorly adapted to the transmission of Bolivian YFV isolates, which have been confined for decades in a jungle cycle involving distinct sylvatic mosquitoes. However, this hypothesis has been tested by others on Brazilian and Bolivian population of A. aegypti collected recently, and a significant competence for virus replication and transmission was observed (Johnson et al. 2002, Mutebi et al. 2004). Another hypothesis refers to herd immunity. It is unlikely that YF vaccination of the urban human population might explain the absence of urban YF in the presence of A. aegypti until 2007, since Bolivia showed low-vaccine immunization coverage (i.e., 35%–40%) in serosurvey studies performed in 1991 and 1997 (Van der Stuyft et al. 1999). By contrast, a YF vaccination campaign was conducted in 2007 and allowed to reach a vaccination coverage of 86% (Pezzoli et al. 2009), which is likely to be associated with significant prevention of YF spread in human communities. Prior to this recent vaccination campaign, the large outbreaks of dengue fever observed since the 80s may have played a role in the limitation of urban YF. Heterologous antibodies to dengue virus may modulate the susceptibility to YFV infection (Mutebi et al. 2004), as previously suspected in the case of Japanese encephalitis (Tarr and Hammon 1974).
In addition, it is possible that rapid changes in sociological behaviors and increasing urbanization may have limited exchanges between sylvatic and urban ecosystems, and therefore, the opportunity for YFV to move out of its sylvatic cycle.
Geographical distribution and virus circulation
In the current study cases of jungle YF reported appear to be related to a range of genetic variants of the virus. Cases involving South American genotype I isolates were observed in the Amazonian region of the Santa Cruz Department and in the tropical area of the La Paz Department. Cases involving South American genotype II isolates were observed south of South American genotype I isolates (Fig. 2). It is difficult from our data to determine the mechanism of persistence and/or emergence of the different variants since most of them were detected only once or during a unique year. Given their sylvatic origin and the absence of identified mechanism that may explain long distance transportation of viruses, it is probable that persistence occurs in a limited geographical zone and iterative introduction of new variants from abroad is unlikely. In support of this hypothesis, South American genotype II group B isolates were observed in a limited area encompassing the western part of the Cochabamba Department and the southern part of the La Paz Department in 1999, 2002, 2005, and 2006. In that specific case, it is clear that the virus was maintained locally in a sylvatic cycle for a 7-year period and this persistence was associated with limited genetic drift (∼0.2% in the PrM region over the complete period, without any nonsynonymous mutation). This is suggestive of the existence of enzootic foci and most likely suggests that YFV is either vertically maintained within arthropod populations or survives in a vertebrate host by a yet unidentified mechanism.
Different viral variants were identified in 1999, during which isolates were collected representing both South American genotype II, group A and B. Group A viruses were isolated in the Santa Cruz region and one group B virus was isolated in the same area, raising the possibility that both variants co-circulated in the same ecosystem. However, genetic analysis revealed that this group B isolate was closely related to isolates from the western region of the country and thus may correspond to an imported case in the Santa Cruz region. In 2002, isolates from both South American genotype I and genotype II were made but this occurred in distant geographical areas.
In conclusion, our study revealed that the genetic diversity of YFV strains circulating in Bolivia is more significant than previously understood. In particular, genotype I viruses were identified for the first time in addition to genotype II isolates previously detected. The different variants of the virus seem to be associated to some extent with their geographical origin and no strong evidence for the co-circulation of different variants in the same area was identified. Finally, all human cases identified by the CENETROP Microbiology Diagnostics Department were associated with an epidemiological context of jungle transmission and no evidence for urban viral circulation could be detected. Recent molecular analysis of samples collected from febrile Bolivian patients from the Cochabamba region with a clinical diagnosis of dengue fever has revealed that some of these cases were YF (authors' personal data). The 10-year follow-up of YF serological survey (enzyme-linked immunosorbent assay detection of specific IgM antibody) by the CENETROP suggests a sustained circulation of YF in Bolivia, with human cases peaking in the first months of the year. Despite its current apparent restriction to jungle transmission cycles, YF remains a serious and probably underestimated public health problem in Bolivia and the threat of the reappearance of an urban viral transmission by A. aegypti mosquitoes calls for future sustained surveillance.
Supplementary Material
Disclosure Statement
No competing financial interests exist.
