Abstract
We recently characterized Sprouty1 (Spry1), a growth factor signaling inhibitor as a regulator of marrow progenitor cells promoting osteoblast differentiation at the expense of adipocytes. Adipose tissue specific Spry1 expression in mice resulted in increased bone mass and reduced body fat while conditional knockout of Spry1 had the opposite effect with decreased bone and increased body fat. Because Spry1 suppresses normal fat development, we tested the hypothesis that Spry1 expression prevents high fat diet-induced obesity, bone loss, and associated lipid abnormalities and demonstrate that Spry1 has a long-term protective effect on mice fed a high caloric diet. We studied diet-induced obesity in mice with fatty acid binding promoter (aP2)-driven expression or conditional knockout of Spry1 in adipocytes. Phenotyping was performed by whole body dual-energy X-ray absorptiometry, microCT, histology and blood analysis. In conditional Spry1 null mice, high fat diet increased body fat by 40%, impaired glucose regulation, and led to liver steatosis. However, over-expression of Spry1 led to 35% lower body fat, reduced bone loss, and normal metabolic function compared to single transgenics. This protective phenotype was associated with decreased circulating insulin (70%) and leptin (54%) compared to controls on a high fat diet. Additionally, Spry1 expression decreased adipose tissue inflammation by 45%. We show that conditional Spry1 expression in adipose tissue protects against high fat diet-induced obesity and associated bone loss.
Keywords: Sprouty, high fat diet, body fat, obesity, bone loss
BACKGROUND
Obesity is a major health problem worldwide with over a billion people overweight and 300,000,000 diagnosed clinically obese. The morbidity surrounding obesity relates to an array of metabolic abnormalities including insulin resistance, hypertension and hyperlipidemia (i.e. “metabolic syndrome”) [1]. In the context of bone, several studies have shown that the metabolic syndrome is associated with a greater risk of fracture [2], and adipocyte accumulation in the bone marrow has been associated with age-related osteoporosis [3-5]. Moreover, abnormalities in glucose, lipid, and fluid homeostasis contribute to cardiovascular disease and type 2 diabetes mellitus [6]. Research over the past decade has shown that obesity is a result of complex interaction among genes, physiology, behavior, socio-cultural factors, and the environment. Yet, diet plays an important role in the pathogenesis of obesity and high fat/caloric diet is the most common factor contributing to rapid weight gain and accumulation of fat tissue. Excessive caloric intake can initiate obesity in susceptible individuals and provoke metabolic and cardiovascular disorders stemming from cellular stress due to chronic low-grade inflammation [1]. However, the molecular and genetic factors that ultimately determine the magnitude of obesity and the development of co-morbid conditions are still not well defined.
While osteoblasts and adipocytes arise from mesenchymal stem cells (MSC), a common progenitor cell type located in bone marrow, adipose tissue and other adult organs [7], the lineage commitment of multipotent MSCs depends on several factors including growth factor signaling. Adipogenesis is regulated by several growth factors starting from determination, commitment to preadipocytes and lipid accumulation stages [8] involving several receptor kinase signaling pathways such as EGF, FGF, VEGF and IGF [9,10]. Sprouty and Spred (Spry-related protein) family proteins are evolutionarily conserved inhibitors of receptor tyrosine kinase (RTK) signaling [11-13]. Spry regulates several signaling pathways, and affects common signal mediators, i.e. MAPK, by suppressing the RAS/MAPK pathway and generating a negative feedback loop during development [14]. Spry1 gene is expressed in virtually all mouse tissues including adipose and bone marrow, there are no reports describing a role for Spry in adipose tissue [15].
Previously, we reported that Sprouty1 (Spry1) functions as a regulatory switch modulating mesenchymal stem cell lineage commitment altering the balance between body fat and bone in mice [16]. In mice, tissue-specific Spry1 deletion using the fatty acid binding protein promoter (aP2Spry1-KO) lowered bone mass, increased body fat, and led to a phenotype analogous to symptoms associated with diabetes, the metabolic syndrome, and osteoporosis. Conversely, over expression of Spry1 using the aP2 promoter (aP2-Spry1) suppressed fat development and improved bone health. Additionally, aP2-Spry1 mice did not lose bone or develop metabolic disorders associated with aging. Given the role of Sprouty in suppressing adipocyte differentiation, we tested if Spry1 expression could prevent high fat diet induced obesity metabolic dysfunction and bone loss.
