Abstract
β1-containing integrins are required for persistent synaptic potentiation in hippocampus and regulate hippocampal-dependent learning. Based largely on indirect evidence, there is a prevailing assumption that β1-integrins are localized at synapses, where they contribute to synapse adhesion and signaling, but this has not been examined directly. Here, we investigate the fine localization of β1-integrin in adult mouse hippocampus using high-resolution immunogold labeling, with a particular emphasis on synaptic labeling patterns. We find that β1-integrins localize to synapses in CA1 and are concentrated postsynaptically. At the postsynaptic membrane, β1-integrins are found more commonly clustered near active zone centers rather than at the peripheral edges. In mice harboring a conditional deletion of β1-integrins, labeling for N-cadherin and Neuroligins increases. Western blots show increased levels of N-cadherin in total lysates and Neuroligins increase selectively in synaptosomes. These data suggest there is a dynamic, compensatory adjustment of synaptic adhesion. Such adjustment is specific only for certain cell adhesion molecules (CAMs), because labeling for SynCAM is unchanged. Together our findings demonstrate unequivocally that β1-integrin is an integral synaptic adhesion protein, and suggest that adhesive function at the synapse reflects a cooperative and dynamic network of multiple CAM families.
Indexing Terms: synaptic cell adhesion molecules, synaptic cleft, CAMs, synapse specificity, cadherin, neuroligins, synCAM
INTRODUCTION
β1-containing integrins (β1-integrins) are required for persistent long-term potentiation (LTP) in hippocampus (Chan et al., 2007; Chan et al., 2003; Chan et al., 2006; Huang et al., 2006; Kramar et al., 2006; Staubli et al., 1998), and coordinate synaptic structural and functional remodeling driven by extracellular metalloprotease activity (Wang et al., 2008). In the absence of β1-integrins working memory is impaired (Chan et al., 2006). Characterizing where, precisely, β1-integrins are localized is important for understanding how and where critical molecular signaling cascades operate to control consolidation of LTP (Kramar et al., 2006), spine dynamics (Wang et al., 2008) and working memory (Chan et al., 2003). Most models of β1 integrin function assume that β1-integrins are found at synapses and are concentrated postsynaptically, but this has never been demonstrated directly. It is possible, for example, that β1-integrins exert their influence indirectly via longer range signaling cascades triggered extrasynaptically in neurons or astrocytes. Distinguishing among these ideas requires ultrastructural localization but this has not been done.
Integrins are classically defined as adhesion receptors, and their interactions with extracellular matrix and cell adhesion molecules have been well-characterized (Chan and Davis, 2008; Humphries et al., 2009; Zaidel-Bar et al., 2007). Ligands such as laminin bind to integrins, which are anchored via intermediaries to the actin cytoskeleton. In this classic mode of action it is reasonable to propose that integrins participate in synaptic adhesion, providing critical structural support. Such adhesion would also be anticipated to be dynamic as integrin binding strength can be modulated by both intracellular and extracellular interactions. Ligand binding also initiates a variety of signalling pathways that can regulate cytoskeletal organization, cell shape, and transcription (Hood and Cheresh, 2002).
Integrin receptors are heterodimeric transmembrane proteins generated by alpha and beta subunits. Multiple combinations can generate more than twenty possible integrin receptors, but in rodent hippocampus in situ hybridization studies suggest the range is more limited (Pinkstaff et al., 1999) with many potential receptors having β1-integrin as an obligatory subunit. When β1-integrin is deleted from mature neurons, synapse size and number are unchanged, but synaptic transmission in response to high frequency stimuli is impaired as is long term potentiation (LTP), and mice show selective cognitive deficits in a working memory task (Chan et al., 2006; Huang et al., 2006). The impact on synaptic transmission and LTP are separable, driven by β1-integrin interactions with particular alpha subunits (Chan et al., 2007; Chun et al., 2001; Kramar et al., 2002).
In addition to integrins, several molecules that contribute to synapse adhesion have been identified, but those that are essential for generating synaptic structure remain unknown. This is due in part to the cooperative nature of synapse adhesion, such that the deletion of any single synapse adhesion protein, including β1-integrins, produces a surprisingly modest impact on synapse number and morphology (Chan et al., 2006; Huang et al., 2006; Robbins et al., 2010; Varoqueaux et al., 2006). In conditional β1-integrin knockout mice, synapse morphology and number are normal in CA1 (Chan et al., 2006; Huang et al., 2006). However, cooperative adhesion would predict that some synaptic cell adhesion molecules (CAMs) may assume alternate roles to compensate for a lost CAM.
In this study, we asked if β1-integrins were localized to hippocampal CA1 synapses, and then we determined the impact of β1-integrin deletion on synaptic cleft organization and the distribution of other synaptic adhesion proteins.
MATERIALS and METHODS
Animals
The conditional β1-integrin knockout mice used here have been described in detail previously (Chan et al., 2006). They were generated by crossing floxed β1-integrin mice (provided by E. Fuchs, Rockefeller University) and CaMKIIα Cre mice (provided by S. Tonegawa, Massachusetss Institute of Technology). Animals were maintained as outcrossed stocks to C57BL/6. Seven β1-integrin conditional knockout mice (Cre/+; floxedβ1/floxedβ1), eight littermate Cre control mice (Cre/+) and four littermate floxed control mice (floxedβ1/floxedβ1) on a C57Bl6 background were used for these studies (3 months of age). Mice were bred as heterozygotes and animals used in the study were littermates. In pilot studies, we found no differences in the distribution of β1-integrin immunolabeling in Cre/+ mice and wild type C57Bl6 mice. Animals were deeply anesthetized and either perfused transcardially with 4% paraformaldehyde/2% glutaraldehyde or sacrificed and their brains dissected for biochemical assays. All animals were treated in accordance with recommendations of the Baylor College of Medicine using protocols approved by the IACUC committee which complies with NIH guidelines.
Tissue Processing
The tissue was processed for immunoelectron microscopy using a freeze substitution protocol that we have described previously (Elste and Benson, 2006). Briefly, the aldehyde fixed brains were cryoprotected in 2.3M sucrose and sectioned on a vibratome at 350μm. CA1 regions were trimmed to 3mm squares and then rapidly frozen in liquid propane at −120°C. Blocks were transferred to methanol with 0.5% uranyl acetate, which was prechilled to −100°C, the temperature was gradually raised to −50°C over 3 days, and the blocks were slowly infiltrated with prechilled Lowicryl HM20 (Electron Microscopy Sciences (EMS), Hatfield, PA). Lowicryl was polymerized at −50°C, and the temperature was gradually raised to RT. Lowicryl blocks were sectioned on a Reichert Ultracut E ultramicrotome at the 70nm setting. Light gold sections were collected on formvar coated nickel grids. Digital images were taken on a Hitachi H7000 TEM at 80kV with an AMT side mount camera.