References
- Barrett AD. Monath TP. Epidemiology and ecology of yellow fever virus. Adv Virus Res. 2003;61:291–315. doi: 10.1016/s0065-3527(03)61007-9. [DOI] [PubMed] [Google Scholar]
- Bevier G. Torres-Munoz N. Doria-Medina J. Yellow fever in Bolivia, its history and epidemiology. Am J Trop Med Hyg. 1953;2:464–482. doi: 10.4269/ajtmh.1953.2.464. [DOI] [PubMed] [Google Scholar]
- Bryant J. Wang H. Cabezas C. Ramirez G, et al. Enzootic transmission of yellow fever virus in Peru. Emerg Infect Dis. 2003;9:926–933. doi: 10.3201/eid0908.030075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant JE. Barrett AD. Comparative phylogenies of yellow fever isolates from Peru and Brazil. FEMS Immunol Med Microbiol. 2003;39:103–118. doi: 10.1016/S0928-8244(03)00238-4. [DOI] [PubMed] [Google Scholar]
- Bryant JE. Vasconcelos PF. Rijnbrand RC. Mutebi JP, et al. Size heterogeneity in the 3' noncoding region of South American isolates of yellow fever virus. J Virol. 2005;79:3807–3821. doi: 10.1128/JVI.79.6.3807-3821.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler DJ. Human arbovirus infections worldwide. Ann N Y Acad Sci. 2001;951:13–24. doi: 10.1111/j.1749-6632.2001.tb02681.x. [DOI] [PubMed] [Google Scholar]
- Gubler DJ. Novak R. Mitchell CJ. Arthropod vector competence-epidemiological, genetic and biological considerations. Proceeding of the International Conference on Genetics of Insect Disease Vectors; Bellagio, Italy. 1982. pp. 343–378. [Google Scholar]
- Johnson BW. Chambers TV. Crabtree MB. Filippis AM, et al. Vector competence of Brazilian Aedes aegypti and Ae. albopictus for a Brazilian yellow fever virus isolate. Trans R Soc Trop Med Hyg. 2002;96:611–613. doi: 10.1016/s0035-9203(02)90326-3. [DOI] [PubMed] [Google Scholar]
- Mutebi JP. Gianella A. Travassos da Rosa A. Tesh RB, et al. Yellow fever virus infectivity for Bolivian Aedes aegypti mosquitoes. Emerg Infect Dis. 2004;10:1657–1660. doi: 10.3201/eid1009.031124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutebi JP. Wang H. Li L. Bryant JE. Barrett AD. Phylogenetic and evolutionary relationships among yellow fever virus isolates in Africa. J Virol. 2001;75:6999–7008. doi: 10.1128/JVI.75.15.6999-7008.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzoli L. Pineda S. Halkyer P. Crespo G, et al. Cluster-sample surveys and lot quality assurance sampling to evaluate yellow fever immunisation coverage following a national campaign, Bolivia, 2007. Trop Med Int Health. 2009;14:355–361. doi: 10.1111/j.1365-3156.2009.02231.x. [DOI] [PubMed] [Google Scholar]
- Posada D. Crandall KA. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- Roca Y. Baronti C. Revollo RJ. Cook S, et al. Molecular epidemiological analysis of dengue fever in Bolivia from 1998 to 2008. Vector Borne Zoonot Dis. 2009;9:337–344. doi: 10.1089/vbz.2008.0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche RR. Alvarez M. Guzman MG. Morier L. Kouri G. Comparison of rapid centrifugation assay with conventional tissue culture method for isolation of dengue 2 virus in c6/36-HT cells. J Clin Microbiol. 2000;38:3508–3510. doi: 10.1128/jcm.38.9.3508-3510.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swofford DL. 4th. Sunderland, MA: Sinauer Associates; 2000. PAUP*. Phylogentic Analysis Using Parsimony (*and Other Methods) [Google Scholar]
- Tamura K. Dudley J. Nei M. Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Tarr GC. Hammon WM. Cross-protection between group B arboviruses: resistance in mice to Japanese B encephalitis and St. Louis encephalitis viruses induced by Dengue virus immunization. Infect Immun. 1974;9:909–915. doi: 10.1128/iai.9.5.909-915.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD. Gibson TJ. Plewniak F. Jeanmougin F. Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Stuyft P. Gianella A. Pirard M. Cespedes J, et al. Urbanisation of yellow fever in Santa Cruz, Bolivia. Lancet. 1999;353:1558–1562. doi: 10.1016/s0140-6736(99)03291-2. [DOI] [PubMed] [Google Scholar]
- Van der Stuyft P. Gianella A. Pirard M. Holzman A, et al. Short communication: dengue serotype 2 subtype III (‘Jamaica’) epidemic in Santa Cruz, Bolivia. Trop Med Int Health. 1998;3:857–858. doi: 10.1046/j.1365-3156.1998.00320.x. [DOI] [PubMed] [Google Scholar]
- Wang E. Weaver SC. Shope RE. Tesh RB, et al. Genetic variation in yellow fever virus: duplication in the 3' noncoding region of strains from Africa. Virology. 1996;225:274–281. doi: 10.1006/viro.1996.0601. [DOI] [PubMed] [Google Scholar]
- Zwickl DJ. Ph.D. dissertation. Austin: The University of Texas at Austin; 2006. Genetic Algorithm Approaches for the Phylogenetic Analysis of Large Biological Sequence Datasets Under the Maximum Likelihood Criterion. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.