METHODS
Mouse models
All experiments involving mice were approved by the Institutional Animal Care and Use Committee (IACUC) at Maine Medical Center Research Institute (MMCRI, 1003). Transgenic aP2-Cre (B6.Cg-Tg(Fabp4-cre)1Rev/J, stock 005069) mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). The Spry1 transgenic mouse [17] and the conditional targeted Spry1 null allele [18] have been previously characterized. The double transgenic aP2-CreSpry1 experimental group is referred to as aP2-Spry1. Controls for comparison were single transgenic aP2-Cre littermates. Genotyping primers and protocols were previously described [16]. The Cre-mediated conditional Spry1 null deletion was obtained by crosses to aP2-Cre, and are referred to as aP2-Spry1-KO, with aP2-Cre mice with wild type Spry1 as control. Since the Spry1 transgenic mouse and conditional targeted Spry1 null allele were on different genetic backgrounds, FVB and C57BL/6 respectively, we maintained separate controls expressing aP2-Cre for the two groups. Consistent with our data showing a higher baseline weight and percentage body fat after high fat feeding (Fig. 1), comparison of strains has shown that compared to the FVB strain, mice on a C57BL/6 background have lower baseline body weight and percentage body fat, and also have lower percentage body fat following a high fat diet (personal communication, J Naggert, KL Svenson, RV Smith, B Paigen and LL Peters). At 5 weeks of age, mice were weaned and grouped based on their gender and genotype as controls and experimental. Each group had a minimum of 15 mice and mice were housed 3-4 /cage and allowed to feed ad libitum until 20 weeks of age. Diets were standard chow diet (10% kJ fat, D12450B, hereto referred to as normal diet) or high fat diet (45% kJ fat, D12451, Research Diets, New Brunswick, NJ, USA), the compositions of which are as follows:
| Normal diet | High Fat diet | |
| Protein | 20 | 20 |
| Carbohydrate | 70 | 35 |
| Fat | 10 | 45 |
| % energy | 100 | 100 |
| Ingredient in kJ (kcal) | ||
| Casein | 3347.2 (800) | 3347.2 (800) |
| L-Cystine | 50.2 (12) | 50.2 (12) |
| Corn Starch | 5271.84 (1260) | 1217.55 (291) |
| Maltodextrin | 585.7 (140) | 1673.6 (400) |
| Sucrose | 5857 (1400) | 2891.1 (691) |
| Soybean Oil | 941.4 (225) | 941.4 (225) |
| Lard | 753.1 (180) | 6686 (1598) |
| Vitamin Mix V10001 | 167.3 (40) | 167.3 (40) |
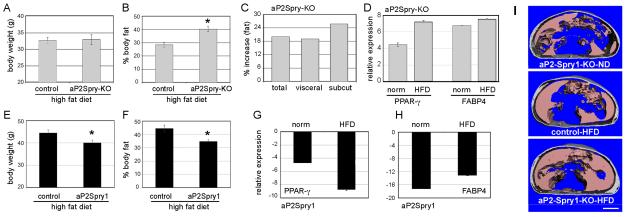
Figure 1. Spry1 expression protects high fat diet induced fat accumulation. Mice were fed a high fat diet for 15 weeks.
(A-D) aP2Spry-KO mice and corresponding controls are shown. Average body weight (A) and percentage body fat (B) for the aP2Spry-KO and corresponding control groups at 20 weeks of age. Body fat calculations were determined by whole-body densitometry (DXA, n≥10). Asterisk indicates p<0.05. C) In vivo microCT image analysis for abdominal fat deposition of the aP2Spry-KO (n≥4) shows increased total, visceral, and subcutaneous (subcut) fat compared to controls on a high fat diet. D) Changes in mRNA transcript levels of adipocyte markers PPARγ and FABP4 were quantified in the abdominal fat depots by quantitative RT-PCR. Shown are the aP2Spry-KO groups on both a normal diet (norm) or high fat diet (HFD). (E-H) aP2Spry1 transgenic and corresponding controls at 20 weeks of age on the high fat diet (n≥10). Total body weight (E) and percentage body fat, quantified by DXA (F), were significantly decreased in the aP2Spry1 transgenics. Asterisks indicate p<0.05. Quantitative RT-PCR analysis was used to assess PPAR-γ (G) and FABP4 (H) expression in the abdominal adipose tissue of aP2Spry1 transgenics compared to corresponding controls. I) transverse sections of microCT imaged abdomen from aP2-Spry1-KO and control mice at 20wk of age showing localization of subcutaneous and visceral adipose tissue. Bar indicates 5mm.
Densitometry
Total body dual energy X-ray absorptiometry scans were conducted using a peripheral instantaneous X-ray imager (GE Lunar PIXImus, GE Healthcare, WI, USA). Mice at 20 weeks were weighed and anesthetized with Avertin (tribromoethanol, 0.2ml of 1.2% solution/10g body wt). Data were analyzed for whole body tissue as well as a region of interest (11 × 35 voxels) fixed at the central diaphysis of the left femur. Each study group of the transgenic or targeted strain was n≥8. Precision of readings was determined and the standard deviation as % of mean was <2%.
MicroCT analysis
The femurs were dissected, cleaned of soft tissue fixed and stored in 70% ethanol at 4°C until analysis. Bones were loaded into 12.3mm diameter scanning tubes and imaged using a vivaCT 40 scanner (SCANCO Medical AG, Brüttisellen, Switzerland). Scans were integrated into three dimensional voxel images (2048 × 2048 pixel matrices for trabecular and 1024 × 1024 pixel matrices for all other individual planar stack). A threshold of 220 was applied to all scans at high resolution (E=55kVp, I =145μA, integration time =300ms). All trabecular measurements were made by drawing contours every 20 slices and using voxel counting for bone volume per tissue volume and sphere filling distance transformation indices. Cortical thickness was measured at the femoral mid-diaphysis. The mice anesthetized with Avertin for densitometry scans were used for in vivo abdominal adiposity scanning to avoid multiple anesthetic administrations. All procedures and protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Maine Medical Center. The torso was scanned at an isotropic voxel size of 76 microns (45kV, 177μA, 300ms integration time) with a vivaCT 40 scanner. Two-dimensional gray-scale image slices were reconstructed into a three-dimensional tomography. Scans were reconstructed between the proximal end of L1 and the distal end of L5. The head and feet were not scanned and/or evaluated because of the relatively low amount of adiposity in these regions, and to allow for a decrease in scan time and radiation exposure to the animals. The region of interest for each animal was defined based on skeletal landmarks from the gray-scale images. Precision of readings was determined and the standard deviation as % of mean was <2%.