Immunolabeling
For immunolabeling, grids with ultrathin sections were immersed in phosphate buffered saline (PBS), pH 7.3, containing 0.1% TritonX-100 (Sigma, St. Louis, MO; PBST) and 0.05M glycine (Sigma) for 15 minutes. Grids were washed in PBST, blocked with Donkey Serum Blocking Solution (EMS) for 30 minutes, washed in incubation buffer (PBSI) containing 0.2% BSA-c (acetylated albumin; AURION BSA; Electron Microscopy Sciences), and incubated in primary antibody (see Table 1) prepared in PBSI overnight at room temperature. Grids were then washed in PBSI, incubated with colloidal gold conjugated secondary antibody, and postfixed with with 2% glutaraldehyde. When silver enhancement was performed, grids were washed in distilled water, incubated in Aurion R gent silver enhancement system according to the package directions for 10 minutes. Grids were then stained sequentially with 4% uranyl acetate (Sigma) prepared in 50% ethanol and 1% phosphotungstic acid (Sigma) prepared in 50% ethanol.
Table 1.
Primary Antibodies
| Antibody (citation) | Immunogen | Manufacturer (catalog No, clone) | Host Species/Isotype | Dilution (for Application) |
|---|---|---|---|---|
| Anti-β1-Integrin (Wilkins, et al. (1996) | whole β1 integrin affinity purified from Jurkat T-leukemic cell line | Millipore (MAB2252, clone N29) | Mouse Monoclonal/IgG | 1 to 20 |
| Anti-β1-integrin (Carter et al, 1990; Van der Pluijm et al, 2001; Nagy et al, 2006) Takada and Puzon, 1993 | epitope (aa 207–218) identified by inhibition of β1- integrin-mediated adhesion and epitope mapping | Millipore (MAB1987Z, clone P4C10) | Mouse Monoclonal/IgG2a | 1 to 60 |
| Anti-Neuroligin 1 (Song et al, 2006) | Recombinant protein of rat neuroligin 1 containing the extracellular sequence (aa1–695) fused to GST. | Synaptic Systems (129–011, clone 4F9) | Mouse Monoclonal/IgG2a | 1 to 750 |
| Anti-Neuroligin 1 (Taniguchi et al, 2007) | Recombinant protein of rat neuroligin 1 C-terminal tail fused to GST | gift of P. Scheiffele | Rabbit polyclonal | 1:1000 |
| Anti-SynCAM (Tsukita, et al., 2001) | SynCAM1 mouse peptide aa431–445 | Sigma (S4945) | Rabbit Polyclonal | 1 to 750 |
| Anti-N-Cadherin (Tanaka et al, 2000) | C-terminus of N-cadherin (aa 735 – 883) | gift of G Phillips, Mount Sinai, New York | Rabbit Polyclonal | 1 to 50 |
| Anti-N-cadherin | Mouse N-cadherin (aa802–819) | BD Biosciences (610921, clone 32N-cadherin | Mouse Monoclonal/IgG1 | 1:5000 |
Antibodies (Tables 1 and 2)
Table 2.
Secondary Anitbodies
| Name | Conjugated to | Manufacturer (catalog No.) | Dilution |
|---|---|---|---|
| Donkey Anti-Mouse | Colloidal Gold, 6nm | Aurion (25812) | 1 to 200 |
| Donkey Anti-Mouse | Colloidal Gold, 10 nm | Aurion (25814) | 1 to 200 |
| Goat anti-rabbit | Colloidal Gold, 6nm | Aurion (25104) | 1 to 200 |
| Goat anti-rabbit | Colloidal Gold, 10 nm | Aurion (25109) | 1 to 200 |
The β1-integrin antibodies that were used have been characterized using several approaches. Western blots reveal a single band of the expected size (e.g. P4C10: van der Pluijm et al., 2001; N29: Conant et al., 2004), an epitope has been mapped in β1-integrin (P4C10: Takada and Puzon, 1993), and functions known to require β1-integrins can be blocked (e.g. (P4C10: Carter et al., 1990; Nagy et al, 2006) or enhanced (N29: Wilkins et al, 1996). Most definitively, we show that immunogold labeling using either of the β1-antibodies was abolished at CA1 synapses taken from the hippocampi of mice in which β1-integrin was conditionally deleted in forebrain excitatory neurons (Chan et al., 2006) (Fig. 2 and text). Substituting primary antibody with an isotype specific IgG2a (matching P4C10) produced no detectable labeling at more than 100 synapses examined.
Figure 2. Immunogold labeling for synaptic CAMs at synapses lacking β1-integrins.
Immunogold labeling for SynCAMs (A, D), N-cadherin (B, E) and Neuroligins (C, F) in control mice (CON, Cre/+; ++) (A–C) and conditional β1-integrin knockouts (cKO) (D–F). As expected immunogold labeling is largely synaptic for all of them and qualitatively, there is no effect of β1-integrin ablation. Magnification bar = 100nm.
The SynCAM antibody used was generated against the C-terminal fragment of SynCAM1, a region that is highly homologous in SynCAM2 and -3 as well (Biederer, 2006). In Western blots of adult mouse hippocampal lysates, three major bands were evident and they were highly enriched in synaptosomes consistent with labeling for SynCAM1 (~100kD), SynCAM2 (~66kD), and SynCAM3 (~49kD) (Fig. 4), and consistent with previous reports in this journal (Fogel et al., 2007). The current study is the first in which this SynCAM antibody has been used for electron microscopy, but the largely synaptic concentrations that we observe are consistent with previous work carried out at the light and EM levels using other pan-SynCAM antibodies (Biederer et al, 2002).
Figure 4.