Metabolic Parameters
Blood glucose tolerance was measured on overnight fasted mice administered 1g glucose/kg body weight by intraperitoneal (i.p) injection and glucose clearance recorded at 0, 15, 30, 60, and 120 minutes. Blood collected terminally was used to analyze plasma levels of insulin and leptin using ELISA based assay kits (Alpco Diagnostics, NH, USA, #80-INSMS-E01 and #22-lepms-E01 respectively).
Tissue processing
Tissue samples were snap frozen for RNA, extracted using TriReagent (Sigma-Aldrich Corp., St. Louis, MO, USA) and reverse transcribed using the qScript cDNA supermix (Quanta BioScience, Gaithersburg, MD, USA) for RT-PCR analysis. For histology, fresh tissue samples were fixed in 4% paraformaldehyde and processed. For immunostaining, paraffin sections were deparaffinized, antigen recovered using DakoCytomation Target Retrieval solution (DAKO, Denmark) and blocked in 2% bovine serum albumin. Primary antibodies were used at recommended dilutions, followed by detection using secondary antibody. Antibodies were anti-F4/80 (BioLegend, San Diego, CA, USA) and anti-PECAM-1 (BD Pharmingen, BD BioScience, USA).
Quantitative RT-PCR
Primer sequences and PCR conditions were previously described [16].
PPARγ1+2: Forward primer 5′ TCATCTCAGAGGGCCAAGGA 3′
Reverse primer 5′ CACCAAAGGGCTTCCGC 3′
FABP4: Forward primer 5′ TGGAAGCTTGTCTCCAGTGA 3′
Reverse primer 5′ AATCCCCATTTACGCTGATG 3′
β-actin: Forward primer 5′ GGAGGAAGAGGATGCGGCA 3′
Reverse primer 5′ GAAGCTGTCCTATGTTGCTCTA 3′
Cyclophilin: Forward primer 5′ CTCGAATAAGTTTGACTTGTGTTT 3′
Reverse primer 5′ CTAGGCATGGGAGGGAACA 3′ Total RNA was extracted from cells using Tri-reagent and reverse transcribed using the qScript cDNA supermix (Quanta BioScience, Gaithersburg, MD). Real time quantitative PCR was performed on the iQCycler (BioRad LifeScience, Hercules, CA, USA) using the iQ-SYBR green supermix (BioRad LifeScience, Hercules, CA, USA). All runs were done in duplicates and results are an average of 3 separate runs and quantification performed by normalizing to β-actin or cycophilin gene expression and further to control tissues.
Statistical analysis
All data are reported as the mean ± standard deviation. Group mean values were compared, as appropriate, by Student’s unpaired two-tailed t test. A p value of ≤0.05 was considered significant.
RESULTS
Spry1 expressing mice have lower body fat on a high fat diet
On the high fat diet, mice in all groups gained weight. The aP2Spry-KO and their control groups had a 5-10% increase in weight over their corresponding normal diet-fed mice with similar average weights (Fig. 1A; control=32.65g, aP2Spry-KO=32.86g). Although the final body weights were not significantly different between the controls and experimental group, percentage body fat was considerably altered. The aP2Spry-KO mice on the high fat diet showed 40% (p<0.05) increase in body fat over the controls (Fig. 1B). In vivo whole-body scan for abdominal fat deposition using microCT confirmed the changes in the aP2Spry-KO mice showing ~20% increase in total, visceral and subcutaneous fat over controls on a high fat diet (Fig. 1C and 1I). This increase in fat accumulation in the aP2Spry-KO was associated with increased expression of adipogenic markers PPAR-γ and FABP4 (Fig. 1D).
In the Spry1 gain-of-function group, the weight gain in the controls and aP2-Spry1 was ~15% (p<0.05) over normal diet-fed mice; however, the weight gain in aP2-Spry1 mice was significantly less than the single transgenic controls (Fig 1E). The difference in body weight and body composition between the control groups for the Spry1 transgenic mouse and conditional targeted Spry1 null allele is due to their different genetic backgrounds and therefore each group has its own control set on the matching genetic background. In the aP2-Spry1 mice, body fat was 35% (p<0.05) lower compared to the single transgenic controls on a high fat diet (Fig. 1F) indicating that mice expressing Spry1 in the fat tissue were protected from excessive fat accumulation. Additionally, there were fewer fat cells in the abdominal fat of Spry1 expressing mice compared to controls suggesting diminished hyperplasia (see also Fig. 4B). Over expression of Spry1 in adipocytes was associated with decreased expression of the adipogenic markers PPAR-γ (Fig. 1G) and FABP4 (Fig. 1H) both on a normal and a high fat diet. Therefore, while loss of Spry1 promotes adipose accumulation, transgenic Spry1 expression in adipocyte lineages has a significant protective effect in reducing hyperplasia and hypertrophy of adipose tissue even during consumption of a high fat diet.