Increased levels of N-cadherin and Neuroligins in β1-integrin cKO. (A) Western blots of hippocampal lysates (L) and synaptosome fractions (S) taken from cKO, CON mice (Cre/+; ++) or floxed β1-integrin mice (floxβ1/floxβ1) (mixed littermates). N-cadherin appears in the lysates only in cKO, Neuroligin levels are increased in synaptosomes from cKOs only, and SynCAM remains unchanged (SynCAM 49 and SynCAM 100 refer to the sizes (kD) of the bands quantified and likely correspond to SynCAMs-3, and -1, respectively). PSD95 labeling was used to confirm the enrichment of synapses in the synaptosome fraction and tubulin was used as a loading control. (B) Film densitometry of synaptosome fractions shows Neuroligins to be significantly enriched in cKOs. Mean density was normalized to tubulin and expressed as a ratio to control. *p < .005 ANOVA, p<.05, Dunnett’s multiple comparison test.
The pan-Neuroligin antibody used recognizes Neuroligins-1, -3 and -4, but not Neuroligin-2. This has been demonstrated by several labs by immunostaining or Western blot using HEK293 or COS7 cells transfected with individual isoforms (Graf et al., 2004; Song et al., 1999). The banding pattern observed in Western blots of lysates from brain or hippocampus are consistent with this interpretation (Petralia et al., 2005; Song et al., 1999). Additionally it was reported that hippocampal neurons infected with lentiviruses expressing shRNAs targeting Neuroligins 1–3 abolished immunoreactivity in Western blots (Belichenko et al., 2009). Previous work has shown that immunogold labeling with this antibody concentrates at neocortical and hippocampal CA1 synapses and our findings are consistent with the previous work (Petralia et al., 2005; Song et al., 1999). Additionally we find that a second antibody against Neuroligins (gift of Peter Scheiffele) yields a similar immunogold labeling pattern and also recognizes a 100KDa band in Western blots (Fig. 4). Substituting primary antibody with an isotype specific secondary (IgG2a) produced no label (see above on Integrin antibodies).
The antibody against N-cadherin recognizes a single band of the appropriate size in Western blots from rat and mouse hippocampus (Phillips et al., 2001; Tanaka et al., 2000; Bozdagi et al, 2010). We have shown previously that immunogold labeling for N-cadherin using the same antibody used here is lost from CA1 synapses in mice having a conditional N-cadherin deletion in mouse forebrain (Bozdagi et al., 2010). Similarly, a band of the appropriate size for N-cadherin is lost in Western blots of hippocampal synaptosomes taken from conditional N-cadherin knockouts (Bozdagi et al., 2010).
To control for specificity of secondary antibodies, tissue was incubated with secondary antibody alone in the absence of primary. None of the secondaries used produced detectable labeling at synapses at the dilutions employed (Table 2).
Western Blots
Synaptoneurosomes were prepared as in (Chen DY et al., 2011) from 12 mice (4 Cre/+, 4 cKO and 4 floxedβ1/floxedβ1; 7 females and 5 males; all bred as hets and six were littermates). Hippocampi were rapidly dissected and frozen on dry ice. Tissue was homogenized in lysis buffer containing 10 mM HEPES, 2 mM EDTA, 2mM EGTA, 0.5 mM DTT, phosphatase and protease inhibitor cocktails (Sigma) using a glass-teflon homogenizer. To one-fifth volume of the homogenates, NaCl was added to the final concentration of 0.2 M, incubated for 30 min and centrifuged at full speed for 30 min. Supernatant was saved and used as total extracts for Western-blotting. Remaining homogenates were filtered through 100μm nylon mesh filter and 5μm nitrocellulose filters sequentially. Filtrates were centrifuged at 1000g for 10 min and pellets were dissolved in lysis buffer. Protein concentrations for synaptoneurosomal fractions and total lystates were determined using Bio-Rad protein assay (Bio-Rad Laboratories). 10 μg protein from each study group were resolved simultaneously on denaturing SDS-PAGE gels and transferred to Immune-Blot PVDF membranes (Bio-Rad) by Semi-Dry electroblotting. Membranes were blocked for 1 hour in 10% NBCS/TBST at room temperature and incubated in primary antibodies overnight at 4°C. Membranes were washed, treated with secondary horseradish peroxidase-labeled antibody 1 hr at room temperature and washed again. Membranes were then incubated with ECL detection reagents (Thermo Scientific) for 30 seconds and exposed to HyBlotCL (Denville Scientific) and developed. Films density was measured in Photoshop.
Staining
The ethanolic phosphotungstic acid (ePTA) stain was performed en bloc using a procedure modified from Bloom and Aghajanian (Bloom and Aghajanian, 1966). Vibratome sections, 350μm thick, were floated in 0.1M phosphate buffer (PB), post-fixed in 6% glutaraldehyde in PB for 2 hours, rinsed in PB, and left at 4°C overnight. They were dehydrated in a graded ethanol series and stained in 1% phosphotungstic acid (Fisher Scientific, Waltham, MA) in absolute ethanol at 50°C for 2 hours, and at RT another hour. They were washed in propylene oxide and incubated in a 1:1 mixture of propylene oxide and embed (E) 812 (Polysciences, Warrington, PA) overnight. They were then incubated in E812 for 2 hours, transferred to fresh E812, and flat embedded and polymerized at 60°C overnight. Sections were cut on a Reichert Ultracut E microtome with a diamond knife (EMS, Diatome) and mounted on copper grids.
The bismuth iodide (Bi) stain was also performed en bloc as described by Pfenninger (Pfenninger, 1971a). Briefly Bi was prepared by combining 0.5 g bismuth subcarbonate (EMS) with 2.5 g potassium iodide (EMS) in 50 ml 0.2 N formic acid at 50°C and then filtered. Sections were washed in 0.1M acetate buffer, pH 5.5 at 4°C then in 0.1M acetate buffer, pH 3.5 at 4°C and then stained overnight in the Bi solution at 4°C. After rinsing with acetate buffer, pH 3.5, sections were dehydrated in graded ethanols, followed by acetone, a 1:1 mixture of acetone:E812 overnight and then embedded and sectioned as described for ePTA. Sections were then stained with uranyl acetate and lead citrate as described previously (Elste and Benson, 2006).
Analysis
Neurolucida (Microbrightfield, Williston, VT) was used to map gold particle distribution along active zones on digital images as described previously (Elste and Benson, 2006). Magnification was calibrated using the scale bar on the image. For twenty synapses from each animal, pre- and postsynaptic membranes and the active zones (defined by the PSD) were traced and all gold particles lying within 30 nm were mapped to account for the displacement caused by the size of antibodies. Position along the length of active zones was extracted using NeuroExplorer (Microbrightfield) and exported to Excel (Microsoft, Redmond, WA). Distributions were compared as described in the text.
Percent synapses labeled was estimated for each animal from a random sample taken in CA1 area at 20,000X. One hundred synapses were counted per animal manually from the EM prints and total label was determined. Percent label was determined by dividing the number of labeled synapses over the number of total synapses X 100. Means were compared using t-tests.
RESULTS
β1-integrins cluster at postsynaptic densities
We used high-resolution, postembedding immunogold electron microscopy to determine the ultrastructural localization of β1-integrins in the CA1 region of mouse hippocampus. Immunogold labeling for β1- integrins was found at about 25% of the synapses (Fig. 1; 24% ± 1.9). Gold particles were most commonly observed in the synaptic cleft or lying over postsynaptic densities, with a lateral distribution that was constrained by active zones (Fig. 1A–C, arrows). On rare occasions, labeling was more broadly distributed along the lengths of synapse appositions (not shown) or within presynaptic terminals (Fig. 1A, arrowheads). The labeling pattern was similar when either of two different monoclonal antibodies against β1-integrin was used (Fig. 1A, B vs. C).
Figure 1. Synaptic distribution of β1-integrin labeling.
Immunogold labeling for β1-integrins in ultrathin sections using monoclonal antibody N29 (A, B) or p4C10 (C). Presynaptic terminals are shaded pink, and postsynaptic terminals, green. Gold particles are most commonly clustered at synaptic clefts (arrows) with a bias toward the postsynaptic density. Occasional particles are also found presynaptically (arrowheads in A). Dot plot (D) shows the distribution of β1-integrin immunogold labeling at synapses in all three control mice with respect to the peripheral edge (0) and center (0.5). Each synapse has been plotted along a line on the y axis. There is a distribution bias toward synapse centers. Magnification bar (A–C) = 500nm.
We verified the specificity of β1-integrin labeling by examining immunogold labeling in ultrathin sections through CA1 taken from adult mice in which β1-integrin was conditionally deleted in forebrain excitatory neurons. As described previously (Chan et al., 2006), these mice were generated by crossing a line of mice carrying a floxed β1-integrin allele with a line of mice expressing Cre recombinase driven by the CaMKIIα promoter. As expected, labeling for β1-integrins was scarce in the hippocampus from such conditional knockout (cKO) mice. Only four percent (± 1) of synapses were labeled, but those synapses that were labeled had typically only 1 particle. Based on these data, synapses labeled by single particles were excluded from all quantitative analyses.
β1-integrin localization along active zones
Like all adhesive junctions, synapses would be expected to have a characteristic organization of cell adhesion molecules such that some concentrate at centers and others at the periphery (Benson and Huntley, 2011). To test whether β1-integrins adopt particular positions along the lengths of active zones, positions of gold particles were mapped using Neurolucida. Distributions among synapses were then compared by plotting all points along a normalized hemisynapse with the edge equal to 0 and the center equal to 0.5. A display of all of the data points shows that β1-integrins can be found anywhere along the synapse (Fig. 1D), but they are more common at the center (67% of the gold particles fall within the central half vs. 33% in the periphery). The mean distance ± S.D. between a gold particle and the edge is 0.3 ± 0.12 μm.
β1-integrins influence synaptic adhesive composition
If synaptic CAMs act cooperatively, deletion of β1-integrins would be expected to affect other CAMs. To test this idea, we compared the relative levels and distribution of some of the prominent synapse cell adhesion molecules, N-cadherin, SynCAMs (using an antibody recognizing SynCAMs-1,-2, and-3) and Neuroligins (using an antibody recognizing Neuroligins-1, -3 and -4), at control synapses and those lacking β1-integrins (Fig. 2A–C vs D–F).
Qualitative patterns of labeling for each of the synaptic CAMs in the β1-integrin cKO synapses were similar to those of control synapses (Fig. 2A–F). Gold particle labeling for N-cadherin, Neuroligins or SynCAMs tends to be clustered within or near synaptic clefts, consistent with previous reports (Biederer et al., 2002; Elste and Benson, 2006; Petralia et al., 2005; Song et al., 1999; Yamagata et al., 1995). However, quantitative analyses showed that the overall percentage of synapses labeled for N-cadherin or for Neuroligins increases significantly in comparison with control synapses (Fig. 3A, control vs. cKO, t-test, p < 0.01, see legend for details). Additionally, at such labeled synapses, the number of gold-particles also increases (Fig. 3C,D). In contrast, there were no detectable differences in SynCAM labeling. In β1-integrin knockout CA1, the overall percentage of SynCAM-labeled synapses and the numbers of gold particles at such labeled synapses were similar in comparison with control CA1 (Fig. 3A,B).
Figure 3. Quantitative analysis of β1-integrin and other synaptic CAM labeling.
(A) The percentage of synapses immunogold-labeled for β1-integrins, SynCAMs, N-cadherin and Neuroligins in CON mice (gray bars) and cKO mice (white bars) ± sem. Labeling in CON and cKO was compared using t-tests (100 synapses/mouse in 3 – 4 mice), *p ≤ 0.01. Histograms (B–D) show percentage of immunolabeled synapses having the given immunogold particle numbers in CON and cKO mice. Antibodies used are indicated in graph titles. Abbreviations: β1-integrins (βint), N-cadherin (Ncad), Neuroligins (Nlgs).
Changes in gold particle density could result from changes in protein levels or in antibody accessability to antigen. To distinguish between these possibilities, we carried out Western blots on lysates and synaptosomes from hippocampi dissected from Cre control, floxed control or β1-integrin cKO brains. The data show no changes in N-cadherin levels in synaptosomes, but they reveal a dramatic increase in the level of N-cadherin in lysate fractions (Fig. 4A). These data suggest that increased numbers of gold particles at synapses may reflect changes in overall levels, but likely also reflect changes in accessability or an altered association of N-cadherin with synapses. In contrast, Neuroligins show a selective increase in synaptosome fractions (Fig. 4A,B), indicating that the increased Neuroligin levels seen by immunogold labeling reflect changes in protein levels.