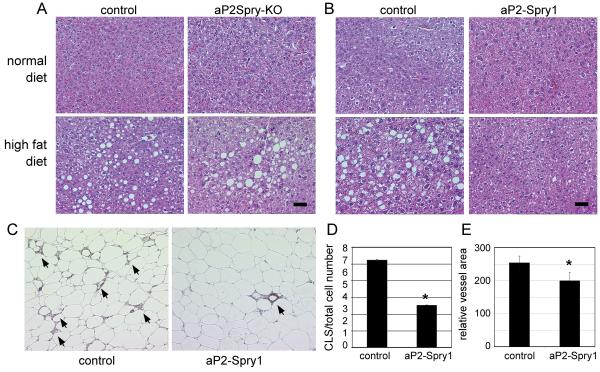
Figure 4. Spry1 expression protects from conditions of fatty liver and inflammation.
(A) Liver tissue samples were collected from the aP2Spry-KO group and respective control on a normal or high fat diet. Histological staining with hematoxylin/eosin shows adipocyte ghost cells, which are remnants of lipid filled adipocytes characteristic of fatty liver condition. Scale bar = 50μm. B) aP2-Spry1 transgenics and single transgenic controls were compared similarly. Scale bar = 50μm. C) Immunohistochemistry using F4/80 antibody to detect macrophage infiltration in abdominal adipose tissue in controls and aP2-Spry1 mice on a high fat diet. Arrows indicate cluster like structure (CLS) formations indicative of macrophage surrounding dead or dying adipocytes. D) CLS were quantified and normalized to total cell numbers for each group indicated. CLS were significantly decreased in aP2-Spry1 adipose tissue (p<0.05). Bars indicate means ± SD. E) Adipose tissue sections were stained for PECAM-1 to quantify the vasculature. aP2-Spry1 transgenic mice had reduced vascular area compared to controls (p<0.05). Bars indicate means ± SD. Asterisks indicates p<0.05.
Spry1 expressing mice have less bone loss on a high fat diet
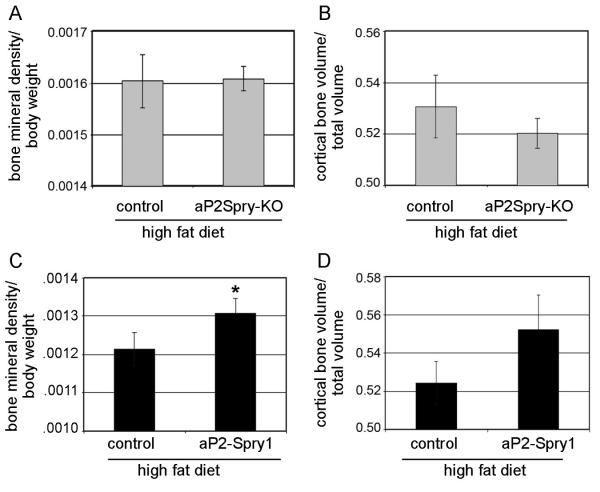
Long-term high fat diet feeding has a negative impact on skeletal remodeling [19,20]. Consistent with previous studies, areal bone mineral density in the high fat diet-fed mice was lower than the corresponding normal diet-fed mice (data not shown). Conditional deletion of Spry1 in the adipose tissue did not influence bone loss, since bone mineral density and bone volume were not significantly different between control and aP2Spry-KO groups on a high fat diet (Fig. 2A-B). However, significant less bone loss (p<0.05) on a high fat diet occurred in the aP2-Spry1 transgenics compared to single transgenics (Fig. 2C). Mice in the aP2Spry1 group retained ~8% higher bone mineral density, with a trend to increased cortical bone volume (Fig. 2D). However, this effect was not seen in the femoral architecture as there was no significant difference in trabecular bone volume or trabecular number between the aP2-Spry1 and corresponding control group (data not shown). These results indicate Spry1 expression in fat influences bone mass positively and reduces bone loss associated with body fat and weight gain typically seen in diet-induced obesity models.
Figure 2. Spry1 expression protects high fat diet induced bone loss.
Bone parameter analysis on 20 week old mice after high fat feeding. (A-B) aP2Spry-KO and corresponding controls had no difference in bone mineral density (A) or cortical bone volume (B). Bone mineral density was quantified by DXA scan and cortical bone volumes were quantified by microCT. The aP2Spry1 transgenic mice (n≥10) lost less bone on high fat diet having higher bone mineral density (C) compared to single transgenics, and a trend towards higher cortical bone volume (D). Bars indicate means ± SD. Asterisk indicates p<0.05.
Spry1 expressing mice are protected from adverse metabolic effects from a high fat diet
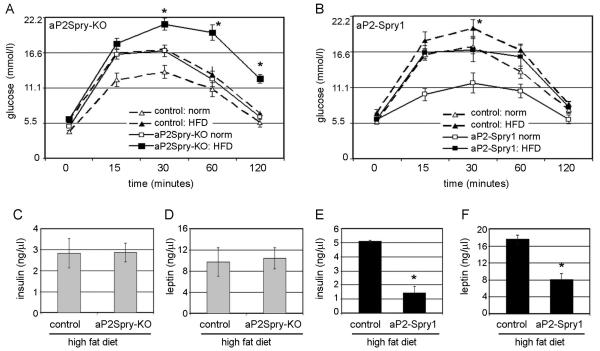
Regardless of the diet, the aP2Spry-KO mice were predisposed to glucose intolerance, showing high blood glucose levels and decreased glucose clearance (Fig. 3A). While the controls developed glucose intolerance following high fat feeding, the condition worsened in aP2Spry-KO mice (p<0.05). In the aP2-Spry1 transgenics, the control mice on the high fat diet alone developed glucose intolerance (p<0.05, Fig. 3B). Interestingly, the glucose clearance in aP2-Spry1 mice on the high fat diet was similar to the control mice on a normal diet (Fig. 3B). Consistent with these findings, blood serum levels of insulin in the aP2Spry-KO (2.83ng/μl) and respective control mice (2.8ng/μl) were elevated (Fig. 3C) compared to mice on normal diet (1.44ng/ul, data not shown). In a pattern similar to insulin, plasma leptin was also elevated in the aP2Spry-KO (10.37ng/μl) and control (9.7ng/μl) groups, both on high fat diet (Fig. 3D). In contrast, in the Spry1 gain-of-function experiment, single transgenic control mice on a high fat diet had markedly elevated insulin plasma levels (5.08ng/μl) that were completely suppressed (70% decrease, p<0.005) in the aP2-Spry1 mice (1.45ng/μl) fed a high fat diet (Fig. 3E). Moreover, leptin was more than two fold higher in the control mice (17.6ng/μl) compared to aP2-Spry1 transgenics (8.11ng/μl) on a high fat diet (p<0.005, Fig. 3F).
Figure 3. Spry1 expression protects from diet induced metabolic abnormalities.
Glucose tolerance test was performed on aP2Spry-KO (A) and aP2Spry1 overexpressing mice (B) fed a normal diet (norm) or high fat diet (HFD) at 20 weeks of age. Blood glucose levels were recorded at 0, 15, 30, 60 and 120 minutes after intraperitoneal injection of glucose to overnight fasted mice (n≥8). All groups underwent blood serum analysis to quantify circulating insulin (C, E) and leptin (D, F) after high fat feeding (n≥8). Bars indicate means ± SD. Asterisks indicates p<0.005.
Spry1 expressing mice on a high fat diet show protective effects on other tissues
Imbalance in energy consumption and combustion results in lipid storage in other organs, most commonly the liver, resulting in fatty liver condition. Histology of the liver following high fat feeding showed excessive deposition of fat indicative of hepatic steatosis in the aP2Spry-KO, their respective controls, and the single transgenic control mice (Fig. 4A-B). However, the aP2-Spry1 overexpressing mice on a high fat diet had no evidence of fatty liver (Fig. 4B). Since severe fatty liver is associated with inflammation, we examined macrophage infiltration in adipose tissue. Immunostaining for macrophages in the abdominal fat tissue of control mice fed a high fat diet showed increased infiltration of macrophages and formation of cluster like structures (CLS) around the dead or dying adipocytes (Fig. 4C). Quantification indicated significantly higher numbers (p<0.05) of CLS in the control single transgenic adipose tissue compared to aP2-Spry1 transgenics after high fat feeding (Fig. 4D). Additionally, quantification of vascularization in the abdominal fat tissue showed significantly lower vessel area (p<0.05) in aP2-Spry1 mice compared to the controls on a high fat diet (Fig. 4E), indicating decrease in angiogenesis in the fat tissue. Spry1 expression reduced vascular density and macrophage infiltration, which are features commonly associated with obesity.
DISCUSSION
This study shows effects of long-term high fat feeding on body composition and metabolism in control or Spry1 conditional targeted or transgenic mice. Previously, we showed that fat tissue-specific expression of Spry1 resulted in reduced body fat, and in this study we further demonstrate that Spry1 expression can prevent fat accumulation even under high fat feeding conditions. Our results confirm the hypothesis that Spry1 expression has long term protective effects on body mass, bone density and metabolic parameters in mice exposed to high dietary fat. In this study, we chose to use the high fat feeding model for inducing obesity as it is an effective and relevant model for the syndrome observed in humans. Indeed, diet induced obesity in mice share many features with human obesity and the resultant metabolic syndrome such as central adiposity, hyperinsulinemia, insulin resistance, and hypertension.
In the high fat diet model, aP2Spry-KO gained more weight and accumulated more body fat compared aP2-Spry1 transgenics. The difference in fat gain demonstrates the localized effect of Spry1 expression in the adipose tissue compartment by repressing adipose tissue accumulation and thereby establish in vivo the protective function of Spry1 expression under conditions of high fat feeding. In growing animal models, high fat diet has deleterious effects on bone mineral status including bone mineral content, structure and mechanical properties [19,20] and leads to increased bone resorption in mice with only modest effects on bone formation [21]. Since osteoblast and adipocytes share a common progenitor, it is somewhat surprising that bone formation is not more significantly altered by high fat feeding in mice although Spry1 play an additional role in lineage allocation of osteogenic progenitors by promoting bone formation as described previously [16]. However, there are several possible mechanisms that may be operative. First, high fat feeding in older mice leads to more marked suppression of bone formation and reduced osteoblast function than in young mice [20]. Second, recent studies have shown that activation of PPAR-γ, which is a critical determinant of cell fate in marrow progenitors, not only causes impaired osteoblast differentiation but also stimulates bone resorption through induction of c-fos [22]. Regardless, Spry1 expression can antagonize the effects of PPAR-γ in mesenchymal stem cells via transcriptional repression brought about by the binding of transcriptional co-activator with PDZ binding motif (TAZ), and thus direct lineage allocation into the osteogenic lineage [16]. Whether, Spry1 also has a direct effect on osteoclast mediated resorption remains to be determined.