Intrasynaptic Distribution of CAMs
Similar to the analysis carried out for β1-integrins, the distribution of labeling for the other CAMs was mapped in control and conditional β1-integrin knockouts. In control animals, N-cadherin immungold labeling showed no bias toward centers or edges similar to what has been reported previously in rats (Elste and Benson, 2006), while immunogold labeling for SynCAMs and Neuroligins showed a very modest bias toward the centers (Fig. 5). Conditional β1-integrin knockout mice showed no significant changes in any of the intrasynaptic distribution patterns, but pan-Neuroligins were modestly reduced at the periphery measured either by an increase in the mean distance between gold particles and the edge of the synapses (0.28 (control), 0.32 (cKO), t-test; p = 0.03) or by a decrease in the ratio of the number of particles at the periphery vs. the central half (0.71 (control), 0.43 (cKO), t-test; p = 0.08), but these changes were not statistically significant.
Figure 5. Stable distribution of gold particles at synapses lacking β1-integrins.
Dot plots permit qualitative comparison of immunogold particle distribution at synapses in con (Cre/+; ++) and cKO mice. Labeling convention is as in Figure 1D and antibodies used are indicated in titles. Neuroligin shows a modest decrease at the synapse periphery and an increase, centrally in cKOs.
β1-integrin-mediated structural changes
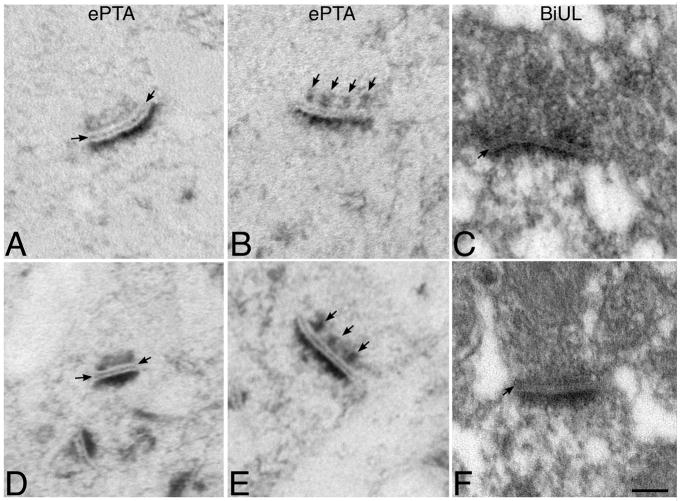
Since the numbers of gold-particles denoting N-cadherin and Neuroligin labeling are increased at synapses in mice lacking β1-integrins, we asked whether there were any corresponding changes in synaptic structure that might reflect such altered adhesive composition. Conventional EM staining protocols (osmium, uranyl acetate and lead citrate) label membranes and PSDs intensely, while cytoskeleton, cleft proteins and presynaptic dense projections are poorly labeled. Previous work has shown that in such conventionally stained EM tissue, postnatal, conditional deletion of β1-integrin in hippocampus produces no overt change in synapse morphology and no effect on synapse size in CA1 (Chan et al., 2006). When tissue is labeled for ethanolic phosphotungstic acid (ePTA), very little in brain is labeled except for synapses and dense projections, synaptic clefts, and PSDs, which stand out in sharp relief (Bloom and Aghajanian, 1966). Nervous system tissue stained with bismuth iodide/uranyl aceate/lead citrate (BiUL) shows more neuropil labeling than ePTA, but also labels synaptic cleft proteins, dense projections and PSDs, while membranes are unlabeled (Pfenninger, 1971a; b). Both BiUL and ePTA are thought to bind to basic residues (Bloom and Aghajanian, 1968; Pfenninger, 1971a). In control and β1-integrin cKO tissue, ePTA clearly concentrates in synaptic cleft lines, presynaptic dense projections and PSDs (Fig. 6A,B,D,E). Every element is not evident at every synapse, but quantitative analyses revealed no differences between groups. Similarly, no differences were observed between control and β1-integrin knockout tissue stained with BiUL (Fig. 6C,F). These data support that overall synapse structure remains intact in the absence of β1-integrins and apparent compensatory changes in other CAM families.
Figure 6. Synaptic structural stability in absence of β1-integrins.
Electron microscopic images of CA1 synapses in control mice (A–C) or conditional β1-integrin knockout mice (D–F) stained with ePTA (A, B, D, E) or BiUL (C, F). Arrows in A and C denote intercleft lines, and those in B and E point out presynaptic dense projections. All of the ePTA stained material shows prominent postsynaptic densities as well. Individual elements are more difficult to see with BiUL staining, but presynaptic densities, an intercleft line (arrow in C and F) and postsynaptic densities are clearly evident. In all images, the presynaptic terminal is oriented above the postsynaptic density. Magnification bar = 100nm.
DISCUSSION
We show here that β1-integrins concentrate postsynaptically at mature hippocampal CA1 synapses. Furthermore, genetic deletion of β1-integrins leads to significant changes in synaptic labeling for N-cadherin and Neuroligins, but no changes in labeling for SynCAMs. These findings suggest a targeted compensation for the loss of β1-integrins by particular CAMs. One speculative implication of these findings is that synaptic CAMs collectively operate more as a dynamic network of inextricably linked CAM families, rather than independent adhesive or signaling units.
While it has long been suspected that β1-integrins are bona fide synaptic proteins, there has been a surprising lack of direct evidence for this. The data presented here show principally postsynaptic immunogold labeling patterns using two different β1-integrin antibodies and that such labeling is eliminated at synapses in which β1-integrin expression has been abolished by a conditional deletion. These data therefore provide strong evidence for a direct, postsynaptic role for β1-integrins in adult hippocampal synaptic physiology and function.
β1-integrins can partner with several different α subunits, many of which are expressed in hippocampus (Pinkstaff et al., 1999). Comparisons between data obtained from conditional β1-integrin knockout mice and from mice lacking α3-integrin, suggest that α3β1 heterodimers can account for β1-integrin-related contributions to LTP and learning and memory (Chan et al., 2007; Chan et al., 2003; Huang et al., 2006). Physiological studies employing function blocking antibodies or using mice lacking both α3- and α5-integrin subunits further support the relevance of α3β1 heterodimers and indicate that α5β1 heterodimers can also contribute to β1-integrin-induced modifications in synaptic function (Chan et al., 2003; Chun et al., 2001). To our knowledge, there are no synaptic localization studies for α3-integrin, but consistent with the β1-integrin localization shown here, light-level studies show α5-integrins to be enriched in CA1 dendrites (Bi et al., 2001). Electron microscopic studies have also shown α8-integrin immunostaining to be localized to postsynaptic sites in dentate gyrus and CA3 regions of hippocampus, suggesting another potential partner for synaptic β1-integrins (Einheber et al., 1996).
Light and confocal level analyses have been used to localize and characterize a variety of synaptic CAMs, but similar data for β1-integrins have been historically absent. Consistent with this, we have failed to detect specific immunolabeling for β1-integrins using a variety of light microscopic techniques (data not shown). Since we can readily detect two different β1-integrin epitopes using a postembedding approach, it seems likely that antigenic epitopes on β1-integrins are commonly buried within synapses and only become accessible following ultrathin sectioning.
Immunogold labeling for β1-integrins is most commonly found nearer to the centers of synapses than to the edges and shows a postsynaptic bias. These data support that activated β1-integrins can signal directly into postsynaptic sites and furthermore, that such signals are likely to be in close proximity to GluAs and GluNs, which also display a more centralized location (Baude et al., 1995; Petralia et al., 1999; Racca et al., 2000) relative to metabotropic glutamate receptors (Luján et al., 1996). These findings are consistent with several indirect lines of evidence supporting a postsynaptic locus for integrin function in mature hippocampus. For example, β1-integrin blockers abrogate LTP-mediated changes in cofilin phosphorylation or F-actin clustering within spines (Kramar et al., 2006; Lin et al., 2005; Wang et al., 2008), and fibronectin stimulation in synaptosome preparations promotes GluN phosphorylation that is presumably postsynaptic (Bernard-Trifilo et al., 2005). The focus of this study was on synapses, however, and as such the data do not exclude the existence or potential relevance of additional extrasynaptic or astrocytic pools.
Labeling for N-cadherin and Neuroligins increased significantly at synapses in β1-integrin conditional knockouts suggesting that these CAMs may expand their functions in response to the loss of β1-integrins. Neuroligin levels are also increased in synaptosomes strongly supporting that increased gold particle labeling reflects changes in protein levels as has been shown previously for other synaptic proteins using similar, quantitative immunogold labeling approaches (Adams et al., 2001; Laake et al., 1999; Somogyi et al., 1986). On the other hand, N-cadherin levels did not increase in synaptosomes, but were more enriched in lysates. Thus, the increased levels seen by immunogold at synapses may reflect an increase in antibody access to antigen or it may be that some synaptic N-cadherin is less tightly affiliated with the synapse such that it fractionates with lysates. N-cadherin levels may also increase at nonsynaptic sites.
While not significant, there is a trend for Neuroligin labeling to be more centrally localized supporting the finding that CAMs compensate for one another. However, low numbers of gold particles per synapse make it difficult to draw strong conclusions about intrasynaptic distribution patterns. We think that this is due to a general limitation of the technique, which displays outstanding spatial resolution, but is less sensitive than other precipitate-based methods. A better approach to examine intrasynaptic distribution would seem to be freeze fracture replica labeling (Fujimoto, 1995; Masugi-Tokita et al., 2007; Rash et al., 2005; Tarusawa et al., 2009), but labeling for synaptic CAMs does not appear to be able to withstand the procedure.
Loss of β1-integrins in conjunction with apparent compensatory changes in N-cadherin and Neuroligins at synapses does not produce overt structural change. Three different synaptic stains, uranyl acetate and lead citrate, ePTA and BiUL indicate that basic synapse structure is intact (Chan et al., 2003; Huang et al., 2006, current study). Together with previous work, these data support a model in which β1-integrins are not necessary to generate or maintain normal synapse structure, but are critical for generating dynamic changes in synapse shape and physiology essential for synaptic plasticity, learning and memory (Chan et al., 2003; Huang et al., 2006; Staubli et al., 1998; Wang et al., 2008).
Acknowledgments
Grant sponsor: National Institutes of Health: NINDS NS037731 (DLB), NIMH MH075783 (GWH), and MH060420 (RLD).
We would like to acknowledge the members of the Benson lab for their helpful criticism and advice.
LITERATURE CITED
- Adams MM, Shah RA, Janssen WG, Morrison JH. Different modes of hippocampal plasticity in response to estrogen in young and aged female rats. Proc Natl Acad Sci U S A. 2001;98(14):8071–8076. doi: 10.1073/pnas.141215898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baude A, Nusser Z, Molnár E, McIlhinney RAJ, Somogyi P. High-resolution immunogold localization of AMPA type glutamate receptor subunits at synaptic and non-synaptic sites in rat hippocampus. Neuroscience. 1995;69:1031–1055. doi: 10.1016/0306-4522(95)00350-r. [DOI] [PubMed] [Google Scholar]
- Belichenko PV, Kleschevnikov AM, Masliah E, Wu C, Takimoto-Kimura R, Salehi A, Mobley WC. Excitatory-inhibitory relationship in the fascia dentata in the Ts65Dn mouse model of Down syndrome. J Comp Neurol. 2009;512(4):453–466. doi: 10.1002/cne.21895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson DL, Huntley GW. Building and remodeling synapses. Hippocampus. 2011 doi: 10.1002/hipo.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard-Trifilo JA, Kramar EA, Torp R, Lin CY, Pineda EA, Lynch G, Gall CM. Integrin signaling cascades are operational in adult hippocampal synapses and modulate NMDA receptor physiology. J Neurochem. 2005;93(4):834–849. doi: 10.1111/j.1471-4159.2005.03062.x. [DOI] [PubMed] [Google Scholar]
- Bi X, Lynch G, Zhou J, Gall CM. Polarized distribution of alpha5 integrin in dendrites of hippocampal and cortical neurons. J Comp Neurol. 2001;435(2):184–193. doi: 10.1002/cne.1201. [DOI] [PubMed] [Google Scholar]
- Biederer T. Bioinformatic characterization of the SynCAM family of immunoglobulin-like domain-containing adhesion molecules. Genomics. 2006;87(1):139–150. doi: 10.1016/j.ygeno.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, Sudhof TC. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297(5586):1525–1531. doi: 10.1126/science.1072356. [DOI] [PubMed] [Google Scholar]