Given the strong interactions and influence between bone, fat and brain, it is not surprising that targeted expression of Spry1 in adipose tissue influences bone metabolism especially by the adipokines, cytokines and growth factors. In addition, aP2 promoter is expressed in other cell types including brown adipose tissue, macrophages, part of the brain, peripheral nervous system and skeletal muscle and active in marrow progenitors suggesting that Spry1 may influence marrow stromal cell fate [23,24]. Insulin resistance is implicated in the metabolic syndrome and is tightly interwoven with obesity. Although insulin has been shown to enhance bone formation [26,27], elevated insulin levels in patients with type 2 diabetes paradoxically have increased fracture risk and in some cases lower bone density [25,28]. Likewise, we see insulin negatively correlated with bone volume in the high fat diet-fed aP2Spry-KO, their respective controls, and the controls for the aP2-Spry1, all of which had elevated serum insulin, while in the aP2-Spry1 transgenic mice there was no relationship between insulin levels and bone mineral density. Lower bone volume in the aP2Spry-KO mice can also be attributed to hyperinsulinemia which is associated with increased bone resorption, high levels of serum leptin and insulin. Leptin and adiponectin are heavily influenced by obesity and can adversely affect bone metabolism in a complex manner [29,30]. In our study, leptin negatively correlated with bone mineral density and bone volume/total volume, consistent with previous reports of leptin negatively influencing bone metabolism via a hypothalamic stimulation of the sympathetic nerve system [31-34]. Put together, high fat diet induced bone loss is complex and could involve several factors: 1) lineage allocation of MSCs; 2) RANKL induced bone loss from immune cell activation; 3) bone resorption from activation of PPARγ and 4) direct effects of free fatty acids on bone formation. So Spry1 has an impact on lineage allocation between adipocytes and osteoblasts but also may impact osteoclast activity through antagonism of PPARγ or via macrophage elaboration of cytokines. In addition free fatty acids may regulate both resorption and formation. Thus, Spry1 over-expression would antagonize PPARγ activation of bone resorption and therefore prevent high fat diet-induced bone loss. On the other hand, deletion of Spry1 may have little effect on bone resorption via PPARγ and therefore may cause little change with a high fat diet but could still impact bone mass through non-cell autonomous effects on osteoblasts.
An additional factor is the excessive lipid storage in the liver brought about as a consequence of peripheral resistance to insulin and transport of fatty acids from adipose tissue to the liver leading to the pathogenesis of fatty liver disease [35]. Histology of the liver in the aP2Spry-KO, their respective controls, and single transgenic Spry1 control mice showed excessive deposition of lipid indicative of non-alcoholic fatty liver or liver steatosis. This condition is also related to insulin resistance, type 2 diabetes mellitus and the metabolic syndrome. Spry1 expression prevented liver steatosis, insulin resistence, diabetes and associated metabolic dysfunction, showing a protective role on multiple levels.
Chronic lipid overload associated with intra-abdominal obesity is an inducer of cellular stress and perpetuates the inflammatory cascade. Clinical and animal studies have demonstrated that obesity, insulin resistance and metabolic dysfunction are associated with a low grade chronic inflammation with increased levels of circulating proinflammatory cytokines and adipokines [1,36]. Adipose tissue in general contains a resident population of innate immune cells, particularly macrophages and T lymphocytes [37] . In obese individuals, infiltration of macrophages disrupts adipocyte homeostasis, acts as an additional trigger for the immune response, and contributes to insulin resistance and hepatic steatosis associated with obesity in mice and humans [35,38,39]. In our study, this immune response was repressed in mice expressing Spry1, demonstrated by fewer CLS in abdominal tissue. These data demonstrate that Spry1 expression suppresses fat mass accumulation and thereby suppresses the associated inflammatory response and macrophage infiltration. Consistent with the role of Spry1 as a feedback inhibitor of receptor tyrosine kinase signaling regulating the RAS/MAPK pathway inhibiting angiogenesis [14,40], there was decreased vessel area in the adipose tissue expressing Spry1. Therefore, Spry1 expression in fat tissue has dual consequences, 1) inhibiting adipocyte differentiation mediated by PPARγ inhibition and 2) inhibiting adipose tissue mediated angiogenesis resulting in decreased macrophage infiltration and inflammation in the adipose tissue.
CONCLUSION
In conclusion, our results demonstrate the protective effects of in vivo Spry1 expression in adipose tissue on bone fat and hepatic tissue, even during consumption of a high fat diet. Future studies in animals should explore the role of Spry1 as a possible intervention in diet induced obesity and ovariectomy and/or age-related bone loss.
ACKNOWLEDGEMENTS
We gratefully acknowledge the support of our Histopathology Core (K. Carrier and V. Lindner), Mouse Transgenic Core (A. Harrington), and animal facility personnel. This work was supported by a mentored research council (Maine Medical Center) grant (to S.U.), NIH grants R01HL070865 (to L.L.), AR54604 (to C.J.R.), DK73871 (to R.E. Friesel) and P20RR1555 (PI: R.E. Friesel). The Histopathology Core Facility was supported by P20RR181789 (PI: D. Wojchowski), and the Mouse Transgenic Facility is supported by NIH grant P20RR1555 (PI: R.E. Friesel), both from the National Center for Research Resources.
LIST OF ABBREVIATIONS
- BMD
Bone mineral density
- BV/TV
Bone volume/total volume
- CLS
Cluster like structure
- DXA
Dual energy X-ray absorptiometry
- HFD
High fat diet
- PPAR
Peroxisome proliferator-activated receptor
- Spry1
Sprouty1
- FABP
Fatty acid binding protein
Footnotes
CONFLICT OF INTERESTS There is no conflict of interest.
AUTHORS’ CONTRIBUTION
S.U. conception and design, financial support, acquisition of data, analysis and interpretation of data, manuscript preparation
T.H. acquisition of data
P.L. acquisition of data
C.J.R. revising it critically for intellectual content
L.L. manuscript preparation, financial support, revising it critically for intellectual content and final approval
REFERENCES
- 1.Iyer A, Fairlie DP, Prins JB, Hammock BD, Brown L. Inflammatory lipid mediators in adipocyte function and obesity. Nat Rev Endocrinol. 6:71–82. doi: 10.1038/nrendo.2009.264. [DOI] [PubMed] [Google Scholar]
- 2.von Muhlen D, Safii S, Jassal SK, Svartberg J, Barrett-Connor E. Associations between the metabolic syndrome and bone health in older men and women: the Rancho Bernardo Study. Osteoporos Int. 2007;18:1337–1344. doi: 10.1007/s00198-007-0385-1. [DOI] [PubMed] [Google Scholar]
- 3.Rosen CJ, Klibanski A. Bone, fat, and body composition: evolving concepts in the pathogenesis of osteoporosis. Am J Med. 2009;122:409–414. doi: 10.1016/j.amjmed.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 4.Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM. Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Expr. 2009;19:109–124. doi: 10.1615/critreveukargeneexpr.v19.i2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosen CJ, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol. 2006;2:35–43. doi: 10.1038/ncprheum0070. [DOI] [PubMed] [Google Scholar]
- 6.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 7.Lin YF, Jing W, Wu L, Li XY, Wu Y, Liu L, Tang W, Long J, Tian WD, Mo XM. Identification of osteo-adipo progenitor cells in fat tissue. Cell Prolif. 2008;41:803–812. doi: 10.1111/j.1365-2184.2008.00542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 9.Newell FS, Su H, Tornqvist H, Whitehead JP, Prins JB, Hutley LJ. Characterization of the transcriptional and functional effects of fibroblast growth factor-1 on human preadipocyte differentiation. Faseb J. 2006;20:2615–2617. doi: 10.1096/fj.05-5710fje. [DOI] [PubMed] [Google Scholar]
- 10.Kakudo N, Shimotsuma A, Kusumoto K. Fibroblast growth factor-2 stimulates adipogenic differentiation of human adipose-derived stem cells. Biochem Biophys Res Commun. 2007;359:239–244. doi: 10.1016/j.bbrc.2007.05.070. [DOI] [PubMed] [Google Scholar]
- 11.Wakioka T, Sasaki A, Kato R, Shouda T, Matsumoto A, Miyoshi K, Tsuneoka M, Komiya S, Baron R, Yoshimura A. Spred is a Sprouty-related suppressor of Ras signalling. Nature. 2001;412:647–651. doi: 10.1038/35088082. [DOI] [PubMed] [Google Scholar]
- 12.Kim HJ, Bar-Sagi D. Modulation of signalling by Sprouty: a developing story. Nat Rev Mol Cell Biol. 2004;5:441–450. doi: 10.1038/nrm1400. [DOI] [PubMed] [Google Scholar]
- 13.Guy G, Jackson RA, Yusoff P, Chow SY. Sprouty proteins: modified modulators, matchmakers or missing links? J Endocrinol. 2009 doi: 10.1677/JOE-09-0110. [DOI] [PubMed] [Google Scholar]
- 14.Cabrita MA, Christofori G. Sprouty proteins, masterminds of receptor tyrosine kinase signaling. Angiogenesis. 2008;11:53–62. doi: 10.1007/s10456-008-9089-1. [DOI] [PubMed] [Google Scholar]
- 15.Novakofski J. Adipogenesis: usefulness of in vitro and in vivo experimental models. J Anim Sci. 2004;82:905–915. doi: 10.2527/2004.823905x. [DOI] [PubMed] [Google Scholar]
- 16.Urs S, Venkatesh D, Tang Y, Henderson T, Yang X, Friesel RE, Rosen CJ, Liaw L. Sprouty1 is a critical regulatory switch of mesenchymal stem cell lineage allocation. Faseb J. 24:3264–3273. doi: 10.1096/fj.10-155127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang X, Harkins LK, Zubanova O, Harrington A, Kovalenko D, Nadeau RJ, Chen PY, Toher JL, Lindner V, Liaw L, Friesel R. Overexpression of Spry1 in chondrocytes causes attenuated FGFR ubiquitination and sustained ERK activation resulting in chondrodysplasia. Dev Biol. 2008;321:64–76. doi: 10.1016/j.ydbio.2008.05.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Basson MA, Akbulut S, Watson-Johnson J, Simon R, Carroll TJ, Shakya R, Gross I, Martin GR, Lufkin T, McMahon AP, Wilson PD, Costantini FD, Mason IJ, Licht JD. Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev Cell. 2005;8:229–239. doi: 10.1016/j.devcel.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Lac G, Cavalie H, Ebal E, Michaux O. Effects of a high fat diet on bone of growing rats. Correlations between visceral fat, adiponectin and bone mass density. Lipids Health Dis. 2008;7:16. doi: 10.1186/1476-511X-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao Y, Cui J, Li YX, Shi YH, Le GW. Expression of genes associated with bone resorption is increased and bone formation is decreased in mice fed a high-fat diet. Lipids. 45:345–355. doi: 10.1007/s11745-010-3397-0. [DOI] [PubMed] [Google Scholar]