- Bloom FE, Aghajanian GK. Cytochemistry of synapses: selective staining for electron microscopy. Science. 1966;154(756):1575–1577. doi: 10.1126/science.154.3756.1575. [DOI] [PubMed] [Google Scholar]
- Bloom FE, Aghajanian GK. Fine structural and cytochemical analysis of the staining of synaptic junctions with phosphotungstic acid. J Ultrastruct Res. 1968;22(5):361–375. doi: 10.1016/s0022-5320(68)90027-0. [DOI] [PubMed] [Google Scholar]
- Bozdagi O, Wang XB, Nikitczuk JS, Anderson TR, Bloss EB, Radice GL, Zhou Q, Benson DL, Huntley GW. Persistence of coordinated long-term potentiation and dendritic spine enlargement at mature hippocampal CA1 synapses requires N-cadherin. J Neurosci. 2010;30(30):9984–9989. doi: 10.1523/JNEUROSCI.1223-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter WG, Wayner EA, Bouchard TS, Kaur P. The role of integrins alpha 2 beta 1 and alpha 3 beta 1 in cell-cell and cell-substrate adhesion of human epidermal cells. J Cell Biol. 1990;110(4):1387–1404. doi: 10.1083/jcb.110.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CS, Davis RL. Integrins and cadherins--extracellular matrix in memory formation. In: Sweatt JD, editor. Molecular Mechanisms of Memory. Oxford: Elsevier; 2008. pp. 721–740. [Google Scholar]
- Chan CS, Levenson JM, Mukhopadhyay PS, Zong L, Bradley A, Sweatt JD, Davis RL. Alpha3-integrins are required for hippocampal long-term potentiation and working memory. Learn Mem. 2007;14(9):606–615. doi: 10.1101/lm.648607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CS, Weeber EJ, Kurup S, Sweatt JD, Davis RL. Integrin requirement for hippocampal synaptic plasticity and spatial memory. J Neurosci. 2003;23(18):7107–7116. doi: 10.1523/JNEUROSCI.23-18-07107.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CS, Weeber EJ, Zong L, Fuchs E, Sweatt JD, Davis RL. Beta 1-integrins are required for hippocampal AMPA receptor-dependent synaptic transmission, synaptic plasticity, and working memory. J Neurosci. 2006;26(1):223–232. doi: 10.1523/JNEUROSCI.4110-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DY, Stern SA, Garcia-Osta A, Saunier-Rebori B, Pollonini G, Bambah-Mukku D, Blitzer RD, Alberini CM. A critical role for IGF-II in memory consolidation and enhancement. Nature. 2011;469(7331):491–497. doi: 10.1038/nature09667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun D, Gall CM, Bi X, Lynch G. Evidence that integrins contribute to multiple stages in the consolidation of long term potentiation in rat hippocampus. Neuroscience. 2001;105(4):815–829. doi: 10.1016/s0306-4522(01)00173-7. [DOI] [PubMed] [Google Scholar]
- Conant K, St Hillaire C, Nagase H, Visse R, Gary D, Haughey N, Anderson C, Turchan J, Nath A. Matrix metalloproteinase 1 interacts with neuronal integrins and stimulates dephosphorylation of Akt. J Biol Chem. 2004;279(9):8056–8062. doi: 10.1074/jbc.M307051200. [DOI] [PubMed] [Google Scholar]
- Einheber S, Schnapp LM, Salzer JL, Cappiello ZB, Milner TA. Regional and ultrastructural distribution of the alpha 8 integrin subunit in developing and adult rat brain suggests a role in synaptic function. Journal of Comparative Neurology. 1996;370(1):105–134. doi: 10.1002/(SICI)1096-9861(19960617)370:1<105::AID-CNE10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Elste AM, Benson DL. Structural basis for developmentally regulated changes in cadherin function at synapses. J Comp Neurol. 2006;495(3):324–335. doi: 10.1002/cne.20876. [DOI] [PubMed] [Google Scholar]
- Fogel AI, Akins MR, Krupp AJ, Stagi M, Stein V, Biederer T. SynCAMs organize synapses through heterophilic adhesion. J Neurosci. 2007;27(46):12516–12530. doi: 10.1523/JNEUROSCI.2739-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto K. Freeze-fracture replica electron microscopy combined with SDS digestion for cytochemical labeling of integral membrane proteins. Application to the immunogold labeling of intercellular junctional complexes. J Cell Sci. 1995;108 ( Pt 11):3443–3449. doi: 10.1242/jcs.108.11.3443. [DOI] [PubMed] [Google Scholar]
- Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119(7):1013–1026. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2(2):91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- Huang Z, Shimazu K, Woo NH, Zang K, Muller U, Lu B, Reichardt LF. Distinct roles of the beta 1-class integrins at the developing and the mature hippocampal excitatory synapse. J Neurosci. 2006;26(43):11208–11219. doi: 10.1523/JNEUROSCI.3526-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries JD, Byron A, Bass MD, Craig SE, Pinney JW, Knight D, Humphries MJ. Proteomic analysis of integrin-associated complexes identifies RCC2 as a dual regulator of Rac1 and Arf6. Sci Signal. 2009;2(87):ra51. doi: 10.1126/scisignal.2000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramar EA, Bernard JA, Gall CM, Lynch G. Alpha3 integrin receptors contribute to the consolidation of long-term potentiation. Neuroscience. 2002;110(1):29–39. doi: 10.1016/s0306-4522(01)00540-1. [DOI] [PubMed] [Google Scholar]
- Kramar EA, Lin B, Rex CS, Gall CM, Lynch G. Integrin-driven actin polymerization consolidates long-term potentiation. Proc Natl Acad Sci U S A. 2006;103(14):5579–5584. doi: 10.1073/pnas.0601354103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laake JH, Takumi Y, Eidet J, Torgner IA, Roberg B, Kvamme E, Ottersen OP. Postembedding immunogold labelling reveals subcellular localization and pathway-specific enrichment of phosphate activated glutaminase in rat cerebellum. Neuroscience. 1999;88(4):1137–1151. doi: 10.1016/s0306-4522(98)00298-x. [DOI] [PubMed] [Google Scholar]
- Lin B, Kramar EA, Bi X, Brucher FA, Gall CM, Lynch G. Theta stimulation polymerizes actin in dendritic spines of hippocampus. J Neurosci. 2005;25(8):2062–2069. doi: 10.1523/JNEUROSCI.4283-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luján R, Nusser Z, Roberts JDB, Shigemoto R, Somogyi P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur J Neurosci. 1996;8:1488–1500. doi: 10.1111/j.1460-9568.1996.tb01611.x. [DOI] [PubMed] [Google Scholar]