- 21.Cao JJ, Gregoire BR, Gao H. High-fat diet decreases cancellous bone mass but has no effect on cortical bone mass in the tibia in mice. Bone. 2009;44:1097–1104. doi: 10.1016/j.bone.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 22.Wei W, Wang X, Yang M, Smith LC, Dechow PC, Sonoda J, Evans RM, Wan Y. PGC1beta mediates PPARgamma activation of osteoclastogenesis and rosiglitazone-induced bone loss. Cell Metab. 11:503–516. doi: 10.1016/j.cmet.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martens K, Bottelbergs A, Baes M. Ectopic recombination in the central and peripheral nervous system by aP2/FABP4-Cre mice: implications for metabolism research. FEBS Lett. 584:1054–1058. doi: 10.1016/j.febslet.2010.01.061. [DOI] [PubMed] [Google Scholar]
- 24.Urs S, Harrington A, Liaw L, Small D. Selective expression of an aP2/Fatty Acid Binding Protein 4-Cre transgene in non-adipogenic tissues during embryonic development. Transgenic Res. 2006;15:647–653. doi: 10.1007/s11248-006-9000-z. [DOI] [PubMed] [Google Scholar]
- 25.Patsch JM, Kiefer FW, Varga P, Pail P, Rauner M, Stupphann D, Resch H, Moser D, Zysset PK, Stulnig TM, Pietschmann P. Increased bone resorption and impaired bone microarchitecture in short-term and extended high-fat diet-induced obesity. Metabolism. 60:243–249. doi: 10.1016/j.metabol.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thrailkill KM, Liu L, Wahl EC, Bunn RC, Perrien DS, Cockrell GE, Skinner RA, Hogue WR, Carver AA, Fowlkes JL, Aronson J, Lumpkin CK., Jr. Bone formation is impaired in a model of type 1 diabetes. Diabetes. 2005;54:2875–2881. doi: 10.2337/diabetes.54.10.2875. [DOI] [PubMed] [Google Scholar]
- 27.Thrailkill KM, Lumpkin CK, Jr., Bunn RC, Kemp SF, Fowlkes JL. Is insulin an anabolic agent in bone? Dissecting the diabetic bone for clues. Am J Physiol Endocrinol Metab. 2005;289:E735–745. doi: 10.1152/ajpendo.00159.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, Ducy P, Karsenty G. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 142:296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams GA, Wang Y, Callon KE, Watson M, Lin JM, Lam JB, Costa JL, Orpe A, Broom N, Naot D, Reid IR, Cornish J. In vitro and in vivo effects of adiponectin on bone. Endocrinology. 2009;150:3603–3610. doi: 10.1210/en.2008-1639. [DOI] [PubMed] [Google Scholar]
- 30.Karsenty G, Oury F. The central regulation of bone mass, the first link between bone remodeling and energy metabolism. J Clin Endocrinol Metab. 95:4795–4801. doi: 10.1210/jc.2010-1030. [DOI] [PubMed] [Google Scholar]
- 31.Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 32.Elefteriou F, Takeda S, Ebihara K, Magre J, Patano N, Kim CA, Ogawa Y, Liu X, Ware SM, Craigen WJ, Robert JJ, Vinson C, Nakao K, Capeau J, Karsenty G. Serum leptin level is a regulator of bone mass. Proc Natl Acad Sci U S A. 2004;101:3258–3263. doi: 10.1073/pnas.0308744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karsenty G. Convergence between bone and energy homeostases: leptin regulation of bone mass. Cell Metab. 2006;4:341–348. doi: 10.1016/j.cmet.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 34.Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 35.Alkhouri N, Gornicka A, Berk MP, Thapaliya S, Dixon LJ, Kashyap S, Schauer PR, Feldstein AE. Adipocyte apoptosis, a link between obesity, insulin resistance, and hepatic steatosis. J Biol Chem. 285:3428–3438. doi: 10.1074/jbc.M109.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herrero L, Shapiro H, Nayer A, Lee J, Shoelson SE. Inflammation and adipose tissue macrophages in lipodystrophic mice. Proc Natl Acad Sci U S A. 107:240–245. doi: 10.1073/pnas.0905310107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suganami T, Ogawa Y. Adipose tissue macrophages: their role in adipose tissue remodeling. J Leukoc Biol. 88:33–39. doi: 10.1189/jlb.0210072. [DOI] [PubMed] [Google Scholar]
- 38.Murano I, Barbatelli G, Parisani V, Latini C, Muzzonigro G, Castellucci M, Cinti S. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J Lipid Res. 2008;49:1562–1568. doi: 10.1194/jlr.M800019-JLR200. [DOI] [PubMed] [Google Scholar]
- 39.Altintas MM, Azad A, Nayer B, Contreras G, Zaias J, Faul C, Reiser J, Nayer A. Mast cells, macrophages, and crown-like structures distinguish subcutaneous from visceral fat in mice. J Lipid Res. 52:480–488. doi: 10.1194/jlr.M011338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee S, Bui Nguyen TM, Kovalenko D, Adhikari N, Grindle S, Polster SP, Friesel R, Ramakrishnan S, Hall JL. Sprouty1 inhibits angiogenesis in association with up-regulation of p21 and p27. Mol Cell Biochem. 338:255–261. doi: 10.1007/s11010-009-0359-z. [DOI] [PMC free article] [PubMed] [Google Scholar]