- Masugi-Tokita M, Tarusawa E, Watanabe M, Molnar E, Fujimoto K, Shigemoto R. Number and density of AMPA receptors in individual synapses in the rat cerebellum as revealed by SDS-digested freeze-fracture replica labeling. J Neurosci. 2007;27(8):2135–2144. doi: 10.1523/JNEUROSCI.2861-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy V, Bozdagi O, Matynia A, Balcerzyk M, Okulski P, Dzwonek J, Costa RM, Silva AJ, Kaczmarek L, Huntley GW. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J Neurosci. 2006;26(7):1923–1934. doi: 10.1523/JNEUROSCI.4359-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Esteban JA, Wang YX, Partridge JG, Zhao HM, Wenthold RJ, Malinow R. Selective acquisition of AMPA receptors over postnatal development suggests a molecular basis for silent synapses. Nat Neurosci. 1999;2(1):31–36. doi: 10.1038/4532. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Sans N, Wang YX, Wenthold RJ. Ontogeny of postsynaptic density proteins at glutamatergic synapses. Mol Cell Neurosci. 2005;29(3):436–452. doi: 10.1016/j.mcn.2005.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfenninger KH. The cytochemistry of synaptic densities. I. An analysis of the bismuth lodide impregnation method. J Ultrastruct Res. 1971a;34(1):103–122. doi: 10.1016/s0022-5320(71)90007-4. [DOI] [PubMed] [Google Scholar]
- Pfenninger KH. The cytochemistry of synaptic densities. II. Proteinaceous components and mechanism of synaptic connectivity. J Ultrastruct Res. 1971b;35(5):451–475. doi: 10.1016/s0022-5320(71)80005-9. [DOI] [PubMed] [Google Scholar]
- Phillips GR, Huang JK, Wang Y, Tanaka H, Shapiro L, Zhang W, Shan WS, Arndt K, Frank M, Gordon RE, Gawinowicz MA, Zhao Y, Colman DR. The presynaptic particle web. ultrastructure, composition, dissolution, and reconstitution. Neuron. 2001;32(1):63–77. doi: 10.1016/s0896-6273(01)00450-0. [DOI] [PubMed] [Google Scholar]
- Pinkstaff JK, Detterich J, Lynch G, Gall C. Integrin subunit gene expression is regionally differentiated in adult brain. J Neurosci. 1999;19(5):1541–1556. doi: 10.1523/JNEUROSCI.19-05-01541.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racca C, Stephenson FA, Streit P, Roberts JD, Somogyi P. NMDA receptor content of synapses in stratum radiatum of the hippocampal CA1 area. J Neurosci. 2000;20(7):2512–2522. doi: 10.1523/JNEUROSCI.20-07-02512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE, Davidson KG, Kamasawa N, Yasumura T, Kamasawa M, Zhang C, Michaels R, Restrepo D, Ottersen OP, Olson CO, Nagy JI. Ultrastructural localization of connexins (Cx36, Cx43, Cx45), glutamate receptors and aquaporin-4 in rodent olfactory mucosa, olfactory nerve and olfactory bulb. J Neurocytol. 2005;34(3–5):307–341. doi: 10.1007/s11068-005-8360-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins EM, Krupp AJ, Perez de Arce K, Ghosh AK, Fogel AI, Boucard A, Sudhof TC, Stein V, Biederer T. SynCAM 1 adhesion dynamically regulates synapse number and impacts plasticity and learning. Neuron. 2010;68(5):894–906. doi: 10.1016/j.neuron.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Halasy K, Somogyi J, Storm-Mathisen J, Ottersen OP. Quantification of immunogold labelling reveals enrichment of glutamate in mossy and parallel fibre terminals in cat cerebellum. Neuroscience. 1986;19(4):1045–1050. doi: 10.1016/0306-4522(86)90121-1. [DOI] [PubMed] [Google Scholar]
- Song JY, Ichtchenko K, Sudhof TC, Brose N. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci U S A. 1999;96(3):1100–1105. doi: 10.1073/pnas.96.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staubli U, Chun D, Lynch G. Time-dependent reversal of long-term potentiation by an integrin antagonist. J Neurosci. 1998;18(9):3460–3469. doi: 10.1523/JNEUROSCI.18-09-03460.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y, Puzon W. Identification of a regulatory region of beta 1 subunit using activating and inhibiting antibodies. J Biol Chem. 1993;268(23):17597–601. [PubMed] [Google Scholar]
- Tanaka H, Shan W, Phillips GR, Arndt K, Bozdagi O, Shapiro L, Huntley GW, Benson DL, Colman DR. Molecular modification of N-cadherin in response to synaptic activity. Neuron. 2000;25(1):93–107. doi: 10.1016/s0896-6273(00)80874-0. [DOI] [PubMed] [Google Scholar]
- Taniguchi H, Gollan L, Scholl FG, Mahadomrongkul V, Dobler E, Limthong N, Peck M, Aoki C, Scheiffele P. Silencing of Neuroligin function by postsynaptic neurexins. J Neurosci. 2007;27:2815–2824. doi: 10.1523/JNEUROSCI.0032-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarusawa E, Matsui K, Budisantoso T, Molnar E, Watanabe M, Matsui M, Fukazawa Y, Shigemoto R. Input-specific intrasynaptic arrangements of ionotropic glutamate receptors and their impact on postsynaptic responses. J Neurosci. 2009;29(41):12896–12908. doi: 10.1523/JNEUROSCI.6160-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Pluijm G, Sijmons B, Vloedgraven H, van der Bent C, Drijfhout JW, Verheijen J, Quax P, Karperien M, Papapoulos S, Lowik C. Urokinase-receptor/integrin complexes are functionally involved in adhesion and progression of human breast cancer in vivo. Am J Pathol. 2001;159(3):971–982. doi: 10.1016/S0002-9440(10)61773-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Sudhof TC, Brose N. Neuroligins determine synapse maturation and function. Neuron. 2006;51(6):741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Wang XB, Bozdagi O, Nikitczuk JS, Zhai ZW, Zhou Q, Huntley GW. Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. Proc Natl Acad Sci U S A. 2008;105(49):19520–19525. doi: 10.1073/pnas.0807248105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins JA, Li A, Ni H, Stupack DG, Shen C. Control of beta1 integrin function. Localization of stimulatory epitopes. J Biol Chem. 1996;271:3046–3051. [PubMed] [Google Scholar]
- Yamagata M, Herman JP, Sanes JR. Lamina-specific expression of adhesion molecules in developing chick optic tectum. Journal of Neuroscience. 1995;15:4556–4571. doi: 10.1523/JNEUROSCI.15-06-04556.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9(8):858–867. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]